Sustainable Co-Production of Xylanase, Cellulase, and Pectinase through Agroindustrial Residue Valorization Using Solid-State Fermentation: A Techno-Economic Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Inoculum Preparation
2.2. Packed-Bed Column Bioreactor Design
2.3. Packed-Bed Column Bioreactor Instrumentation
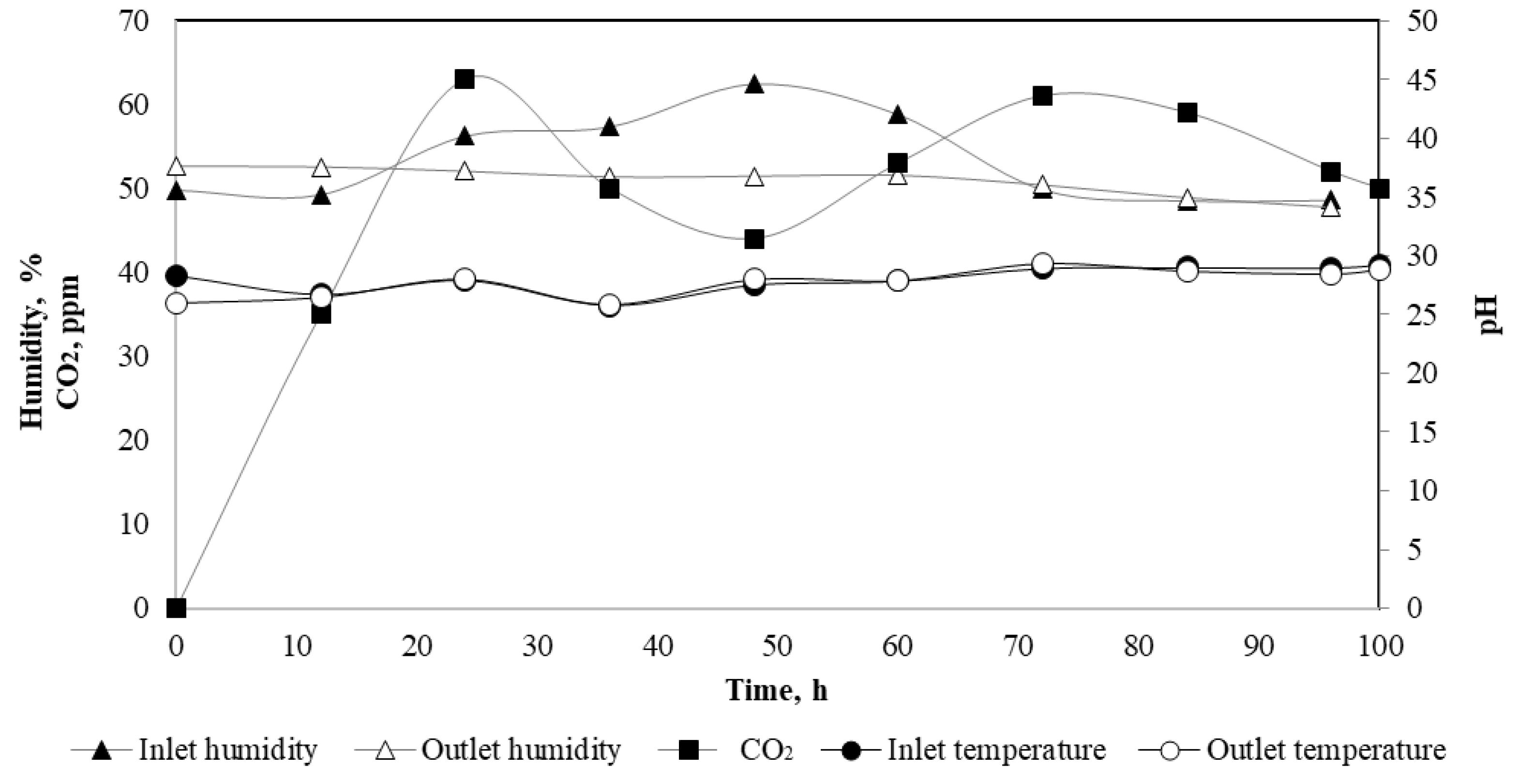
2.4. Cultivation Conditions in a Packed-Bed Column
2.5. Simulation Description
2.6. Process Description
- Scenario I. ECEX (U mL−1): X = 582.39, P = 22.86, and C = 26.10. Optimum conditions to enhance xylanase activity, using a BSG/AP ratio of 81/19 and an initial humidity of 50%.
- Scenario II. ECEP (U mL−1): X = 135.46, P = 61.73, and C = 10.54. Optimum conditions to enhance pectinase, using a BSG/AP ratio of 72/28 and an initial humidity of 66%.
