Study on Sustainable Application of Low-Carbon Supersulfated Cement with Alkanolamines
Abstract
:1. Introduction
2. Materials and Methods
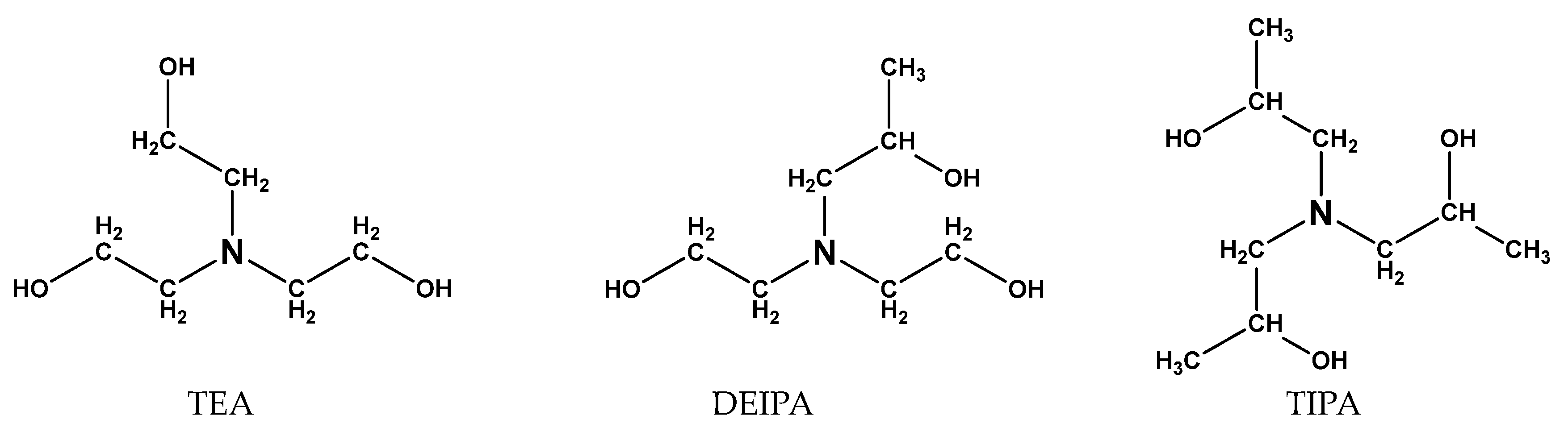
2.1. Materials
2.2. Mix Proportion
2.3. Methodology
2.3.1. Mechanical Strength
2.3.2. Isothermal Calorimetry
2.3.3. XRD and QXRD
2.3.4. TGA
2.3.5. Degree of Hydration
2.3.6. SEM
3. Results
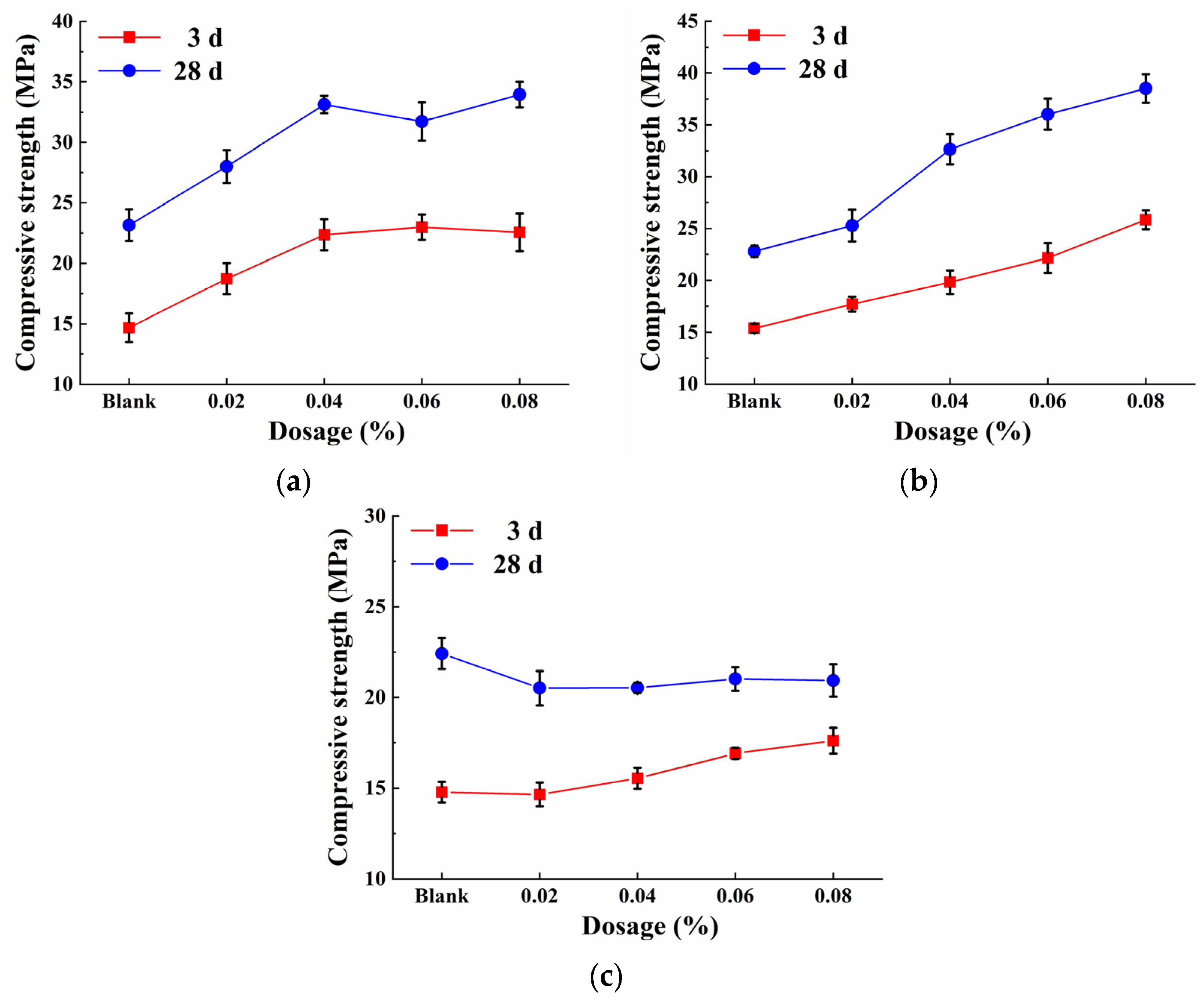
3.1. Mechanical Properties
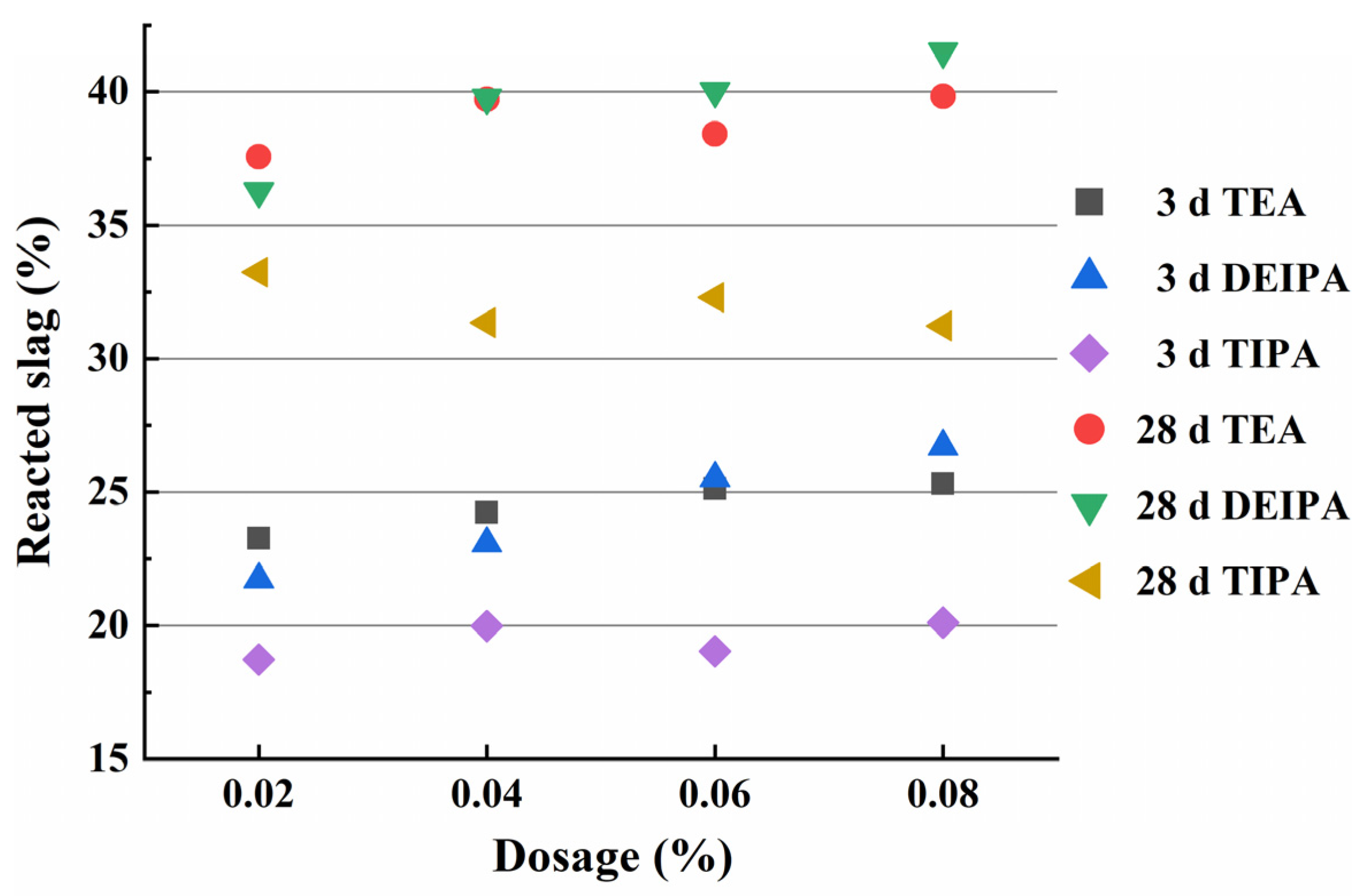
3.2. Degree of Slag Hydration
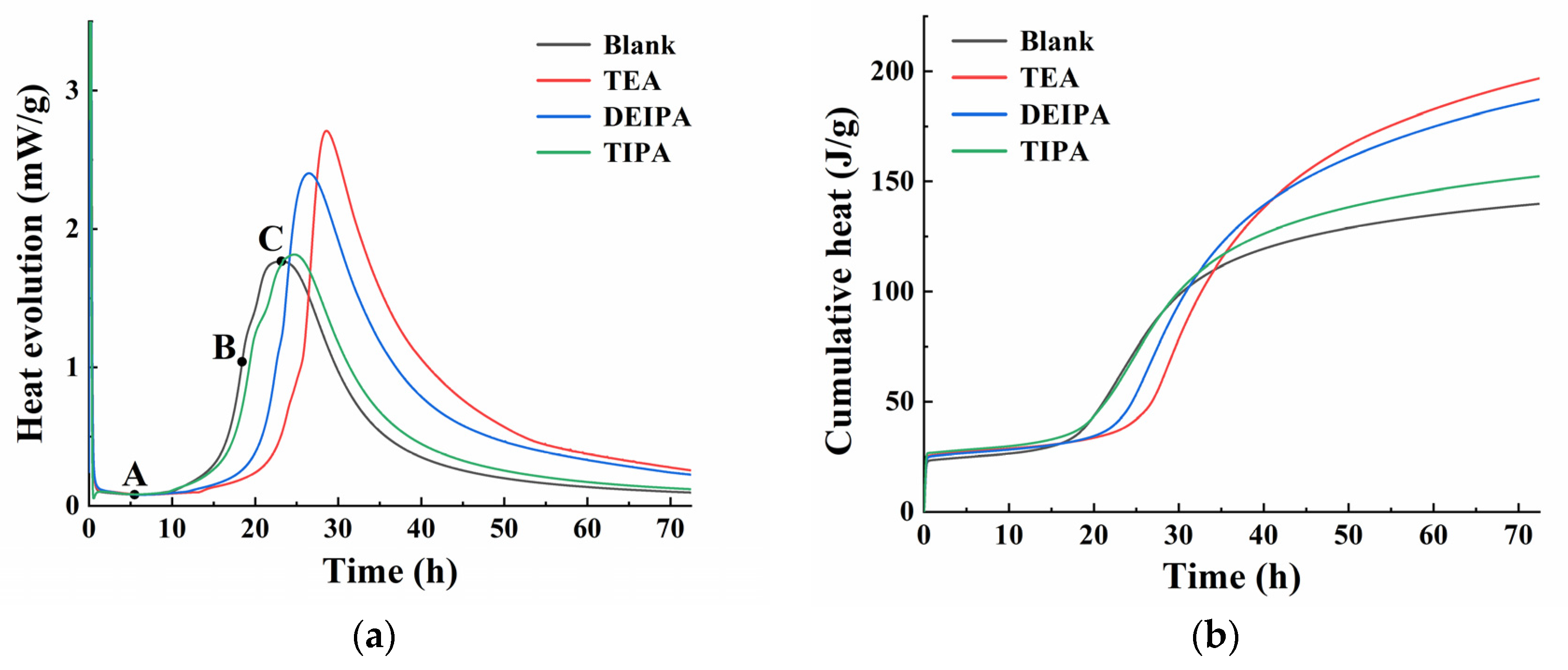
3.3. Heat Evolution
3.4. Hydration Products
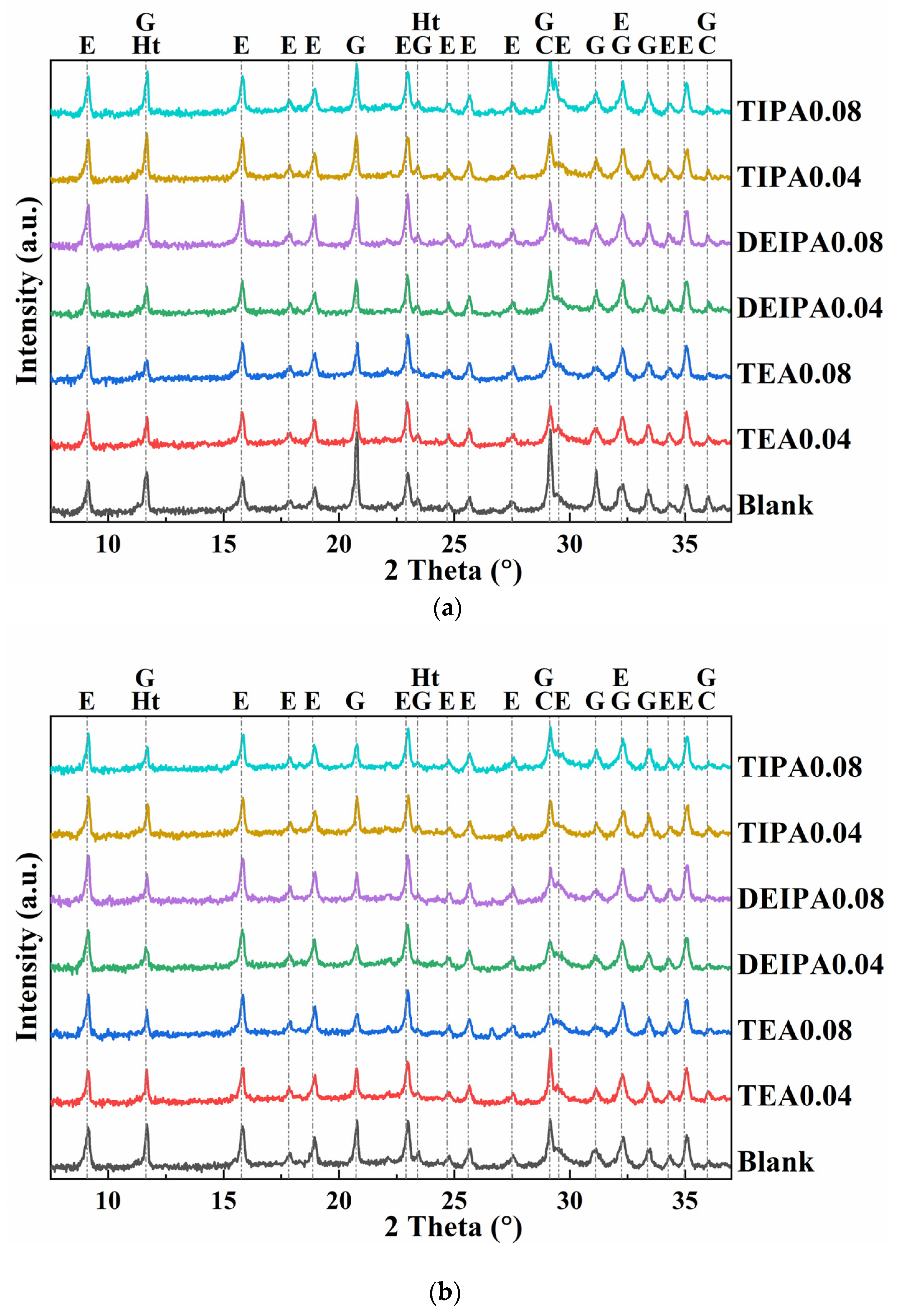
3.4.1. XRD Analysis
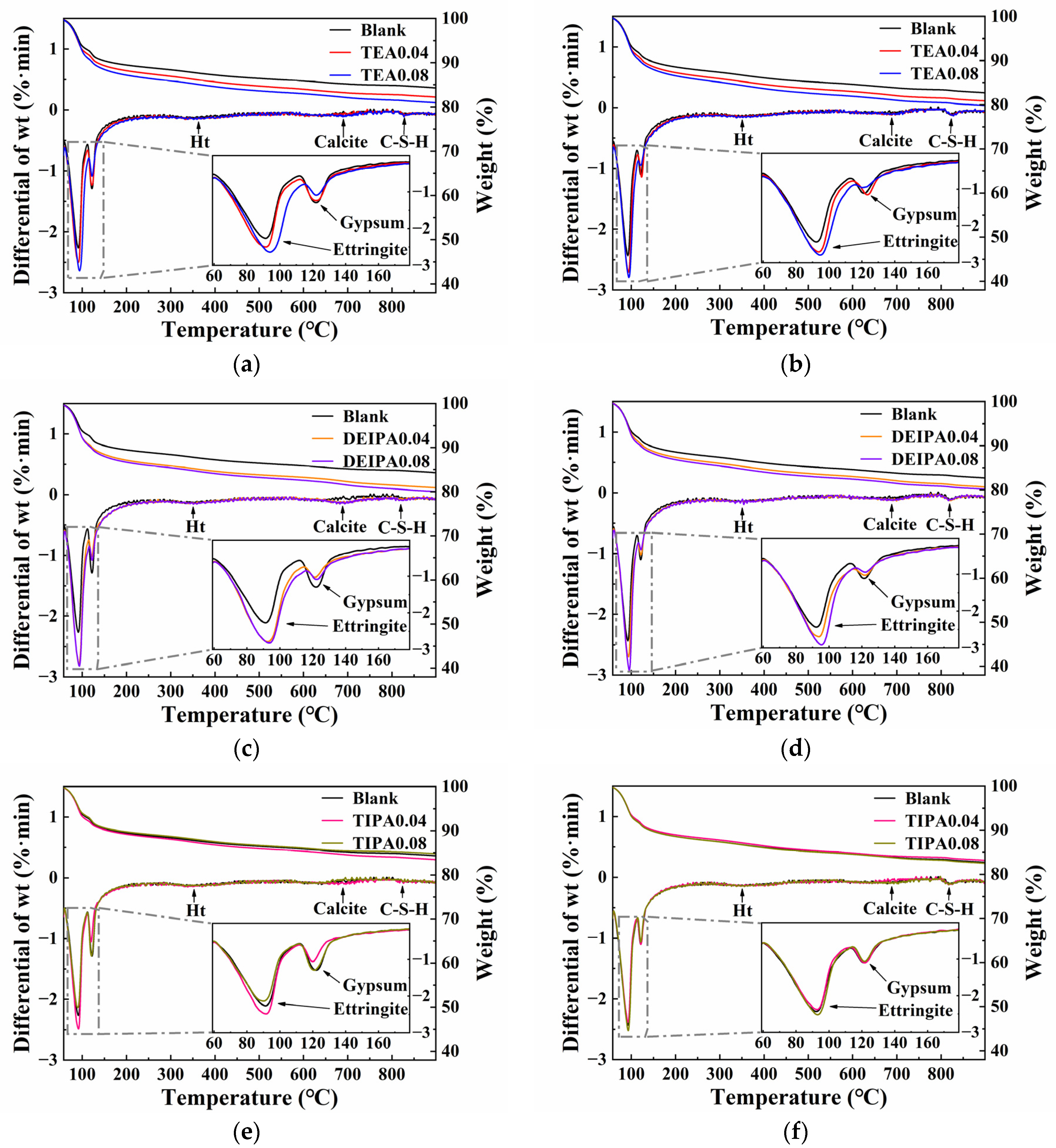
3.4.2. TGA
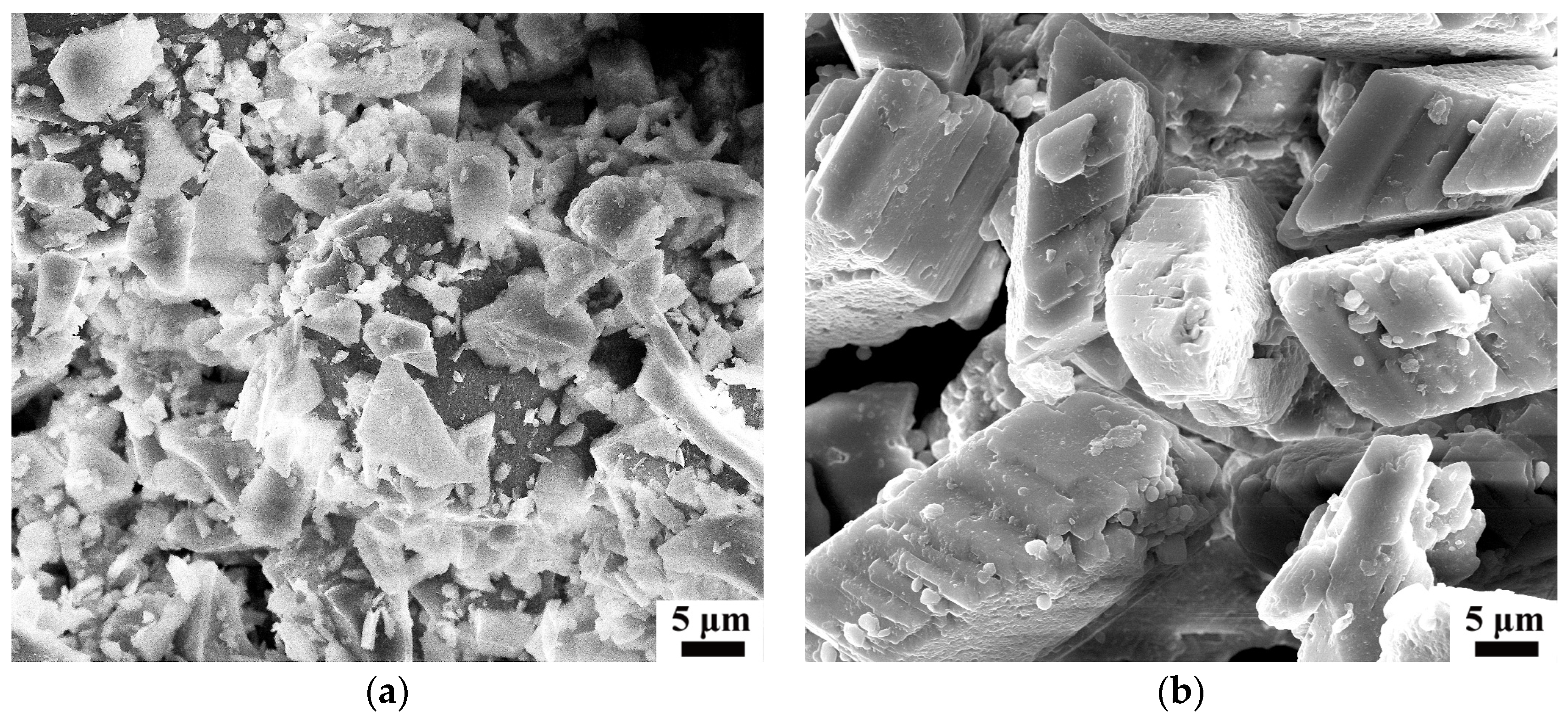
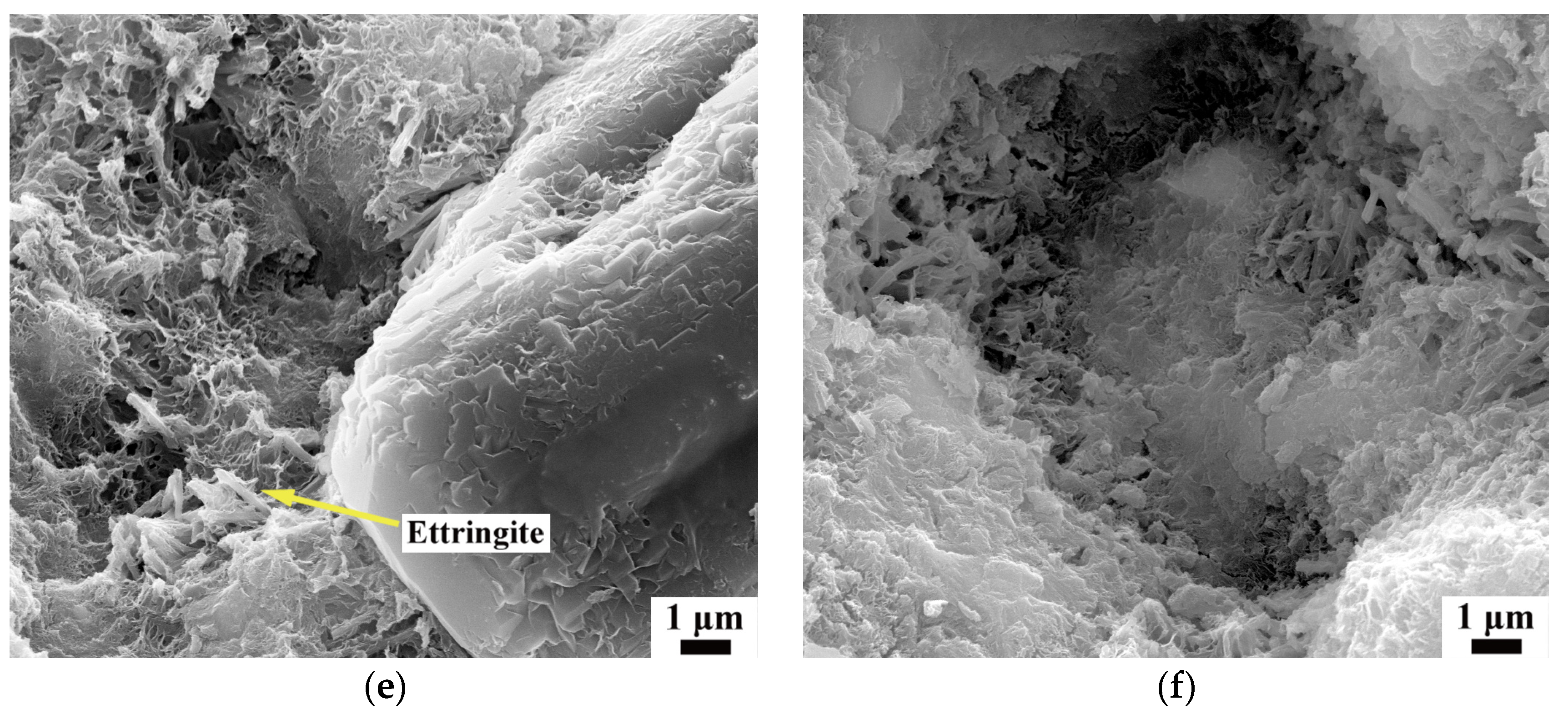
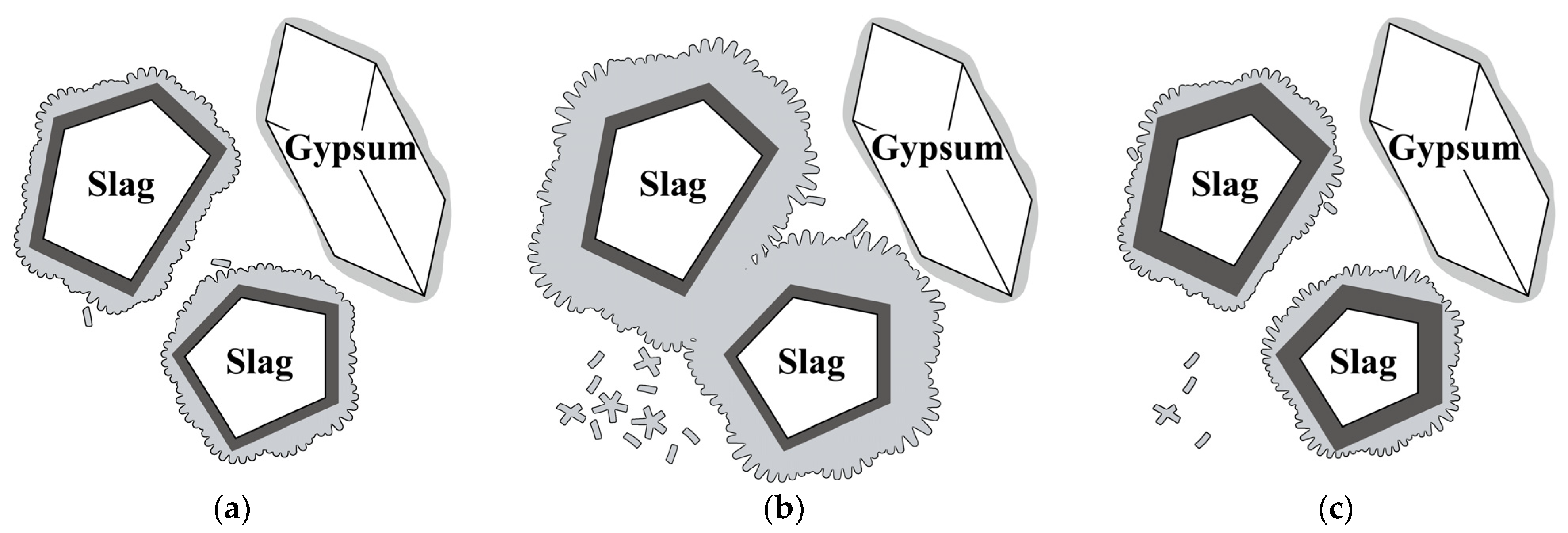
3.5. Morphology
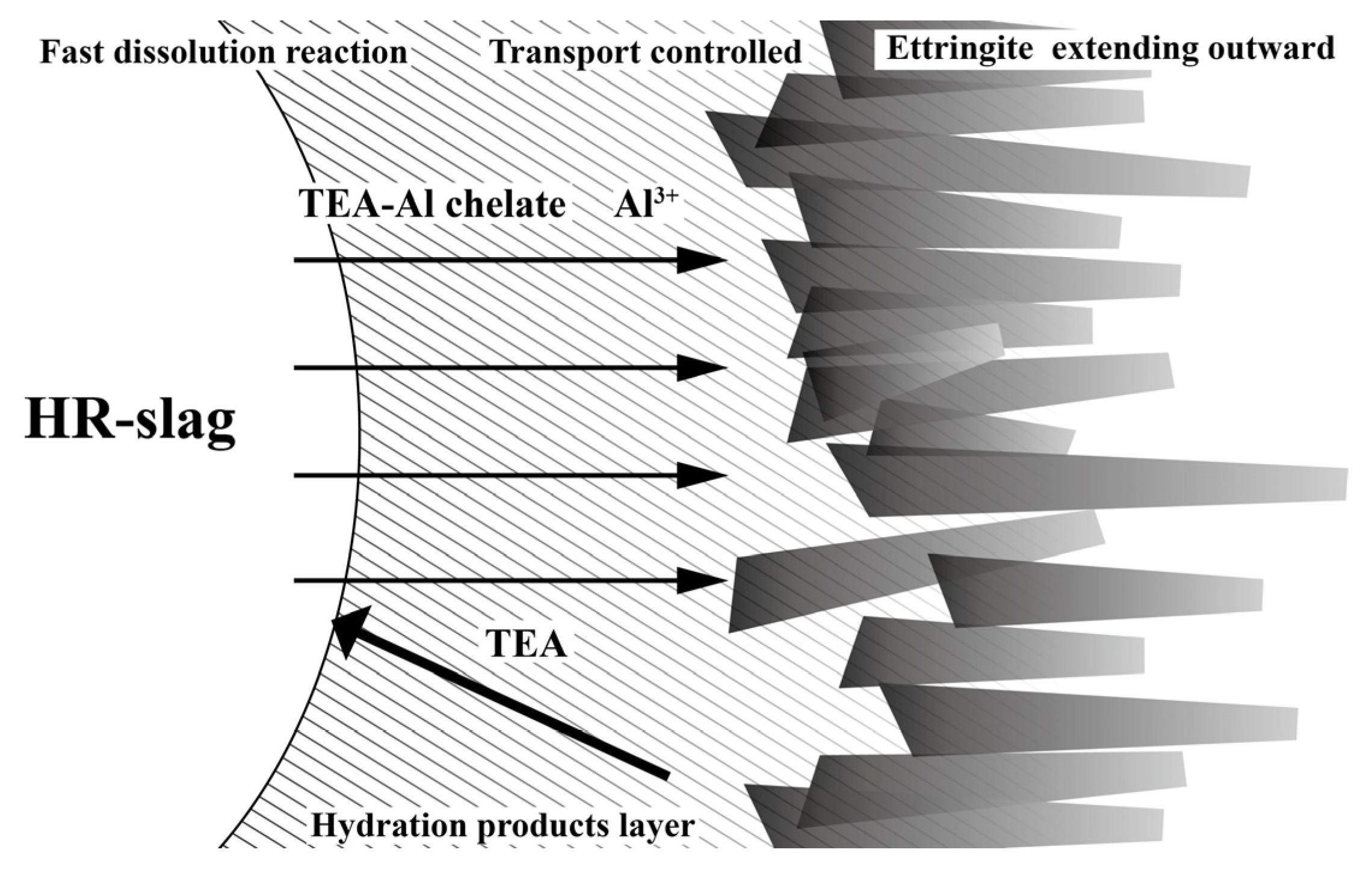
4. Discussion

5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hafez, H.; Kassim, D.; Kurda, R.; Silva, R.V.; de Brito, J. Assessing the sustainability potential of alkali-activated concrete from electric arc furnace slag using the ECO2 framework. Constr. Build. Mater. 2021, 281, 122559. [Google Scholar] [CrossRef]
- Shahane, H.A.; Patel, S. Influence of curing method on characteristics of environment-friendly angular shaped cold bonded fly ash aggregates. J. Build. Eng. 2021, 35, 101997. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—Present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Trung, N.T.; Alemi, N.; Haido, J.H.; Shariati, M.; Baradaran, S.; Yousif, S.T. Reduction of cement consumption by producing smart green concretes with natural zeolites. Smart Struct. Syst. 2019, 24, 415–425. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Hooton, R.D.; Zhang, X.; He, S. Effects of TEA on rheological property and hydration performance of lithium slag-cement composite binder. Constr. Build. Mater. 2022, 318, 125757. [Google Scholar] [CrossRef]
- Kong, X.-M.; Lu, Z.-B.; Liu, H.; Wang, D.-M. Influence of triethanolamine on the hydration and the strength development of cementitious systems. Mag. Concr. Res. 2013, 65, 1101–1109. [Google Scholar] [CrossRef]
