Sorption-Desorption of Phosphorus on Manure- and Plant-Derived Biochars at Different Pyrolysis Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Collection
2.2. Biochar Production
2.3. Biochar Characterization
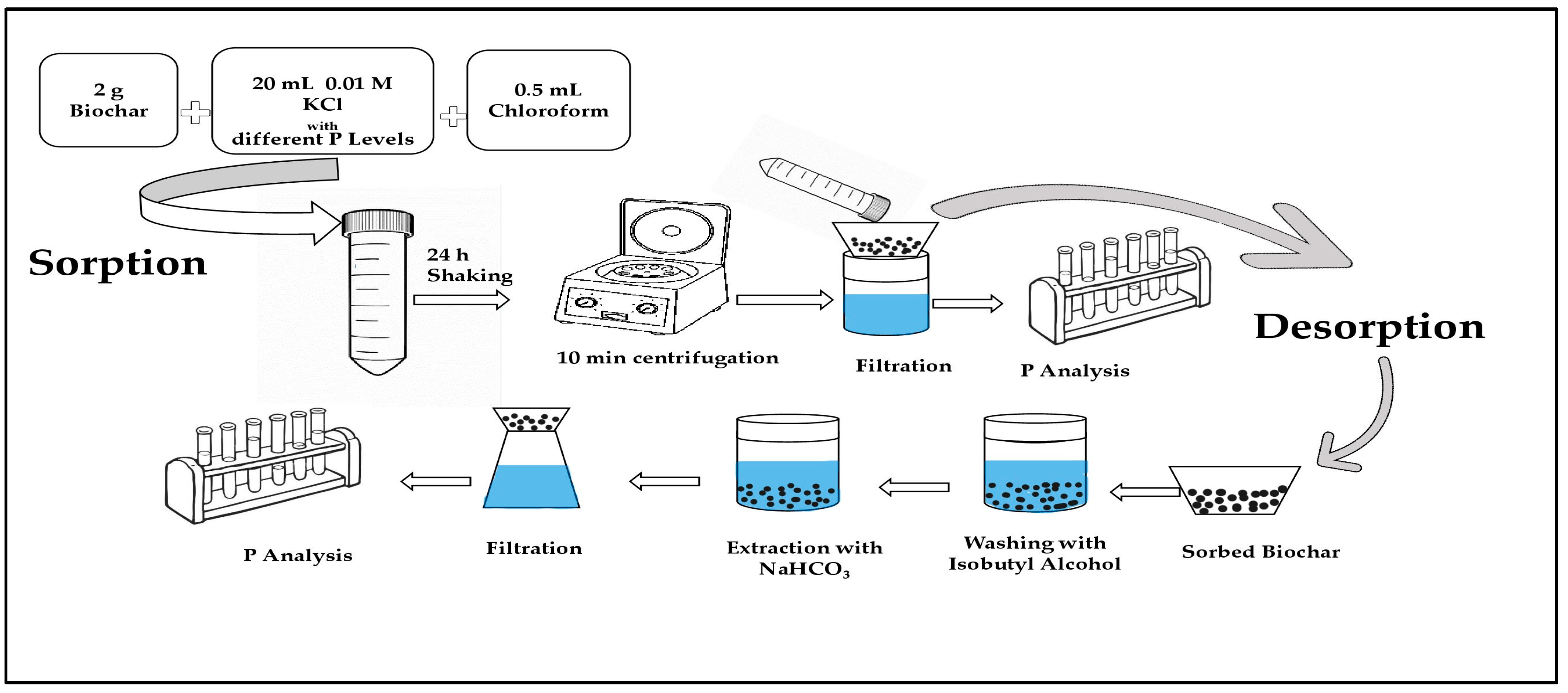
2.4. Phosphorus Sorption Experiment
2.5. Phosphorus Desorption Experiment
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Properties
3.2. Elemental Composition
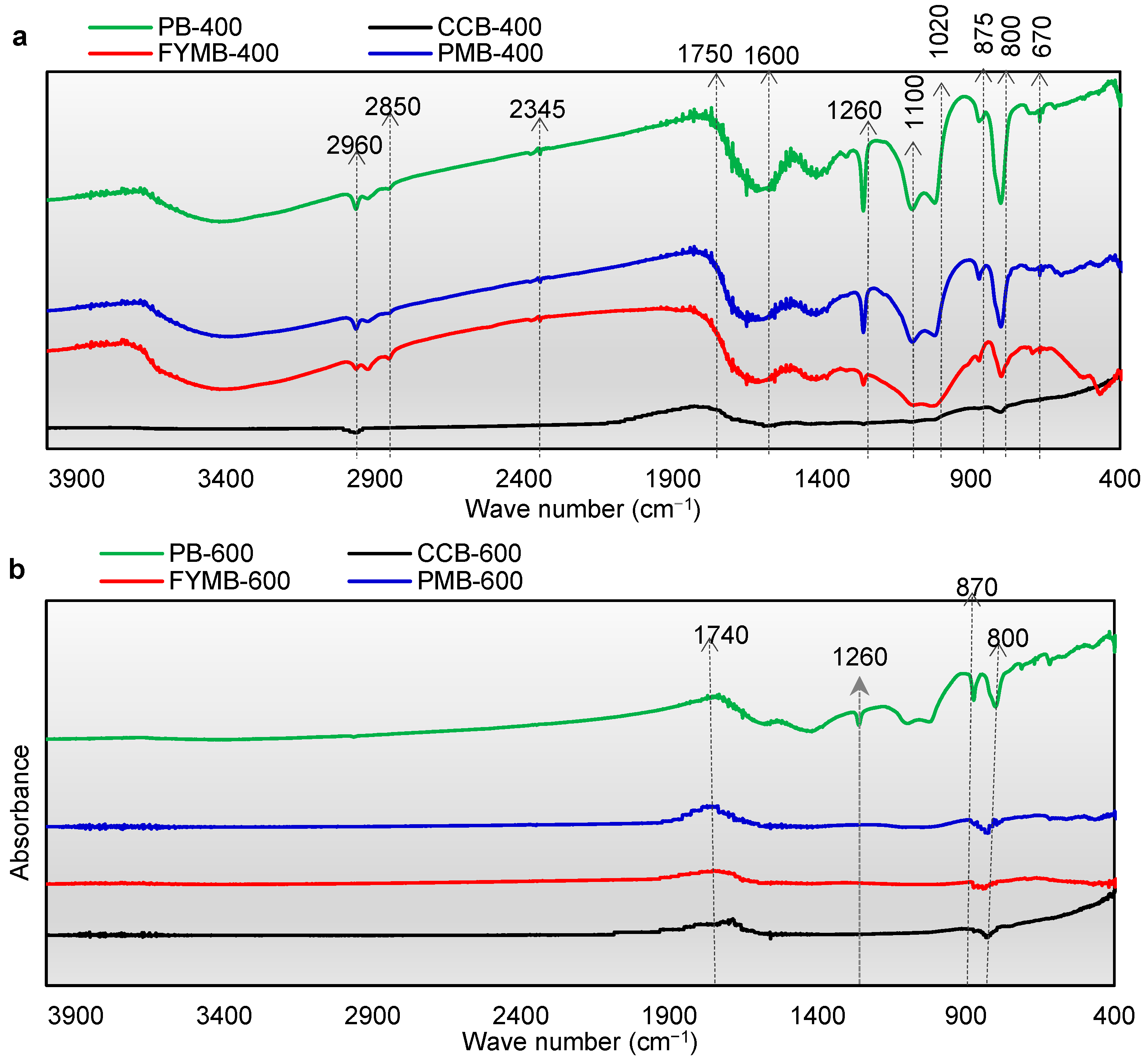
3.3. FTIR Spectra Analysis
3.4. Scanning Electron Microscopy and SSA
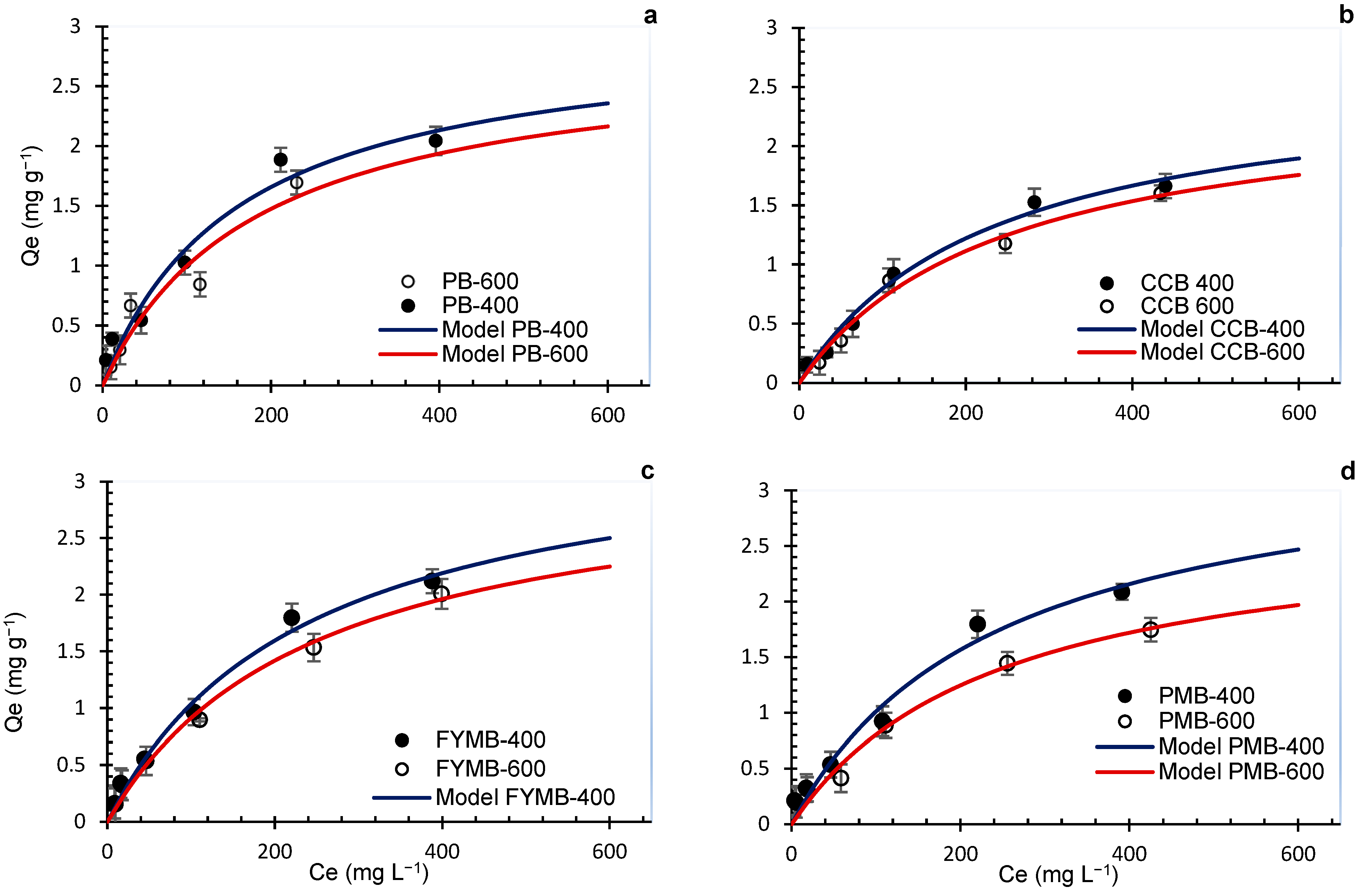
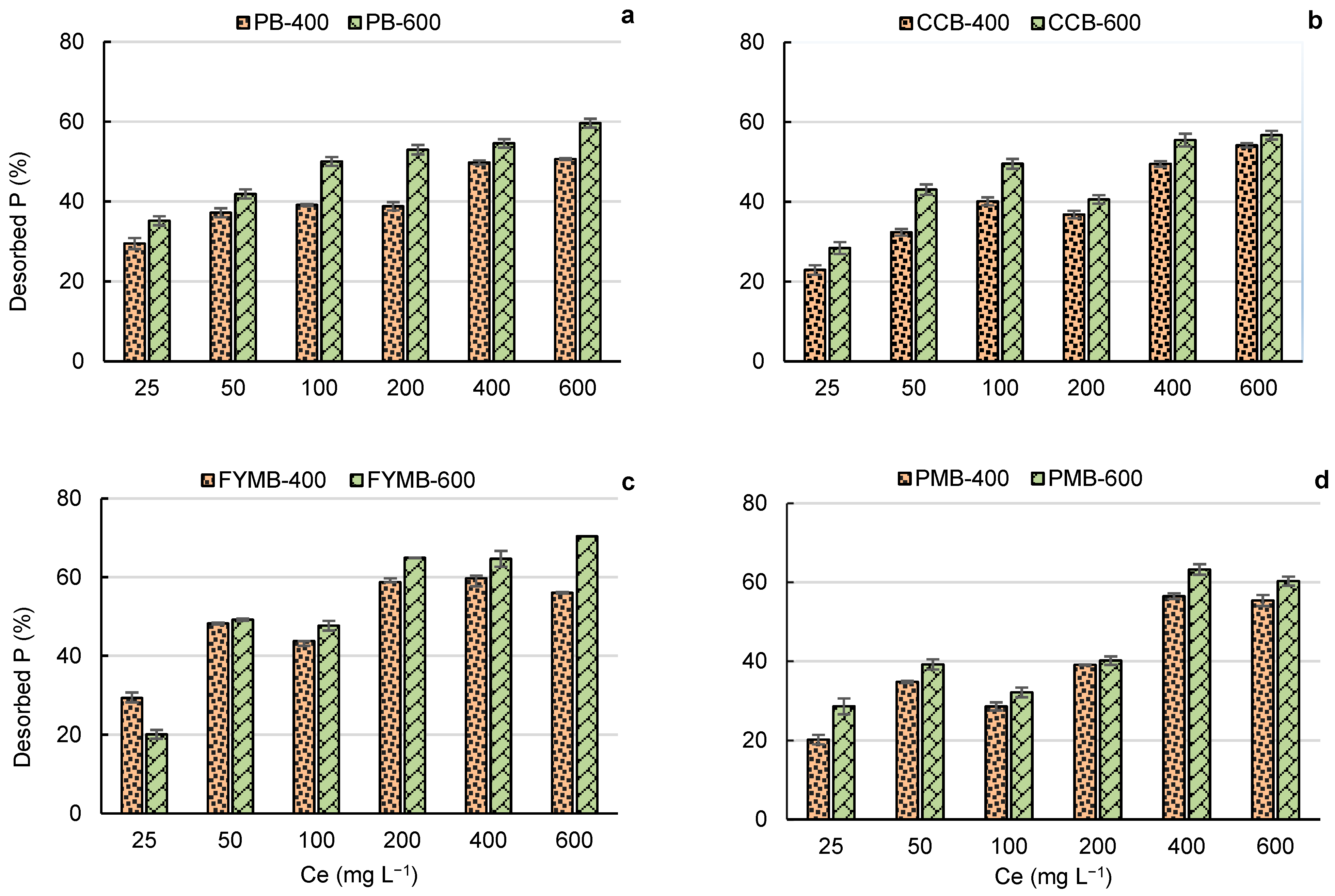
3.5. Effect of Feedstock Type on Phosphorus Sorption and Desorption
3.6. Effect of Pyrolysis Temperature on Phosphorus Sorption and Desorption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Ma, H.; Meng, Y.; Wei, T.; Wang, B. Phosphorus recovery and reuse in water bodies with simple ball-milled Ca-loaded biochar. Sci. Total Environ. 2023, 860, 160502. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.; Ahmad, Z.; Akhtar, N.; Iqbal, A.; Mujeeb, F.; Shakir, M. Role of phosphate solubilizing bacteria (PSB) in enhancing P availability and promoting cotton growth. J. Anim. Plant Sci. 2012, 22, 204–210. [Google Scholar]
- Cordell, D.; White, S. Life’s bottleneck: Sustaining the world’s phosphorus for a food secure future. Annu. Rev. Environ. Resour. 2014, 39, 161–188. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Huo, J.; Zhang, X.; Wen, H.; Zhang, D.; Zhao, Y.; Kang, D.; Guo, W.; Ngo, H.H. Adsorption recovery of phosphorus in contaminated water by calcium modified biochar derived from spent coffee grounds. Sci. Total Environ. 2024, 909, 168426. [Google Scholar] [CrossRef]
- Han, Y.; Choi, B.; Chen, X. Adsorption and desorption of phosphorus in biochar-amended black soil as affected by freeze-thaw cycles in Northeast China. Sustainability 2018, 10, 1574. [Google Scholar] [CrossRef]