- Scenario III. ECEC (U mL−1): X = 0, P = 1.01, and C = 69.90. Optimum conditions to enhance cellulase, using a BSG/AP ratio of 88/12 and an initial humidity of 66%.
2.7. Techno-Economic Assessment
2.8. Sensitivity Analysis
- BSG cost: USD 19, USD 28, and USD 36 per ton.
- AP cost: USD 70, USD 100, and USD 130 per ton.
- Inoculum media cost: USD 5, USD 30, and USD 60 per kilogram.
- Selling price of cellulase: USD 20, USD 45, USD 55, and USD 80 per 1000 units.
- Selling price of pectinase: USD 11, USD 15, and USD 38 per 1000 units.
- Selling price of xylanase: USD 10, USD 12, USD 24, and USD 30 per 1000 units.
3. Results
3.1. Hydrolytic Enzyme Production in a Packed-Bed Column
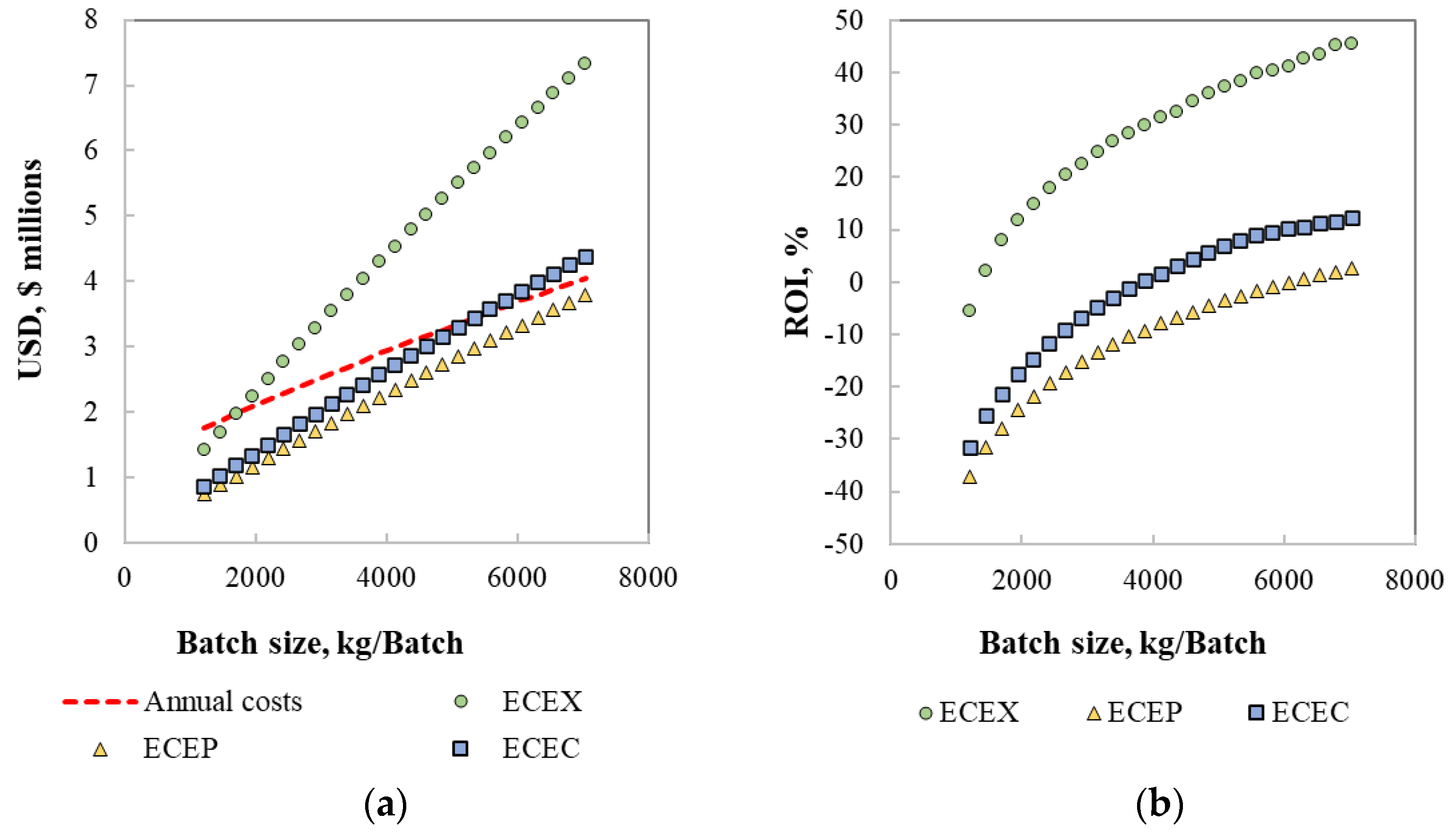
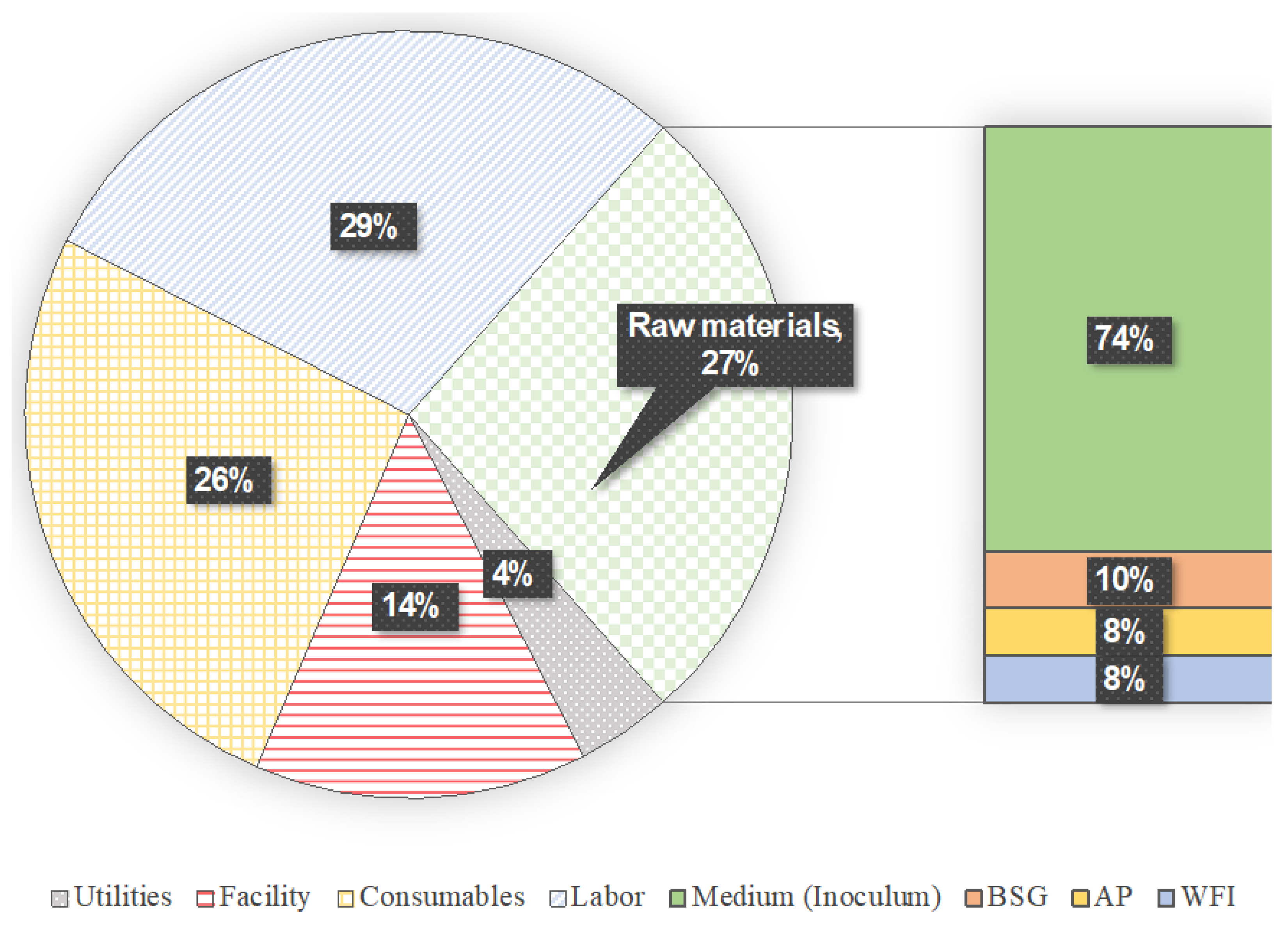
3.2. Techno-Economic Assessment
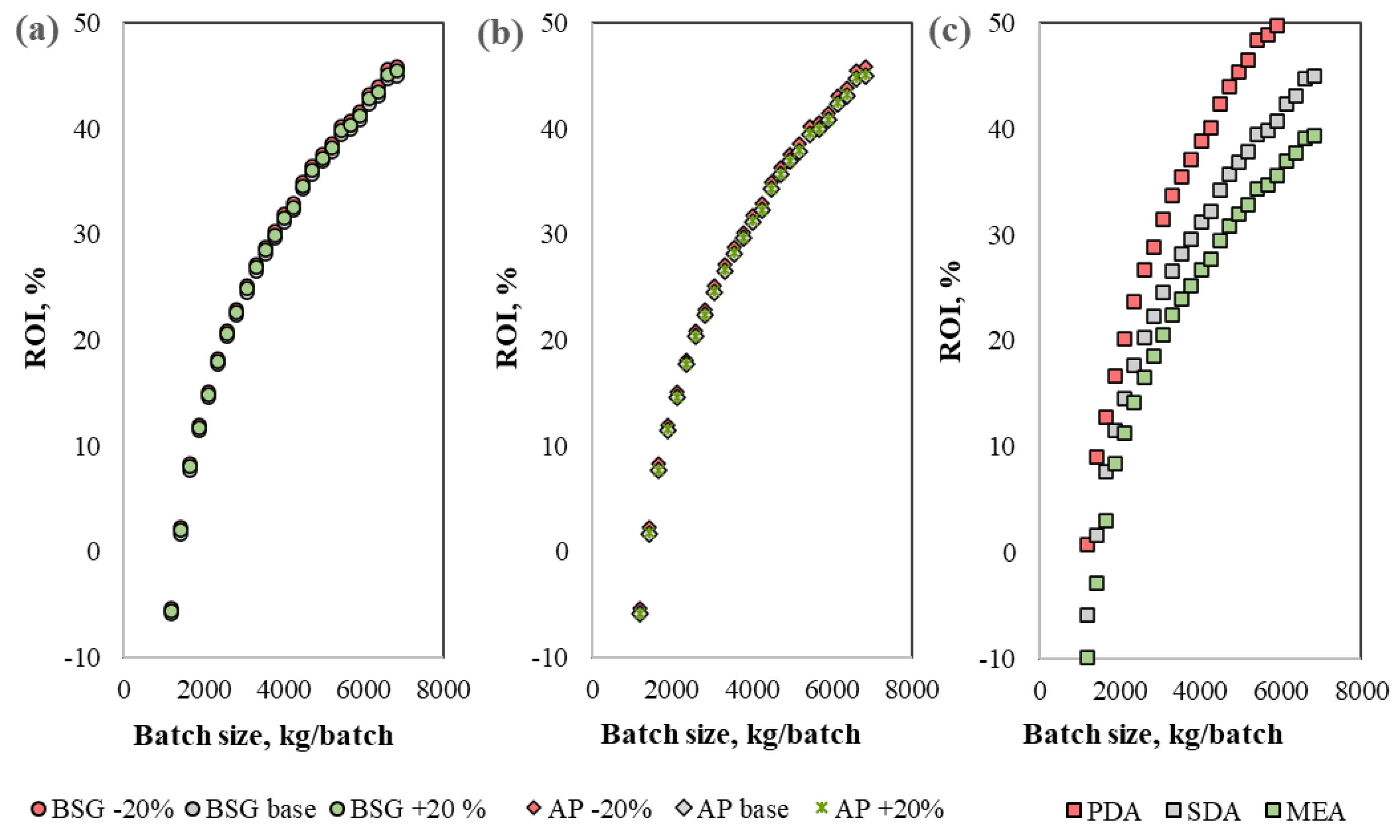
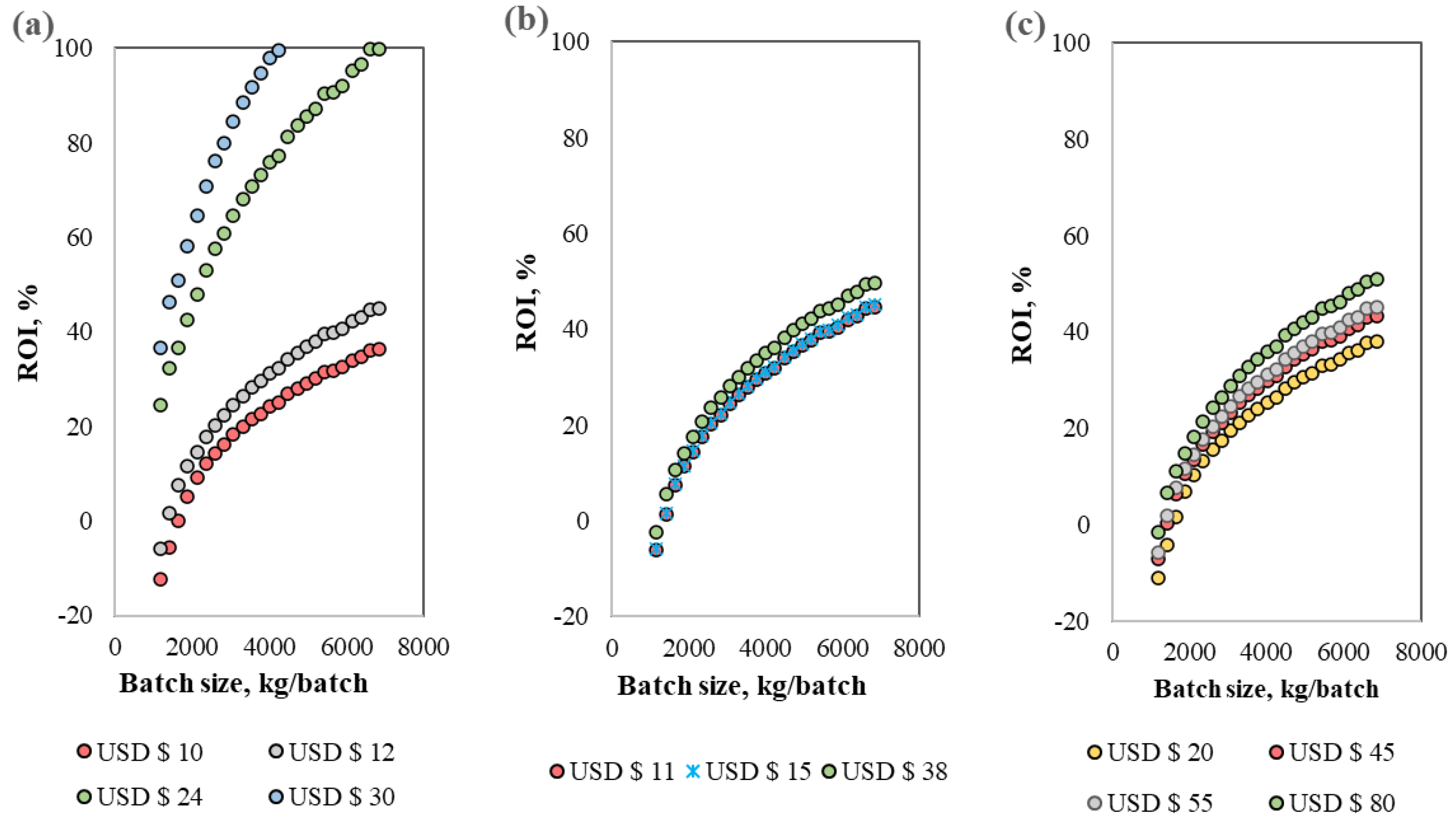
3.3. Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pellis, A.; Cantone, S.; Ebert, C.; Gardossi, L. Evolving Biocatalysis to Meet Bioeconomy Challenges and Opportunities. New Biotechnol. 2018, 40, 154–169. [Google Scholar] [CrossRef]
- Nezhad, N.G.; Rahman, R.N.Z.R.A.; Yahaya, N.M.; Oslan, S.N.; Shariff, F.M.; Leow, T.C. Recent Advances in Simultaneous Thermostability-Activity Improvement of Industrial Enzymes through Structure Modification. Int. J. Biol. Macromol. 2023, 232, 123440. [Google Scholar] [CrossRef]
- Tarafdar, A.; Sirohi, R.; Gaur, V.K.; Kumar, S.; Sharma, P.; Varjani, S.; Pandey, H.O.; Sindhu, R.; Madhavan, A.; Rajasekharan, R.; et al. Engineering Interventions in Enzyme Production: Lab to Industrial Scale. Bioresour. Technol. 2021, 326, 124771. [Google Scholar] [CrossRef]
- Ergun, S.O.; Urek, R.O. Production of Ligninolytic Enzymes by Solid State Fermentation Using Pleurotus Ostreatus. Ann. Agrar. Sci. 2017, 15, 273–277. [Google Scholar] [CrossRef]
