Rest Grazing from the Critical Period of Soil Thawing in Alpine Meadow of Tibetan Plateau Is Conducive to the Sexual Reproduction of Polygonum viviparum
Abstract
:1. Introduction
2. Materials and Methods
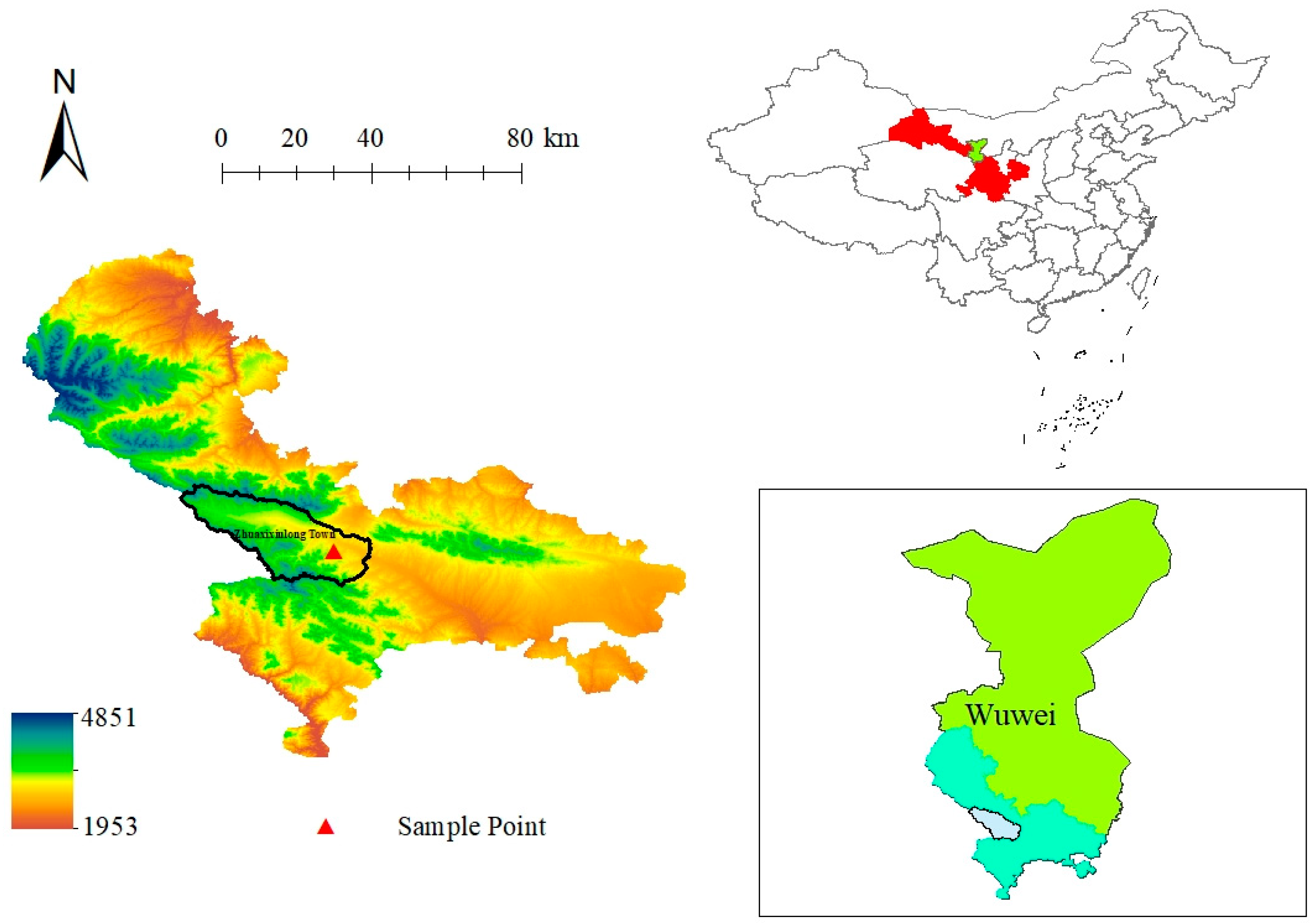
2.1. Test Plot Overview
2.2. Plot Setting
2.3. Determination Indices and Methods
2.4. Data Analysis
3. Results
3.1. Effects of Different Periods of Rest Grazing on the Biomass of Each Component of P. viviparum
3.1.1. Aboveground Biomass and Underground Biomass
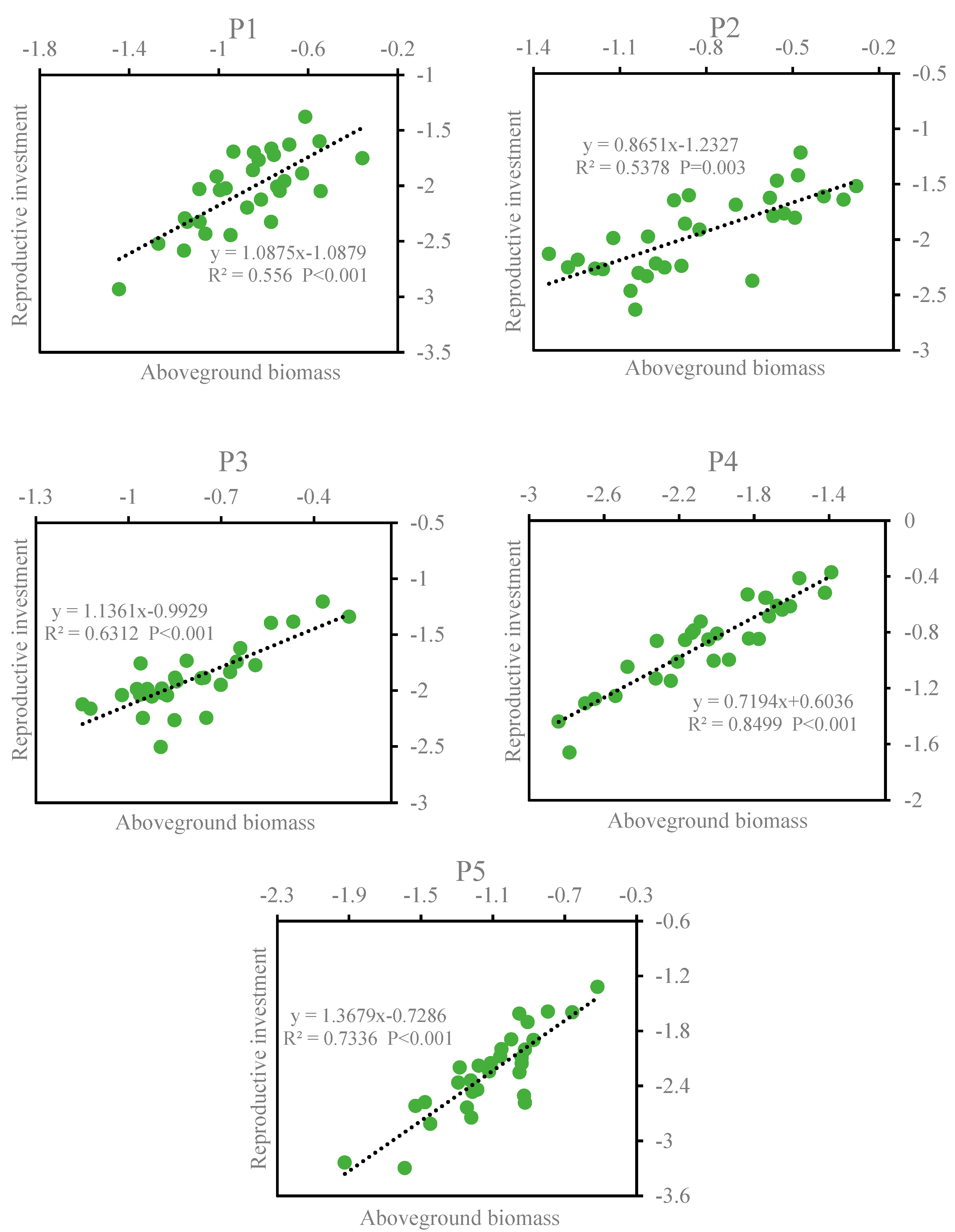
3.1.2. The Relationship between Individual Size and Reproductive Investment
3.1.3. The Plant Biomass Allocation Ratio
3.2. Effects of Rest Grazing at Different Periods on Reproductive Characteristics of P. viviparum
3.2.1. The Flower and Bulbil Related Indicators
3.2.2. The Seed Yield and Its Components
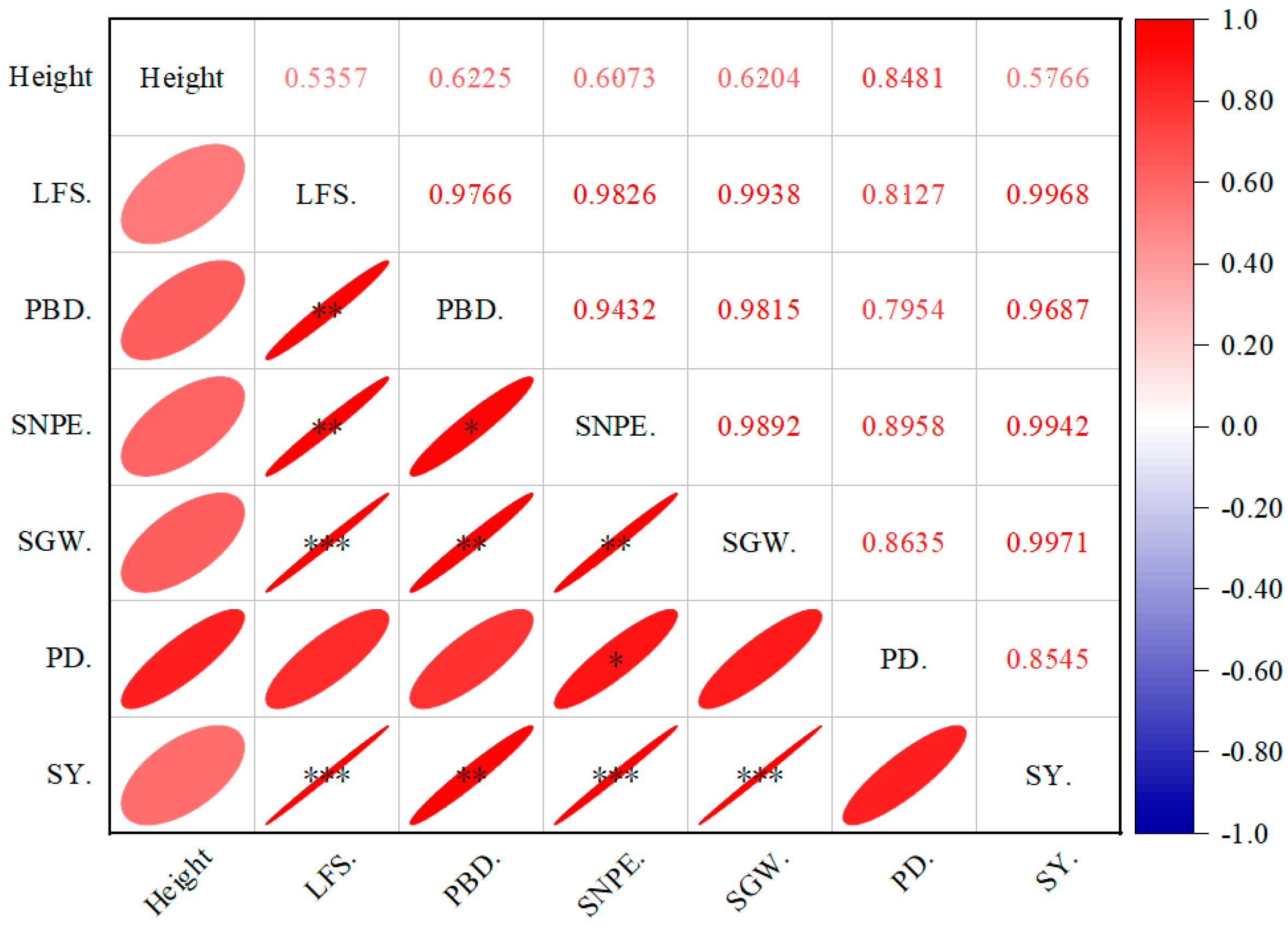
3.2.3. The Seed Yield and Related Indexes of P. viviparum
3.2.4. Effects of Different Periods of Rest Grazing on Seed Quality of P. viviparum
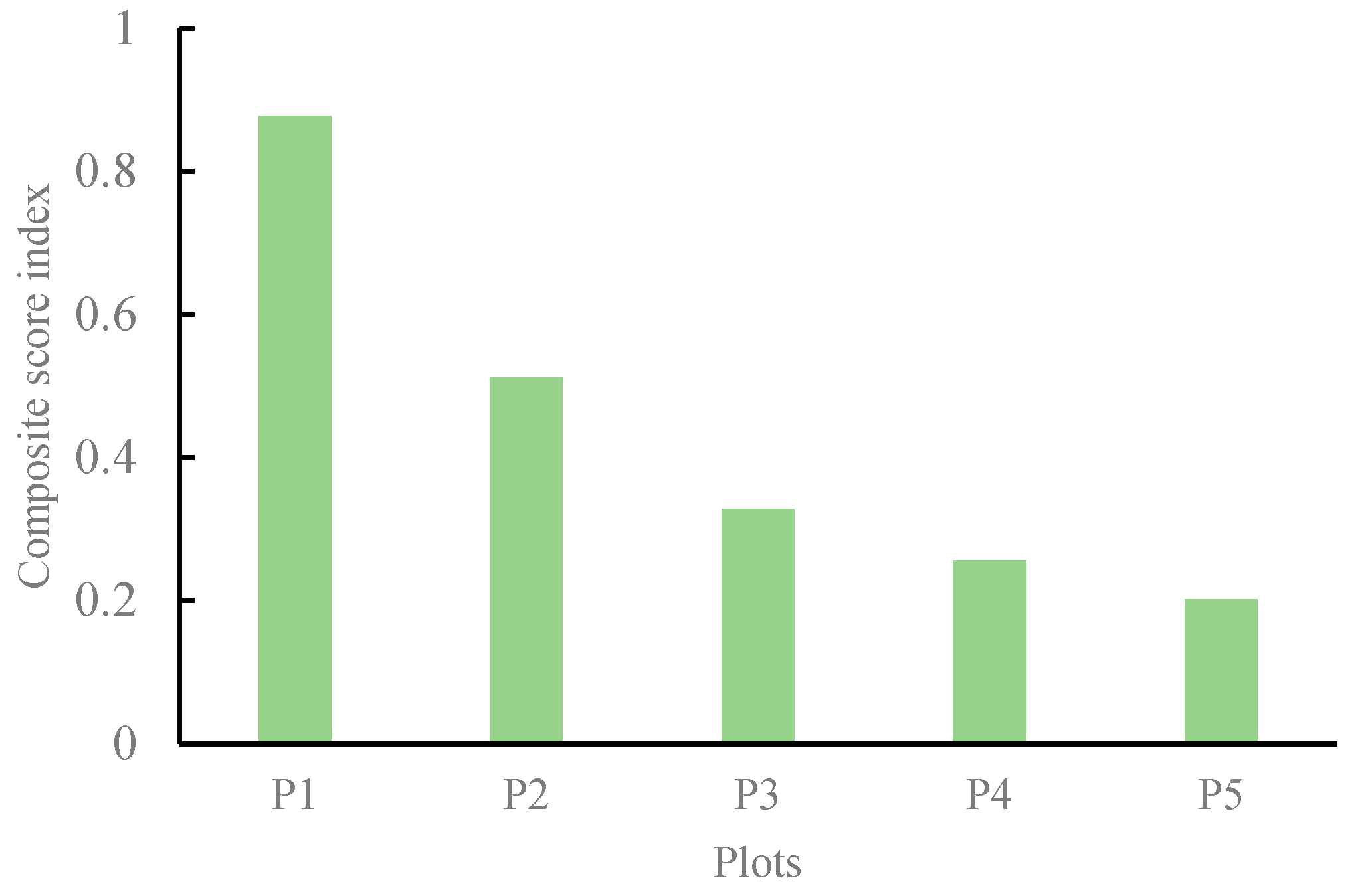