- Almanassra, I.W.; McKay, G.; Kochkodan, V.; Ali Atieh, M.; Al-Ansari, T. A state of the art review on phosphate removal from water by biochars. Chem. Eng. J. 2021, 409, 128211. [Google Scholar] [CrossRef]
- Novais, S.V.; Zenero, M.D.O.; Tronto, J.; Conz, R.F.; Cerri, C.E.P. Poultry manure and sugarcane straw biochars modified with MgCl2 for phosphorus adsorption. J. Environ. Manag. 2018, 214, 36–44. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Wang, Y.; An, W.; Jin, J.; Sun, K.; Wang, X. Effects of biochar addition on the abundance, speciation, availability, and leaching loss of soil phosphorus. Sci. Total Environ. 2020, 758, 143657. [Google Scholar] [CrossRef]
- Mukherjee, B.; Ahn, J.; Gruber, S.B.; Chatterjee, N. Respond to “GE-Whiz! Ratcheting Up Gene-Environment Studies”. Am. J. Epidemiol. 2012, 175, 208. [Google Scholar] [CrossRef]
- Qian, T.; Zhang, X.; Hu, J.; Jiang, H. Effects of environmental conditions on the release of phosphorus from biochar. Chemosphere 2013, 93, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Jones, D.L. Biochar effects on soil nutrient transformations. In Biochar for Environmental Management; Routledge: London, UK, 2015; pp. 453–486. [Google Scholar]
- Shepherd, J.G.; Joseph, S.; Sohi, S.P.; Heal, K.V. Biochar and enhanced phosphate capture: Mapping mechanisms to functional properties. Chemosphere 2017, 179, 57–74. [Google Scholar] [CrossRef]
- Tran, H.N.; Tomul, F.; Ha, N.T.H.; Nguyen, D.T.; Lima, E.C.; Le, G.T.; Chang, C.-T.; Masindi, V.; Woo, S.H. Innovative spherical biochar for pharmaceutical removal from water: Insight into adsorption mechanism. J. Hazard. Mater. 2020, 394, 122255. [Google Scholar] [CrossRef] [PubMed]
- Bruun, S.; Harmer, S.L.; Bekiaris, G.; Christel, W.; Zuin, L.; Hu, Y.; Jensen, L.S.; Lombi, E. The effect of different pyrolysis temperatures on the speciation and availability in soil of P in biochar produced from the solid fraction of manure. Chemosphere 2017, 169, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, 00570. [Google Scholar] [CrossRef] [PubMed]
