Insights into the Roles of Surface Functional Groups and Micropores in the Sorption of Ofloxacin on Banana Pseudo-Stem Biochars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Biochars
2.3. Characterization of Biochars
2.4. Sorption Experiment
2.5. Quantification of Dissolvable K in Biochars
2.6. Detection of OFL
2.7. Fourier-Transform Infrared Spectroscopy Characterization of Biochar and OFL-Loaded Biochar
2.8. Data Analysis
3. Results and Discussion
3.1. Biochar Characterization
3.1.1. Elemental Characterization of Biochars
3.1.2. BET Analysis and Pore Distribution
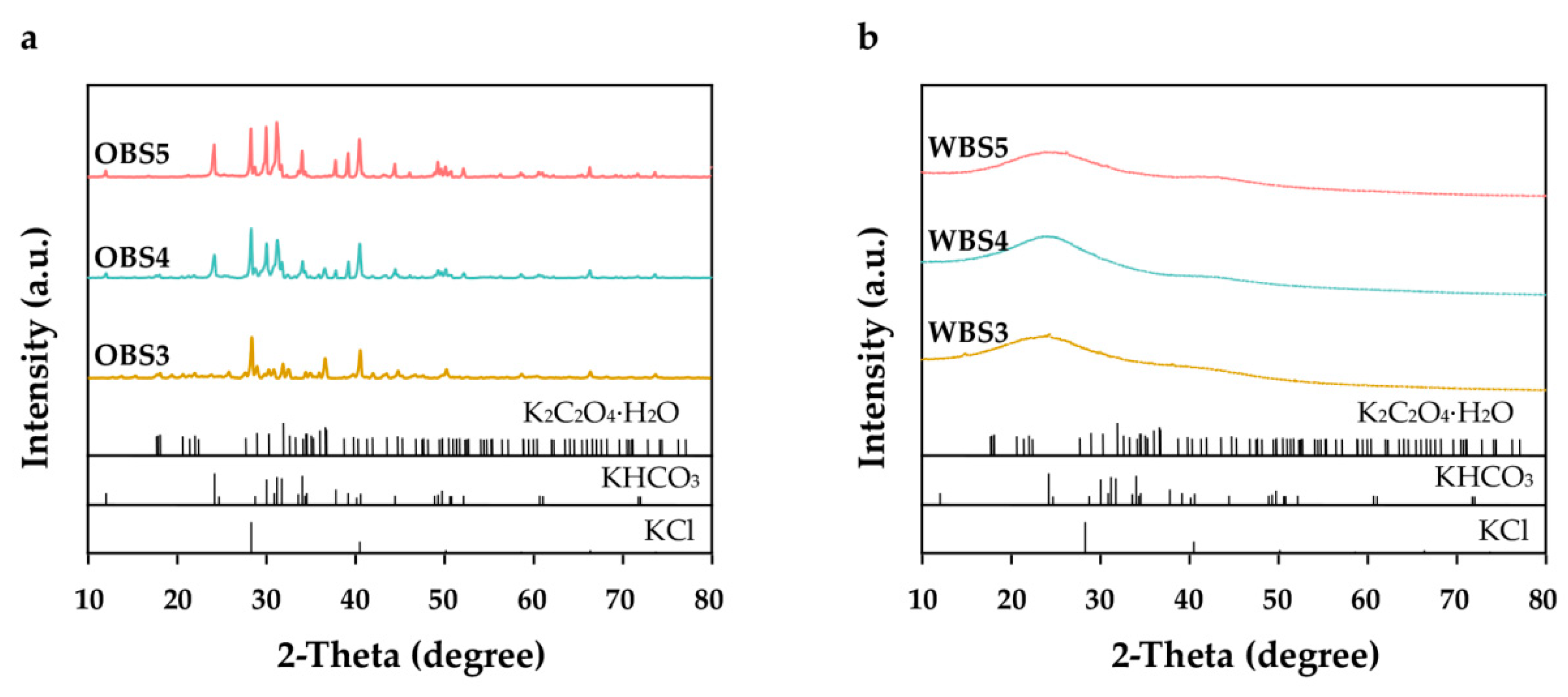
3.1.3. The Inorganic Composition of Biochars Identified by X-ray Diffraction
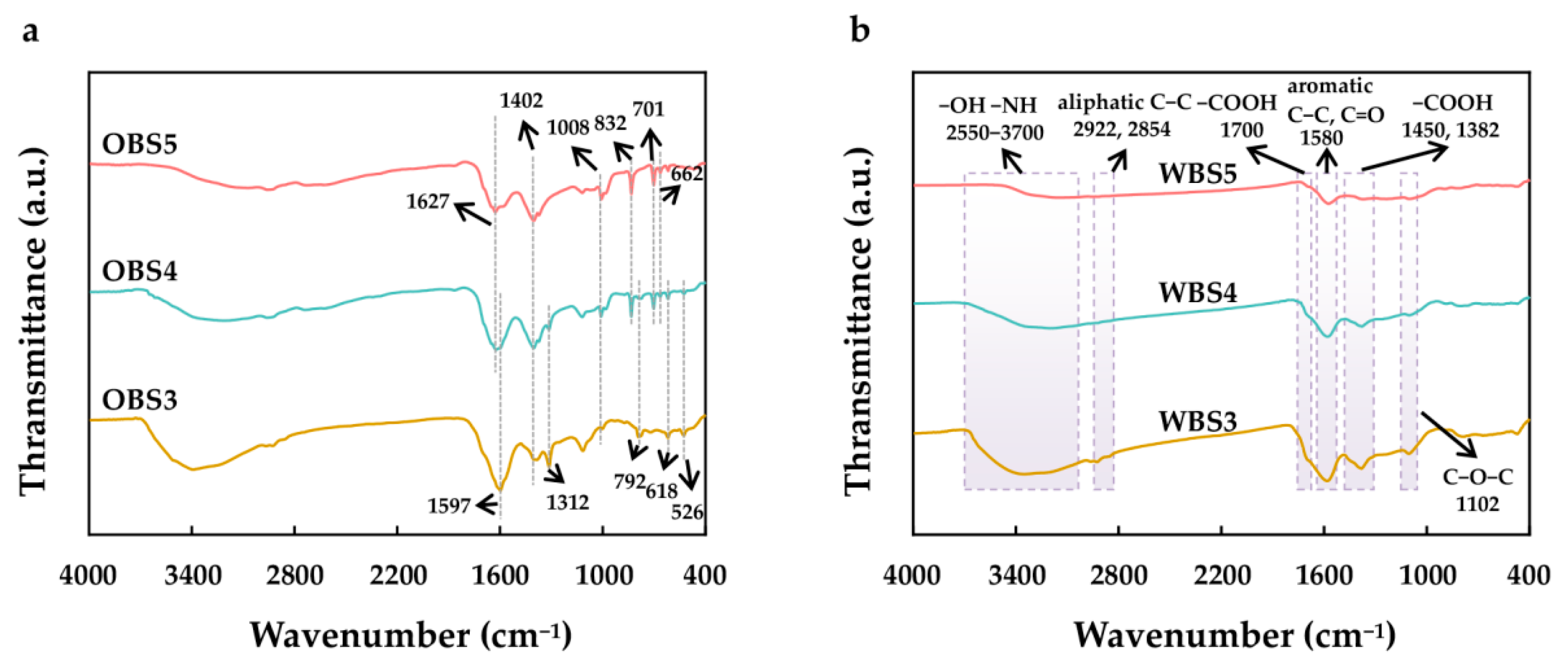
3.1.4. The Surface Functional Groups Analyzed by FT-IR
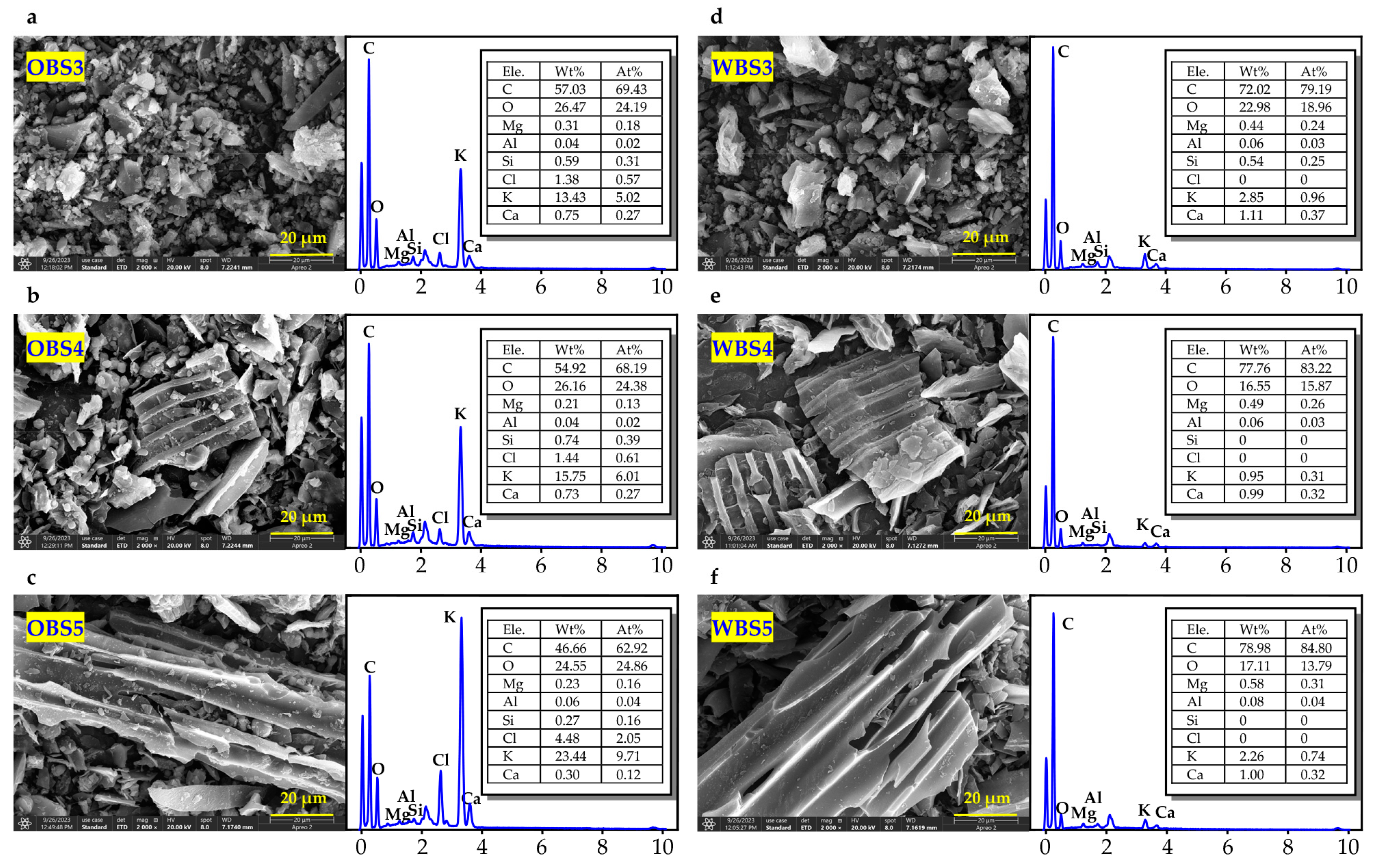
3.1.5. The Surface Morphology and EDX Analysis
3.1.6. Raman Analysis
3.2. Sorption of OFL on Banana-Pseudo-Stem-Derived Biochars
3.2.1. Sorption Kinetics
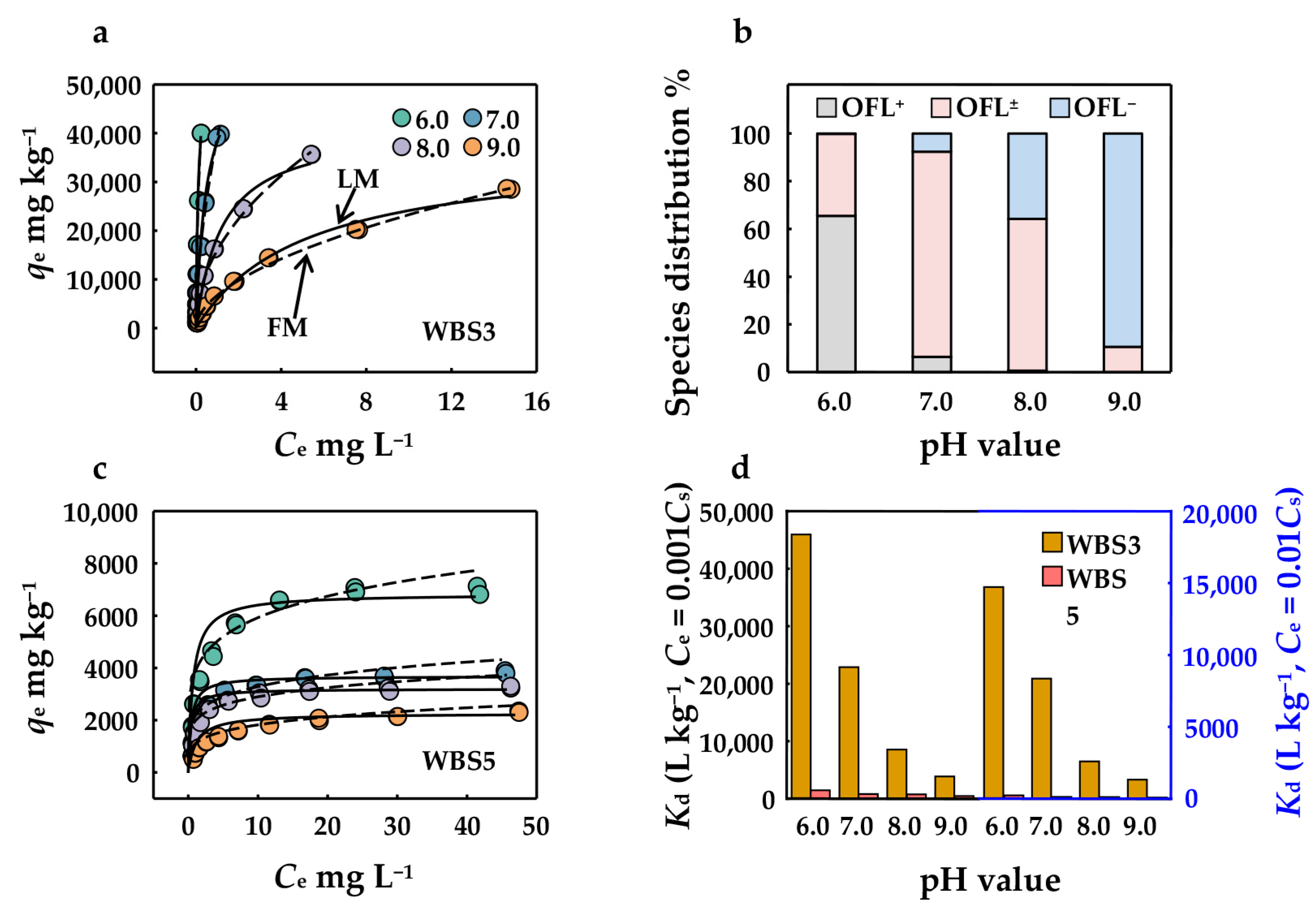
3.2.2. Sorption Isotherms and Potential Sorption Mechanisms
- (i)
- Electronic interaction
- (ii)
- Cation exchange
- (iii)
- Hydrophobic effect
- (iv)
- Pore-filling effect
- (v)
- π–π EDA interaction
- (vi)
- Hydrogen bond
3.2.3. FT-IR Analysis of Biochars before and after OFL Sorption
3.2.4. Effect of pH, Surface Functional Groups, and Micropores on OFL Sorption
3.2.5. Effect of K-Containing Salts on OFL Sorption
4. Conclusions and Environmental Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Li, G.; Chen, C.; Zhang, X.; Zhou, K.; Long, X. Banana Stem and Leaf Biochar as an Effective Adsorbent for Cadmium and Lead in Aqueous Solution. Sci. Rep. 2022, 12, 1584. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Mohd Taib, R.; Mohamad Aziz, N.S.; Omar, M.R.; Md Disa, N. Banana Pseudo-Stem Biochar Derived from Slow and Fast Pyrolysis Process. Heliyon 2023, 9, e12940. [Google Scholar] [CrossRef] [PubMed]
- Ding, E.; Jiang, J.; Lan, Y.; Zhang, L.; Gao, C.; Jiang, K.; Qi, X.; Fan, X. Optimizing Cd2+ Adsorption Performance of KOH Modified Biochar Adopting Response Surface Methodology. J. Anal. Appl. Pyrolysis 2023, 169, 105788. [Google Scholar] [CrossRef]
- Oelofse, S.H.; Nahman, A. Estimating the Magnitude of Food Waste Generated in South Africa. Waste Manag. Res. 2013, 31, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Xing, B. Black Carbon (Biochar) In Water/Soil Environments: Molecular Structure, Sorption, Stability, and Potential Risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef] [PubMed]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar Based Removal of Antibiotic Sulfonamides and Tetracyclines in Aquatic Environments: A Critical Review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Su, L.; Cheng, C.; Cheng, H.; Chang, M.; Liu, F.; Liu, N.; Oh, K. A New Type of Calcium-Rich Biochars Derived from Spent Mushroom Substrates and Their Efficient Adsorption Properties for Cationic Dyes. Front. Bioeng. Biotechnol. 2022, 10, 1007630. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in Climate Change Mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Sial, T.A.; Khan, M.N.; Lan, Z.; Kumbhar, F.; Ying, Z.; Zhang, J.; Sun, D.; Li, X. Contrasting Effects of Banana Peels Waste and Its Biochar on Greenhouse Gas Emissions and Soil Biochemical Properties. Process Saf. Environ. Prot. 2019, 122, 366–377. [Google Scholar] [CrossRef]
