Synthesis and Accumulation of Phytocompounds in Field-, Tissue-Culture Grown (Stress) Root Tissues and Simultaneous Defense Response Activity in Glycyrrhiza glabra L.
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Culture Establishment Conditions
2.2. Callus Induction and Proliferation
2.3. Axillary Shoot Sprouting and Growth
2.4. Root of Axillary Shoots
2.5. Preparation of Extracts
2.6. GC–MS Analyses
2.7. Biochemical Analyses
2.7.1. Estimation of Total Phenolic Content
2.7.2. Estimation of Total Flavonoid Content (TFC)
2.7.3. Determination of Free Radical Scavenging Activity by DPPH Assay
2.7.4. Determination of Peroxidase (POD; EC: 1.11.1.7) Activity
2.7.5. Determination of Superoxide Dismutase (SOD; EC: 1.15.1.1) Activity
2.8. Statistical Analysis
3. Results
3.1. Callus Induction and Proliferation
3.2. Axillary Shoot Sprouting and Growth
3.3. Rooting of Axillary Shoots
3.4. GC–MS Analysis
3.5. Total Phenolic Content (TPC), Total Flavonoid Content (TFC) and DPPH Scavenging Activity
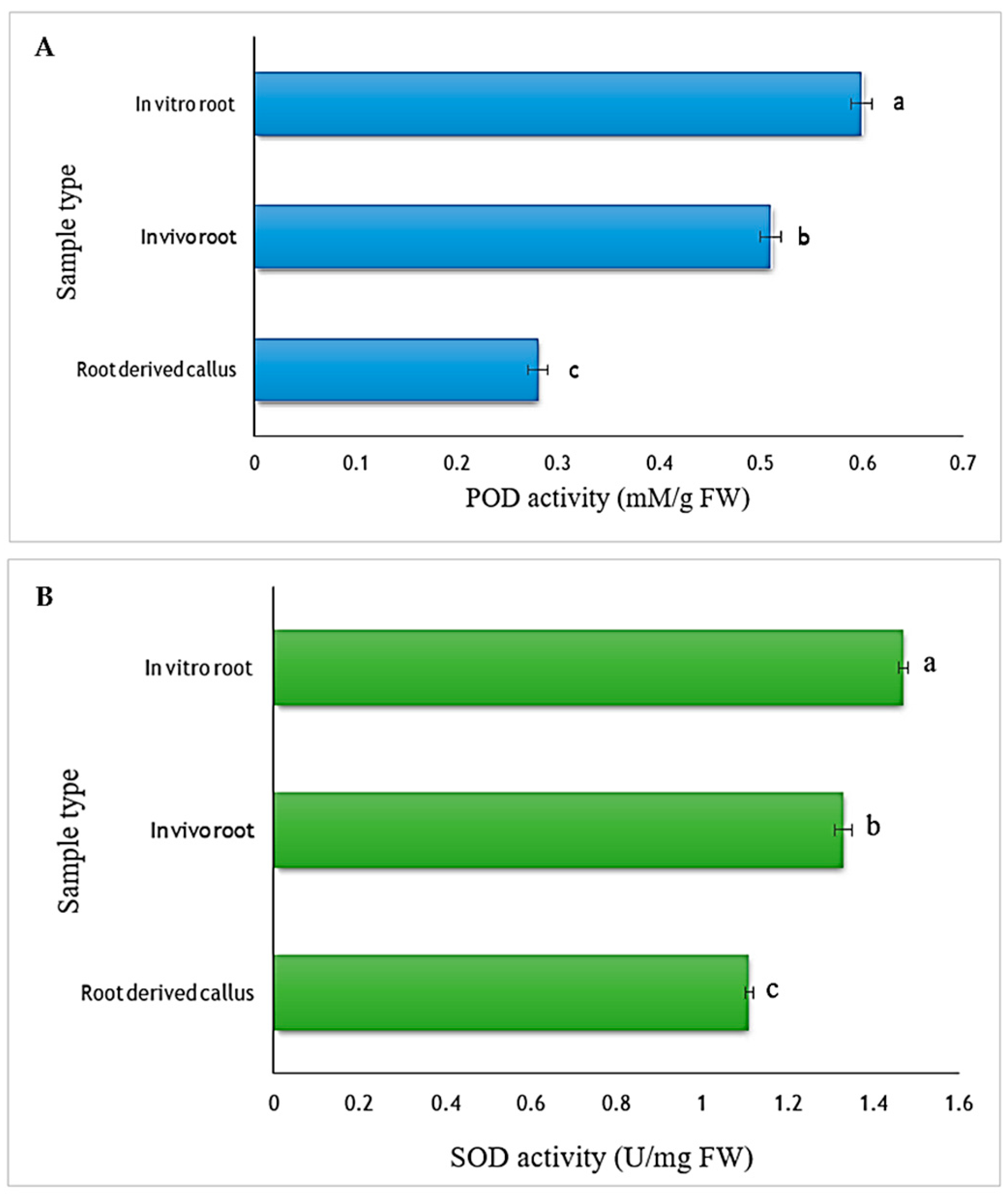
3.6. Antioxidant Enzyme Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, K.; Singh, N. Factors influencing in vitro plant regeneration of liquorice (Glycyrrhiza glabra L.). Iran. J. Biotechnol. 2012, 10, 161–167. [Google Scholar]
- Akhtar, R.; Shahzad, A. Alginate Encapsulation in Glycyrrhiza glabra L. with Phyto-Chemical Profiling of Root Extracts of In Vitro Converted Plants Using GC-MS Analysis. Asian Pac. J. Trop. Biomed. 2017, 7, 855–861. [Google Scholar] [CrossRef]
- Khan, S.; Pandotra, P.; Manzoor, M.M.; Kushwaha, M.; Sharma, R.; Jain, S.; Ahuja, A.; Amancha, V.; Bhushan, S.; Guru, S.K.; et al. Terpenoid and Flavonoid Spectrum of In Vitro Cultures of Glycyrrhiza glabra Revealed High Chemical Heterogeneity: Platform to Understand Biosynthesis. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 124, 507–516. [Google Scholar] [CrossRef]
- Srivastava, M.; Singh, G.; Sharma, S.; Shukla, S.; Misra, P. Elicitation Enhanced the Yield of Glycyrrhizin and Antioxidant Activities in Hairy Root Cultures of Glycyrrhiza glabra L. J. Plant Growth Regul. 2019, 38, 373–384. [Google Scholar] [CrossRef]
- Jaiswal, N.; Verma, Y.; Misra, P. High Frequency In Vitro Callogenesis and Plant Regeneration of Glycyrrhiza glabra L. Vegetos 2021, 34, 495–504. [Google Scholar] [CrossRef]
- Shaheen, A.; Ali, M.; Ahmad, N.; Dewir, Y.H.; El-Hendawy, S.; Abd-El Gawad, A.M. Micropropagation of licorice (Glycyrrhiza glabra L.) by using intermediate nodal explants. Chil. J. Agric. Res. 2020, 80, 326–333. [Google Scholar] [CrossRef]
- Bansal, Y.; Mujib, A.; Siddiqui, Z.H.; Mamgain, J.; Syeed, R.; Ejaz, B. Ploidy Status, Nuclear DNA Content and Start Codon Targeted (SCoT) Genetic Homogeneity Assessment in Digitalis purpurea L., Regenerated In Vitro. Genes 2022, 13, 2335. [Google Scholar] [CrossRef]
- Mujib, A.; Ali, M.; Isah, T. Somatic Embryo Mediated Mass Production of Catharanthus roseus in Culture Vessel (Bioreactor)—A Comparative Study. Saudi J. Biol. Sci. 2014, 21, 442–449. [Google Scholar] [CrossRef]
