Abstract
Loess disintegration is a significant physicochemical and mechanical dissolution process that occurs when loess comes into contact with water. This phenomenon contributes to geological disasters such as loess cave erosion, landslides, and debris flows. The disintegration of loess can be influenced by both internal and external factors. Research on internal factors of loess disintegration has been widely recorded, but the research progress on external environmental factors that affect loess disintegration is not well summarized. This review summarizes the impacts of external water environmental factors on loess disintegration and reveals that six external water environmental factors, namely the temperature of the aqueous solution, hydrodynamic conditions, solution pH, salt concentration and type in the solution, freeze–thaw cycles, and dry–wet cycles, can significantly impact loess disintegration. Furthermore, this review delves into three key research areas in loess disintegration under the influence of these water environmental factors: experimental research on loess disintegration, the disintegration parameters used in such research and their variations, and the water–soil chemical reactions and microstructural changes during loess disintegration. It concludes that current experimental research on loess disintegration suffers from inadequate studies, with existing research associated with poor comparability and weak representativeness, and a lack of comprehensive, systematic analysis of its regularities of influence and response mechanisms from both microscopic and macroscopic perspectives. This paper can provide valuable insights for the prevention of loess geological disasters and engineering safety construction.
1. Introduction
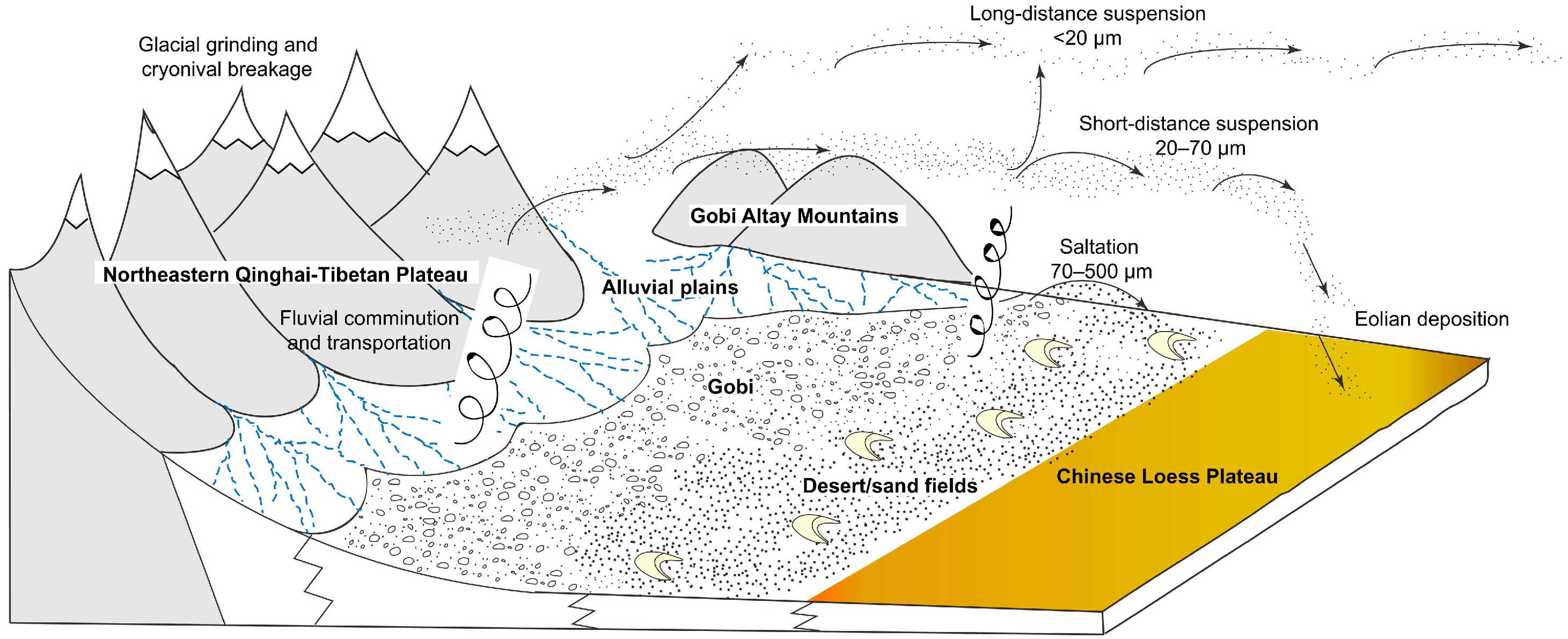
Loess, a loose deposit formed during the Quaternary period under arid and semi-arid climate conditions [1], is most typical in China’s Loess Plateau [2,3]. Regarding the formation of loess, there have been many arguments. However, the hypothesis of aeolian origin for loess has been accepted by more people in recent years [4,5]. The genetic hypothesis of loess is shown in Figure 1 [6].
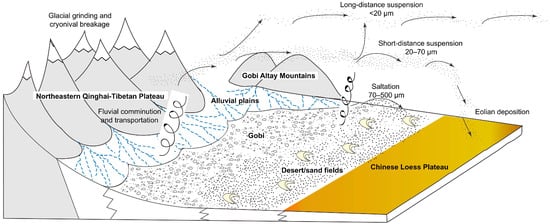
Figure 1.
The genetic hypothesis of loess formation and origin [6].
Loess consists mainly of silt with a certain amount of fine sand and clay [7,8]. The color is yellowish gray or brownish yellow. It is homogeneous, unconsolidated, and slightly clayey, and it contains a high proportion of calcium and other mineral nutrients, which is advantageous for farming. In addition, clay minerals in loess have a strong adsorption and ion exchange capability and can be used as adsorbents and chemical catalysts, which is conducive to sewage purification and chemical production. However, characterized by high vertical permeability, water sensitivity, collapsibility, and disintegration [9], loess is susceptible to external environmental factors and human activities. These vulnerabilities often lead to various natural disasters, such as loess landslides, collapses, ground subsidence, and soil erosion [10]. In recent years, due to the increase in human activities [11,12] and environmental degradation, these disasters have happened frequently, severely impacting socioeconomic development and the safety of people’s lives and property [13,14,15]. As a result, they have gained significant attention. From the 1950s to the early 21st century, the Chinese government implemented a series of projects on the Chinese Loess Plateau to bolster loess geological disaster prevention and to recover the eco-environment there.
Loess disintegration, defined as the process of loess softening and dispersing upon water contact [16], is a key characteristic of loess. It contributes to geologic hazards such as loess cave erosion, landslides, and debris flows. Research into loess disintegration is crucial for soil and water conservation and disaster prevention. The disintegration process is triggered when water immersion weakens the cementation force between soil grains, reducing it to less than the disintegration force (Figure 2) [17].
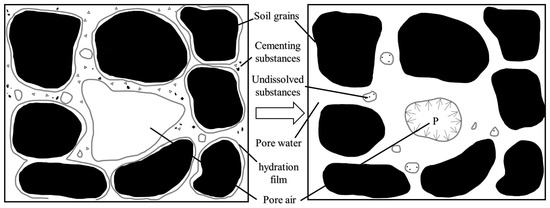
Figure 2.
Loess disintegration process [18].
Factors causing loess disintegration can be classified into internal and external categories. Internal factors primarily include mineral composition, grain size composition, water content, and loess structure [19,20]. Studies have revealed that the mineral composition that determines the strength of disintegration of loess is the hydrophilic and expansive clay minerals contained in loess (such as illite, kaolinite, montmorillonite), and a higher amount of clay minerals in loess causes stronger disintegration. However, the disintegration of loess may decrease sharply with the increase in natural water content in loess. The grain size affects the disintegration of loess by determining the porosity, permeability, and cementation state of the loess. Coarser particles can usually induce stronger disintegration. The pores and fractures in loess determine the disintegration mode when loess encounters water, thus determining the strength of disintegration. Developed pores and fissures usually produce more severe rapid disintegration of loess. External factors encompass water environmental elements such as the temperature of the aqueous solution [21,22,23,24,25], hydrodynamic conditions [26], solution pH [27,28], salt concentration and type in the solution [21,29], and freeze–thaw and dry–wet cycles [30,31].
Given the unique climate characteristics (arid and semi-arid) and environmental contamination (including industrial, agricultural, and domestic waste pollution) in China’s loess area [32,33], loess disintegration is noticeably affected by varying external environments. Therefore, it is crucial to understand loess disintegration under different water environments. Through literature retrieval, it is found that research on loess disintegration under varying water environments is relatively limited, and no unified research results have been obtained. This review focuses on the following: (1) the research progress in loess disintegration experiments; (2) experimental research on water environmental factors affecting loess disintegration; and (3) the mechanisms by which water environmental factors induce loess disintegration. Through this review, we aim to clarify the current research progress and challenges in experimental research on loess disintegration induced by water environmental factors and provide insights for future research, aiding in the prevention of loess geological disasters.
2. Research Progress in Loess Disintegration Experiments
2.1. Adopted Methods in Disintegration Experiments
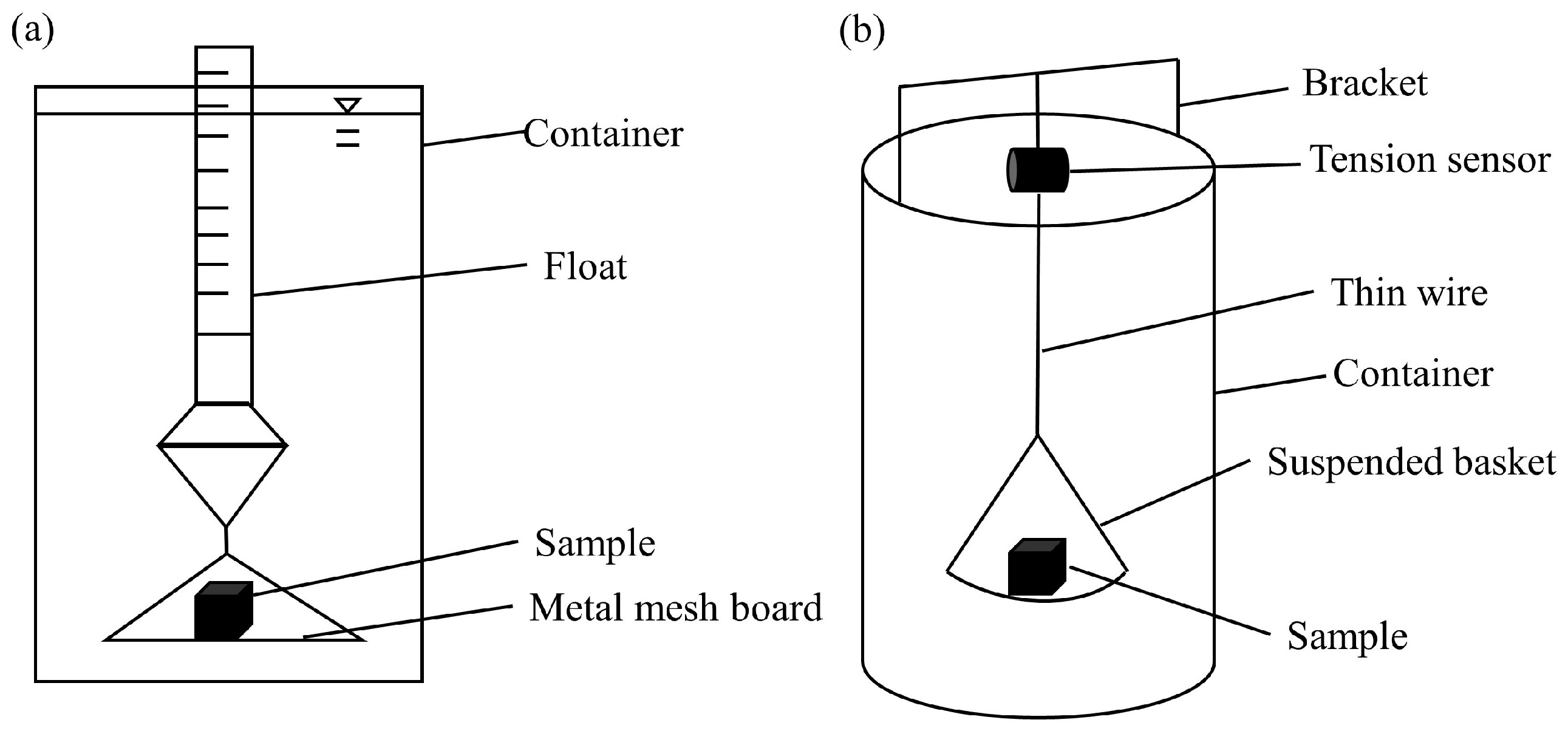
The methods used in loess disintegration experiments can be broadly classified into the float method (volume method) and the mass method. The device used for the traditional float method is relatively simple and easy to operate (Figure 3a). However, its reliance on manual reading can lead to significant errors, affecting the accuracy of test results and making it challenging to track the dynamic process of loess disintegration. To improve its accuracy and to capture disintegration dynamics, an improved mass method was proposed by improvements in test device modifications (Figure 3b) [18,21,23,24,25,26]. The original float is replaced with a tension meter, a balance, or a tension sensor. This method can yield higher accuracy than the float method.