3.3. Comprehensive Evaluation of P. viviparum Traits under Different Periods of Rest Grazing Treatments
4. Discussion
4.1. Effects of Rest Grazing at Different Stages on Biomass of Each Component of P. viviparum
4.2. Effects of Rest Grazing at Different Stages on Reproductive Characteristics of P. viviparum
4.3. The Effects of Different Periods of Rest Grazing on P. viviparum Seeds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griscom, B.W. Natural climate solutions. Proc. Natl. Acad. Sci. USA 2019, 116, 2776. [Google Scholar] [CrossRef]
- Li, P.; Zhu, Q.; Peng, C.H.; Zhang, J.; Wang, M.; Zhang, J.J.; Ding, J.H.; Zhou, X.L. Change in autumn vegetation phenology and the climate controls from 1982 to 2012 on the Qinghai-Tibet Plateau. Front. Plant Sci. 2020, 10, 1677. [Google Scholar] [CrossRef]
- Nie, Z.N.; Zollinger, R.P.; Behrendt, R. Impact of deferred grazing and fertilizer on herbage production, soil seed reserve and nutritive value of native pastures in steep hill country of southern Australia. Grass Forage Sci. 2015, 70, 394–405. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.X.; Ding, X.M.; Zhang, N.; Mu, C.S. Research Advances of Plant Reproductive Strategy Response to Global Climate Change. North. Hortic. 2014, 19, 194–198. [Google Scholar]