- Malik, M.; Wachol, M.; Pawlowska, B. Liquid Culture Systems Affect Morphological and Biochemical Parameters during Rosa canina Plantlets In Vitro Production. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 58–64. [Google Scholar] [CrossRef]
- Khan, H.; Khan, T.; Ahmad, N.; Zaman, G.; Khan, T.; Ahmad, W.; Batool, S.; Hussain, Z.; Drouet, S.; Hano, C.; et al. Chemical Elicitors-Induced Variation in Cellular Biomass, Biosynthesis of Secondary Cell Products, and Antioxidant System in Callus Cultures of Fagonia indica. Molecules 2021, 26, 6340. [Google Scholar] [CrossRef]
- Mamgain, J.; Mujib, A.; Syeed, R.; Ejaz, B.; Malik, M.Q.; Bansal, Y. Genome Size and Gas Chromatography-Mass Spectrometry (GC–MS) Analysis of Field-Grown and In Vitro Regenerated Pluchea lanceolata Plants. J. Appl. Genet. 2023, 64, 1–21. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ahmad, N.; Anis, M.; Alatar, A.A. Influence of Meta-Topolin on In Vitro Organogenesis in Tecoma stans L., Assessment of Genetic Fidelity and Phytochemical Profiling of Wild and Regenerated Plants. Plant Cell Tissue Organ Cult. 2019, 138, 339–351. [Google Scholar] [CrossRef]
- Khan, A.; Shah, A.H.; Ali, N. In-Vitro Propagation and Phytochemical Profiling of a Highly Medicinal and Endemic Plant Species of the Himalayan Region (Saussurea costus). Sci. Rep. 2021, 11, 23575. [Google Scholar] [CrossRef]
- Moghbel, N.; Borujeni, M.K.; Bernard, F. Colchicine Effect on the DNA Content and Stomata Size of Glycyrrhiza glabra var. Glandulifera and Carthamus tinctorius L. Cultured In Vitro. J. Genet. Eng. Biotechnol. 2015, 13, 1–6. [Google Scholar] [CrossRef]
- Badkhane, Y.; Yadav, A.S.; Bajaj, A. effect of explant sources and different concentrations of plant growth regulators on in vitro micropropagation of Glycyrrhiza glabra L. Indo Am. J. Pharma Res. 2016, 6, 5830–5840. [Google Scholar]
- Tahoori, F.; Majd, A.; Nejadsattari, T.; Ofoghi, H.; Iranbakhsh, A. Qualitative and Quantitative Study of Quercetin and Glycyrrhizin in In Vitro Culture of Liquorice (Glycyrrhiza glabra L.) and Elicitation with AgNO3. Not. Bot. Horti Agrobot. Cluj Napoca 2018, 47, 143–151. [Google Scholar] [CrossRef]
- Sathish, D.; Vasudevan, V.; Theboral, J.; Elayaraja, D.; Appunu, C.; Siva, R.; Manickavasagam, M. Efficient direct plant regeneration from immature leaf roll explants of sugarcane (Saccharum officinarum L.) using polyamines and assessment of genetic fidelity by SCoT markers. In Vitro Cell. Dev. Biol.-Plant 2018, 54, 399–412. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Haida, Z.; Hakiman, M. A Comprehensive Review on the Determination of Enzymatic Assay and Nonenzymatic Antioxidant Activities. Food Sci. Nutr. 2019, 7, 1555–1563. [Google Scholar] [CrossRef]
- Mujib, A.; Fatima, S.; Malik, M.Q. Gamma Ray–Induced Tissue Responses and Improved Secondary Metabolites Accumulation in Catharanthus roseus. Appl. Microbiol. Biotechnol. 2022, 106, 6109–6123. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple Range and Multiple F Tests. Biometrics 1955, 11, 1. [Google Scholar] [CrossRef]