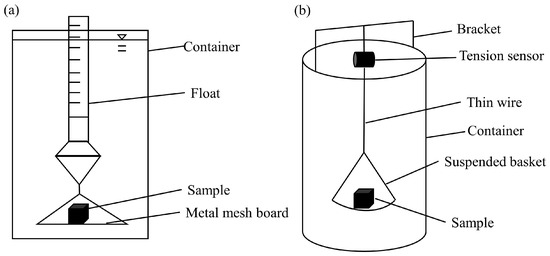
Figure 3.
Methods in disintegration experiments [18,24]. (a) Float method and (b) mass method.
Furthermore, it has been observed that disintegration experiments using the mass method involve both saturated and unsaturated stages. Loess transitions from an unsaturated state (absorbing water period and softening period) to a saturated state (disintegration period and stable period) during disintegration. Thus, both saturated soil disintegration and unsaturated soil disintegration occur [19,24,26].
Moreover, to systematically characterize loess disintegration properties and describe the dynamic process of loess disintegration in detail, several disintegration parameters are deduced based on the principles of the float method and mass method. Currently, commonly used loess disintegration parameters include disintegration amount [16,34], disintegration time, disintegration rate [18,21,22,24], disintegration velocity [18,22,25,35], disintegration resistance coefficient [36], accumulative disintegration modulus [37], and accumulative percentage of disintegration mass [38]. These disintegration parameters are mainly derived, modified, and verified according to the mechanical equilibrium relationship during loess disintegration.
2.2. Development Trend of Loess Disintegration Experimental Research
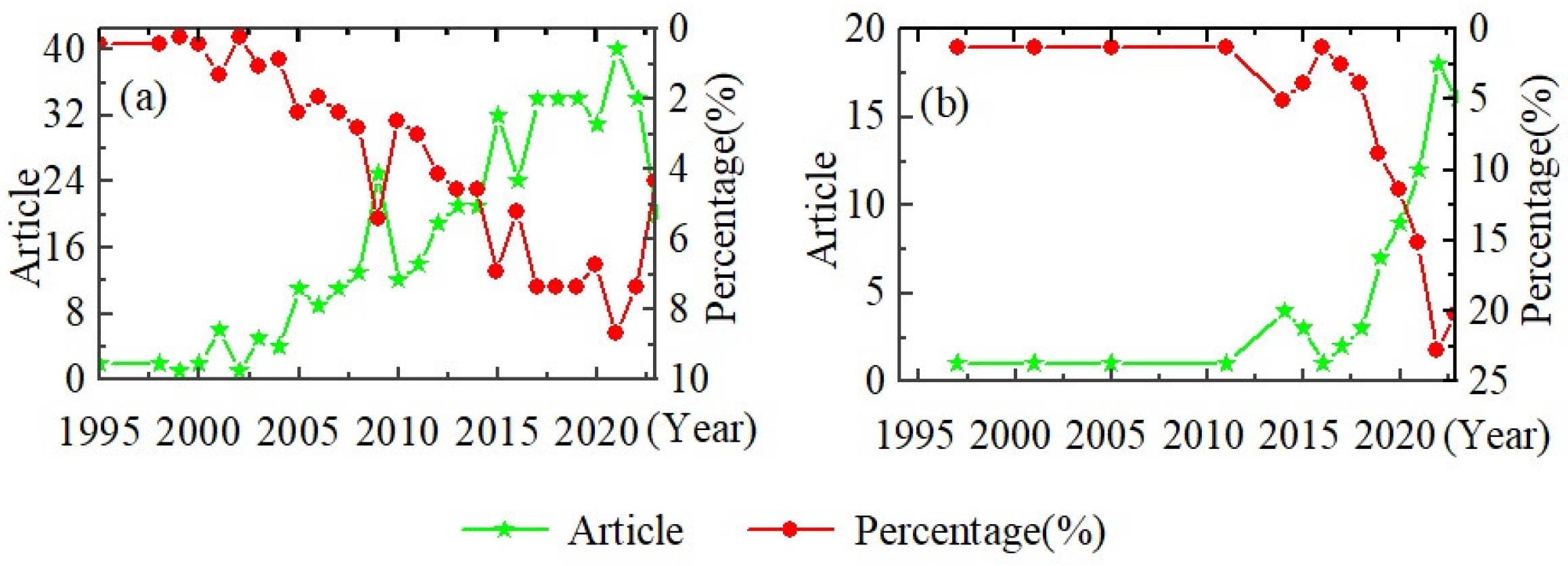
At present, most of the research on loess disintegration characteristics is conducted in the loess region of China. However, within the broader research field of loess characteristics, the proportion of studies on loess disintegration and loess disintegration experiments is relatively small. According to the Wanfang database and Web of Science, the number of articles on the topic of loess disintegration experiments is less than 100 and less than 40, respectively (Table 1). This indicates that within the vast research field of loess, the understanding of the erodibility characteristics of loess disintegration remains somewhat unclear. The limited number of disintegration tests has resulted in a lack of research efforts and depth on loess disintegration.

Table 1.
Articles on loess disintegration research and loess disintegration experiments.
Despite the overall scarcity of experimental studies on loess disintegration, the number of publications on loess disintegration and its percentage of the total number of publications have been increasing year by year since the end of the 20th century to 2023, and the rate of increase is accelerating based on the Wanfang database and Web of Science (Table 1 and Figure 4). This trend indicates that an increasing number of scholars are beginning to research the disintegration characteristics of loess. It is expected that research on the disintegration characteristics of loess will become increasingly popular in the future.
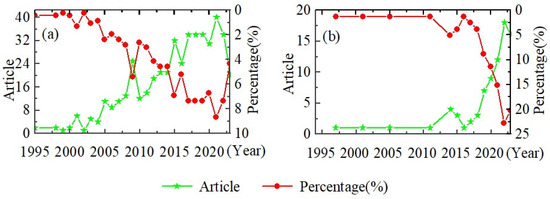
Figure 4.
Research trend of loess disintegration. (a) Publications in Wanfang database and (b) publications in Web of Science.
3. Water Environmental Factors Influencing Loess Disintegration
In many loess disintegration experiments, scholars have investigated the influence of different water environmental factors on loess disintegration. They have done this by observing experimental phenomena, calculating and analyzing disintegration parameters, and clarifying the mechanisms and causes of these influences from both microscopic and macroscopic perspectives. This work lays a foundation for further research on loess disintegration characteristics and the prevention of geological disasters.
Through the review and analysis of numerous articles, six major water environmental factors that influence the disintegration of loess were identified: temperature of the aqueous solution, hydrodynamic conditions, pH of the aqueous solution, salt concentration and type of salt in the aqueous solution, freeze–thaw cycles, and dry–wet cycles. The following sections will provide a detailed introduction to the experimental research on loess disintegration induced by these water environmental factors.
3.1. Temperature of the Aqueous Solution
The temperature of the aqueous solution is often an important factor in researching the disintegration of loess. It generally impacts the disintegration of loess by affecting the physical (chemical) reaction rate between soil and aqueous solution [39]. Based on a review of relevant domestic and international articles, the experimental research on the effect of aqueous solution temperature on loess disintegration is summarized in the following table (Table 2).

Table 2.
Experimental research on the effect of solution temperature on loess disintegration.
As can be seen from the table above, many scholars [21,23,24,25] have used undisturbed Q3 Malan loess as the subject of laboratory disintegration experiments to explore the influence of the aqueous solution temperature on loess disintegration. They took the disintegration rate and the average disintegration velocity as the disintegration parameters and adopted the principle of a single variable to observe the variation in each disintegration parameter. The results consistently showed that within a certain range, the average disintegration velocity of loess accelerated as the temperature of the aqueous solution rose, and the instantaneous disintegration rate increased. This indicates that an increase in the temperature of the aqueous solution can promote loess disintegration.
3.2. Hydrodynamic Conditions
Currently, most loess disintegration experiments are conducted under static water conditions. However, the disintegration of loess or other soils under natural conditions occurs in dynamic water environments such as rainfall, scouring erosion, and runoff. Therefore, the disintegration of loess under hydrostatic conditions does not fully reflect the actual situation. Compared with hydrostatic conditions, the disintegration of loess in dynamic water can better reflect the erosion characteristics of loess under hydrodynamic action, which is more practical. At present, there are few experimental studies on loess disintegration under hydrodynamic action, and reports on whether different hydrodynamic conditions also affect other internal and external water environmental factors of loess disintegration are almost non-existent.
Li et al. used loess at different depths from the same location as test soil samples and studied the effect of hydrodynamic action on the disintegration of loess by observing its disintegration time and volume disintegration velocity in static water and dynamic water with a flow velocity of 1.0 m/s [40]. The results showed that the time of complete disintegration of all loess at the same depth in dynamic water was less than that in static water, and the volume disintegration velocity in dynamic water was also greater than that in static water. This suggests that hydrodynamic action promotes the disintegration of loess in water.
