In-Line Co-Processing of Stainless Steel Pickling Sludge Using Argon Oxygen Decarburization Slag Bath: Behavior and Mechanism
Abstract
1. Introduction
2. Experimental
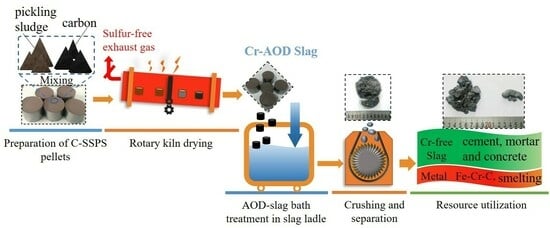
2.1. Technical Route
- (a)
- Stainless steel pickling sludge (SSPS) is initially mixed with a reducing agent and extruded into briquettes with diameters ranging from 15–20 mm.
- (b)
- The briquettes are calcined at temperatures up to 800 °C to achieve adequate strength, with each briquette exhibiting a compressive strength exceeding 1800 N. The density of the briquette is 1.45 g/cm3.
- (a)
- The calcined briquettes are pre-loaded into a slag pot.
- (b)
- The slag discharged from the AOD furnace creates a high-temperature mixed slag flushing pool within the slag bag.
- (c)
- At elevated temperatures, the sludge briquettes cooperatively reduce valuable metals present in the chromium-containing slag.
- (d)
- The cooled slag is crushed, and metal particles are recovered via magnetic separation for use in the smelting of stainless steel products.
- (e)
- The chromium-free residue can be recycled/reused as part of building materials such as cement and concrete.
2.2. Materials
2.3. Pre-Reduction and Slag Bath Reduction
2.4. Characterization and Evaluation
2.4.1. Thermodynamic Analysis
2.4.2. Composition and Phase Analysis
2.4.3. TG-MS Analysis
2.4.4. Slag Bath Reduction Analysis
3. Results and Discussion
- A.
- Thermodynamic model of pre-reduction and slag-bath smelting
- B.
- Pre-reduction stage of C-SSPS
- Thermal analysis and gas release
- 2.
- Mineral phase formation of the product
- C.
- Slag bath reduction stage
- Sulfide release and reduction rate
- 2.
- Phase transformation at the interface between briquettes and slag
- D.
- Slag Bath Reduction Mechanism and Model Establishment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, Z.; Ding, X.; Yan, X.; Dong, Y.; Liu, C. Recovery of Iron, Chromium, and Nickel from Pickling Sludge Using Smelting Reduction. Metals 2018, 8, 936. [Google Scholar] [CrossRef]
- Junxue, Z.; Zhongyu, Z.; Ruimeng, S.; Xiaoming, L.; Yaru, C. Issues relevant to recycling of stainless-steel pickling sludge. JOM 2018, 70, 2825–2836. [Google Scholar] [CrossRef]
- Yang, C.; Pan, J.; Zhu, D.; Guo, Z.Q.; Li, X.M. Pyrometallurgical recycling of stainless-steel pickling sludge: A review. J. Iron Steel Res. Int. 2019, 26, 547–557. [Google Scholar] [CrossRef]
- Li, X.; Lv, M.; Yin, W.D.; Zhao, J.X.; Cui, Y.R. Desulfurization thermodynamics experiment of stainless-steel pickling sludge. J. Iron Steel Res. Int. 2019, 26, 519–528. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. National Hazardous Waste Directory (New Version), Reference No. 000014672/2016-00564. Available online: http://www.mee.gov.cn/gkml/hbb/bl/201606/t20160621_354852.htm (accessed on 21 June 2016).