- Verma, S.K.; Yucesan, B.; Sahin, G.; Gurel, E. Embryogenesis, Plant Regeneration and Cardiac Glycoside Determination in Digitalis ferruginea subsp. ferruginea L. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 119, 625–634. [Google Scholar] [CrossRef]
- Dang, S.; Gao, R.; Zhang, Y.; Feng, Y. In Vitro Regeneration and Its Histological Characteristics of Dioscorea nipponica Makino. Sci. Rep. 2022, 12, 18436. [Google Scholar] [CrossRef]
- Nowakowska, M.; Pavlović, Ž.; Nowicki, M.; Boggess, S.L.; Trigiano, R.N. In Vitro Propagation of an Endangered Helianthus verticillatus by Axillary Bud Proliferation. Plants 2020, 9, 712. [Google Scholar] [CrossRef]
- Nazirah, A.; Nor-Hasnida, H.; Mohd-Saifuldullah, A.W.; Muhammad-Fuad, Y.; Ahmad-Zuhaidi, Y.; Rozidah, K. Development of an efficient micropropagation protocol for eucalyptus hybrid (E. urophylla × E. grandis) through axillary shoot proliferation. J. Trop. For. Sci. 2021, 33, 391–397. [Google Scholar] [CrossRef]
- Tikendra, L.; Dey, A.; Jamir, I.; Sahoo, M.R.; Nongdam, P. Cytokinin Influence on In Vitro Shoot Induction and Genetic Stability Assessment of Dendrocalamus latiflorus Munro: A Commercially Important Bamboo in Manipur, North-East India. Vegetos 2022, 35, 1085–1095. [Google Scholar] [CrossRef]
- Novikova, T.I.; Asbaganov, S.V.; Ambros, E.V.; Zaytseva, Y.G. TDZ-Induced Axillary Shoot Proliferation of Rhododendron mucronulatum Turcz and Assessment of Clonal Fidelity Using DNA-Based Markers and Flow Cytometry. In Vitro Cell. Dev. Biol.-Plant 2020, 56, 307–317. [Google Scholar] [CrossRef]
- Jena, S.; Ray, A.; Sahoo, A.; Sahoo, S.; Dash, B.; Kar, B.; Nayak, S. Rapid Plant Regeneration in Industrially Important Curcuma zedoaria Revealing Genetic and Biochemical Fidelity of the Regenerants. 3 Biotech 2020, 10, 17. [Google Scholar] [CrossRef]
- Kumar, S.S.; Giridhar, P. In Vitro Micropropagation of Basella rubra L. through Proliferation of Axillary Shoots. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 144, 477–483. [Google Scholar] [CrossRef]
- Wardani, I.B. Effect of BAP (6-Benzyl Amino Purine) and NAA (Naphtalen Acetic Acid) on the Induction of Axillary Shoots in Sandalwood (Santalum album L.). J. Sci. Technol. Educ. 2022, 1, 23–30. [Google Scholar]
- Ayangla, N.W.; Dwivedi, P.; Dey, A.; Pandey, D.K. In vitro propagation, genetic and phytochemical fidelity in Glycyrrhiza glabra L., a potent glycyrrhizin yielding endangered plant. Nucleus 2022, 65, 369–377. [Google Scholar] [CrossRef]
- Mujib, A.; Aslam, J.; Bansal, Y. low colchicine doses improved callus induction, biomass growth, and shoot regeneration in in vitro culture of Dracaena sanderiana sander ex mast. Prop. Ornam. Plants 2023, 23, 81–87. [Google Scholar]
- Guo, Y.X.; Zhao, Y.Y.; Zhang, M.; Zhang, L.Y. Development of a novel in vitro rooting culture system for the micropropagation of highbush blueberry (Vaccinium corymbosum) seedlings. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 139, 615–620. [Google Scholar] [CrossRef]
- Chirumamilla, P.; Gopu, C.; Jogam, P.; Taduri, S. Highly Efficient Rapid Micropropagation and Assessment of Genetic Fidelity of Regenerants by ISSR and SCoT Markers of Solanum khasianum Clarke. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 144, 397–407. [Google Scholar] [CrossRef]
