Spatial Distribution, Ecological Risk Assessment, and Source Identification of Metals in Sediments of the Krka River Estuary (Croatia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sediment Sampling and Analysis
2.3. Diffusive Gradients in Thin Films and Supporting Pore Water Analysis
2.4. Sediment Contamination Assessment
2.4.1. Enrichment Factor (EF)
2.4.2. Geoaccumulation Index (Igeo)
2.4.3. Pollution Load Index (PLI)
2.4.4. Mean ERM Quotient (m-ERM-Q)
2.5. Statistical Analysis
3. Results and Discussion
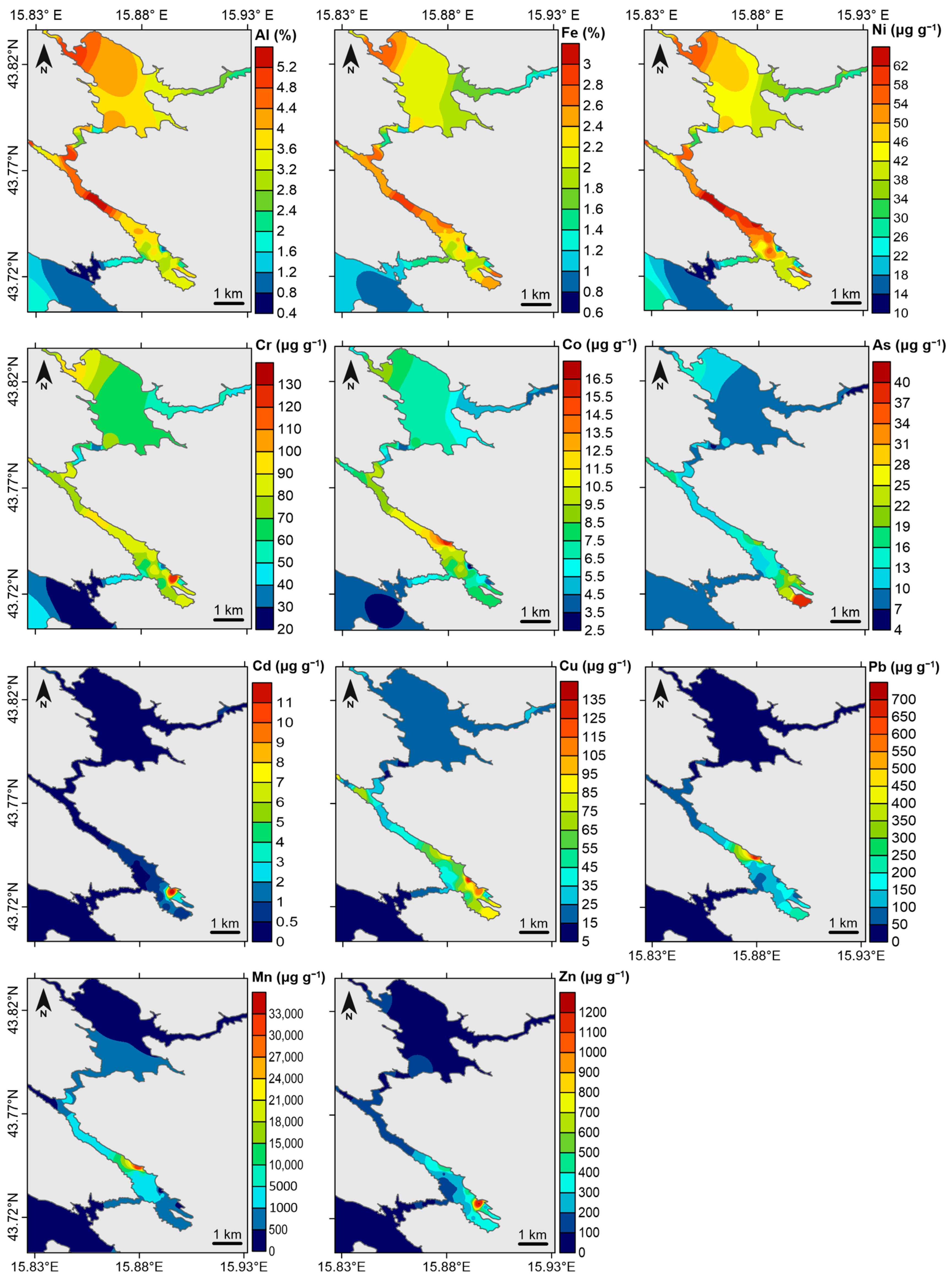
3.1. Contents of Metals in Surface Sediments
3.2. Risk Assessment
3.2.1. Enrichment Factor
3.2.2. Geoaccumulation Index
3.2.3. The Pollution Load Index (PLI)
3.2.4. Mean ERM Quotients (m-ERM-Q)
3.3. Diffusive Gradient in Thin Films
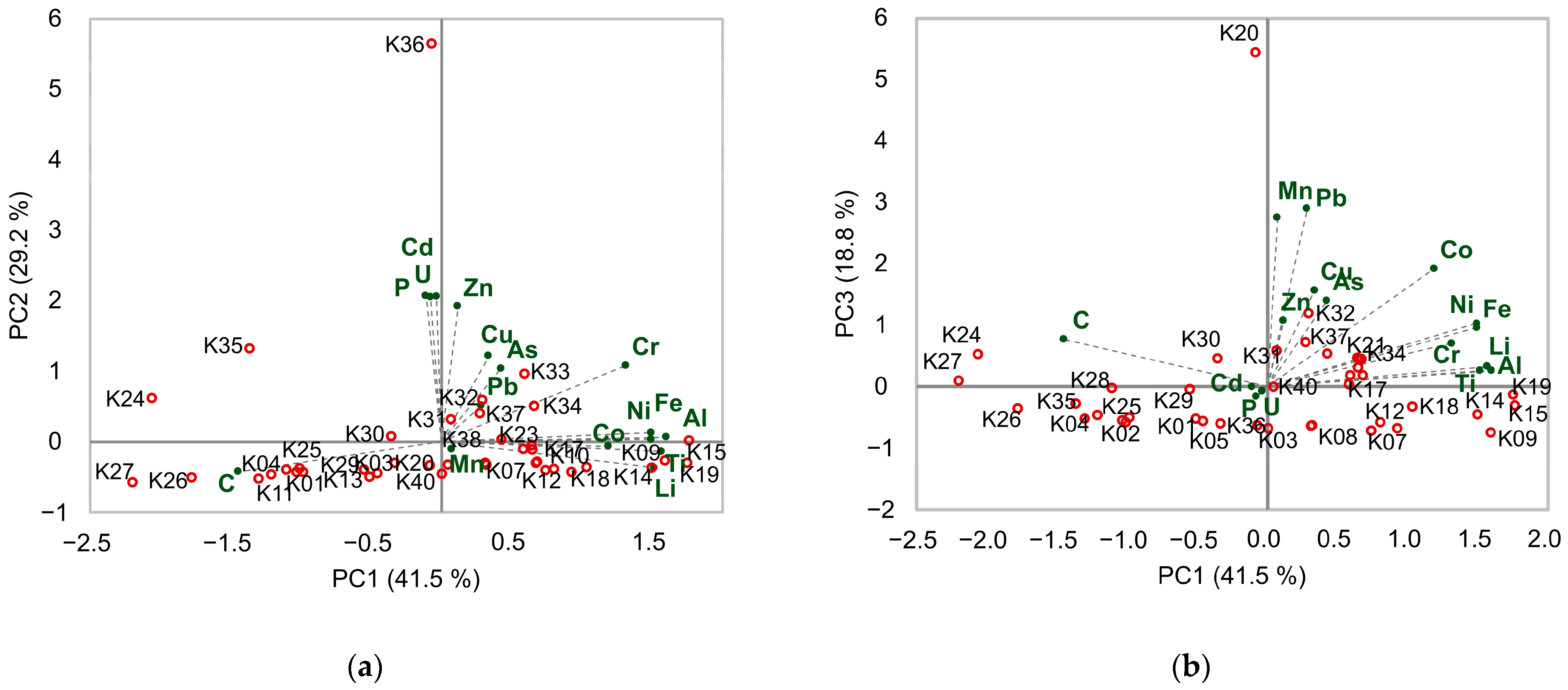
3.4. Source Identification of Metals in Surface Estuarine Sediments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ridgway, J.; Shimmield, G. Estuaries as repositories of historical contamination and their impact on shelf seas. Estuar. Coast. Shelf Sci. 2002, 55, 903–928. [Google Scholar] [CrossRef]
- Di Leonardo, R.; Tranchida, G.; Bellanca, A.; Neri, R.; Angelone, M.; Mazzola, S. Mercury levels in sediments of central Mediterranean Sea: A 150+ year record from box-cores recovered in the Strait of Sicily. Chemosphere 2006, 65, 2366–2376. [Google Scholar] [CrossRef] [PubMed]
- Petranich, E.; Croce, S.; Crosera, M.; Pavoni, E.; Faganeli, J.; Adami, G.; Covelli, S. Mobility of metal(loid)s at the sediment-water interface in two tourist port areas of the Gulf of Trieste (northern Adriatic Sea). Environ. Sci. Pollut. Res. 2018, 25, 26887–26902. [Google Scholar] [CrossRef] [PubMed]
- Tessier, E.; Garnier, C.; Mullot, J.-U.; Lenoble, V.; Arnaud, M.; Raynaud, M.; Mounier, S. Study of the spatial and historical distribution of sediment inorganic contamination in the Toulon bay (France). Mar. Pollut. Bull. 2011, 62, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Birch, G. Determination of sediment metal background concentrations and enrichment in marine environments—A critical review. Sci. Total Environ. 2017, 580, 813–831. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Song, J.; Liang, X.; Yin, M.; Yuan, H.; Li, X.; Ren, C.; Zhou, B.; Kang, X.; Yin, X. Dynamics and diagenesis of trace metals in sediments of the Changjiang Estuary. Sci. Total Environ. 2019, 675, 247–259. [Google Scholar] [CrossRef]
- Förstner, U.; Salomons, W. Trace metal analysis on polluted sediments: Part I: Assessment of sources and intensities. Environ. Technol. Lett. 1980, 1, 494–505. [Google Scholar] [CrossRef]
