Reduction of Nitrous Oxide Emissions from Urine Patches from Grazed Dairy Pastures in New Zealand: A Preliminary Assessment of ORUN® as an Alternative to the Use of Nitrification Inhibitor Dicyandiamide (DCD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Site
2.2. Treatments and Experimental Design
2.3. Climate Data
2.4. Soil Mineral N
2.5. Herbage Analysis
2.6. Measurement of Nitrous Oxide Flux
2.7. Statistical Analysis
3. Results and Discussion
3.1. Climatic Data
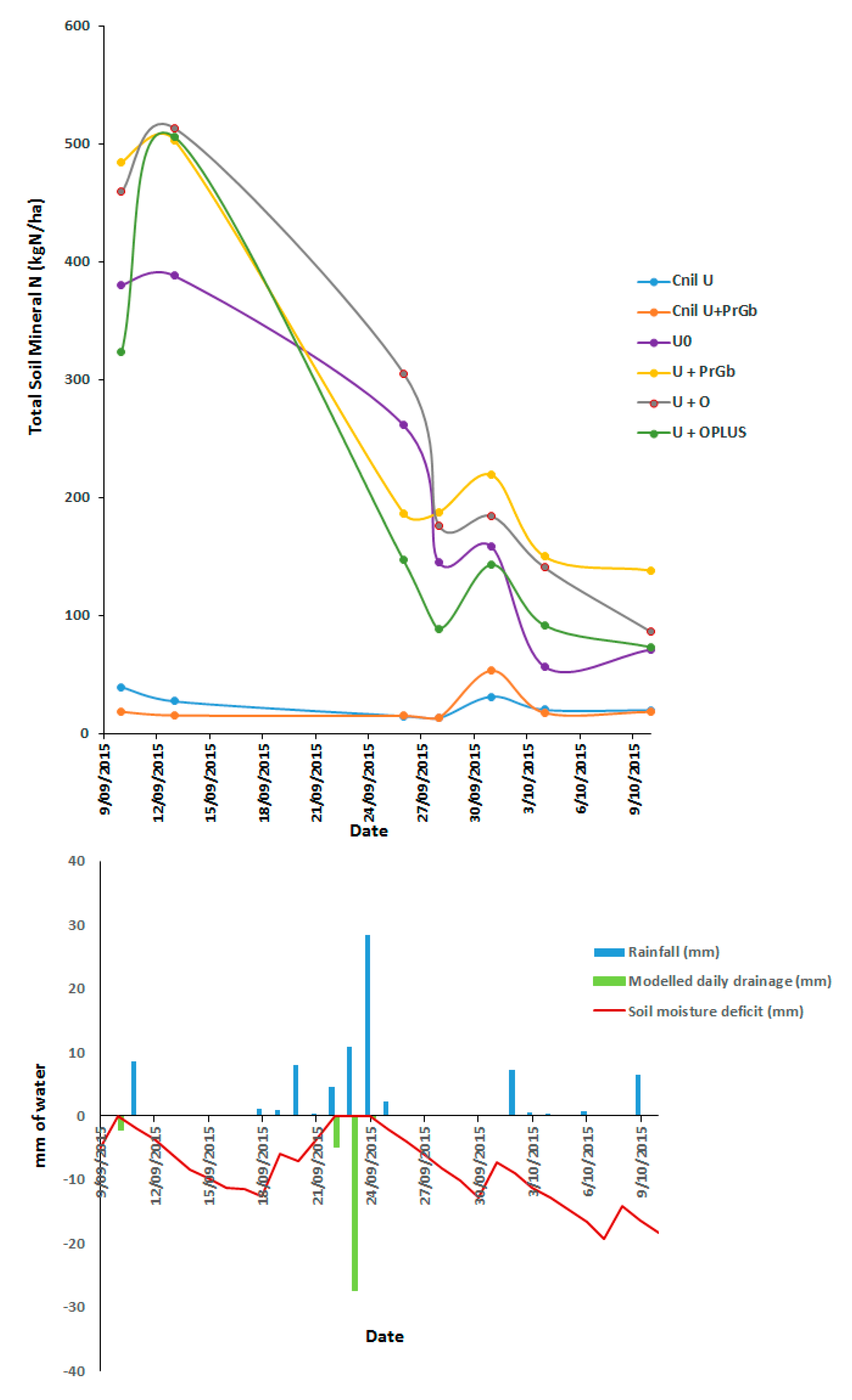
3.2. Soil Mineral N Concentration
3.2.1. Ammonium N and Nitrate N (0–5 cm Depth)
3.2.2. Total (NH4+ N + NO3− N) Soil Mineral N (0–5 cm and 0–60 cm)
3.3. Herbage Analysis from Spring Urine Patches Treated with and without ProGibb®, ORUN®, and ORUN® PLUS from Manawatu Mottled Fine Sandy Loam
3.3.1. Dry Matter Yields
3.3.2. Herbage N
3.3.3. Herbage P
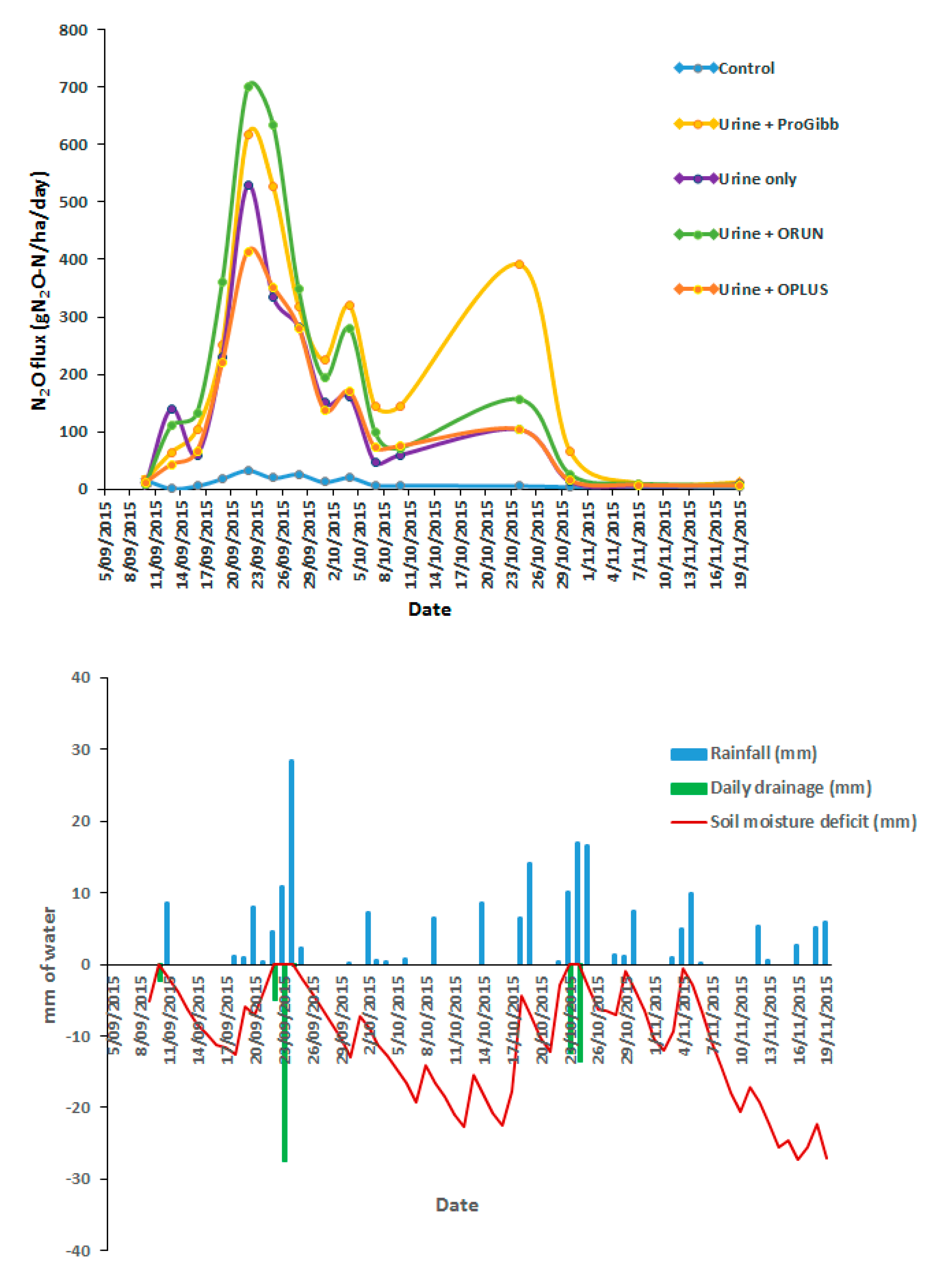
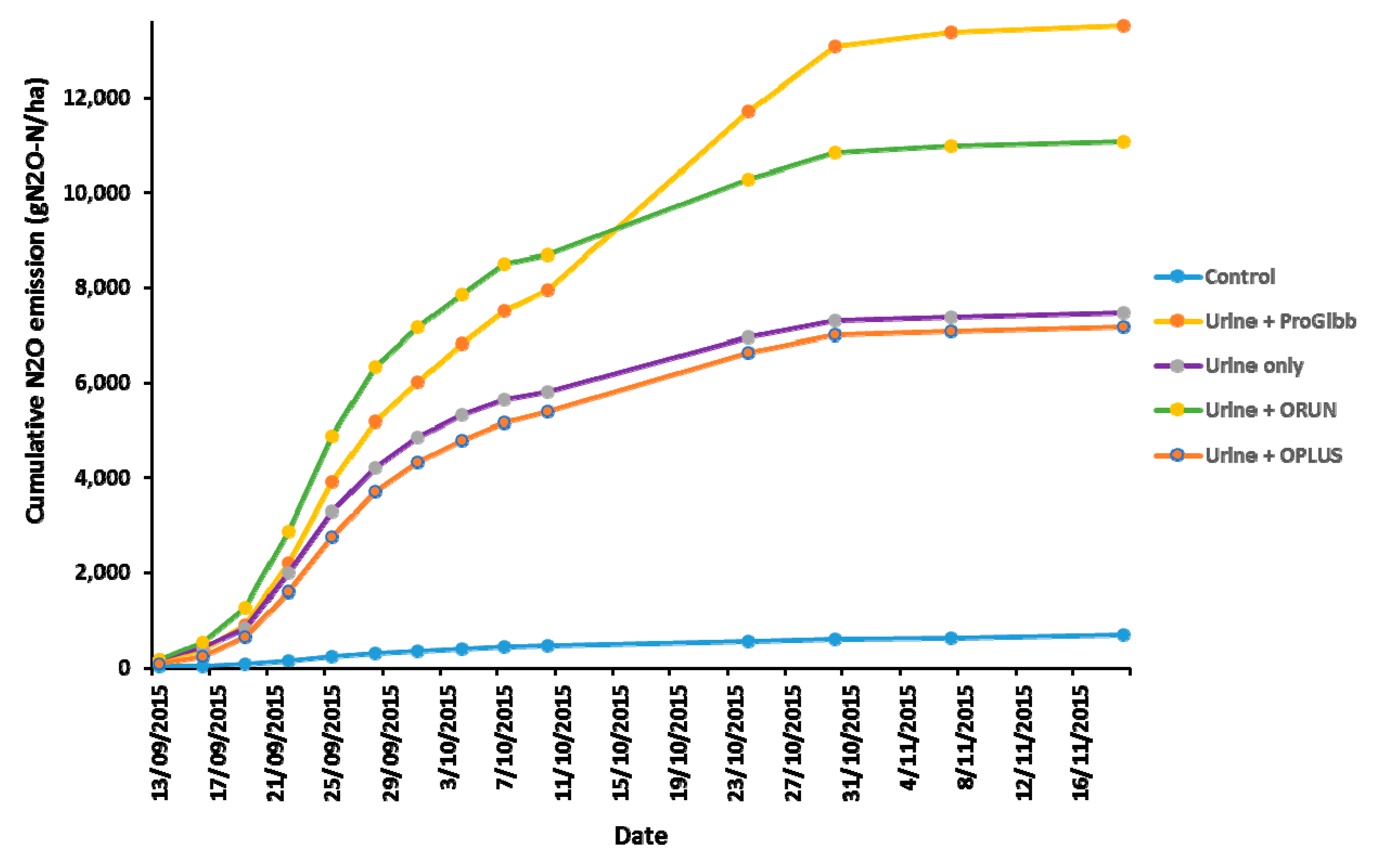
3.4. Nitrous Oxide Emissions from Combined Effects of Climatic Data of Trial Site and the Application of ProGibb, ORUN®, and ORUN® PLUS to Spring Urine Patches from Manawatu Mottled Fine Sandy Loam
3.4.1. N2O Flux
3.4.2. Cumulative N2O Flux
4. General Discussion
Fate of Urine-Applied N
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araújo, R.G.; Chavez-Santoscoy, R.A.; Parra-Saldívar, R.; Melchor-Martínez, E.M.; Iqbal, H.M. Agro-food systems and environment: Sustaining the unsustainable. Curr. Opin. Environ. Sci. Health 2023, 31, 100413. [Google Scholar] [CrossRef]