- Reyhanitabar, A.; Frahadi, E.; Ramezanzadeh, H.; Oustan, S. Effect of pyrolysis temperature and feedstock sources on physicochemical characteristics of biochar. J. Agri. Sci. Technol. 2020, 22, 547–561. [Google Scholar]
- Wang, M.; Yan, J.; Xu, Y.; Zhou, X.; Diao, Y.; Wang, H.; Bian, J.; Liu, C.; Quan, G. Mechanochemical modified nitrogen-rich biochar derived from shrimp shell: Dominant mechanism in pyridinic-N for aquatic methylene blue removal. J. Environ. Manag. 2023, 329, 117049. [Google Scholar] [CrossRef] [PubMed]
- Eduah, J.; Nartey, E.; Abekoe, M.; Breuning-Madsen, H.; Andersen, M. Phosphorus retention and availability in three contrasting soils amended with rice husk and corn cob biochar at varying pyrolysis temperatures. Geoderma 2019, 341, 10–17. [Google Scholar] [CrossRef]
- Matin, N.H.; Jalali, M.; Antoniadis, V.; Shaheen, S.M.; Wang, J.; Zhang, T.; Wang, H.; Rinklebe, J. Almond and walnut shell-derived biochars affect sorption-desorption, fractionation, and release of phosphorus in two different soils. Chemosphere 2020, 241, 124888. [Google Scholar] [CrossRef]
- Kumawat, T.K.; Sharma, V.; Kumawat, V.; Pandit, A.; Biyani, M. Chapter 10—Agricultural and agro-wastes as sorbents for remediation of noxious pollutants from water and wastewater. In Sustainable Materials for Sensing and Remediation of Noxious Pollutants; Tyagi, I., Goscianska, J., Dehghani, M.H., Karri, R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 161–176. [Google Scholar]
- Mao, R.; Shabbir, A.; Adkins, S. Parthenium hysterophorus: A tale of global invasion over two centuries, spread and prevention measures. J. Environ. Manag. 2021, 279, 111751. [Google Scholar] [CrossRef]
- Kumar, N.; Aggarwal, N.K. A review on valorization, management, and applications of the hazardous weed Parthenium hysterophorus. Syst. Microbiol. Biomanufacturing 2024, 4, 607–619. [Google Scholar] [CrossRef]