- Sosa-Martínez, J.D.; Montañez, J.; Contreras-Esquivel, J.C.; Balagurusamy, N.; Gadi, S.K.; Morales-Oyervides, L. Agroindustrial and Food Processing Residues Valorization for Solid-State Fermentation Processes: A Case for Optimizing the Co-Production of Hydrolytic Enzymes. J. Environ. Manag. 2023, 347, 119067. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Chio, C.; Khatiwada, J.R.; Kognou, A.L.M.; Qin, W. Formulation of the Agro-Waste Mixture for Multi-Enzyme (Pectinase, Xylanase, and Cellulase) Production by Mixture Design Method Exploiting Streptomyces sp. Bioresour. Technol. Rep. 2022, 19, 101142. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalysilic Acid Reagent for Determination of Reducing Sugar. Anal. Chem 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Ferreira, R.D.G.; Azzoni, A.R.; Freitas, S. Techno-Economic Analysis of the Industrial Production of a Low-Cost Enzyme Using E. Coli: The Case of Recombinant β-Glucosidase. Biotechnol. Biofuels 2018, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.G.; Azzoni, A.R.; Freitas, S. On the Production Cost of Lignocellulose-Degrading Enzymes. Biofuels Bioprod. Biorefin. 2021, 15, 85–99. [Google Scholar] [CrossRef]
- Villegas-Méndez, M.Á.; Montañez, J.; Contreras-Esquivel, J.C.; Salmerón, I.; Koutinas, A.; Morales-Oyervides, L. Coproduction of Microbial Oil and Carotenoids within the Circular Bioeconomy Concept: A Sequential Solid-State and Submerged Fermentation Approach. Fermentation 2022, 8, 258. [Google Scholar] [CrossRef]
- Coral-Velasco, D.A.; Correa, L.F.; Sánchez, Ó.J.; Gómez, J.A. Process Design and Techno-Economic Assessment of Cellulolytic Enzymes Production from Coffee Husk through Process Simulation. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Taiwo, A.E.; Tom-James, A.; Falowo, O.A.; Okoji, A.; Adeyi, O.; Olalere, A.O.; Eloka-Eboka, A. Techno-Economic Analysis of Cellulase Production by Trichoderma Reesei in Submerged Fermentation Processes Using a Process Simulator. S. Afr. J. Chem. Eng. 2022, 42, 98–105. [Google Scholar] [CrossRef]
