Assessment of the Hydrochemical Characteristics of the Carbon Observational Site ‘Carbon-Sakhalin’ (Aniva Bay, Sea of Okhotsk)
Abstract
1. Introduction
- -
- Creating a network of carbon observational sites, which include terrestrial and marine areas in different climatic zones of Russia;
- -
- Investigation of the physical and biochemical processes that define the carbon cycle;
- -
- Measuring of emission and absorption of greenhouse gases (GHGs) using the standard equipment and methods;
- -
- Assessments of the regional biogeochemical carbon cycle and human impacts;
- -
- Investigation of the potential of GHG sequestration by natural ecosystems;
- -
- Development and testing of carbon balance control and technologies of GHG sequestration, and anthropogenic increase in the sequestration capacity of natural ecosystems within the framework of the “green” and “blue” economy.
2. Review of Hydrochemical Regime of the Sea of Okhotsk and Aniva Bay Waters
3. Data and Methods
3.1. Field Measurement Data
3.2. Methods of Monitoring of Hydrochemical Parameters
4. Results
4.1. Analysis of Seasonal Variability of Hydrochemical Parameters in Aniva Bay
4.1.1. Dissolved Oxygen
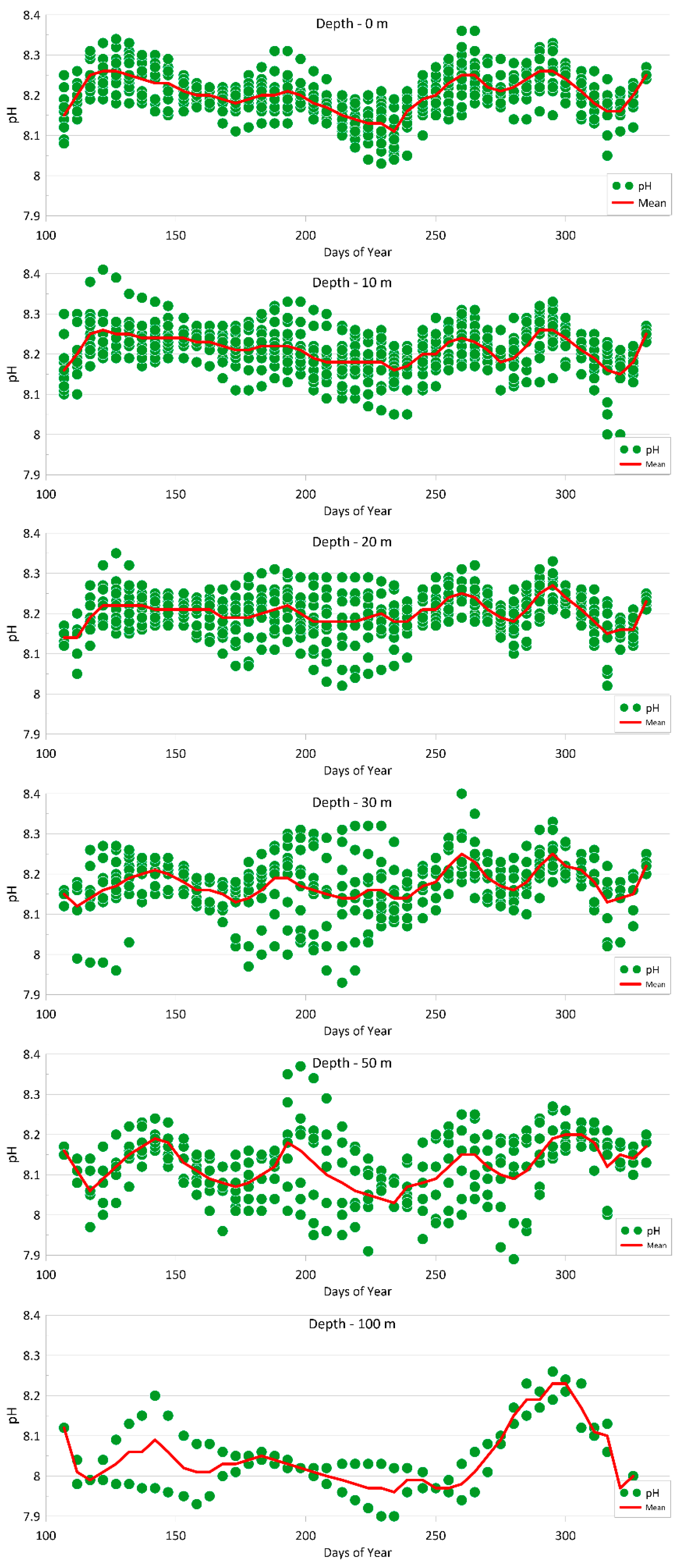
4.1.2. pH Value
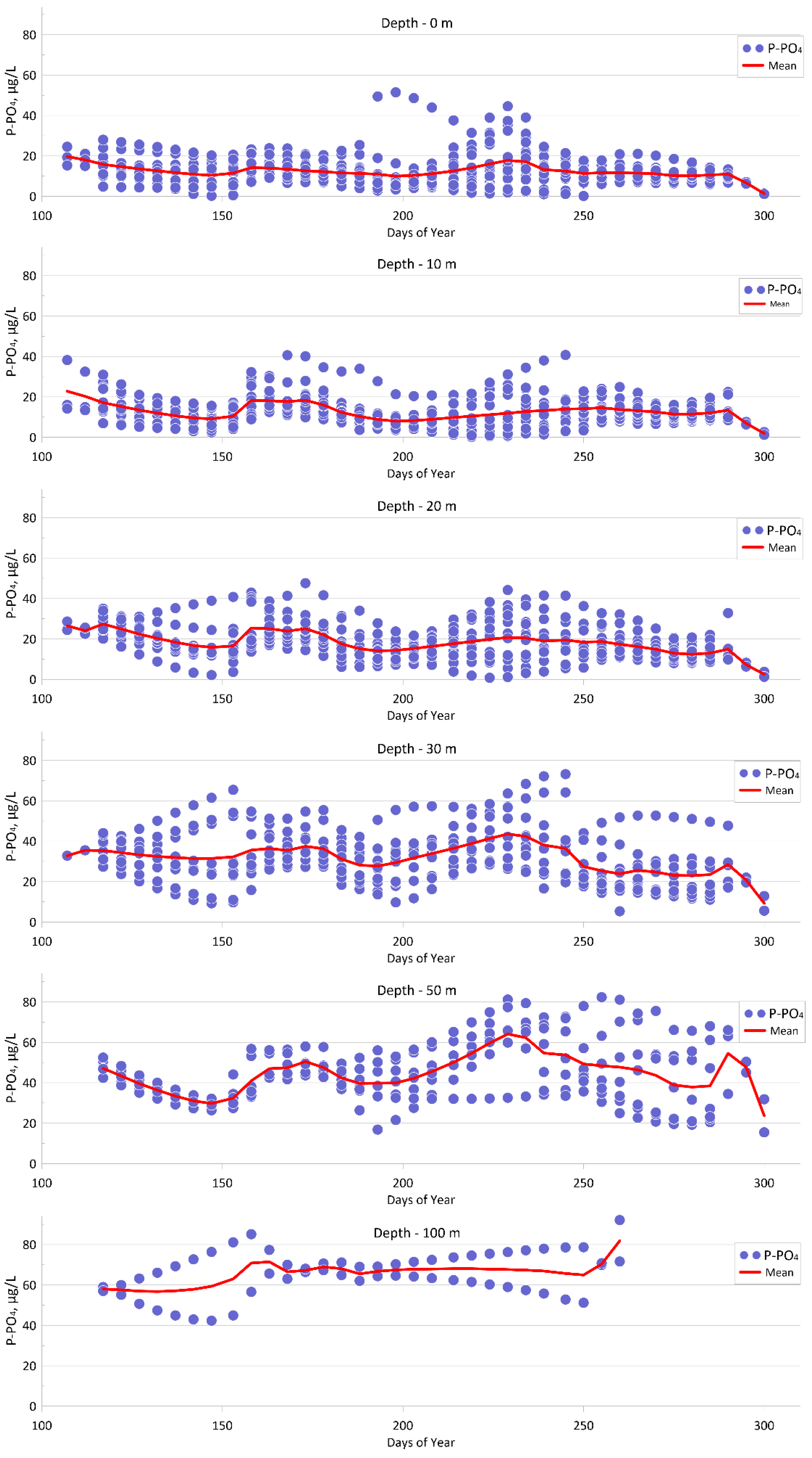
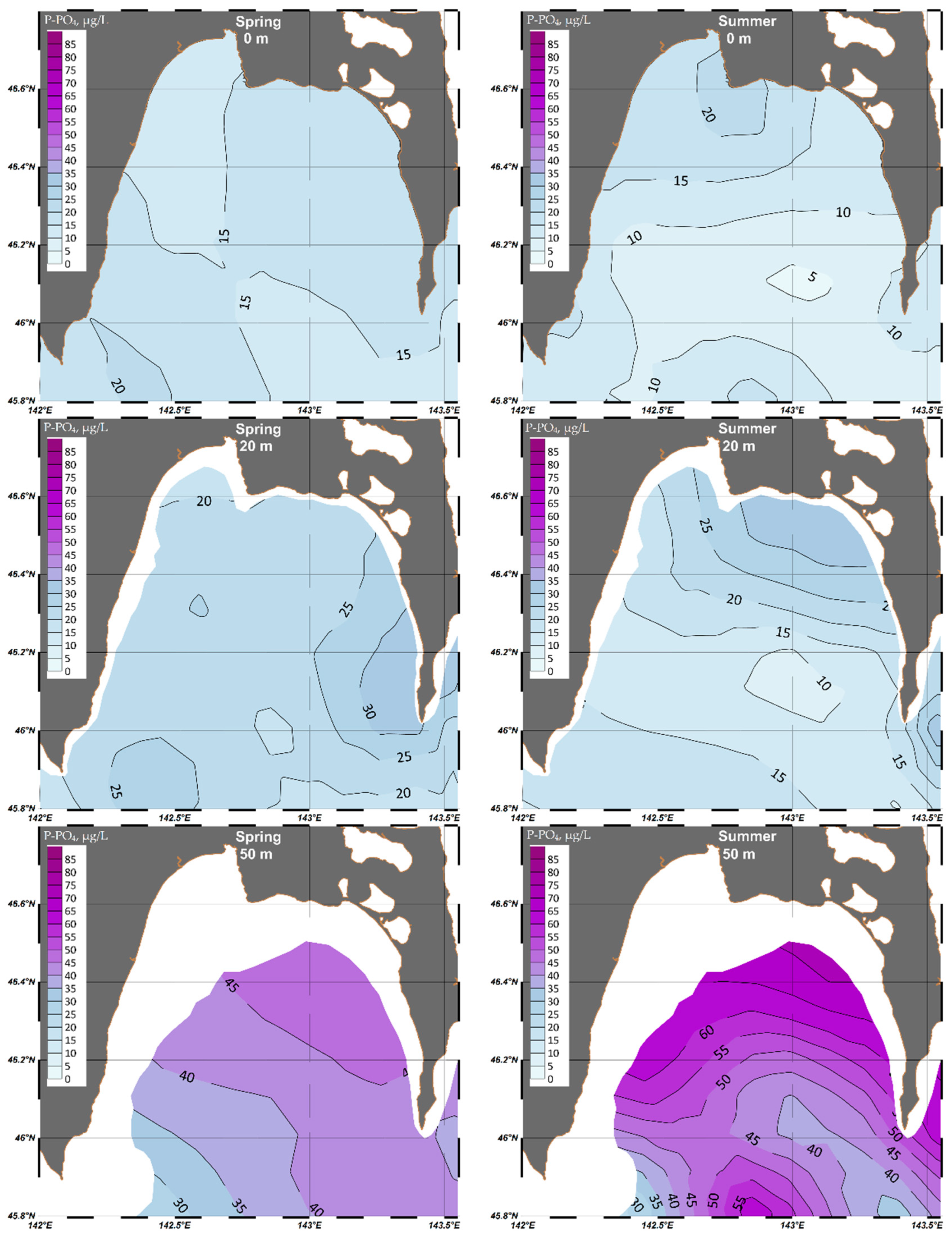