- Cordeiro, N.; Belgacem, M.N.; Torres, I.C.; Moura, J.C.V.P. Chemical Composition and Pulping of Banana Pseudo-Stems. Ind. Crops Prod. 2004, 19, 147–154. [Google Scholar] [CrossRef]
- Krasucka, P.; Pan, B.; Sik Ok, Y.; Mohan, D.; Sarkar, B.; Oleszczuk, P. Engineered Biochar–A Sustainable Solution for the Removal of Antibiotics from Water. Chem. Eng. J. 2021, 405, 126926. [Google Scholar] [CrossRef]
- Hacıosmanoğlu, G.G.; Mejías, C.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Antibiotic Adsorption by Natural and Modified Clay Minerals as Designer Adsorbents for Wastewater Treatment: A Comprehensive Review. J. Environ. Manag. 2022, 317, 115397. [Google Scholar] [CrossRef]
- Xiao, R.; Huang, D.; Du, L.; Song, B.; Yin, L.; Chen, Y.; Gao, L.; Li, R.; Huang, H.; Zeng, G. Antibiotic Resistance in Soil-Plant Systems: A Review of the Source, Dissemination, Influence Factors, and Potential Exposure Risks. Sci. Total Environ. 2023, 869, 161855. [Google Scholar] [CrossRef]
- Singh, A.; Chaurasia, D.; Khan, N.; Singh, E.; Chaturvedi Bhargava, P. Efficient Mitigation of Emerging Antibiotics Residues from Water Matrix: Integrated Approaches and Sustainable Technologies. Environ. Pollut. 2023, 328, 121552. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ondon, B.S.; Ho, S.-H.; Li, F. Emerging Soil Contamination of Antibiotics Resistance Bacteria (ARB) Carrying Genes (ARGs): New Challenges for Soil Remediation and Conservation. Environ. Res. 2023, 219, 115132. [Google Scholar] [CrossRef]
- Zou, M.; Tian, W.; Zhao, J.; Chu, M.; Song, T. Quinolone Antibiotics in Sewage Treatment Plants with Activated Sludge Treatment Processes: A Review on Source, Concentration and Removal. Process Saf. Environ. Prot. 2022, 160, 116–129. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone Antibiotics: An Emerging Class of Environmental Micropollutants. Sci. Total Environ. 2014, 500–501, 250–269. [Google Scholar] [CrossRef]
- Patel, A.K.; Katiyar, R.; Chen, C.-W.; Singhania, R.R.; Awasthi, M.K.; Bhatia, S.; Bhaskar, T.; Dong, C.-D. Antibiotic Bioremediation by New Generation Biochar: Recent Updates. Bioresour. Technol. 2022, 358, 127384. [Google Scholar] [CrossRef]
- Kah, M.; Sigmund, G.; Xiao, F.; Hofmann, T. Sorption of Ionizable and Ionic Organic Compounds to Biochar, Activated Carbon and Other Carbonaceous Materials. Water Res. 2017, 124, 673–692. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and Inorganic Contaminants Removal from Water with Biochar, a Renewable, Low Cost and Sustainable Adsorbent–A Critical Review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; He, L.; Liang, N.; Wang, L.; Zhao, J.; Pan, B. Sorption of Sulfamethoxazole on Biochars of Varying Mineral Content. Environ. Sci. Proc. Impacts 2020, 22, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Ge, C.; Feng, D.; Yu, H.; Luo, J.; Li, J.; Strong, P.J.; Sarmah, A.K.; Bolan, N.S.; Wang, H. Effects of Metal Ions and pH on Ofloxacin Sorption to Cassava Residue-Derived Biochar. Sci. Total Environ. 