Similarly, to explore the influence of hydrodynamic action on loess disintegration, Fan and Li conducted laboratory disintegration experiments with a dynamic and static water disintegration device [26]. In the experiment, they used root-containing undisturbed Malan loess and rootless undisturbed Malan loess from Taiyuan, Shanxi Province, as the soil samples and took the disintegration rate as the disintegration parameter. The rotational speed of the sieve barrel, which was used to hold soil samples, was set to 20 r/min to hit the water and achieve dynamic water conditions. The results showed that both root-containing soil and rootless soil had greater disintegration rates under dynamic water conditions and were easier to disintegrate. Both of these studies researched the influence of dynamic water on the disintegration of stable soil, but the difference is that the former study was conducted in moving water with a steady flow rate, representing disintegration in a natural situation, while the latter study was realized under a complex moving water condition, including different stresses through a sieve barrel, which makes the research more comprehensive.
3.3. pH of Aqueous Solution
The mechanical properties and structural forms of loess are greatly affected by the pollution of acid and alkali pollutants, which can easily lead to geological disasters such as foundation deformation and slope collapse, causing serious safety hazards to engineering quality [41,42]. The main sources of acidification and alkalization of loess are the pollution from agricultural fertilizers [43] and sewage irrigation [44,45,46,47]. Since loess disintegration is one of the factors that induce geological disasters and engineering quality safety hazards, its research under the conditions of acid and alkali pollution is very important for engineering construction and disaster prevention in loess areas.
Through a review of relevant domestic and international articles, the experimental research on the effect of the pH of the aqueous solution on loess disintegration is summarized in the following table (Table 3).

Table 3.
Experimental research on the effect of pH of aqueous solution on loess disintegration.
To explore the disintegration characteristics of loess under acidic conditions, Gao et al. and Lin et al. used solutions with different concentrations of HCl as an acid-contaminated water environment for loess disintegration [27,28], observing the variation in disintegration parameters. The results showed that as the pH value of the disintegration acid solution decreased, the disintegration rate of the soil increased at the same moment, and the average disintegration velocity increased nonlinearly, with the increasing range going from slow to fast and then to slow. Gu and Liu et al. used Q3-contaminated loess samples that were contaminated with solutions with different concentrations of HNO3 for laboratory disintegration experiments [18,35]. The results showed that the average disintegration velocity of contaminated loess samples increased nonlinearly with the increase in HNO3 concentration and eventually became stable. However, at a given moment, the disintegration rate of acid-contaminated loess first increased and then decreased under the increase in the concentration of HNO3 in the solution, finally decreasing to be close to the disintegration rate of uncontaminated loess samples. This indicated that acidic conditions have a certain promoting effect on the disintegration of loess, but when the pH value is reduced to a certain extent, the promoting effect is weakened, and the effect of acidity on loess disintegration becomes smaller.
To study the disintegration characteristics of loess under alkaline conditions, Li conducted a laboratory disintegration experiment on loess mixed with different mass percentages of quicklime (CaO) [48]. The results showed that with the increase in CaO mixing content, the time for complete disintegration of soil first became shorter, then became longer, and finally tended to be stable. The disintegration time showed a trend towards lengthening overall. This showed that the disintegration of loess was weakened in the alkaline environment where CaO reacts with water to yield Ca(OH)2. Gu and Yang mixed different mass percentages of slaked lime (Ca(OH)2) into loess for a disintegration test [35,49]. It was found that compared with the original loess, the loess mixed with slaked lime was stable in water and almost did not disintegrate, which greatly inhibited the loess disintegration.
It is worth noting that to study the disintegration characteristics of loess under both acidic and alkaline conditions, Gu et al. analyzed the disintegration of Q3 undisturbed loess under different pH solutions [23]. They found that the disintegration velocity could not be fitted by a monotonic function, indicating that the effect of pH on loess disintegration was non-monotonic. Additionally, by observing the variation in disintegration rate and disintegration velocity with pH value, it was found that there was an optimum range of pH value for soil disintegration. When the pH value was in the ranges of 5.5–6.5 and 9.5–10.5, the disintegration rate and disintegration velocity were generally higher, and the disintegration was more intense. Under other acid and alkali conditions, the effect of pH on disintegration was weak or had a slight inhibitory effect, and the disintegration rate and disintegration velocity were significantly lower than the values within the optimum range.
3.4. Salt Concentration and Type in Aqueous Solution
Most of the loess area is located in arid and semi-arid regions, with large annual evaporation and significant soil salinization [50,51]. About one-third of the world’s soil is salinized, and saline–alkali lands are widely distributed in China. Among them, the Ningxia Hetao Plain is one of the areas with severe soil salinization in China. According to relevant data, the total area of salinized farmland in the Ningxia Plain is about 183,300 hm2, accounting for about 33% of the Yellow River irrigation area; the area of salinized farmland in the Hetao Irrigation District of Inner Mongolia is 323,000 hm2, accounting for about 45% of the Yellow River irrigation area [52,53]. In addition, due to early-stage unreasonable irrigation methods (Yellow River diversion and broad irrigation, high-salinity groundwater irrigation) and agricultural fertilizer pollution in some areas [54], loess salinization has become more pronounced. Salinization alters the physicochemical properties of loess, which in turn affects its disintegration characteristics.
Upon reviewing relevant domestic and international articles, it is found that there is limited experimental research on the effect of the salt concentration and salt type of the aqueous solution on loess disintegration. Wang et al. [21], Gu et al. [23], and Yuan [25] used undisturbed Malan loess from Heifangtai, Gansu Province, as the soil sample, and used a NaCl solution to configure salt solutions with different total dissolved solids (TDS) for laboratory disintegration experiments. The disintegration rate and average disintegration velocity were taken as disintegration parameters to explore the effect of the salinity of the aqueous solution on loess disintegration. It was found that with the increase in TDS, the disintegration rate of the loess at the same moment showed a slight downward trend. Although the average disintegration velocity increased slowly with the increase in TDS, its value was generally low, even lower than that of loess in pure water under the same conditions, indicating that the salinity of the aqueous solution has a slight inhibitory effect on the disintegration of loess within a certain range.
Similarly, to research the influence of the salinity of the aqueous solution on loess disintegration, Gu, Zhao, and Xiang et al. used remolded Malan loess from Lanzhou City, Gansu Province, as the soil sample [29,55,56] and used sodium silicate aqueous solutions with different modulus and Bé values for laboratory disintegration experiments. The modulus and Bé are used to characterize the content of Na2O·SiO2, the active ingredient in sodium silicate aqueous solution. The lower the modulus, the higher the Bé, the more its content. Through the observation of the disintegration rate, it was found that the lower the modulus and the higher the Bé of the sodium silicate aqueous solution, the lower the disintegration rate of the loess at the same moment, and the weaker the disintegration.
In addition, in the study of loess disintegration, Li et al. [40] concluded that the variation in loess disintegration is related to the type and concentration of exchange cations in the aqueous solution. When the exchange cations in the aqueous solution are monovalent Na+ and K+, the loess disintegrates more fully, and the disintegration is independent of the natural water content of the loess. If the exchange cations in the aqueous solution are divalent Mg2+ and Ca2+, the disintegration characteristics are related to the original water content, and dry loess disintegrates quickly while wet loess is relatively stable. When the exchange cations in water are trivalent Fe3+ and Al3+, the soil does not easily disintegrate. A low concentration of aqueous solution is beneficial to soil disintegration, while a high concentration makes disintegration more difficult.
3.5. Freeze–Thaw Cycle and Dry–Wet Cycle
Most of the loess area is located in arid and semi-arid regions, with significant freeze–thaw cycles due to temperatures fluctuating around 0 °C. Freeze–thaw erosion, a common type of soil erosion, occurs frequently in the Loess Plateau region [57,58,59]. The microstructure and mechanical strength of loess will be affected by freeze–thaw action [57,60,61,62], and the disintegration of loess is closely related to the microstructure and mechanical strength of soil. Therefore, the freeze–thaw cycle is one of the important water environment factors affecting the disintegration of loess.
Similarly, the dry–wet cycle is also a typical physical geological action and phenomenon in the loess area. The arid and rainless climate causes a large area of loess to be in an unsaturated state. However, due to the periodicity and concentration period of rainfall, the surface loess and even the deep loess undergo a saturated–unsaturated dry–wet cycle during rainfall–evaporation [63,64]. According to a large number of research data [65,66], dry–wet cycles can deteriorate the mechanical properties of loess and change the microstructure of loess, resulting in a decrease in loess strength, destruction, and deformation, which impacts the disintegration of loess.
According to a review of relevant articles, the experimental research on the effect of freeze–thaw cycles and dry–wet cycles on loess disintegration is summarized in Table 4.

Table 4.
Experimental research on the effect of freeze–thaw cycles and dry–wet cycles on loess disintegration.
To research the effect of freeze–thaw cycles on the disintegration of loess, Zhang used aeolian sandy soil, loessial soil, and lou soil from the Loess Plateau for laboratory disintegration experiments under different freeze–thaw cycle times [67]. It was found that the average disintegration velocity of the three soils increased significantly with the increase in the number of freeze–thaw cycles. Similarly, Li et al. used original loess and the loess treated with roots before and after freezing and thawing for laboratory disintegration experiments [68]. The results also showed that the average disintegration velocity of the two types of loess increased significantly after freezing and thawing. These results showed that freeze–thaw cycles promote the disintegration of the loess.
Considering the effect of dry–wet cycles on the disintegration of loess, the experiment results of Wang et al. and Yang et al. were slightly different [31,69]. Wang et al. [31] used the surface loess of farmland in Yangling, Shaanxi Province, as the soil sample and conducted laboratory disintegration experiments under one to four dry–wet cycles. The results showed that the disintegration amount and average disintegration velocity of loess at the same moment decrease monotonously with the increase in the number of dry–wet cycles, indicating that dry–wet cycles inhibit loess disintegration. On the contrary, Yang et al. [69] used the loess collected in the Fengqi Tableland in Xi’an, Shaanxi Province, for a disintegration experiment under 0 to 10 dry–wet cycles in the laboratory. The results showed that the change in the average disintegration velocity of loess samples was non-monotonic with the increase in the number of dry–wet cycles. When the number of dry–wet cycles is not more than four, the average disintegration velocity increases first and then decreases, with the overall trend being decreasing. When the number of dry–wet cycles is greater than four, the average disintegration velocity begins to increase gradually. When the number of dry–wet cycles reaches eight, the overall average disintegration velocity increases compared with no cycles. On the whole, dry–wet cycles promote the disintegration of loess. The reason why the results of the two tests are slightly different may be mainly related to the physical properties of the soil samples used and the method and number of dry–wet cycles. The physical properties of soil samples are different, and the microstructure and mechanical properties of soil are also different, resulting in the difference in disintegration results. The initial conditions and process of soil disintegration are different due to the different methods and numbers of dry–wet cycles, which leads to different disintegration results [70].
Additionally, Yan et al. [30] used the remolded loess in Yan’an, Shaanxi Province, for laboratory disintegration experiments under dry–wet and freeze–thaw conditions. Their results showed that the average disintegration velocity of loess increased nonlinearly with the increase in dry–wet and freeze–thaw times and gradually stabilized, indicating that dry–wet cycles and freeze–thaw cycles promote the disintegration of loess.
