Abstract
Silicon is known to be an effective salt stress attenuator for crops, and evaluating its application effectiveness in combination with other salt stress attenuators is essential for crops and soils. This work aimed to assess whether applying organic matter (OM) and Trichoderma (T) potentiates silicon (Si) in mitigating soil salinization and promoting quinoa growth under salt stress. Quinoa plants were grown in pots under saline irrigation (3.12 dS m−1) and subjected to the following treatments: quinoa only; quinoa + Si; quinoa + Si + OM; quinoa + Si + T; and quinoa + Si + OM + T, at two levels of soil moisture—30 and 80% of the available water content (AWC). Sixty days after transplanting, soil and quinoa plants were collected from the pots. At 80% AWC, Si + OM and Si + OM + T promoted the highest fresh mass for quinoa—301.54 and 247.26 g, respectively. Si + OM + T significantly mitigated saline parameters (EC = 9.82 dS m−1; ESP = 32.27%). Si combined with OM and T was the most effective way to attenuate salt stress in quinoa and soil salinization and promote a more sustainable way to manage saline irrigation in semiarid regions.
1. Introduction
Indications that the global population will exceed 9.5 billion people by 2050 [1] imply a significant increase in the demands for water and food [2]. This represents a substantial challenge for agricultural production in semiarid regions with serious limiting factors for food production, among which water scarcity and salinity are the main ones [3].
In the Brazilian semiarid region, groundwater is commonly used in irrigation to meet the water demand of crops due to the scarcity of good-quality water for irrigation. The drawback is that the vast majority of these waters have high salt contents, mainly high sodium and chlorine concentrations, and represent low-quality water for irrigated agriculture [4]. Consequently, the inadequate management of these waters has been the leading cause of secondary salinization, degrading and decertifying Brazilian semiarid soils [5]. Therefore, to ensure the sustainability of agriculture in several semiarid regions worldwide, it is necessary to find ways to grow crops and minimize the negative impacts of using saline irrigation.
In addition to soil degradation, saline irrigation negatively affects crop growth, production, and quality through several means: a reduction in the net photosynthesis rate [6]; morphological and anatomical changes decreasing fresh and dry matter of plants [7]; nutritional imbalance; osmotic inhibition; and specific saline ion toxicity [1]. Adding salt stress attenuators—nutrients and growth regulators—has received significant attention for attenuating the adverse effects of salt stress in plants [8]. In this sense, silicon (Si) has stood out for being considered a low-cost and easy-to-apply nutrient, serving as an alternative to improve the tolerance of crops to salt stress [9].
The exogenous application of Si has been considered an eco-friendly practice [10], which maintains an optimal K+/Na+ ratio and nutrient balance and regulates the production of reactive oxygen species (ROS) [11], as well as increases the activity of H+-ATPase in the plasma membrane and tonoplast, facilitating the extrusion of Na+ from the cell [12]. Several studies have reported the effectiveness of Si in mitigating salt stress in different crops [13,14], such as quinoa (Chenopodium quinoa Willd.) [15], which is a promising crop for semiarid regions because it is known to be tolerant to water and saline stress [16] and is an important food source due to its high nutritional value [17]. However, most of these studies have focused on the application of silicon in isolation and have yet to test its effectiveness in combination with other salt stress attenuators, and information on its effects on soils is also scarce or non-existent. Thus, evaluating its application effectiveness in combination with other salt stress attenuators is essential for crops and soils.
In addition to crop damage, salinity and sodicity seriously affect soils’ physical and chemical properties, such as an increase in clay dispersion, a reduction in hydraulic conductivity, the water retention capacity, an increase in the percentage of exchangeable sodium, and electrical conductivity [18]. Strategies to recover soils’ physical and chemical properties subjected to saline irrigation, such as manure application, are essential to mitigate the salt accumulation in the root zone and maintain or improve soil attributes to favor crop growth [19,20].
The salt tolerance of crops can also be improved depending on the root-colonizing organisms. Among these, fungi of the Trichoderma genus are commonly found in soils colonizing plant roots [21]. The agricultural use of Trichoderma has demonstrated several benefits for crops, such as promoting root growth [22]; increasing water and nutrient uptake and increasing nutrient utilization efficiency by crops [1]; improving soil structure [23]; and improving plant tolerance when subjected to abiotic stresses, such as drought and salt stress [24].
Studies addressing the production potential of quinoa under abiotic stresses in the Brazilian semiarid regions are still rare and incipient. However, investigating the application of salt stress attenuators, such as silicon alone or in interaction with Trichoderma and organic matter, can promote a hopeful alternative to alleviate some of the harmful effects of salts on quinoa cultivation. However, the attenuators’ effectiveness in relieving salinity’s adverse effects on quinoa must be evaluated under different soil moisture conditions, as in semiarid soils, moisture contents vary very quickly. It is also essential to monitor the saline attributes of soils under the application of Si alone or combined with other saline stress attenuators to verify its effects on the soil’s chemical properties and to guarantee the sustainability of crop production in semiarid regions as a function of the agricultural practices adopted.
Thus, we hypothesize that Si applied with organic matter and Trichoderma potentiates salt stress attenuation on quinoa and is a more effective strategy for attenuating soil salinization due to saline irrigation. The objectives of the present study were (1) to evaluate whether applying organic matter and Trichoderma potentiate silicon to attenuate salt stress in quinoa and favors its growth in different soil moisture contents under saline irrigation and (2) to verify if the application of Si in combination with organic matter and Trichoderma is a more effective strategy for mitigating the impacts of saline irrigation on soil chemical properties. Thus, this study contributes to improving the effectiveness of management strategies for saline stress attenuators on crops and attenuating soil salinity to promote the sustainability of irrigated agriculture in semiarid regions.
2. Materials and Methods
2.1. Experimental Set Up
The present study was carried out in a greenhouse at the Parnamirim Irrigated Agriculture Station—Rural Federal University of Pernambuco (8°08’379” S; 39°57’42” W), located in the municipality of Parnamirim, a semiarid part of the state of Pernambuco, Northeast Brazil. The climate in the region is semiarid and of the BSwh type, according to the Köppen classification. During the experiment, the average temperature inside the greenhouse was 34.9 °C, and the average relative humidity was 42%.
For the experiment, we used Fluvisol—saline soil, according to Richards [25]. The saline soil was collected from the 0–20 cm layer, air-dried, homogenized, and sieved (4 mm sieve) to set up the experiment. Subsequently, the sieved soil was transferred to pots, with 15 kg of soil per pot. The soils’ chemical and physical characteristics are shown in Table 1, measured according to Richards [25] and Embrapa [26].

Table 1.
Chemical properties and textural analysis of the saline soil used in the experiment.
2.2. Treatments and Experimental Design
Quinoa plants (Chenopodium quinoa Willd.)—cultivar BRS Piabiru—were grown in pots (20 L in volume, 37 cm in height, and 32 cm in diameter) on the collected soil (Table 1). The transplantation of quinoa plants occurred 15 days after germination. To verify if the effectiveness of silicon (Si) in attenuating salt stress on quinoa is potentiated, we tested the use of applied Si alone and in association with Trichoderma (Trichoderma harzianum) and organic matter, corresponding to the following mixtures of saline stress attenuator: (A) control (without the application of saline stress attenuator); (B) Si; (C) Si + organic matter; (D) Si + Trichoderma; and (E) Si + organic matter + Trichoderma. Cultivated quinoa plants were grown at two levels of soil moisture to verify the efficacy of the tested salt stress attenuators under low- and high-soil moisture content conditions. In this study, we induced 30 and 80% of the available water content as low and high soil moisture content, respectively. Thus, the experiment had a completely randomized design, a 5 × 2 factorial arrangement, and four replications.
After weighing the pots, quinoa plants were irrigated with saline water sufficient for maintaining moisture levels at 30% and 80% of the soil field capacity. During the entire experimental period, the quinoa plants were irrigated with saline water collected from a well, classified as C4S1 according to Richards [25], every two days, totaling an irrigation interval of 48 h. The chemical characteristics of the saline water used in the experiment are shown in Table 2.

Table 2.
Chemical characteristics of the saline water used in the experiment.