- Habib, A.; Sarwar, S.; Ahson, U.; Idrees, A.S. Measuring green growth in agriculture: A comparative analysis of world economies. Qual. Quant. 2023, 57, 5491–5511. [Google Scholar] [CrossRef]
- Vannier, C.; Cochrane, T.A.; Zawar-Reza, P.; Bellamy, L. Development of a Systems Model for Assessing Pathways to Resilient, Sustainable, and Profitable Agriculture in New Zealand. Land 2022, 11, 2334. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Working Group 3. Climate Change 2007: Mitigation: Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change: Summary for Policymakers and Technical Summary; Metz, B., Davidson, O., Bosch, P., Dave, R., Meyer, L., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Li, D.; Watson, C.J.; Yan, M.J.; Lalor, S.; Rafique, R.; Hyde, B.; Humphreys, J. A review of nitrous oxide mitigation by farm nitrogen management in temperate grassland-based agriculture. J. Environ. Manag. 2013, 128, 893–903. [Google Scholar] [CrossRef]
- Gaugler, T.; Stoeckl, S.; Rathgeber, A.W. Global climate impacts of agriculture: A meta-regression analysis of food production. J. Clean. Prod. 2020, 276, 122575. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Miller, H.L. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. 2007. Available online: https://unfccc.int/process-and-meetings/transparency-and-reporting/greenhouse-gas-data/frequently-asked-questions/global-warming-potentials-ipcc-fourth-assessment-report (accessed on 19 February 2024).
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nat. Clim. Chang. 2012, 2, 410–416. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Yao, Y.A. comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Wrage, N.; Velthof, G.L.; Van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Bio. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Baral, K.R.; Thomsen, A.G.; Olesen, J.E.; Petersen, S.O. Controls of nitrous oxide emission after simulated cattle urine deposition. Agric. Ecosyst. Environ. 2014, 188, 103–110. [Google Scholar] [CrossRef]
- De Klein, C.A.M.; Eckard, R.J. Targeted technologies for nitrous oxide abatement from animal agriculture. Anim. Prod. Sci. 2008, 48, 14–20. [Google Scholar] [CrossRef]
- Caradus, J.R.; Goldson, S.L.; Moot, D.J.; Rowarth, J.S.; Stewart, A.V. Pastoral agriculture, a significant driver of New Zealand’s economy, based on an introduced grassland ecology and technological advances. J. R. Soc. N. Z. 2023, 53, 259–303. [Google Scholar] [CrossRef]
- Ministry of Primary Industries. Situation and Outlook for Primary Industries. 2021. p. 64. Available online: https://www.mpi.govt.nz/dmsdocument/45451-Situation-and-Outlook-for-Primary-Industries-SOPI-June-2021 (accessed on 14 July 2023).