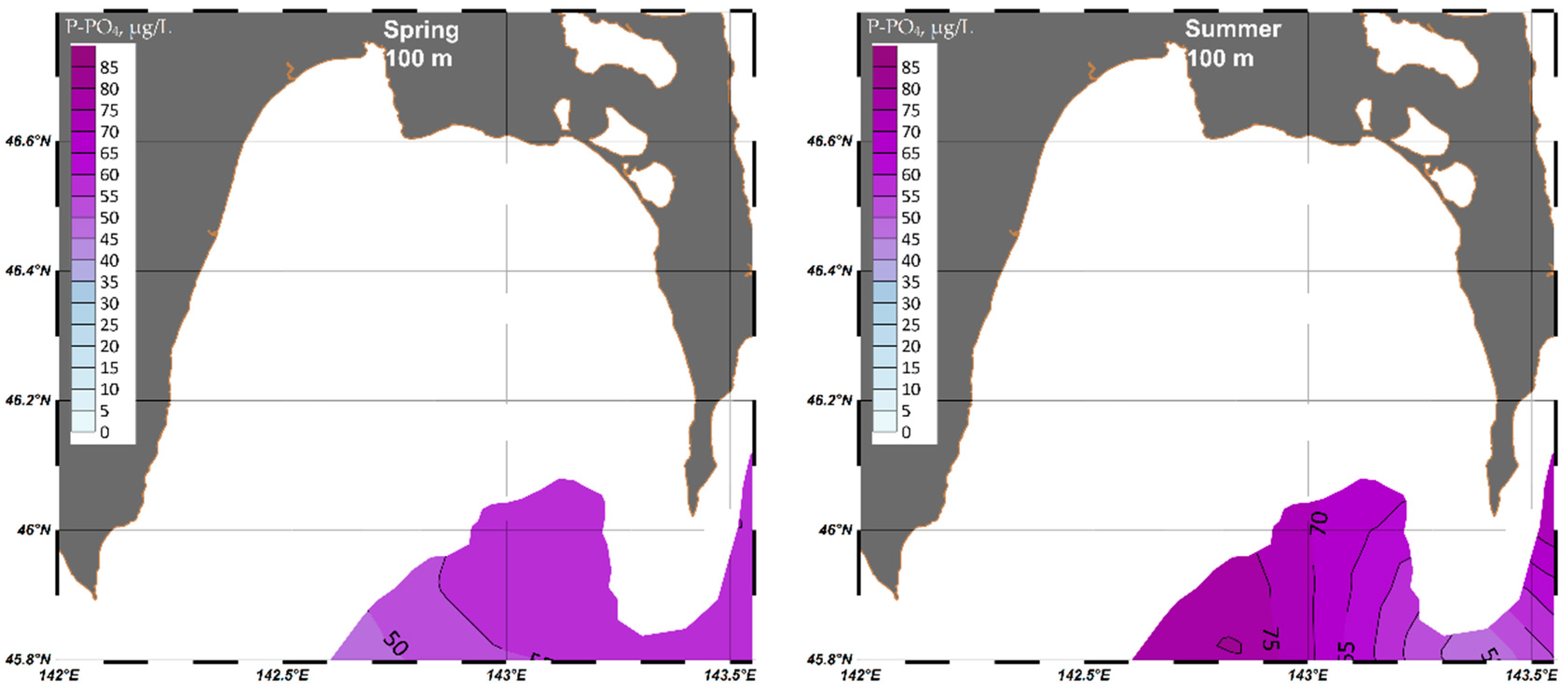
4.1.3. Phosphate (P-PO4)
4.1.4. Silicate (Si-SiO3)
4.1.5. Nitrite (N-NO2)
5. Discussion
- -
- In situ gas sensors and continuous monitoring systems (including eddy covariance techniques) on ocean platforms, vessels, and ferries can provide real-time data on gas emissions more effectively than discrete sampling;
- -
- Biomolecular marker techniques like DNA-based analyses or genetic markers can identify specific microbial communities responsible for greenhouse gas production, offering insights into potential emission sources;
- -
- Isotope tracing methods can trace the origin and transformation of greenhouse gases, providing insights into their production pathways and helping quantify emissions more accurately;
- -
- Remote sensing and imaging spectral investigations by unmanned aerial vehicles and satellite-based remote sensing technologies offer broad coverage and continuous monitoring of large marine areas;
- -
- Integrated modeling approaches with machine learning techniques.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shcherbanin, Y.A.; Golyzhnikova, D.Y. Export Cargo Flows via Russian Maritime Ports: Oil Cargo—Some Considerations) (30 April 2019). Russ. Foreign Econ. J. 2019, 4, 18–36. Available online: https://ssrn.com/abstract=3973394 (accessed on 20 February 2024).