The source of Si used in the study was potassium silicate—K2SiO3—at a dose of 10 mL of K2SiO3 L−1. The Si applications were made using soil—at the transplantation and 20 days after transplanting—and foliar, at intervals of 15 days after transplanting, in a total of 6 applications. The fungus T. harzianum was obtained from the commercial product Trichodermil SC 1306—Strain ESALQ-1306—and was applied to the soil during transplantion and 20 days after transplanting, at a concentration of 2 × 109 viable conidia m L−1, at a dose equivalent to 1 L ha−1. The source of organic matter used was bovine manure (Table 3), applied at 60 t ha−1, representing the dose used in the study region.

Table 3.
Chemical characteristics of the organic matter (goat manure) used in the experiment.
2.3. Quinoa Growth and Shoot Ions Accumulation
The experiment was conducted between August and November 2021. Sixty days after transplanting, the quinoa plants were collected from the pots, and the fresh mass of the aerial part was weighed. In our study, salt stress on quinoa was evaluated concerning the relative biomass of quinoa, which was calculated using the ratio between the biomass of the plant produced under the applications of Si treatments (alone and in association with T. harzianum and organic matter) and the biomass of the plant obtained by the control (without Si alone or in association) at the two moisture levels studied:
where RB = relative biomass of quinoa plants; SFMx = shoot fresh matter at x treatment; and SFMc = shoot fresh matter at control treatment.
RB (%) = [(SFMx/SFMc) × 100]
Subsequently, the collected quinoa plants were deposited in a forced circulation oven at 65 °C until they reached constant weight for element measurement. The aerial part of the dried quinoa was ground in a Wiley-type mill, and 0.05 g was weighed to determine the elements Na+ and K+. We added 10 mL of distilled water and boiled it in a water bath for one hour. The extracts were filtered, and the Na+ and K+ were measured via flame emission photometry [27]. An accurate 0.1 g of ground dry matter was weighed to determine Cl−, and 10 mL of distilled water was added, followed by boiling. After cooling the extract, we used 5 mL of the extract to determine the Cl− in quinoa plants. We added 1 mL of K2CrO4 solution at 50 g L−1 to the extract and titrated it with AgNO3 solution at 0.0125 mol L−1 [28].
2.4. Soil Chemical Analysis
The collected soil samples were air-dried and passed through a 2 mm mesh sieve for chemical analyses, which were performed according to Richards [25]. The soil pH was determined in a soil–water suspension of 1:2.5 directly from the pH meter. Exchangeable Na+ and K+ were extracted with ammonium acetate solution (1 mol L−1) at pH 7 after pre-washing the salts and then measured via flame emission photometry. The CEC was measured through extraction with sodium acetate (1 mol L−1) at pH 8.2 and measured via flame emission photometry. The percentage of exchangeable sodium (ESP) was calculated using the ratio of exchangeable Na+ (cmolc kg−1) to CEC (cmolc kg−1), according to the following equation [25]:
ESP (%) = Na+/CEC × 100.
We prepared the saturated paste extract to determine electrical conductivity and soluble elements—Na+, K+, and Cl−. For this, the saturated paste extract was obtained by adding distilled water to 500 g of soil with the material mixture until complete saturation was reached. The electrical conductivity of the paste extract (at 25 °C) was measured directly using a conductivity meter (model Digimed DM-32) with a relative precision of 0.05%. Na+ and K+ were measured via flame emission photometry, and Cl− was measured via titration with AgNO3−.
2.5. Statistical Analysis
The data were statistically analyzed using SPSS software (SPSS 16.0 for Windows, IBM Company, Inc., Chicago, IL, USA). We used Kolmogorov–Smirnov and Levene tests to verify the assumption of normality and homogeneity of the variance regarding the experiment’s studied parameters. The tests indicated that the variance and normal distribution were homogeneous for all investigated cases.
Plant and soil parameters responding to the tested treatments were analyzed as dependent variables, while moisture levels and saline attenuators were fixed factors. A two-way analysis of variance (ANOVA), at a significance level of p < 0.05 followed by Tukey’s test for a comparison of the means, was used to evaluate if the plant and soil parameters were affected by the tested treatments.
3. Results
The two-way ANOVA test examined the effect of the two studied factors (salt stress attenuators and available water content) on plant and soil parameters (Table 4). According to the obtained data, it was notable that the interactive effect between salt stress attenuators and the available water content significantly influenced the plant and soil parameters, indicating significant differences (p < 0.05) for all studied variables. Thus, in this study, we emphasized the results obtained based on the interactive effect. This finding implies that the effectiveness of Si, when applied alone or in combination with other salt stress attenuators, is strongly influenced by the available water content.

Table 4.
Summary of the two-way variance analysis of the studied plant and soil parameters.
3.1. Quinoa Growth and Shoot Ion Accumulation
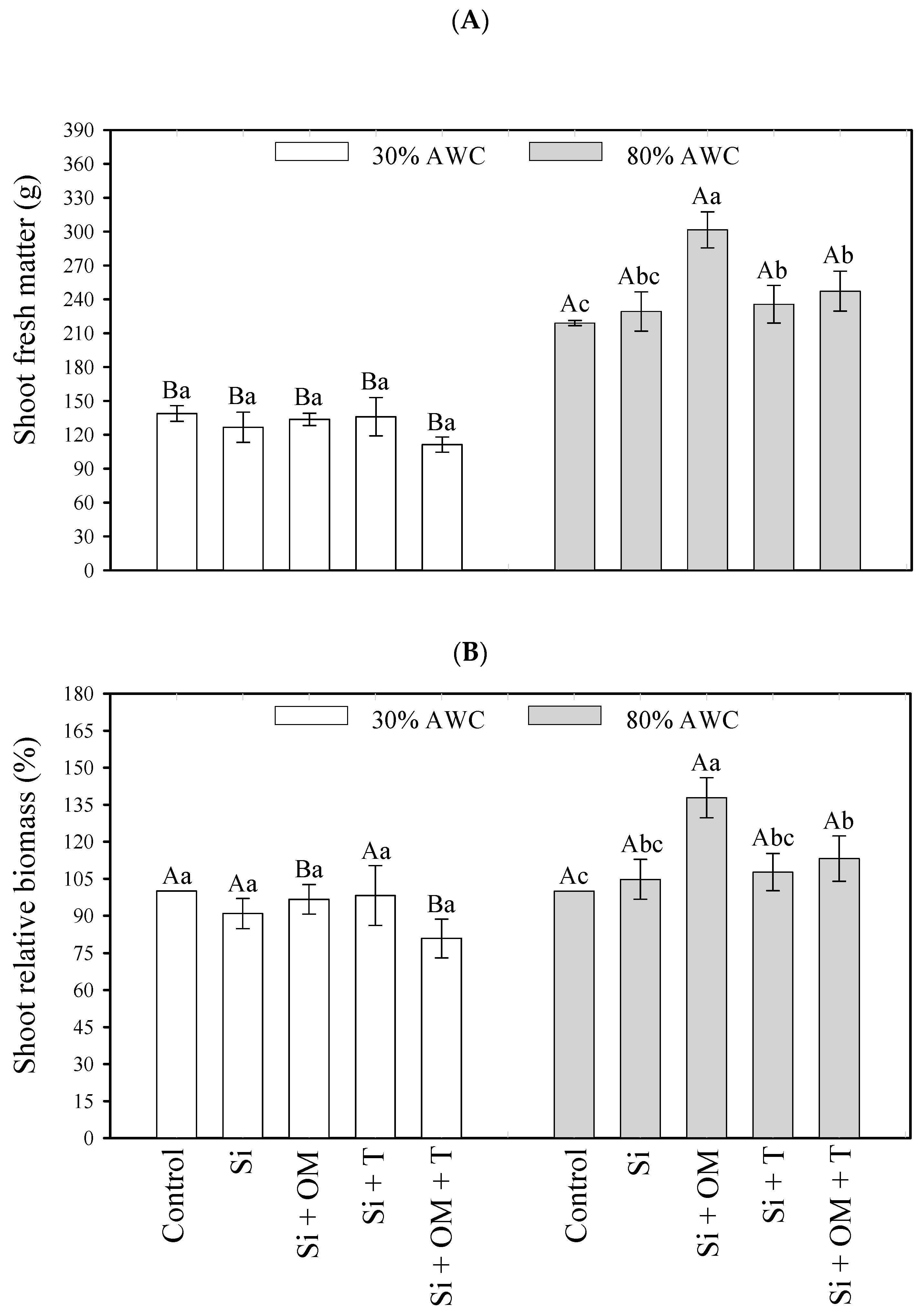
The interactive effect between saline stress attenuators and available water content on quinoa plant growth is shown in Figure 1. Applying salt stress attenuators at 80% AWC promoted significant increases (p < 0.05) in the growth of quinoa, as observed by the increases in shoot fresh matter and relative biomass concerning the control (Figure 1). Saline stress attenuators were ineffective at low soil available water contents (30% AWC).