- Jovanovic, M.; Vucorovic, D.; Dodic, S.; Bajic, B.; Dodic, J.; Vlajkov, V.; Jevit-Mumibacic, R. Simulation Model Comparison of Submerged and Solid-State Hydrolytic Enzymes Production from Wheat Chaff. Rom. Biotechnol. Lett. 2020, 25, 1938–1948. [Google Scholar] [CrossRef]
- Amraini, S.Z.; Surya, E.A.; Limoes, S.; Setyahadi, S.; Abd-Aziz, S.; Gozan, M. Techno-Economic Analysis of Recombinant Endo-β-1,4-Glucanase Production from Escherichia Coli Eg-RK2 Culture Using Oil Palm Empty Fruit Bunch. IOP Conf. Ser. Earth Environ. Sci. 2021, 926, 012070. [Google Scholar] [CrossRef]
- Hafid, H.S.; Baharuddin, A.S.; Mokhtar, M.N.; Omar, F.N.; Mohammed, M.A.P.; Wakisaka, M. Enhanced Laccase Production for Oil Palm Biomass Delignification Using Biological Pretreatment and Its Estimation at Biorefinary Scale. Biomass Bioenergy 2021, 144, 105904. [Google Scholar] [CrossRef]
- Noman, E.; Al-Gheethi, A.A.; Talip, B.A.; Mohamed, R.; Kassim, A.H. Oxidative Enzymes from Newly Local Strain Aspergillus Iizukae EAN605 Using Pumpkin Peels as a Production Substrate: Optimized Production, Characterization, Application and Techno-Economic Analysis. J. Hazard. Mater. 2020, 386, 121954. [Google Scholar] [CrossRef]
- de Lima, E.A.; Mandelli, F.; Kolling, D.; Matsusato Souza, J.; de Oliveira Filho, C.A.; Ribeiro da Silva, M.; Lobo de Mesquita Sampaio, I.; Lopes Junqueira, T.; Ferreira Chagas, M.; Teodoro, J.C.; et al. Development of an Economically Competitive Trichoderma-Based Platform for Enzyme Production: Bioprocess Optimization, Pilot Plant Scale-up, Techno-Economic Analysis and Life Cycle Assessment. Bioresour. Technol. 2022, 364, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; O’Keefe, S.F.; Stewart, A.C.; Neilson, A.P.; Kim, Y.T.; Huang, H. Techno-Economic Analysis of a Grape Pomace Biorefinery: Production of Seed Oil, Polyphenols, and Biochar. Food Bioprod. Process. 2021, 127, 139–151. [Google Scholar] [CrossRef]