- Aminina, N.M.; Vishnevskaya, T.I.; Galanin, D.A.; Repnikova, A.R.; Guruleva, O.N. Description of Saccharina japonica commercial stock in the Aniva Bay (Okhotsk Sea). Izvestiya TINRO 2014, 178, 116–123. [Google Scholar] [CrossRef]
- Maslennikov, S.I. Marine Biological Resources in the Far Eastern Coast: Their Rational Use from Ecological and Economic Viewpoints. In Energy and Environment in Slavic Eurasia: Toward the Establishment of the Network of Environmental Studies in the Pan-Okhotsk Region; Slavik Research Center, Hokkaido University: Sapporo, Japan, 2008; pp. 89–125. [Google Scholar]
- Ricour, F.; Guidi, L.; Gehlen, M.; DeVries, T.; Legendre, L. Century-scale carbon sequestration flux throughout the ocean by the biological pump. Nat. Geosci. 2023, 16, 1105–1113. [Google Scholar] [CrossRef]
- Claustre, H.; Legendre, L.; Boyd, P.W.; Levy, M. The Oceans’ Biological Carbon Pumps: Framework for a Research Observational Community Approach. Front. Mar. Sci. 2021, 8, 780052. [Google Scholar] [CrossRef]
- Pipko, I.I.; Pugach, S.P.; Luchin, V.A.; Francis, O.P.; Savelieva, N.I.; Charkin, A.N.; Dudarev, O.V.; Semiletov, I.P. Surface CO2 system dynamics in the Gulf of Anadyr during the open water season. Cont. Shelf Res. 2021, 217, 104371. [Google Scholar] [CrossRef]
- Carbon Supersites. Available online: https://carbon-polygons.ru/en/ (accessed on 20 February 2024).
- Report on the Inventory of Anthropogenic Emissions by Sources and Removals by Sinks of Greenhouse Gases in the Sakhalin Region for 2019–2021. IGC&E. 2021. Available online: https://ecology.sakhalin.gov.ru/fileadmin/user_upload/Doklad_o_kadastre_Sakhalinskoi_oblasti_za_2019_g.pdf (accessed on 20 February 2024).
- Abakumov, E.; Polyakov, V. Carbon polygons and carbon offsets: Current state, key challenges and pedological aspects. Agronomy 2021, 11, 2013. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S.D.; Craft, C.B.; Fourqurean, J.W.; Kauffman, J.B.; Marbà, N.; et al. Estimating Global “Blue Carbon” Emissions from Conversion and Degradation of Vegetated Coastal Ecosystems. PLoS ONE 2012, 7, e043542. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Change 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Sondak, C.F.A.; Chung, I.K. Potential blue carbon from coastal ecosystems in the Republic of Korea. Ocean Sci. J. 2015, 50, 1–8. [Google Scholar] [CrossRef]
- Pessarrodona, A.; Moore, P.J.; Sayer, M.D.J.; Smale, D.A. Carbon assimilation and transfer through kelp forests in the NE Atlantic is diminished under a warmer ocean climate. Glob. Change Biol. 2018, 24, 4386–4398. [Google Scholar] [CrossRef]
- Afanas’ev, V.V.; Latkovskaya, E.M.; Uba, A.V.; Levitsky, A.I. Carbon Sequestration in the Coastal Marine Biomorpholithosystems of the Salmon Bay of Aniva Bay. In Physical and Mathematical Modeling of Earth and Environment Processes—2022, PMMEEP 2022; Karev, V.I., Ed.; Springer Proceedings in Earth and Environmental Sciences; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Zhong, C.; Li, T.; Bi, R.; Sanganyado, E.; Huang, J.; Jiang, S.; Zhang, Z.; Du, H. A systematic overview, trends and global perspectives on blue carbon: A bibliometric study (2003–2021). Ecol. Indic. 2023, 148, 110063. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Cai, Y.; Fang, Z.; Zhao, X.; Zhang, Q.; Yuan, H.; Lin, N.; Zou, C.; Zheng, M. Macroalgae culture–induced carbon sink in a large cultivation area of China. Environ. Sci. Pollut. Res. 2023, 30, 107693–107702. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zhang, J.; Fang, J. Shellfish and seaweed mariculture increase atmospheric CO2 absorption by coastal ecosystems. Mar. Ecol. Prog. Ser. 2011, 424, 97–104. [Google Scholar] [CrossRef]
- Lai, Q.; Ma, J.; He, F.; Zhang, A.; Pei, D.; Yu, M. Current and Future Potential of Shellfish and Algae Mariculture Carbon Sinks in China. Int. J. Environ. Res. Public Health 2022, 19, 8873. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Shin, S.K.; Wu, H.; Yarish, C.; Yoo, H.I.; Kim, J.K. Evaluation of nutrient bioextraction by seaweed and shellfish aquaculture in Korea. J. World Aquac. Soc. 2021, 52, 1118–1134. [Google Scholar] [CrossRef]
- Myers, C.R.; Subban, C.V. Ocean Deacidification Technologies for Marine Aquaculture. J. Mar. Sci. Eng. 2022, 10, 523. [Google Scholar] [CrossRef]
- Troell, M.; Henriksson, P.J.G.; Buschmann, A.H.; Chopin, T.; Quahe, S. Farming the Ocean—Seaweeds as a Quick Fix for the Climate? Rev. Fish. Sci. Aquac. 2023, 31, 285–295. [Google Scholar] [CrossRef]
- Gu, Y.; Lyu, S.; Wang, L.; Chen, Z.; Wang, X. Assessing the carbon sink capacity of coastal mariculture shellfish resources in China from 1981–2020. Front. Mar. Sci. 2022, 9, 981569. [Google Scholar] [CrossRef]
- Campbell, I.; Macleod, A.; Sahlmann, C.; Neves, L.; Funderud, J.; Øverland, M.; Hughes, A.D.; Stanley, M. The Environmental Risks Associated With the Development of Seaweed Farming in Europe—Prioritizing Key Knowledge Gaps. Front. Mar. Sci. 2019, 6, 107. [Google Scholar] [CrossRef]
- Romanyuk, V.A.; Pishchalnik, V.M.; Shumilov, I.V. Features of the ice regime of Aniva Bay (Sea of Okhotsk) as a risk factor in the planning of mariculture farms. Process. GeoMedia 2022, 4, 1820–1826. [Google Scholar]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.S. Ecological risks associated with seaweed cultivation and identifying risk minimization approaches. Algal Res. 2023, 69, 102967. [Google Scholar] [CrossRef]
- Aldridge, J.N.; Mooney, K.; Dabrowski, T.; Capuzzo, E. Modelling effects of seaweed aquaculture on phytoplankton and mussel production. Application to Strangford Lough (Northern Ireland). Aquaculture 2021, 536, 736400. [Google Scholar] [CrossRef]
- Cakmak, E.K.; Hartl, M.; Kisser, J.; Cetecioglu, Z. Phosphorus mining from eutrophic marine environment towards a blue economy: The role of bio-based applications. Water Res. 2022, 219, 118505. [Google Scholar] [CrossRef] [PubMed]
- Orekhova, N.A.; Konovalov, S.K.; Polukhin, A.A.; Seliverstova, A.M. Oxygen and pCO2 in the Surface Waters of the Atlantic Southern Ocean in 2021–2022. Water 2023, 15, 1642. [Google Scholar] [CrossRef]