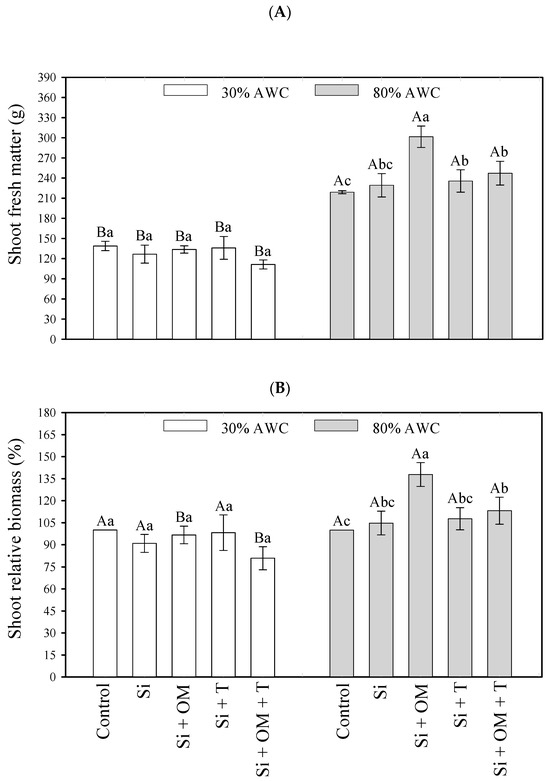
Figure 1.
Interactive effects of salt stress attenuators and available water content on quinoa growth parameters—shoot fresh matter (A) and shoot relative biomass (B). Capital letters indicate the growth plant parameters between the available water content for each salt stress attenuator. Lowercase letters indicate the plant growth parameters between the salt stress attenuators for each amount of available water content (AWC). Different letters represent significant differences among treatments based on the Tukey test (p < 0.05). Si = Silicon; OM = Organic Matter; T = Trichoderma.
At 80% AWC, the Si + OM attenuators obtained the highest fresh mass values with a production of 301.54 g, followed by Si + OM + T (247.26 g) and Si + T (235.68 g) (Figure 1A). Regarding the relative biomass, the attenuator Si + OM was superior to the others, promoting an increase of 37.81% in the biomass of quinoa and an increase of 13.11% when applied Si + OM + T, at 80% AWC (Figure 1B).
Soil water content had a significant effect on quinoa growth, promoting an increase in fresh mass from 138.93 g (30% AWC) to 218.97 g (80% AWC) for the control, while with the application of Si + OM, the fresh mass gain went from 133.64 g (30% AWC) to 301.54 g (80% AWC) (Figure 1A). Still, in comparing the water availability, it was evidenced that in the highest water availability (80% AWC), where organic matter was applied (Si + OM, Si + OM + T), the relative biomass yield increased (Figure 1B).
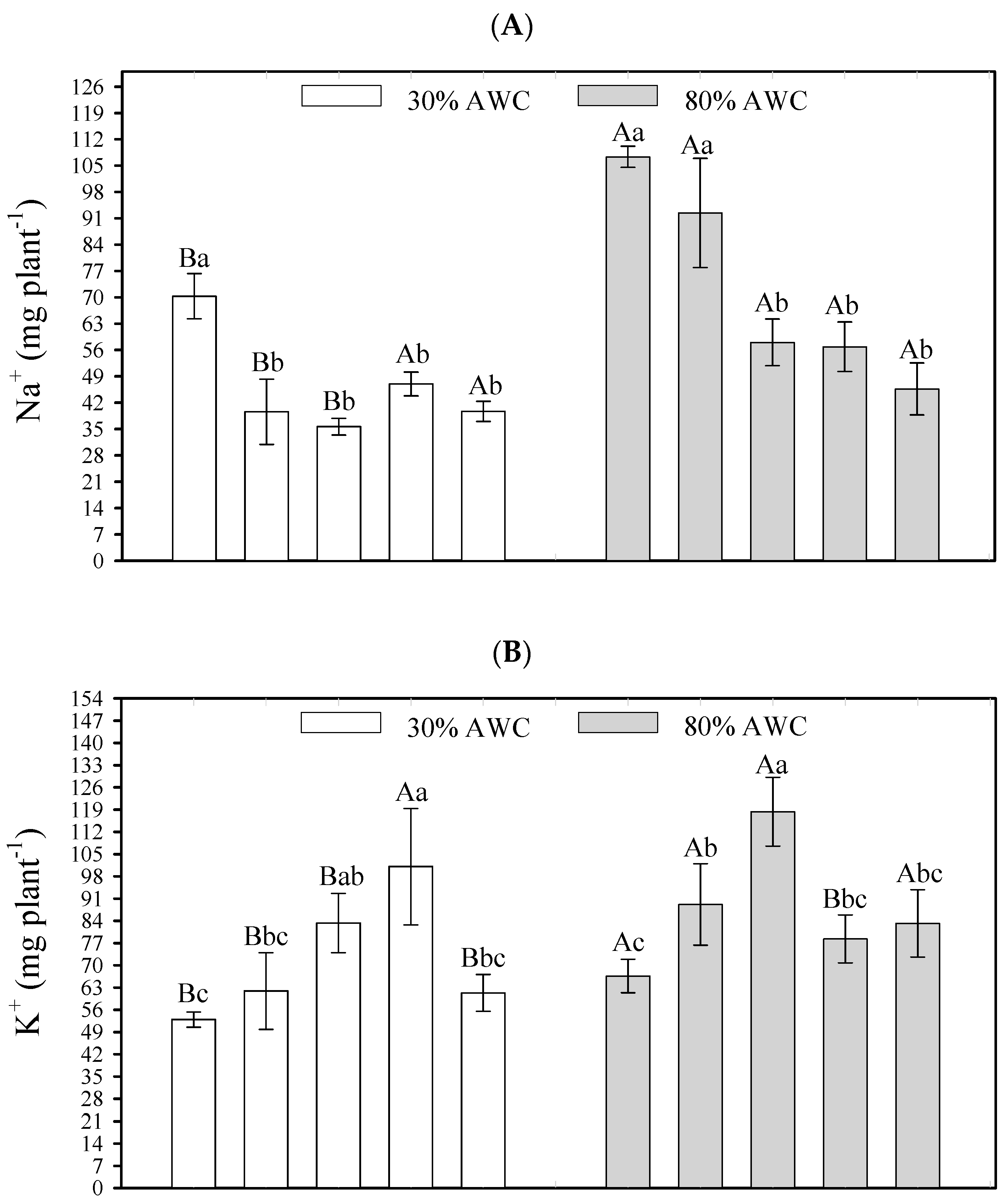
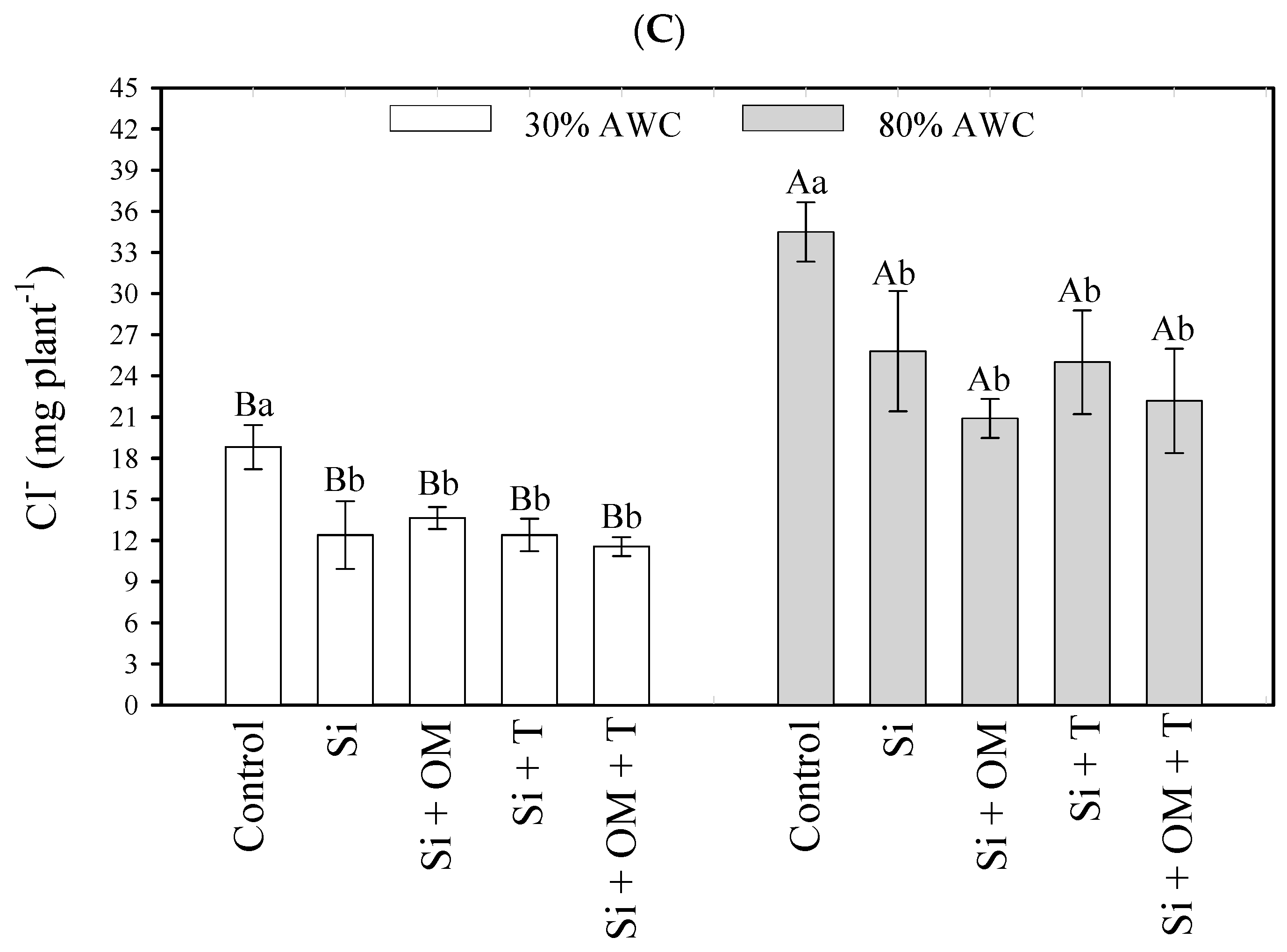
Element content in the plant was significantly influenced (p < 0.05) by the interaction of salt stress attenuators with different available soil water contents. A higher available water content in the soil promoted a greater absorption of the elements Na+, K+, and Cl−, concerning the lower available water content—30% (Figure 2). Na+ content gains in quinoa shoots ranged from 35.62 mg to 58.03 mg when Si + OM was applied and from 70.29 mg to 107.55 mg in the control. Using Si + T and Si + OM + T did not promote increased Na+ content with increased soil water availability (Figure 2A). For K+, the gains were from 52.93 mg to 66.62 mg (control) and 83.31 mg to 118.36 mg (Si + OM) (Figure 2B). Regarding Cl−, the increases ranged from 13.64 mg to 20.90 mg (Si + OM) and from 18.81 mg to 34.49 mg (control) (Figure 2C).
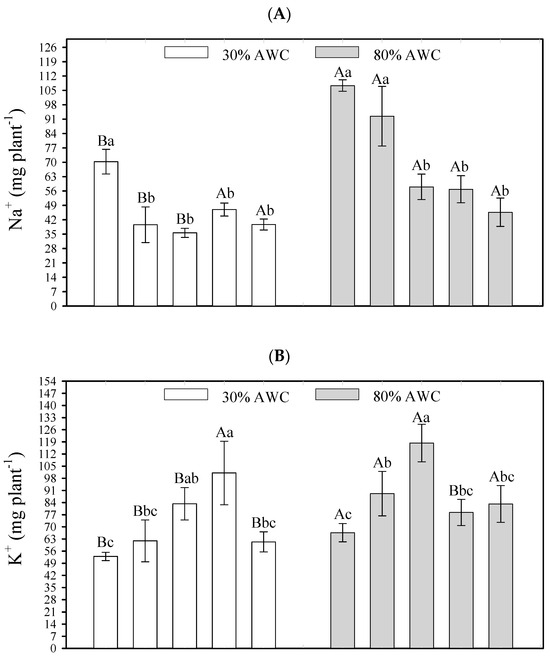
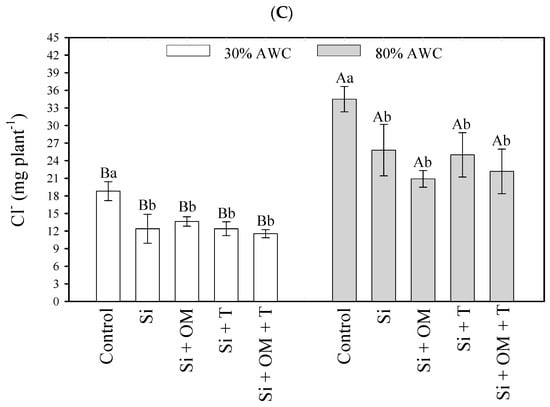
Figure 2.