- Khootama, A.; Putri, D.N.; Hermansyah, H. Techno-Economic Analysis of Lipase Enzyme Production from Aspergillus Niger Using Agro-Industrial Waste by Solid State Fermentation. Energy Procedia 2018, 153, 143–148. [Google Scholar] [CrossRef]
- De Castro, A.M.H.; Carvalho, D.F.; Freire, D.M.G.; Castilho, L.D.R. Economic Analysis of the Production of Amylases and Other Hydrolases by Aspergillus Awamori in Solid-State Fermentation of Babassu Cake. Enzyme Res. 2010, 2010, 576872. [Google Scholar] [CrossRef]
- Gómez-Corona, C.; Escalona-Buendía, H.B.; García, M.; Chollet, S.; Valentin, D. Craft vs. Industrial: Habits, Attitudes and Motivations towards Beer Consumption in Mexico. Appetite 2016, 96, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Naibaho, J.; Korzeniowska, M. The Variability of Physico-Chemical Properties of Brewery Spent Grain from 8 Different Breweries. Heliyon 2021, 7, e06583. [Google Scholar] [CrossRef] [PubMed]
- Le Féon, S.; Benezech, T.; Yannou-Le Bris, G.; Aubin, J.; Sampers, I.; Herreman, D.; Pénicaud, C. Life Cycle Assessment of a Small-Scale and Low-Input Organic Apple Value Chain Including Fresh Fruit, Juice and Applesauce. Clean. Environ. Syst. 2023, 11, 100141. [Google Scholar] [CrossRef]
- Ramos-Aguilar, A.L.; Victoria-Campos, C.I.; Ochoa-Reyes, E.; Ornelas-Paz, J.d.J.; Zamudio-Flores, P.B.; Rios-Velasco, C.; Reyes-Hernández, J.; Pérez-Martínez, J.D.; Ibarra-Junquera, V. Physicochemical Properties of Apple Juice during Sequential Steps of the Industrial Processing and Functional Properties of Pectin Fractions from the Generated Pomace. LWT 2017, 86, 465–472. [Google Scholar] [CrossRef]