- Zhang, D.Y. Evolution of Plant Life History and Reproductive Ecology; Science Press: Beijing, China, 2004. [Google Scholar]
- Niu, C.J.; Lou, A.R.; Sun, R.Y.; Li, Q.F. Basic Ecology, 3rd ed.; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Zhang, C.; An, Y.M.; Yun, J.; Wang, L.L.; Zhou, Z.L.; Wang, L.P.; Yang, Y.P.; Duan, Y.W. Processes on reproductive ecology of plant species in the Qinghai-Xizang Plateau and adjacent highlands. Chin. J. Plant Ecol. 2020, 44, 1–21. [Google Scholar] [CrossRef]
- Bai, X. Effects of Enclosure Duration on Offspring Recruitment and Bud Bank in Semiarid Steppe on the Loess Plateau. Master’s Thesis, Henan University of Science and Technology, Luoyang, China, 2017. [Google Scholar] [CrossRef]
- Wei, X.J.; Du, J.; Wang, W.; Pei, S.Q. Effect of Different Treatments on Seed Germination of Polygonum viviparum in Alpine Areas. J. Grassl. Forage Sci. 2022, 2, 28–35. [Google Scholar]
- Qian, Z.M.; Chen, L.; Wu, M.Q.; Li, D.Q. Rapid screening and characterization of natural antioxidants in Polygonum viviparum by an on-line system integrating the pressurised liquid micro-extraction, HPLC-DAD-QTOF-MS/MS analysis and antioxidant assay. J. Chromatogr. B 2020, 1137, 121926. [Google Scholar] [CrossRef]
- Martin, R.B. Genetic Diversity and Ecotypic Differentiation in Arctic and Alpine Populations of Polygonum viviparum. Arct. Alp. Res. 2018, 28, 190–195. [Google Scholar] [CrossRef]
- Song, X.Y.; Nie, J.L.; Yang, M.H.; Yu, M.C.; Tao, L.M.; Feng, H.Y.; Pan, J.B. Photosynthetic characteristics and reproductive strategies of Polygonum viviparium at different altitudes in the Qilian Mountains. Chin. J. Appl. Environ. Biol. 2022, 28, 1527–1533. [Google Scholar] [CrossRef]
- Wang, C.; Lu, J. Research progress in response of plant reproductive strategies to altitude. Hubei Agric. Sci. 2021, 60, 11–14+50. [Google Scholar] [CrossRef]
- Wang, B.H. Effects of Different Sites and Habitats on Growth, Reproduction and Pest Infection of Polygonum viviparum. Nongcun Shiyong Jishu 2022, 1, 76–78. [Google Scholar]
