Abstract
The management of water resources is a complex issue, and the conservation of fish and fishery resources is a growing challenge given the increase in the number of hydroelectric projects in Brazilian river basins. This study describes the fragmentation of the Tocantins–Araguaia River Basin resulting from the installation of hydroelectric plants in operation and planned by the electricity sector until 2050, as well as its relationship with the conservation of fish fauna, especially long-distance migratory species. The hydroelectric projects in operation and planned were analyzed using data obtained from the National Electric Energy Agency. A literature review was carried out to assess the fish species of the Tocantins–Araguaia ecoregion, with an emphasis on long-distance migrants. In general, 75 hydroelectric dams are in operation, and a further 119 projects are in the electricity sector’s plans for construction by 2050 in the Tocantins–Araguaia ecoregion, including a stretch of the basin above the Tucurui dam, which will accentuate the fragmentation in the area. Of the 702 species found in the region, 31.1% are endemic, 6.1% are endangered, and several long-distance migratory species have had their populations restricted. Analysis of this information highlights the widespread impact on the ichthyofauna, affecting both species with a restricted distribution and migratory species with a long-distance distribution. The studies associated with the projects contribute to the advancement of knowledge, but they are only carried out after the works have been defined, which makes it difficult to plan conservation in advance. Transformations in the basin are imminent given the current changes and those expected in the coming years because of the electricity sector’s planning for the region. Considering the inseparable relationship between biodiversity and socio-environmental and cultural diversity, fish conservation is intrinsically linked to the conservation of socio-diversity and the effective participation of local communities from the start of the process. Fish depend on water, and people need both water and aquatic diversity. In conclusion, a well-structured and adaptative conservation plan, combined with the integration of effective fish routes, can contribute to the sustainable development of hydroelectric projects while safeguarding the biodiversity and ecological integrity of the Tocantins–Araguaia Basin.
1. Introduction
The management of fishery resources is dynamic, and the challenges are escalating in the face of the growing expansion of hydroelectric plants, driven by technological, infrastructural, and social advancements that create competition for water resources. With the increasing number of hydroelectric projects and the accumulation of their effects, whether synergistic or not, it has become increasingly difficult to reconcile the production of hydroelectricity with the conservation of socio-environmental diversity, such as fishing. This process becomes more complex as dependence on energy increases and areas of knowledge become more specialized, making dialogue more difficult [1].
Fish communities are characterized by their dynamism and ability to adapt to biotic and abiotic pressures, regardless of their origin. Their composition is influenced by the history of connections between individuals and populations in the aquatic environment, as well as by environmental variations, including the types of environments (lentic and lotic) and climatic conditions (droughts, floods, and temperature variations) of the systems where they evolved [2,3]. The ability of these populations to adapt to environmental changes, regardless of their cause, is influenced by the geological history of the river basins and the evolution of the ichthyofauna. Evolutionary factors influencing fish communities include environmental diversity and abiotic and biotic factors [4,5], which are interconnected and have relations with the socio-environmental and cultural diversity of each region.
In the context of water resources management, energy planning conflicts with the conservation of fishery resources and fishing, especially concerning the electric sector. While management for biodiversity conservation seeks to maintain the diversity of species associated with systems with a view to their resilience [6], in the electricity sector, maximizing the extraction and conversion of hydraulic energy into electricity leads to the fragmentation of rivers and the homogenization of large stretches of river basins. While energy planning addresses the basin as a whole, the conservation of fishery resources is, in most cases, compartmentalized, and the cumulative and synergistic impacts of hydroelectric projects on fishery resources are postponed or disregarded.
Reservoirs that accumulate water and homogenize the environment upstream of dams can act as filters and select species that are adapted to lentic flows to the detriment of others [7,8]. On the other hand, the desiccation of the downstream stretches has an effect on the abundance of the ichthyofauna due to the reduction in connectivity with the lateral environments to the river and the irregularity of the level due to the operation of the projects. Therefore, the location of dams in river basins has different effects on the characteristics of the environment, the ichthyofauna and, consequently, the fishing systems [1,9].
Migratory fish populations are the main ones impacted by hydroelectric dams. These species are the main components traded in freshwater fisheries and leave thousands of people who depend on this activity for their livelihoods with no alternative worldwide [10]. Thus, the rate of the implementation of hydroelectric projects in water bodies has accelerated in recent decades, and concern about the conservation of fisheries resources and socio-environmental issues is pressing.
In South America, specifically in the Tocantins–Araguaia Basin, located in Brazilian territory, hydroelectric dams have been advancing rapidly, similar to the processes occurring in the Paraná and São Francisco river basins [8]. On Tocantins River, there are seven large dams in operation, Tucuruí (1984), Serra da Mesa (1998), Lajeado (2001), Cana Brava (2002), Peixe Angical (2006), São Salvador (2009), and Estreito (2011), the last five of which were built in about a decade, as well as several other generating 30 MW or less, especially on smaller rivers and in the higher reaches of the basin.
The intensification of the occupation process in the Tocantins–Araguaia Basin [11] has increased the complexity of water resource management and the conservation of fishery resources, with the increase in the number of hydroelectric dams being one of the main factors. This study describes the fragmentation of the Tocantins–Araguaia Basin resulting from the installation of hydroelectric dams that are currently in operation and those planned by the electricity sector until 2025, as well as its relationship with the conservation of fish fauna, especially large migratory fish.
2. Materials and Methods
2.1. Study Area
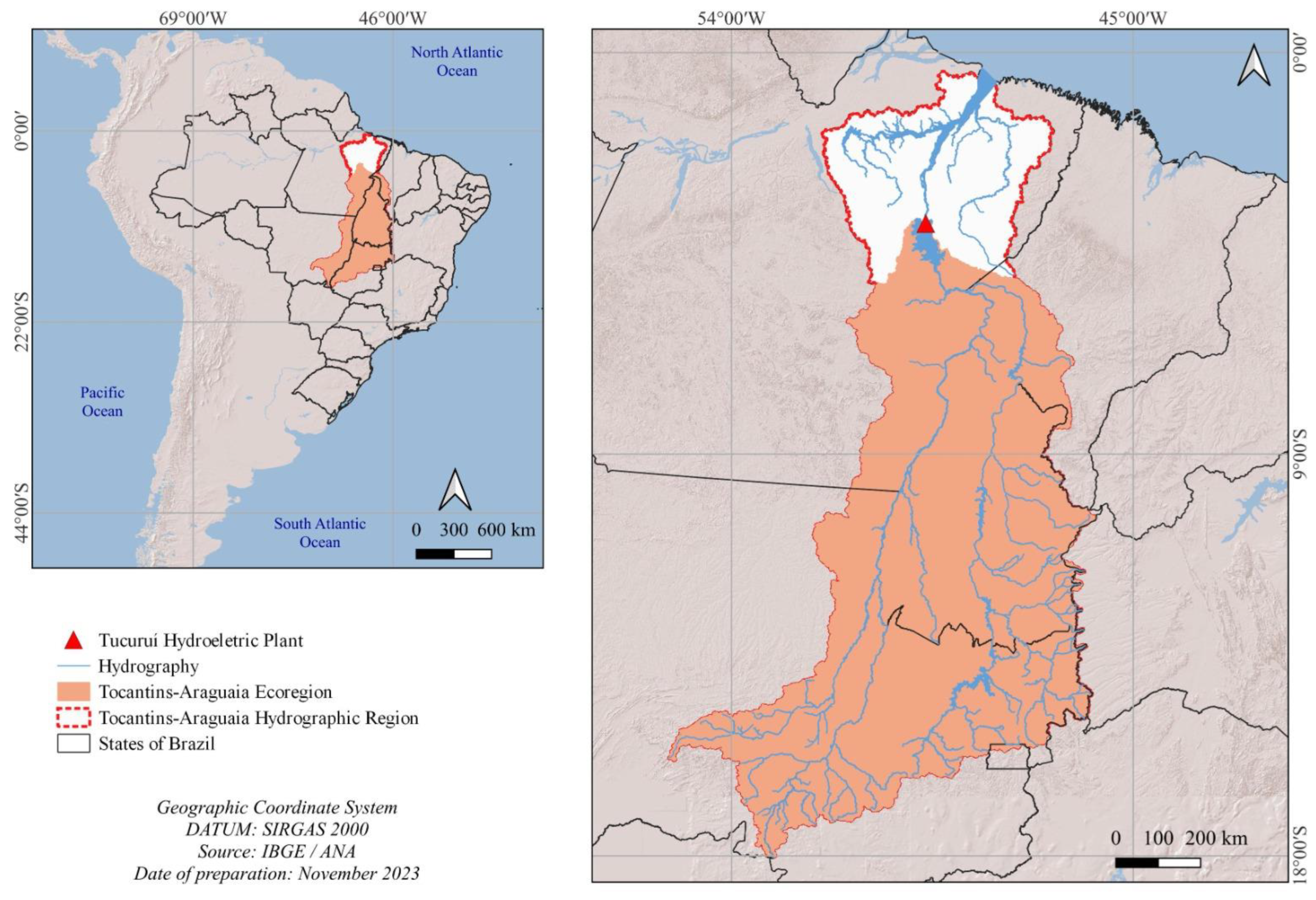
The Tocantins–Araguaia Hydrographic Region has an elongated configuration and is entirely located in Brazilian territory, covering 938,368 km2 [12], encompassing the states of Goiás (21.4%), Tocantins (30.2%), Pará (30.3%), Maranhão (3.3%), Mato Grosso (14.7%), and Distrito Federal (0.1%) [13]. The total area of the ecoregion is 717,332 km2 [14] and corresponds to 76.4% of the Tocantins–Araguaia Hydrographic Region, representing the stretch of the basin upstream of the Tucuruí hydroelectric plant, according to [15], as shown in Figure 1.
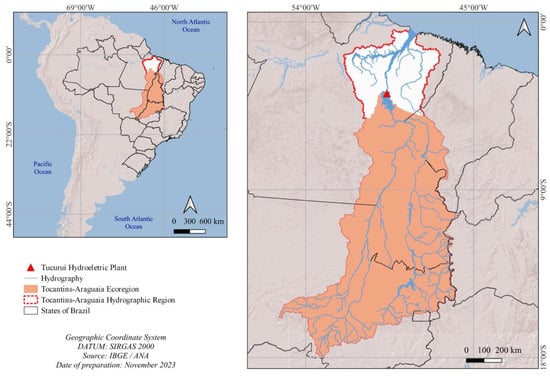
Figure 1.
Location of the Tocantins–Araguaia Hydrographic Region and the Tocantins–Araguaia ecoregion.
The area of the basin is mainly in the Cerrado morphoclimatic domain in the southern and central portions and by the Amazon Forest biome in the northern portion [16], with a gradient from higher and drier regions, with well-defined rainy periods, in the areas of the sources of the former and flatter and with a lower slope in the lower stretch, characterized by a great diversity of environments [17].
The Tocantins River is approximately 2400 km long and is formed by the confluence of the Almas and Maranhão rivers. The headwaters of the rivers are located on the Central Plateau, at an altitude of around 1000 m, occupying a drainage area of around 764,996 km2 up to its mouth, of which 385,060 km2 belong to the Araguaia River. The Araguaia River’s headwaters in the Serra do Caiapó are at an altitude of around 850 m. It runs for 2600 km to its mouth on the Tocantins River, of which 76.92% (2000 km) runs at an elevation of 90 m [13], forming a wide and productive floodplain.
The differences in slope, with stretches of backwaters interspersed with waterfalls and rapids, have diversified the environments and, consequently, the ichthyofauna, especially in the upper and middle reaches. However, these same characteristics are of interest to the electric sector, with several projects operating in the basin. According to [11] (2021), this and other anthropogenic activities have resulted in the large-scale degradation of the basin.
2.2. Ichthyofauna of the Tocantins–Araguaia Basin
The ichthyofauna of the Tocantins–Araguaia Basin comprises 751 species [18], with emphasis on 30.5% (229) of the species that have restricted occurrence or are endemic to the basin.
Since the construction of the Tucuruí Hydroelectric Power Plant, the first large hydroelectric power station built on the lower Tocantins River and which began operating in 1984, the longitudinal connectivity of the aquatic system has been interrupted, leading to the reduction in important fisheries in the region [16]. The dam altered the regional dynamics of the water and fish fauna and blocked connectivity with the basin. Fragmentation in the lower stretches of the basin is the main factor impacting aquatic system connectivity [19,20]. In view of this, this study specifically addresses the ichthyofauna in the stretch of the Tocantins–Araguaia Basin located upstream of the Tucuruí dam.