- Ministry for the Environment (MFE). New Zealand’s Emissions Reduction Targets: Doing Our Fair Share. Available online: https://www.climatechange.govt.nz/ (accessed on 24 August 2016).
- New Zealand Agricultural Greenhouse Gas Research Centre (NZAGRC). Highlights 2014. 2015. p. 19. Available online: www.nzagrc.org.nz (accessed on 24 August 2016).
- Ministry for the Environment. New Zealand’s Greenhouse Gas Inventory 1990–2013: Fulfilling reporting requirements under the United Nations Framework Convention on Climate Change and the Kyoto Protocol. Available online: http://www.mfe.govt.nz (accessed on 13 August 2015).
- de Klein, C.A.; Luo, J.; Woodward, K.B.; Styles, T.; Wise, B.; Lindsey, S.; Cox, N. The effect of nitrogen concentration in synthetic cattle urine on nitrous oxide emissions. Agric. Ecosyst. Environ. 2014, 188, 85–92. [Google Scholar] [CrossRef]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.S.; Luo, J.; Giltrap, D.L.; Tillman, R.W. Denitrification and N2O: N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci. Total Environ. 2013, 465, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Saggar, S.; Giltrap, D.L.; Hedley, C.B.; Lambie, S.M.; Tate, K.R.; Li, C.; Amstel, A.V. Nitrous oxide emissions from grazed perennial pastures in New Zealand. In Proceedings of the 4th International Symposium on non-CO2 Greenhouse Gases (NCGG-4), Science, Control, Policy and Implementation, Utrecht, The Netherlands, 4–6 July 2005; Millpress Science Publishers: Rotterdam, The Netherlands, 2005; pp. 113–122. [Google Scholar]
- Selbie, D.R.; Buckthought, L.E.; Shepherd, M.A. The Challenge of the Urine Patch for Managing Nitrogen in Grazed Pasture Systems. Adv. Agron. 2015, 129, 229–292. [Google Scholar]
- Saggar, S.; Andrew, R.M.; Tate, K.R.; Hedley, C.B.; Rodda, N.J.; Townsend, J.A. Modelling nitrous oxide emissions from dairy-grazed pastures. Nutr. Cycl. Agroecosystems 2004, 68, 243–255. [Google Scholar] [CrossRef]
- van der Weerden, T.J.; Manderson, A.; Kelliher, F.M.; de Klein, C.A. Spatial and temporal nitrous oxide emissions from dairy cattle urine deposited onto grazed pastures across New Zealand based on soil water balance modelling. Agric. Ecosyst. Environ. 2014, 189, 92–100. [Google Scholar] [CrossRef]
- Whitehead, D.; Edwards, G.R. Assessment of the application of gibberellins to increase productivity and reduce nitrous oxide emissions in grazed grassland. Agric. Ecosyst. Environ. 2015, 207, 40–50. [Google Scholar] [CrossRef]
- Cheng, L.; Judson, H.G.; Bryant, R.H.; Mowat, H.; Guinot, L.; Hague, H.; Edwards, G.R. The effects of feeding cut plantain and perennial ryegrass-white clover pasture on dairy heifer feed and water intake, apparent nutrient digestibility and nitrogen excretion in urine. Ani. Feed Sci. Technol. 2017, 229, 43–46. [Google Scholar] [CrossRef]
- de Klein, C.A.; van der Weerden, T.J.; Luo, J.; Cameron, K.C.; Di, H.J. A review of plant options for mitigating nitrous oxide emissions from pasture-based systems. N. Z. J. Agric. Res. 2020, 63, 29–43. [Google Scholar] [CrossRef]
- Earl-Goulet, S.; Talbot, W.D.; Cameron, K.C.; Di, H.J. Effects of plantain in pasture on nitrous oxide emissions from cattle urine patches, as affected by urine deposition timing and soil type. N. Z. J. Agric. Res. 2023, 66, 44–60. [Google Scholar] [CrossRef]
- Gardiner, C.A.; Clough, T.J.; Cameron, K.C.; Di, H.J.; Edwards, G.R.; de Klein, C.A. Potential inhibition of urine patch nitrous oxide emissions by Plantago lanceolata and its metabolite aucubin. N. Z. J. Agric. Res. 2018, 61, 495–503. [Google Scholar] [CrossRef]
- Gardiner, C.A.; Clough, T.J.; Cameron, K.C.; Di, H.J.; Edwards, G.R. Efficacy of aucubin as a nitrification inhibitor assessed in two Canterbury field trials. N. Z. J. Agric. Res. 2020, 63, 73–86. [Google Scholar] [CrossRef]
- Luo, J.; Balvert, S.F.; Wise, B.; Welten, B.; Ledgard, S.F.; de Klein, C.A.M.; Judge, A. Using alternative forage species to reduce emissions of the greenhouse gas nitrous oxide from cattle urine deposited onto soil. Sci. Total Environ. 2018, 610, 1271–1280. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Navarrete, S.; Horne, D.J.; Hanly, J.A.; Bishop, P.; Kemp, P.D. Can secondary metabolites of plantain reduce N losses from urine patches? N. Z. J. Agric. Res. 2023, 66, 83–100. [Google Scholar] [CrossRef]