Interactive effects of salt stress attenuators and available water content on element contents in shoot quinoa plants—Na (A), K (B), and Cl (C). Capital letters indicate the growth plant parameters between the available water content for each salt stress attenuator. Lowercase letters indicate the plant growth parameters between the salt stress attenuators for each available water content (AWC). Different letters represent significant differences among treatments based on the Tukey test (p < 0.05). Si = Silicon; OM = Organic Matter; T = Trichoderma.
At 30% AWC, all saline stress attenuators had a significant effect on reducing the quinoa’s Na+ absorption (Si + OM—35.62 mg, Si—39.59 mg, Si + OM + T—39.69 mg, Si + T—46.98 mg) when compared to the control (70.29 mg). At 80% AWC, except for Si, the other saline stress attenuators had a significant effect on reducing the quinoa′s Na+ absorption (Si + OM + T—45.68 mg, Si + T—56.82 mg, Si + OM—58.03 mg) when compared to the control (107.55 mg) (Figure 2A).
For K+, at 30% AWC, the attenuators Si + T and Si + OM promoted greater element absorption, presenting a content of 101.08 and 83.31 mg, respectively, compared to the control (52.93 mg). At 80% AWC, the Si + OM and Si attenuators promoted a greater K+ absorption—118.36 and 89.14 mg, respectively—compared to the control (66.62 mg) (Figure 2B).
Regarding Cl− in both conditions of soil moisture, all stress attenuators significantly reduced the quinoa’s absorption of the element compared to the control (Figure 2C). At 30% AWC, the control quinoa plants obtained a content of 18.81 mg of Cl−, while the quinoa plants cultivated with Si + OM + T, Si, Si + T, and Si + OM obtained 11.55, 12.39, 12.39, and 13.64 mg of Cl−, respectively. At 80% AWC, the Cl− content was as follows—control (34.49 mg), Si (25.80 mg), Si + T (25.00 mg), Si + OM + T (22.18 mg), and Si + OM (20.90 mg).
3.2. Soluble and Exchangeable Ions
The concentrations of soluble and exchangeable elements for soil available water content and the saline stress attenuators are in Table 5. We verified that the soluble K+ was higher (p < 0.05) at 30% AWC compared to 80% AWC. In contrast, the concentration of soluble Na+ showed the opposite behavior to that of K+—higher at 80% AWC. For Cl−, it was observed that the available water content in the soil effectively differentiated the concentrations of the ion in solution only in the control, being greater than 80% AWC.

Table 5.
Interactive effects of salt stress attenuators vs. soil available water content on soil exchangeable and soluble element contents.
At 30% AWC, the amendments with organic matter (Si + OM and Si + OM + T) had the highest concentrations of soluble K+, followed by the Trichoderma application (Si + T) and isolated silicon (Si). At 80% AWC, the joint application of Si + OM + T resulted in a higher soluble K+.