4. Mechanism of Loess Disintegration Induced by Water Environmental Factors
4.1. Loess Disintegration at Different Temperatures of Aqueous Solution
The influence of temperature on the disintegration of loess in an aqueous environment is significant because temperature can affect many other environmental factors such as chemical reactions occurring between the loess and aqueous solution, which are important for loess disintegration [71,72]. There are few experimental studies on the disintegration of loess under varying temperatures, and the experimental theory under different temperatures requires further verification. Therefore, the influence of this factor on the disintegration of loess is controversial. The main reasons may be as follows:
First, as the temperature of the aqueous solution increases, molecular thermal motion intensifies. This transforms the water tightly bound around soil grains into loosely bound and free water. As a result, the hydration film and diffusion layer around the soil grains thicken, reducing the binding force between the grains and making disintegration more likely [73,74]. In addition, loess is packed with hydrophilic and expansive clay minerals. When the temperature of the aqueous solution rises, it expedites the dissolution and expansion of these clay minerals within the loess [75].
Second, a higher temperature in the aqueous solution accelerates the release of gas from the soil’s pores. This creates favorable conditions for the dispersion and disintegration of soil grains, leading to further soil softening [76].
Third, a rise in the temperature of the aqueous solution also prompts the rapid dissolution of soluble and semi-soluble salts within the loess [71,72]. This enhances the water–soil chemical reaction, thereby accelerating the disintegration rate of the loess and increasing the overall amount of disintegration.
4.2. Loess Disintegration under Different Hydrodynamic Conditions
Hydrodynamic conditions affect the disintegration of loess. The reasons for this are detailed as follows:
Firstly, from a macroscopic perspective, loess under dynamic water conditions is subject not only to water soaking and infiltration but also to scouring. This leads to a rapid dispersion, disintegration, and dissociation of soil grains [77]. It also enhances the scouring strength of the soil, thereby intensifying disintegration.
Secondly, considering hydrodynamics, dynamic water exerts a drag force (permeability) on the soil grain skeleton. Compared to static water, this results in more pronounced changes in soil stress and deformation, encourages soil grain migration, and causes more severe damage to the soil structure [78], all of which enhance disintegration.
Thirdly, under the influence of dynamic water, water rapidly infiltrates the soil via pore channels, and the gas pressure within the soil cannot be released in time. The outward gas extrusion effect is stronger than that in static water, and the bubbles formed exert greater pressure on the soil sample exterior [79]. Due to the effect of water surface tension, the dispersion and disintegration of soil grains with higher water content in the outer layer of the soil sample are more intense, thereby enhancing disintegration.
Lastly, the rapid infiltration of water accelerates the dissolution of clay minerals and soluble salts in the soil, causing a rapid decrease in cementing substance contents, which results in more intense disintegration.
4.3. Water–Soil Chemical Interactions and Microstructural Changes of Soil during Loess Disintegration under Different pH Values of Aqueous Solution
The variation in disintegration parameters of loess under different pH values of an aqueous solution is intimately related to the water–soil chemical interactions that occur during loess disintegration and the resulting microstructural changes. Understanding the water–soil chemical interactions and microstructural changes can shed light on the influence of acids and alkalis on loess disintegration.
4.3.1. Water–Soil Chemical Interactions
From the perspective of water–soil chemical interactions, loess is rich in soluble salts and clay minerals, which are crucial components of soil cementing substances. Their content directly influences the disintegration, solubility, swelling, adhesion, permeability, and stability of loess [80,81]. The primary water–soil chemical interactions during loess disintegration under acidic conditions are dissolution [18,27,28,35,82] and cation exchange and adsorption [83].
Free oxides (such as Al2O3, CaO, Fe2O3, MgO) and acid-soluble minerals (like calcite, dolomite, illite, kaolinite, montmorillonite) in loess can easily undergo chemical reactions with acids in an acidic environment. These reactions destroy the original soil structure and alter its mechanical properties. Loess grains carry a negative charge on their surface, which can adsorb cations. Under certain conditions, loess grains can adsorb cations from aqueous solutions, displacing some cations originally adsorbed and diffusing them into the solution [84]. The cation exchange and adsorption during loess disintegration under acidic conditions are mainly reflected in the replacement of K+ by high-valence cations due to the increased content of high-valent cations such as Al3+, Fe3+, Ca2+, and Mg2+ in the loess–water solution system under acid dissolution.
Conversely, in loess disintegration experiments under alkaline conditions, the alkali solutions used are primarily NaOH and Ca(OH)2. The main soil–water chemical interactions that occur are precipitation or crystallization, carbonation, pozzolanic reactions [35], dedolomitization [85,86], and the reaction of amphoteric oxides with alkali solution [87].
Precipitation or crystallization mainly refers to the chemical reaction between the easily soluble salts containing Ca2+ and Mg2+ in loess and OH− in the aqueous solution to produce colloids such as Ca(OH)2 and Mg(OH)2 [35]. When the alkali solution is Ca(OH)2 or when the colloids such as Ca(OH)2 and Mg(OH)2 produced by the above precipitation or crystallization react with CO2 at the interface or in the air, which results in carbonation, they produce insoluble crystalline minerals such as CaCO3 and MgCO3, which bind the soil grains more closely.
When the alkali solution is Ca(OH)2, Al2O3 and SiO2 in the loess react with Ca(OH)2 to produce gels such as calcium aluminate hydrate (C-A-H) and calcium silicate hydrate (C-S-H), which promote the bonding of soil grains and enhance the strength and stability of the soil [35]. Dedolomitization primarily refers to the chemical reaction between the dolomite-type carbonate minerals in the loess and the OH− in the alkali solution to produce calcite, magnesium hydroxide, and CO32− [86].
When the alkali solution is NaOH, free oxides such as Al2O3 and Fe2O3 in loess can react with NaOH to produce alkaline soluble salts such as NaAlO2 and NaFeO2. Furthermore, NaOH can react with SiO2 to produce Na2SiO3. As the OH− concentration increases, these soluble salts gradually transform into soft-plastic gel materials [87].
4.3.2. Microstructural Changes of Soil Induced by Water–Soil Chemical Interactions
The disintegration of loess is influenced by water–soil chemical interactions. These interactions, particularly under acidic and alkaline conditions, can induce microstructural changes in the loess. However, these microstructural changes vary under different conditions.
Under acidic conditions, the loess cementing substances, which include expansive clay minerals with strong hydrophilicity, ultrafine carbonates, easily soluble salts, and amorphous free oxides, are rapidly dissolved by the acid. This dissolution reduces the content of cementing substances, weakens the cementation between soil grains, and reduces the cementation force. Concurrently, these cementing substances react with the acid, forming holes that gradually penetrate the internal pores of the soil. This process changes the soil from a dense structure to a loose one, providing channels for the aqueous solution to enter the soil [88,89]. Additionally, the mutual contact area between soil grains decreases, and the connection strength between grains is reduced, leading to decreased soil stability and enhanced disintegration.
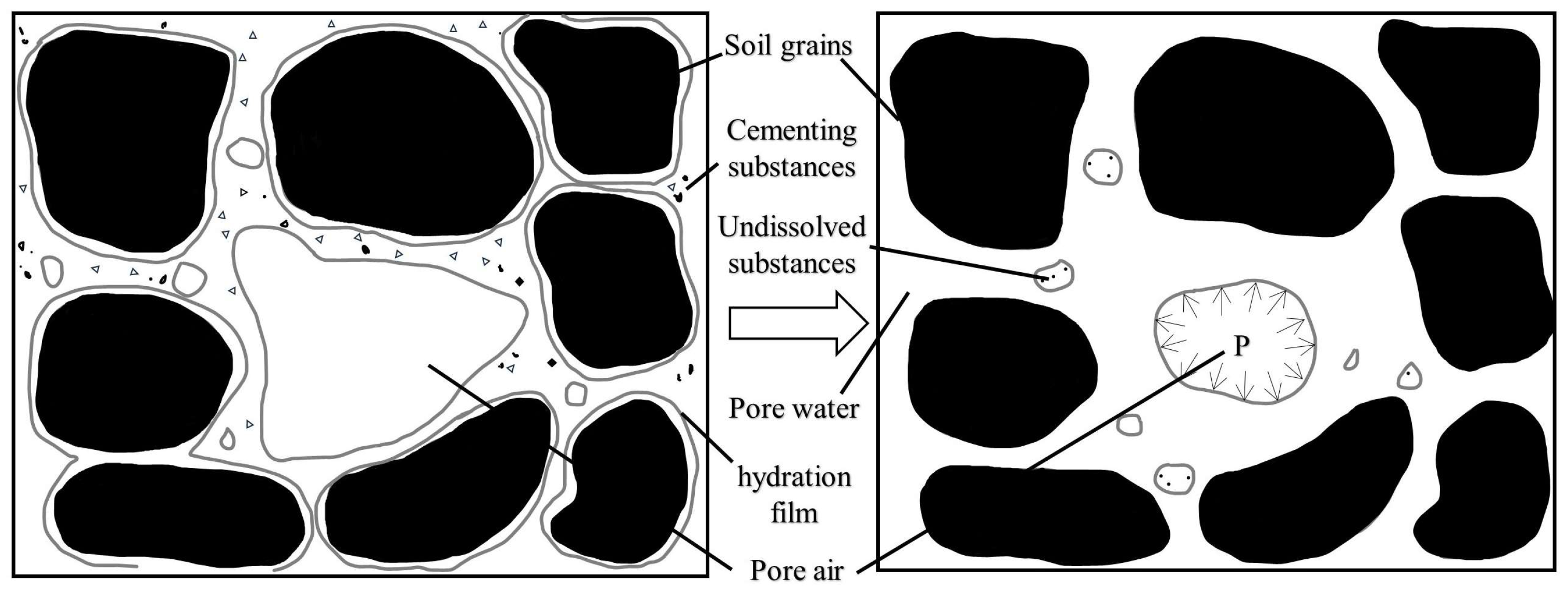
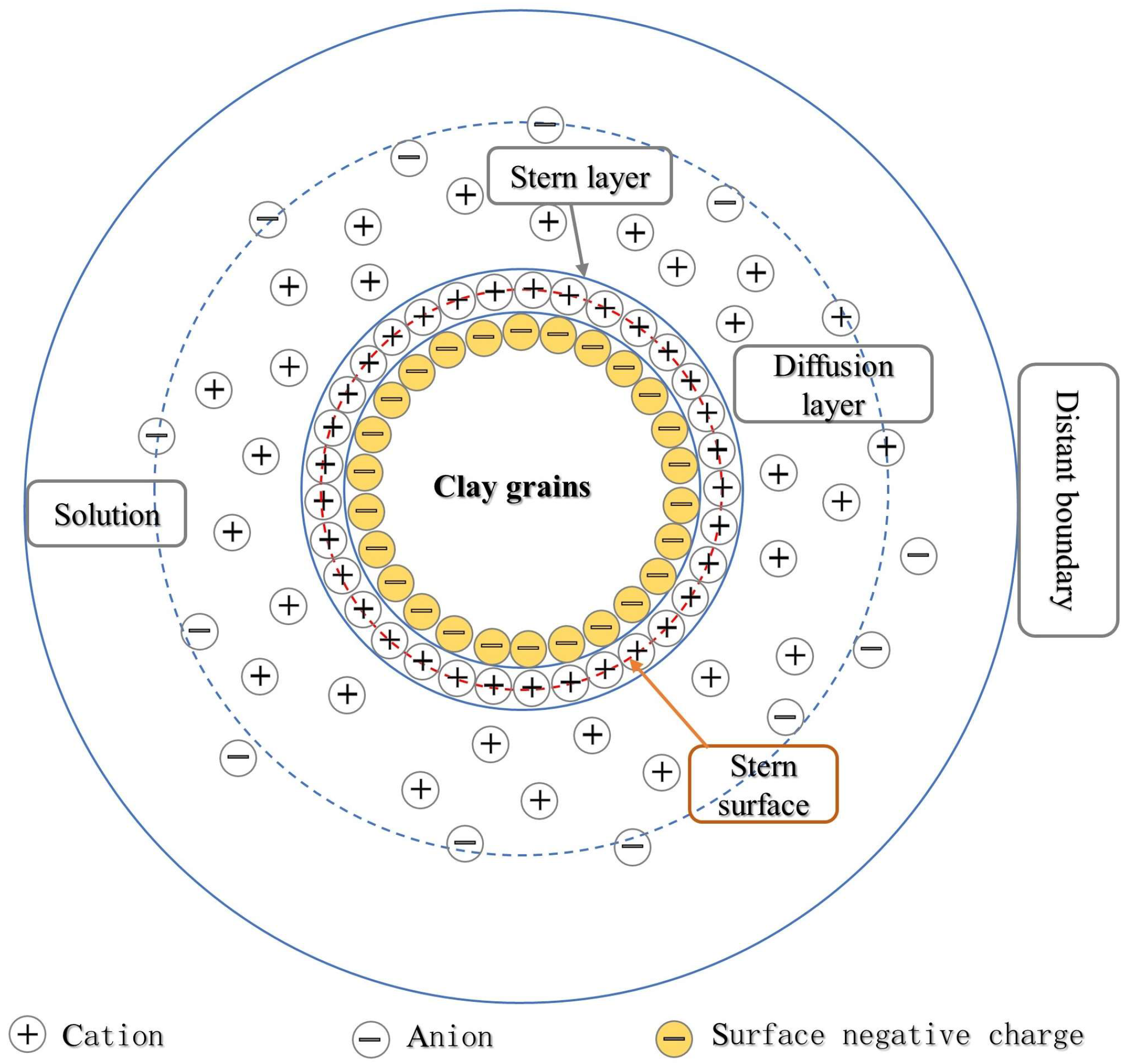
Theoretically, with the increase in acidity, the disintegration of loess is also enhanced. However, there is an optimum range of pH in acidity that promotes the disintegration of loess. Under the dissolution of acid, the content of high valence cations such as Ca2+, Mg2+, Al3+, and Fe3+ in the pore water around the loess grains gradually increases. Under the effect of cation exchange and adsorption, the surface of loess grains will mainly adsorb these high-valent cations and replace the original adsorbed low-valence cations such as Na+ and K+. This exchange affects loess stability by affecting the thickness of the diffuse double layer (Figure 5) [90].