- Pan, L.M.; Meng, L.H. Effect of fenced enclosure on the reproductive performance of Polygonum viviparum. J. Gansu Agric. Univ. 2021, 56, 103–108. [Google Scholar] [CrossRef]
- Bai, M.M.; Wei, K.T.; Ma, K.K.; Xu, C.L.; Yu, X.J. Rest grazing from the critical period of soil thawing promotes the propagation of Kobresia humilis in alpine meadow. Ecol. Eng. 2022, 179, 106634. [Google Scholar] [CrossRef]
- Peng, Z.; Bai, M.M.; Xu, C.L.; Yu, X.J. Effects of different rest grazing periods on the reproduction and root characteristics of Carex capillifolia in subalpine meadow. Glob. Ecol. Conserv. 2022, 38, e02248. [Google Scholar] [CrossRef]
- Ma, K.K.; Xu, C.L.; Yu, X.J.; Liu, Y.Y.; Yang, H.; Wei, K.T.; Jing, Y.Y.; Jiang, J.C.; Wang, H. Rest grazing start from the critical period of soil thawing optimizes plant community characteristics and grassland grazing capacity in alpine meadows. Ecol. Eng. 2022, 183, 106763. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 9th ed.; United States Department of Agriculture and Natural Resources Conservation Service: Washington, DC, USA, 2003.