- Regel-Rosocka, M. A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Rao, M.; Luo, J.; Zhang, X.; You, J.; Jiang, T. Coprocessing of Stainless-Steel Pickling Sludge with Laterite Ore via Rotary Kiln-Electric Furnace Route: Enhanced Desulfurization and Metal Recovery. Process Saf. Environ. Prot. 2020, 142, 92–98. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Chou, K. Preparation of Low-Carbon and Low-Sulfur Fe-Cr-Ni-Si Alloy by Using CaSO4-Containing Stainless-steel Pickling Sludge. Metall. Mater. Trans. B 2020, 51, 2057–2067. [Google Scholar] [CrossRef]
- Wu, M.; Li, Y.L.; Guo, Q.; Shao, D.W.; He, M.M.; Qi, T. Harmless treatment and resource utilization of stainless-steel pickling sludge via direct reduction and magnetic separation. J. Clean. Prod. 2019, 240, 118187. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.; Wang, Z.; Yang, F.; Lu, X. Metals droplet assembling mechanism during carbon reduction of stainless-steel pickling sludge. J. Clean. Prod. 2020, 247, 119580. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, B.; Ding, Y.; Zhang, J.; Wen, Q.; Ekberg, C.; Zhang, S. Study on glass-ceramics made from MSWI fly ash, pickling sludge and waste glass by one-step process. J. Clean. Prod. 2020, 271, 122674. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, S.G.; Pan, D.A.; Liu, B.; Wu, C.L.; Volinsky, A.A. Treatment method of hazardous pickling sludge by reusing as glass–ceramics nucleation agent. Rare Met. 2016, 35, 269–274. [Google Scholar] [CrossRef]
- Pan, D.A.; Li, L.J.; Yang, J.; Bu, J.B.; Guo, B.; Liu, B.; Zhang, S.G.; Volinsky, A.A. Production of Glass–Ceramics from Heavy Metal Gypsum and Pickling Sludge. Int. J. Environ. Sci. Technol. 2015, 12, 3047–3052. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E.; Nordström, U. Physicochemical and mineralogical properties of stainless-steel slags oriented to metal recovery. Resour. Conserv. Recycl. 2004, 40, 245–271. [Google Scholar] [CrossRef]
- Tossavainen, M.; Engstrom, F.; Yang, Q.; Menad, N.; Larsson, M.L.; Bjorkman, B. Characteristics of steel slag under different cooling conditions. Waste Manag. 2007, 27, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Kriskova, L.; Pontikes, Y.; Cizer, Ö.; Mertens, G.; Veulemans, W.; Geysen, D.; Blanpain, B. Effect of mechanical activation on the hydraulic properties of stainless steel slags. Cem. Concr. Res. 2012, 42, 778–788. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Wang, Z.; Zeng, Y.; Ren, Q. Long-term leaching characterization and geochemical modeling of chromium released from AOD slag. Environ. Sci. Pollut. Res. 2020, 27, 921–929. [Google Scholar] [CrossRef]
- Kriskova, L.; Pontikes, Y.; Zhang, F.; Cizer, Ö.; Jones, P.T.; Van Balen, K.; Blanpain, B. Influence of mechanical and chemical activation on the hydraulic properties of gamma dicalcium silicate. Cem. Concr. Res. 2014, 55, 59–68. [Google Scholar] [CrossRef]
- Park, B.; Choi, Y.C. Investigation of carbon-capture property of foam concrete using stainless-steel AOD slag. J. Clean. Prod. 2021, 288, 125621. [Google Scholar] [CrossRef]
- Moon, E.J.; Choi, Y.C. Development of carbon-capture binder using stainless-steel argon oxygen decarburization slag activated by carbonation. J. Clean. Prod. 2018, 180, 642–654. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zeng, Y.N.; Li, J.G.; Zhang, Y.Z.; Zhang, Y.J.; Zhao, Q.Z. Carbonation of argon oxygen decarburization stainless-steel slag and its effect on chromium leachability. J. Clean. Prod. 2020, 256, 120377. [Google Scholar] [CrossRef]