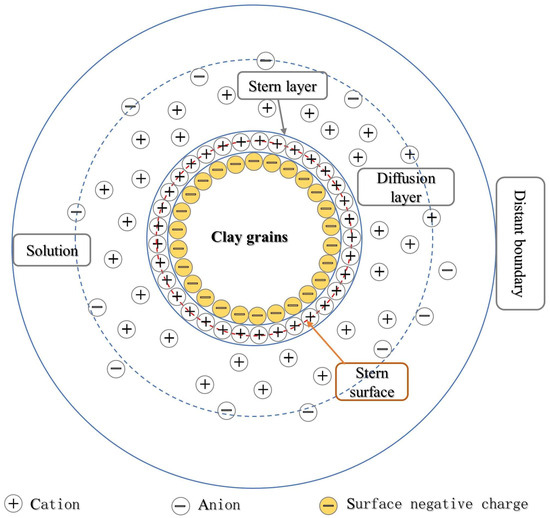
Figure 5.
Diffuse double-layer structure model [91].
When the pH value is relatively low, indicating a weak acidic environment, the content of high valence cations such as Ca2+, Mg2+, Al3+, and Fe3+ generated by the reaction with H+ is relatively less, and the cation exchange effect is weak. The surface of loess grains mainly adsorbs low-valence cations such as Na+ and K+. At this time, the amount of charge neutralized by cations in the adsorption layer is little, the electric potential is high, the diffusion layer and the hydration film are thick, and the loess grains tend to disperse [92], promoting the dissolution of minerals and enhancing disintegration.
When the acidity is further enhanced, the content of H+ in the pore water around the loess grains increases, and then the content of high-valence cations such as Ca2+, Mg2+, Al3+, and Fe3+ generated by the reaction with H+ increases, and the cation exchange effect is strengthened. The surface of loess grains begins to adsorb a large number of these high-valence cations and replace low-valence cations such as Na+ and K+. Due to the large charge of a high-valence cation, when the total charge on the surface of the soil grains is constant, the amount of charge neutralized by the cation in the adsorption layer is large, so the electric potential becomes lower, and the diffusion layer and the hydration film on the surface of the soil grains become thinner [93,94], which results in the increase in the bonding strength between the soil grains. At the same time, the increase in H+ concentration will cause the compression of the diffuse double layer and further thinning of the diffusion layer and the hydration film [95]. Their combined effect slows down the decrease in cement bond strength, which is manifested by the gradual weakening of the influence of acid concentration on the quantitative indices of disintegration, and the values of the quantitative indices of disintegration tend to be stable.
Under alkaline conditions, free oxides such as SiO2, Al2O3, and Fe2O3, which are most abundant in loess, react with the alkaline solution to produce alkaline soluble salts such as sodium aluminate and sodium silicate. This reaction reduces the cementing substances content, develops pores, and weakens the cementation force. However, with the increase in the concentration of the alkaline solution, the high concentration of OH− will inhibit the dissolution of alkaline soluble salts such as Na2CO3 and NaHCO3 in loess and other alkaline soluble salts produced by the reaction of free oxides with the alkaline solution. Moreover, when the alkali solution is Ca(OH)2 (commonly known as lime water), precipitation or crystallization, carbonization, and volcanic ash will occur during the disintegration, generating insoluble crystalline substances or gels. These substances fill in the pores to form new cementing substances or wrap up the soil grains to play a bonding and reinforcement role, resulting in enhanced cementation between soil grains, increased bonding strength, enhanced stability, and weakened disintegration.
4.4. Loess Disintegration under Different Salt Solutions
The disintegration of loess under varying salt solution conditions can be attributed to water–soil chemical reactions, which are primarily influenced by the acidity and alkalinity of the salt solution [96].
When the salt is a strong acid and weak base (e.g., CuSO4, FeCl3, AlCl3), its aqueous solution is acidic. This leads to dissolution, cation exchange, and adsorption with free oxides (e.g., Al2O3, CaO, Fe2O3, MgO) and acid-soluble minerals (e.g., calcite, dolomite) present in the soil.
Conversely, when the salt is a strong alkali and weak acid (e.g., Na2CO3, NaHCO3, Ca(HCO3)2), its aqueous solution is alkaline. The OH− ions produced by the ionization reaction will undergo precipitation or crystallization with easily soluble salts containing Ca2+ and Mg2+ in the loess. They also react with free oxides such as SiO2 and Al2O3 to produce gel materials like meta-aluminate and silicate. This scenario also includes the dedolomitization effect.
In the case of neutral salts (e.g., NaCl, Na2SO4, CaCl2), there is minimal chemical reaction with the soil, with only the dissolution of soluble salts in water and the swelling and softening of clay minerals occurring.
The salt concentration of the aqueous solution and the type of exchange cations predominantly inhibit the disintegration of loess. The increase in the concentration of counter-ions involved in ion exchange in the solution compresses the diffuse double layer, resulting in the thinning of the diffusion layer and the hydration film. This strengthens the bonding strength between soil particles, enhances soil stability, and weakens disintegration [91,97]. Furthermore, a higher valence of exchange cations in the salt solution leads to a lower electric potential of the diffuse double layer and a thinner diffusion layer and hydration film, resulting in weaker loess disintegration [98].
4.5. Microstructural Analysis of Loess Disintegration under Freeze–Thaw Cycle and Dry–Wet Cycle
The impact of freeze–thaw cycles on soil microstructure is contingent upon four primary variables: the number of freeze–thaw cycles, the soil’s water content prior to freezing–thawing, the duration of freezing–thawing, and the temperature of freezing–thawing [99,100].
An analysis of existing tests on the influence of freeze–thaw cycles on loess disintegration, along with relevant literature, reveals that freeze–thaw cycles primarily promote loess disintegration by increasing soil porosity and weakening soil structure stability. Under freeze–thaw conditions, water within the loess undergoes repeated freezing and thawing cycles due to temperature changes. During freezing, pore water between soil grains solidifies into ice, generating various forms of ice intrusion, which induces relative movement between soil grains and increases soil volume, resulting in a freezing and swelling phenomenon [101]. As the temperature rises, the ice in the pores begins to melt, forming larger pores. The soil transitions from a denser structure to a looser one, the bonding strength between grains decreases, the soil structure becomes less stable, and disintegration is more pronounced when exposed to water. With an increase in the number of freeze–thaw cycles, the cumulative effects of the frost heave–thaw phenomenon occur, leading to further development of soil pore space, continuously weakening the stability of the soil structure [102,103], and enhancing disintegration.
Existing experiments and relevant literature on the influence of dry–wet cycles on loess disintegration reveal that dry–wet cycles affect the structural stability of the soil by altering grain size distribution, pore structure characteristics, grain arrangement, bonding mode, and the state of soluble salts in the soil, in turn affecting its disintegration [104,105].
When the number of dry–wet cycles is small, changes in grain size distribution, pore structure characteristics, grain arrangement, and bonding mode are not significant. However, soluble salts in the soil may continue to undergo dissolution–crystallization changes under repeated dry–wet action. With water migration, these salts are continuously aggregated and crystallized on the upper surface of the soil sample, filling the gaps between the grains [106]. This makes the soil sample denser and enhances its resistance to disintegration in the short term.
As the number of dry–wet cycles increases, the content of large-sized soil grains gradually increases, and the content of small-sized grains gradually decreases due to adherence to large-sized grains under the effect of dry–wet action. This results in the arrangement of soil grains and the contact between grains gradually changing from the initial solid arrangement and face contact to an overhead arrangement and point contact. Consequently, the distance between grains becomes larger, the bonding force decreases continuously, and the structure becomes loose and less stable.
In addition, as the number of dry–wet cycles increases, the micro and small pores in the soil gradually connect into medium and large pores, and the pore structure continues to expand. The interconnectivity between pores is enhanced under repeated dry–wet action. Meanwhile, cementing substances such as clay minerals and soluble salts in the loess repeatedly dissolve and migrate under dry–wet cycles, so the cementation force between grains is continuously weakened [107]. These multiple effects lead to a continuous decrease in the stability of soil structure, resulting in enhanced disintegration.
5. Challenges and Future Prospects
As one of the important processes and phenomena of loess, disintegration is one of the causes of many geological disasters such as loess cave erosion, landslides, and debris flows. Its research has experienced rapid development over the past 25 years, as evidenced by the increasing number of scholars focusing on loess disintegrations. In particular, in the past 15 years, the number of publications in national and international journals has grown significantly, suggesting that the study of loess disintegration is becoming increasingly popular.