In this stretch of the Tocantins–Araguaia Basin, characterized as an ecoregion [15], 701 valid species have been documented, of which 270 (38.5%) have a type locality within this ecoregion [21].
Since 2000, there has been an increase in the number of fish species descriptions due to the intensified sampling efforts, and an increase is expected in the coming years due to the large number of genera with species that still need to be described, among other factors [21].
The Tocantins–Araguaia ecoregion is an area that is subject to the construction of dams as established in the National Energy Plan 2050, approved by the Ministry of Mines and Energy [22], which is regularly updated.
2.3. Data Collection and Analysis
Information on hydroelectric plants in operation and planned was obtained from the National Electric Energy Agency database in February 2022 [23] for the Tocantins–Araguaia Hydrographic Region. To check which plants were in operation, information available on the World Wide Web (Internet) was sought. In the list of planned plants, those described in the “Hydroelectric Development Phase—AHE” field as “canceled” and “deactivated” were disregarded.
The plants were grouped using the power criteria established in Resolução Normativa No. 875 of 10 March 2020 [24]. According to this resolution, a Power Generation Plant (PGP) is defined as having a power of 5000 kW or less (≤5 MW); a Small Hydroelectric Plant (SHP) has a power of more than 5000 kW (>5 MW) and 30,000 kW or less (≤30 kW) and a reservoir of up to 13 km2; and a Hydroelectric Power Plant (HPP) with an installed power of more than 50,000 kW (>50 MW). The use of power was chosen to facilitate the grouping of projects, without discussing the licensing process itself.
The coordinates of hydroelectric dams in operation and planned, including those under construction, were obtained through the georeferenced information system of the electric sector [25]. The data obtained were processed in a Shapefile format by the Geographical Information System (GIS) QGIS 3.22 [26], using the World Geographical System (WGS 84).
For the fish species, the list provided by [21] for the Tocantins–Araguaia ecoregion was used as a basis. This list was updated but only took into account the fish fauna upstream of the Tucuruí Hydroelectric Power Plant, which represents the first major project to fragment the aquatic environment within the basin.
The fish species were categorized by threat level based on the National List of Endangered Species, contained in Portaria GM/MMA No. 300 of 13 December 2022 [27], in the categories of Vulnerable (VU), Endangered (EN), Critically Endangered (CR), and Critically Endangered (probably extinct; CR (PEX)).
The survey of the occurrence of long-distance migratory species was based on studies carried out in the basin containing a list of the species surveyed and the area covered by the collections made. This category included 27 species (Supplementary Material—Table S1), defined based on [28,29] and the authors’ experience with ichthyofauna studies in the basin.
3. Results
Aneel’s database [23] lists 200 hydroelectric projects, 46 Power Generation Plants, 113 Small Hydroelectric Plants, and 41 Hydroelectric Power Plants. The analysis included 194 projects, with the exclusion of six Power Generation Plants (four are listed as canceled and two as deactivated).
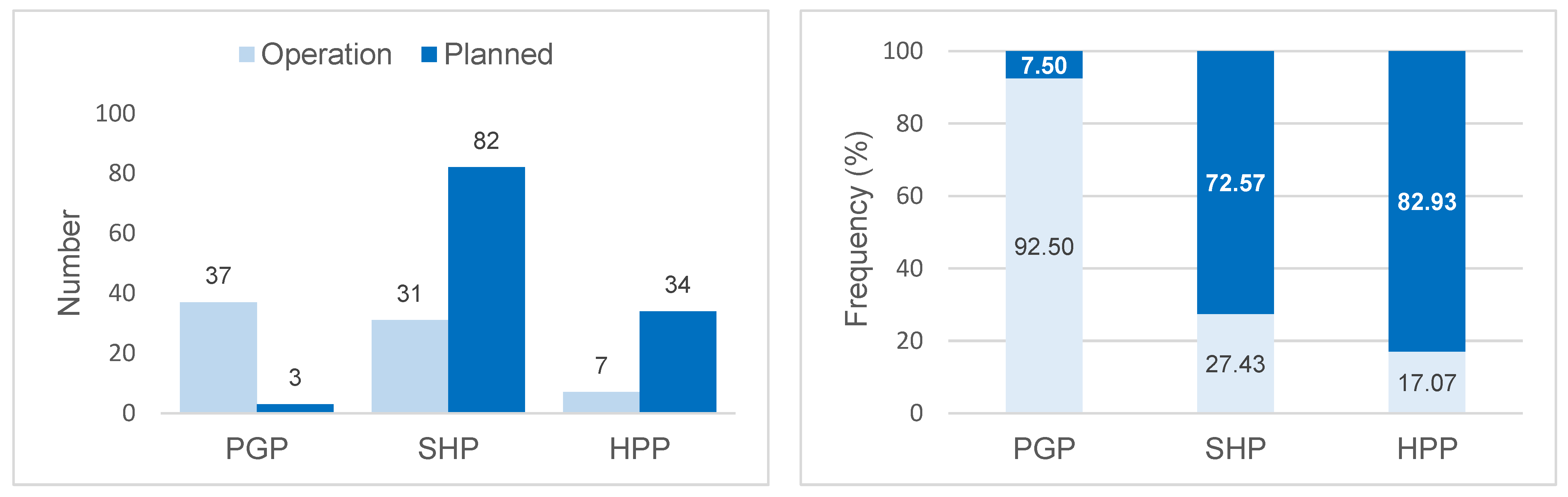
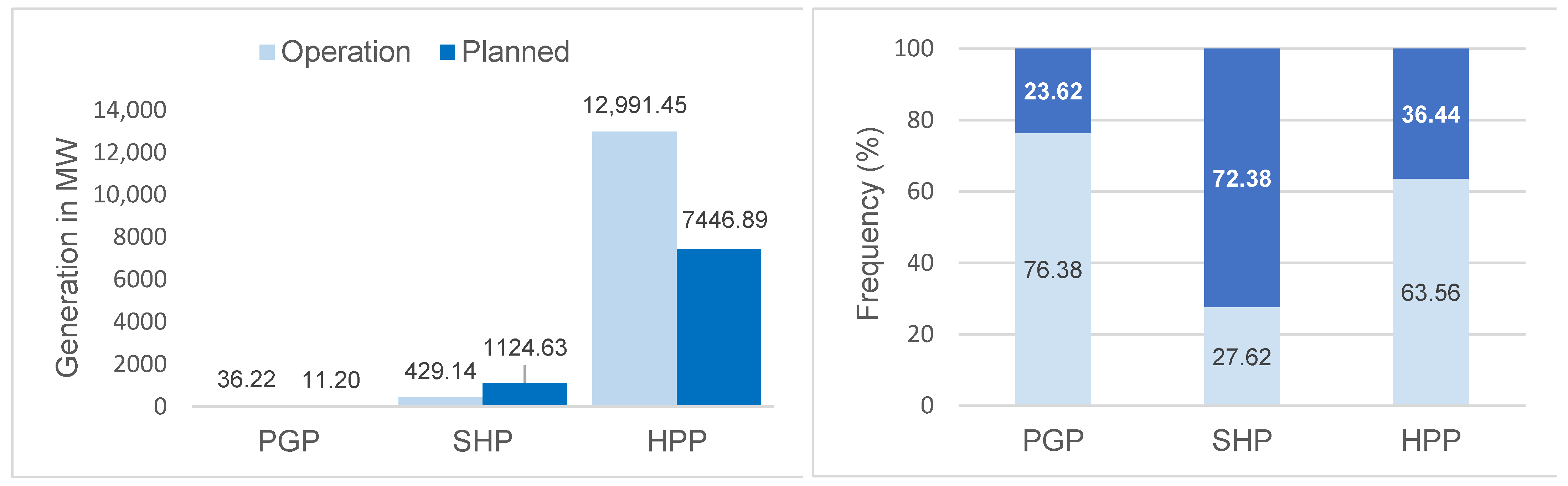
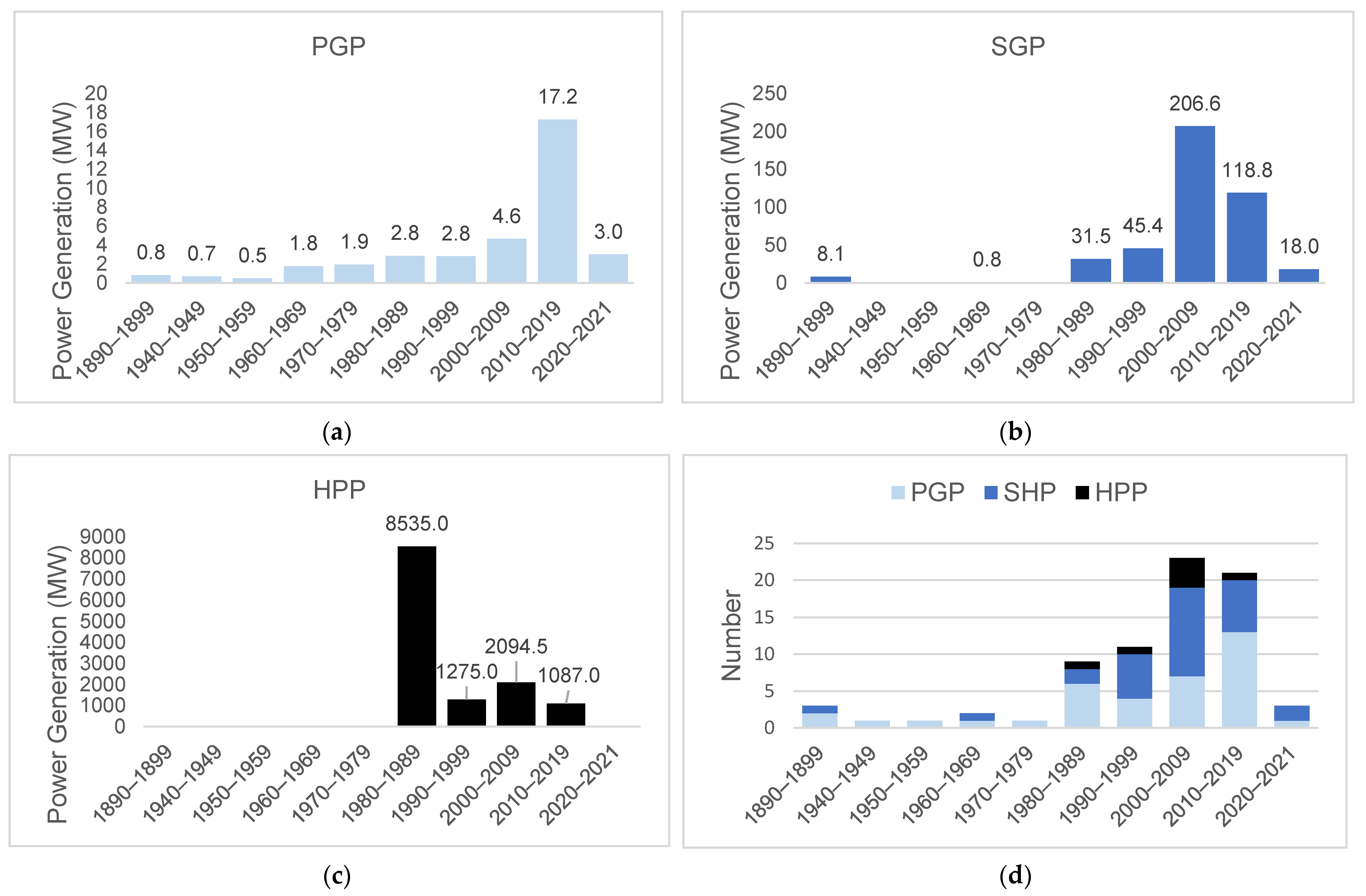
The projects in operation in the basin correspond to 75 hydroelectric plants, of which 37 (92.5%) are Power Generation Plants, 31 (27.4%) Small Hydroelectric Plants, and 7 (17.1%) Hydroelectric Power Plants. However, by 2050, 119 projects are expected (3 PGP; 82 SHP; 34 HPP) (Figure 2). The hydroelectric plants in operation contribute 13,456.81 MW (around 179 MW per plant), with an expected increase of 8582.72 MW (around 72 MW per plant) to the energy system, especially through Hydroelectric Power Plants (Figure 3). These results indicate the potential for the fragmentation of the basin both in terms of the number and size (power) of the projects.