- Perez, C.L.; Casciatori, F.P.; Thoméo, J.C. Strategies for Scaling-up Packed-Bed Bioreactors for Solid-State Fermentation: The Case of Cellulolytic Enzymes Production by a Thermophilic Fungus. Chem. Eng. J. 2019, 361, 1142–1151. [Google Scholar] [CrossRef]




| Project Indices | Scenario Xylanase | Scenario Pectinase | Scenario Cellulase |
|---|---|---|---|
| Enzyme production, U/mL | X = 582.39 P = 22.86 C = 26.10 | X = 135.46 P = 61.73 C = 10.54 | X = 0 P = 1.01 C = 69.90 |
| Investment, USD | 4,523,678 | 4,425,109 | 4,481,762 |
| Annual operating cost, USD/yr | 3,365,109 | 3,357,599 | 3,316,886 |
| Unit production cost, USD/kg | 2.25 | 2.31 | 2.30 |
| Unit production revenue, USD/kg | 3.72 | 1.92 | 2.22 |
| Annual revenues, USD/yr | 5,544,254 | 2,800,335 | 3,201,315 |
| Gross margin, % | 39.90 | −19.90 | −3.61 |
| ROI, % | 37.59 | −3.92 | 6.10 |
| Payback time, yr | 2.66 | N/A | 16.39 |
| IRR (After tax) | 26.80 | N/A | N/A |
| NPV at 7.00%, USD | 7,325,537 | −5,791,384 | −2,813,314 |
| Enzyme | Microorganism | Biowaste/Carbon Source | Production Scale/Plant Capacity | Economic Index/Product Cost | Sensitivity Analysis | Reference |
|---|---|---|---|---|---|---|
| Cellulase | * Trichoderma reesei | Coffee husk | 1893 tons/year and 5.7 tons/batch (processed biowaste); 20,000 L (fermenter). | PBT: 2.27 years ROI: 44.08% NPV: USD 32,958,000 Cost: 28.68 USD/kg Selling price: 42 USD/kg | - | [11] |
| Cellulase | Trichoderma reesei | Glucose | 12,785.97 tons/year (processed biowaste). | PBT: 1.88 years IRR: 36.95% NPV: USD 140,328,000 Cost: 20.71 USD/kg | Aeration rate Yield Energy demand Productivity | [12] |
| Amylase Cellulase Xylanase | Trichoderma reesei | Wheat chaff | 375 tons/batch (processed biowaste); 2803 m3 (fermenter). | PBT: 2.75 years IRR: 27.93% NPV: USD 29,895,000 | - | [13] |