However, despite the growing number of studies, the overall research remains relatively inadequate, and this is particularly so for experimental research on loess disintegration induced by water environmental factors. Based on a literature review, we believe that the following research priorities require further attention:
Temperature of the aqueous solution: As indicated in the previous review in this paper, the current research lacks quantitative studies correlating water temperature with disintegration parameters. Existing studies mostly analyze phenomena through experiments, without establishing whether there is a linear or nonlinear relationship between water temperature and loess disintegration indices [21,22,23,24,25]. Establishing a linear or nonlinear relationship between water temperature and loess disintegration can provide parameters with higher reliability for models. The relationship established can also be beneficial for quantitatively understanding the optimal temperature range, facilitating many engineering projects in loess areas. Therefore, the optimal range or value of disintegration water temperature for loess disintegration should be determined using batch experiments, and this research topic should be promoted.
Hydrodynamic conditions: Current research only explores the impact of hydrodynamic action on loess disintegration by comparing results under static water conditions and dynamic water conditions of certain flow velocities or rotational speeds [26,40]. However, there is a lack of research on the influence of other hydrodynamic conditions, such as pressure, water flow rate, and vibration, on loess disintegration. Therefore, the mechanism of how hydrodynamic conditions influence loess disintegration also needs systematic analysis. In particular, natural environmental changes and human activities can alter hydrodynamic conditions significantly, thus exerting unpredictable impacts on the loess disintegration. For example, changing rainfall patterns and seasonal irrigation plans can rapidly change hydrodynamics, and rapid changes in hydrodynamics will further cause changes in loess disintegration. Therefore, when conducting experimental research on loess disintegration under varying hydrodynamic conditions, particular attention should be paid to climate change and human activities in different climate zones and regions in which human activities may be diverse.
The pH of the aqueous solution: While the overall effect of acidic conditions on promoting loess disintegration is greater than the inhibitory effect, and the inhibitory effect of alkaline conditions is greater than the promotion effect, the variation in disintegration parameters under acid and alkali conditions is nonlinear and non-monotonic with pH changes [18,27,28,35]. However, the optimal pH range for promoting loess disintegration is yet to be comprehensively experimentally verified. Similar to water temperature, pH is also critical in affecting the chemical reactions occurring in loess. The determination of the optimal pH range can benefit not only the understanding of the mechanisms of loess disintegration, but also the practical engineering projects in loess areas, contributing significantly to the security insurance of humans and properties in loess areas.
Based on the preceding discussion regarding research on loess disintegration experiments under varying water environmental factors, the following suggestions are also proposed for enhancing the experiments:
Diversify the types of loess used in the experiments: Currently, the disintegration experiments primarily focus on Holocene loess and Malan loess samples, while experiments on Wucheng loess and Lishi loess are quite limited [108]. By conducting disintegration experiments on various types of loess under different water environments, researchers can observe how the distinct properties of loess can impact the experimental outcomes. In addition, disintegration parameters for different types of loess can be obtained, which is vital for engineering construction in loess areas.
Enhance the disintegration experiment apparatus: The majority of the simple disintegration devices in current use struggle to accommodate research on diverse external water environmental factors [21,22,23,24,25,26,27,28,29,30,31]. To address this limitation, improvements to the disintegration device are imperative. Examples include the following:
For controlling the temperature of the aqueous solution, a temperature control system (such as a temperature regulator) can be incorporated to manage the solution’s temperature during disintegration, thereby facilitating experimentation under varied temperature conditions.
To regulate hydrodynamic conditions, dynamic control systems (e.g., vibration controllers, flow regulators) can be integrated to control water flow rates and fluctuations, enabling the simulation of different hydrodynamic scenarios.
For monitoring the pH level and salt concentration in the aqueous solution, a water chemical sensing system (e.g., pH detector, TDS salinity sensor) can be added to accurately track changes in the aqueous environment during disintegration experiments.
6. Conclusions
Despite the relative scarcity of studies on loess disintegration, there has been an increase in scholarly interest in this topic in recent years, as indicated by the increasing number of publications on this topic. The trend suggests that research on the characteristics of loess disintegration will continue to gain popularity.
The methods employed in loess disintegration experiments can be broadly divided into the float method (volume method) and the mass method. The parameters characterizing loess disintegration include disintegration amount, disintegration time, disintegration rate, disintegration velocity, disintegration resistance coefficient, accumulative disintegration modulus, and accumulative percentage of disintegration mass. Currently, disintegration experiments are primarily based on the mass method, with a focus on laboratory remolded soil. There is a lack of experimental research on the disintegration of undisturbed loess in the field.
The water environmental factors that influence loess disintegration include the temperature of the aqueous solution, hydrodynamic conditions, pH of the aqueous solution, salt concentration and salt type in the aqueous solution, and freeze–thaw and dry–wet cycles.
There is a lack of experimental research on loess disintegration under different water environmental factors, and the experimental methods need further and more systematic improvements. The research results lack comparability and representativeness. There is also a lack of a more comprehensive and systematic analysis of the influence patterns and response mechanisms from both microscopic and macroscopic perspectives. Therefore, promotions in loess disintegration research should be enhanced. In addition, the types of loess used in the experiments should be diverse, covering all types of loess. The experimental apparatus for loess disintegration should also be improved by adding a parameter control system and an in situ parameter monitoring system.
Author Contributions
Conceptualization, Y.C. and P.L.; methodology, Y.C.; investigation, Y.C.; writing—original draft preparation, Y.C. and P.L.; writing—review and editing, Y.W. and J.L.; visualization, Y.C.; supervision, P.L.; project administration, P.L.; funding acquisition, P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (42072286 and 41761144059), the National Key Research and Development Program of China (2023YFC3706901), and the Qinchuangyuan “Scientist + Engineer” Team Development Program of the Shaanxi Provincial Department of Science and Technology (2022KXJ-005).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
During the peer review processes, five anonymous reviewers provided us with lots of useful and constructive comments, which helped us improve the quality of our review. We appreciate that so much. We are also extremely grateful for the suggestion rendered by Wanfang Zhou from ZeoEnvironmental, USA, during his visit to our university. His encouragement of writing a monograph, Loess Hydrogeology, which will be published soon by Springer, is highly appreciated. The various funding agencies listed in the Funding section are also acknowledged for financially supporting us in conducting research in the Chinese Loess Plateau.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shen, Y.; Yan, H.; Wang, R.; Song, P.; He, Y. Review: Progress with Functional Materials Based on Loess Particles. Clays Clay Miner. 2021, 69, 301–314. [Google Scholar] [CrossRef]
- Sun, J. Provenance of loess material and formation of loess deposits on the Chinese Loess Plateau. Earth Planet. Sci. Lett. 2002, 203, 845–859. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, M.; Jia, X.; Zhang, C. Particle size distribution of soils (0–500 cm) in the Loess Plateau, China. Geoderma Reg. 2016, 7, 251–258. [Google Scholar] [CrossRef]
- D’Amico, M.E.; Casati, E.; Andreucci, S.; Martini, M.; Panzeri, L.; Sechi, D.; Abu EI Khair, D.; Previtali, F. New dates of a Northern Italian loess deposit (Monte Orfano, Southern pre-Alps, Brescia). J. Soils Sediments 2021, 21, 832–841. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.; Fitzsimmons, K.E.; Chen, X.; Prud’homme, C.; Zong, X. Origin of loess deposits in the North Tian Shan piedmont, Central Asia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 559, 109972. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, H.; He, J.; Xie, W.; Wang, H.; Zhang, H.; Breecker, D.; Bird, A.; Stevens, T.; Nie, J.; et al. Large-number detrital zircon U-Pb ages reveal global cooling caused the formation of the Chinese Loess Plateau during Late Miocene. Sci. Adv. 2022, 8, eabq2007. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Peng, J.; Wang, S.; Lu, F. Development of water sensitivity index of loess from its mechanical properties. Eng. Geol. 2021, 280, 105918. [Google Scholar] [CrossRef]
- Li, P.; Qian, H. Water in Loess. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2018; pp. 1–17. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Zhang, D.; Gu, T. Vibration-induced acceleration of infiltration in loess. Sci. China-Earth Sci. 2021, 64, 611–630. [Google Scholar] [CrossRef]
- Gao, C.; Du, G.; Zhuang, Z.; Zeng, B.; Chen, X.; Cheng, F. Disintegration characteristics of collapsible loess after vibration compaction. Proc. Inst. Civ. Eng.-Geotech. Eng. 2023, 176, 86–98. [Google Scholar] [CrossRef]