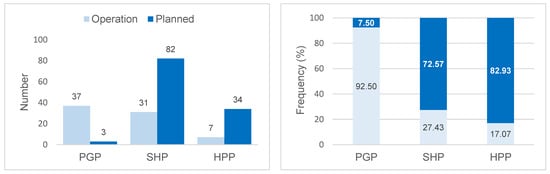
Figure 2.
Number and percentage frequency of Power Generation Plants (PGPs). Small Hydroelectric Plants (SHPs) and Hydroelectric Power Plants (HPPs) in operation in 2021 and planned for the Tocantins–Araguaia Hydrographic Basin until 2050.
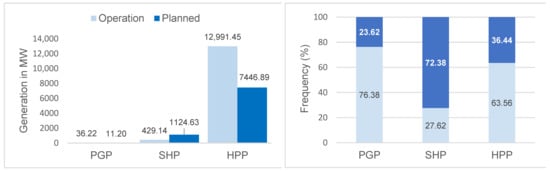
Figure 3.
Total generation power and percentage of Power Generation Plants (PGPs). Small Hydroelectric Plants (SHPs) and Hydroelectric Power Plants (HPPs) in operation in 2021 and planned for the Tocantins–Araguaia Hydrographic Basin until 2050.
Before 1900, three small hydroelectric plants started operating: the Primavera and Matula I, Small Hydroelectric Plants in sub-basin 26, and Mãe Benta in sub-basin 20 (Figure 4; Table 1). Recently, in 2021, the Diamantino Small Hydroelectric Plants in sub-basin 24 and Manuel Alves were commissioned. Although these projects have been operating since 1890, it was after 1980 that the number of hydroelectric plants increased, with 23 units coming into operation from 2000 to 2009 (Figure 5d), when the Lajeado, Cana Brava, Peixe Angical, and São Salvador Hydroelectric Power Plants came into operation, and a further 21 hydroelectric plants from 2010 to 2019, including the Estreito Power Plant and several other Small Hydroelectric Plants and Power Generation Plants (45 projects in 20 years, as shown in Figure 6).
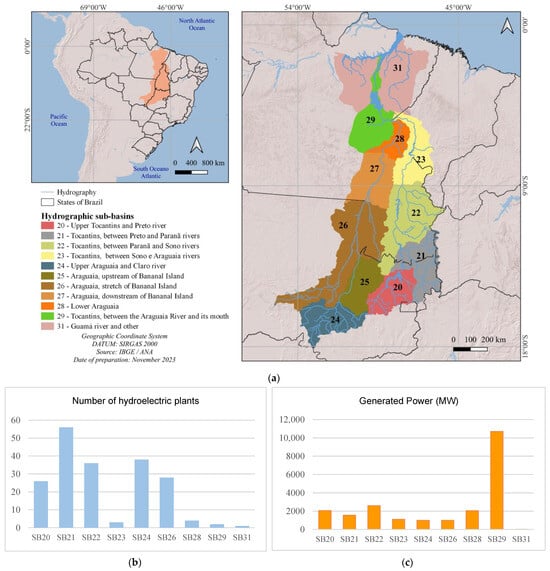
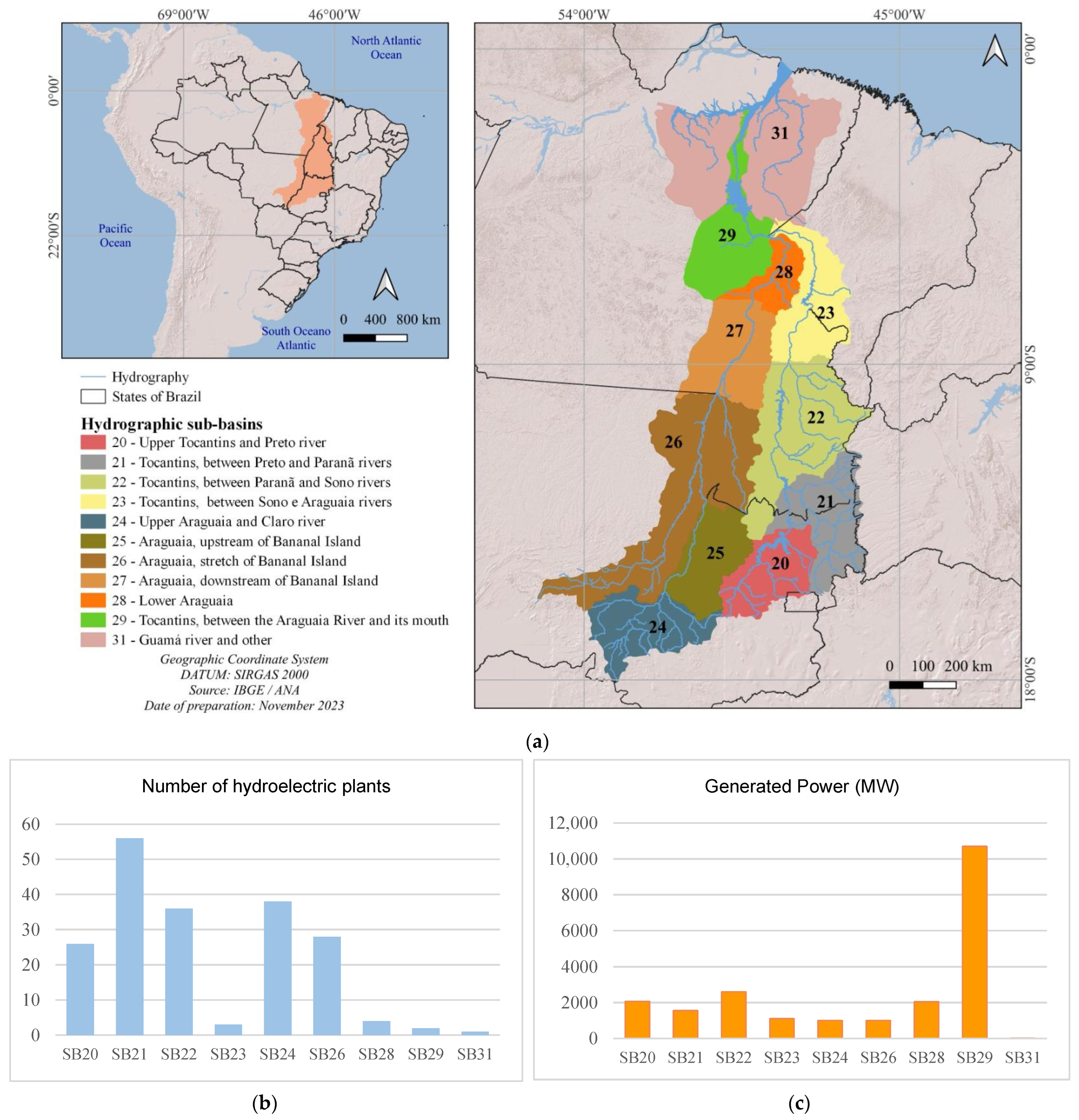
Figure 4.
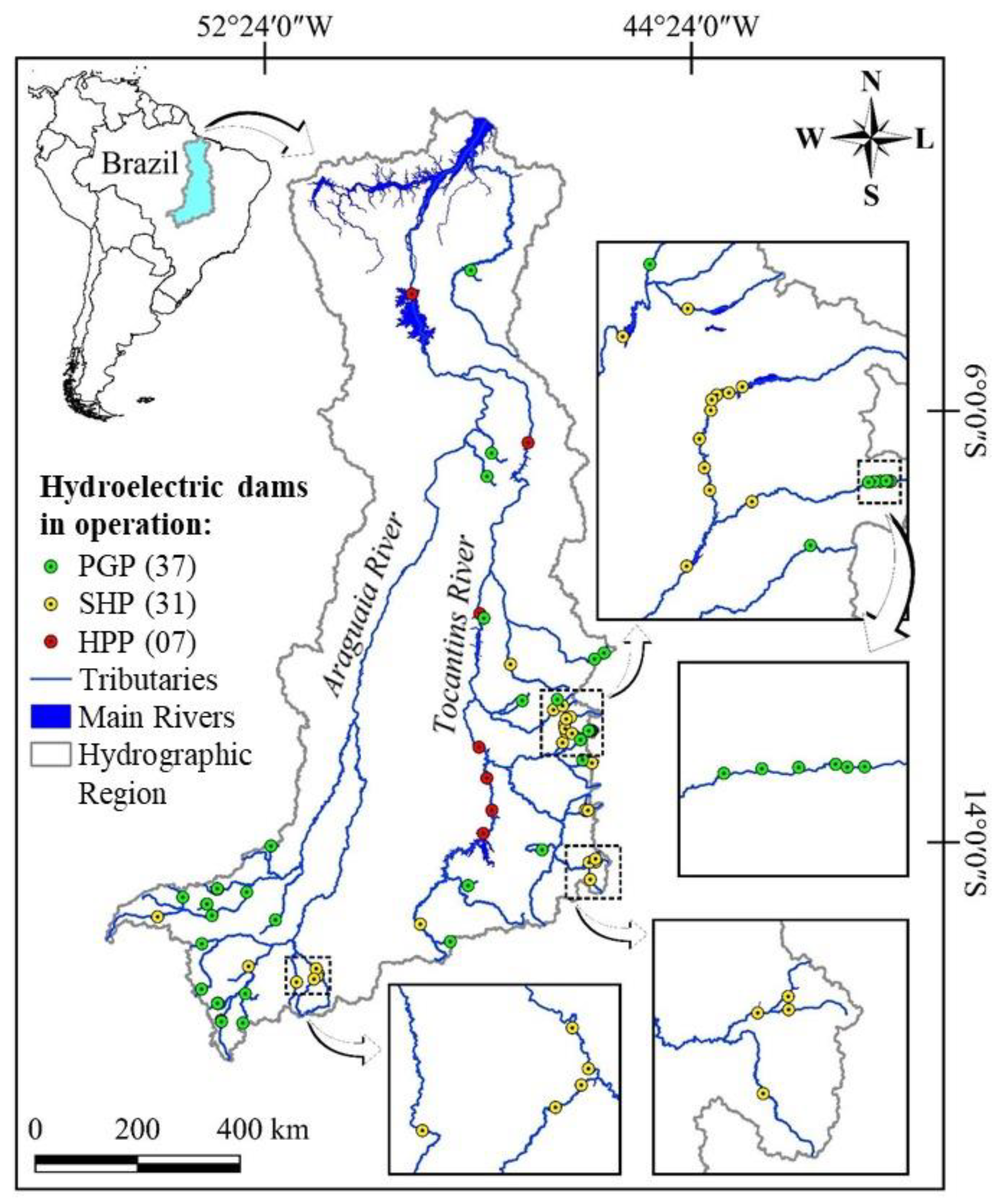
Location (a), number (b), and generated power (c) of hydroelectricity production units in operation and/or planned in the Tocantins–Araguaia Hydrographic Region by sub-basin. Sub-basin code (SB): 20. Upper Tocantins and Preto river; 21. Tocantins between Preto and Paranã rivers; 22. Tocantins between Paranã and Sono rivers; 23. Tocantins between Sono and Araguaia rivers; 24. Upper Araguaia and Claro river; 26. Araguaia, stretch of Bananal Island; 28. Lower Araguaia; 29. Tocantins, between the Araguaia River and its mouth; 31. Guamá River and others.

Table 1.
Number (N) and power (MW) of hydroelectric plants in operation and planned by sub-basin in the Tocantins–Araguaia Hydrographic Region. Sub-basin code (SB): 20. Upper Tocantins and Preto river; 21. Tocantins between Preto and Paranã rivers; 22. Tocantins between Paranã and Sono rivers; 23. Tocantins between Sono and Araguaia rivers; 24. Upper Araguaia and Claro river; 26. Araguaia, stretch of Bananal Island; 28. Lower Araguaia; 29. Tocantins between the Araguaia River and its mouth. Power Generation Plants (PGPs), Small Hydroelectric Plants (SHPs), and Hydroelectric Power Plants (HPPs).
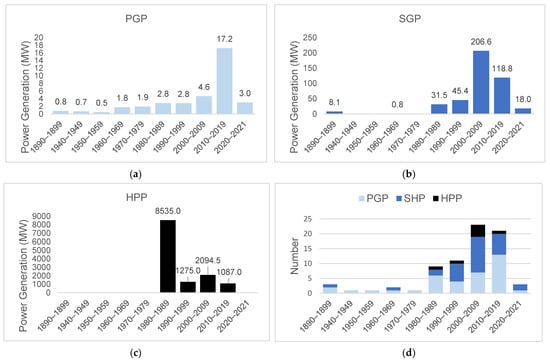
Figure 5.