- Guo, N.; Wang, A.D.; Degen, A.A.; Deng, B.; Shang, Z.H.; Ding, L.M.; Long, R.J. Grazing exclusion increases soil CO2 emission during the growing season in alpine meadows on the Tibetan Plateau. Atmos. Environ. 2018, 174, 92–98. [Google Scholar] [CrossRef]
- Xu, P. Grassland Resource Inventory and Planning; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Wang, Y.P.; He, W.L.; Cheng, L.X. Changes of pigment contents and photosynthetic electron transport activities of thylakoid membranes of Polygonum viviparum grown at different altitudes. Acta Prataculturae Sin. 2011, 20, 75–81. [Google Scholar] [CrossRef]
- Pan, T.T. Effect of Simulated Tibetan Sheep and Yak Trampling in Different Periods on the Reproductive Characteristics, Underground Morphology and Carbohydrate Content of Kobresia humilis. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2019. [Google Scholar] [CrossRef]
- Meng, J.L. Reproductive Strategies of Three Common Species of Ranunculaceae at Alpine Meadow in Qinghai Tibetan Plateau. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2010. [Google Scholar]
- Wang, Y.P.; Gao, H.H.; Zhang, F.; Chen, L.X.; Sun, W.B. Altitudinal phenotypic plasticity of leaf characteristics of Polygonum viviparum. J. Appl. Ecol. 2021, 32, 2070–2078. [Google Scholar] [CrossRef]
- Weppler, T.; Stöcklin, J. Variation of sexual and clonal reproduction in the alpine Geum reptans in contrasting altitudes and successional stages. Basic Appl. Ecol. 2005, 6, 305–316. [Google Scholar] [CrossRef]
- Teng, X. Effects of Sheep Ingesting and Tramping in Leymus chinensis Grassland. Ph.D. Thesis, Northeast Normal University, Changchun, China, 2010. [Google Scholar]
- Chen, T.; Christensen, M.; Nan, Z.B.; Hou, F.J. The effects of different intensities of long-term grazing on the direction and strength of plant-soil feedback in a semiarid grassland of Northwest China. Plant Soil 2017, 413, 303–317. [Google Scholar] [CrossRef]
- Gifford, R.M.; Thorne, J.H.; Hitz, W.D.; Giaquinta, R.T. Crop productivity and photoassimilate partitioning. Science 1984, 225, 801–808. [Google Scholar] [CrossRef] [PubMed]

| Treatments | Rest Grazing Settings | Actual Rest Grazing Period | Actual Grazing Period | Number of Grazing Livestock (Adults) | Sample Area (m2) |
|---|---|---|---|---|---|
| P1 | Critical period of soil thawing–plant withering period | 19 March~28 February of the following year | 1 March~18 March | 4 (Yaks + Tibetan Sheep) | 1881 |
| P2 | Late period of soil Thawing plant withering period | 2 April~28 February of the following year | 1 March~1 April | 4 (Yaks + Tibetan Sheep) | 3344 |
| P3 | Early period of grass revival–plant withering period | 16 April~28 February of the following year | 1 March~15 April | 4 (Yaks + Tibetan Sheep) | 4807 |