- Xu, Z.; Li, W.; Sun, J.; Hu, Y.; Xu, K.; Ma, S.; Shen, X. Hydration of Portland cement with alkanolamines by thermal analysis. J. Therm. Anal. Calorim. 2017, 131, 37–47. [Google Scholar] [CrossRef]
- Xu, Z.; Li, W.; Sun, J.; Hu, Y.; Xu, K.; Ma, S.; Shen, X. Research on cement hydration and hardening with different alkanolamines. Constr. Build. Mater. 2017, 141, 296–306. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, L.; Cai, X.; Li, Q.; Kong, X. Influences of triethanolamine on the performance of cement pastes used in slab track. Constr. Build. Mater. 2020, 238, 117670. [Google Scholar] [CrossRef]
- Yaphary, Y.L.; Yu, Z.; Lam, R.H.W.; Lau, D. Effect of triethanolamine on cement hydration toward initial setting time. Constr. Build. Mater. 2017, 141, 94–103. [Google Scholar] [CrossRef]
- Perez, J.-P.; Nonat, A.; Garrault, S.; Pourchet, S.; Mosquet, M. Influence of triisopropanolamine on the physico-chemical and mechanical properties of pure cement pastes and mortars. In Proceedings of the 11th ICCC, Durban, South Africa, 11–16 May 2003; Volume 28, pp. 454–463. [Google Scholar]
- Ramachandran, V.S. Action of triethanolamine on the hydration of tricalcium aluminate. Cem. Concr. Res. 1973, 3, 41–54. [Google Scholar] [CrossRef]
- Han, J.; Wang, K.; Shi, J.; Wang, Y. Mechanism of triethanolamine on Portland cement hydration process and microstructure characteristics. Constr. Build. Mater. 2015, 93, 457–462. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Jansen, D.; Zhang, C.; Wang, J.; Pang, X.; Yin, J. Towards a further understanding of cement hydration in the presence of triethanolamine. Cem. Concr. Res. 2020, 132, 106041. [Google Scholar] [CrossRef]
- Yan-Rong, Z.; Xiang-Ming, K.; Zi-Chen, L.; Zhen-Bao, L.; Qing, Z.; Bi-Qin, D.; Feng, X. Influence of triethanolamine on the hydration product of portlandite in cement paste and the mechanism. Cem. Concr. Res. 2016, 87, 64–76. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Zhang, X.; Liu, W.; Liao, G.; Xu, M. Influence of triethanolamine on mechanical strength and hydration performance of blended cement containing fly ash, limestone and slag. J. Build. Eng. 2021, 44, 102879. [Google Scholar] [CrossRef]