In conditions of low soil moisture (30% AWC), the saline stress attenuators did not result in significant differences (p < 0.05) in the concentrations of soluble Na+ and Cl−. In contrast, at 80% AWC, only the Si + OM + T application reduced the concentrations of these soluble elements compared to the control (Table 5).
For the soil exchange complex, soil water content resulted in a significant source of variation (p < 0.05) for Na+ concentrations but not for K+. Na+ concentrations increased when more saline water was added to the soil (80% AWC) (Table 5).
Regarding saline stress attenuators, they were effective in promoting changes in the Na+ and K+ concentrations (p < 0.05). For K+, the behavior was the same regardless of the water content in the soil. Organic matter application (Si + OM + T and Si + OM) resulted in high element concentrations on the soil exchange complex, followed by Si + T and Si. The application of Si + T caused the lower concentration of Na+, followed by Si + OM + T, Si + OM, and Si at 30% AWC. At 80% AWC, only the Si + OM + T and Si + T applications had lower Na+ concentrations than the control (Table 5).
3.3. Soil Salinity and Sodicity Parameters
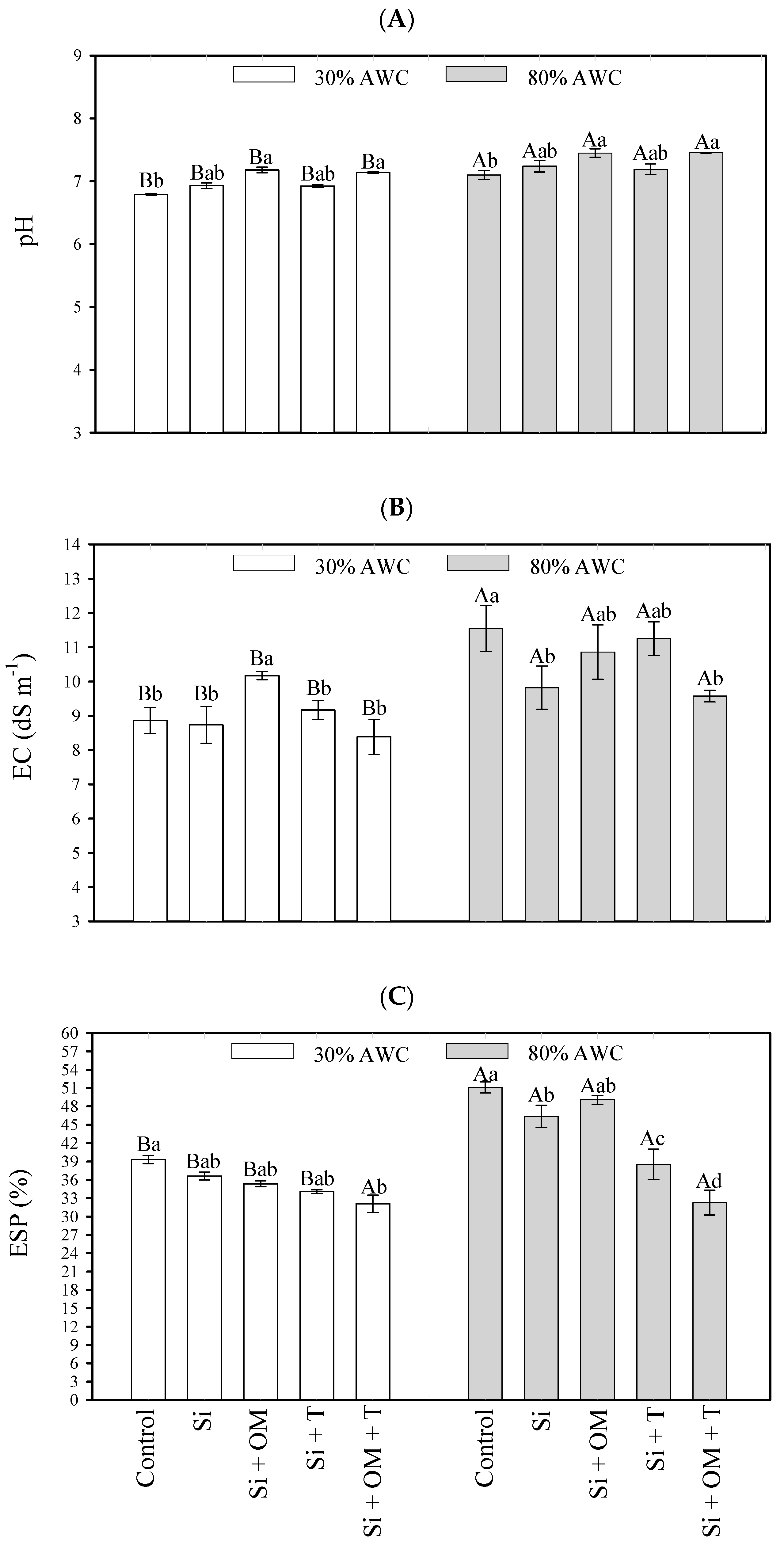
The parameters referring to soil salinity and sodicity (pH, EC, ESP) were significantly influenced (p < 0.05) by the interaction of saline stress attenuators in different contents of available water in the soil (Figure 3).
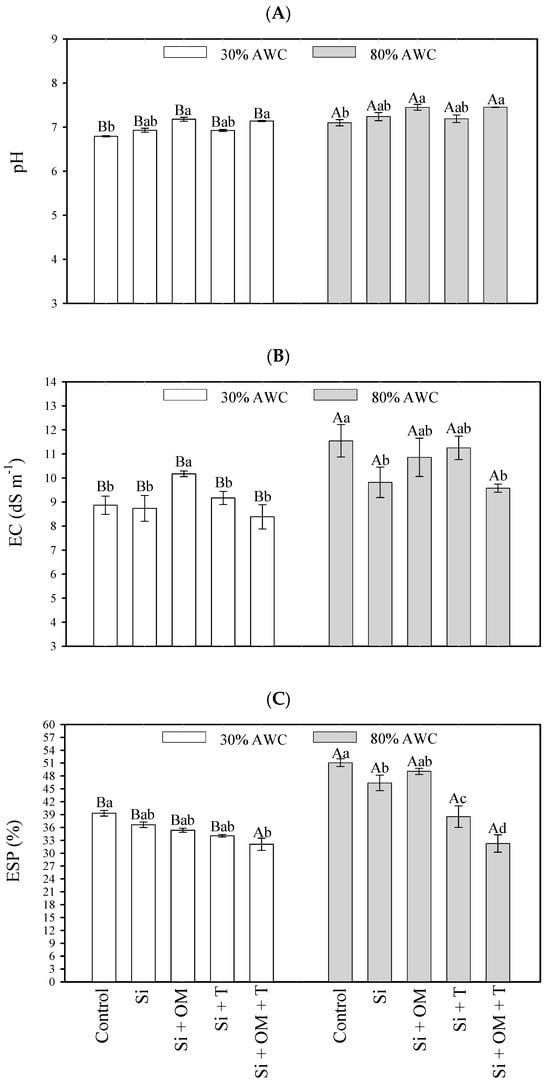
Figure 3.
Interactive effects of salt stress attenuators and available water content on soil salinity parameters—pH (A), electrical conductivity (B), and exchangeable sodium percentage (C). Capital letters indicate the soil salinity parameters between the available water content for each salt stress attenuator. Lowercase letters indicate the soil salinity parameters between the salt stress attenuators for each available water content (AWC). Different letters represent significant differences among treatments based on the Tukey test (p < 0.05). Si = Silicon; OM = Organic Matter; T = Trichoderma.
An 80% AWC promoted an increase in all variables compared to 30% AWC. The pH increased from 6.79 to 7.1 in the control and from 7.14 to 7.45 for Si + OM + T. For EC, this increase ranged from 8.38 to 9.57 dS m−1 due to the Si + OM + T application and from 8.87 to 11.54 dS m−1 for the control treatment, which had the most significant increase for EC. The increases in ESP ranged from 34.08 to 38.52% for Si + T, and the most significant increase occurred in the control, ranging from 39.32 to 51.08%. Only the application of Si + OM + T did not promote an increase in ESP with the rise in the soil’s water content.