- Schulz, K.G.; Maher, D.T. Atmospheric Carbon Dioxide and Changing Ocean Chemistry. In Marine Pollution—Monitoring, Management and Mitigation. Springer Textbooks in Earth Sciences, Geography and Environment; Reichelt-Brushett, A., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Xue, Z.; He, R.; Fennel, K.; Cai, W.-J.; Lohrenz, S.; Huang, W.-J.; Tian, H.; Ren, W.; Zang, Z. Modeling pCO2 variability in the Gulf of Mexico. Biogeosciences 2016, 13, 4359–4377. [Google Scholar] [CrossRef]
- Luo, X.; Wei, H.; Liu, Z.; Zhao, L. Seasonal variability of air-SEA CO2 fluxes in the yellow and east China Seas: A case study of continental shelf SEA carbon cycle model. Cont. Shelf Res. 2015, 107, 69–78. [Google Scholar] [CrossRef]
- Lu, Z.; Gan, J.; Dai, M. Modeling seasonal and diurnal pCO2 variations in the northern South China Sea. J. Mar. Syst. 2012, 92, 30–41. [Google Scholar] [CrossRef]
- Leonov, A.V.; Pishchal’nik, V.M.; Petukhov, V.I.; Petrova, E.A.; Chicherina, O.V. Biohydrochemical Studies of the Intra-Annual Nutrient Dynamics in the Northeastern Shelf Waters of the Sakhalin Island. IOP Conf. Ser. Earth Environ. Sci. 2019, 272, 032241. [Google Scholar] [CrossRef]
- Ostrovskii, A.G.; Emelianov, M.V.; Kochetov, O.Y.; Kremenetskiy, V.V.; Shvoev, D.A.; Volkov, S.V.; Zatsepin, A.G.; Korovchinsky, N.M.; Olshanskiy, V.M.; Olchev, A.V. Automated Tethered Profiler for Hydrophysical and Bio-Optical Measurements in the Black Sea Carbon Observational Site. J. Mar. Sci. Eng. 2022, 10, 322. [Google Scholar] [CrossRef]
- State Standard GOST 17.1.3.08-82; State Committee of the USSR for Standards. Nature Protection. Hydrosphere. Procedures for Quality Control of Marine Waters. IPK Izdatelstvo Standartov: Moscow, Russia, 1982; 6p. (In Russian)
- Hydrometeorological Conditions and Oceanological Basis of the Formation of Bioproductivity. In Hydrometeorology and Hydrochemistry of the Seas, Vol. 9: Sea of Okhotsk, Issue 2; (In Russian). Terziev, F.S., Ed.; Gidrometeoizdat: St. Petersburg, Russia, 1993. [Google Scholar]
- Sapozhnikov, V.V. New perceptions of the functioning of the Okhotsk Sea ecosystem. In Complex Studies of the Okhotsk Sea Ecosystem; VNIRO: Moscow, Russia, 1997; pp. 5–7. (In Russian) [Google Scholar]
- Pishchalnik, V.M.; Bobkov, A.O. Author’s Certificate for Software Application “ATLAS”; No. 930078; RosAPO: Moscow, Russia, 1993. (In Russian) [Google Scholar]
- Pischalnik, V.M.; Bobkov, A.O. Oceanographic Atlas of Sakhalin Shelf. Part 1 and 2; SakhGU: Yuzhno-Sakhalinsk, Russia, 2000. [Google Scholar]
- Pishchal’nik, V.M.; Bobkov, A.O. Modeling Natural Processes Based on “Sakhalinskii Shel’f” GIS; SakhGU: Yuzhno-Sakhalinsk, Russia, 2008. [Google Scholar]
- Pischalnik, V.M.; Bobkov, A.O. Digital Technologies for Modeling Natural Processes; SakhGU: Yuzhno-Sakhalinsk, Russia, 2020; p. 164. [Google Scholar]
- Koreneva, T.G.; Latkovskaya, E.M. Characterization of the variability of waters in Aniva Bay based on phytoplankton pigment content. Voda Khim. Ekol. 2013, 10, 68–78. (In Russian) [Google Scholar]
- Koreneva, T.G.; Latkovskaya, E.M.; Chastikov, V.N. Seasonal dynamics of hydrological and hydrochemical characteristics and chlorophyll a concentration in Aniva Bay in 2003. Voda Khim. Ekol. 2014, 4, 33–45. (In Russian) [Google Scholar]
- Mogilnikova, T.A.; Polteva, A.V.; Latkovskaya, E.M.; Leonov, A.V.; Pokrashenko, S.A.; Pishchalnik, V.M. Sea-Ice and Under-Ice Microalgae and Heterotrophic Bacteria: Evolution in Sakhalin Island Coastal Waters; REA Special Issue №1; Dalnauka: Vladivostok, Russia, 2009; pp. 129–145. (In Russian) [Google Scholar]
- Kantakov, G.A.; Stonik, I.V.; Selina, M.S.; Orlova, T.Y. Advection, vertical stability of waters and features of phytoplankton existential distribution in Aniva Bay of the Okhotsk Sea during 2001–2003. Trudy SakhNIRO. 2007, 9, 295–324. (In Russian) [Google Scholar]
- Kas’kova, V.O.; PoIteva, A.V.; Latkovskaya, E.M. Seasonal dynamics of petroleum products concentration and petroleum-oxidizing microorganisms abundance in the coastal zone of Aniva Bay. Trudy SakhNIRO. 2005, 7, 375–384. (In Russian) [Google Scholar]
- Propp, L.N.; Gavrina, L.Y.H. Seasonal variations of compound nutrients and productional characteristics in the Aniva Bay waters by the results of expeditional reserches in 2001–2021. Trudy SakhNIRO. 2005, 7, 111–155. (In Russian) [Google Scholar]
- Chen, C.-T.; Yu, S.; Huang, T.-H.; Bai, Y.; He, X. Changes in Temperature, Chlorophyll Concentration, and Secchi Disk Depth in the Okhotsk Sea from 1998 to 2016. In Changing Asia-Pacific Marginal Seas; Chen, C.T.A., Guo, X.Y., Eds.; Springer: Singapore, 2020; pp. 57–68. [Google Scholar] [CrossRef]
- Chen, C.-T.; Yu, S.; Huang, T.-H.; Bai, Y.; He, X.; Lui, H.-K. Temperature and Secchi Disk Depth Increase More Rapidly in the Subpolar Bering/Okhotsk Seas Than in the Subtropical South China Sea. Water 2023, 15, 98. [Google Scholar] [CrossRef]
- Nakanowatari, T.; Mitsudera, H. Long-term trend and interannual to decadal variability in the Sea of Okhotsk. In Changing Asia-Pacific Marginal Seas; Chen, C.T.A., Guo, X.Y., Eds.; Springer: Singapore, 2020; pp. 19–56. [Google Scholar] [CrossRef]
- Nishioka, J.; Mitsudera, H.; Yasuda, I.; Liu, H.; Nakatsuka, T.; Volkov, Y.N. Biogeochemical and physical processes in the Sea of Okhotsk and the linkage to the Pacific ocean. Prog. Oceanogr. 2014, 126, 1–7. [Google Scholar] [CrossRef]
- Sato, T.; Shiozaki, T.; Taniuchi, Y.; Kasai, H.; Takahashi, K. Nitrogen fixation and diazotroph community in the subarctic Sea of Japan and Sea of Okhotsk. J. Geophys. Res. Ocean. 2021, 126, e2020JC017071. [Google Scholar] [CrossRef]
- Granskog, M.A.; Nomura, D.; Müller, S.; Krell, A.; Toyota, T.; Hattori, H. Evidence for significant protein-like dissolved organic matter accumulation in Sea of Okhotsk sea ice. Ann. Glaciol. 2015, 56, 1–8. [Google Scholar] [CrossRef]
