Contribution of Photosynthetic, Root and Phenotypic Traits to Soybean Plant Height
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Site and Growth Conditions
2.3. Measurement Indexes
2.3.1. Phenotypic Trait Indicators
2.3.2. Root Trait Indicators
2.3.3. Determination of Photosynthetic Indicators
2.4. Statistical Analysis
3. Results
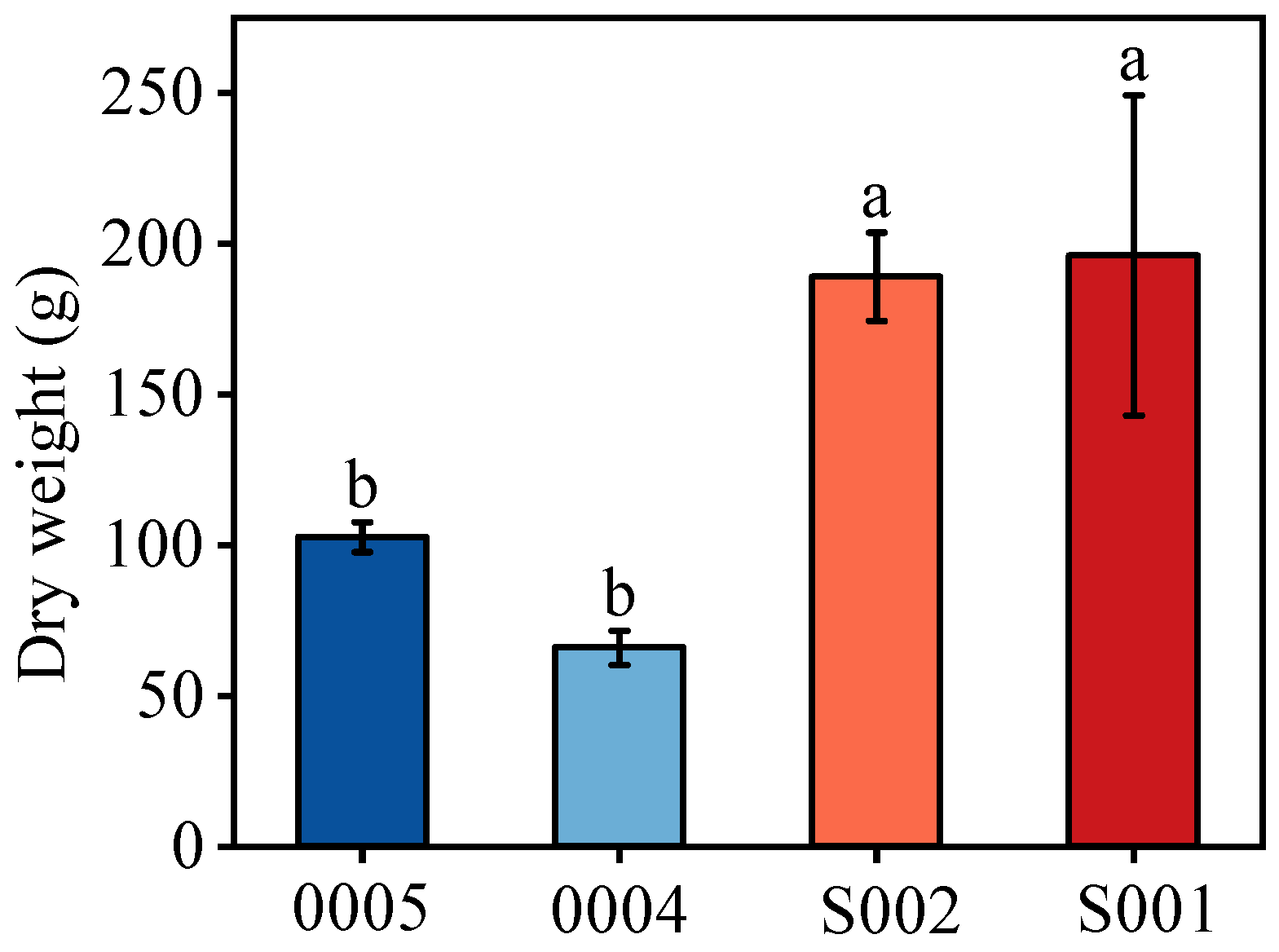
3.1. Dry Weight per Plant Differences between Tall and Short Soybean Cultivars
3.2. Aboveground Phenotypic Differences between Tall and Short Soybean Cultivars
3.3. Root Traits of Different Soybean Cultivars
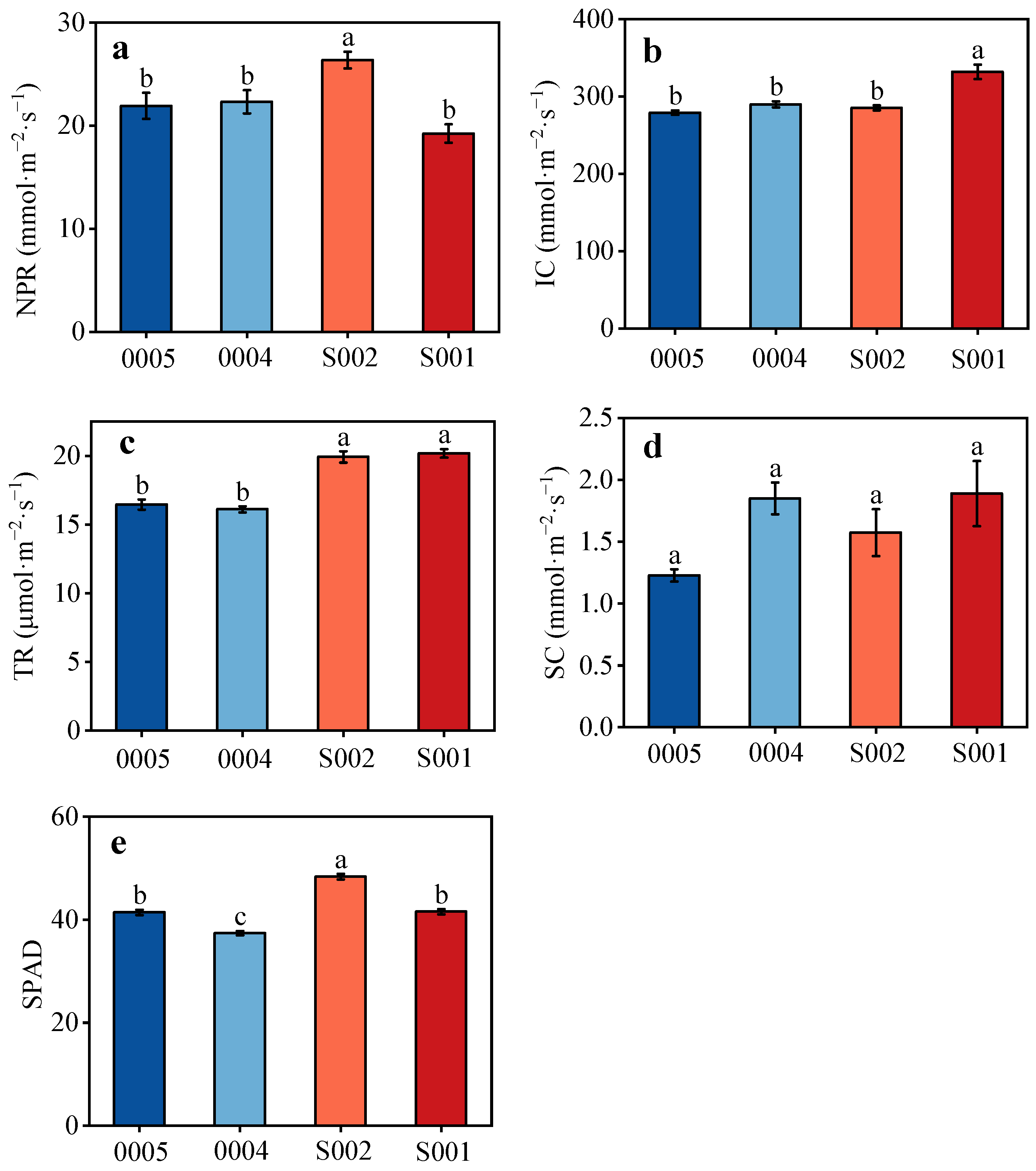
3.4. Photosynthesis Characteristics of Different Soybean Cultivars
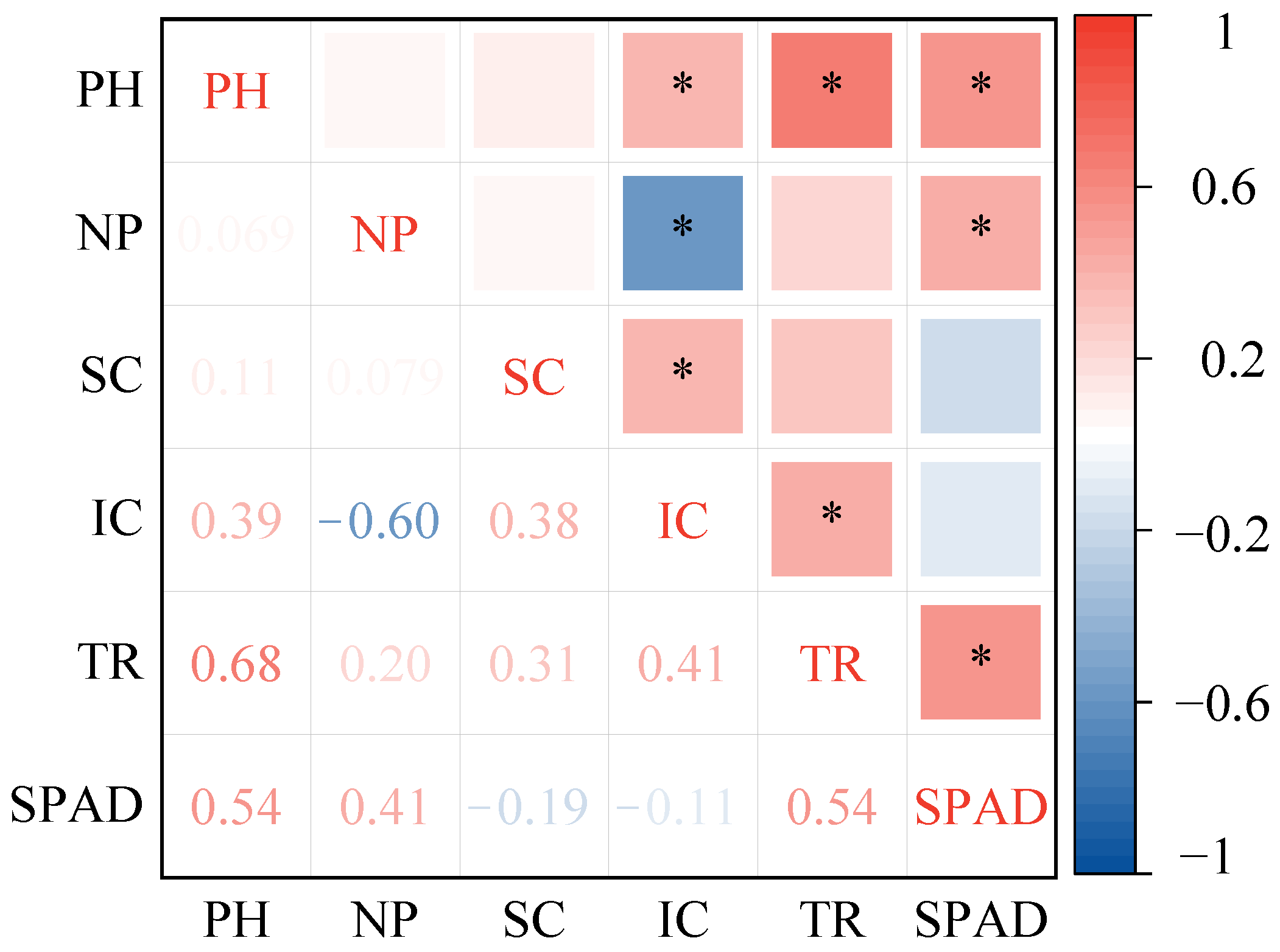
3.5. Correlation of Soybean Plant Height with Phenotypic and Photosynthetic Indicators
3.5.1. Correlation of Soybean Plant Height with Phenotypic Characteristics
3.5.2. Correlation of Soybean Plant Height with Root Traits
3.5.3. Correlation of Soybean Plant Height with Photosynthetic Characteristics
3.6. Principal Component Analysis of Soybean Plant Height, Phenotypic Traits, and Photosynthetic Characteristics
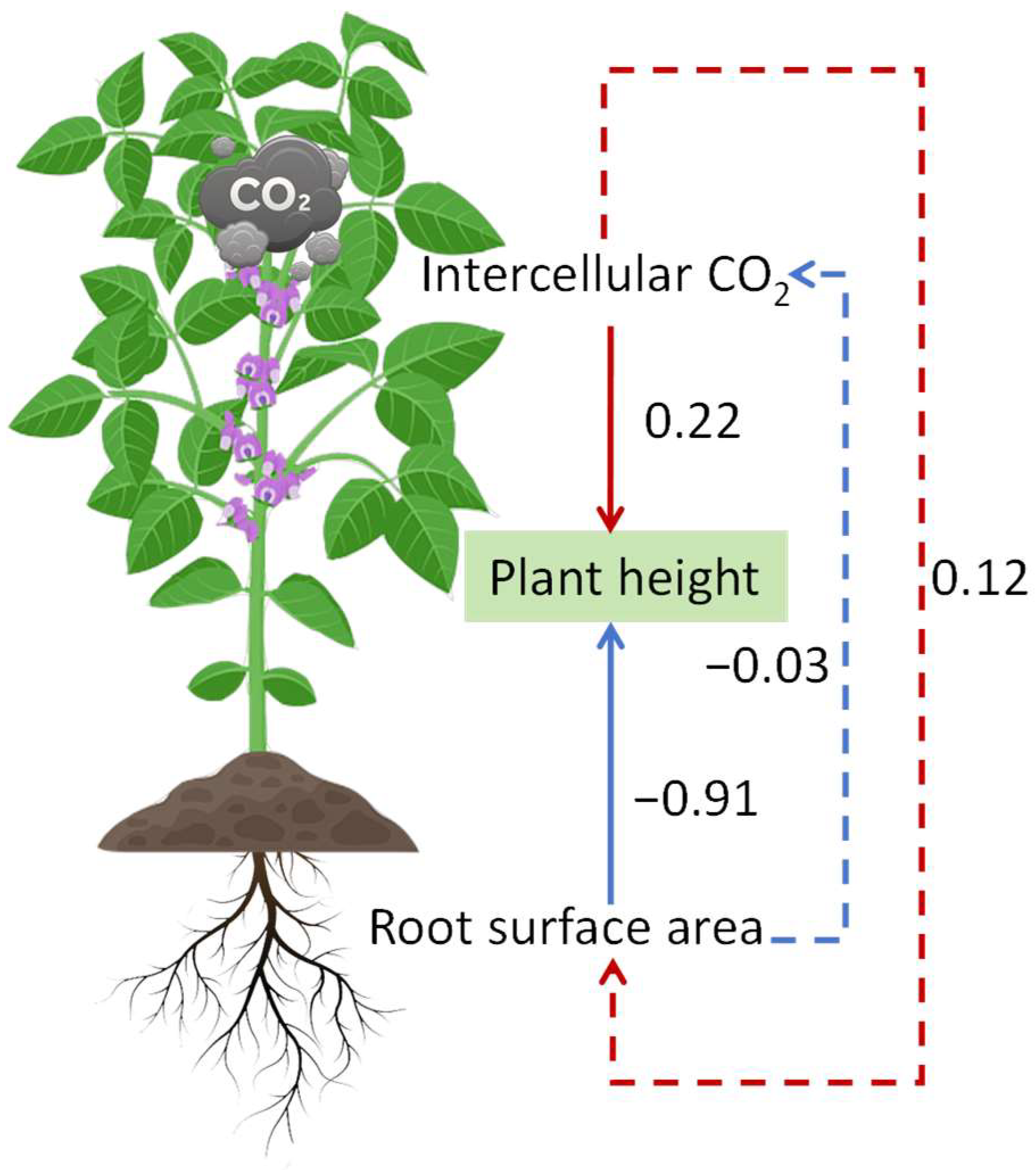
3.7. Role of Phenotypic Traits and Photosynthetic Characteristics in the Height of Soybean
4. Discussion
4.1. The Phenotypic Traits Affecting Forage Soybean Plant Height
4.2. The Root Traits Affecting Forage Soybean Plant Height
4.3. The Photosynthetic Indicators Affecting Forage Soybean Plant Height