In comparing salt stress attenuators vs. the control, it was observed that the Si + OM and Si + OM + T applications had gains in pH values at both humidities (30% AWC—7.18 and 7.14; 80% AWC—7.45 and 7.45, respectively) (Figure 3A). Under low water contents (30% AWC), the Si + OM application increased soil EC values (10.17 dS m−1) concerning the control treatment (8.87 dS m−1). The results of other attenuators did not differ significantly from each other. At 80% AWC, the Si and Si + OM + T applications promoted the lowest average values for soil EC, 9.57 and 9.82 dS m−1, respectively, compared to the control treatment (11.54 dS m−1) (Figure 3B).
Regarding ESP, at 30% AWC, the Si + OM + T, Si + T, and Si + OM attenuators promoted the lowest mean values (32.09, 34.08, and 35.35%, respectively), and the control, the highest (39.32%). At 80% AWC, the Si + OM + T, Si + T, and Si attenuators had the lowest ESP values (32.27, 38.52, and 46.38%) compared to the control (51.08%) (Figure 3C).
4. Discussion
4.1. Quinoa Growth and Nutritional Status
Water stress harms quinoa, decreasing plant biomass. Maestro-Gaitán et al. [29] studied four quinoa cultivars, and they found that when quinoa was cultivated with 75% soil water available, fresh mass production was more significant than when grown with 35% soil water available, where the reductions in leaf biomass, stems, and seeds, also demonstrated this effect. Manaa et al. [30] also found reduced quinoa growth under water stress.
Losses in quinoa growth occur due to water stress, which promotes decreases in chlorophyll content, leaf osmotic, and water potential and induces significant changes in the chloroplast organization. The stress decreases the growth of the quinoa mainly by reducing leaf expansion and net photosynthesis. However, it is noteworthy that when subjected to stress recovery irrigation, the loss of structural integrity and chloroplast arrangement disorder are quickly recovered, suggesting that quinoa can withstand drought conditions well [30].
Under adequate soil moisture conditions (80% AWC), saline stress attenuators increased the fresh mass production in quinoa, mainly with the organic matter application (Si + OM). Rekaby et al. [31] studied organic compost and humic acid application in quinoa, and they also observed that plant growth variables increased. The authors justify that the organic matter addition mitigates the harm of salts as a direct effect and that these compounds help the plant to tolerate salts through metabolic changes. The organic compounds added to salt-affected soils reduce salt stress and improve plant growth by increasing nutrient availability, water retention, soil structure, and microorganism bioactivity [32,33]. The Trichoderma applicationd (Si + T and Si + OM + T) increased fresh mass, which may be associated with the fungus’s ability to improve soil nutrient availability by secreting organic acids into the rhizosphere [34].
The toxic ion accumulation in the tissues under salt stress can affect the total plant biomass. What can be observed in this work is that the treatments that promoted a more significant production of fresh mass (Si + OM, Si + T, and Si + OM + T) are the same ones that favored a lower toxic ions absorption—Na+ and Cl−. At the same time, the lower fresh mass production (control) is due to higher absorptions of Na+ and Cl− instead of K+. It is essential to realize that in quinoa grown at 80% AWC, there was a higher salt entry into the soil, mainly Na+ and Cl−, due to the irrigation water being rich in these ions and having the capacity to salinize the soil. In addition, even with a higher soil salt concentration, the salinity attenuators effectively controlled the salt effect stress, decreasing the plants absorption of Na+ and Cl−. In other research, Silva et al. [35], by testing the same attenuators and combinations as this study in sorghum (Si + OM, Si + T, and Si + OM + T), showed that the Si combinations reduced toxic ion absorption (Na+ and Cl−) and favored nutrient ion absorption (K+), resulting in significant gains in biomass.
Potassium is a many-cytoplasmic enzyme activator necessary for photosynthesis and respiration. Still, as Na+ and K+ ions have similar physicochemical properties, they compete for the primary binding sites in these essential metabolic processes in the cytoplasm [36]. Therefore, K+ deficiency suppresses photosynthesis and reduces plant growth. Increasing K+ uptake alleviates the harmful effects of salinity [37]. In addition, K+ is an essential ion for maintaining and creating turgor pressure and adjusting the plant’s water balance [36]. Even when quinoa is cultivated with low soil available water content (30% AWC), salt stress attenuators effectively decreased the Na+ and Cl− absorption and increased the K+ absorption of the quinoa plants, with a low fresh mass production associated with multiple stress occurrences (water + salt).
Silicon (Si) and organic matter (Si + OM) applications promoted significant K+ absorption. The Si source was potassium silicate, which may explain this increase in K+ absorption. However, Si applied in isolation did not reduce the plant Na+ absorption, requiring further studies with different doses or other Si sources. Regarding OM, as previously discussed, OM is a nutrient source, increasing its availability and promoting other soil improvements that may justify the better absorption of this element by quinoa.
Terletskaya et al. [38] evaluated the influence of osmotic and saline stress on quinoa growth and found that a saline level between 100 and 200 mM NaCl was not crucial for its development. Still, with increasing salinity (300 mM NaCl), biomass was reduced due to higher Na+ absorption in the aerial part of the plant. This corroborates the results found in this work, as quinoa cultivated without adding salt stress attenuator (control) absorbed more Na+ and reduced its fresh mass production. The Na+ abundance in a saline environment results in its greater root uptake, increasing its concentration and reducing the other ions concentration, mainly K+, in the plant [36].
4.2. Soil Properties
Regarding elements’ content in the soil (soluble and exchangeable), the reduction in soluble K+ in the highest available water content occurred as a consequence of the increase in soluble Na+, which presented higher levels for the highest available water content (80% AWC), due to the water quality used in the experiment, which had a high range of this element (Table 2). As these elements present antagonistic interactions on soil [39], increasing soluble Na+ reduces soluble K+. Although no differences were verified for Cl−, according to the data obtained in our study, it was evident that greater amounts of this saline water tend to increase Cl− accumulation. As there was no drainage in the experiment, this contributed to the fact that the application of this water in more significant amounts resulted in higher contents of soluble Na+ and Cl−.
In this study, we verified that the exchangeable Na+ content also increased significantly for the highest available soil water content (80%) due to the application of higher saline irrigation depths. Na+ in saline water passes to the soil solution, increasing its levels. Increasing the concentration of this element in the soil solution promotes its passage to the exchangeable phase of the soil, thus increasing the levels of exchangeable Na+ [40].
The highest levels of elements verified for the highest available water content in the soil (80%) increased the soil pH values, electrical conductivity, and percentage of exchangeable sodium. According to Black and Campbell [41], more ions in the soil solution increase the soil solution’s ionic strength and thus its electrical conductivity values—soil salinization. On the other hand, as exchangeable Na+ is directly related to the percentage of exchangeable sodium [42], increasing its exchangeable levels, promoted by 80% AWC, resulted in higher ESP values. Increases in soil pH through saline irrigation occur because of the imbalance in the ionic composition due to sodium addition [43], and the predominance of carbonate and bicarbonate ions and hydroxide compounds, contributing to the increase in the soil pH [44]. Thus, the increase in pH observed at 80% AWC also occurred due to a greater contribution of HCO32− ions, which is present in the composition of saline water used for irrigation (Table 2).
In the Brazilian semiarid region, saline waters with high levels of Na+ and Cl− are commonly used to irrigate crops grown in soils without drainage systems [5]. As a consequence, increasing soil pH and electrical conductivity values and increasing exchangeable Na+ and ESP tend to deteriorate soil physical properties, such as its structure and permeability [18]. This represents the need for studies that assess plant growth and the effects on soil properties by using saline water for irrigation to find sustainable management strategies for using these waters.
Applying saline stress attenuators effectively increased the soluble and exchangeable K+ levels compared to the control, where no source of K+ was used. As there was a combined application of potassium silicate through the soil and application of organic matter, which were both sources of K+, this contributed to the increases in its soluble and exchangeable contents. In addition, Trichoderma is efficient in solubilizing nutrients and improving soil fertility and nutrient utilization efficiency of plants [45]. Significant reductions verified for the exchangeable Na+ contents indicate that Si, organic matter, and Trichoderma applications tend to regulate the Na+ balance in the soil, attenuating its adsorption in the exchange complex in higher available water contents (80% AWC), and consequently reducing the ESP values.
Our results show, notably, that where saline stress attenuators—isolated or combined with silicon—were applied, soil pH increased, and significant reductions in electrical conductivity and the percentage of exchangeable sodium occurred. Some studies have shown increases in soil pH values due to silicon source applications [46,47,48,49]. According to Haynes [50], OH− ions are released during the dissolution of silicate fertilizers, contributing to the increase in soil pH.