- Cowie, B.W.; Byrne, M.J.; Witkowski, E.T.; Strathie, L.W.; Goodall, J.M.; Venter, N. Parthenium avoids drought: Understanding the morphological and physiological responses of the invasive herb Parthenium hysterophorus to progressive water stress. Environ. Exp. Bot. 2020, 171, 103945. [Google Scholar] [CrossRef]
- Zareen, S.; Khan, N.; Rahman, S. Distributions of invasive weed Parthenium (Parthenium hysterophorus L.) in the University campus Peshawar KPK. Acta Ecol. Sin. 2021, 41, 10–17. [Google Scholar] [CrossRef]
- Kumar, S.; Masto, R.E.; Ram, L.C.; Sarkar, P.; George, J.; Selvi, V.A. Biochar preparation from Parthenium hysterophorus and its potential use in soil application. Ecol. Eng. 2013, 55, 67–72. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, M.; Shimanto, M.H.; Ferdous, J.; Rahman, A.A.-N.S.; Sagor, P.S.; Chowdhury, T. A global analysis on the effect of temperature, socio-economic and environmental factors on the spread and mortality rate of the COVID-19 pandemic. Environ. Dev. Sustain. 2021, 23, 9352–9366. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- ASTM D3172-13(2021)e1; Standard Practice for Proximate Analysis of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2010. [CrossRef]
- Sahin, O.; Taskin, M.; Kaya, E.; Atakol, O.; Emir, E.; Inal, A.; Gunes, A. Effect of acid modification of biochar on nutrient availability and maize growth in a calcareous soil. Soil Use Manag. 2017, 33, 447–456. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil Sci. 1962, 93, 68. [Google Scholar] [CrossRef]
- Brake, J.D. A practical guide for composting poultry litter. Bull.-Miss. Agric. For. Exp. Stn. 1992, 981, 8. [Google Scholar]
- Cerato, A.B.; Lutenegger, A.J. Determination of surface area of fine-grained soils by the ethylene glycol monoethyl ether (EGME) method. Geotech. Test J. 2002, 25, 315–321. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis, Part 1, 2nd ed.; Klute, A., Ed.; ASA and SSSA: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Pastor-Villegas, J.; Durán-Valle, C.J.; Valenzuela-Calahorro, C.; Gómez-Serrano, V. Organic chemical structure and structural shrinkage of chars prepared from rockrose. Carbon 1998, 36, 1251–1256. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Gao, Q.; Gao, Y.; Liu, S. Adsorption of phosphorus by different biochars. Spectrosc Lett. 2017, 50, 73–80. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Chintala, R.; Schumacher, T.E.; McDonald, L.M.; Clay, D.E.; Malo, D.D.; Papiernik, S.K.; Clay, S.A.; Julson, J.L. Phosphorus Sorption and Availability from Biochars and Soil/B iochar Mixtures. CLEAN–Soil Air Water 2014, 42, 626–634. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, K.; Kim, K.-H.; Ok, Y.S.; Tsang, D.C.W.; Tsang, Y.F.; Giri, B.S.; Singh, R.S. Engineered/designer biochar for the removal of phosphate in water and wastewater. Sci. Total Environ. 2018, 616, 1242–1260. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, F.; Zhang, P.; Guo, J.; Sun, H. Aging effect of minerals on biochar properties and sorption capacities for atrazine and phenanthrene. Chemosphere 2018, 206, 51–58. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Pérez, M. The role of biochar porosity and surface functionality in augmenting hydrologic properties of a sandy soil. Sci. Total Environ. 2017, 574, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, T.; Huang, H.; Zhao, D.; Kobayashi, N.; Chen, Y. Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge. J. Anal. Appl. Pyrolysis 2015, 112, 284–289. [Google Scholar] [CrossRef]
- Angın, D. Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef]
- Yue, Y.; Lin, Q.; Xu, Y.; Li, G.; Zhao, X. Slow pyrolysis as a measure for rapidly treating cow manure and the biochar characteristics. J. Anal. Appl. Pyrolysis 2017, 124, 355–361. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S.; Wu, J. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 2010, 89, 510–514. [Google Scholar] [CrossRef]