- Saulnier, I.; Mucci, A. Trace metal remobilization following the resuspension of estuarine sediments: Saguenay Fjord, Canada. Appl. Geochem. 2000, 15, 191–210. [Google Scholar] [CrossRef]
- Sakellari, A.; Plavšić, M.; Karavoltsos, S.; Dassenakis, M.; Scoullos, M. Assessment of copper, cadmium and zinc remobilization in Mediterranean marine coastal sediments. Estuar. Coast. Shelf Sci. 2011, 91, 1–12. [Google Scholar] [CrossRef]
- Santschi, P.; Höhener, P.; Benoit, G.; Brink, M.B.-T. Chemical processes at the sediment-water interface. Mar. Chem. 1990, 30, 269–315. [Google Scholar] [CrossRef]
- Dou, Y.; Li, J.; Zhao, J.; Hu, B.; Yang, S. Distribution, enrichment and source of heavy metals in surface sediments of the eastern Beibu Bay, South China Sea. Mar. Pollut. Bull. 2013, 67, 137–145. [Google Scholar] [CrossRef]
- Garmendia, M.; de Vallejuelo, S.F.-O.; Liñero, O.; Gredilla, A.; Arana, G.; Soto, M.; de Diego, A. Long term monitoring of metal pollution in sediments as a tool to investigate the effects of engineering works in estuaries. A case study, the Nerbioi-Ibaizabal estuary (Bilbao, Basque Country). Mar. Pollut. Bull. 2019, 145, 555–563. [Google Scholar] [CrossRef]
- Ip, C.C.; Li, X.-D.; Zhang, G.; Wai, O.W.; Li, Y.-S. Trace metal distribution in sediments of the Pearl River Estuary and the surrounding coastal area, South China. Environ. Pollut. 2007, 147, 311–323. [Google Scholar] [CrossRef]
- Pan, K.; Wang, W.-X. Trace metal contamination in estuarine and coastal environments in China. Sci. Total Environ. 2012, 421, 3–16. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X. Heavy metals in surface sediments of the intertidal Laizhou Bay, Bohai Sea, China: Distributions, sources and contamination assessment. Mar. Pollut. Bull. 2015, 98, 320–327. [Google Scholar] [CrossRef]
- Zhao, G.; Ye, S.; Yuan, H.; Ding, X.; Wang, J. Surface sediment properties and heavy metal pollution assessment in the Pearl River Estuary, China. Environ. Sci. Pollut. Res. 2017, 24, 2966–2979. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Strezov, V. Comparison of pollution indices for the assessment of heavy metals in the sediments of seaports of NSW, Australia. Mar. Pollut. Bull. 2018, 128, 295–306. [Google Scholar] [CrossRef]
- Burton, G.A. Sediment quality criteria in use around the world. Limnology 2002, 3, 65–76. [Google Scholar] [CrossRef]
- Long, E.R.; MacDonald, D.D. Recommended Uses of Empirically Derived, Sediment Quality Guidelines for Marine and Estuarine Ecosystems. Hum. Ecol. Risk Assess. Int. J. 1998, 4, 1019–1039. [Google Scholar] [CrossRef]