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizzo, G.; Luciana, B. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 1, 43. [Google Scholar] [CrossRef]
- Islam, I.; Adam, Z.; Shahidul, I. Soybean (Glycine max): Alternative Sources of Human Nutrition and Bioenergy for the 21st Century. J. Food Sci. Technol. 2019, 7, 1–6. [Google Scholar] [CrossRef]
- Pretto, C.D.; Giordano, R.d.L.C.; Tardioli, P.W.; Caliane, B.B.C. Possibilities for Producing Energy, Fuels, and Chemicals from Soybean: A Biorefinery Concept. Waste Biomass Valorization 2018, 9, 1703–1730. [Google Scholar] [CrossRef]
- Azam, M.; Zhang, S.; Qi, J.; Abdelghany, A.M.; Shaibu, A.S.; Ghosh, S.; Feng, Y.; Huai, Y.; Gebregziabher, B.S.; Li, J.; et al. Profiling and associations of seed nutritional characteristics in Chinese and USA soybean cultivars. J. Food Compos. Anal. 2021, 98, 103803. [Google Scholar] [CrossRef]
- Nielsen, D.C. Forage Soybean Yield and Quality Response to Water Use. Field Crop Res. 2011, 3, 400–407. [Google Scholar] [CrossRef]
- Rogers, J.; Florez-Palacios, L.; Chen, P.Y.; Orazaly, M.; Jaureguy, L.M.; Zeng, A.L.; Wu, C.J. Evaluation of Diverse Soybean Germplasm for Forage Yield and Quality Attributes. Crop Sci. 2017, 2, 1020–1026. [Google Scholar] [CrossRef]
- Lee, E.J.; Choi, H.J.; Shin, D.H.; Kwon, C.H.; Shannon, J.G.; Lee, J.D. Evaluation of Forage Yield and Quality in Wild Soybeans (Glycine Soja Sieb. and Zucc.). Plant Breed. Biotechnol. 2014, 2, 71–79. [Google Scholar] [CrossRef]