2018, 616–617, 1384–1391. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, P.; Zhang, H.; Zhang, D.; Zhou, Y. Sorption Kinetics of Ofloxacin by Carbonaceous Sorbents with Different Characteristics. Environ. Chem. 2016, 35, 651–657. [Google Scholar] [CrossRef]
- Akhtar, L.; Ahmad, M.; Iqbal, S.; Abdelhafez, A.A.; Mehran, M.T. Biochars’ Adsorption Performance towards Moxifloxacin and Ofloxacin in Aqueous Solution: Role of Pyrolysis Temperature and Biomass Type. Environ. Technol. Innov. 2021, 24, 101912. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Wang, Z.; Wen, Y.; Liu, L.; Wang, T.; Xie, X. Removal of Ofloxacin from Water by Natural Ilmenite-Biochar Composite: A Study on the Synergistic Adsorption Mechanism of Multiple Effects. Bioresour. Technol. 2022, 363, 127938. [Google Scholar] [CrossRef] [PubMed]
- Gurav, R.; Bhatia, S.K.; Choi, T.-R.; Park, Y.-L.; Park, J.Y.; Han, Y.-H.; Vyavahare, G.; Jadhav, J.; Song, H.-S.; Yang, P.; et al. Treatment of Furazolidone Contaminated Water Using Banana Pseudostem Biochar Engineered with Facile Synthesized Magnetic Nanocomposites. Bioresour. Technol. 2020, 297, 122472. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Pittman, C.U.; Mohan, D. Ciprofloxacin and Acetaminophen Sorption onto Banana Peel Biochars: Environmental and Process Parameter Influences. Environ. Res. 2021, 201, 111218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Chen, K.; Shen, S.; Hu, H.; Chang, M.; Chen, D.; Wu, Y.; Yuan, H.; Wang, Y. Engineering Banana-Peel-Derived Biochar for the Rapid Adsorption of Tetracycline Based on Double Chemical Activation. Resour. Conserv. Recycl. 2023, 190, 106821. [Google Scholar] [CrossRef]
- Hu, Z.-T.; Ding, Y.; Shao, Y.; Cai, L.; Jin, Z.-Y.; Liu, Z.; Zhao, J.; Li, F.; Pan, Z.; Li, X.; et al. Banana Peel Biochar with Nanoflake-Assembled Structure for Cross Contamination Treatment in Water: Interaction Behaviors between Lead and Tetracycline. Chem. Eng. J. 2021, 420, 129807. [Google Scholar] [CrossRef]
- Peng, H.; Pan, B.; Wu, M.; Liu, R.; Zhang, D.; Wu, D.; Xing, B. Adsorption of Ofloxacin on Carbon Nanotubes: Solubility, pH and Cosolvent Effects. J. Hazard. Mater. 2012, 211–212, 342–348. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, B.; Chiou, C.T. Fast and Slow Rates of Naphthalene Sorption to Biochars Produced at Different Temperatures. Environ. Sci. Technol. 2012, 46, 11104–11111. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of Feedstock Type, Production Method, and Pyrolysis Temperature on Biochar and Hydrochar Properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Singh, G.; Maria Ruban, A.; Geng, X.; Vinu, A. Recognizing the Potential of K-Salts, Apart from KOH, for Generating Porous Carbons Using Chemical Activation. Chem. Eng. J. 2023, 451, 139045. [Google Scholar] [CrossRef]