- Birch, G.; Lee, J.-H. The use of sedimentary metal data in predictive modelling of estuarine contamination, assessment of environmental condition and pollutant source identification (Narrabeen Lagoon, Sydney, Australia). Environ. Sci. Pollut. Res. 2020, 27, 43685–43699. [Google Scholar] [CrossRef] [PubMed]
- Birch, G. A review of chemical-based sediment quality assessment methodologies for the marine environment. Mar. Pollut. Bull. 2018, 133, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davison, W.; Miller, S.; Tych, W. In situ high resolution measurements of fluxes of Ni, Cu, Fe, and Mn and concentrations of Zn and Cd in porewaters by DGT. Geochim. Cosmochim. Acta 1995, 59, 4181–4192. [Google Scholar] [CrossRef]
- Davison, W.; Zhang, H. In situ speciation measurements of trace components in natural waters using thin-film gels. Nature 1994, 367, 546–548. [Google Scholar] [CrossRef]
- Amato, E.D.; Wadige, C.P.M.; Taylor, A.M.; Maher, W.A.; Simpson, S.L.; Jolley, D.F. Field and laboratory evaluation of DGT for predicting metal bioaccumulation and toxicity in the freshwater bivalve Hyridella australis exposed to contaminated sediments. Environ. Pollut. 2018, 243, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Amato, E.D.; Simpson, S.L.; Jarolimek, C.V.; Jolley, D.F. Diffusive gradients in thin films technique provide robust prediction of metal bioavailability and toxicity in estuarine sediments. Environ. Sci. Technol. 2014, 48, 4485–4494. [Google Scholar] [CrossRef]
- Ren, J.; Williams, P.N.; Luo, J.; Ma, H.; Wang, X. Sediment metal bioavailability in Lake Taihu, China: Evaluation of sequential extraction, DGT, and PBET techniques. Environ. Sci. Pollut. Res. 2015, 22, 12919–12928. [Google Scholar] [CrossRef]
- Xie, M.; Simpson, S.L.; Huang, J.; Teasdale, P.R.; Wang, W.-X. In Situ DGT Sensing of Bioavailable Metal Fluxes to Improve Toxicity Predictions for Sediments. Environ. Sci. Technol. 2021, 55, 7355–7364. [Google Scholar] [CrossRef]
- Cindrić, A.-M.; Garnier, C.; Oursel, B.; Pižeta, I.; Omanović, D. Evidencing the natural and anthropogenic processes controlling trace metals dynamic in a highly stratified estuary: The Krka River estuary (Adriatic, Croatia). Mar. Pollut. Bull. 2015, 94, 199–216. [Google Scholar] [CrossRef]
- Elbaz-Poulichet, F.; Guan, D.M.; Martin, J.-M. Trace-metal behavior in a highly stratified mediterranean estuary—The Krka (Yugoslavia). Mar. Chem. 1991, 32, 211–224. [Google Scholar] [CrossRef]