- Simon, P.L.; de Klein, C.A.; Worth, W.; Rutherford, A.J.; Dieckow, J. The efficacy of Plantago lanceolata for mitigating nitrous oxide emissions from cattle urine patches. Sci. Total Environ. 2019, 691, 430–441. [Google Scholar] [CrossRef]
- Adler, A.A.; Doole, G.J.; Romera, A.J.; Beukes, P.C. Managing greenhouse gas emissions in two major dairy regions of New Zealand: A system-level evaluation. Agric. Syst. 2015, 135, 1–9. [Google Scholar] [CrossRef]
- Fleming, D.; Kerr, S.; Lou, E. Cows, cash and climate: Low stocking rates, high-performing cows, emissions and profitability across New Zealand farms. High-Perform. Cows Emiss. Profitab. N. Z. Farms 2019, 1124-2019-2294. [Google Scholar]
- Leahy, S.C.; Kearney, L.; Reisinger, A.; Clark, H. Mitigating greenhouse gas emissions from New Zealand pasture-based livestock farm systems. J. New Zealand Grassl. 2019, 81, 101–110. [Google Scholar] [CrossRef]
- Reisinger, A.; Clark, H.; Journeaux, P.; Clark, D.; Lambert, G. On-Farm Options to Reduce Agricultural GHG Emissions in New Zealand; New Zealand Agricultural Greenhouse Gas Research Centre: Palmerston North, New Zealand, 2017. [Google Scholar]
- Van der Weerden, T.; Beukes, P.; De Klein, C.; Hutchinson, K.; Farrell, L.; Stormink, T.; Dynes, R. The effects of system changes in grazed dairy farmlet trials on greenhouse gas emissions. Animals 2018, 8, 234. [Google Scholar] [CrossRef]
- Christensen, C.L.; Hedley, M.J.; Hanly, J.A.; Horne, D.J. Duration-controlled grazing of dairy cows. 2: Nitrogen losses in sub-surface drainage water and surface runoff. N. Z. J. Agric. Res. 2019, 62, 48–68. [Google Scholar] [CrossRef]
- Romera, A.J.; Cichota, R.; Beukes, P.C.; Gregorini, P.; Snow, V.O.; Vogeler, I. Combining restricted grazing and nitrification inhibitors to reduce nitrogen leaching on New Zealand dairy farms. J. Environ. Qual. 2017, 46, 72–79. [Google Scholar] [CrossRef]
- Van der Weerden, T.J.; Laurenson, S.; Vogeler, I.; Beukes, P.C.; Thomas, S.M.; Rees, R.M.; De Klein, C.A.M. Mitigating nitrous oxide and manure-derived methane emissions by removing cows in response to wet soil conditions. Agric. Syst. 2017, 156, 126–138. [Google Scholar] [CrossRef]
- de Klein, C.A.; Shepherd, M.A.; van der Weerden, T.J. Nitrous oxide emissions from grazed grasslands: Interactions between the N cycle and climate change—A New Zealand case study. Curr. Opin. Environ. Sust. 2014, 9, 131–139. [Google Scholar] [CrossRef]
- Edwards, G.R.; De Ruiter, J.M.; Dalley, D.E.; Pinxterhuis, J.B.; Cameron, K.C.; Bryant, R.H.; Chapman, D.F. Urinary nitrogen concentration of cows grazing fodder beet, kale and kale-oat forage systems in winter. In Proceedings of the 5th Australasian Dairy Science Symposium, Hamilton, New Zealand, 19–21 November 2014; pp. 144–147. [Google Scholar]
- Luo, J.; Sun, X.Z.; Pacheco, D.; Ledgard, S.F.; Lindsey, S.B.; Hoogendoorn, C.J.; Watkins, N.L. Nitrous oxide emission factors for urine and dung from sheep fed either fresh forage rape (Brassica napus L.) or fresh perennial ryegrass (Lolium perenne L.). Animals 2015, 9, 534–543. [Google Scholar] [CrossRef]
- Malcolm, B.J.; Cameron, K.C.; Edwards, G.R.; Di, H.J. Nitrogen leaching losses from lysimeters containing winter kale: The effects of urinary N rate and DCD application. N. Z. J. Agric. Res. 2015, 58, 13–25. [Google Scholar] [CrossRef]
- Gardiner, C.A.; Clough, T.J.; Cameron, K.C.; Di, H.J.; Edwards, G.R.; de Klein, C.A.M. Potential for forage diet manipulation in New Zealand pasture ecosystems to mitigate ruminant urine derived N2O emissions: A review. N. Z. J. Agric. Res. 2016, 59, 301–317. [Google Scholar] [CrossRef]
- Adhikari, K.P.; Chibuike, G.; Saggar, S.; Simon, P.L.; Luo, J.; de Klein, C.A. Management and implications of using nitrification inhibitors to reduce nitrous oxide emissions from urine patches on grazed pasture soils–A review. Sci. Total Environ. 2021, 791, 148099. [Google Scholar] [CrossRef]
- Chibuike, G.; Saggar, S.; Palmada, T.; Luo, J. The persistence and efficacy of nitrification inhibitors to mitigate nitrous oxide emissions from New Zealand pasture soils amended with urine. Geoderma Reg. 2022, 30, e00541. [Google Scholar] [CrossRef]
- Grace, P.; De Rosa, D.; Shcherbak, I.; Strazzabosco, A.; Rowlings, D.; Scheer, C.; Bell, M. Revised emission factors for estimating direct nitrous oxide emissions from nitrogen inputs in Australia’s agricultural production systems: A meta-analysis. Soil Res. 2023, 62, SR23070. [Google Scholar] [CrossRef]