- Li, J.; Liang, N.; Jin, X.; Zhou, D.; Li, H.; Wu, M.; Pan, B. The Role of Ash Content on Bisphenol A Sorption to Biochars Derived from Different Agricultural Wastes. Chemosphere 2017, 171, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Thabithal, J.; Ravi, N. Effect of Laser Induced Potassium Oxalate (C2K2O4) Nanopowder. J. Nanosci. Technol. 2019, 5, 713–715. [Google Scholar] [CrossRef]
- Gasc, F.; Thiebaud-Roux, S.; Mouloungui, Z. Methods for Synthesizing Diethyl Carbonate from Ethanol and Supercritical Carbon Dioxide by One-Pot or Two-Step Reactions in the Presence of Potassium Carbonate. J. Supercrit. Fluids 2009, 50, 46–53. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, X.; Chen, B.; Zhu, L. Quantification of Chemical States, Dissociation Constants and Contents of Oxygen-Containing Groups on the Surface of Biochars Produced at Different Temperatures. Environ. Sci. Technol. 2015, 49, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jin, L.; Guo, C.; Min, L.; Zhang, P.; Sun, H.; Zhu, H.; Zhang, C. Enhanced Heavy Metals Sorption by Modified Biochars Derived from Pig Manure. Sci. Total Environ. 2021, 786, 147595. [Google Scholar] [CrossRef]
- Inoue, J.; Yoshie, A.; Tanaka, T.; Onji, T.; Inoue, Y. Disappearance and Alteration Process of Charcoal Fragments in Cumulative Soils Studied Using Raman Spectroscopy. Geoderma 2017, 285, 164–172. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Chu, G. Reactivity of Aged Biochars to the Degradation of Adsorbed P-Nitrophenol: Role of Intensity and Species of Persistent Free Radicals. Chemosphere 2023, 344, 140362. [Google Scholar] [CrossRef]
- Tsaneva, V.N.; Kwapinski, W.; Teng, X.; Glowacki, B.A. Assessment of the Structural Evolution of Carbons from Microwave Plasma Natural Gas Reforming and Biomass Pyrolysis Using Raman Spectroscopy. Carbon 2014, 80, 617–628. [Google Scholar] [CrossRef]
- Zuo, X.; Yi, P.; Chen, Q.; Wu, M.; Zhang, L.; Pan, B.; Xing, B. Inter-Molecular Interactions of Phthalic Acid Esters and Multi-Stage Sorption Revealed by Experimental Investigations and Computation Simulations. Chem. Eng. J. 2022, 431, 134018. [Google Scholar] [CrossRef]
- Huang, J.; Zimmerman, A.R.; Chen, H.; Gao, B. Ball Milled Biochar Effectively Removes Sulfamethoxazole and Sulfapyridine Antibiotics from Water and Wastewater. Environ. Pollut. 2020, 258, 113809. [Google Scholar] [CrossRef]
- Ogunleye, D.T.; Akpotu, S.O.; Moodley, B. Adsorption of Sulfamethoxazole and Reactive Blue 19 Using Graphene Oxide Modified with Imidazolium Based Ionic Liquid. Environ. Technol. Innov. 2020, 17, 100616. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Z.; Li, J.; Du, H.; Li, Z. Efficient with Low-Cost Removal and Adsorption Mechanisms of Norfloxacin, Ciprofloxacin and Ofloxacin on Modified Thermal Kaolin: Experimental and Theoretical Studies. J. Hazard. Mater. 2022, 430, 128500. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Li, P.; Yang, R.; Li, A.; Yang, H. Investigation of Multiple Adsorption Mechanisms for Efficient Removal of Ofloxacin from Water Using Lignin-Based Adsorbents. Sci. Rep. 2019, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, Y.; Du, C.; Wang, Y.; Wang, L.; Li, X. A Novel Role of Various Hydrogen Bonds in Adsorption, Desorption and Co-Adsorption of PPCPs on Corn Straw-Derived Biochars. Sci. Total Environ. 2023, 861, 160623. [Google Scholar] [CrossRef]