- Kwokal, Ž.; Frančišković-Bilinski, S.; Bilinski, H.; Branica, M. A comparison of anthropogenic mercury pollution in Kaštela Bay (Croatia) with pristine estuaries in Öre (Sweden) and Krka (Croatia). Mar. Pollut. Bull. 2002, 44, 1152–1157. [Google Scholar] [CrossRef]
- Mikac, N.; Kwokal, Z.; May, K.; Branica, M. Mercury distribution in the krka river Estuary (Eastern Adriatic Coast). Mar. Chem. 1989, 28, 109–126. [Google Scholar] [CrossRef]
- Bogner, D.; Ujević, I.; Zvonarić, T.; Barić, A. Distribution of selected trace metals in coastal surface sediments from the middle and south Adriatic. Fresenius Environ. Bull 2004, 13, 1281–1287. [Google Scholar]
- Martinčić, D.; Kwokal, Ž.; Branica, M. Distribution of zinc, lead, cadmium and copper between different size fractions of sediments II. The Krka River Estuary and the Kornati Islands (Central Adriatic Sea). Sci. Total Environ. 1990, 95, 217–225. [Google Scholar] [CrossRef]
- Martinčić, D.; Kwokal, Ž.; Stoeppler, M.; Branica, M. Trace metals in sediments from the adriatic sea. Sci. Total Environ. 1989, 84, 135–147. [Google Scholar] [CrossRef]
- Carić, H.; Cukrov, N.; Omanović, D. Nautical tourism in marine protected areas (MPAs): Evaluating an impact of copper emission from antifouling coating. Sustainability 2021, 13, 11897. [Google Scholar] [CrossRef]
- Prohić, E.; Juračić, M. Heavy metals in sediments—Problems concerning determination of the anthropogenic influence. Study in the Krka River estuary, eastern Adriatic coast, Yugoslavia. Environ. Geol. 1989, 13, 145–151. [Google Scholar] [CrossRef]
- Prohić, E.; Kniewald, G. Heavy metal distribution in recent sediments of the Krka River Estuary—An example of sequential extraction analysis. Mar. Chem. 1987, 22, 279–297. [Google Scholar] [CrossRef]
- Bonacci, O.; Andrić, I.; Roje-Bonacci, T. Hydrological analysis of Skradinski Buk tufa waterfall (Krka River, Dinaric karst, Croatia). Environ. Earth Sci. 2017, 76, 669. [Google Scholar] [CrossRef]
- Cukrov, N. Metal Dynamics in the Sediments of the Krka River Estuary. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 2021. [Google Scholar]
- Juračić, M.; Prohić, E. Mineralogy, Sources of Particles, and Sedimentation in the Krka River Estuary (Croatia). Geološki Vjesnik 1991, 44, 195–200. [Google Scholar]
- Cukrov, N.; Barišić, D.; Juračić, M. Calculated sedimentation rate in the Krka River Estuary using vertical Distribution of 137Cs. Rapp. Comm. Int. Mer. Medit. 2007, 38, 81. [Google Scholar]
- Prohić, E. Distribution of Trace Elements in Recent Sediments of the Krka Estuary. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 1984. (In Croatian). [Google Scholar]