- Li, P.; Kou, X.; Wang, Y.; Niu, L. Building a More Sustainable Chinese Loess Plateau. J. Earth Sci. 2024, 35, 283–287. [Google Scholar] [CrossRef]
- Cao, Z.; Sun, Q.; Li, Z.; Du, F. Abnormal ore pressure mechanism of working face under the influence of overlying concentrated coal pillar. Sci. Rep. 2024, 14, 626. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Wang, Q.; Liu, Q.; Yang, D.; Xing, M.; Pei, Y.; Yan, S. Landslide susceptibility assessment using frequency ratio, statistical index and certainty factor models for the Gangu County, China. Arab. J. Geosci. 2016, 9, 84. [Google Scholar] [CrossRef]
- Zhuang, J.; Peng, J.; Wang, G.; Javed, I.; Wang, Y.; Li, W. Distribution and characteristics of landslide in Loess Plateau: A case study in Shaanxi province. Eng. Geol. 2018, 236, 89–96. [Google Scholar] [CrossRef]
- Wu, J.; Yang, N.; Li, P.; Yang, C. Influence of moisture content and dry density on the compressibility of disturbed loess: A case study in Yan’an City, China. Sustainability 2023, 15, 6212. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, N.; Li, Y.; He, S.; Guo, D. Water-induced disintegration behaviour of Malan loess. Earth Surf. Process. Landf. 2022, 47, 1891–1901. [Google Scholar] [CrossRef]
- Piccarreta, M.; Faulkner, H.; Bentivenga, M.; Capolongo, D. The influence of physico-chemical material properties on erosion processes in the badlands of Basilicata, Southern Italy. Geomorphology 2006, 81, 235–251. [Google Scholar] [CrossRef]
- Liu, H.; Gu, H.; Hu, P.; Hu, W.; Niu, Z. Experimental study on disintegration characteristics of compacted loess induced by acid pollution of pore water. J. Xi’an Univ. Archit. Technol. (Nat. Sci. Ed.) 2021, 53, 178–185. (In Chinese) [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Yan, Y.; Hong, B.; Li, L. Experimental study on the disintegration of loess in the Loess Plateau of China. Bull. Eng. Geol. Environ. 2019, 78, 4907–4918. [Google Scholar] [CrossRef]
- Yuan, L.; Gu, T.; Hu, W.; Zhu, L.; Wang, X. Experimental Study on Disintegration of Loess in Different Regions. Sci. Technol. Eng. 2017, 17, 93–100. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.; Gu, T.; Zhang, M.; Xu, Y.; Kong, J. Experimental study of loess disintegration characteristics. Earth Surf. Process. Landf. 2019, 44, 1317–1329. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, H.; Yang, X.; Gao, C.; Liu, J. Experimental Study on the Effect of Thermal Treatment on the Water Stability of Compacted Loess. Soil Mech. Found. Eng. 2022, 59, 15–22. [Google Scholar] [CrossRef]
- Gu, T.; Yuan, L.; Hu, W.; Zhu, L.; Wang, X. Experimental research on disintegration of the Heifangtai loess. Hydrogeol. Eng. Geol. 2017, 44, 62–70. (In Chinese) [Google Scholar] [CrossRef]
- Liu, M. Study on the Disintegration and Collapsibility of Loess of Gully Land Consolidation under Eluviation in Yan’an Area. Master’s Thesis, Chang’an University, Xi’an, China, 2021. (In Chinese). [Google Scholar] [CrossRef]
- Yuan, L. The Experimental Study on Disintegration of Loess. Master’s Thesis, Northwest University, Xi’an, China, 2017. (In Chinese). [Google Scholar]
- Fan, N.; Li, Y. Preliminary Research on the Disintegration of Malan Loess with Roots in Different Water Environments. China Rural Water Hydropower 2021, 461, 6–12. (In Chinese) [Google Scholar] [CrossRef]
- Gao, C.; Du, G.; Wu, Y.; Liu, S.; Zhang, D.; Yang, Y. Experimental Investigation of Remolded Loess Mixed with Different Concentrations of Hydrochloric Acid. J. Mater. Civ. Eng. 2022, 34, 04021391. [Google Scholar] [CrossRef]
- Lin, H.; Wang, J.; Xu, Y. Synergistic effects and mechanism of locomotive vibration and chemical dissolution on loess disintegration. J. Soils Sediments 2022, 22, 3106–3118. [Google Scholar] [CrossRef]
- Gu, L.; Lv, Q.; Wang, S.; Xiang, J.; Guo, L.; Jiang, J. Effect of sodium silicate on the properties of loess stabilized with alkali-activated fly ash-based. Constr. Build. Mater. 2021, 280, 122515. [Google Scholar] [CrossRef]
- Yan, C.; An, N.; Wang, Y.; Sun, W. Effect of Dry-Wet Cycles and Freeze-Thaw Cycles on the Antierosion Ability of Fiber-Reinforced Loess. Adv. Mater. Sci. Eng. 2021, 2021, 8834598. [Google Scholar] [CrossRef]
- Wang, J.; Ma, F.; Zhang, P.; Meng, Q.; Zhang, Q.; Zhou, M. Effect of wet-dry alternation on loess disintegration rate. Acta Pedol. Sin. 2015, 52, 1273–1279. (In Chinese) [Google Scholar] [CrossRef]
- He, X.; Li, P. Surface Water Pollution in the Middle Chinese Loess Plateau with Special Focus on Hexavalent Chromium (Cr6+): Occurrence, Sources and Health Risks. Expo. Health 2020, 12, 385–401. [Google Scholar] [CrossRef]
- Hu, W.; Cheng, W.; Wen, S.; Rahman, M.M. Effects of chemical contamination on microscale structural characteristics of intact loess and resultant macroscale mechanical properties. Catena 2021, 203, 105361. [Google Scholar] [CrossRef]
- Jiang, D.; Li, X.; Fan, X.; Zhang, H. Research on the Law of Soil Disintegration Rate Change and its Effect Factors on the Loess Plateau. Bull. Soil Water Conserv. 1995, 1995, 20–27. (In Chinese) [Google Scholar]
- Gu, H. Study on Disintegration Characteristics and Mechanical Parameters Evolution of Modified Contaminated Loess. Master’s Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2021. (In Chinese). [Google Scholar] [CrossRef]
- Zhu, L.; Pei, X.; Zhang, X.; Ren, T.; Yang, Q. A study of water retention and ecological effects of loess improved by double polymers. Hydrogeol. Eng. Geol. 2020, 47, 158–166. (In Chinese) [Google Scholar] [CrossRef]
- Li, J.; Cui, S.; Tian, W. Erosion characteristic of road slope and test of soil disintegration. J. Chang. Univ. (Nat. Sci. Ed.) 2007, 117, 23–26. (In Chinese) [Google Scholar] [CrossRef]
- Ma, X.; Zhu, S. Soil Disintegration Characteristics of Terrace Hedgerows in Loess Hilly-gully Areas. J. Northwest For. Univ. 2013, 28, 21–25. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, S.; Sun, Q.; Zhang, H.; Meng, H.; Gao, Q.; Zhou, Y. Elemental dissolution characteristics of granite and gabbro under high-temperature water-rock interactions. Sci. Total Environ. 2023, 897, 165455. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Ye, W. Engineering Geological Research on Tunnel-Erosion in Loess, 1st ed.; Tongji University Press: Shanghai, China, 2010; pp. 60–62. (In Chinese) [Google Scholar]
- Liu, H.; Zhang, S.; Niu, F.; Shao, Z.; Niu, Z.; Lu, J. Experimental study on one-dimensional compression characteristics of Q3 loess contaminated by acid or alkali solutions. Rock Soil Mech. 2019, 40, 210–216. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Chen, W.; Liu, F. Strength of Recompacted Loess Affected by Coupling between Acid-Base Pollution and Freeze-Thaw Cycles. J. Cold Reg. Eng. 2020, 34, 04020024. [Google Scholar] [CrossRef]
- Xiao, J.; Qin, Z.; Zhao, J. Status and Countermeasures of Farmland Soil Polluted by Chemical Fertilizer. Environ. Prot. Sci. 2005, 2005, 36–38. (In Chinese) [Google Scholar] [CrossRef]
- Ma, J.; Pan, F.; He, J.; Chen, L.; Fu, S.; Jia, B. Petroleum pollution and evolution of water quality in the Malian River Basin of the Longdong Loess Plateau, Northwestern China. Environ. Earth Sci. 2012, 66, 1769–1782. [Google Scholar] [CrossRef]
- Wei, Y.; Fan, W.; Wang, W.; Deng, L. Identification of nitrate pollution sources of groundwater and analysis of potential pollution paths in loess regions: A case study in Tongchuan region, China. Environ. Earth Sci. 2017, 76, 423. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Z.; Li, Y.; Dong, S.; Li, L.; Long, S.; Wu, Y.; Wang, S. The changes in the physicochemical properties of calcareous soils and the factors of arsenic (As) uptake by wheat were investigated after the cessation of effluent irrigation for nearly 20 years. Sci. Total Environ. 2023, 859, 160171. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Lei, X.; Gou, J.; Gao, D.; Wang, H.; Nan, Z. Leaching-induced migration and compositional form change of Cu, Zn, and Cd from sludge to loess. Toxicol. Environ. Chem. 2015, 97, 439–453. [Google Scholar] [CrossRef]
- Li, Z. Improving Physical and Chemical Properties of Lime-Treated Loess. Master’s Thesis, Lanzhou University, Lanzhou, China, 2017. (In Chinese). [Google Scholar]
- Yang, B. Mechanical properties and microscopic analysis of lime-improved loess. China Water Transp. 2016, 16, 320–321. (In Chinese) [Google Scholar]
- Wei, X.; Dong, L.; Chen, X.; Zhou, Y. Influence of Soluble Salt NaCl on Cracking Characteristics and Mechanism of Loess. Sustainability 2023, 15, 5268. [Google Scholar] [CrossRef]
- Zhao, X.; Hao, Q.; Sun, Y. Spatial heterogeneity of soil salinization and its influencing factors in the typical region of the Mu Us Desert-Loess Plateau transitional zone Northwest China. Chin. J. Appl. Ecol. 2017, 28, 1761–1768. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Jia, H. Analysis of Spatial Variation of Soil Salinization Using a Hydrochemical and Stable Isotopic Method in a Semiarid Irrigated Basin, Hetao Plain, Inner Mongolia, North China. Environ. Process. 2016, 3, 723–733. [Google Scholar] [CrossRef]
- Li, G.; Huang, H.; Su, C.; Hu, T.; Xie, X. Dynamic Changes and Driving Forces of Soil Salinization in Hetao lrrigation District from 1986 to 2019. Saf. Environ. Eng. 2022, 29, 162–174,183. (In Chinese) [Google Scholar] [CrossRef]
- Han, S.; Li, F.; Wang, S.; Li, H.; Yuan, L.; Liu, J.; Shen, H.; Zhang, X.; Li, C.; Wu, X.; et al. Groundwater resource and eco-environmental problem of the Yellow River Basin. Geol. China 2021, 48, 1001–1019. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, Y.; Xiang, J.; Lv, Q.; Shan, X.; Chen, Y. Effect of Alkali Activator on Engineering Properties of Geopolymer-solidified Loess. J. Beijing Univ. Technol. 2021, 47, 636–643. (In Chinese) [Google Scholar] [CrossRef]
- Xiang, J. Effects of Sodium Silicate on Engineering Properties of Alkali-Activated Geopolymer-Solidified Loess. Master’s Thesis, Lanzhou University, Lanzhou, China, 2020. (In Chinese). [Google Scholar] [CrossRef]
- Lu, J.; Sun, B.; Ren, F.; Li, H.; Jiao, X. Effect of Freeze-Thaw Cycles on Soil Detachment Capacities of Three Loamy Soils on the Loess Plateau of China. Water 2021, 13, 342. [Google Scholar] [CrossRef]
- Sun, B.; Liu, J.; Ren, F.; Li, H.; Zhang, G.; Ma, J.; Ma, B.; Li, Z. Effects of seasonal freeze-thaw and wind erosion on runoff and sediment yields of three loamy slopes of Loess Plateau, China. Catena 2022, 215, 106309. [Google Scholar] [CrossRef]
- Wang, S. Characteristics of Freeze and Thaw Weathering and lts Contribution to Sediment Yield in Middle Yellow River Basin. Bull. Soil Water Conserv. 2004, 24, 1–5. (In Chinese) [Google Scholar] [CrossRef]