- Hao, J.; Wu, L.; Lu, X.; Zeng, Y.; Jia, B.; Luo, T.; He, S.; Liang, L. A Stable Fe/Co Bimetallic Modified Biochar for Ofloxacin Removal from Water: Adsorption Behavior and Mechanisms. RSC Adv. 2022, 12, 31650–31662. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Ramos, C.G.; Piccilli, D.G.A.; Lima, E.C.; Sher, F. A Review of the Antibiotic Ofloxacin: Current Status of Ecotoxicology and Scientific Advances in Its Removal from Aqueous Systems by Adsorption Technology. Chem. Eng. Res. Des. 2023, 193, 99–120. [Google Scholar] [CrossRef]
- Li, Y.; Bi, E.; Chen, H. Sorption Behavior of Ofloxacin to Kaolinite: Effects of pH, Ionic Strength, and Cu(II). Water Air Soil Pollut. 2017, 228, 46. [Google Scholar] [CrossRef]
- Li, C.; Zhu, X.; He, H.; Fang, Y.; Dong, H.; Lü, J.; Li, J.; Li, Y. Adsorption of Two Antibiotics on Biochar Prepared in Air-Containing Atmosphere: Influence of Biochar Porosity and Molecular Size of Antibiotics. J. Mol. Liq. 2019, 274, 353–361. [Google Scholar] [CrossRef]
- Zhu, X.; Li, C.; Li, J.; Xie, B.; Lü, J.; Li, Y. Thermal Treatment of Biochar in the Air/Nitrogen Atmosphere for Developed Mesoporosity and Enhanced Adsorption to Tetracycline. Bioresour. Technol. 2018, 263, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, A.; Zhou, Q.; Shuang, C.; Zhou, W.; Wang, M. Effect of Pore Size Distribution on Tetracycline Adsorption Using Magnetic Hypercrosslinked Resins. Microporous Mesoporous Mater. 2014, 184, 105–111. [Google Scholar] [CrossRef]
- Antonelli, R.; Martins, F.R.; Malpass, G.R.P.; Da Silva, M.G.C.; Vieira, M.G.A. Ofloxacin Adsorption by Calcined Verde-Lodo Bentonite Clay: Batch and Fixed Bed System Evaluation. J. Mol. Liq. 2020, 315, 113718. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Shang, H.; Cao, Y.; Yang, C.; Hu, W.; Feng, Y.; Yu, Y. Influence of Adsorption Sites of Biochar on Its Adsorption Performance for Sulfamethoxazole. Chemosphere 2023, 326, 138408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xiao, X.; Xing, B.; Chen, B. pH-Dependent Sorption of Sulfonamide Antibiotics onto Biochars: Sorption Mechanisms and Modeling. Environ. Pollut. 2019, 248, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, C.C.; Sahoo, S.; Behera, P.K. Characterization of an Ofloxacin/Carbopol 940 Mucoadhesive Polymeric Suspension. Int. J. Curr. Pharm. Res. 2012, 4, 92–100. [Google Scholar]
- Vashisth, P.; Raghuwanshi, N.; Srivastava, A.K.; Singh, H.; Nagar, H.; Pruthi, V. Ofloxacin Loaded Gellan/PVA Nanofibers–Synthesis, Characterization and Evaluation of Their Gastroretentive/Mucoadhesive Drug Delivery Potential. Mater. Sci. Eng. C 2017, 71, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, H.; Li, X.; Li, N.; Liu, Y.; Li, T.; Wang, Y.; Xing, B. Direct Spectroscopic Evidence for Charge-Assisted Hydrogen-Bond Formation between Ionizable Organic Chemicals and Carbonaceous Materials. Environ. Sci. Technol. 2022, 56, 9356–9366. [Google Scholar] [CrossRef]