- Sutherland, R.A. Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ. Geol. 2000, 39, 611–627. [Google Scholar] [CrossRef]
- Müller, G. Schwermetalle in den Sedimenten des Rheins—Veränderungen seit. Umschau 1979, 79, 133–149. [Google Scholar]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresunters 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Long, E.R.; Macdonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Long, E.R.; MacDonald, D.D.; Severn, C.G.; Hong, C.B. Classifying probabilities of acute toxicity in marine sediments with empirically derived sediment quality guidelines. Environ. Toxicol. Chem. 2000, 19, 2598–2601. [Google Scholar] [CrossRef]
- Juračić, M.; Pravdić, V. The Role of Suspended Matter in Assessing the Assimilative Capacity Case Study of Two Estuaries in the Adriatic Sea. Chem. Ecol. 1991, 5, 241–248. [Google Scholar] [CrossRef]
- de Vallejuelo, S.F.-O.; Arana, G.; de Diego, A.; Madariaga, J.M. Risk assessment of trace elements in sediments: The case of the estuary of the Nerbioi–Ibaizabal River (Basque Country). J. Hazard. Mater. 2010, 181, 565–573. [Google Scholar] [CrossRef]
- Jurina, I.; Ivanić, M.; Vdović, N.; Troskot-Čorbić, T.; Lojen, S.; Mikac, N.; Sondi, I. Deposition of trace metals in sediments of the deltaic plain and adjacent coastal area (the Neretva River, Adriatic Sea). J. Geochem. Explor. 2015, 157, 120–131. [Google Scholar] [CrossRef]
- Komar, D.; Dolenec, M.; Belak, Ž.L.; Matešić, S.S.; Lojen, S.; Kniewald, G.; Vrhovnik, P.; Dolenec, T.; Šmuc, N.R. Geochemical characterization and environmental status of Makirina bay sediments (Northern Dalmatia, Republic of Croatia). Geol. Croat. 2015, 68, 79–92. [Google Scholar] [CrossRef]
- Oreščanin, V.; Barišić, D.; Lovrenčić Mikelić, I.; Rožmarić, M. Environmental contamination assessment of the sur-roundings of the ex-Šibenik’s ferro-manganese Smelter, Croatia. J. Environ. Sci. Health Part A 2004, 39, 2493–2506. [Google Scholar] [CrossRef] [PubMed]
- Cukrov, N.; Frančišković-Bilinski, S.; Hlača, B.; Barišić, D. A recent history of metal accumulation in the sediments of Rijeka harbor, Adriatic Sea, Croatia. Mar. Pollut. Bull. 2011, 62, 154–167. [Google Scholar] [CrossRef]
- Acquavita, A.; Predonzani, S.; Mattassi, G.; Rossin, P.; Tamberlich, F.; Falomo, J.; Valic, I. Heavy Metal Contents and Distribution in Coastal Sediments of the Gulf of Trieste (Northern Adriatic Sea, Italy). Water Air Soil Pollut. 2010, 211, 95–111. [Google Scholar] [CrossRef]