| Endo-β-1,4-Glucanase | * Escherichia coli | Oil palm empty fruit bunch | 8.5 tons/batch (processed biowaste); 82.27 m3 (fermenter). | PBT: 3.29 years IRR: 31.64% NPV: USD 32,121,000 Selling price: 250 USD/kg | Raw material cost Cellulase price Labor cost Overall operating cost | [14] |
| Lacasse | Pycnoporus sanguineus | Oil palm empty fruit bunch | 50 tons/batch. | ROI: 28.70% NPV: USD 2.86 million Cost: 14.256 USD/kg | Raw materials Direct-fixed capital | [15] |
| Laccase Manganese Peroxidase Lignin peroxidase | Aspergillus iizukae | Pumpkin peels | 66.6 kg/batch (processed biowaste); 2.7 m3/batch (fermenter). | IRR: 50% Cost: 0.107 USD/cm3 Selling price: 1 USD/cm3 | - | [16] |
| β-glucosidase β-xylo- sidase, endo-β-1,4-glucanase Endo-β-1,4-xylanase | * Trichoderma reesei | Sugarcane molasses | 300 m3 (fermenter). | Cost: 2.86 to 3.59 USD/kg protein. Selling price: 4.24 USD/kg | - | [17] |
| Cellulase Pectinase Xylanase | Aspergillus sp. | Brewery spent grain/apple pomace | 6825 kg/residue per batch 8 m3 (fermenter). | PBT: 2.2 years ROI: 45% IRR: 26.80% NPV: USD 7,325,537.00 Cost: 2.25 USD/kg | Raw material cost Inoculum medium cost Selling price | This work |
| Quantity | Name | Description | Unit Cost, USD | Cost, USD |
|---|---|---|---|---|
| 1 | MB1 | Blending tank vessel (1227.39 L) | 14,000 | 14,000 |
| 1 | HS1 | Heat sterilizer (276.16 L/h) | 3000 | 3000 |
| 2 | TD1 | Tray dryer (281.57 m2) | 10,000 | 20,000 |
| 1 | G1 | Grinder (4050.00 kg/h) | 27,000 | 27,000 |
| 1 | TD2 | Tray dryer (69.36 m2) | 5000 | 5000 |
| 1 | S2 | Solids drum (1.40 m3) | 1000 | 1000 |
| 1 | S1 | Solids drum (5.97 m3) | 1000 | 1000 |
| 20 | SSF1 | Packed-bed column (8.03 m3) | 36,000 | 720,000 |
| 3 | S3 | Solids tote (1.7 m3) | 3000 | 9000 |
| 1 | L1 | Solids mixer (4.40 m3/h) | 16,000 | 16,000 |