- Zhang, J.; Zhai, J.; Zheng, H.; Li, X.; Wang, Y.; Li, X.; Xing, B. Adsorption, Desorption and Coadsorption Behaviors of Sulfamerazine, Pb(II) and Benzoic Acid on Carbon Nanotubes and Nano-Silica. Sci. Total Environ. 2020, 738, 139685. [Google Scholar] [CrossRef]
- Feng, D.; Yu, H.; Deng, H.; Li, F.; Ge, C. Adsorption Characteristics of Norfloxacin by Biochar Prepared by Cassava Dreg: Kinetics, Isotherms, and Thermodynamic Analysis. Bioresources 2015, 10, 6751–6768. [Google Scholar] [CrossRef]
- He, S.; Chen, Q.; Chen, G.; Shi, G.; Ruan, C.; Feng, M.; Ma, Y.; Jin, X.; Liu, X.; Du, C.; et al. N-Doped Activated Carbon for High-Efficiency Ofloxacin Adsorption. Microporous Mesoporous Mater. 2022, 335, 111848. [Google Scholar] [CrossRef]
- Song, X.; Zhang, H.; Zhang, J.; Sun, R.; Zhao, J.; Zhao, H.; Hu, J.; Liu, Y. Removal of Ciprofloxacin from Water by a Potassium Carbonate-Activated Sycamore Floc-Based Carbonaceous Adsorbent: Adsorption Behavior and Mechanism. Langmuir 2023, 39, 5323–5332. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Fang, Y.; Xiang, H.; Liu, H.; Yan, H.; Wang, B.; Lin, X.; Liang, J.; Qian, W. Preparation and modification of bagasse biochar unveiling ofloxacin wastewater adsorption. Environ. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Yang, C.; Miao, S.; Li, T. Influence of water washing treatment on Ulva prolifera-derived biochar properties and sorption characteristics of ofloxacin. Sci. Rep. 2021, 11, 1797. [Google Scholar] [CrossRef]
- Zhu, C.; Lang, Y.; Liu, B.; Zhao, H. Ofloxacin Adsorption on Chitosan/Biochar Composite: Kinetics, Isotherms, and Effects of Solution Chemistry. Polycycl. Aromat. Compd. 2019, 39, 287–297. [Google Scholar] [CrossRef]
- Singh, V.; Srivastava, V.C. Transformation of textile dyeing industrial sludge into economical biochar for sorption of ofloxacin: Equilibrium, kinetic, and cost analysis. Biomass Convers. Biorefin. 2024, 14, 1881–1893. [Google Scholar] [CrossRef]
- Ma, Y.; Li, P.; Yang, L.; Wu, L.; He, L.; Gao, F.; Qi, X.; Zhang, Z. Iron/zinc and phosphoric acid modified sludge biochar as an efficient adsorbent for fluoroquinolones antibiotics removal. Ecotoxicol. Environ. Saf. 2020, 196, 110550. [Google Scholar] [CrossRef]
- Wu, M.; Pan, B.; Zhang, D.; Xiao, D.; Li, H.; Wang, C.; Ning, P. The sorption of organic contaminants on biochars derived from sediments with high organic carbon content. Chemosphere 2013, 90, 782–788. [Google Scholar] [CrossRef]
- Na, P.T.L.; Tuyen, N.D.K.; Dang, B.-T. Sorption of four antibiotics onto pristine biochar derived from macadamia nutshell. Bioresour. Technol. 2024, 394, 130281. [Google Scholar] [CrossRef]