| P4 | Late period of grass revival–plant withering period | 2 May~28 February of the following year | 1 March~1 May | 4 (Yaks + Tibetan Sheep) | 6478 |
| P5(CK) | Period of local traditional rest grazing–plant withering period | 21 May~28 February of the following year | 1 March~20 May | 16 (Yaks + Tibetan Sheep) | 33,855 |
| Treatments | Biomass of Flowers/mg | Biomass of Bulbils/mg | Biomass of Stalks/g | Biomass of Leaves/mg | Underground Biomass/mg |
|---|---|---|---|---|---|
| P1 | 19.44 ± 3.18 a | 153.93 ± 9.15 a | 119.82 ± 8.64 a | 198.23 ± 10.57 a | 553.00 ± 50.41 a |
| P2 | 8.04 ± 2.37 b | 143.82 ± 10.40 ab | 115.62 ± 6.04 ab | 193.95 ± 10.27 a | 574.52 ± 61.34 a |
| P3 | 3.18 ± 1.05 bc | 135.37 ± 9.65 abc | 109.24 ± 3.61 ab | 186.39 ± 12.96 a | 564.68 ± 64.90 a |
| P4 | 2.19 ± 0.82 c | 117.94 ± 9.24 bc | 100.68 ± 6.52 b | 174.89 ± 11.26 ab | 563.37 ± 51.88 a |
| P5(CK) | 2.02 ± 0.62 c | 112.37 ± 10.33 c | 76.81 ± 4.16 c | 149.96 ± 8.10 b | 549.55 ± 44.81 a |
| p-value | 0.000 | 0.019 | 0.000 | 0.014 | 0.998 |
| Treatments | Biomass Proportion of Asxeual Reproduction Organs (%) | Biomass Proportion of Sexual Reproduction Organs (%) | Proportion of Aboveground Biomass (%) | Proportion of Underground Biomass (%) | Root–Crown Ratio |
|---|---|---|---|---|---|
| P1 | 15.68 ± 1.11 a | 1.93 ± 0.34 a | 46.28 ± 2.63 ab | 53.72 ± 2.63 ab | 1.39 ± 0.15 a |
| P2 | 15.21 ± 1.30 a | 0.76 ± 0.22 b | 47.22 ± 2.56 ab | 52.78 ± 2.56 ab | 1.36 ± 0.17 a |
| P3 | 14.54 ± 1.16 a | 0.33 ± 0.12 b | 48.65 ± 2.79 a | 51.35 ± 2.79 b | 1.31 ± 0.16 a |
| P4 | 12.82 ± 1.04 a | 0.27 ± 0.11 b | 45.96 ± 2.74 ab | 54.04 ± 2.74 ab | 1.48 ± 0.19 a |
| P5 | 13.12 ± 1.33 a | 0.24 ± 0.08 b | 40.16 ± 2.42 b | 59.84 ± 2.42 a | 1.81 ± 0.20 a |
| p-value | 0.179 | 0.000 | 0.199 | 0.199 | 0.271 |
| Treatments | Length of the Flower Spike(cm) | Proportion of Flower Spike Length per Plant (%) | Length of Bulbous Spike (cm) | Proportion of Bulbils Spike Length per Plant (%) | Number of Flowers | Proportion of Flower Number per Plant (%) | Number of Bulbils | Proportion of Bulbil Number per Plant (%) | Flower Size (mg) | Bulbil Size (mg) |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 2.34 ± 0.27 a | 8.62 ± 0.99 a | 5.46 ± 1.27 a | 10.70 ± 0.89 b | 48.67 ± 7.67 a | 39.76 ± 4.63 a | 49.73 ± 2.17 a | 60.24 ± 4.63 c | 0.47 ± 0.14 a | 3.39 ± 0.14 a |
| P2 | 0.97 ± 0.20 b | 3.61 ± 0.75 b | 5.25 ± 1.67 a | 15.50 ± 0.92 b | 17.10 ± 4.25 b | 21.14 ± 4.59 b | 49.50 ± 3.10 a | 78.86 ± 4.59 b | 0.20 ± 0.05 b | 2.95 ± 0.13 ab |
| P3 | 0.48 ± 0.14 bc | 1.32 ± 0.41 b | 4.03 ± 0.21 a | 17.30 ± 4.64 b | 8.03 ± 2.48 b | 13.00 ± 3.49 bc | 45.87 ± 2.23 a | 87.00 ± 3.49 ab | 0.107 ± 0.03 b | 2.40 ± 0.12 ab |
| P4 | 0.48 ± 0.13 bc | 2.86 ± 1.09 b | 3.42 ± 0.23 a | 25.73 ± 6.36 b | 6.37 ± 1.79 b | 12.20 ± 3.44 bc | 45.43 ± 3.14 a | 87.91 ± 3.44 ab | 0.17 ± 0.04 b | 2.32 ± 0.10 ab |