Total power and number of hydroelectric plants added per decade. from 1899 to 2021. in the Tocantins–Araguaia Basin: (a) Power Generation Plants (PGPs), (b) Small Hydroelectric Plants (SHPs), (c) Hydroelectric Power Plants (HPPs), and (d) number of hydroelectric plants.
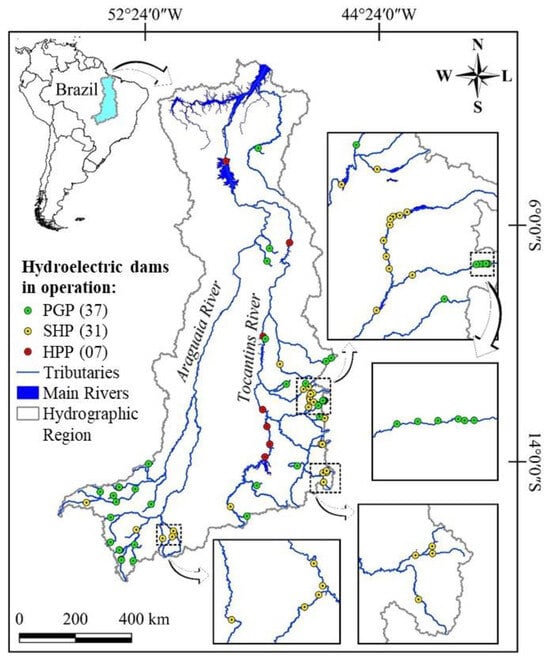
Figure 6.
Location of the Power Generation Plants (PGPs). Small Hydroelectric Plants (SHPs) and Hydroelectric Power Plants (HPPs) in operation in 2022 in the Tocantins–Araguaia Hydrographic Region.
The most fragmented stretches in the coming years will be the upper Tocantins River from the Sono River sub-basin upstream (sub-basins 20, 21, and 22) and the upper Araguaia BRiver (sub-basin 24). The stretches between the Preto and Paranã rivers (sub-basin 21) and the Upper Araguaia and Rio Claro (sub-basin 24) are fragmented, with 29 and 15 plants in operation, respectively (Figure 5; Table 1). The location of the Small Hydroelectric Power Plants in the headwaters regions contributes to habitat fragmentation. In terms of power, large rivers are the main contributors, both in operation and in terms of forecasts for the coming years (Table 1).
The Small Hydroelectric Power Plants in operation are in the upper reaches of the basins that form the Paranã River (Palmeiras and Buritis rivers, for example), a tributary of the Tocantins River, and in the upper reaches of the Araguaia River (Araguainha and Das Mortes rivers, for example). On the other hand, Hydroelectric Power Plants, which are larger projects, are all on the Tocantins River (Figure 6). Fish passages are present at the Lajeado and Peixe Angical hydroelectric plants and at the Tamboril Small Hydroelectric Plants, with an installed capacity of 29.33 MW, located on the Bonito River in the Upper Araguaia River.
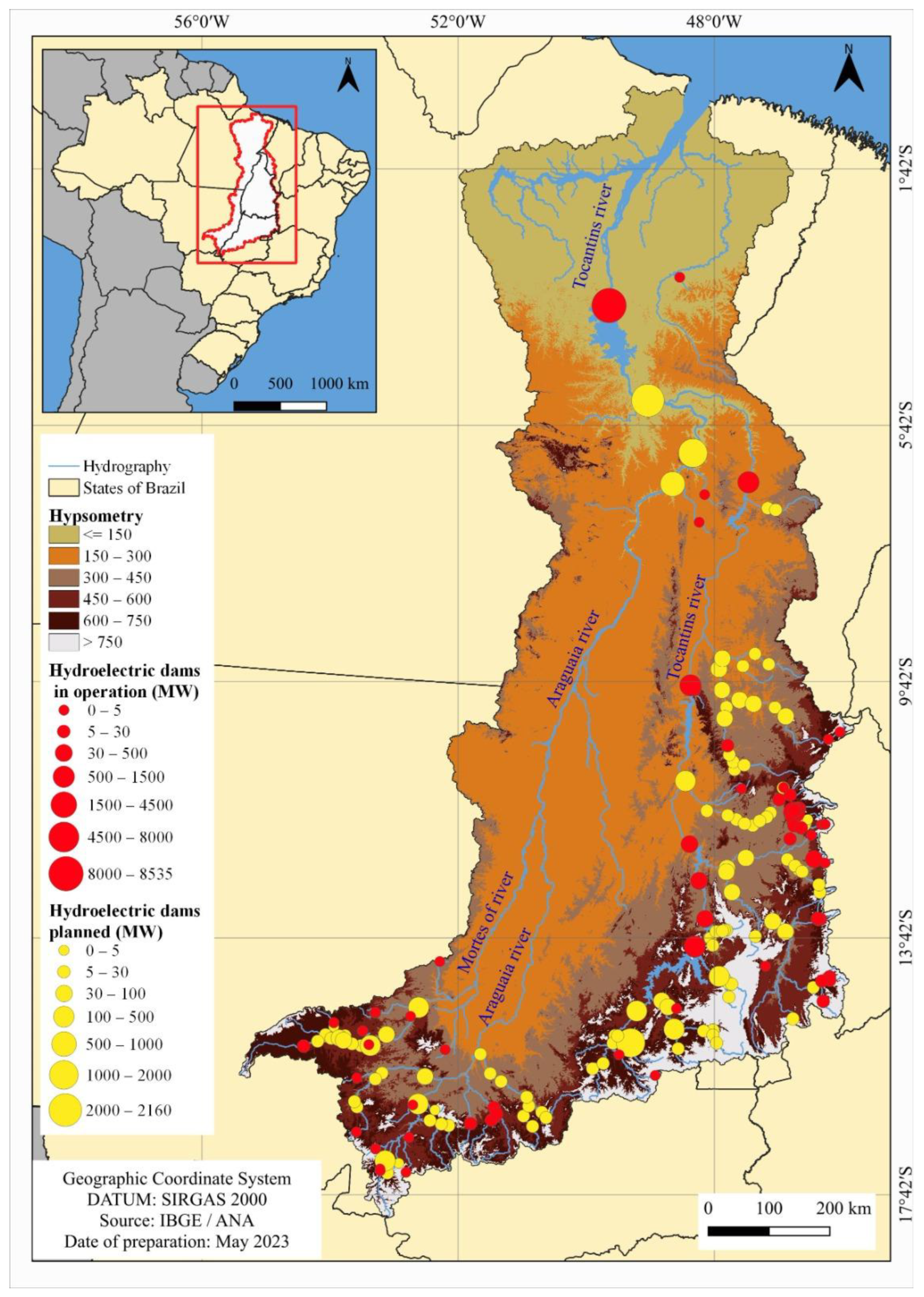
The 119 hydroelectric plants planned or under construction in the electricity sector plan should accentuate fragmentation in the lower stretches of the Araguaia River, the lower and middle stretches of the Tocantins River with the construction of Hydroelectric Power Plants, and the upper stretches of various sub-basins where most of the Small Hydroelectric Plants are planned (Figure 7; Table 2), these being the main areas of endemism for the ichthyofauna [18]. All the planned plants are within the Tocantins–Araguaia ecoregion.
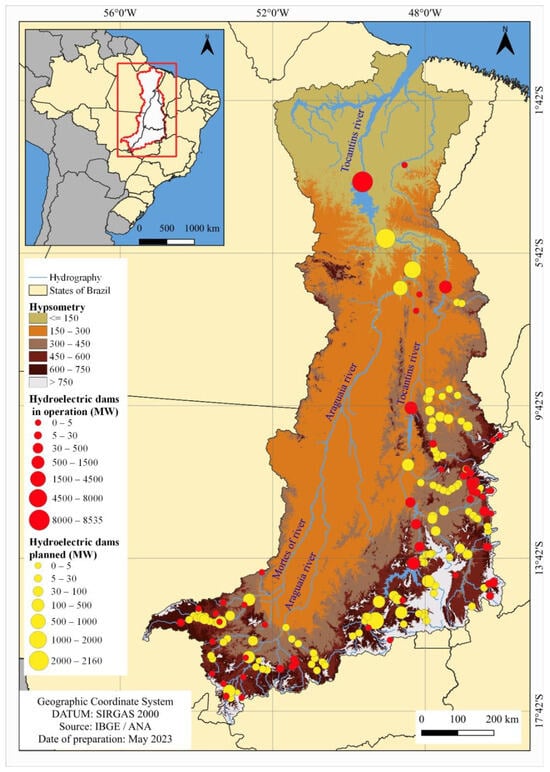
Figure 7.
Total generation capacity and percentage of operational hydropower plants in 2022 and planned for the Tocantins–Araguaia Hydrographic Region until 2050.
In the Tocantins–Araguaia ecoregion upstream of the Tucuruí dam, 702 species of fish have been recorded, of which the dourada Brachyplatystma rousseauxii (previously erroneously called Brachyplatystma flavicans) was added to the list by [21] based on records in the ecoregion by [16] and fishermen from the upper middle Tocantins River (personal information). Of these species, 218 (31.1%) are endemic. Endangered species accounted for 43 (6.1%), most of which, 41 species, are endemic, except for Hasemania crenuchoides (EN) and Scobinancistrus pariolispos (VU), which can be found outside the ecoregion. Of the species at risk of extinction (threatened), 8 species are categorized as critically endangered (CR), 15 as endangered (EN), 19 as vulnerable (VU), and 1 as critically endangered (probably extinct CR (PEX))—See Supplementary Material Table S1.
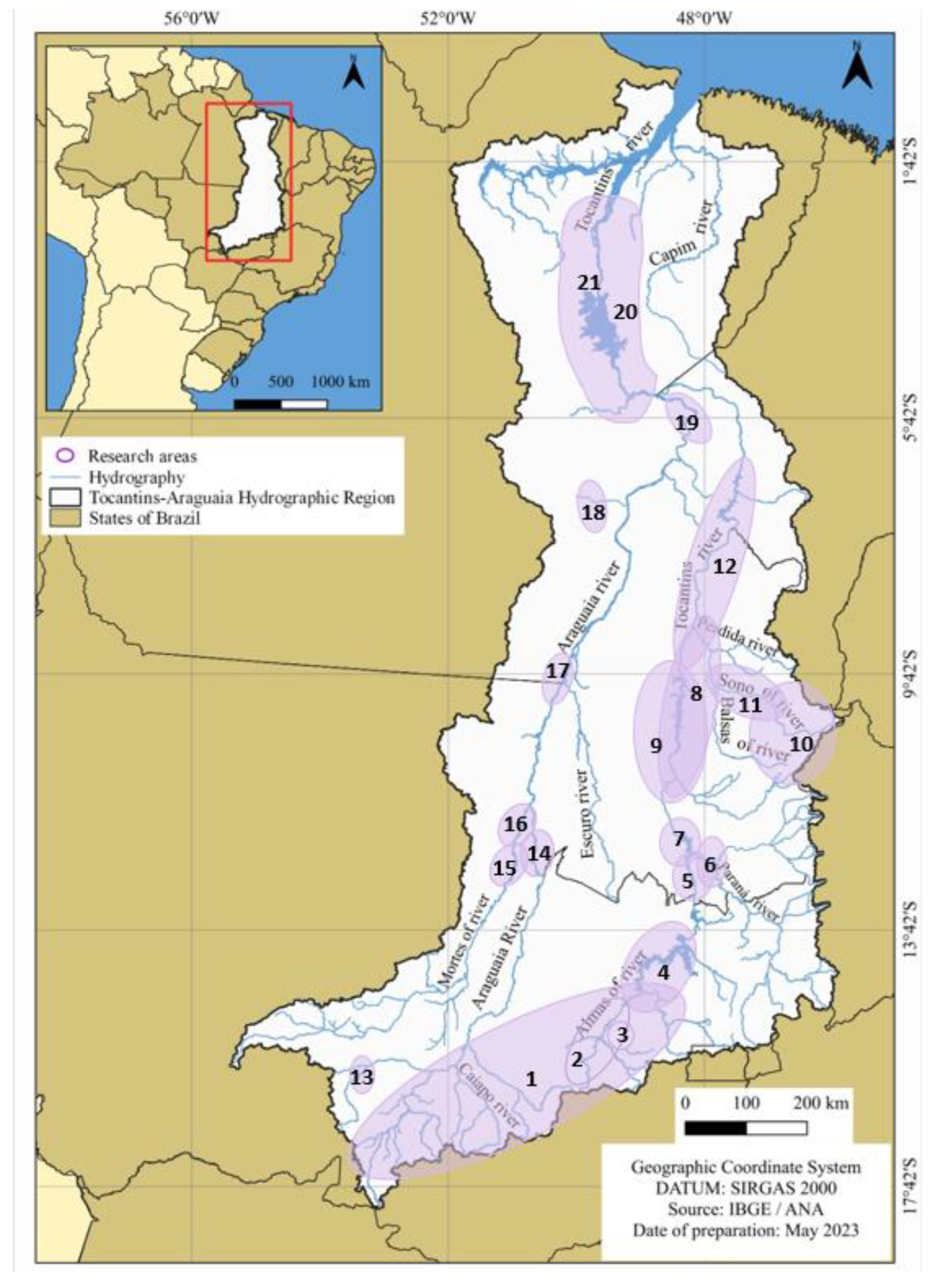
Among the studies that report the occurrence of species, those with greater spatial and temporal coverage are generally associated with diagnosis and/or monitoring in areas where large hydroelectric plants are being implemented, such as [30,31], related to the Serra da Mesa Hydroelectric Power Plant; ref. [32] the São Salvador Hydroelectric Power Plant; ref. [33] the Peixe Angical Hydroelectric Power Plant; refs. [34,35] the Lajeado Hydroelectric Power Plant; refs. [36,37] the Estreito Hydroelectric Power Plant; refs. [38,39] the Tucuruí Hydroelectric Power Plant; and refs. [40,41] which overlap the area planned for the construction of the Santa Isabel Power Plant. Other studies, such as [2,42], are related to the implementation of Conservation Units (Table 2 and Figure 8).