- Soares, J.R.; Souza, B.R.; Mazzetto, A.M.; Galdos, M.V.; Chadwick, D.R.; Campbell, E.E.; Jaiswal, D.; Oliveira, J.C.; Monteiro, L.A.; Vianna, M.S.; et al. Mitigation of nitrous oxide emissions in grazing systems through nitrification inhibitors: A meta-analysis. Nutr. Cycl. Agroecosystems 2023, 125, 359–377. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. Nitrous oxide emissions from two dairy pasture soils as affected by different rates of a fine particle suspension nitrification inhibitor, dicyandiamide. Biol. Fertil. Soils 2006, 42, 472–480. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C.; Sherlock, R.R. Comparison of the effectiveness of a nitrification inhibitor, dicyandiamide, in reducing nitrous oxide emissions in four different soils under different climatic and management conditions. Soil Use Man. 2007, 23, 1–9. [Google Scholar] [CrossRef]
- Hoogendoorn, C.J.; de Klein, C.A.; Rutherford, A.J.; Letica, S.; Devantier, B.P. The effect of increasing rates of nitrogen fertiliser and a nitrification inhibitor on nitrous oxide emissions from urine patches on sheep grazed hill country pasture. Aust. Prod. Sci. 2008, 48, 147–151. [Google Scholar] [CrossRef]
- Ravensdown. N-Protect®. Available online: https://www.ravensdown.co.nz/products/fertiliser/n-protect (accessed on 19 February 2024).
- O’Neill, M.; Richards, K.A.; Bates, G.; Smith, D.; Forrestal, P.J. Performance of “Spikey” in locating and detecting freshly deposited urine aptches in livestock grazed pasture soils of Ireland. In Nutrient Management in Farmed Landscapes; Christensen, C.L., Horne, D.J., Singh, R., Eds.; Occasional Report No. 33; Farmed Landscapes Research Centre, Massey University: Palmerston North, New Zealand, 2020; 11p, Available online: http://flrc.massey.ac.nz/publications.html (accessed on 19 February 2024).
- Rodriguez, M.J.; Saggar, S.; Berben, P.; Palmada, T.; Lopez-Villalobos, N.; Pal, P. Use of a urease inhibitor to mitigate ammonia emissions from urine patches. Environ. Technol. 2021, 42, 20–31. [Google Scholar] [CrossRef]
- Ray, A.; Nkwonta, C.; Forrestal, P.; Hogan, S.; Cummins, E. Current knowledge on urease and nitrification inhibitors technology and their safety. Rev. Environ. Health 2021, 36, 477–491. [Google Scholar] [CrossRef]
- Bishop, P.A.; Quin, B.F. Modelling of the Effect of Combined DCD and Urease Inhibitors on the Post-Doposition Size of Urine Patches–Implications for Decreased N losses and Increased Pasture Production Using the ‘Taurine’ Tail-Attached Dispenser. In Proceedings of the Workshop “Farming’s Future: Minimising Footprints and Maximising Margins, Palmerston North, New Zealand, 10–11 February 2010; pp. 268–273. [Google Scholar]
- Cowie, J.D.; Rijkse, W.C. Soils of Manawatu County, North Island, New Zealand. N. Z. Soil Surv. Rep. 1977, 30, 34–41. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA, Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Kamphake, L.J.; Hannah, S.A.; Cohen, J.M. Automated analysis for nitrate by hydrazine reduction. Water Res. 1967, 1, 205–216. [Google Scholar] [CrossRef]
- Blakemore, L.C.; Searle, P.L.; Daly, B.K. Methods for chemical analysis of soils. N. Z. Soil Bur. Scient. Rep. 1987, 80, 21–23. [Google Scholar]
- Zaman, M.; Saggar, S.; Blennerhassett, J.D.; Singh, J. Effect of urease and nitrification inhibitors on N transformation, gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system. Soil Biol. Biochem. 2009, 41, 1270–1280. [Google Scholar] [CrossRef]
- Rolston, D.E. Gases flux. In Methods of Soil Analysis Part 1 Physical and Mineralogical Methods; Klute, A., Ed.; Soil Science Society of America: Madison, WI, USA, 1986; pp. 1103–1119. [Google Scholar]
- Hensen, A.; Skiba, U.; Famulari, D. Low cost and state of the art methods to measure nitrous oxide emissions. Environ. Res. Lett. 2013, 8, 025022. [Google Scholar] [CrossRef]
- Adhikari, K.P.; Saggar, S.; Hanly, J.A.; Guinto, D.F. Urease inhibitors reduced ammonia emissions from cattle urine applied to pasture soil. Nutr. Cycl. Agroecosystems 2020, 117, 317–335. [Google Scholar] [CrossRef]
- Hill Laboratories Limited, R.J. Crop Guide-Mixed Pasture. KB Item: 3472v3. Available online: http://www.hill-laboratories.com/file/fileid/38620 (accessed on 24 August 2016).