The most significant differences for the variable electrical conductivity, verified with a higher content of available water in the soil (80% AWC), indicate a greater effectiveness of the saline stress attenuators in reducing soil salinity under conditions of a higher soil moisture content. The results verified in our study for EC values also suggest that saline stress attenuators are ineffective in promoting reductions in soil salinity at low soil available water content (30% AWC). Khan et al. [51] stated that exogenous silicon regulates electrical conductivity. In the present study, the salt stress attenuators were applied with silicon, alone or in combination. Thus, our findings are in agreement with those of previous studies [52,53], which have also found that exogenous silicon application reduces soil electrical conductivity and favors crop growth.
5. Conclusions
The data obtained in the present study allow us to conclude that although the increase in the available water content in the soil increased the electrical conductivity, the Na+ content (exchangeable and soluble), and the exchangeable Na+ percentage, 80% of the available water content is the moisture content that provides the most significant growth of quinoa under saline irrigation.
In the future, additional field-scale investigations that verify the effectiveness of these salt stress attenuators under quinoa growth and yield using drainage systems are essential for a better assessment of the use of these waters on soil properties and thus for establishing sustainable soil and saline water management strategies.
The tested saline stress attenuators’ application favored quinoa growth subjected to saline irrigation only in conditions of higher soil moisture contents (80% AWC). However, their applications should be recommended due to their effectiveness in reducing the absorption of elements (Na+ and Cl−) by quinoa plants under low and high soil water content conditions.
The present study’s results reveal that the application of silicon combined with organic matter and Trichoderma Harzianum is more effective in mitigating salinity’s harmful effects on quinoa by reducing Na+ and Cl− contents in soils and attenuating soil salinization and sodification through the use of saline irrigation. Our study suggests that applying silicon combinations promotes a more sustainable way of managing saline irrigation in semiarid regions.
Author Contributions
Conceptualization, E.M.d.A. and L.G.M.P.; methodology, E.M.d.A., L.G.M.P. and M.B.G.d.S.F.; field work and laboratory analyses, E.M.d.A., J.O.N.d.S. and L.F.d.S.S.; data curation, M.B.G.d.S.F. and A.C.d.O.; writing—original draft, E.M.d.A., L.G.M.P. and E.M.d.S.; writing—review and editing, J.O.N.d.S.; supervision, E.L.d.N.A. and L.G.M.P.; funding acquisition, L.G.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development—CNPQ Brazil) and obtained through the Call CNPq/MCTI/FNDCT nº 18/2021 (UNIVERSAL), Track A—Emerging Groups (Project Number: 409937/2021-5).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors acknowledge the Embrapa—Empresa Brasileira de Pesquisa Agropecuária, Brasília—for supplying the quinoa seeds used in this study—BRS Piabiru.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Daliakopoulos, I.N.; Apostolakis, A.; Wagner, K.; Deligianni, A.; Koutskoudis, D.; Stamatakis, A.; Tsanis, I.K. Effectiveness of Trichoderma harzianum in soil and yield conservation of tomato crops under saline irrigation. CATENA 2019, 175, 144–153. [Google Scholar] [CrossRef]
- Ragab, R.; Battilani, A.; Matovic, G.; Stikic, R.; Psarras, G.; Chartzoulakis, K. SALTMED model as an integrated management tool for water, crop, soil and N-Fertilizer water management strategies and productivity: Field and simulation study. Irrig. Drain. 2015, 64, 13–28. [Google Scholar] [CrossRef]
- Talebnejad, R.; Sepaskhah, A.R. Effect of different saline groundwater depths and irrigation water salinities on yield and water use of quinoa in lysimeter. Agric. Water Manag. 2015, 148, 177–188. [Google Scholar] [CrossRef]
- Silva Júnior, L.G.A.; Gheyi, H.R.; Medeiros, J.F. Composição química de águas do cristalino do nordeste brasileiro. Rev. Bras. Eng. Agrícola e Ambient. 1999, 3, 11–17. [Google Scholar] [CrossRef]
- Pessoa, L.G.M.; Freire, M.B.G.S.; Green, C.H.M.; Miranda, M.F.A.; Filho, J.C.A.; Pessoa, W.R.L.S. Assessment of soil salinity status under different land-use conditions in the semiarid region of Northeastern Brazil. Ecol. Indic. 2022, 141, 109139. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in portulaca oleracea. Plants 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Juraimi, A.S.; Rafii, M.Y.; Abdul Hamid, A. Effect of salinity on biomass yield and physiological and stem-root anatomical characteristics of purslane (Portulaca oleracea L.) accessions. BioMed Res. Int. 2015, 2015, 105695. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.V.; Silva, T.I.D.; Lopes, M.D.F.D.Q.; Leal, M.P.D.S.; Basilio, A.G.S.; Leal, Y.H.; Dias, T.J. Salinity stress and plant growth regulator in basil: Effects on plant and soil. DYNA 2021, 88, 75–83. [Google Scholar] [CrossRef]
- Thorne, S.J.; Hartley, S.E.; Maathuis, F.J.M. Is silicon a Panacea for alleviating drought and salt stress in crops? Front. Plant Sci. 2020, 11, 543584. [Google Scholar] [CrossRef]
- Dhiman, P.; Rajora, N.; Bhardwaj, S.; Sudhakaran, S.S.; Kumar, A.; Raturi, G.; Chakraborty, K.; Guspta, O.P.; Devana, B.N.; Tripathi, D.K.; et al. Fascinating role of silicon to combat salinity stress in plants: An updated overview. Plant Physiol. Biochem. 2021, 162, 110–123. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, W.; Chen, Q.; Liu, Y.; Ding, R. Effect of exogenous silicon (Si) on H+-ATPase activity, phospholipids and fluidity of plasma membrane in leaves of salt-stressed barley (Hordeum vulgare L.). Environ. Exp. Bot. 2006, 57, 212–219. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; Prado, R.M.; Junior, G.D.S.S.; Gratao, P.L.; Felisberto, G.; Viciedo, D.O.; Santos, D.M.M. Different methods of silicon application attenuate salt stress in sorghum and sunflower by modifying the antioxidative defense mechanism. Ecotoxicol. Environ. Saf. 2020, 203, 110964. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.R.; Nunes, L.R.L.; Pinheiro, C.L.; Abud, H.F.; Torres, S.B.; Dutra, A.S. Potassium silicate as an inducer of abiotic stress resistance in grain sorghum seeds. Rev. Cienc. Agron. 2022, 53, e20218136. [Google Scholar] [CrossRef]
- Sales, A.C.; Campos, C.N.S.; Souza Junior, J.P.; Silva, D.L.; Oliveira, K.S.; Prado, R.M.; Teodoro, P.E. Silicon mitigates nutritional stress in quinoa (Chenopodium quinoa Willd.). Sci. Rep. 2021, 11, 14665. [Google Scholar] [CrossRef] [PubMed]
- Choukr-Allah, R.; Rao, N.K.; Hirich, A.; Shahid, M.; Alshankiti, A.; Toderich, K.; Butt, K.U.R. Quinoa for marginal environments: Toward future food and nutritional security in MENA and central Asia regions. Front. Plant Sci. 2016, 7, 183664. [Google Scholar] [CrossRef] [PubMed]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional value and use of the andean crops quinoa (Chenopodium quinoa) and kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Miranda, M.F.A.; Freire, M.B.G.S.; Almeida, B.G.; Freire, A.G.; Freire, F.J.; Pessoa, L.G.M. Improvement of degraded physical attributes of a saline-sodic soil as influenced by phytoremediation and soil conditioners. Arch. Agron. Soil Sci. 2018, 64, 1207–1221. [Google Scholar] [CrossRef]