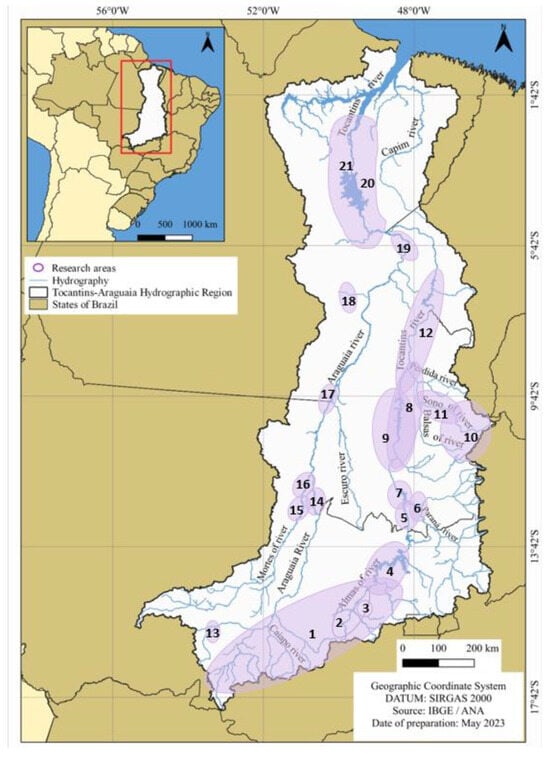
Figure 8.
Tocantins–Araguaia Hydrographic Region and indication of the collection areas of the studies surveyed in the literature. 1. [43]. 2. [44]. 3. [45]. 4. [30]. 5. [31]. 6. [32]. 7. [33]. 8. [34]. 9. [35]. 10. [2]. 11. [46]. 12. [36,37]. 13. [47]. 14. [48]. 15. [49]. 16. [50]. 17. [42]. 18. [51]. 19. [40,41]. 20. [38]. 21. [39].

Table 2.
List of studies carried out in the field based on a survey of primary ichthyofauna data for the Tocantins–Araguaia Basin.
Table 2.
List of studies carried out in the field based on a survey of primary ichthyofauna data for the Tocantins–Araguaia Basin.
| Authors | Number of Taxa | Period, Hydroelectric Plant, River or Watershed | Data Source and Observation |
|---|---|---|---|
| [43] | 62 species | 2008 (dry season from May to September); 25 streams; headwaters of Tocantins and Araguaia rivers | Sampling with experimental seine net |
| [44] | 82 species; 6 orders; 26 families | 2016 (dry season from April to May); 4 points in Almas River; a tributary of Tocantins River; 29 contributing streams | Sampling with experimental gill net |
| [45] | 67 species; 5 orders; 19 families | 2010 (July); municipalities of Pirenópolis, Barro Alto and Goianésia, and Almas and Maranhão rivers; upper Tocantins River | Sampling with seine net, cast net, and sieve; 60 min at each site |
| [30] | 233 species; 9 orders; 39 families | 1995 to 2000; 2001–2002 and 2008 to 2010; Serra da Mesa Reservoir influence region | Sampling with experimental multi-gear fishing before, during, and after the dam |
| [31] | 264 species; 11 orders; 35 families | April 1998 to September 2001; Paranã municipality; upper Tocantins River; downstream of Serra da Mesa Reservoir | Sampling with experimental multi-gear fishing |
| [32] | 258 species; 8 orders; 37 families | August 2007 to August 2008, December 2009 to November 2011, and November 2012 to May 2014; Paranã, São Salvador, and Palmeirópolis municipalities; upper Tocantins River; São Salvador Reservoir influence area | Sampling with experimental multi-gear fishing, subsistence, commercial, and sport fishing |
| [33] | 288 species; 11 orders; 38 families | 2004 to 2007; upper Tocantins River between Peixe and São Salvador municipalities; Peixe Angical Reservoir influence area | Sampling with experimental multi-gear fishing |
| [34] | 343 species; 12 orders; 42 families | 1999 to 2004; middle and upper Tocantins River; between Pedro Afonso and Peixe municipalities; Lajeado Reservoir influence area | Sampling with experimental multi-gear fishing; 38 endemic species |
| [35] | 194 species; 10 orders; 38 families | 2013 (August) to 2015 (May); upper and middle Tocantins River; tributaries and streams; Lajeado Reservoir influence area | Sampling with experimental multi-gear fishing |
| [2] | 85 species ** | 2008 (January–February); Serra Geral do Tocantins Ecological Station and surrounding (records of over hundred species) | Fishing with seine net, complementing the list with specimens deposited in museums |
| [46] | 111 species; 7 orders; 25 families | 2016 (January, April, and June); Novo Acordo and Rio Sono municipalities; Do Sono River; Monte Santo Hydroelectric Plant influence area. | Sampling with experimental multi-gear fishing |
| [36,37] | 215 species; 10 orders; 40 families | 2009 to 2012; between Tupiratins and Imperatriz municipalities; Estreito Reservoir influence area | Sampling with experimental multi-gear fishing and commercial fishing |
| [47] | 89 species; 5 orders; 21 families | 2008 (July–August); Aragarças and Barra do Garças municipalities; Serra Azul and Serra do Roncador area; upper and middle Araguaia River | Sieve, seine net, and cast net; effort of 3 people; 60 min at each site; in the dry season |
| [48] | 92 species; 6 orders; 22 families | 1994 (April and September, beginning and end of the drought); region close to Luís Alves | Sampling with experimental gill net and trap; 12 lagoons |
| [49] | 80 species; 5 orders; 19 families | 2007? (March, July, October, and December); lower Das Mortes River, Bananal Floodplain, and Araguaia River watershed | Sampling with experimental gill net; inlet and river channel |
| [50] | 72 species; 5 orders; 17 families | 2004 (October) and 2005 (March); lower Das Mortes River; Bananal Floodplain; Araguaia River watershed | Sampling with experimental gill net |
| [42] | 271 species; 12 orders; 41 families; 183 genera | 2000–2001 (?); Caseara and Pium municipalities; Cantão State Park; Middle Araguaia River | Sampling with experimental gill net and sport fishing |
| [51] | 37 species; 4 orders; 14 families; 33 genera | 2009 (December) and 2010 (January); Redenção and Santa Maria das Barreiras municipalities; three streams in the Araguaia River watershed, downstream Bananal Island, and Carajás Mountains | Rapid assessment protocol with interviews, field observations, and sampling with nets |
| [40,41] | 208 species; 11 orders; 40 families | 2008–2009; Aragominas and Araguatins municipalities; lower Araguaia River * | Sampling with experimental multi-gear fishing and commercial fishing |
| [38] # | 123 commercial species; 26 families; the authors documented more than 300 species and covered reaches downstream from the ecoregion | 1980 and 1983; lower Tocantins River. between Cametá and Marabá municipalities; Tucuruí Reservoir influence areas; reservoir pre-filling phase | Commercial fishing |
| [39] # | 217 species; 13 orders; 41 families | 1999 to 2003; lower Tocantins River, between Cametá and Marabá municipalities, including Tucuruí Reservoir area | Commercial fishing and scientific collections |
* Area similar to the influence area of the Santa Isabel Hydroelectric Plant. ** 35 species were collected by the authors. and the remaining ones were recorded from other sources. # The area covered by these studies exceeds the Tocantins–Araguaia ecoregion.
Among the migratory species is Brycon gouldingi, a large-sized species (over 40 cm) with a restricted distribution and threat of extinction. The small-sized species, categorized as restricted and threatened, occur in small tributaries, particularly in the upper reaches of the basin, where most of the Small Hydroelectric Power Plants are concentrated (see Supplementary Material Table S1; Figure 6 and Figure 7).
Within the group of long-distance migratory species are the large migratory catfish of the Brachyplatystoma genus. The dourada Brachyplatystoma rousseauxii has been documented in the Tucuruí area and in the Paranã River region but before the construction of the large dams in the middle Tocantins River. The piramutaba Brachyplatystoma vaillantti, in the same region, was observed in the Almas River and, more recently, in the Estreito area (Table 3). The filhote or piraíba Brachyplatystoma filamentosum occurred in the upper, middle, and lower Tocantins region in the Serra da Mesa, Lajeado, Estreito, and Tucuruí areas but with a time lag in the studies. Platynematichthys notatus was only recorded in upper Tocantins, downstream of Serra da Mesa, and in the Tucuruí area (Table 3).

Table 3.
Record of the capture of long-distance migratory species in studies carried out in the Tocantins–Araguaia ecoregion. The studies that recorded each species are highlighted in grey.
The group of long-distance migratory species also includes the pirarara Phractocephalus hemioliopterus; the jaú Zungaro zungaro; the surubim Pseudoplatystoma fasciatum; the chicote Sorubimichthys planiceps; the barbado Pinirampus pirinampu; the cuiús-cuiús and abotoados Oxydoras niger and Pterodoras granulosus; the scaled caranha fish Piaractus brachypomus; the cachorras of the genus Hydrolycus; the piracanjuba Brycon gouldingi and the papa-terra or curimatá Prochilodus nigricans, large and medium-sized species that are important for fishing; and Piaractus mesopotamicus and Colossoma macropomum, considered ‘non-native’ species for the basin, occurred in the stretch between the São Salvador and Lajeado reservoirs. The last mentioned species also occurred in the lower Araguaia River (Table 3).
4. Discussion
In the Brazilian electricity sector, plans undergo periodic adjustments which can involve changes in the location, size, and operation of the projects. The inclusion of hydroelectric plants in the Ten-Year Energy Expansion Plans (PDE) varies according to the interests of investors and the government. The Ipueiras plant was included in the PDE until 2019 [53] (annexes; p. 84), the Tupiratins plant until the 2021 plan [54], and the Marabá plant until the 2024 plan [55], disappearing in subsequent PDEs until the year 2031 [56]. However, this exclusion may only be a postponement in its implementation, and the plants can be resumed at any time. From 2022 onwards, the PDEs began to consider socio-environmental concerns [56], and the creation of “energy well-being” indices is being studied [57].
The time lag between the planning of the electricity sector, which, in Brazil, is scheduled for 2050, and the discussion or dissemination of the diagnoses is linked to the projects, occurs practically when they are about to be approved and/or implemented, especially regarding the basic environmental plans. This scenario relegates the conservation of fish and, consequently, fishing resources to a secondary priority. Despite acknowledging the plasticity, adaptability, and socio-environmental resilience of communities and fish, the fishing activity predominantly centered on migratory species is jeopardized, posing a threat to socio-environmental diversity.
The ecological impacts of hydroelectric plants are disproportionately high compared to their economic and rentier benefits, yielding minimal social returns. The imbalance between the number and production capacity of hydroelectric plants calls into question the benefits in relation to the social and environmental costs. In Brazil, operational hydroelectric plants constitute 218 projects, generating 94.3% of the hydroelectric power produced, whereas Small Hydroelectric Plants represents 427 projects, contributing 5.0%, and Power Generation Plants account for 692 projects, contributing 0.7% [58]. This situation holds significant implications for the conservation of the landscape and aquatic biodiversity.