- Bell, M.J.; Rees, R.M.; Cloy, J.M.; Topp, C.F.E.; Bagnall, A.; Chadwick, D.R. Nitrous oxide emissions from cattle excreta applied to a Scottish grassland: Effects of soil and climatic conditions and a nitrification inhibitor. Sci. Total Environ. 2015, 508, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Brüggemann, N.; Gasche, R.; Shen, Z.; Wolf, B.; Butterbach-Bahl, K. Environmental controls over soil-atmosphere exchange of N2O, NO, and CO2 in a temperate Norway spruce forest. Glob. Biogeochem. Cycles 2010, 2, 24. [Google Scholar] [CrossRef]
- Luo, G.J.; Brüggemann, N.; Wolf, B.; Gasche, R.; Grote, R.; Butterbach-Bahl, K. Decadal variability of soil CO2, NO, N2O, and CH4 fluxes at the Höglwald Forest, Germany. Biogeoscience 2012, 9, 1741–1763. [Google Scholar] [CrossRef]
- Alves, B.J.; Smith, K.A.; Flores, R.A.; Cardoso, A.S.; Oliveira, W.R.; Jantalia, C.P.; Boddey, R.M. Selection of the most suitable sampling time for static chambers for the estimation of daily mean N2O flux from soils. Soil Biol. Biochem. 2012, 46, 129–135. [Google Scholar] [CrossRef]
- Hargreaves, P.R.; Rees, R.M.; Horgan, G.W.; Ball, B.C. Size and Persistence of Nitrous Oxide Hot-Spots in Grazed and Ungrazed Grassland. Environ. Nat. Res. Res. 2015, 5, 1. [Google Scholar] [CrossRef][Green Version]
- Müller, C.; Sherlock, R.R. Nitrous oxide emissions from temperate grassland ecosystems in the Northern and Southern Hemispheres. Glob. Biogeochem. Cycles 2004, 1, 18. [Google Scholar] [CrossRef]
- Ding, K.; Luo, J.; Clough, T.J.; Lindsey, S.; Di, H.J. In situ nitrous oxide and dinitrogen fluxes from a grazed pasture soil following cow urine application at two nitrogen rates. Sci. Total Environ. 2022, 838, 156473. [Google Scholar] [CrossRef]

| Treatment | 10 September 2015 | 13 September 2015 | 26 September 2015 | 28 September 2015 | 1 October 2015 | 4 October 2015 | 10 October 2015 | 16 October 2015 | 24 November 2015 |
|---|---|---|---|---|---|---|---|---|---|
| Days after Application of Treatment (DAT) | 1 | 4 | 17 | 19 | 22 | 25 | 31 | 37 | 76 |
| Cnil U | 28.9 b | 20.84 b | 10.65 c | 6.55 | 21.11 | 14.72 | 13.17 | 6.22 | 13.11 |
| CnilU + PrGb | 15.9 b | 12.82 b | 13.15 c | 10.24 | 19.88 | 14.36 | 13.78 | nd | nd |
| U0 | 377.8 a | 384.29 a | 195.52 ab | 54.57 | 31 | 14.12 | 11.91 | 6.05 | 11.76 |
| U + PrGb | 481.9 a | 501.05 a | 130.90 ab | 86.1 | 53.18 | 27.78 | 16.63 | 8.62 | 8.13 |
| U + O | 457.2 a | 509.93 a | 231.85 a | 66.52 | 22.02 | 29.47 | 11.27 | 8.49 | 8.8 |
| U + OPLUS | 320.7 a | 502.63 a | 98.11 bc | 31.24 | 51.4 | 14.95 | 18.45 | 8.41 | 9.65 |
| LSD | 184.89 | 164.98 | 114.57 | 67.32 | 48.945 | 18.579 | 6.804 | 3.862 | 4.804 |
| SEM | 78.1 | 61.8 | 42.1 | 26.7 | 20.37 | 6.98 | 2.6 | 1.532 | 1.906 |
| p value | 0.0005 | <0.0001 | 0.0152 | 0.2891 NS | 0.7046 NS | 0.4861 NS | 0.4383 NS | 0.593 NS | 0.362 NS |
| Treatment | 10 September 2015 | 13 September 2015 | 26 September 2015 | 28 September 2015 | 1 October 2015 | 4 October 2015 | 10 October 2015 | 16 October 2015 | 24 November 2015 |
|---|---|---|---|---|---|---|---|---|---|
| Days after Application of Treatment (DAT) | 1 | 4 | 17 | 19 | 22 | 25 | 31 | 37 | 76 |
| Cnil U | 10.06 | 6.11 | 3.67 b | 6.30 c | 10.14 b | 4.95 c | 6.16 cd | 4 | 4.84 |
| CnilU + PrGb | 2.2 | 2.2 | 1.47 b | 2.92 c | 33.20 b | 2.78 c | 4.24 d | nd | nd |
| U0 | 2.26 | 3.58 | 65.72 a | 90.21 ab | 126.77 a | 42.08 b | 48.15 bcd | 67 | 4.04 |
| U + PrGb | 2.39 | 2.06 | 55.56 a | 101.36 a | 165.82 a | 121.92 a | 121.40 a | 72 | 2.23 |
| U + O | 1.8 | 3.27 | 73.34 a | 109.62 a | 161.75 a | 110.86 a | 74.86 ab | 89 | 4.84 |