- Cukrov, N.; Frančišković-Bilinski, S.; Bogner, D. Metal contamination recorded in the sediment of the semi-closed Bakar Bay (Croatia). Environ. Geochem. Health 2013, 36, 195–208. [Google Scholar] [CrossRef]
- Coynel, A.; Gorse, L.; Curti, C.; Schafer, J.; Grosbois, C.; Morelli, G.; Ducassou, E.; Blanc, G.; Maillet, G.M.; Mojtahid, M. Spatial distribution of trace elements in the surface sediments of a major European estuary (Loire Estuary, France): Source identification and evaluation of anthropogenic contribution. J. Sea Res. 2016, 118, 77–91. [Google Scholar] [CrossRef]
- Montuori, P.; Aurino, S.; Garzonio, F.; Nardone, A.; Triassi, M. Estimation of heavy metal loads from Tiber River to the Tyrrhenian Sea and environmental quality assessment. Environ. Sci. Pollut. Res. 2016, 23, 23694–23713. [Google Scholar] [CrossRef]
- Cukrov, N.; Doumandji, N.; Garnier, C.; Tucaković, I.; Dang, D.H.; Omanović, D.; Cukrov, N. Anthropogenic mercury contamination in sediments of Krka River estuary (Croatia). Environ. Sci. Pollut. Res. 2020, 27, 7628–7638. [Google Scholar] [CrossRef] [PubMed]
- Bilinski, H.; Kozar, S.; Plavšić, M.; Kwokal, Ž.; Branica, M. Trace metal adsorption on inorganic solid phases under estuarine conditions. Mar. Chem. 1991, 32, 225–233. [Google Scholar] [CrossRef]
- Jokinen, S.A.; Jilbert, T.; Tiihonen-Filppula, R.; Koho, K. Terrestrial organic matter input drives sedimentary trace metal sequestration in a human-impacted boreal estuary. Sci. Total Environ. 2020, 717, 137047. [Google Scholar] [CrossRef]
- Prajith, A.; Rao, V.P.; Chakraborty, P. Distribution, provenance and early diagenesis of major and trace metals in sediment cores from the Mandovi estuary, western India. Estuarine Coast. Shelf Sci. 2016, 170, 173–185. [Google Scholar] [CrossRef]
- Santos-Echeandia, J.; Prego, R.; Cobelo-García, A.; Millward, G. Porewater geochemistry in a Galician Ria (NW Iberian Peninsula): Implications for benthic fluxes of dissolved trace elements (Co, Cu, Ni, Pb, V, Zn). Mar. Chem. 2009, 117, 77–87. [Google Scholar] [CrossRef]
- Tankere-Muller, S.; Zhang, H.; Davison, W.; Finke, N.; Larsen, O.; Stahl, H.; Glud, R.N. Fine scale remobilisation of Fe, Mn, Co, Ni, Cu and Cd in contaminated marine sediment. Mar. Chem. 2007, 106, 192–207. [Google Scholar] [CrossRef]
- Gao, L.; Li, R.; Liang, Z.; Wu, Q.; Yang, Z.; Li, M.; Chen, J.; Hou, L. Mobilization mechanisms and toxicity risk of sediment trace metals (Cu, Zn, Ni, and Pb) based on diffusive gradients in thin films: A case study in the Xizhi River basin, South China. J. Hazard. Mater. 2021, 410, 124590. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.-X. Trace metal behavior in sediments of Jiulong River Estuary and implication for benthic exchange fluxes. Environ. Pollut. 2017, 225, 598–609. [Google Scholar] [CrossRef]