- Faisal, M.; Qahtan, A.A.; Alatar, A.A. Thidiazuron Induced In Vitro Plant Regeneration, Phenolic Contents, Antioxidant Potential, GC-MS Profiles and Nuclear Genome Stability of Plectranthus amboinicus (Lour.) Spreng. Horticulturae 2023, 9, 277. [Google Scholar] [CrossRef]
- Qahtan, A.A.; Faisal, M.; Alatar, A.A.; Abdel-Salam, E.M. Callus-Mediated High-Frequency Plant Regeneration, Phytochemical Profiling, Antioxidant Activity and Genetic Stability in Ruta chalepensis L. Plants 2022, 11, 1614. [Google Scholar] [CrossRef]
- Vijayalakshmi, U.; Shourie, A. Comparative gc-ms analysis of secondary metabolites from leaf, stem and callus of Glycyrrhiza glabra. World J. Pharm. Res. 2019, 8, 1915–1923. [Google Scholar] [CrossRef]
- Adel, R.; Abdel-Ghani, A.E.; Abouelenein, D.D.; El-Dahmy, S.I. Variation in the Volatile Constituents of Wild and In Vitro Propagated Tanacetum sinaicum Del. Ex DC through GC-MS Chemical Fingerprint. Ind. J. Nat. Prod. Res. 2021, 12, 238–246. [Google Scholar]
- Bansal, Y.; Mujib, A.; Mamgain, J.; Dewir, Y.H.; Rihan, H.Z. Phytochemical Composition and Detection of Novel Bioactives in Anther Callus of Catharanthus roseus L. Plants 2023, 12, 2186. [Google Scholar] [CrossRef]
- Konappa, N.; Udayashankar, A.C.; Krishnamurthy, S.; Pradeep, C.K.; Chowdappa, S.; Jogaiah, S. GC–MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Sci. Rep. 2020, 10, 16438. [Google Scholar] [CrossRef]
- Bhat, M.P.; Rudrappa, M.; Hugar, A.; Gunagambhire, P.V.; Suresh Kumar, R.; Nayaka, S.; Almansour, A.I.; Perumal, K. In-Vitro Investigation on the Biological Activities of Squalene Derived from the Soil Fungus Talaromyces pinophilus. Heliyon 2023, 9, e21461. [Google Scholar] [CrossRef]
- Li, C.; Li, T.; Zhu, M.; Lai, J.; Wu, Z. Pharmacological Properties of Glabridin (a Flavonoid Extracted from Licorice): A Comprehensive Review. J. Funct. Foods 2021, 85, 104638. [Google Scholar] [CrossRef]
- Sharma, N.; Palia, P.; Chaudhary, A.; Shalini; Verma, K.; Kumar, I. A Review on Pharmacological Activities of Lupeol and Its Triterpene Derivatives. J. Drug Deliv. Ther. 2020, 10, 325–332. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Ganesan, T.; Subban, M.; Christopher Leslee, D.B.; Kuppannan, S.B.; Seedevi, P. Structural characterization of n-hexadecanoic acid from the leaves of Ipomoea eriocarpa and its antioxidant and antibacterial activities. Biomass Convers Biorefinery 2022. [Google Scholar] [CrossRef]
- Ghosh, A.; Igamberdiev, A.U.; Debnath, S.C. Thidiazuron-Induced Somatic Embryogenesis and Changes of Antioxidant Properties in Tissue Cultures of Half-High Blueberry Plants. Sci. Rep. 2018, 8, 16978. [Google Scholar] [CrossRef]