- Ardyna, M.; Mundy, C.J.; Mayot, N.; Matthes, L.C.; Oziel, L.; Horvat, C.; Leu, E.; Assmy, P.; Hill, V.; Matrai, P.A.; et al. Under-Ice Phytoplankton Blooms: Shedding Light on the “Invisible” Part of Arctic Primary Production. Front. Mar. Sci. 2020, 7, 608032. [Google Scholar] [CrossRef]
- Yan, D.; Yoshida, K.; Nishioka, J.; Ito, M.; Toyota, T.; Suzuki, K. Response to Sea Ice Melt Indicates High Seeding Potential of the Ice Diatom Thalassiosira to Spring Phytoplankton Blooms: A Laboratory Study on an Ice Algal Community From the Sea of Okhotsk. Front. Mar. Sci. 2020, 7, 613. [Google Scholar] [CrossRef]
- Wakita, M.; Watanabe, Y.W.; Watanabe, S.; Noriki, S.; Wakatsuchi, M. Oceanic uptake rate of anthropogenic CO2 in a subpolar marginal sea: The Sea of Okhotsk. Geophys. Res. Lett. 2003, 30, 2252. [Google Scholar] [CrossRef]
- Tishchenko, P.Y.; Lobanov, V.B.; Semkin, P.Y.; Sergeev, A.F.; Anisimova, E.V.; Barabanshchikov, Y.A.; Melnikov, V.V.; Ryumina, A.A.; Sagalaev, S.G.; Ulanova, O.A.; et al. Hydrochemical Study of Academy Bay (Sea of Okhotsk). Oceanology 2022, 62, 80–92. [Google Scholar] [CrossRef]
- Sapozhnikov, V. Hydrochemical Atlas of the Sea of Okhotsk, International Ocean Atlas Series, Volume 3, Russian Federal Institute of Fisheries and Oceanography; Bremerhaven, PANGAEA: Moscow, Russia, 2001. [Google Scholar]
- Nedashkovsky, A.P.; Khen, G.V.; Savelieva, N.I. Chemical patterns of the TINRO Basin (Okhotsk Sea) in abnormally cold years. Izvestiya TINRO 2018, 194, 86–98. (In Russian) [Google Scholar] [CrossRef]
- Sasano, D.; Takatani, Y.; Kosugi, N.; Nakano, T.; Midorikawa, T.; Ishii, M. Multidecadal trends of oxygen and their controlling factors in the western North Pacific. Glob. Biogeochem. Cycles 2015, 29, 935–956. [Google Scholar] [CrossRef]
- Gordeev, E.I.; Girina, O.A.; Lupyan, E.A.; Sorokin, A.A.; Kramareva, L.S.; Efremov, V.Y.; Kashnitskii, A.V.; Uvarov, I.A.; Burtsev, M.A.; Romanova, I.M.; et al. The VolSatView information system for Monitoring the Volcanic Activity in Kamchatka and on the Kuril Islands. J. Volcanolog. Seismol. 2016, 10, 382–394. [Google Scholar] [CrossRef]
- Apollaro, C.; Fuoco, I.; Gennaro, E.; Giuliani, L.; Iezzi, G.; Marini, L.; Radica, F.; Di Luccio, F.; Ventura, G.; Vespasiano, G. Advanced argillic alteration at Cave di Caolino, Lipari, Aeolian Islands (Italy): Implications for the mitigation of volcanic risks and the exploitation of geothermal resources. Sci. Total Environ. 2023, 889, 164333. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Y.; Liu, S.; Zhao, C.; Dong, S.; Song, Y. CO2 transport and carbonate precipitation in the coupled diffusion-reaction process during CO2 storage. Fuel 2023, 334, 126805. [Google Scholar] [CrossRef]
- Kawasaki, K.; Tachibana, Y.; Nakamura, T.; Yamazaki, K. Role of the cold Okhotsk sea on the climate of the North Pacific subtropical high and Baiu precipitation. J. Clim. 2021, 34, 495–507. [Google Scholar] [CrossRef]
- Sohrin, R.; Imanishi, K.; Suzuki, Y.; Kuma, K.; Yasuda, I.; Suzuki, K.; Nakatsuka, T. Distributions of dissolved organic carbon and nitrogen in the western Okhotsk Sea and their effluxes to the North Pacific. Prog. Oceanogr. 2014, 126, 168–179. [Google Scholar] [CrossRef]
- Pishchalnik, V.M.; Bobkov, A.O. Author’s Certificate for Software Application “ATLAS-2018”; No. 2019665674; RosAPO: Moscow, Russia, 2019. [Google Scholar]
- Pishchal’nik, V.M.; Leonov, A.V. Studying the Ecosystem Functioning Conditionsin Aniva Bay—La Perouse Strait. Water Resour. 2003, 30, 569–588. [Google Scholar] [CrossRef]
- Leonov, A.V.; Pishchal’nik, V.M. Biotransformation of Organogenic Substances in the Aniva Bay Waters: Assessment with the Use of Mathematical Modeling. Water Resour. 2005, 32, 508–523. [Google Scholar] [CrossRef]
- Leonov, A.V.; Pishchal’nik, V.M. Analysis of Conditions of Petroleum Hydrocarbon Transformations in Seawater and Simulation of This Process in Aniva Bay. Water Resour. 2005, 32, 653–666. [Google Scholar] [CrossRef]
- Pishchal’nik, V.M.; Arkhipkin, V.S.; Leonov, A.V. Thermohaline Analysis of the La Perouse Strait Water. Water Resour. 2005, 32, 13–22. [Google Scholar] [CrossRef]
- Propp, L.N.; Gavrina, L.Y. Seasonal variations of hydrological and hydrochemical parameters and chlorophyll-a in the La Perouse (Soya) Strait and adjacent regions of the Sea of Okhotsk and Japan Sea in 1996–1997). Trudy SahNIRO 2019, 15, 243–279. [Google Scholar]
- RD 52.10.243-92. Guidance Document 52.10.243-92: Guidelines for the Chemical Analysis of Sea Waters; SG Oradovsky, Ed.; SPb.: Gidrometeoizdat, Russia, 1993; 264p. (In Russian) [Google Scholar]
- Alekin, O.A.; Lyahin, Y.I. Ocean Chemistry; Gidrometeoizdat Publ.: Leningrad, Russia, 1984; 343p. (In Russian) [Google Scholar]
- Research and Applied USSR Climate Reference Book No 3. Part 1–6, V. 13; Gidrometeoizdat: Leningrad, Russia, 1990; 724p. (In Russian)
- Alekin, O.A. Basics of Hydrochemistry; Gidrometeoizdat Publ.: Leningrad, Russia, 1970; 444p. (In Russian) [Google Scholar]
- Arzhanova, N.V.; Zubarevich, V.L. Chemical Basis of Bioproductivity of the Sea of Okhotsk. In Complex Studies of the Okhotsk Sea Ecosystem; VNIRO: Moscow, Russia, 1997; pp. 86–92. [Google Scholar]
- Redfield, S. The Influence of Organisms on the Composition of Seawater; Redfield, S., Ketchum, B.H., Richards, F.A., Eds.; The Sea New York: New York, NY, USA, 1963; Volume 2, pp. 26–77. [Google Scholar]
- Arzhanova, N.V.; Zubarevich, V.L. Seasonal Changes in Nutrient Contents in the Sea of Okhotsk as a Base for Estimation of the Phytoplankton Production. In Complex Studies of the Ecosystem of the Sea of Okhotsk; Sapozhnikov, V.V., Ed.; VNIRO: Moscow, Russia, 1997; pp. 92–97. (In Russian) [Google Scholar]
- Bruevich, S.V. Chemistry of the Pacific Ocean; Nauka: Moscow, Russia, 1966; 360p. (In Russian) [Google Scholar]
- Sapozhnikov, V.V. Formation of the Basic Salt Composition of Seawater and Its Physico-Chemical Properties in “The Pacific Ocean”; Mysl’: Moscow, Russia, 1982. (In Russian) [Google Scholar]
- WMO. Guidelines on the Calculation of Climate Normals; WMO: Geneva, Switzerland, 2017; Available online: https://library.wmo.int/opac/doc_num.php?explnum_id=4166 (accessed on 18 January 2018).