On the other hand, it is necessary to direct attention and pro-conservation attitudes towards valuing socio-environmental diversity as an alternative to projects justified purely by economic issues. This is particularly important in stretches of high diversity, such as in the region of the confluence of the Araguaia and Tocantins rivers, a site with a crucial role in the reproduction and growth of fish larvae and juveniles [16,40,59] (personal information of the author E. E. Marques). In this context, plans to build the Marabá Hydroelectric Power Plant are underway, a project expected to have a significant impact on fish fauna in the basin [59].
In sensitive regions with high endemism, such as the headwaters [18,52] and the Do Sono River Basin [2] and in places like Cachoeira da Velha and several other equally sensitive areas of the basin, hydroelectric dams are also expected to be built. Even though some of these hydroelectric plants are not included in the most recent plans for the electricity sector [56], the pressures for their implementation persist.
The definition of conservation areas and plans must precede the implementation of projects in order to ensure fairness in negotiations. However, concerning the conservation of the ichthyofauna in the Tocantins–Araguaia Basin, there is currently no integrated plan comparable to that of the electricity sector. Despite several surveys conducted in the basin, information remains fragmented, often associated with specific hydroelectric projects or the establishment of conservation units, lacking a comprehensive approach to the entire basin. Additionally, when monitoring does occur, it is conducted in a segmented and discontinuous manner.
Planning conservation based on data from the entire ecoregion prior to projects approvals is essential for both conservation efforts and the advancement of the electricity sector. The integration of information from the entire basin, with the aim of balancing energy production with the maintenance of biological and socio-environmental diversity, includes exploring potential optimizations in economic investment. This approach also entails careful consideration of the concept of ‘sustainable development’.
Along the Mekong River, a sequence of political and economic incentives for renewable energies and impact mitigation strategies have provided justification for the establishment of additional hydroelectric plants [60], thereby intensifying the impacts on fish and fisheries. Entrepreneurs and other stakeholders in the sector need to integrate knowledge and appreciation for the value of diversity. The prevailing perception suggests that priority is accorded to energy production at the expense of the conservation of socio-environmental diversity, which is a crucial factor in the resilience of systems.
The chronology of hydroelectric dam construction in the basin, starting with the first major project, the Tucuruí Dam, near the mouth in 1984, followed by the Serra da Mesa Dam in the upper stretch in 1998 and the Lajeado Dam in the middle stretch in 2001, along with subsequent constructions, has restricted the movement of fish, making free stretches increasingly scarce. Even if some migratory species, especially long-distance ones, complete their life cycle in smaller stretches, this does not guarantee the maintenance of genetic diversity.
The reduction in the populations of large migratory catfish, such as filhote, piraíba, and dourada of the Brachyplatystoma genus, is notable, particularly in the middle reaches of the Tocantins River, where they were once an important component of fishing but are now sporadically caught [61], as anticipated since the construction of Tucuruí [16]. Alternatives to mitigate and compensate for the impacts are often suggested for short periods and generally disregard the cumulative effects and those that are noticeable in the long term. Even for long-distance migratory species recorded in several areas, a reduction in their abundance is observed over time.
The flow of the river without dams allows for the free movement of fish and genetic flow. With the introduction of dams without the construction of adequate connectivity mechanisms, such as fishways and bypasses, it is evident that populations will become isolated, especially in watercourses with cascading dams, potentially leading to regional extinctions over the long term. Bidirectional connectivity must be considered in hydroelectric projects to conserve migratory species [62,63,64]. Despite exhibiting selectivity [8], these mechanisms can contribute to connectivity. However, it is essential to test management strategies in tropical regions and conduct a study on their applicability, considering that they may be unsuitable in naturally isolated areas.
With the advancement of hydroelectric projects in tropical regions, problems related to the subsistence of people who rely directly on freshwater fish are intensified while simultaneously simplifying alternatives for ecosystem services by reducing species diversity. Given the dynamics and complexity of aquatic systems, strategies to maximize the possibilities of adjustment become important attributes for water conservation in the face of climate change. Faced with emerging threats and persistent challenges to freshwater biodiversity conservation [65], the knowledge held by local populations, which represents a heritage of intangible socio-environmental and cultural diversity, must be considered and respected. This constitutes a component to be protected, conserved, and restored in an integrated way with freshwater diversity and the services and solutions upon which people depend, as outlined by [66]. In order to reconcile economic development with conservation, it is necessary to rethink the construction of new hydroelectric dams.
In the Tocantins–Araguaia River Basin, the number of species categorized as endangered has increased from 12 [21], according to [67] Portaria No. 445 from 17 December 2014, to 43 [27], according to Portaria GN/MMA No. 300 from 13 December 2022, in the Tocantins–Araguaia ecoregion, with an increase of more than 3.5 times in about eight years. The low resistance of species to environmental variations, coupled with activities such as tourism, mining, commercial and sports fishing, and especially hydroelectric dams, are factors that threaten the species in the basin. Habitat modification and the interruption of migration routes contribute to this scenario. In addition, the formation of reservoirs has created opportunities for the development of cage aquaculture, allowing the entry of exotic species for breeding. Ref. [68] conducted an extensive survey of non-native species in the Amazon region and recorded six non-native species for the Tocantins–Araguaia Basin.
The authors emphasized that the phenomenon of species invasion in the Amazon region began in 1939 but has intensified since the 2000s. The opening for tilapia farming in cage aquaculture systems [69] accidentally led to the release of thousands of individuals into the reservoir of the Lajeado Hydroelectric Power (personal information from the authors).
Two species, Aguarunichthys tocantinsensis, known as Pernambuco, and Brycon gouldingi, are matrinchã or piracanjuba, rheophilic, and endemic to the Tocantins–Araguaia Basin and classified as “endangered” on the Red List of Threatened Species, exemplify the need for distinct conservation strategies for migratory and non-migratory fish populations. The pernambuco prefers areas with strong currents and rocky bottoms, with an average size of around 35 cm in standard body length and an estimated generation time of nine years. On the other hand, the piracanjuba, a larger species (the largest recorded specimen measured 48 cm), is migratory, inhabits river channels and adjacent plains, primarily feeds on plants and invertebrates, and is target of commercial and sport fishing, with an estimated generational time of 7.5 years [70]. These two species illustrate the significant impacts of dam construction in the basin: the reduction in riffles habitats, the interruption of migratory routes, and changes in the relationship with riparian vegetation, either through the control of the level that modifies the rate of flooding and/or the drowning of vegetation in the reservoir.
Vegetation is a primary component of the hydrological cycle, and the high rates of the deforestation and conversion of natural areas into crops, pastures, and sugarcane plantations trigger hydrological, geomorphological, and biochemical changes in rivers of all sizes, leading to alterations in land surface temperatures. The changes resulting from the construction of cascade dams have adverse effects on the diversity of riparian systems [71] and the desiccation of downstream riverbanks by controlling the flow of water [72]. Both changes have implications for fish fauna.
Freshwater fish have restrictions imposed by their physiology, limiting their presence to rivers and lakes, and they generally have limited abilities to overcome barriers. As a result, there is often a close correspondence between the evolutionary history of river basins and the groups of fish found in them. In the case of the Tocantins–Araguaia Basin, the results indicate a high diversity of species, generally of small size, restricted to the upper reaches of the basin. There are several species that are distinguished between the streams of the upper reaches of the Tocantins and Araguaia rivers [18,73]. These areas are key locations for the installation of smaller hydroelectric plants (Small Hydroelectric Plants and Power Generation Plants). Some of these projects may not represent an effective barrier to migration due to their design or the installation in locations where natural barriers already exist and are taken advantage, such as natural waterfalls. Even in such cases, the installation of these hydroelectric plants requires attention due to other alterations in the hydrological system related to these projects, such as deforestation, river channel diversion, reservoir formation, and others that impact the ichthyofauna directly and indirectly.
On the other hand, the lower stretches of the basins and their main tributaries have essential sites for migratory species to complete their life cycle. However, these areas also coincide with locations identified for the installation of larger hydroelectric plants. Thus, if pressures on the environment continue at the same pace, ecosystems may never recover, resulting in the loss of both fish diversity and the socio-environmental diversity that has adapted to these systems over time.
The freshwater fish fauna of South America has been influenced by numerous geological and climatic changes throughout its evolution. These changes have involved the formation of hydrographic basins, as well as large-scale landscape configurations, shaped by prevailing temperature and humidity conditions over time. Rivers form continuous systems with longitudinal and lateral connections along their course, regulating the flow of matter and energy [74] and influence the entire system. Gradients of altitude and water temperature, channel depth, turbidity, and conductivity are factors that influence the functional attributes of fish assemblages in streams in the upper reaches of the Tocantins–Araguaia Basin, a region that is still poorly understood but seriously threatened [73].
The conservation units, which cover 7.4% of the Tocantins–Araguaia hydrographic basin [18] (Supplementary Material), are particularly associated with headwater areas and/or established as compensation measures due to hydroelectric projects. However, these units prove insufficient to conserve the species within the basin and maintain the hydrogeomorphological and ecological functionality of its rivers. Among several ways to mitigate or compensate for these impacts, Ref. [75] tested a tool to quantify the impacts of large hydroelectric dams in the Tocantins–Araguaia Hydrographic Region on vertebrate species considered threatened or endemic, aiming at systematic territorial planning and conservation. Considering the hydroelectric plants in operation, the authors indicated that an addition of 450% (6.1 million hectares) would be needed in Integral Protection areas in the hydrographic region. For the future scenario, they estimated that almost 100,000 additional hectares would be required beyond this value [75].
Freshwater fish are geographically limited to hydrographic basins, but in the Amazon Basin, their distributions often extend beyond the boundaries of modern hydrographic basins. This is due to a complex and interconnected history of drainage, where geomorphological processes are often more informative than current basin divisions [52]. In this context, the fragmentation of the Tocantins–Araguaia Basin could have effects on both the extinction of species with restricted distributions and the maintenance of viable population sizes of long-distance migratory fish due to the speed of changes in the watercourses and the basin itself.
Based on the knowledge available about the region, a detailed analysis at the ecoregion scale, aiming to relate what is intended to be extracted (energy, minerals, soil, water, biodiversity, and others) with a thorough understanding of the complexities of the environment in each region is fundamental to advance conservation. This applies to both the composition and interactions among species and socio-environmental relationships. The costs of replacing historically established regulatory mechanisms with those designed to meet the demands of the ‘market’ are significant. The responsibility for the commons is collective, requiring the integration of the information.
The transformations in the ichthyofauna, particularly in the populations of endemic and migratory species, as well as in socio-environmental diversity, are imminent given the current changes and those expected for the coming years, resulting from the planning of the hydroelectric sector for the Tocantins–Araguaia ecoregion. In this context, negotiations and efforts to conserve diversity require a systemic approach, involving the development of short-, medium-, and long-term plans covering the entire ecoregion, as well as for the Tocantins–Araguaia Hydrographic Region, similar to the Electricity Sector Development Plans, incorporating the effective participation and engagement of local societies.
The strengthening of institutions related to socio-environmental planning and conservation, together with local communities, before approving new projects associated with aquatic environments, is fundamental to promote equity in discussions, negotiations, and integration in favor of the common good and the community. Considering that both fish and people depend on water and aquatic diversity, as highlighted by [66], prioritizing the conservation of ichthyofauna with equity is important for energy production with socio-environmental justice, a commitment worth embracing.
Considering the implications for planning and conservation of fish species, especially concerning the fish routes in the context of hydroelectric projects, we recommend the following:
- Integrated Conservation Planning: Implement comprehensive and integrated conservation plans for the entire Tocantins–Araguaia Basin, taking into account the diverse ichthyofauna, knowledge that is continuously evolving and currently available and the potential impacts of hydroelectric projects. This approach ensures the prevention of fragmented conservation efforts, enabling a holistic strategy to protect the socio-environmental diversity of the basin.
- Fish Route Implementation: Prioritize, whenever possible, the incorporation of fish routes, such as fishways and bypasses, in the design and construction of hydroelectric projects. Fish routes facilitate the movement of fish past dams, aiding in maintaining genetic diversity and preventing population isolation, which is crucial for the long-term conservation of migratory species.
- Adaptive Management Strategies: Develop and test adaptive management strategies specifically tailored to tropical regions, considering the unique characteristics and challenges of the local ecosystems. Tailoring strategies to the region’s specific conditions ensures the effectiveness of conservation efforts and the applicability of fish routes in naturally isolated areas.