- Leal, L.D.S.G.; Pessoa, L.G.M.; Oliveira, J.P.; Santos, N.A.; Silva, L.F.D.S.; Júnior, G.B.; Souza, E.S. Do applications of soil conditioner mixtures improve the salt extraction ability of Atriplex nummularia at early growth stage? Int. J. Phytoremediation 2020, 22, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.F.A.; Freire, M.B.G.S.; Almeida, B.G.; Freire, F.J.; Pessoa, L.G.M.; Freire, A.G. Phytodesalination and chemical and organic conditioners to recover the chemical properties of saline-sodic soil. Soil Sci. Soc. Am. J. 2021, 85, 132–145. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Al Huqail, A.A.; Egamberdieva, D. Alleviation of abiotic salt stress in Ochradenus baccatus (Del.) by Trichoderma hamatum (Bonord.) Bainier. J. Plant Interact. 2014, 9, 857–868. [Google Scholar] [CrossRef]
- HARMAN, G.E.; Howell, C.R.; Viterbo, A.; Che, I. Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Singh, P.C.; Mishra, A.; Chauhan, P.S.; Dwivedi, S.; Bais, R.T.; Tripathi, R.D. Trichoderma: A potential bioremediator for environmental clean up. Clean Technol. Environ. Policy 2013, 15, 541–550. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.A. Diagnosis and improvement of saline and alkali soils. Soil Sci. Soc. Am. J. 1954, 18. [Google Scholar] [CrossRef]
- Embrapa. Sistema Brasileiro de Classificação de Solos; Centro Nacional: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Silva, M.M.A.; Santos, H.R.B.; Silva, E.N.; Neto, J.B.; Hermínio, P.J.; Ramalho, T.L.; Ferreira-Silva, S.L. Higher control of Na+ and Cl− transport to the shoot along with K+/Na+ selectivity is determinant for differential salt resistance in grapevine rootstocks. J. Plant Growth Regul. 2023, 42, 5713–5726. [Google Scholar] [CrossRef]
- Ferreira-Silva, S.L.; Silveira, J.A.G.; Voigt, E.L.; Soares, L.S.P.; Viégas, R.A. Changes in physiological indicators associated with salt tolerance in two contrasting cashew rootstocks. Brazilian J. Plant Physiol. 2008, 20, 51–59. [Google Scholar] [CrossRef]
- Maestro-Gaitán, I.; Granado-Rodríguez, S.; Orús, M.I.; Matías, J.; Cruz, V.; Bolaños, L.; Reguera, M. Genotype-dependent responses to long-term water stress reveal different water-saving strategies in Chenopodium quinoa Willd. Environ. Exp. Bot. 2022, 201, 104976. [Google Scholar] [CrossRef]
- Manaa, A.; Goussi, R.; Derbali, W.; Cantamessa, S.; Essemine, J.; Barbato, R. Photosynthetic performance of quinoa (Chenopodium quinoa Willd.) after exposure to a gradual drought stress followed by a recovery period. Biochim. Biophys. Acta—Bioenerg. 2021, 1862, 148383. [Google Scholar] [CrossRef] [PubMed]
- Rekaby, S.A.; AL-Huqail, A.A.; Gebreel, M.; Alotaibi, S.S.; Ghoneim, A.M. Compost and humic acid mitigate the salinity stress on quinoa (Chenopodium quinoa Willd L.) and improve some sandy soil properties. J. Soil Sci. Plant Nutr. 2023, 23, 2651–2661. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L. Vermiculite and humic acid improve the quality of green waste compost as a growth medium for Centaurea cyanus L. Environ. Technol. Innov. 2021, 24, 101945. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L. The effectiveness of composted green waste amended with vermiculite and humic acid powders as an alternative cultivation substrate for cornflower cultivation. Commun. Soil Sci. Plant Anal. 2021, 52, 2945–2957. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Esparza-Reynoso, S.; Garnica-Vergara, A.; López-Bucio, J.; Herrera-Estrella, A. Trichoderma-induced acidification is an early trigger for changes in arabidopsis root growth and determines fungal phytostimulation. Front. Plant Sci. 2017, 8, 270021. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.O.N.; Pessoa, L.G.M.; Silva, E.M.; Silva, L.R.; Freire, M.B.G.S.; Souza, E.S.; Ferreira-Silva, S.L.; França, J.G.E.; Silva, T.G.F.; Alencar, E.L.D.N. Effects of silicon alone and combined with organic matter and Trichoderma harzianum on sorghum yield, ions accumulation and soil properties under saline irrigation. Agriculture 2023, 13, 2146. [Google Scholar] [CrossRef]
- Karimi, G.; Pourakbar, L.; Moghaddam, S.S.; Danesh, Y.R.; Popovi´c-Djordjevi´c, J. Effectiveness of fungal bacterial biofertilizers on agrobiochemical attributes of quinoa (Chenopodium quinoa willd.) under salinity stress. Int. J. Environ. Sci. Technol. 2022, 19, 11989–12002. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Terletskaya, N.V.; Erbay, M.; Zorbekova, A.N.; Prokofieva, M.Y.; Saidova, L.T.; Mamirova, A. Influence of osmotic, salt, and combined stress on morphophysiological parameters of Chenopodium quinoa photosynthetic organs. Agriculture 2023, 13, 1. [Google Scholar] [CrossRef]
- Wakeel, A. Potassium-sodium interactions in soil and plant under saline-sodic conditions. J. Plant Nutr. Soil Sci. 2013, 176, 344–354. [Google Scholar] [CrossRef]
- Robbins, C.W. Sodium adsorption ratio-exchangeable sodium percentage relationships in a high potassium saline-sodic soil. Irrig. Sci. 1984, 5, 173–179. [Google Scholar] [CrossRef]
- Black, A.S.; Campbell, A.S. Ionic strength of soil solution and its effect on charge properties of some New Zealand soils. J. Soil Sci. 1982, 33, 249–262. [Google Scholar] [CrossRef]
- Irakoze, W.; Prodjinoto, H.; Nijimbere, S.; Bizimana, J.B.; Bigirimana, J.; Rufyikiri, G.; Lutts, S. Nacl-and na2 so4-induced salinity differentially affect clay soil chemical properties and yield components of two rice cultivars (Oryza sativa L.) in Burundi. Agronomy 2021, 11, 571. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Yao, R.; Wang, X.; Xie, W. Short-term effects of biochar and gypsum on soil hydraulic properties and sodicity in a saline-alkali soil. Pedosphere 2020, 30, 694–702. [Google Scholar] [CrossRef]
- Sunkari, E.D.; Abu, M.; Zango, M.S. Geochemical evolution and tracing of groundwater salinization using different ionic ratios, multivariate statistical and geochemical modeling approaches in a typical semi-arid basin. J. Contam. Hydrol. 2021, 236, 103742. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xiao, Y.; Wang, Y.; Liu, Z.; Yang, K. Saline–alkaline stress in growing maize seedlings is alleviated by Trichoderma asperellum through regulation of the soil environment. Sci. Rep. 2021, 11, 11152. [Google Scholar] [CrossRef]
- Miles, N.; Manson, A.D.; Rhodes, R.; Antwerpen, R.V.; Weigel, A. Extractable silicon in soils of the south african sugar industry and relationships with crop uptake. Commun. Soil Sci. Plant Anal. 2014, 45, 2949–2958. [Google Scholar] [CrossRef]
- Yanai, J.; Taniguchi, H.; Nakao, A. Evaluation of available silicon content and its determining factors of agricultural soils in Japan. Soil Sci. Plant Nutr. 2016, 62, 511–518. [Google Scholar] [CrossRef]
- Camargo, M.S.; Pereira, H.S.; Korndörfer, G.H.; Queiroz, A.A.; Reis, C.B. Soil reaction and absorption of silicon by rice. Sci. Agric. 2007, 64, 176–180. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon influences soil availability and accumulation of mineral nutrients in various plant species. Plants 2018, 7, 41. [Google Scholar] [CrossRef]
- Haynes, R.J. What effect does liming have on silicon availability in agricultural soils? Geoderma 2019, 337, 375–383. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Huang, L. Effects of silicon on heavy metal uptake at the soil-plant interphase: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef]
- Wang, B.; Chu, C.; Wei, H.; Zhang, L.; Ahmad, Z.; Wu, S.; Xie, B. Ameliorative effects of silicon fertilizer on soil bacterial community and pakchoi (Brassica chinensis L.) grown on soil contaminated with multiple heavy metals. Environ. Pollut. 2020, 267, 115411. [Google Scholar] [CrossRef] [PubMed]
- Veras, M.L.M.; Alves, L.S.; Silva, T.I.; Silva, I.N.; Costa, A.S.D.N.; Melo, E.N.; Dias, T.J. Silicon as mitigator of salt stress in mango tree seedlings. Aust. J. Crop Sci. 2021, 15, 1146–1150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).