- Sandberg, P.J.; Doncaster, F. On the mechanism of strength enhancement of cement paste and mortar with triisopropanolamine. Cem. Concr. Res. 2004, 34, 973–976. [Google Scholar] [CrossRef]
- Gartner, E.; Myers, D. Influence of tertiary alkanolamines on portland-cement hydration. J. Am. Ceram. Soc. 1993, 76, 1521–1530. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.-r.; Shen, X.-d. Hydration of ternary cement in the presence of triisopropanolamine. Constr. Build. Mater. 2016, 111, 513–521. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, T.; Tan, H.; Liu, X.; Mei, J.; Qi, H.; Jiang, W.; Zou, F. Effect of triisopropanolamine on compressive strength and hydration of cement-fly ash paste. Constr. Build. Mater. 2018, 179, 89–99. [Google Scholar] [CrossRef]
- Zhang, F.; Bai, Y.; Cai, Y.; Chen, B.; Ning, F. Effect of Triisopropanolamine on the Compressive Strength and Early Hydration of Cement at Low Temperature. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2020, 35, 611–619. [Google Scholar] [CrossRef]
- Wang, J.; Chang, L.; Yue, D.; Zhou, Y.; Liu, H.; Wang, Y.; Yang, S.; Cui, S. Effect of chelating solubilization via different alkanolamines on the dissolution properties of steel slag. J. Clean. Prod. 2022, 365, 132824. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Jansen, D.; Zhang, C. A comparative study of the effects of two alkanolamines on cement hydration. Adv. Cem. Res. 2022, 34, 47–56. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, L.; Hu, X.; Liu, Y.; Shi, C. Effect of diethanolisopropanolamine and ethyldiisopropylamine on hydration and strength development of Portland cement. Cem. Concr. Res. 2022, 162, 132824. [Google Scholar] [CrossRef]
- Ma, S.; Li, W.; Zhang, S.; Hu, Y.; Shen, X. Study on the hydration and microstructure of Portland cement containing diethanol-isopropanolamine. Cem. Concr. Res. 2015, 67, 122–130. [Google Scholar] [CrossRef]
- Lu, X.; Ye, Z.; Zhang, L.; Hou, P.; Cheng, X. The influence of ethanol-diisopropanolamine on the hydration and mechanical properties of Portland cement. Constr. Build. Mater. 2017, 135, 484–489. [Google Scholar] [CrossRef]
- Heinz, D.; Göbel, M.; Hilbig, H.; Urbonas, L.; Bujauskaite, G. Effect of TEA on fly ash solubility and early age strength of mortar. Cem. Concr. Res. 2010, 40, 392–397. [Google Scholar] [CrossRef]