- Monitoring and Research: Conduct continuous and thorough monitoring of fish populations and their movements, both before and after the implementation of fish routes. Regular monitoring yields valuable data on the success of fish routes, helping researchers and conservationists make informed decisions and adapt strategies as needed.
- Stakeholder Engagement: Involve local communities, fisheries, and indigenous populations in the planning and implementation of fish routes, considering their traditional knowledge and practices. Local communities often have valuable insights into fish behavior and ecosystems. Involving them fosters a sense of ownership and cooperation in conservation initiatives.
- Mitigation of Economic Impact: Explore and implement measures to mitigate the potential economic impact on communities relying on freshwater fish for subsistence, taking into account the potential disruption caused by hydroelectric projects. Addressing economic concerns ensures a more balanced approach, considering both conservation goals and the well-being of local populations.
- Holistic Approach to Conservation: Integrate the conservation of fish species with broader ecosystem preservation efforts, considering the interconnectedness of aquatic systems and the role of fish in maintaining ecological balance. A holistic approach recognizes the importance of preserving entire ecosystems, acknowledging the intricate relationships between different species and their environments.
In conclusion, a well-structured and adaptive conservation plan, coupled with the incorporation of effective fish routes, can contribute to the sustainable development of hydroelectric projects while safeguarding the biodiversity and ecological integrity of the Tocantins–Araguaia Basin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su16062303/s1, Table S1. List of species that occur in the Tocantins–Araguaia ecoregion, with emphasis on species restricted to the Tocantins–Araguaia ecoregion (R), threatened with extinction according to the Official National List of Endangered Fauna Species (categories: Endangered (EN), Vulnerable (VU), Critically Endangered (CR) and Critically Endangered, Possibly Extinct (CRPEX)), in accordance with Portaria GM/MMA No. 300 of 13 December 2022 [27], and Long-Distance Migratory species (LDM). * Added based on information from fishermen and [31].
Author Contributions
Conceptualization, M.P.O., E.E.M., T.L.d.O.G. and M.C.M.; Methodology, M.P.O., J.F.M.d.S., A.d.G.d.C. and S.M.; Software, M.P.O. and J.F.M.d.S.; Validation, M.P.O., E.E.M., T.L.d.O.G., J.F.M.d.S., M.C.M. and S.M.; Formal analysis, M.P.O., E.E.M., T.L.d.O.G., M.C.M. and S.M.; Investigation, M.P.O., E.E.M., T.L.d.O.G. and A.d.G.d.C.; Resources, M.P.O., E.E.M., M.C.M. and S.M.; Data curation, M.P.O., T.L.d.O.G. and J.F.M.d.S.; Writing—original draft, M.P.O. and E.E.M.; Writing—review & editing, T.L.d.O.G., M.C.M., A.d.G.d.C. and S.M.; Visualization, M.P.O. and S.M.; Supervision, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tundisi, J.G. Gerenciamento integrado de bacias hidrográficas e reservatórios: Estudos de caso e perspectivas. In Ecologia de Reservatórios: Impactos Potenciais. Ações de Manejo e Sistemas em Cascata, 2nd ed.; Nogueira, M.G., Henry, R., Jorcin, A., Eds.; RiMa: São Carlos, Brazil, 2005; ISBN 85-7656-092-5. [Google Scholar]
- Lima, F.C.T.D.; Caires, R.A. Peixes da Estação Ecológica Serra Geral do Tocantins. bacias dos rios Tocantins e São Francisco. com observações sobre as implicações biogeográficas das” águas emendadas” dos rios Sapão e Galheiros. Biota Neotrop. 2011, 11, 231–250. [Google Scholar] [CrossRef]
- Albert, J.S.; Reis, R.E. (Eds.) Historical Biogeography of Neotropical Freshwater Fishes; University of California Press: London, UK, 2011; ISBN 0520948505. [Google Scholar]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa. biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- Su, G.; Tedesco, P.A.; Toussaint, A.; Villéger, S.; Brosse, S. Contemporary environment and historical legacy explain functional diversity of freshwater fishes in the world rivers. Glob. Ecol. Biogeogr. 2022, 31, 700–713. [Google Scholar] [CrossRef]
- Folke, C. Resilience: The emergence of a perspective for social–ecological systems analyses. Glob. Environ. Chang. 2006, 16, 253–267. [Google Scholar] [CrossRef]
- Ferrareze, M.; Casatti, L.; Nogueira, M.G. Spatial heterogeneity affecting fish fauna in cascade reservoirs of the Upper Paraná Basin, Brazil. Hydrobiologia 2014, 738, 97–109. [Google Scholar] [CrossRef]
- Agostinho, A.A.; Gomes, L.C.; Pelicice, F.M. Ecologia e Manejo de Recursos Pesqueiros em Reservatórios do Brasil, 1st ed.; Eduem: Maringa, Brazil, 2007; ISBN 978-85-7628-095-8. [Google Scholar]
- Barbosa, F.A.R.; Padisák, J.; Espíndola, E.L.G.; Borics, G.; Rocha, O. The cascading reservoir continuum concept (CRCC) and its application to the river Tietê-basin, São Paulo State, Brazil. In Theoretical Reservoir Ecology and Its Applications; Tundisi, J.G., Strakraba, M., Eds.; International Institute of Ecology: Oldendorf, Germany; Academy of Sciences and Backhuys Publishers: Philadelphia, PA, USA, 1999; pp. 425–437. [Google Scholar]
- Winemiller, K.O.; McIntyre, P.B.; Castello, L.; Fluet-Chouinard, E.; Giarrizzo, T.; Nam, S.; Baird, I.G.; Darwall, W.; Lujan, N.K.; Harrison, I.; et al. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 2016, 351, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Pelicice, F.M.; Agostinho, A.A.; Akama, A.; Andrade Filho, J.D.; Azevedo-Santos, V.M.; Barbosa, M.V.M.; Bini, L.M.; Brito, M.F.G.; dos Anjos Candeiro, C.R.; Caramaschi, É.P.; et al. Large-scale degradation of the Tocantins-Araguaia River basin. Environ. Manag. 2021, 68, 445–452. [Google Scholar] [CrossRef] [PubMed]
- IBGE—Instituto Brasileiro de Geografia e Estatística. Bacias e Divisões Hidrográficas do Brasil/IBGE, Coordenação de Recursos Naturais e Estudos Ambientais; IBGE: Rio de Janeiro, Brazil, 2021; Volume 48, p. 160. ISSN 0101-2843.
- ANA—Agência Nacional de Águas. Plano Estratégico de Recursos Hídricos da Bacia Hidrográfica dos Rios Tocantins e Araguaia: Relatório Síntese; Agência Nacional de Águas: Brasília, Brazil, 2009; p. 256. ISBN 978-85-89629-55-3.
- Albert, J.S.; Petry, P.; Reis, R.E. Major Biogeographic and Phylogenetic Patterns. In Historical Biogeography of Neotropical Freshwater Fishes; Albert, J.S., Reis, R.E., Eds.; University of California Press: Los Angeles, CA, USA, 2011; pp. 21–57. [Google Scholar]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Ribeiro, M.C.L.D.B.; Petrere, M.; Juras, A.A. Ecological integrity and fisheries ecology of the Araguaia—Tocantins River Basin, Brazil. Regul. Rivers Res. Manag. 1995, 11, 325–350. [Google Scholar] [CrossRef]
- Tocantins; Secretaria da Fazenda e Planejamento (SEFAZ); Subsecretaria do Planejamento e Orçamento; Superintendência de Planejamento Governamental; Diretoria de Gestão de Informações Territoriais e Socioeconômicas; Gerência de Zoneamento Territorial (GZT). Projeto de Desenvolvimento Regional Integrado e Sustentável, Elaboração das Cartas Climáticas do Estado do Tocantins; SEFAZ/GZT: Palmas, Brazil, 2020; p. 464.
- Chamon, C.C.; Serra, J.P.; Camelier, P.; Zanata, A.M.; Fichberg, I.; Marinho, M.M.F. Building knowledge to save species: 20 years of ichthyological studies in the Tocantins-Araguaia River basin. Biota Neotrop. 2022, 22, e20211296. [Google Scholar] [CrossRef]
- Cote, D.; Kehler, D.G.; Bourne, C.; Wiersma, Y.F. A new measure of longitudinal connectivity for stream networks. Landsc. Ecol. 2008, 24, 101–113. [Google Scholar] [CrossRef]
- Díaz, G.; Arriagada, P.; Górski, K.; Link, O.; Karelovic, B.; Gonzalez, J.; Habit, E. Fragmentation of Chilean Andean rivers: Expected effects of hydropower development. Rev. Chil. Hist. Nat. 2019, 92, 1. [Google Scholar] [CrossRef]
- Guedes, T.L.O. A Ictiofauna da Ecorregião Tocantins-Araguaia: Diversidade, Redes de Pesquisa e Construção do Conhecimento; Programa de Ciências do Ambiente/UFT: Palmas, Brazil, 2021; p. 172. [Google Scholar]
- MME-EPE—Ministério de Minas e Energia, Empresa de Pesquisa Energética. Plano Nacional de Energia 2050; Ministério de Minas e Energia, Empresa de Pesquisa Energética, MME/EPE: Brasília, Brazil, 2020. [Google Scholar]
- Aneel—Agência Nacional de Energia Elétrica. AHE—Mapa dos Empreendimentos de Aproveitamento Hidrelétricos (Estágio da Usina). Available online: https://sigel.aneel.gov.br/portal/home/ (accessed on 12 March 2021).
- MME—Ministério de Minas e Energia—Agência Nacional de Energia Elétrica/Diretoria/Aneel. Resolução Normativa nº 875, de 10 de Março de 2020; Diário Oficial da União: Brasília, Brazil, 2020.
- Aneel—Agência Nacional de Energia Elétrica. Aplicação Desenvolvida Utilizando Web AppBuilder para Downloads dos Dados Nos Formatos Shapefile e KMZ. Available online: https://sigel.aneel.gov.br/portal/home/ (accessed on 26 February 2022).
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2015. Available online: http://www.qgis.org/ (accessed on 23 May 2022).
- Brasil. MMA—Ministério do Meio Ambiente. Portaria GM/MMA Nº 300. de 13 de dezembro de 2022. Reconhece a Lista Nacional de Espécies Ameaçadas de Extinção. D.O.U., 14 December 2022. Available online: https://www.sindipi.com.br/uploads/repositorio/files/300.pdf (accessed on 1 February 2024).