| P5 | 0.37 ± 0.11 c | 3.42 ± 1.40 b | 2.76 ± 0.20 a | 49.32 ± 5.10 a | 5.87 ± 2.20 b | 8.95 ± 2.81 c | 43.80 ± 3.22 a | 91.06 ± 2.81 a | 0.105 ± 0.03 b | 1.77 ± 0.12 b |
| p-value | 0.000 | 0.000 | 0.209 | 0.005 | 0.000 | 0.000 | 0.482 | 0.000 | 0.003 | 0.083 |
| Treatments | Height (cm) | Length of Flower Spike (cm) | Panicle Basal Diameter (mm) | Seed Number per Ear | Single Grain Weight (mg) | Plant Density (m2) | Seed Yield (kg·hm−2) |
|---|---|---|---|---|---|---|---|
| P1 | 26.81 ± 0.75 a | 2.34 ± 0.27 a | 2.00 ± 1.48 a | 21.64 ± 2.56 a | 0.73 ± 0.37 a | 39.20 ± 3.57 a | 6.16 ± 0.14 a |
| P2 | 26.59 ± 0.64 a | 0.97 ± 0.20 b | 1.06 ± 0.03 a | 16.00 ± 1.13 ab | 0.45 ± 0.03 a | 36.80 ± 4.04 ab | 2.62 ± 0.41 ab |
| P3 | 26.32 ± 1.21 a | 0.483 ± 0.14 bc | 1.02 ± 0.04 a | 11.92 ± 2.20 b | 0.35 ± 0.03 a | 26.40 ± 4.46 bc | 0.97 ± 0.10 b |
| P4 | 24.11 ± 1.12 a | 0.48 ± 0.13 bc | 0.94 ± 0.04 a | 11.24 ± 2.40 b | 0.31 ± 0.03 a | 21.60 ± 2.73 c | 0.78 ± 0.21 b |
| P5 (CK) | 19.07 ± 1.20 b | 0.37 ± 0.11 c | 0.70 ± 0.06 a | 10.80 ± 0.42 b | 0.27 ± 0.02 a | 15.80 ± 3.77 c | 0.45 ± 0.11 b |
| p-value | 0.000 | 0.000 | 0.685 | 0.000 | 0.329 | 0.001 | 0.000 |
| Treatments | Seed Length/mm | Seed Edge Length/mm | Seed Size/mm3 | Seed Setting Rate/% | Plump Grain Weight Ratio/% | Plump Grain Number Ratio/% |
|---|---|---|---|---|---|---|
| P1 | 2.89 ± 0.04 a | 1.43 ± 0.04 a | 3.85 ± 0.19 a | 61.49 ± 2.72 a | 33.81 ± 1.78 a | 31.62 ± 9.93 a |
| P2 | 2.90 ± 0.05 a | 1.40 ± 0.03 a | 3.71 ± 0.24 a | 49.09 ± 2.94 ab | 33.17 ± 0.94 a | 26.27 ± 1.03 ab |
| P3 | 2.70 ± 0.05 b | 1.40 ± 0.03 a | 3.44 ± 0.17 a | 39.89 ± 4.18 bc | 32.98 ± 5.05 a | 25.30 ± 1.32 ab |
| P4 | 2.83 ± 0.07 ab | 1.39 ± 0.03 a | 3.58 ± 0.16 a | 39.84 ± 7.54 bc | 31.22 ± 7.21 a | 17.02 ± 3.83 ab |
| P5 (CK) | 2.77 ± 0.04 ab | 1.38 ± 0.03 a | 3.44 ± 0.18 a | 33.64 ± 2.90 c | 29.77 ± 4.72 a | 13.29 ± 1.90 b |
| p-value | 0.029 | 0.842 | 0.411 | 0.003 | 0.969 | 0.095 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, M.; Xu, J.; Wei, K.; Jing, Y.; Xu, C.; Yu, X. Rest Grazing from the Critical Period of Soil Thawing in Alpine Meadow of Tibetan Plateau Is Conducive to the Sexual Reproduction of Polygonum viviparum. Sustainability 2024, 16, 1984. https://doi.org/10.3390/su16051984
Bai M, Xu J, Wei K, Jing Y, Xu C, Yu X. Rest Grazing from the Critical Period of Soil Thawing in Alpine Meadow of Tibetan Plateau Is Conducive to the Sexual Reproduction of Polygonum viviparum. Sustainability. 2024; 16(5):1984. https://doi.org/10.3390/su16051984
Chicago/Turabian StyleBai, Meimei, Jingjing Xu, Kongtao Wei, Yuanyuan Jing, Changlin Xu, and Xiaojun Yu. 2024. "Rest Grazing from the Critical Period of Soil Thawing in Alpine Meadow of Tibetan Plateau Is Conducive to the Sexual Reproduction of Polygonum viviparum" Sustainability 16, no. 5: 1984. https://doi.org/10.3390/su16051984