- Kökten, K.; Seyithan, S.; Mahmut, K.; Erkan, B. Forage Nutritive Value of Soybean Varieties. Legume Res. 2011, 37, 201–206. [Google Scholar] [CrossRef]
- Darmosarkoro, W.; Harbur, M.M.; Buxton, D.R.; Moore, K.J.; Devine, T.E.; Anderson, I.C. Growth, Development, and Yield of Soybean Lines Developed for Forage. Agron. J. 2001, 5, 1028–1034. [Google Scholar] [CrossRef]
- Koivisto, J.; Devine, T.; Lane, G.; Sawyer, C.; Brown, H. Forage Soybeans (Glycine max (L.) Merr.) in the United Kingdom: Test of New Cultivars. Agronomie 2003, 4, 287–291. [Google Scholar] [CrossRef]
- Asekova, S.; Shannon, J.G.; Lee, J.D. The current status of forage soybean. Plant Breed. Biotechnol. 2014, 2, 334–341. [Google Scholar] [CrossRef]
- Liu, S.L.; Zhang, M.; Feng, F.; Tian, Z.X. Toward a Green Revolution for Soybean. Mol. Plant. 2020, 5, 688–697. [Google Scholar] [CrossRef]
- Huang, Z.W.; Wang, W.; Xu, X.J.; Wen, Z.X.; Li, H.C.; Li, J.Y.; Lu, W.G. Relationship of Dynamic Plant Height and Its Relative Growth Rate with Yield Using Recombinant Inbred Lines of Soybean. Acta Agron. Sin. 2011, 3, 559–562. [Google Scholar] [CrossRef]
- Josie, J.; Alcivar, A.; Rainho, J.; Kassem, A. Genomic Regions Containing QTL for Plant Height, Internodes Length, and Flower Color in Soybean [Glycine max (L.) Merr]. Bios. 2007, 4, 119–126. [Google Scholar] [CrossRef]
- Acikgoz, E.; Sincik, M.; Oz, M.; Albayrak, S.; Wietgrefe, G.; Turan, Z.M.; Goksoy, A.T.; Bilgili, U.; Karasu, A.; Tongel, O. Forage Soybean Performance in Mediterranean Environments. Field Crop Res. 2007, 3, 239–247. [Google Scholar] [CrossRef]