- Riding, K.; Silva, D.A.; Scrivener, K. Early age strength enhancement of blended cement systems by CaCl2 and diethanol-isopropanolamine. Cem. Concr. Res. 2010, 40, 935–946. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Novak, R.; Schneider, W.; Lang, E. New knowledge regarding the supersulphated cement Slagstar. ZKG Int. 2005, 58, 70–78. [Google Scholar]
- Dutta, D.K.; Borthakur, P.C. Activation of low lime high alumina granulated blast-furnace slag by anhydrite. Cem. Concr. Res. 1990, 20, 711–722. [Google Scholar] [CrossRef]
- Bijen, J.; Niel, E. Supersulfated cement from blastfurnace slag and chemical gypsum available in the netherlands and neighboring countries. Cem. Concr. Res. 1981, 11, 307–320. [Google Scholar] [CrossRef]
- Gruskovnjak, A.; Lothenbach, B.; Winnefeld, F.; Münch, B.; Figi, R.; Ko, S.-C.; Adler, M.; Mäder, U. Quantification of hydration phases in supersulfated cements: Review and new approaches. Adv. Cem. Res. 2011, 23, 265–275. [Google Scholar] [CrossRef]
- Rubert, S.; Angulski da Luz, C.; Varela, M.V.F.; Pereira Filho, J.I.; Hooton, R.D. Hydration mechanisms of supersulfated cement. J. Therm. Anal. Calorim. 2018, 134, 971–980. [Google Scholar] [CrossRef]
- Gruskovnjak, A.; Lothenbach, B.; Winnefeld, F.; Figi, R.; Ko, S.C.; Adler, M.; Mäder, U. Hydration mechanisms of super sulphated slag cement. Cem. Concr. Res. 2008, 38, 983–992. [Google Scholar] [CrossRef]
- Matschei, T.; Bellmann, E.; Stark, J. Hydration behaviour of sulphate-activated slag cements. Adv. Cem. Res. 2005, 17, 167–178. [Google Scholar] [CrossRef]
- Grounds, T.; Nowell, D.V.; Wilburn, F.W. The influence of temperature and different storage-conditions on the stability of supersulphated cement. J. Therm. Anal. 1994, 41, 687–699. [Google Scholar] [CrossRef]
- Erdem, E.; Olmez, H. The mechanical-properties of supersulphated cement containing phosphogypsum. Cem. Concr. Res. 1993, 23, 115–121. [Google Scholar] [CrossRef]
- Grounds, T.; Nowell, D.V.; Wilburn, F.W. Resistance of supersulfated cement to strong sulfate solutions. J. Therm. Anal. Calorim. 2003, 72, 181–190. [Google Scholar] [CrossRef]