- Soinne, H.; Hovi, J.; Tammeorg, P.; Turtola, E. Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 2014, 219–220, 162–167. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, K.; Jeong, T.-U.; Ahn, K.-H. Influence of pyrolysis temperature on characteristics and phosphate adsorption capability of biochar derived from waste-marine macroalgae (Undaria pinnatifida roots). Bioresour. Technol. 2016, 200, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Sarkhot, D.V.; Ghezzehei, T.A.; Berhe, A.A. Effectiveness of biochar for sorption of ammonium and phosphate from dairy effluent. J. Environ. Qual. 2013, 42, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater. 2011, 190, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Lawrinenko, M.; Laird, D.A. Anion exchange capacity of biochar. Green Chem. 2015, 17, 4628–4636. [Google Scholar] [CrossRef]
- Yao, W.; Millero, F.J. Adsorption of phosphate on manganese dioxide in seawater. Environ. Sci. Technol. 1996, 30, 536–541. [Google Scholar] [CrossRef]
- Tang, Q.; Shi, C.; Shi, W.; Huang, X.; Ye, Y.; Jiang, W.; Kang, J.; Liu, D.; Ren, Y.; Li, D. Preferable phosphate removal by nano-La (III) hydroxides modified mesoporous rice husk biochars: Role of the host pore structure and point of zero charge. Sci. Total Environ. 2019, 662, 511–520. [Google Scholar] [CrossRef]
- Dugdug, A.A.; Chang, S.X.; Ok, Y.S.; Rajapaksha, A.U.; Anyia, A. Phosphorus sorption capacity of biochars varies with biochar type and salinity level. Environ. Sci. Pollut. Res. Int. 2018, 25, 25799–25812. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Hwang, M.-J.; Ahn, K.-H.; Ok, Y.-S. Kinetic study on phosphate removal from aqueous solution by biochar derived from peanut shell as renewable adsorptive media. Int. J. Environ. Sci. Technol. 2015, 12, 3363–3372. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Biochar derived from anaerobically digested sugar beet tailings: Characterization and phosphate removal potential. Bioresour. Technol. 2011, 102, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Tan, Z.; Zhang, L.; Huang, Q. Preparation of biochar with high absorbability and its nutrient adsorption–desorption behaviour. Sci. Total Environ. 2019, 694, 133728. [Google Scholar] [CrossRef] [PubMed]