- Sheaffer, C.C.; Orf, J.H.; Devine, T.E.; Jewett, J.G. Yield and Quality of Forage Soybean. Agron. J. 2001, 1, 99–106. [Google Scholar] [CrossRef]
- Xiong, S.S.; Guo, D.D.; Wan, Z.; Quan, L.; Lu, W.T.; Xue, Y.; Liu, B.; Zhai, H. Regulation of soybean stem growth habit: A ten-year progress report. Crop J. 2023, 11, 1642–1648. [Google Scholar] [CrossRef]
- Kim, J.H.; Scaboo, A.; Pantalone, V.; Li, Z.; Bilyeu, K. Utilization of Plant Architecture Genes in Soybean to Positively Impact Adaptation to High Yield Environments. Front. Plant Sci. 2022, 13, 891587. [Google Scholar] [CrossRef]
- Zhang, L.; Allen, L.H., Jr.; Vaughan, M.M.; Hauser, B.A.; Boote, K. Solar Ultraviolet Radiation Exclusion Increases Soybean Internode Lengths and Plant Height. Agric. Meteorol. 2014, 184, 170–178. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, G.; Lv, H.Y.; Wang, C.H.; Yang, Y.Q.; Liao, H. Environmental and Genetic Regulation of Plant Height in Soybean. BMC Plant Biol. 2021, 21, 63. [Google Scholar] [CrossRef]
- Mustapha, Y.; Biwe, E.R.; Salem, A. Effects of Moisture Stress on the Growth Parameters of Soybean Genotypes. Discourse J. Agr. Food Sci. 2014, 2, 142–148. [Google Scholar]
- Servani, M.; Mobasser, H.; Sobhkhizi, A.; Adibian, M.; Noori, M. Effect of Phosphorus Fertilizer on Plant Height, Seed Weight and Number of Nodes in Soybean. Int. J. Plant Anim. Environ. Sci. 2014, 2, 696–700. [Google Scholar]