- Pinto, S.R.; da Luz, C.A.; Munhoz, G.S.; Medeiros, R.A. Durability of phosphogypsum-based supersulfated cement mortar against external attack by sodium and magnesium sulfate. Cem. Concr. Res. 2020, 136, 106172. [Google Scholar] [CrossRef]
- Pinto, S.R.; da Luz, C.A.; Munhoz, G.S.; Medeiros-Junior, R.A. Resistance of phosphogypsum-based supersulfated cement to carbonation and chloride ingress. Constr. Build. Mater. 2020, 263, 120640. [Google Scholar] [CrossRef]
- Cabrera-Luna, K.; Perez-Cortes, P.; Garcia, I.E. Pumice-based supersulfated cements in mortars: Effects of pumice fineness and activator ratio on physical and environmental characteristics. Constr. Build. Mater. 2022, 342, 127947. [Google Scholar] [CrossRef]
- Goetz-Neunhoeffer, F.; Neubauer, J. Refined ettringite (Ca6Al2(SO4)3(OH)12•26H2O structure for quantitative X-ray diffraction analysis. Powder Diffr. 2006, 21, 4–11. [Google Scholar] [CrossRef]
- Boeyens, J.C.A.; Ichharam, V.V.H. Redetermination of the crystal structure of calcium sulphate dihydrate, CaSO4•2H2O. Z. Fur Krist.-New Cryst. Struct. 2002, 217, 9–10. [Google Scholar] [CrossRef]
- Markgraf, S.A.; Reeder, R.J. High-temperature structure refinements of calcite and magnesite. Am. Mineral. 1985, 70, 590–600. [Google Scholar]
- Burdett, J.K.; Hughbanks, T.; Miller, G.J.; Richardson, J.W., Jr.; Smith, J.V. Structural-electronic relationships in inorganic solids: Powder neutron diffraction studies of the rutile and anatase polymorphs of titanium dioxide at 15 and 295 K. J. Am. Chem. Soc. 1987, 109, 3639–3646. [Google Scholar] [CrossRef]
- Kocaba, V.; Gallucci, E.; Scrivener, K.L. Methods for determination of degree of reaction of slag in blended cement pastes. Cem. Concr. Res. 2012, 42, 511–525. [Google Scholar] [CrossRef]
- Luke, K.; Glasser, F.P. Selective dissolution of hydrated blast-furnace slag cements. Cem. Concr. Res. 1987, 17, 273–282. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; He, X.; Su, Y.; Strnadel, B.; Miao, W. Hydration and compressive strength of supersulfated cement with low-activity high alumina ferronickel slag. Cem. Concr. Compos. 2023, 136, 104892. [Google Scholar] [CrossRef]