| Biochar | Feedstock | Pyrolysis Temperature |
|---|---|---|
| PB-400 | Parthenium biochar | 400 °C |
| CCB-400 | Corn cob biochar | 400 °C |
| FYMB-400 | Farmyard manure biochar | 400 °C |
| PMB-400 | Poultry manure biochar | 400 °C |
| PB-600 | Parthenium biochar | 600 °C |
| CCB-600 | Corn cob biochar | 600 °C |
| FYMB-600 | Farmyard manure biochar | 600 °C |
| PMB-600 | Poultry manure biochar | 600 °C |
| Biochars | pH (1:20) | SSA (m2g−1) | EC (dS m−1) | CEC (cmolc kg−1) | Bulk Density (g cm3) | Porosity (%) |
|---|---|---|---|---|---|---|
| PB-400 | 8.33 ± 0.02 | 719 ± 6.89 | 0.93 ± 0.00 | 68.7 ± 0.43 | 0.27 ± 0.01 | 72.7 ± 2.40 |
| PB-600 | 10.14 ± 0.01 | 361 ± 4.60 | 1.01 ± 0.01 | 53.9 ± 1.70 | 0.59 ± 0.01 | 46.8 ± 4.25 |
| CCB-400 | 6.93 ± 0.02 | 934 ± 3.28 | 0.0 6± 0.00 | 77.6 ± 0.29 | 0.22 ± 0.02 | 76.6 ± 2.55 |
| CCB-600 | 9.87 ± 0.05 | 352 ± 7.81 | 0.33 ± 0.02 | 50.7 ± 0.51 | 0.474 ± 0.02 | 48.5 ± 5.20 |
| FYMB-400 | 8.07 ± 0.03 | 344 ± 3.75 | 0.18 ± 0.00 | 44.4 ± 1.84 | 0.48 ± 0.03 | 58.9 ± 4.02 |
| FYMB-600 | 9.61 ± 0.01 | 238 ± 2.52 | 0.17 ± 0.00 | 41.7 ± 0.95 | 0.54 ± 0.02 | 50.3 ± 2.27 |
| PMB-400 | 8.26 ± 0.01 | 551 ± 5.06 | 0.60 ± 0.00 | 58.8 ± 0.50 | 0.55 ± 0.02 | 53.3 ± 2.11 |
| PMB-600 | 9.87 ± 0.03 | 300 ± 2.71 | 0.44 ± 0.02 | 46.8 ± 0.75 | 0.54 ± 0.02 | 48.4 ± 5.62 |
| Biochars | C (%) | N (g kg−1) | P (g kg−1) | K (g kg−1) | Ca (g kg−1) | Mg (g kg−1) |
|---|---|---|---|---|---|---|
| PB-400 | 64.5 ± 0.21 | 3.11 ± 0.05 | 1.84 ± 0.04 | 50.1 ± 0.25 | 11.4 ± 0.40 | 4.46 ± 0.24 |
| PB-600 | 74.3 ± 0.19 | 2.81 ± 0.02 | 4.42 ± 0.14 | 58.8 ± 0.95 | 19.3 ± 0.24 | 9.94 ± 0.33 |
| CCB-400 | 57.1± 0.34 | 2.91 ± 0.02 | 1.07 ± 0.01 | 12.3 ± 0.35 | 2.72 ± 0.04 | 1.82 ± 0.01 |