- Liu, L.; Shang, D.; Zhao, Y.; Zhao, Q.; Guo, Y.; Zhang, F.; Kong, Q.; Zhang, H.; Wang, Q.; Zhao, C. Preparation of Chladophora-Based Biochar and Its Adsorption Properties for Antibiotics. Chem. Select. 2024, 9, e202303598. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Zhan, L.; Wu, D.; Zhang, S.; Pang, R.; Xie, B. Removal of emerging contaminants (bisphenol A and antibiotics) from kitchen wastewater by alkali-modified biochar. Sci. Total Environ. 2022, 805, 150158. [Google Scholar] [CrossRef] [PubMed]
- Dang, B.T.; Gotore, O.; Ramaraj, R.; Unpaprom, Y.; Whangchai, N.; Bui, X.T.; Maseda, H.; Itayama, T. Sustainability and application of corncob-derived biochar for removal of fluoroquinolones. Biomass Convers. Biorefin. 2022, 12, 913–923. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.e.k.; Bouhfid, R. Microwave-assisted synthesis of MIL–53(Fe)/biochar composite from date palm for ciprofloxacin and ofloxacin antibiotics removal. Sep. Purif. Technol. 2023, 308, 122850. [Google Scholar] [CrossRef]
- Wang, Y.B.; Lu, J.; Wu, J.; Liu, Q.; Zhang, H.; Jin, S. Adsorptive Removal of Fluoroquinolone Antibiotics Using Bamboo Biochar. Sustainability 2015, 7, 12947–12957. [Google Scholar] [CrossRef]
- Sulaiman, N.S.; Mohamad Amini, M.H.; Danish, M.; Sulaiman, O.; Hashim, R.; Demirel, S.; Demirel, G.K. Characterization and Ofloxacin Adsorption Studies of Chemically Modified Activated Carbon from Cassava Stem. Materials 2022, 15, 5117. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, Q.; Zhou, X.; Chen, J.; Chen, Z.; Liu, Z.; Yan, J.; Wang, J. Removal of fluoroquinolone antibiotics by adsorption of dopamine-modified biochar aerogel. Korean J. Chem. Eng. 2023, 40, 215–222. [Google Scholar] [CrossRef]






| Samples | Composition, wt% | Ash/% | Atomic Ratio | BET-N2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | H/C | (O + N)/C | O/C | SSA * (m2 g−1) | Vt * (cm3 g−1) | Dw * (nm) | ||
| OBS3 | 42.2 | 3.38 | 26.6 | 1.38 | 0.45 | 33.7 | 0.96 | 0.50 | 0.47 | 3.51 | 0.019 | 21.5 |
| WBS3 | 51.8 | 3.69 | 25.6 | 1.92 | 0.26 | 10.3 | 0.85 | 0.40 | 0.37 | 4.75 | 0.026 | 21.7 |
| OBS4 | 36.7 | 2.24 | 27.3 | 1.03 | 0.29 | 33.5 | 0.73 | 0.58 | 0.56 | 6.30 | 0.030 | 24.3 |
| WBS4 | 59.9 | 2.83 | 21.5 | 2.05 | 0.24 | 9.5 | 0.57 | 0.30 | 0.27 | 9.53 | 0.035 | 14.7 |
| OBS5 | 37.9 | 1.87 | 27.9 | 1.12 | 0.44 | 35.6 | 0.59 | 0.58 | 0.55 | 6.78 | 0.058 | 33.9 |
| WBS5 | 70.3 | 2.59 | 22.1 | 2.32 | 0.26 | 7.69 | 0.46 | 0.20 | 0.25 | 390 | 0.198 | 2.03 |
| Biochar | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
| qe (mg kg−1) | k1 (h−1) | r2 | qe (mg kg−1) | k2 ([(mg kg−1) h−1]) | r2adj | |
| OBS3 | 33,027.1 | 10.9 | 0.996 | 33,293.0 | 0.0015 | 0.999 |
| OBS4 | 6191.5 | 4.8 | 0.893 | 6411.9 | 0.0013 | 0.940 |
| OBS5 | – | – | – | – | – | – |
| WBS3 | 38,699.6 | 10.5 | 0.997 | 39,016.7 | 0.0012 | 1.000 |
| WBS4 | 11,114.5 | 2.2 | 0.773 | 11,785.8 | 0.0002 | 0.874 |
| WBS5 | 4369.7 | 12.6 | 0.978 | 4384.2 | 0.0220 | 0.978 |
| Biochar | Two-Compartment First-Order Model | |||||
| qe (mg kg−1) | Ffast | Fslow | kfast (h−1) | kslow (h−1) | r2adj | |
| OBS3 | 33,509.3 | 0.953 | 0.047 | 13.9 | 0.191 | 0.999 |
| OBS4 | 6957.4 | 0.751 | 0.249 | 9.3 | 0.061 | 0.995 |