- Bazaldua-Medellin, M.E.; Fuentes, A.F.; Gorokhovsky, A.; Escalante-Garcia, J.I. Early and late hydration of supersulphated cements of blast furnace slag with fluorgypsum. Mater. De Constr. 2015, 65, e043. [Google Scholar] [CrossRef]
- Tan, H.; Nie, K.; He, X.; Guo, Y.; Zhang, X.; Deng, X.; Su, Y.; Yang, J. Effect of organic alkali on compressive strength and hydration of wet-grinded granulated blast-furnace slag containing Portland cement. Constr. Build. Mater. 2019, 206, 10–18. [Google Scholar] [CrossRef]
- Masoudi, R.; Hooton, R.D. Examining the hydration mechanism of supersulfated cements made with high and low-alumina slags. Cem. Concr. Compos. 2019, 103, 193–203. [Google Scholar] [CrossRef]
- Lothenbach, B.; Durdziński, P.; De Weerdt, K. Thermogravimetric analysis. In A Practical Guide to Microstructural Analysis of Cementitious Materials; Scrivener, K., Snellings, R., Lothenbach, B., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 178–208. [Google Scholar]
- Cassagnabere, F.; Mouret, M.; Escadeillas, G. Early hydration of clinker-slag-metakaolin combination in steam curing conditions, relation with mechanical properties. Cem. Concr. Res. 2009, 39, 1164–1173. [Google Scholar] [CrossRef]
- Kalpokaite-Dickuviene, R.; Baltusnikas, A.; Levinskas, R.; Cesniene, J. Incinerator residual ash-metakaolin blended cements: Effect on cement hydration and properties. Constr. Build. Mater. 2019, 206, 297–306. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L. Hydration products of alkali-activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Angulo-Ramirez, D.E.; de Gutierrez, R.M.; Puertas, F. Alkali-activated Portland blast-furnace slag cement: Mechanical properties and hydration. Constr. Build. Mater. 2017, 140, 119–128. [Google Scholar] [CrossRef]
- Nied, D.; Enemark-Rasmussen, K.; L’Hopital, E.; Skibsted, J.; Lothenbach, B. Properties of magnesium silicate hydrates (M-S-H). Cem. Concr. Res. 2016, 79, 323–332. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Chang, T.P.; Shih, J.Y.; Chen, C.T. Influence of low calcium fly ash on compressive strength and hydration product of low energy super sulfated cement paste. Cem. Concr. Compos. 2019, 99, 40–48. [Google Scholar] [CrossRef]