| CCB-600 | 74.7 ± 0.12 | 1.91 ± 0.02 | 2.30 ± 0.02 | 18.1 ± 1.02 | 3.73 ± 0.03 | 2.16 ± 0.03 |
| FYMB-400 | 55.3 ± 1.73 | 4.22 ± 0.03 | 1.88 ± 0.02 | 13.0 ± 0.58 | 21.3 ± 0.29 | 10.0 ± 0.59 |
| FYMB-600 | 45.6 ± 0.99 | 3.12 ± 0.03 | 4.44 ± 0.03 | 21.8 ± 0.33 | 39.1 ± 0.67 | 20.3 ± 0.18 |
| PMB-400 | 54.5 ± 1.01 | 6.33 ± 0.03 | 4.46 ± 0.08 | 34.3 ± 0.45 | 20.9 ± 0.54 | 15.1 ± 0.21 |
| PMB-600 | 48.8 ± 0.45 | 4.82 ± 0.02 | 7.24 ± 0.14 | 44.9 ± 0.58 | 40.4 ± 0.13 | 24.8 ± 0.70 |
| Biochar | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| KL | Qmax | R2L | KF | n | R2F | |
| PB-400 | 0.006 | 2.99 | 0.95 | 0.100 | 1.95 | 0.95 |
| PB-600 | 0.005 | 2.82 | 0.94 | 0.072 | 1.79 | 0.92 |
| CCB-400 | 0.004 | 2.62 | 0.98 | 0.066 | 1.84 | 0.95 |
| CCB-600 | 0.004 | 2.47 | 0.98 | 0.029 | 1.51 | 0.95 |
| FYMB-400 | 0.004 | 3.49 | 0.99 | 0.058 | 1.63 | 0.97 |
| FYMB-600 | 0.004 | 3.18 | 0.98 | 0.055 | 1.66 | 0.99 |
| PMB-400 | 0.004 | 3.47 | 0.97 | 0.064 | 1.80 | 0.96 |
| PMB-600 | 0.004 | 2.77 | 0.97 | 0.073 | 1.00 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, N.; Khan, K.S.; Blankinship, J.C.; Ijaz, S.S.; Akram, Z.; Alwahibi, M.S.; Ali, M.A.; Yousra, M. Sorption-Desorption of Phosphorus on Manure- and Plant-Derived Biochars at Different Pyrolysis Temperatures. Sustainability 2024, 16, 2755. https://doi.org/10.3390/su16072755
Musa N, Khan KS, Blankinship JC, Ijaz SS, Akram Z, Alwahibi MS, Ali MA, Yousra M. Sorption-Desorption of Phosphorus on Manure- and Plant-Derived Biochars at Different Pyrolysis Temperatures. Sustainability. 2024; 16(7):2755. https://doi.org/10.3390/su16072755
Chicago/Turabian StyleMusa, Nighet, Khalid Saifullah Khan, Joseph C. Blankinship, Shahzada Sohail Ijaz, Zahid Akram, Mona S. Alwahibi, Mohammad Ajmal Ali, and Munazza Yousra. 2024. "Sorption-Desorption of Phosphorus on Manure- and Plant-Derived Biochars at Different Pyrolysis Temperatures" Sustainability 16, no. 7: 2755. https://doi.org/10.3390/su16072755