| OBS5 | – | – | – | – | – | – |
| WBS3 | 39,087.3 | 0.945 | 0.055 | 14.1 | 0.478 | 1.000 |
| WBS4 | 13,075.9 | 0.578 | 0.422 | 8.2 | 0.075 | 0.990 |
| WBS5 | 4440.2 | 0.961 | 0.039 | 15.4 | 0.108 | 0.979 |
| Freundlich Model | Langmuir Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biochar | N | KF ([(mg kg−1)/(mg L−1) N]) | r2adj b | SEE b | Kd (L kg−1) | Q0 (mg kg−1) | KL (L kg−1) | r2adj b | SEE b | |
| 0.001 CS | 0.01 CS | |||||||||
| OBS3 | 0.342 | 9297.5 | 0.983 | 1049.3 | 4155.8 | 913.4 | 24,904.6 | 0.630 | 0.950 | 1823.3 |
| WBS3 | 0.563 | 39,058.3 | 0.982 | 1692.4 | 22,880.1 | 8364.8 | 56,455.1 | 2.11 | 0.988 | 1389.2 |
| OBS4 | 0.176 | 1394.5 | 0.942 | 257.4 | 508.7 | 76.3 | 2450.9 | 1.48 | 0.937 | 162.2 |
| WBS4 | 0.198 | 7615.8 | 0.958 | 873.9 | 2854.1 | 450.3 | 13,041.9 | 3.12 | 0.920 | 1204.6 |
| OBS5 | 0.145 | 1050.9 | 0.909 | 107.7 | 369.1 | 51.5 | 1633.2 | 2.26 | 0.934 | 91.6 |
| WBS5 | 0.179 | 2172.7 | 0.966 | 244.9 | 795.5 | 120.1 | 3667.4 | 2.56 | 0.944 | 314.4 |
| WBS3-pH 6.0 | 0.506 | 84,155.1 | 0.994 | 1007.1 | 45,947.7 | 14,715.1 | 55,107.7 | 10.49 | 0.966 | 2293.3 |
| WBS3-pH 7.0 | 0.563 | 39,058.3 | 0.982 | 1692.4 | 22,880.1 | 8364.8 | 56,455.1 | 2.11 | 0.988 | 1389.2 |
| WBS3-pH 8.0 | 0.484 | 16,000.6 | 0.992 | 979.9 | 8507.2 | 2591.7 | 41,373.7 | 0.82 | 0.985 | 1380.8 |
| WBS3-pH 9.0 | 0.539 | 6733.5 | 0.996 | 570.97 | 3828.4 | 1323.2 | 36,473.9 | 0.20 | 0.986 | 1048.8 |
| WBS5-pH 6.0 | 0.187 | 3856.5 | 0.937 | 602.9 | 1425.9 | 219.3 | 6843.3 | 1.51 | 0.856 | 908.3 |
| WBS5-pH 7.0 | 0.180 | 2180.6 | 0.948 | 259.0 | 799.4 | 121.0 | 3700.0 | 2.48 | 0.914 | 332.2 |
| WBS5-pH 8.0 | 0.166 | 1982.9 | 0.930 | 247.3 | 714.6 | 104.7 | 3207.3 | 2.95 | 0.898 | 299.7 |
| WBS5-pH 9.0 | 0.219 | 1112.1 | 0.984 | 77.8 | 427.6 | 70.8 | 2272.6 | 0.880 | 0.885 | 205.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yang, Y.; Wang, M.; Yuan, R.; Song, W.; Wang, L.; Liang, N.; Shi, J.; Li, J. Insights into the Roles of Surface Functional Groups and Micropores in the Sorption of Ofloxacin on Banana Pseudo-Stem Biochars. Sustainability 2024, 16, 2629. https://doi.org/10.3390/su16072629
Wang H, Yang Y, Wang M, Yuan R, Song W, Wang L, Liang N, Shi J, Li J. Insights into the Roles of Surface Functional Groups and Micropores in the Sorption of Ofloxacin on Banana Pseudo-Stem Biochars. Sustainability. 2024; 16(7):2629. https://doi.org/10.3390/su16072629
Chicago/Turabian StyleWang, Haifeng, Yang Yang, Mengping Wang, Runjiao Yuan, Wenyi Song, Lin Wang, Ni Liang, Jiayi Shi, and Jing Li. 2024. "Insights into the Roles of Surface Functional Groups and Micropores in the Sorption of Ofloxacin on Banana Pseudo-Stem Biochars" Sustainability 16, no. 7: 2629. https://doi.org/10.3390/su16072629
APA StyleWang, H., Yang, Y., Wang, M., Yuan, R., Song, W., Wang, L., Liang, N., Shi, J., & Li, J. (2024). Insights into the Roles of Surface Functional Groups and Micropores in the Sorption of Ofloxacin on Banana Pseudo-Stem Biochars. Sustainability, 16(7), 2629. https://doi.org/10.3390/su16072629