- Xing, J.; Zhou, Y.; Peng, Z.; Wang, J.; Jin, Y.; Jin, M. The influence of different kinds of weak acid salts on the macro-performance, micro-structure, and hydration mechanism of the supersulfated cement. J. Build. Eng. 2023, 66, 105937. [Google Scholar] [CrossRef]
- Li, Y.; Qiao, C.Y.; Ni, W. Green concrete with ground granulated blast-furnace slag activated by desulfurization gypsum and electric arc furnace reducing slag. J. Clean. Prod. 2020, 269, 122212. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1996; Volume 25, p. 1162. [Google Scholar]
- Pinkas, J.; Verkade, J.G. Alumatrane, Al(OCH2CH2)3N: A reinvestigation of its oligomeric behavior. Inorg. Chem. 1993, 32, 2711–2716. [Google Scholar] [CrossRef]
- Fernandez, L.; Viruela-Martin, P.; Latorre, J.; Guillem, C.; Beltrán, A.; Amorós, P. Theoretical study of oligomeric alumatranes present in the chemistry of materials from micro to mesoporous molecular sieves and alumina composites. J. Mol. Struct. 2008, 850, 94–104. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Avet, F.; Hanpongpun, W.; Scrivener, K. Strength-promoting mechanism of alkanolamines on limestone-calcined clay cement and the role of sulfate. Cem. Concr. Res. 2021, 147, 106527. [Google Scholar] [CrossRef]



| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Loss | |
|---|---|---|---|---|---|---|---|
| Cement | 22.16 | 5.18 | 3.95 | 66.25 | 1.26 | 0.36 | 0.09 |
| GGBS | 34.5 | 17.7 | 1.03 | 34 | 6.01 | 1.64 | 0.84 |
| Desulfurization gypsum | 2.18 | 0.91 | 0.49 | 30.07 | 0.25 | 42.38 | 22.71 |
| ID | SSC | TEA | DEIPA | TIPA |
|---|---|---|---|---|
| Blank | 100 | 0 | 0 | 0 |
| TEA0.02 | 100 | 0.02 | 0 | 0 |
| TEA0.04 | 100 | 0.04 | 0 | 0 |
| TEA0.06 | 100 | 0.06 | 0 | 0 |
| TEA0.06 | 100 | 0.08 | 0 | 0 |
| DEIPA0.02 | 100 | 0 | 0.02 | 0 |
| DEIPA0.04 | 100 | 0 | 0.04 | 0 |
| DEIPA0.06 | 100 | 0 | 0.06 | 0 |
| DEIPA0.08 | 100 | 0 | 0.08 | 0 |
| TIPA0.02 | 100 | 0 | 0 | 0.02 |
| TIPA0.04 | 100 | 0 | 0 | 0.04 |
| TIPA0.06 | 100 | 0 | 0 | 0.06 |
| TIPA0.08 | 100 | 0 | 0 | 0.08 |
| Phase | Formula | Crystal System | ICSD Cods | Reference |
|---|---|---|---|---|
| Ettringite | 3CaO·Al2O3·3CaSO4·32H2O | Trigonal | 155395 | [44] |
| Gypsum | CaSO4·2H2O | Monoclinic | 409581 | [45] |