| 2 | C1 | Centrifuge (1.74 m3/h) | 6000 | 12,000 |
| 1 | DEF1 | Dead-end filter (42.72 m2) | 1000 | 1000 |
| 2 | DF1 | Diafilter (60.06 m2) | 11,000 | 22,000 |
| 20 | GC1 | Centrifugal compressor (1.79 kW) | 2000 | 40,000 |
| 20 | AF2 | Air filter (15.34 m3/h) | 1000 | 20,000 |
| TOTAL | 1,126,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosa-Martínez, J.D.; Morales-Oyervides, L.; Montañez, J.; Contreras-Esquivel, J.C.; Balagurusamy, N.; Gadi, S.K.; Salmerón, I. Sustainable Co-Production of Xylanase, Cellulase, and Pectinase through Agroindustrial Residue Valorization Using Solid-State Fermentation: A Techno-Economic Assessment. Sustainability 2024, 16, 1564. https://doi.org/10.3390/su16041564
Sosa-Martínez JD, Morales-Oyervides L, Montañez J, Contreras-Esquivel JC, Balagurusamy N, Gadi SK, Salmerón I. Sustainable Co-Production of Xylanase, Cellulase, and Pectinase through Agroindustrial Residue Valorization Using Solid-State Fermentation: A Techno-Economic Assessment. Sustainability. 2024; 16(4):1564. https://doi.org/10.3390/su16041564
Chicago/Turabian StyleSosa-Martínez, Jazel Doménica, Lourdes Morales-Oyervides, Julio Montañez, Juan Carlos Contreras-Esquivel, Nagamani Balagurusamy, Suresh Kumar Gadi, and Ivan Salmerón. 2024. "Sustainable Co-Production of Xylanase, Cellulase, and Pectinase through Agroindustrial Residue Valorization Using Solid-State Fermentation: A Techno-Economic Assessment" Sustainability 16, no. 4: 1564. https://doi.org/10.3390/su16041564
APA StyleSosa-Martínez, J. D., Morales-Oyervides, L., Montañez, J., Contreras-Esquivel, J. C., Balagurusamy, N., Gadi, S. K., & Salmerón, I. (2024). Sustainable Co-Production of Xylanase, Cellulase, and Pectinase through Agroindustrial Residue Valorization Using Solid-State Fermentation: A Techno-Economic Assessment. Sustainability, 16(4), 1564. https://doi.org/10.3390/su16041564