- Jeong, B.R.; Sivanesan, I. Direct Adventitious Shoot Regeneration, In Vitro Flowering, Fruiting, Secondary Metabolite Content and Antioxidant Activity of Scrophularia takesimensis Nakai. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 123, 607–618. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Kumaria, S.; Tandon, P. High Frequency Regeneration Protocol for Dendrobium Nobile: A Model Tissue Culture Approach for Propagation of Medicinally Important Orchid Species. S. Afr. J. Bot. 2016, 104, 232–243. [Google Scholar] [CrossRef]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-Proline Biosynthesis, Signal Transduction, Transport, Accumulation and Its Vital Role in Plants during Variable Environmental Conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef]
- Khorasani Esmaeili, A.; Mat Taha, R.; Mohajer, S.; Banisalam, B. Antioxidant Activity and Total Phenolic and Flavonoid Content of Various Solvent Extracts from In Vivo and In Vitro Grown Trifolium pratense L. (Red Clover). BioMed Res. Int. 2015, 2015, 643285. [Google Scholar] [CrossRef]
- Marshall, D.; Siow, Y.; Debnath, S. Somatic Embryogenesis in Vaccinium vitis-Idaea and Genetic Delity Evaluation of Regenerants Using Molecular Markers Together with Their Phytochemical Prole. Sayani Kundu Agric. Agric.-Food Can. Rajesh Barua Agric. Agric.-Food Can. 2023. [Google Scholar] [CrossRef]
- Ma, N.; Hu, C.; Wan, L.; Hu, Q.; Xiong, J.; Zhang, C. Strigolactones Improve Plant Growth, Photosynthesis, and Alleviate Oxidative Stress under Salinity in Rapeseed (Brassica napus L.) by Regulating Gene Expression. Front. Plant Sci. 2017, 8, 1671. [Google Scholar] [CrossRef] [PubMed]
- Zayova, E.; Nikolova, M.; Dimitrova, L.; Petrova, M. Comparative Study of In Vitro, Ex Vitro and In Vivo Propagated Salvia hispanica (Chia) Plants: Morphometric Analysis and Antioxidant Activity. AgroLife Sci. J. 2016, 5, 166–173. [Google Scholar]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total Phenolic and Flavonoid Contents and Antioxidant Activity of Ginger (Zingiber officinale Rosc.) Rhizome, Callus and Callus Treated with Some Elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Mamgain, J.; Mujib, A.; Bansal, Y.; Gulzar, B.; Zafar, N.; Syeed, R.; Alsughayyir, A.; Dewir, Y.H. Elicitation Induced α-Amyrin Synthesis in Tylophora indica In Vitro Cultures and Comparative Phytochemical Analyses of In Vivo and Micropropagated Plants. Plants 2024, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.K.; Pandey, S.; Niranjan, A.; Misra, P. Comparative analysis of phenolic compounds from wild and in vitro propagated plant Thalictrum foliolosum and antioxidant activity of various crude extracts. Chem. Pap. 2021, 75, 4873–4885. [Google Scholar] [CrossRef]





| PGRs | Concentration (mg/L) | Callusing Frequency (%) | Fresh Biomass (g) |
|---|---|---|---|
| Control | 0 | 0 e | 0 d |
| BAP + 2,4-D | 1.0 + 0.5 | 72.21 ± 5.56 ab | 3.9 ± 0.6 ab |
| 1.0 + 2.0 | 61.11 ± 5.55 bc | 3.6 ± 0.4 ab | |
| 2.0 + 0.5 | 88.89 ± 11.11 a | 4.4 ± 0.7 a | |
| BAP + NAA | 1.0 + 0.5 | 27.77 ± 5.56 de | 2.1 ± 0.1 c |
| 1.0 + 2.0 | 11.11 ± 5.55 e | 0.8 ± 0.1 d | |
| 2.0 + 1.0 | 44.44 ± 5.56 cd | 2.9 ± 0.2 bc |
| PGRs | Concentration (mg/L) | Shooting Frequency (%) | Shoot Length (cm) | Mean Shoot Number |
|---|---|---|---|---|
| Control | 0 | 0 e | 0 f | 0 d |
| BAP | 0.5 | 77.77 ± 5.56 ab | 8.7 ± 0.4 b | 3.33 ± 0.67 ab |
| 1.0 | 94.44 ± 5.56 a | 10.5 ± 0.3 a | 4.67 ± 0.88 a | |