- Murray, J.W.; Dillard, J.G. The oxidation of cobalt(II) adsorbed on manganese dioxide. Geochim. Cosmochim. Acta 1979, 43, 781–787. [Google Scholar] [CrossRef]
- Alkhader, A.M.F. The Impact of Phosphorus Fertilizers on Heavy Metals Content of Soils and Vegetables Grown on Selected Farms in Jordan. Agrotechnology 2015, 5, 137. [Google Scholar] [CrossRef]
- Carnelo, L.G.L.; de Miguez, S.R.; Marbán, L. Heavy metals input with phosphate fertilizers used in Argentina. Sci. Total Environ. 1997, 204, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Schipper, L.A.; Sparling, G.P.; Fisk, L.M.; Dodd, M.B.; Power, I.L.; Littler, R.A. Rates of accumulation of cadmium and uranium in a New Zealand hill farm soil as a result of long-term use of phosphate fertilizer. Agric. Ecosyst. Environ. 2011, 144, 95–101. [Google Scholar] [CrossRef]
- Schnug, E.; Haneklaus, N. Uranium, the hidden treasure in phosphates. Procedia Eng. 2014, 83, 265–269. [Google Scholar] [CrossRef]
- Mandić, J.; Tronczyński, J.; Kušpilić, G. Polycyclic aromatic hydrocarbons in surface sediments of the mid-Adriatic and along the Croatian coast: Levels, distributions and sources. Environ. Pollut. 2018, 242, 519–527. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cukrov, N.; Cindrić, A.-M.; Omanović, D.; Cukrov, N. Spatial Distribution, Ecological Risk Assessment, and Source Identification of Metals in Sediments of the Krka River Estuary (Croatia). Sustainability 2024, 16, 1800. https://doi.org/10.3390/su16051800
Cukrov N, Cindrić A-M, Omanović D, Cukrov N. Spatial Distribution, Ecological Risk Assessment, and Source Identification of Metals in Sediments of the Krka River Estuary (Croatia). Sustainability. 2024; 16(5):1800. https://doi.org/10.3390/su16051800
Chicago/Turabian StyleCukrov, Nuša, Ana-Marija Cindrić, Dario Omanović, and Neven Cukrov. 2024. "Spatial Distribution, Ecological Risk Assessment, and Source Identification of Metals in Sediments of the Krka River Estuary (Croatia)" Sustainability 16, no. 5: 1800. https://doi.org/10.3390/su16051800
APA StyleCukrov, N., Cindrić, A.-M., Omanović, D., & Cukrov, N. (2024). Spatial Distribution, Ecological Risk Assessment, and Source Identification of Metals in Sediments of the Krka River Estuary (Croatia). Sustainability, 16(5), 1800. https://doi.org/10.3390/su16051800