- Bale, C.; Belisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Zhao, J.; Su, H.; Zuo, H.; Wang, J.; Xue, Q. The Mechanism of Preparation Calcium Ferrite from Desulfurization Gypsum Produced in Sintering. J. Clean. Prod. 2020, 267, 122002. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y.; Zhou, S.; Jiang, J.; Huang, X.; Hua, J. Status of research on the resource utilization of stainless steel pickling sludge in China: A review. Environ. Sci. Pollut. Res. 2023, 30, 90223–90242. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.; Huang, Y.; Zhang, Z.; He, F.; Yang, H. A Sustainable Process for the Resource Utilization and Stabilization Disposal of Stainless Steel Pickling Sludge. JOM 2022, 74, 3910–3920. [Google Scholar] [CrossRef]
- Pickles, C.A. Thermodynamic analysis of the selective carbothermic reduction of electric arc furnace dust. J. Hazard. Mater. 2008, 150, 265–278. [Google Scholar] [CrossRef]
- Al-Harahsheh, M. Thermodynamic analysis on the thermal treatment of electric arc furnace dust-PVC blends. Arab. J. Sci. Eng. 2018, 43, 5757–5769. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Y.; Zhao, Z.; Yuan, F. Effects of Fe2O3 on Reduction Process of Cr-Containing Solid Waste Self-Reduction Briquette and Relevant Mechanism. Metals 2019, 9, 51. [Google Scholar] [CrossRef]
- Mori, T.; Yang, J.; Kuwabara, M. Mechanism of Carbothermic Reduction of Chromium Oxide. ISIJ Int. 2007, 47, 1387–1393. [Google Scholar] [CrossRef]







| Raw Material | F | Na | S | Cr | Ca | Fe | Ni | Si | Al | O |
|---|---|---|---|---|---|---|---|---|---|---|
| SSPS | 0.22 | 0.74 | 13.98 | 2.88 | 18.6 | 21 | 0.1 | 1.05 | 0.29 | 40.4 |
| Raw Material | CaO | SiO2 | Al2O3 | MgO | Cr2O3 | MnO | CaF2 | Fe2O3 | S |
|---|---|---|---|---|---|---|---|---|---|
| AOD slag | 50.6 | 31.1 | 1.2 | 5.9 | 0.5 | 3.7 | 5.4 | 0.21 | 0.19 |
| No. | Phase |
| 1 | C |
| 2 | CaSO4 |
| 3 | FeCr2O4-CaSO4-CaS-FeS |
| 4 | CaS |
| No. | Phase |
|---|---|
| 7, 11 | C |
| 6, 9 | CaS |
| 5, 12 | C-Fe-Cr |
| 8 | CaF2-Ca2SiO4 |
| 10, 13 | Fe-Cr |
| I | FeCr2O4-CaS-(Fe-Cr-C) |
| II | Ca3SiO5-Mg(Fe,Cr)2O4 |
| III, VI | Ca2MgSi2O7 |
| IV | (Fe-Cr-C)-CaS |
| V | Mg(Al,Cr)O4-Ca2MgSi2O7 |
| VII | Ca2MgSi2O7-Ca2Al2SiO7-CaS-FeS-MnS-MgCr2O4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Zhang, Y.; Yuan, F.; Wu, T. In-Line Co-Processing of Stainless Steel Pickling Sludge Using Argon Oxygen Decarburization Slag Bath: Behavior and Mechanism. Sustainability 2024, 16, 1895. https://doi.org/10.3390/su16051895
Zhao Z, Zhang Y, Yuan F, Wu T. In-Line Co-Processing of Stainless Steel Pickling Sludge Using Argon Oxygen Decarburization Slag Bath: Behavior and Mechanism. Sustainability. 2024; 16(5):1895. https://doi.org/10.3390/su16051895
Chicago/Turabian StyleZhao, Zheng, Yanling Zhang, Fang Yuan, and Tuo Wu. 2024. "In-Line Co-Processing of Stainless Steel Pickling Sludge Using Argon Oxygen Decarburization Slag Bath: Behavior and Mechanism" Sustainability 16, no. 5: 1895. https://doi.org/10.3390/su16051895
APA StyleZhao, Z., Zhang, Y., Yuan, F., & Wu, T. (2024). In-Line Co-Processing of Stainless Steel Pickling Sludge Using Argon Oxygen Decarburization Slag Bath: Behavior and Mechanism. Sustainability, 16(5), 1895. https://doi.org/10.3390/su16051895