| 2.0 | 38.88 ± 14.70 cd | 4.4 ± 0.6 d | 2.67 ± 0.88 bc | |
| 4.0 | 22.21 ± 5.55 de | 2.1 ± 0.2 e | 1.33 ± 0.33 cd | |
| BAP + IAA | 1.0 + 0.25 | 55.55 ± 11.11 bcd | 6.2 ± 0.4 c | 3.33 ± 0.33 ab |
| 1.0 + 0.50 | 61.12 ± 14.69 abc | 6.8 ± 0.8 c | 3.33 ± 0.33 ab |
| PGRs | Concentration (mg/L) | Rooting Frequency (%) | Mean Root Numbers/Shoot |
|---|---|---|---|
| Control | 0 | 0 c | 0 d |
| IBA | 1.0 | 72.22 ± 14.70 ab | 6.67 ± 1.20 ab |
| 2.0 | 88.89 ± 11.11 a | 7.33 ± 1.33 a | |
| 3.0 | 61.12 ± 14.70 ab | 4.67 ± 0.88 abc | |
| IAA | 1.0 | 49.99 ± 9.62 b | 3.67 ± 0.33 bc |
| 2.0 | 38.89 ± 11.11 b | 2.33 ± 0.67 c | |
| 3.0 | 0 c | 0 d |
| S.No. | Name of the Compound | Retention Time (min) | Peak Area % | Molecular Formula | Molecular Weight | ||
|---|---|---|---|---|---|---|---|
| In Vivo Root | In Vitro Root | In Vivo Root | In Vitro Root | ||||
| 1 | Pyranone | 5.089 | 4.971 | 0.68 | 1.89 | C6H8O4 | 144 |
| 2 | 2-piperidinemethanol | 5.670 | - | 0.47 | - | C6H13NO | 115.00 |
| 3 | Guanosine | 9.835 | 9.915 | 4.80 | 8.59 | C10H13N5O5 | 283 |
| 4 | Xanthosine | 10.440 | - | 0.37 | - | C10H12N4O6 | 284 |
| 5 | 1-Propylpentyl butyrate | 10.832 | - | 0.28 | - | C12H24O2 | 200 |
| 6 | Hexadecanoic acid | - | 10.843 | - | 0.71 | C16H32O2 | 256 |
| 7 | cis-Sesquisabinene hydrate | - | 11.757 | - | 0.28 | C15H26O | 222 |
| 8 | 4-Methylmannitol | - | 12.271 | - | 0.55 | C7H16O6 | 196 |
| 9 | Mome inositol | 13.03 | 12.594 | 27.79 | 0.27 | C7H14O6 | 194 |
| 10 | Ethyl 3-(4-fluorophenyl)-3-oxopropanoate | - | 13.056 | - | 0.35 | C11H11FO3 | 210 |
| 11 | 4-octanol | 13.221 | - | 1.12 | - | C8H18O | 130 |
| 12 | 1-pentadecanol | 14.144 | - | 0.2 | - | C15H32O | 228 |
| 13 | Butylated Hydroxytoluene | - | 14.454 | - | 0.21 | C15H24O | 220 |
| 14 | Palmitic acid, methyl ester | 14.55 | 14.549 | 3.03 | 5.09 | C17H34O2 | 270 |
| 15 | Isopropyl palmitate | 15.48 | - | 0.15 | - | C19H38O2 | 298 |
| 16 | Linoleic acid, methyl ester | 16.173 | 16.179 | 1.92 | 3.19 | C19H34O2 | 294 |
| 17 | Oleic acid, methyl ester | 16.234 | 16.236 | 10.01 | 16.26 | C19H36O2 | 296 |
| 18 | Methyl elaidate | 16.285 | 16.286 | 0.12 | 0.17 | C19H36O2 | 296 |
| 19 | Linolenic acid, methyl ester | 16.366 | 16.372 | 0.72 | 1.21 | C19H32O2 | 292 |
| 20 | Stearic acid, methyl ester | 16.468 | 16.47 | 2.46 | 4.48 | C19H38O2 | 298 |
| 21 | Glycol myristate | - | 17.53 | - | 0.15 | C16H32O3 | 272 |
| 22 | Tributyl acetylicitrate | 17.544 | - | 0.3 | - | C20H34O8 | 402 |
| 23 | 2-Monopalmitin | - | 17.598 | - | 0.16 | C19H38O4 | 330 |
| 24 | Glycidyl palmitate | 17.993 | 17.996 | 1.65 | 2.72 | C19H36O3 | 312 |
| 25 | Eicosanoic acid, methyl ester | 18.233 | 18.235 | 0.25 | 0.69 | C21H42O2 | 326 |
| 23 | 9-octadecenamide | 18.703 | 18.702 | 0.59 | 0.21 | C18H35NO | 281 |
| 27 | Oleoyl chloride | 19.099 | 19.094 | 0.89 | 1.58 | C18H33ClO | 300 |
| 28 | alpha-Monostearin | - | 19.296 | - | 0.35 | C21H42O4 | 358 |
| 29 | Glycidyl oleate | 19.484 | 19.479 | 7.51 | 11.64 | C21H38O3 | 338 |
| 30 | cis-8,11,14-Eicosatrienoic Acid | 19.622 | - | 0.26 | - | C20H34O2 | 306 |