- Pishchal’nik, V.M.; Arkhipkin, V.S.; Leonov, A.V. Reconstruction of the Annual Variations of Thermohaline Characteristics and Water Circulation on the Northeastern Sakhalin Shelf. Water Resour. 2014, 41, 385–395. [Google Scholar] [CrossRef]
- Pishchalnik, V.M.; Arkhipkin, V.S.; Leonov, A.V. Modeling mean month thermohaline patterns and water circulation on southwestern shelf of Sakhalin. Izvestiya TINRO 2017, 191, 160–175. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Leonov, A.V.; Arkhipkin, V.S.; Pishchalnik, V.M.; Latkovskaya, E.M. Modeling the dynamics of dissolved oxygen concentration in waters of the Aniva bay (the Sea of Okhotsk). Lomonosov Geogr. J. 2023, 78, 77–85. [Google Scholar] [CrossRef]
- Leonov, A.V. Modeling of Natural Processes Based on the Simulation Hydrogeoecological Model of Transformations of S, N, P, and Si Compounds: An Educational Aid; SakhGU: Yuzhno-Sakhalinsk, Russia, 2012. (In Russian) [Google Scholar]
- Blumberg, A.F.; Mellor, G.L. A Description of a Three-Dimensional Coastal Ocean Circulation Model. In Coastal and Estuarine Sciences; AGU: Washington, DC, USA, 1987; pp. 1–16. [Google Scholar]
- Khan, I.; Yuan, A.; Khan, S.; Khan, A.; Khan, S.; Shah, S.; Luo, M.S.; Yaseen, W.; Shen, X.; Yaseen, M. Graphitic Carbon Nitride Composites with Gold and ZIF-67 Nanoparticles as Visible-Light-Promoted Catalysts for CO2 Conversion and Bisphenol A Degradation. ACS Appl. Nano Mater. 2022, 5, 13404–13416. [Google Scholar] [CrossRef]