| Calcite | CaCO3 | Trigonal | 40545 | [46] |
| Rutile (standard) | TiO2 | Tetragonal | 202241 | [47] |
| tA (h) | (dQ/dt)A (mW/g) | tC (h) | (dQ/dt)C (mW/g) | Q (J/g) | kB | |
|---|---|---|---|---|---|---|
| Blank | 5.5 | 0.08119 | 23.18333 | 1.76642 | 139.89844 | 0.42955 |
| TEA | 7.56667 | 0.08109 | 28.66667 | 2.70791 | 196.85867 | 0.89248 |
| DEIPA | 6.35833 | 0.07967 | 26.49167 | 2.4025 | 187.21136 | 0.68707 |
| TIPA | 5.96667 | 0.08271 | 24.71667 | 1.81567 | 152.32699 | 0.39886 |
| 3 d | Blank | TEA0.04 | TEA0.08 | DEIPA0.04 | DEIPA0.08 | TIPA0.04 | TIPA0.08 |
| Ettringite | 0.1118 | 0.1162 | 0.1226 | 0.1131 | 0.1343 | 0.1094 | 0.1061 |
| Gypsum | 0.1115 | 0.0611 | 0.0463 | 0.0529 | 0.0679 | 0.0729 | 0.0747 |
| Calcite | 0.0122 | 0.0075 | 0.0081 | 0.0089 | 0.0060 | 0.0148 | 0.0121 |
| Amorphous | 0.7644 | 0.8151 | 0.8230 | 0.8091 | 0.7918 | 0.8029 | 0.8071 |
| 28 d | Blank | TEA0.04 | TEA0.08 | DEIPA0.04 | DEIPA0.08 | TIPA0.04 | TIPA0.08 |
| Ettringite | 0.1248 | 0.1259 | 0.1304 | 0.1320 | 0.1407 | 0.1152 | 0.1172 |
| Gypsum | 0.0700 | 0.0479 | 0.0349 | 0.0421 | 0.0359 | 0.0549 | 0.0477 |
| Calcite | 0.0107 | 0.0112 | 0.0070 | 0.0090 | 0.0089 | 0.0074 | 0.0104 |
| Amorphous | 0.7945 | 0.8150 | 0.8277 | 0.8170 | 0.8145 | 0.8225 | 0.8247 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Jin, B.; Wu, S.; Fan, S.; Hang, F.; Chen, H. Study on Sustainable Application of Low-Carbon Supersulfated Cement with Alkanolamines. Sustainability 2024, 16, 3008. https://doi.org/10.3390/su16073008
Zhou R, Jin B, Wu S, Fan S, Hang F, Chen H. Study on Sustainable Application of Low-Carbon Supersulfated Cement with Alkanolamines. Sustainability. 2024; 16(7):3008. https://doi.org/10.3390/su16073008
Chicago/Turabian StyleZhou, Runduo, Bingxin Jin, Shuanglei Wu, Shujing Fan, Fafu Hang, and Huxing Chen. 2024. "Study on Sustainable Application of Low-Carbon Supersulfated Cement with Alkanolamines" Sustainability 16, no. 7: 3008. https://doi.org/10.3390/su16073008
APA StyleZhou, R., Jin, B., Wu, S., Fan, S., Hang, F., & Chen, H. (2024). Study on Sustainable Application of Low-Carbon Supersulfated Cement with Alkanolamines. Sustainability, 16(7), 3008. https://doi.org/10.3390/su16073008