| 31 | Glycidyl stearate | 19.671 | 19.678 | 1.14 | 3.01 | C21H40O3 | 340 |
| 32 | Dipalmitin | 19.847 | 19.852 | 1.69 | 7.92 | C35H68O5 | 568 |
| 33 | Pentadecyl hexanoate | 20.563 | 20.57 | 0.29 | 0.51 | C21H42O2 | 326 |
| 34 | .beta.-Monoolein | 21.222 | 21.225 | 15.13 | 16.53 | C21H40O4 | 356 |
| 35 | Methyl 12-oxo-9-dodecenoate | 21.389 | 21.396 | 1.48 | 2.21 | C13H22O3 | 226 |
| 36 | Oleic acid, 3-hydroxypropyl ester | 21.857 | 21.861 | 1.02 | 0.89 | C21H40O3 | 340 |
| 37 | Squalene | 21.969 | - | 0.76 | - | C30H50 | 410 |
| 38 | Isopropyl linoleate | - | 22.638 | - | 0.13 | C21H38O2 | 322 |
| 39 | 4′-O-Methylglabridin | 23.842 | - | 0.32 | - | C21H22O4 | 338 |
| 40 | Stigmasta-3,5-diene | 24.14 | 24.14 | 0.25 | 0.11 | C29H48 | 396 |
| 41 | Heneicosanoic acid, 3-ethyl-3-methyl-, methyl ester | 24.787 | - | 0.18 | - | C25H50O2 | 382 |
| 42 | 24-Epicampesterol | 25.413 | - | 0.30 | - | C28H48O | 400 |
| 43 | (22E)-Stigmasta-4,22-dien-3-ol | 25.663 | 25.676 | 1.20 | 1.35 | C29H48O | 412 |
| 44 | gamma.-Sitosterol | 26.323 | 26.338 | 1.04 | 0.74 | C29H50O | 414 |
| 45 | Lupeol | 27.438 | - | 0.70 | - | C30H50O | 426 |
| 46 | Ethylcyclodocosane | - | 27.913 | - | 0.65 | C24H48 | 336 |
| 47 | alpha-Spinosterol acetate | 28.619 | 28.543 | 4.65 | 0.79 | C31H50O2 | 454 |
| 48 | N,N-Dimethylcholestan-6-amine | 30.995 | - | 0.75 | - | C29H53N | 415 |
| 49 | N-[2-(tetradecyloxy)phenyl]acetamide | - | 31.008 | - | 1.2 | C22H37NO2 | 347 |
| 50 | cis-15-Tetracosenoic acid, propyl ester | - | 31.414 | - | 0.68 | C27H52O2 | 408 |
| S.No. | RT (min) | Peak Area % | Name of the Compound | Molecular Formula | Molecular Weight |
|---|---|---|---|---|---|
| 1 | 10.014 | 2.44 | Guanosine | C10H13N5O5 | 283 |
| 2 | 12.189 | 10.73 | Methyl galactoside | C7H14O6 | 194 |
| 3 | 14.526 | 1.59 | Palmitic acid, methyl ester | C17H34O2 | 270 |
| 4 | 15.076 | 0.88 | Palmitinic acid | C16H32O2 | 256 |
| 5 | 16.164 | 0.90 | Linoleic acid, methyl ester | C19H34O2 | 294 |
| 6 | 16.227 | 5.18 | Oleic acid, methyl ester | C19H36O2 | 296 |
| 7 | 16.356 | 0.45 | Linolenic acid, methyl ester | C19H32O2 | 292 |
| 8 | 16.458 | 1.26 | Stearic acid, methyl ester | C19H38O2 | 298 |
| 9 | 16.839 | 2.26 | cis-9,cis-12-Octadecadienoic acid | C18H32O2 | 280 |
| 10 | 17.581 | 0.15 | 2-Monopalmitin | C19H38O4 | 330 |
| 11 | 17.990 | 5.19 | Glycidyl palmitate | C19H36O3 | 312 |
| 12 | 18.171 | 0.19 | Palmidrol | C18H37NO2 | 299 |
| 13 | 18.231 | 0.31 | Eicosanoic acid, methyl ester | C21H42O2 | 326 |
| 14 | 18.680 | 0.68 | 9-octadecenamide | C18H35NO | 281 |
| 15 | 19.076 | 0.14 | Oleoyl chloride | C18H33ClO | 300 |
| 16 | 19.289 | 0.09 | 2-Formylhexadecane | C17H34O | 254 |
| 17 | 19.433 | 1.05 | 1,3,14,16-Nonadecatetraene | C19H32 | 260 |
| 18 | 19.478 | 3.88 | Glycidyl oleate | C21H38O3 | 338 |
| 19 | 19.604 | 0.18 | Ethyl. alpha.-linolenate | C20H34O2 | 306 |
| 20 | 19.663 | 2.75 | 1-Monostearin | C21H42O4 | 358 |
| 21 | 19.842 | 5.96 | 2,3-dihydroxypropyl laurate | C15H30O4 | 274 |
| 22 | 20.552 | 0.48 | Pentadecyl hexanoate | C21H42O2 | 326 |
| 23 | 20.854 | 0.84 | Linalool oxide, trimethylsilyl ether | C13H26O2Si | 242 |