- He, H.F.; Yan, C.H.; Wu, N.; Liu, J.L.; Jia, Y.H. Effects of Different Nitrogen Levels on Photosynthetic Characteristics and Drought Resistance of Switchgrass (Panicum virgatum). Acta Prataculturae Sin. 2021, 30, 107. [Google Scholar] [CrossRef]
- Bengough, A.G.; McKenzie, B.M.; Hallett, P.D.; Valentine, T.A. Root Elongation, Water Stress, and Mechanical Impedance: A Review of Limiting Stresses and Beneficial Root Tip Traits. J. Exp. Bot. 2011, 1, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Fried, H.G.; Narayanan, S.; Fallen, B. Characterization of a Soybean (Glycine max L. Merr.) Germplasm Collection for Root Traits. PLoS ONE 2018, 7, e0200463. [Google Scholar] [CrossRef] [PubMed]
- Xue, A.; Zhao, M.H.; Qian, Z.; Jie, L.; Zhang, H.J.; Wang, H.Y.; Yu, C.M.; Li, C.H.; Yao, X.D.; Xie, F.T. Study on Plant Morphological Traits and Production Characteristics of Super High-Yielding Soybean. J. Integr. Agric. 2013, 7, 1173–1182. [Google Scholar] [CrossRef]
- Lu, Y.P.; Zhang, J.M.; Guo, X.Y.; Chen, J.J.; Chang, R.Z.; Guan, R.X.; Qiu, L.J. Identification of Genomic Regions Associated with Vine Growth and Plant Height of Soybean. Int. J. Mol. Sci. 2022, 10, 5823. [Google Scholar] [CrossRef] [PubMed]
- Tsukaya, H. The Leaf Index: Heteroblasty, Natural Variation, and the Genetic Control of Polar Processes of Leaf Expansion. Plant Cell Physiol. 2002, 43, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Hallauer, A.R. History, Contribution, and Future of Quantitative Genetics in Plant Breeding: Lessons from Maize. Crop Sci. 2007, 27, S4–S19. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.J.; Cheng, T.T.; Tong, C.C.; Xue, W. Study on Leaf Characteristics and Yield Effect of Alfalfa with Different Nitrogen Efficiency. Acta Agrestia Sin. 2021, 9, 1941. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Xu, F.; Zhang, Y.Q.; Hai, G.; Yang, C.Y.; Wu, Z.; Wang, B.; Yu, Z. Comparison and Correlation of Agronomic Characteristics and Fermentation Quality of Different Types of Hybrid Corn. Acta Agrestia Sin. 2019, 1, 250. [Google Scholar] [CrossRef]
- Sujata, B.; Basavaraja, G.T.; Salimath, P.M. Evaluation of Narrow Leaflet Genotypes and Genetic Variability in Segregating Generation of Soybean (Glycine max (L) Merrill). Electron. J. Plant Breed. 2011, 2, 124–131. [Google Scholar]
- Virdi, K.S.; Sreekanta, S.; Dobbels, A.; Haaning, A.; Jarquin, D.; Stupar, R.M.; Lorenz, A.J.; Muehlbauer, G. Branch Angle and Leaflet Shape Are Associated with Canopy Coverage in Soybean. Plant Genome 2023, 16, e20304. [Google Scholar] [CrossRef] [PubMed]
- Haile, F.J.; Higley, L.G.; Specht, J.E.; Spomer, S.M. Soybean Leaf Morphology and Defoliation Tolerance. Agron. J. 1998, 3, 353–362. [Google Scholar] [CrossRef]
- Salim, M.; Chen, Y.L.; Ye, H.; Nguyen, H.T.; Solaiman, Z.M.; Siddique, K.H.M. Screening of Soybean Genotypes Based on Root Morphology and Shoot Traits Using the Semi-Hydroponic Phenotyping Platform and Rhizobox Technique. Agronomy 2021, 1, 56. [Google Scholar] [CrossRef]