- Li, G.; Ma, W.; Mu, Y.; Wang, F.; Fan, S.; Wu, Y. Effects of freeze-thaw cycle on engineering properties of loess used as road fills in seasonally frozen ground regions, North China. J. Mt. Sci. 2017, 14, 356–368. [Google Scholar] [CrossRef]
- Xiao, L.; Yao, K.; Li, P.; Liu, Y.; Zhang, Y. Effects of freeze-thaw cycles and initial soil moisture content on soil aggregate stability in natural grassland and Chinese pine forest on the Loess Plateau of China. J. Soils Sediments 2020, 20, 1222–1230. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Lan, W.; Wang, S. Shear strength and damage mechanism of saline intact loess after freeze-thaw cycling. Cold Reg. Sci. Technol. 2019, 164, 102779. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Ren, C.; Lan, W. Damage of saline intact loess after dry-wet and its interpretation based on SEM and NMR. Soils Found. 2020, 60, 911–928. [Google Scholar] [CrossRef]
- Zhang, Y.; Hassan, M.A.; King, L.; Fu, X.; Istanbulluoglu, E.; Wang, G. Morphometrics of China’s Loess Plateau: The spatial legacy of tectonics, climate, and loess deposition history. Geomorphology 2020, 354, 107043. [Google Scholar] [CrossRef]
- Lian, B.; Wang, X.; Zhan, H.; Wang, J.; Peng, J.; Gu, T.; Zhu, R. Creep mechanical and microstructural insights into the failure mechanism of loess landslides induced by dry-wet cycles in the Heifangtai platform, China. Eng. Geol. 2022, 300, 106589. [Google Scholar] [CrossRef]
- Liu, K.; Gu, T.; Wang, X.; Wang, J. Time-Dependence of the Mechanical Behavior of Loess after Dry-Wet Cycles. Appl. Sci. 2022, 12, 1212. [Google Scholar] [CrossRef]
- Zhang, Z. Experimental Study on the Effect of Freeze-Thaw on Water Erosion Processes of Three Soil Types in the Loess Plateau. Master’s Thesis, Northwest A & F University, Yangling, China, 2021. (In Chinese). [Google Scholar] [CrossRef]
- Li, Q.; Liu, G.; Xu, M.; Sun, H.; Zhang, Z.; Gao, L. Effect of seasonal freeze-thaw on soil anti-scouribility and its related physical property in hilly Loess Plateau. Trans. Chin. Soc. Agric. Eng. 2013, 29, 105–112. (In Chinese) [Google Scholar] [CrossRef]
- Yang, Q.; Li, C. Research on the lmpact of Drying and Wetting Cycle of Capillary Water on Weathering of Soil Sites. Chin. J. Undergr. Space Eng. 2012, 8, 517–525. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, Z.; Bai, Y.; Wu, Y.; Chen, Y.; Guo, Z.; Cheng, W. Multiscale study on the microstructural evolution and macromechanical deterioration of expansive soil under dry-wet cycles. J. Mech. 2022, 38, 610–620. [Google Scholar] [CrossRef]
- Wang, R.; Wu, X.; Zhai, Y.; Su, Y.; Liu, C. An Experimental Study on the Sources of Strontium in Mineral Water and General Rules of Its Dissolution—A Case Study of Chengde, Hebei. Water 2021, 13, 699. [Google Scholar] [CrossRef]
- Choi, J.; Ichikawa, Y.; Kimoto, K.; Chae, B.G. A new theoretical mineral dissolution rate equation for physicochemical factors. Geochem. J. 2015, 49, 549–557. [Google Scholar] [CrossRef]
- Alizadeh, A.; Wang, M. Temperature effects on electrical double layer at solid-aqueous solution interface. Electrophoresis 2020, 41, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Villar, M.V.; Lloret, A. Influence of temperature on the hydro-mechanical behaviour of a compacted bentonite. Appl. Clay Sci. 2004, 26, 337–350. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, C.; Yun, J.; Hu, Q.; Yang, G. Compositional transformations as well as thermodynamics and mechanism of dissolution for clay minerals. Chem. Geol. 2018, 494, 109–116. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, Y.; Liu, J.; Chen, R. A Temperature-Controlled Apparatus for Gas Permeability under Low Gas Pressure. Appl. Sci. 2023, 13, 10943. [Google Scholar] [CrossRef]
- Xing, H.; Huang, Y.; Chen, X.; Luo, B.; Mi, H. Comparative study of soil erodibility and critical shear stress between loess and purple soils. J. Hydrol. 2018, 558, 625–631. [Google Scholar] [CrossRef]
- Mandal, K.K.; Maity, D. Transient Response of Concrete Gravity Dam Considering Dam-Reservoir-Foundation Interaction. J. Earthq. Eng. 2018, 22, 211–233. [Google Scholar] [CrossRef]
- Liu, G.; Zha, X.; Guan, J.; Tong, F. Field experiment of rainfall infiltration on a soil slope and simulations based on a water-air two-phase flow model. J. Mt. Sci. 2021, 18, 2159–2167. [Google Scholar] [CrossRef]
- Bing, H.; Zhang, Y.; Ma, M. Impact of Desalination on Physical and Mechanical Properties of Lanzhou Loess. Eurasian Soil Sci. 2017, 50, 1444–1449. [Google Scholar] [CrossRef]
- Drewnik, M.; Skiba, M.; Szymanski, W.; Zyla, M. Mineral composition vs. soil forming processes in loess soils—A case study from Krakow (Southern Poland). Catena 2014, 119, 166–173. [Google Scholar] [CrossRef]
- Liu, H.; He, J.; Zhao, Q.; Wang, T. An experimental investigation on engineering properties of undisturbed loess under acid contamination. Environ. Sci. Pollut. Res. 2021, 28, 29845–29858. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Yang, H.; Xiao, J.; Xu, Y. Soil-water chemical tests and action mechanism of acid rain infiltration into expansive soil. Chin. J. Geotech. Eng. 2022, 44, 1483–1492. (In Chinese) [Google Scholar] [CrossRef]
- Szatanik-Kloc, A.; Horn, R.; Lipiec, J.; Siczek, A.; Szerement, J. Soil compaction-induced changes of physicochemical properties of cereal roots. Soil Tillage Res. 2018, 175, 226–233. [Google Scholar] [CrossRef]
- Li, W.; Deng, M.; Mo, L.; Panesar, D.K.; Mao, Z. Alkali carbonate reaction (ACR): Investigations on mechanism of dedolomitization of dolomite in dolostones. Constr. Build. Mater. 2022, 351, 128942. [Google Scholar] [CrossRef]
- Stukovnik, P.; Bosiljkov, V.B.; Marinsek, M. Alkali-dolomite reaction in air lime mortar—Implications for increased strength and water resistance. J. Cult. Herit. 2020, 45, 160–168. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Zhang, Q. Compressive strength properties of soil polluted by acid and alkali consolidated by SH. J. Guilin Univ. Technol. 2017, 37, 422–428. (In Chinese) [Google Scholar] [CrossRef]
- Meng, J.; Li, X. Effects of carbonate on the structure and properties of loess and the corresponding mechanism: An experimental study of the Malan loess, Xi’an area, China. Bull. Eng. Geol. Environ. 2019, 78, 4965–4976. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, L.; Zhang, J.; Peng, J.; Chen, L.; Zhang, Y. Pore characteristics and micro-structure change of undisturbed loess induced by acid corrosion. Eng. Geol. 2022, 310, 106880. [Google Scholar] [CrossRef]
- Ishiguro, M. Water, solute transport, and interfacial electric phenomena in soils. Soil Sci. Plant Nutr. 2019, 65, 223–227. [Google Scholar] [CrossRef]
- Shang, F.; Yang, C.; Zhang, L.; Zhou, C.; Han, D.; Shi, Y. Analysis of influencing factors of clay particle diffusion in electric double layer. J. Glaciol. Geocryol. 2022, 44, 495–505. (In Chinese) [Google Scholar] [CrossRef]
- Ding, W.; Liu, X.; Hu, F.; Zhu, H.; Luo, Y.; Li, S.; Li, H. How the particle interaction forces determine soil water infiltration: Specific ion effects. J. Hydrol. 2019, 568, 492–500. [Google Scholar] [CrossRef]
- Mahanta, K.K.; Mishra, G.C.; Kansal, M.L. Estimation of the electric double layer thickness in the presence of two types of ions in soil water. Appl. Clay Sci. 2014, 87, 212–218. [Google Scholar] [CrossRef]
- Xu, R.; Xiao, S.; Jiang, J.; Wang, Y. Effects of Amorphous Al(OH)3 on the Desorption of Ca2+, Mg2+, and Na+ from Soils and Minerals As Related to Diffuse Layer Overlapping. J. Chem. Eng. Data 2011, 56, 2536–2542. [Google Scholar] [CrossRef]
- Agbenin, J.O.; Modisaemang, L. Charge Distribution and the Interactive Effect of pH and Ionic Strength on Phosphate Adsorption Properties of Two Benchmark Soils from Botswana. Commun. Soil Sci. Plant Anal. 2015, 46, 2821–2836. [Google Scholar] [CrossRef]
- Di Pietro, S.A.; Emerson, H.P.; Katsenovich, Y.; Qafoku, N.P.; Szecsody, J.E. Phyllosilicate mineral dissolution upon alkaline treatment under aerobic and anaerobic conditions. Appl. Clay Sci. 2020, 189, 105520. [Google Scholar] [CrossRef]
- Yao, C.; Wei, C.; Ma, T.; Chen, P.; Tian, H. Experimental Investigation on the Influence of Thermochemical Effect on the Pore-Water Status in Expansive Soil. Int. J. Geomech. 2021, 21, 04021080. [Google Scholar] [CrossRef]
- Sridharan, A.; Satyamurty, P.V. Potential-Distance Relationships of Clay-Water Systems Considering the Stern Theory. Clays Clay Miner. 1996, 44, 479–484. [Google Scholar] [CrossRef]
- Henry, H.A.L. Soil freeze–thaw cycle experiments: Trends, methodological weaknesses and suggested improvements. Soil Biol. Biochem. 2007, 39, 977–986. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, F.; Li, H.; Cheng, D.; Sun, B. The Influence Mechanism of Freeze-Thaw on Soil Erosion: A Review. Water 2021, 13, 1010. [Google Scholar] [CrossRef]
- Gao, Z.; Zhong, X.; Ma, H.; Liu, F.; Ma, J.; Wang, Q. Effect of Freeze-Thaw Cycles on Shear Strength Properties of Loess Reinforced with Lignin Fiber. Geofluids 2022, 2022, 8685553. [Google Scholar] [CrossRef]
- Wang, L.; Xue, Y.; Cao, Z.; Kong, H.; Han, J.; Zhang, Z. Experimental Study on Mode I Fracture Characteristics of Granite after Low Temperature Cooling with Liquid Nitrogen. Water 2023, 15, 3442. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Cao, Z.; Xue, Y.; Liu, J.; Zhou, Y.; Duan, C.; Chen, T. Effect of weakening characteristics of mechanical properties of granite under the action of liquid nitrogen. Front. Ecol. Evol. 2023, 11, 1249617. [Google Scholar] [CrossRef]
- Liu, K.; Ye, W.; Jing, H. Multiscale evaluation of the structural characteristics of intact loess subjected to wet/dry cycles. Nat. Hazards 2023, 120, 1215–1240. [Google Scholar] [CrossRef]
- Nie, Y.; Ni, W.; Lv, X. Effects of dry-wet cycles on compacted loess: From macroscopic to microscopic investigation. Eur. J. Environ. Civ. Eng. 2023. [Google Scholar] [CrossRef]
- Fan, Y.; Zheng, M.; Wu, J. A study on the shear strength characteristics and microscopic mechanism of coal-bearing soil under dry-wet cycles. Front. Earth Sci. 2023, 10, 1096980. [Google Scholar] [CrossRef]
- Li, X.; Di, S.J.; Shi, L.; Zhang, Y.; Huang, P.; Mu, Q. Effects of In-Situ Drying-Wetting Cycles on the Stress-Dependent Water Retention Behavior of Intact Loess. Adv. Civ. Eng. 2023, 2023, 2994986. [Google Scholar] [CrossRef]
- Liang, Q.; Li, J.; Wu, X.; Zhou, A. Anisotropy of Q2 loess in the Baijiapo Tunnel on the Lanyu Railway, China. Bull. Eng. Geol. Environ. 2016, 75, 109–124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).