- Khan, I.; Kang, K.; Khan, A.; Jiyuan, G.; Khan, S.; Khan, S.; Basir, A.; Sadiq, S. Efficient CO2 conversion and organic pollutants degradation over Sm3+ doped and rutile TiO2 nanorods decorated-GdFeO3 nanorods. Int. J. Hydrogen Energy 2023, 48, 32756–32770. [Google Scholar] [CrossRef]
- Spillias, S.; Valin, H.; Batka, M.; Sperling, F.; Havlík, P.; Leclère, D.; Cottrell, R.S.; O’brien, K.R.; McDonald-Madden, E. Reducing global land-use pressures with seaweed farming. Nat. Sustain. 2023, 6, 380–390. [Google Scholar] [CrossRef]
- Belmesov, A.A.; Glukhov, A.A.; Kayumov, R.R.; Podlesniy, D.N.; Latkovskaya, E.M.; Repina, M.A.; Ivanov, N.P.; Tsvetkov, M.V.; Shichalin, O.O. Using Aquatic Plant-Derived Biochars as Carbon Materials for the Negative Electrodes of Li-Ion Batteries. Coatings 2023, 13, 2075. [Google Scholar] [CrossRef]
- Tsvetkov, M.; Zaichenko, A.; Podlesniy, D.; Latkovskaya, E.; Tsvetkova, Y.; Kislov, V. Thermogravimetric analysis of gasification and pyrolysis of algae biomass. E3S Web Conf. 2024, 498, 02002. [Google Scholar] [CrossRef]
- Tsvetkov, M.; Zaichenko, A.; Podlesniy, D. Thermodynamic assessment of gasification and pyrolysis of marine algae to produce hydrogen-containing gas. E3S Web Conf. 2023, 419, 01010. [Google Scholar] [CrossRef]