- Narayanan, S.; Mohan, A.; Gill, K.S.; Prasad, P.V.V. Variability of Root Traits in Spring Wheat Germplasm. PLoS ONE 2014, 6, e100317. [Google Scholar] [CrossRef]
- Shen, Y.; Gilbert, G.S.; Li, W.; Fang, M.; Lu, H.P.; Yu, S.X. Linking Aboveground Traits to Root Traits and Local Environment: Implications of the Plant Economics Spectrum. Front. Plant Sci. 2019, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Ryser, P.; Eek, L. Consequences of Phenotypic Plasticity vs. Interspecific Differences in Leaf and Root Traits for Acquisition of Aboveground and Belowground Resources. Am. J. Bot. 2000, 3, 402–411. [Google Scholar] [CrossRef]
- Schulze, E.D.; Caldwell, M.M. Overview: Perspectives in Ecophysiological Research of Photosynthesis. In Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1995; pp. 553–564. [Google Scholar] [CrossRef]
- Lan, J.H.; Chu, D. Study on the Genetic Basis of Plant Height and Ear Height in Maize (Zea mays L.) by QTL Dissection. Hereditas 2005, 6, 925–934. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.Z.; Wang, X.P.; You, Z.M.; Chen, C.S. Comparison of Leaf Functional and Photosynthetic Characteristics in Different Tea Cultivars. J. Tea Sci. Res. 2016, 3, 285–292. [Google Scholar] [CrossRef]
- Sakoda, K.; Tanaka, Y.; Long, S.P.; Shiraiwa, T. Genetic and Physiological Diversity in the Leaf Photosynthetic Capacity of Soybean. Crop Sci. 2016, 5, 2731–2741. [Google Scholar] [CrossRef]