| 24 | 21.106 | 1.20 | .beta.-Stigmasterol | C29H48O | 412 |
| 25 | 21.281 | 35.65 | Oleic anhydride | C36H66O3 | 546 |
| 26 | 21.417 | 2.43 | 2-Stearoylglycerol | C21H42O4 | 358 |
| 27 | 21.864 | 1.27 | Oleic acid, 2-hydroxyethyl ester | C20H38O3 | 326 |
| 28 | 21.906 | 0.25 | Methyl 2-hydroxy-octadeca-9,12,15-trienoate | C19H32O3 | 308 |
| 29 | 21.968 | 0.15 | Squalene | C30H50 | 410 |
| 30 | 22.028 | 0.35 | Octadecyl hexanoate | C24H48O2 | 368 |
| 31 | 23.032 | 0.19 | Dodecyl 3-(trifluoromethyl)benzoate | C20H29F3O2 | 358 |
| 32 | 24.127 | 0.26 | Stigmasta-3,5-diene | C29H48 | 396 |
| 33 | 25.407 | 0.43 | 24-Epicampesterol | C28H48O | 400 |
| 34 | 25.652 | 1.11 | (22E)-Stigmasta-4,22-dien-3-ol | C29H48O | 412 |
| 35 | 26.301 | 1.51 | gamma.-Sitosterol | C29H50O | 414 |
| 36 | 26.772 | 0.19 | beta.-Saccharostenone | C29H46O | 410 |
| 37 | 27.889 | 0.51 | Ethylcyclodocosane | C24H48 | 336 |
| 38 | 28.823 | 0.19 | Dihydroagarofuran | C15H26O | 222 |
| 39 | 29.032 | 1.18 | 3-Oxo-9.beta.-lanosta-7,22,24-trien-26,23-olide | C30H42O3 | 450 |
| 40 | 30.983 | 1.02 | N-[2-(tetradecyloxy)phenyl]acetamide | C22H37NO2 | 347 |
| 41 | 31.223 | 0.30 | Methyl hexadecatrienoate | C17H28O2 | 264 |
| 42 | 31.384 | 0.47 | cis-15-Tetracosenoic acid, propyl ester | C27H52O2 | 408 |
| 43 | 33.416 | 1.95 | 2-Methylpregn-4-ene-3,20-dione | C22H32O2 | 328 |
| 44 | 34.062 | 0.45 | 24-Epibrassicasterol | C28H46O | 398 |
| Sample Type | TPC (mg GAE/g DW) | TFC (mg QE/g DW) | DPPH Scavenging Activity (%) |
|---|---|---|---|
| Root derived callus | 3.64 ± 0.45 c | 0.72 ± 0.36 b | 22.9 ± 0.31 c |
| In vivo root | 7.49 ± 0.54 b | 1.44 ± 0.15 a | 31.34 ± 1.35 b |
| In vitro root | 9.76 ± 0.21 a | 1.58 ± 0.67 a | 40.63 ± 2.11 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bansal, Y.; Mujib, A.; Mamgain, J.; Kumar, S.; Dewir, Y.H.; Magyar-Tábori, K. Synthesis and Accumulation of Phytocompounds in Field-, Tissue-Culture Grown (Stress) Root Tissues and Simultaneous Defense Response Activity in Glycyrrhiza glabra L. Sustainability 2024, 16, 1613. https://doi.org/10.3390/su16041613
Bansal Y, Mujib A, Mamgain J, Kumar S, Dewir YH, Magyar-Tábori K. Synthesis and Accumulation of Phytocompounds in Field-, Tissue-Culture Grown (Stress) Root Tissues and Simultaneous Defense Response Activity in Glycyrrhiza glabra L. Sustainability. 2024; 16(4):1613. https://doi.org/10.3390/su16041613
Chicago/Turabian StyleBansal, Yashika, Abdul Mujib, Jyoti Mamgain, Shubham Kumar, Yaser Hassan Dewir, and Katalin Magyar-Tábori. 2024. "Synthesis and Accumulation of Phytocompounds in Field-, Tissue-Culture Grown (Stress) Root Tissues and Simultaneous Defense Response Activity in Glycyrrhiza glabra L." Sustainability 16, no. 4: 1613. https://doi.org/10.3390/su16041613
APA StyleBansal, Y., Mujib, A., Mamgain, J., Kumar, S., Dewir, Y. H., & Magyar-Tábori, K. (2024). Synthesis and Accumulation of Phytocompounds in Field-, Tissue-Culture Grown (Stress) Root Tissues and Simultaneous Defense Response Activity in Glycyrrhiza glabra L. Sustainability, 16(4), 1613. https://doi.org/10.3390/su16041613