| U + OPLUS | 2.17 | 3.07 | 48.47 a | 57.34 b | 91.82 ab | 61.98 b | 54.56 bc | 36 | 2.59 |
| LSD | 5.942 | 3.185 | 40.139 | 42.664 | 83.305 | 37.647 | 49.638 | 58.8 | 4.087 |
| SEM | 2.397 | 1.285 | 13.63 | 15.27 | 34.9 | 14.32 | 20.02 | 23.3 | 1.622 |
| p value | 0.1686 NS | 0.3900 NS | 0.0188 | 0.0006 | 0.0174 | <0.0001 | 0.0041 | 0.142 NS | 0.683 NS |
| Treatment | 16 October 2015 | 24 November 2015 | ||||
|---|---|---|---|---|---|---|
| Days after Application of Treatment (DAT) | 37 | 76 | ||||
| NH4+ N | NO3− N | Total Mineral N (kg/ha) | NH4+ N | NO3– N | Total Mineral N (kg/ha) | |
| Cnil U | 20.4 | 13.3 b | 33.7 b | 61.7 | 6.4 | 68.1 |
| U0 | 40.6 | 285.6 a | 326.2 a | 60.6 | 13.5 | 74.1 |
| U + PrGb | 40.6 | 204.8 a | 245.4 a | 54.4 | 18.3 | 72.7 |
| U + O | 44.4 | 248.8 a | 293.2 a | 61.7 | 10.5 | 72.2 |
| U + OPLUS | 64.8 | 240.4 a | 305.2 a | 62.5 | 12 | 74.5 |
| LSD | 51.33 | 134.3 | 180.8 | 7.59 | 9.3 | 6.77 |
| SEM | 20.36 | 53.3 | 71.7 | 3.01 | 3.69 | 2.69 |
| p value | 0.671 NS | 0.026 | 0.076 | 0.357 NS | 0.292 NS | 0.499 NS |
| Treatment | Dry Matter (kg/ha) | Herbage N (kgN/ha) | Herbage P (kgP/ha) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| First Harvest | Second Harvest | Cumulative Harvest | First Harvest | Second Harvest | Cumulative N | First Harvest | Second Harvest | Cumulative P | |
| Cnil U | 1820 c | 2262.6 b | 3881 c | 55.82 c | 50.72 b | 106.54 c | 8.046 | 8.80 ab | 16.85 ab |
| CnilU + PrGb | 2186 ab | 1448 c | 3634 c | 59.09 c | 35.29 c | 90.43 d | 8.65 | 5.83 c | 14.48 c |
| U0 | 2052 bc | 2801 a | 4854 a | 80.61 ab | 67.11 a | 147.72 a | 8.69 | 9.60 a | 18.29 a |
| U + PrGb | 2183 ab | 2263 b | 4446 b | 73.15 b | 58.14 ab | 131.29 b | 8.21 | 7.45 bcd | 15.65 bc |
| U + O | 2312 ab | 2126 b | 4438 b | 80.51 ab | 55.25 b | 135.76 ab | 9.2 | 7.60 bc | 16.79 ab |
| U + OPLUS | 2386 a | 2086 b | 4471 b | 85.18 a | 53.29 b | 138.47 ab | 9 | 7.01 cd | 16.00 bc |
| LSD | 284.7 | 351.6 | 327 | 7.79 | 11.61 | 13.39 | 1.18 | 1.71 | 2.027 |
| SEM | 114.8 | 161.6 | 152.9 | 3.58 | 4.68 | 5.4 | 0.435 | 0.69 | 0.818 |
| p value | 0.042 | <0.001 | <0.001 | <0.001 | 0.007 | <0.0001 | 0.5324 NS | 0.022 | 0.079 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otene, I.J.J.; Hedley, M.J.; Bishop, P. Reduction of Nitrous Oxide Emissions from Urine Patches from Grazed Dairy Pastures in New Zealand: A Preliminary Assessment of ORUN® as an Alternative to the Use of Nitrification Inhibitor Dicyandiamide (DCD). Sustainability 2024, 16, 2843. https://doi.org/10.3390/su16072843
Otene IJJ, Hedley MJ, Bishop P. Reduction of Nitrous Oxide Emissions from Urine Patches from Grazed Dairy Pastures in New Zealand: A Preliminary Assessment of ORUN® as an Alternative to the Use of Nitrification Inhibitor Dicyandiamide (DCD). Sustainability. 2024; 16(7):2843. https://doi.org/10.3390/su16072843
Chicago/Turabian StyleOtene, Iduh Jonathan Joseph, Mike J. Hedley, and Peter Bishop. 2024. "Reduction of Nitrous Oxide Emissions from Urine Patches from Grazed Dairy Pastures in New Zealand: A Preliminary Assessment of ORUN® as an Alternative to the Use of Nitrification Inhibitor Dicyandiamide (DCD)" Sustainability 16, no. 7: 2843. https://doi.org/10.3390/su16072843
APA StyleOtene, I. J. J., Hedley, M. J., & Bishop, P. (2024). Reduction of Nitrous Oxide Emissions from Urine Patches from Grazed Dairy Pastures in New Zealand: A Preliminary Assessment of ORUN® as an Alternative to the Use of Nitrification Inhibitor Dicyandiamide (DCD). Sustainability, 16(7), 2843. https://doi.org/10.3390/su16072843