- Sawada, S.; Kuninaka, M.; Watanabe, K.; Sato, A.; Kawamura, H.; Komine, K.; Sakamoto, T.; Kasai, M. The Mechanism to Suppress Photosynthesis through End-Product Inhibition in Single-Rooted Soybean Leaves During Acclimation to CO2 Enrichment. Plant Cell Physiol. 2001, 10, 1093–1102. [Google Scholar] [CrossRef] [PubMed]





| Test Material | Origin | Height Class | 2016 Height (cm) | 2017 Height (cm) | 2018 Height (cm) |
|---|---|---|---|---|---|
| Neinong S001 | Inner Mongolia, China | Tall | 189 | 172.4 | 175.9 |
| Neinong S002 | Inner Mongolia, China | Tall | 186 | 180.2 | 176.5 |
| Neinong 0004 | Inner Mongolia, China | Short | 79.2 | 72.3 | 82.3 |
| Neinong 0005 | Inner Mongolia, China | Short | 78.6 | 79.9 | 78.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suo, R.; Wang, M.; Zhao, T. Contribution of Photosynthetic, Root and Phenotypic Traits to Soybean Plant Height. Sustainability 2024, 16, 2886. https://doi.org/10.3390/su16072886
Suo R, Wang M, Zhao T. Contribution of Photosynthetic, Root and Phenotypic Traits to Soybean Plant Height. Sustainability. 2024; 16(7):2886. https://doi.org/10.3390/su16072886
Chicago/Turabian StyleSuo, Rongzhen, Mingjiu Wang, and Tianqi Zhao. 2024. "Contribution of Photosynthetic, Root and Phenotypic Traits to Soybean Plant Height" Sustainability 16, no. 7: 2886. https://doi.org/10.3390/su16072886
APA StyleSuo, R., Wang, M., & Zhao, T. (2024). Contribution of Photosynthetic, Root and Phenotypic Traits to Soybean Plant Height. Sustainability, 16(7), 2886. https://doi.org/10.3390/su16072886