- Carolsfeld, J.; Harvey, B.; Ross, C.; Baer, A. Migratory Fish of South America, Biology, Fisheries and Conservation Status World; Fisheries Trust: Victoria, BC, Canada, 2003; ISBN 0-9683958-2-12. [Google Scholar]
- Neuberger, A.L.; Marques, E.E.; Agostinho, C.S.; Pelicice, F.M. Variações espaciais na atividade reprodutiva de peixes na área de influência do reservatório de Peixe Angical. In Reservatório de Peixe Angical: Bases Ecológicas para o Manejo da Ictiofauna; Agostinho, C.S., Pelicice, M.P., Marques, E.E., Eds.; RiMa Editora: São Carlos, Brazil, 2009. [Google Scholar]
- Bartolette, R.; Souza-Lima, R.; Figueiredo, C.A.A.; Moraes, D.F., Jr.; Caramaschi, E.P. Composição taxonômica da ictiofauna da área da UHE Serra da Mesa. In Usina Hidrelétrica de Serra da Mesa: 15 Anos de Estudos da Ictiofauna do Alto Rio Tocantins; Mazzoni, R., Caramaschi, E.P., Iglesias-Rios, R., Eds.; Furnas: Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Agostinho, C.S.; Marques, E.E.; Oliveira, R.J.; Lucinda, P.H.F.; Freitas, I.S.; Neuberger, A.L.; De Melo, J.R.B.; Soares, A.B.; Monteiro, A.S. Estudos da Ictiofauna a Jusante da Usina Hidrelétrica de Serra da Mesa; Final Report; Unitins/Fundação Universidade do Tocantins: Palmas, Brazil, 2005; 110p. [Google Scholar]
- Agostinho, K.D.G.L.; Abujanra, F.; Oliveira, C.R.C.; Latini, J.D.; Ortega, J.; Gomes, L.C.; Agostinho, A.A. Monitoramento e Conservação da Ictiofauna da UHE São Salvador Durante a Fase de Operação do Reservatório; Thecnical Report; Tractebel Energía and Limnobios Consultoria em Ambientes Aquáticos: Maringá, Brazil, 2015; 373p. [Google Scholar]
- Soares, A.B.; Pelicice, F.M.; Lucinda, P.H.; Akama, A.; Agostinho, C.S. Diversidade de peixes na área de influência da barragem de Peixe Angical, antes e após a formação do reservatório. In Reservatório de Peixe Angical: Bases Ecológicas para o Manejo da Ictiofauna; Agostinho, C.S., Pelicice, M.P., Marques, E.E., Eds.; RiMa Editora: São Carlos, Brazil, 2009. [Google Scholar]
- Lucinda, P.H.; Freitas, I.S.; Soares, A.B.; Marques, E.E.; Agostinho, C.S.; de Oliveira, R.J. Fish, Lajeado reservoir, rio Tocantins drainage, state of Tocantins, Brazil. Check List 2007, 3, 70–83. [Google Scholar] [CrossRef]
- Bartolette, R.; Vieira, C.S.; Santos, C.D.C.; Luduvice, J.S.V.; Passos, T.S.; D’avilla, T.; Nascimento, B.O.; Ernesto, D.; Argolo, F.H.; Aguiar, A.J.M.; et al. The ichthyofauna in the influence area of the Lajeado reservoir, Tocantins state, Brazil. Check List 2017, 13, 1–14. [Google Scholar] [CrossRef]
- FECD—Fundação Educacional Ciência e Desenvolvimento; Contrato Consórcio Estreito Energia—CESTE. Programa de Con-servação da Ictiofauna do AHE de Estreito—Fase Reservatório; Final Report; FECD: Rio de Janeiro, Brazil, 2013; 249p. [Google Scholar]
- Biota-Ceste, Biota Projetos e Consultoria Ambiental Ltd.; Consórcio Estreito Energía; Usina Hidrelétrica de Estreito. Moni-Toramento e Conservação da Ictiofauna (Maio a Dezembro de 2016); Thecnical Report; Biota Projetos e Consultoria Ambiental: Goiânia, Brazil, 2017; 125p. [Google Scholar]
- Santos, G.; Jegu, M.; Merona, B.D.; do Brasil, C.E.D.N. Catálogo de Peixes Comerciais do Baixo Rio Tocantins; Eletronorte: Brasília, Brazil, 1984. [Google Scholar]
- Santos, G.M.D.; Juras, A.A.; Mérona, B.D.; Jégue, M. Peixes do Baixo Rio Tocantins, 20 Anos Depois da Usina Hidrelétrica Tucuruí; Eletronorte: Brasília, Brazil, 2004. [Google Scholar]
- Orsi, C.H.; Message, H.J.; Debona, T.; Baumgartner, D.; Baumgartner, G. Hydrological seasonality dictates fish fauna of the lower Araguaia River, Tocantins-Araguaia basin. Environ. Biol. Fishes 2018, 101, 881–897. [Google Scholar] [CrossRef]
- Zacarkim, C.E.; Piana, P.A.; Baumgartner, G.; Aranha, J.M.R. The panorama of artisanal fisheries of the Araguaia River, Brazil. Fish. Sci. 2015, 81, 409–416. [Google Scholar] [CrossRef]
- Ferreira, E.J.G.; Zuanon, J.; dos Santos, G.M.; Amadio, S.A. The fish fauna of the Parque Estadual do Cantão, Araguaia River, State of Tocantins, Brazil. Biota Neotrop. 2011, 11, 277–284. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Tejerina-Garro, F.L. Relationships between taxonomic and functional components of diversity: Implications for conservation of tropical freshwater fishes. Freshw. Biol. 2015, 60, 1854–1862. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Teresa, F.B.; Tejerina-Garro, F.L. The effect of riverine networks on fish β-diversity patterns in a Ne-otropical system. Hydrobiologia 2021, 848, 515–529. [Google Scholar] [CrossRef]
- Claro-García, A.; Shibatta, O.A. The fish fauna of streams from the upper rio Tocantins basin, Goiás State, Brazil. Check List 2013, 9, 28–33. [Google Scholar] [CrossRef][Green Version]
- Torres, T.P. Aspectos Ecológicos da Ictiofauna da Drenagem do Rio do Sono, Bacia do Rio Tocantins, Brasil. Marter’s Thesis, Universidade Federal do Oeste do Pará, Santarém, Brazil, 2018. Available online: https://repositorio.ufopa.edu.br/jspui/handle/123456789/305 (accessed on 10 July 2023).
- Jarduli, L.R.; Claro-García, A.; Shibatta, O.A. Ichthyofauna of the rio Araguaia basin, states of Mato Grosso and Goiás, Brazil. Check List 2014, 10, 483–515. [Google Scholar] [CrossRef]
- Tejerina-Garro, F.L.; Fortin, R.; Rodríguez, M.A. Fish community structure in relation to environmental variation in floodplain lakes of the Araguaia River, Amazon Basin. Environ. Biol. Fishes 1998, 51, 399–410. [Google Scholar] [CrossRef]
- da Silva, E.F.; de Melo, C.E.; Vênere, P.C. Fatores que influenciam a comunidade de peixes em dois ambientes no baixo Rio das Mortes, Planície do Bananal, Mato Grosso, Brasil. Rev. Bras. Zool. 2007, 24, 482–492. [Google Scholar] [CrossRef][Green Version]
- Melo, T.L.; Tejerina-Garro, F.L.; Melo, C.E. Diversidade biológica da comunidade de peixes no baixo Rio das Mortes, Mato Grosso, Brasil. Rev. Bras. Zool. 2007, 24, 657–665. [Google Scholar] [CrossRef]
- Giongo, P.; Sampaio, W.M.S.; Belei, F.; de Carvalho, F.K.; Fernandes, A.; Dergam, J.A. Ichthyofauna of the Carrapato. Mutum and Caba Saco streams (Araguaia River Basin), Serra dos Carajás region, southeastern Pará, Brazil. Check List 2011, 7, 517–521. [Google Scholar] [CrossRef]
- Dagosta, F.C.; de Pinna, M. The fishes of the Amazon: Distribution and biogeographical patterns. with a comprehensive list of species. Bull. Am. Mus. Nat. Hist. 2019, 431, 1–163. [Google Scholar] [CrossRef]
- MME-EPE, Ministério de Minas e Energia, Empresa de Pesquisa Energética. Plano Decenal de Expansão de Energia 2019; Ministério de Minas e Energia, Empresa de Pesquisa Energética, MME/EPE: Brasília, Brazil, 2010. [Google Scholar]
- MME-EPE, Ministério de Minas e Energia, Empresa de Pesquisa Energética. Plano Decenal de Expansão de Energia 2021; Ministério de Minas e Energia, Empresa de Pesquisa Energética, MME/EPE: Brasília, Brazil, 2012. [Google Scholar]
- MME-EPE, Ministério de Minas e Energia, Empresa de Pesquisa Energética. Plano Decenal de Expansão de Energia 2024; Ministério de Minas e Energia, Empresa de Pesquisa Energética, MME/EPE: Brasília, Brazil, 2015. [Google Scholar]
- MME-EPE, Ministério de Minas e Energia, Empresa de Pesquisa Energética. Plano Decenal de Expansão de Energia 2031; Ministério de Minas e Energia, Empresa de Pesquisa Energética, MME/EPE: Brasília, Brazil, 2022. [Google Scholar]
- EPE E DIVERSAS—Sustentabilidade. SIEMAS—Sistema de Indicadores de Energia. Meio Ambiente e Sociedade. Bem-Estar. Indicadores de Bem-Estar Energético. Texto Base: Subsídios para as Oficinas. 2022. Available online: https://www.epe.gov.br (accessed on 6 May 2023).
- Aneel—Agência Nacional de Energia Elétrica. Informações Gerenciais. 2018. Available online: https://antigo.aneel.gov.br/documents/656877/14854008/Boletim+de+Informa%C3%A7%C3%B5es+Gerenciais+-+3%C2%BA+trimestre+de+2018/9658018c-b292-4e62-5612-02452e28e959 (accessed on 6 May 2023).
- Akama, A. Impacts of the hydroelectric power generation over the fish fauna of the Tocantins River, Brazil: Marabá dam, the final blow. Oecologia Aust. 2017, 21, 222–231. [Google Scholar] [CrossRef]
- Middleton, C. Moving Beyond ‘Sustainable Hydropower’ in the Mekong Basin. 2022. Available online: https://www.water-alternatives.org/index.php/blog/mekong (accessed on 3 May 2023).
- Prysthon, A.; da Cunha, C.V.; Dias, C.R.G. The Fishing Productivity Assessement Upstream and Downstream of Tucuruí Hydroeletric Dam, Tocantins-Araguaia basin, Brazil. Int. J. Adv. Eng. Res. Sci. IJAERS 2019, 6, 85–92. [Google Scholar] [CrossRef]
- Dodd, J.R.; Cowx, I.G.; Bolland, J.D. Efficiency of a nature-like bypass channel for restoring longitudinal connectivity for a river-resident population of brown trout. J. Environ. Manag. 2017, 204, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Celestino, L.F.; Sanz-Ronda, F.J.; Miranda, L.E.; Makrakis, M.C.; Dias, J.H.P.; Makrakis, S. Bidirectional connectivity via fish ladders in a large Neotropical river. River Res. Appl. 2019, 35, 236–246. [Google Scholar] [CrossRef]
- Makrakis, S.; Bertão, A.P.S.; Silva, J.F.M.; Makrakis, M.C.; Sanz-Ronda, F.J.; Celestino, L.F. Hydropower Development and Fishways: A Need for Connectivity in Rivers of the Upper Paraná Basin. Sustainability 2019, 11, 3749. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- Lynch, A.J.; Cooke, S.J.; Arthington, A.H.; Baigun, C.; Bossenbroek, L.; Dickens, C.; Harrison, I.; Kimirei, I.; Langhans, S.D.; Murchie, K.J.; et al. People need freshwater biodiversity. WIRESs Water 2023, 10, e1633. [Google Scholar] [CrossRef]
- Ministério de Meio Ambiente. Portaria MMA Nº 445. de 17 de dezembro de 2014. Reconhece a Lista Nacional Oficial de Espécies da Fauna Ameaçadas de Extinção—Peixes e Invertebrados Aquáticos. In Official Gazette of the Union; Ministério de Meio Ambiente: Brasília, Brasil, 2014; Volume 1, p. 126. [Google Scholar]
- Doria, C.R.D.C.; Agudelo, E.; Akama, A.; Barros, B.S.F.; Bonfim, M.; Carneiro, L.; Briglia-Ferreira, S.R.; Carvalho, L.N.; Bonilla, C.A.; Charvet, P.; et al. The silent threat of non-native fish in the Amazon: ANNF database and review. Front. Ecol. Evol. 2021, 9, 646702. [Google Scholar] [CrossRef]
- Tocantins. Conselho Estadual do Meio Ambiente. Resolução COEMA-TO. N. 88. de 5 dezembro de 2018. Dispõe sobre o licenciamento ambiental da aquicultura no estado do Tocantins. Official Gazette of the State of Tocantins, 7 December 2018. Available online: https://www.legisweb.com.br/legislacao/?id=370992 (accessed on 1 February 2024).
- ICMBio—Instituto Chico Mendes de Conservação da Biodiversidade. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Volume VI—Peixes. In Instituto Chico Mendes de Conservação da Biodiversidade (org.). Livro Vermelho da Fauna Brasileira Ameaçada de Extinção; ICMBio: Brasília, Brazil, 2018; 1232p. [Google Scholar]
- Yi, Y.J.; Zhou, Y.; Song, J.; Zhang, S.; Cai, Y.; Yang, W.; Yang, Z. The effects of cascade dam construction and operation on riparian vegetation. Adv. Water Resour. 2019, 131, 103206. [Google Scholar] [CrossRef]
- Swanson, A.C.; Kaplan, D.; Toh, K.B.; Marques, E.E.; Bohlman, S.A. Changes in floodplain hydrology following serial damming of the Tocantins River in the eastern Amazon. Sci. Total Environ. 2021, 800, 149494. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.A.; Tejerina-Garro, F.L. The influence of environmental variables on the functional structure of headwater stream fish assemblages: A study of two tropical basins in Central Brazil. Neotrop. Ichthyol. 2015, 13, 349–360. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Choueri, R.B.; Azevedo, J.A.R. Biodiversidade e Impacto de Grandes Empreendimentos Hidrelétricos na Bacia Tocantins-Araguaia: Uma Análise Sistêmica. Soc. Nat. 2017, 29, 443–457. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).