- Han, T.; Shi, R.; Qi, Z.; Huang, H.; Gong, X. Impacts of large-scale aquaculture activities on the seawater carbonate system and air-sea CO2 flux in a subtropical mariculture bay, southern China. Aquac. Environ. Interact. 2021, 13, 199–210. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Liang, Y.; Li, H.; Li, G.; Chen, X.; Zhao, P.; Jiang, Z.; Zou, D.; Liu, X.; et al. Carbon sequestration processes and mechanisms in coastal mariculture environments in China. Sci. China Earth Sci. 2017, 60, 2097–2107. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Wu, W.; Zhong, Y.; Li, H.; Wang, X.; Yang, J.; Zhang, Y. Effects of Shellfish and Macro-Algae IMTA in North China on the Environment, Inorganic Carbon System, Organic Carbon System, and Sea–Air CO2 Fluxes. Front. Mar. Sci. 2022, 9, 864306. [Google Scholar] [CrossRef]
- Shi, X.; Xu, Y.; Dong, B.; Nishino, N. Mariculture carbon sequestration efficiency in China: Its measurement and socio-economic factor analysis. Sustain. Prod. Consum. 2023, 40, 101–121. [Google Scholar] [CrossRef]
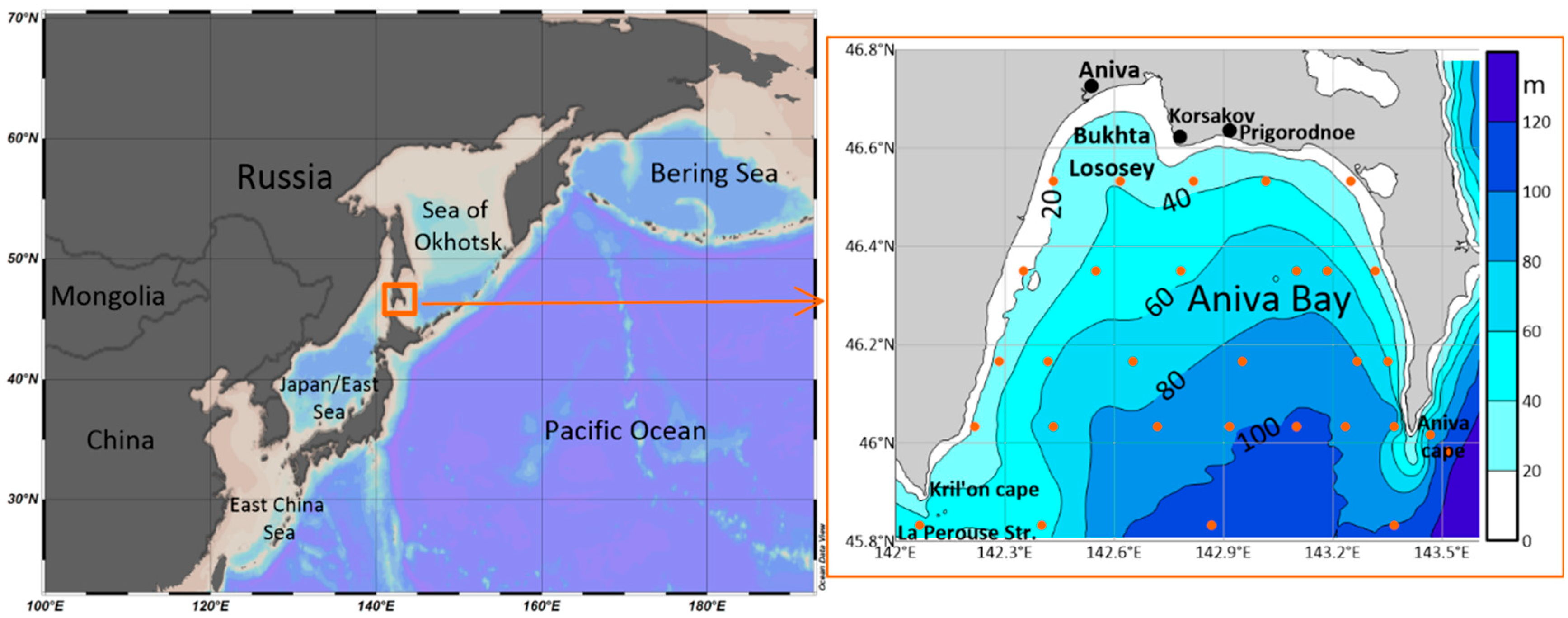
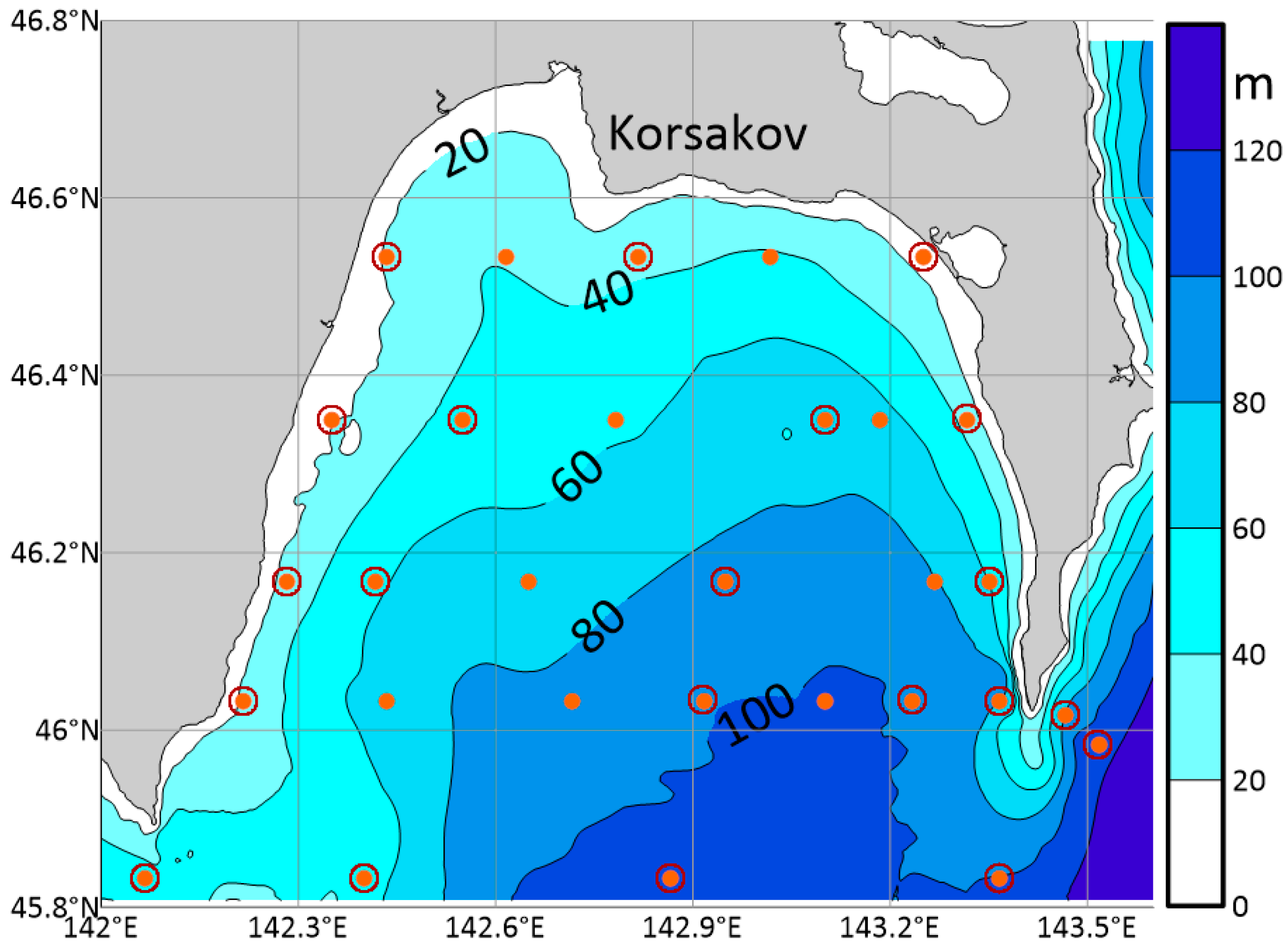
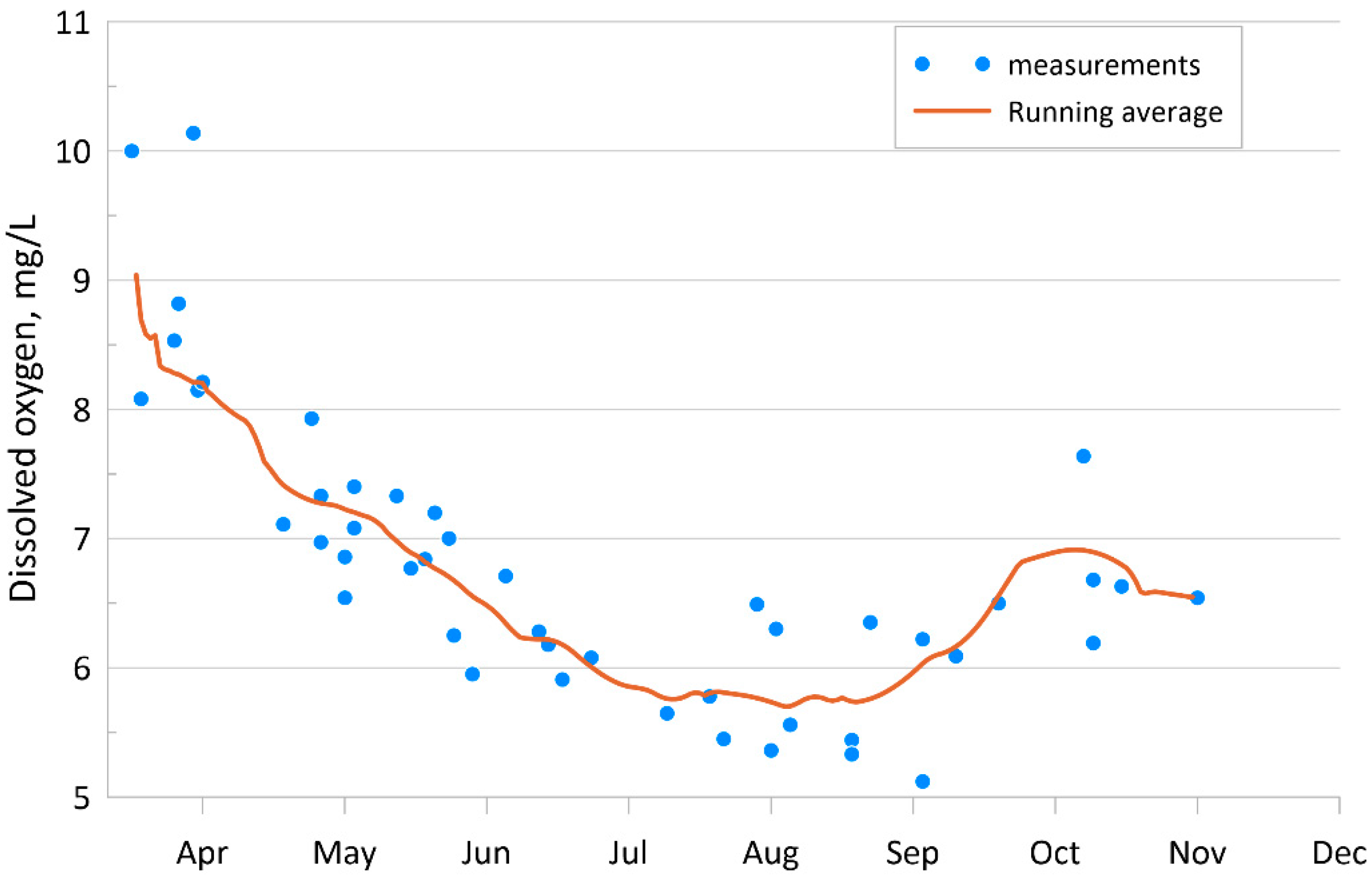
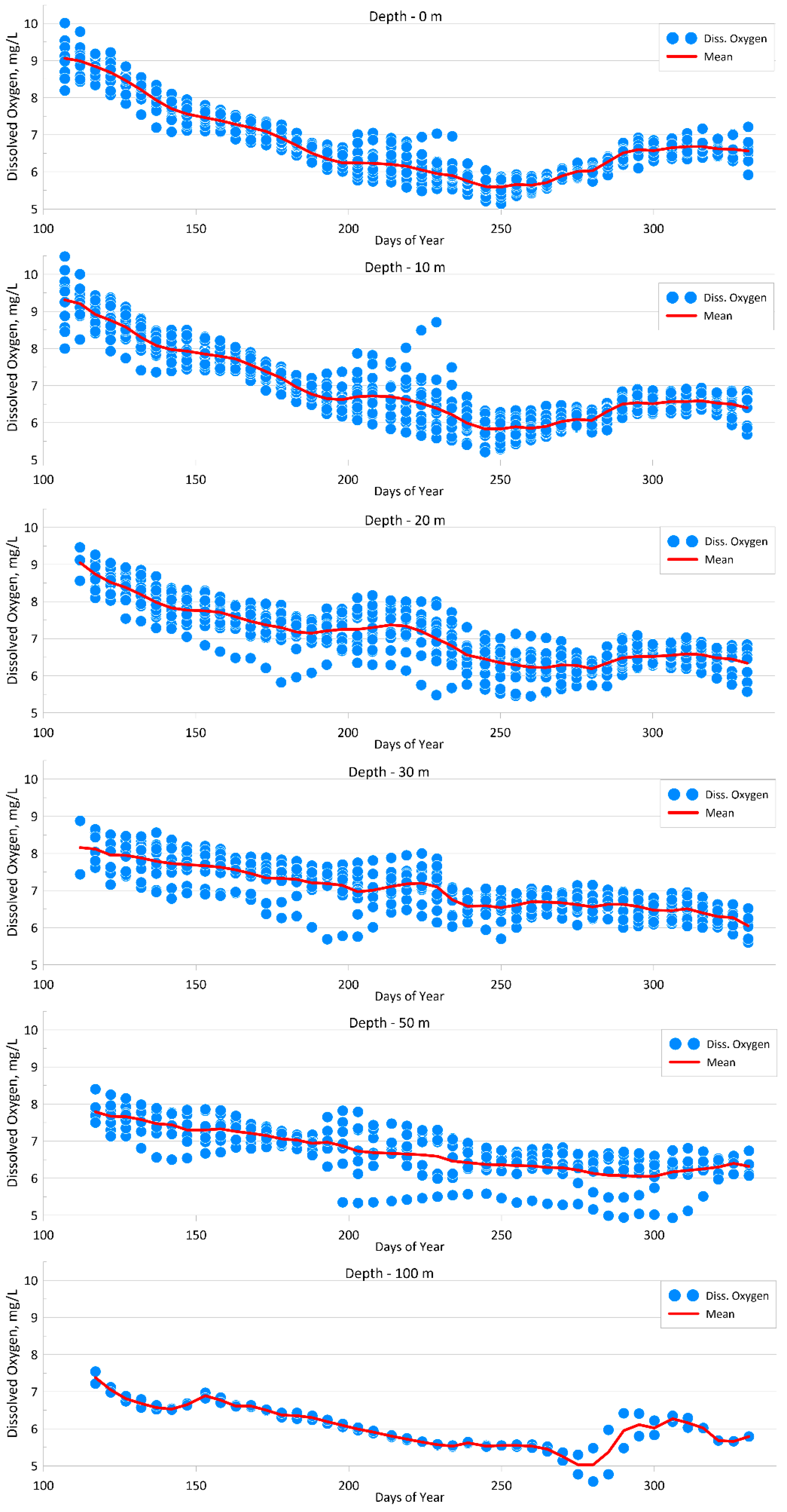
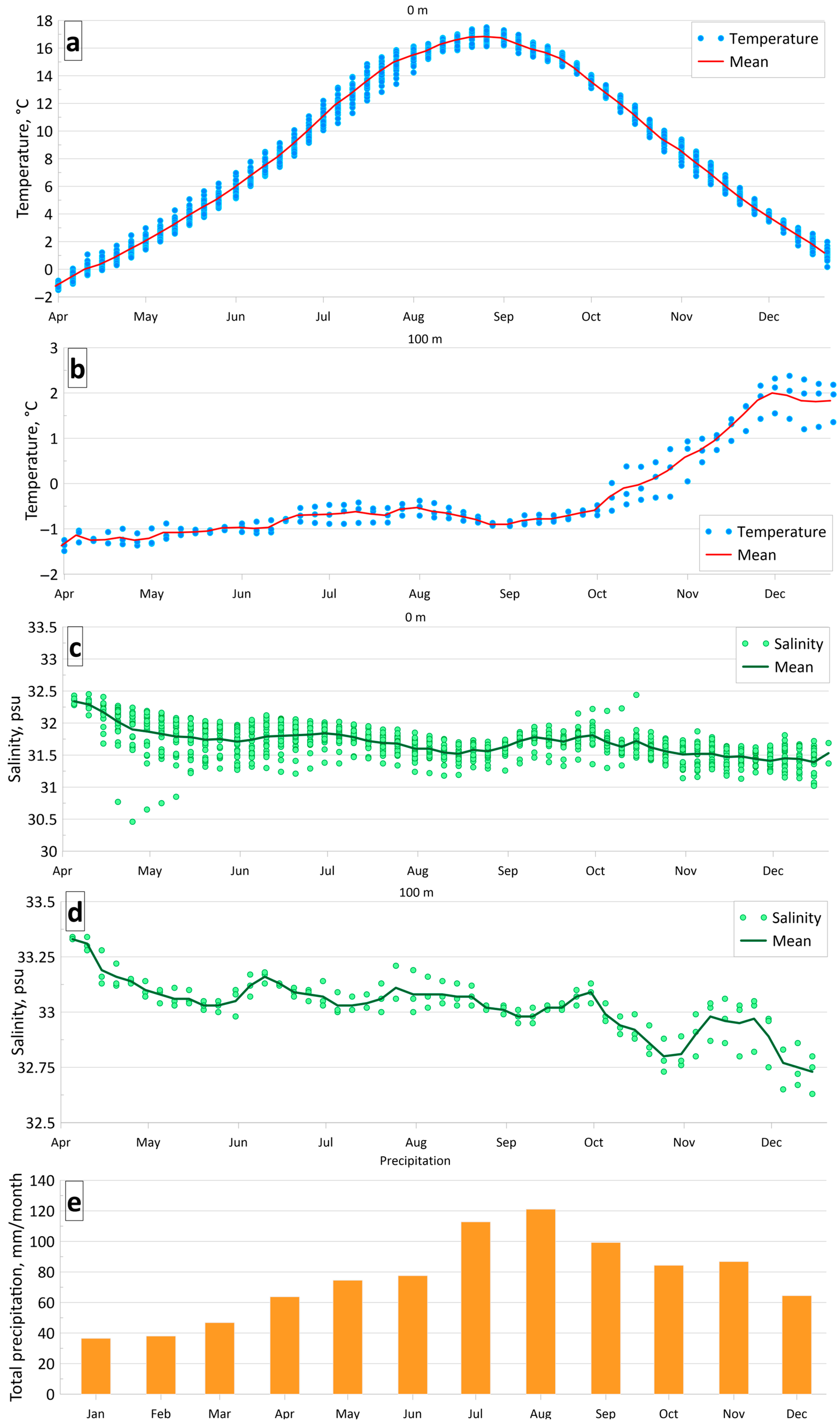


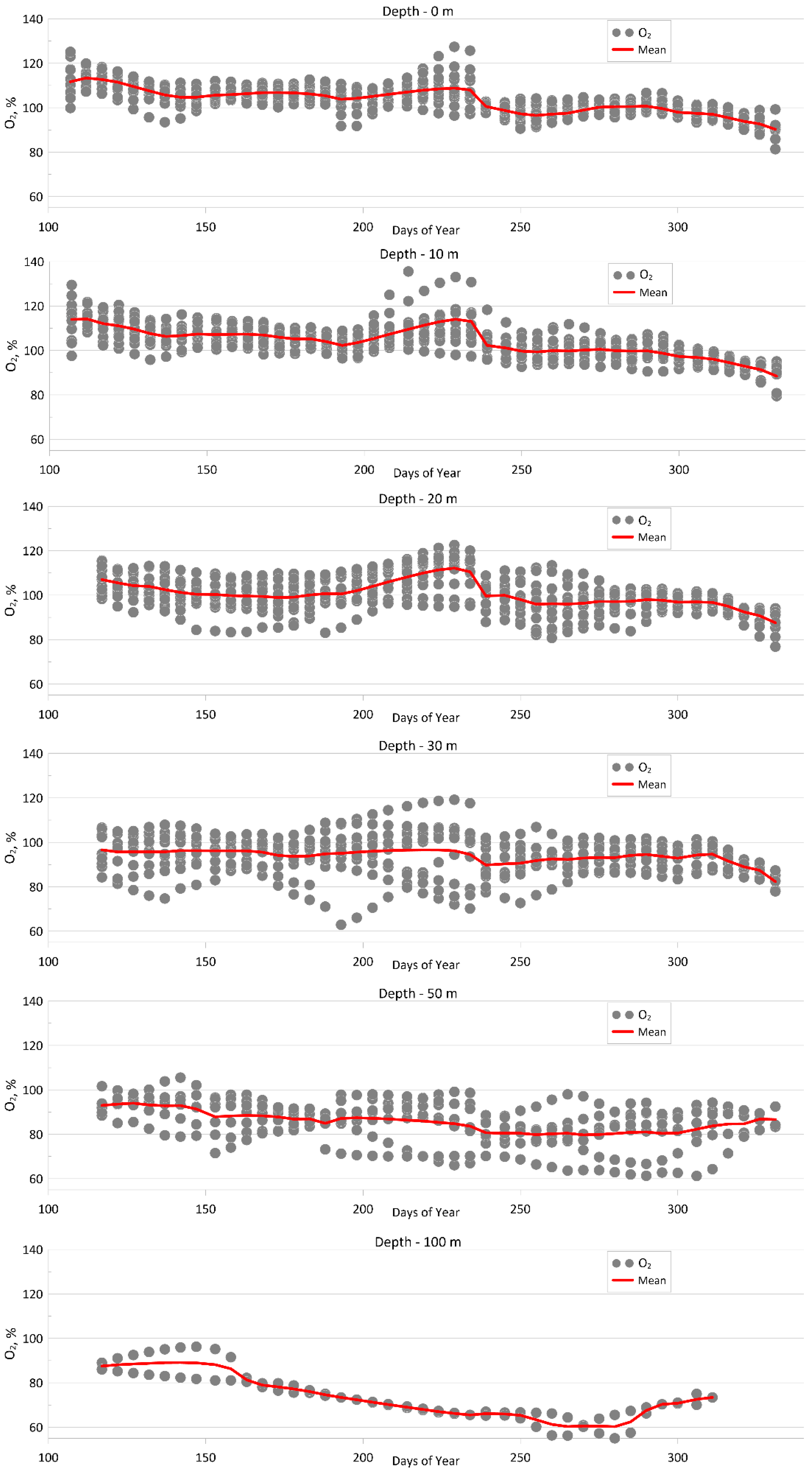
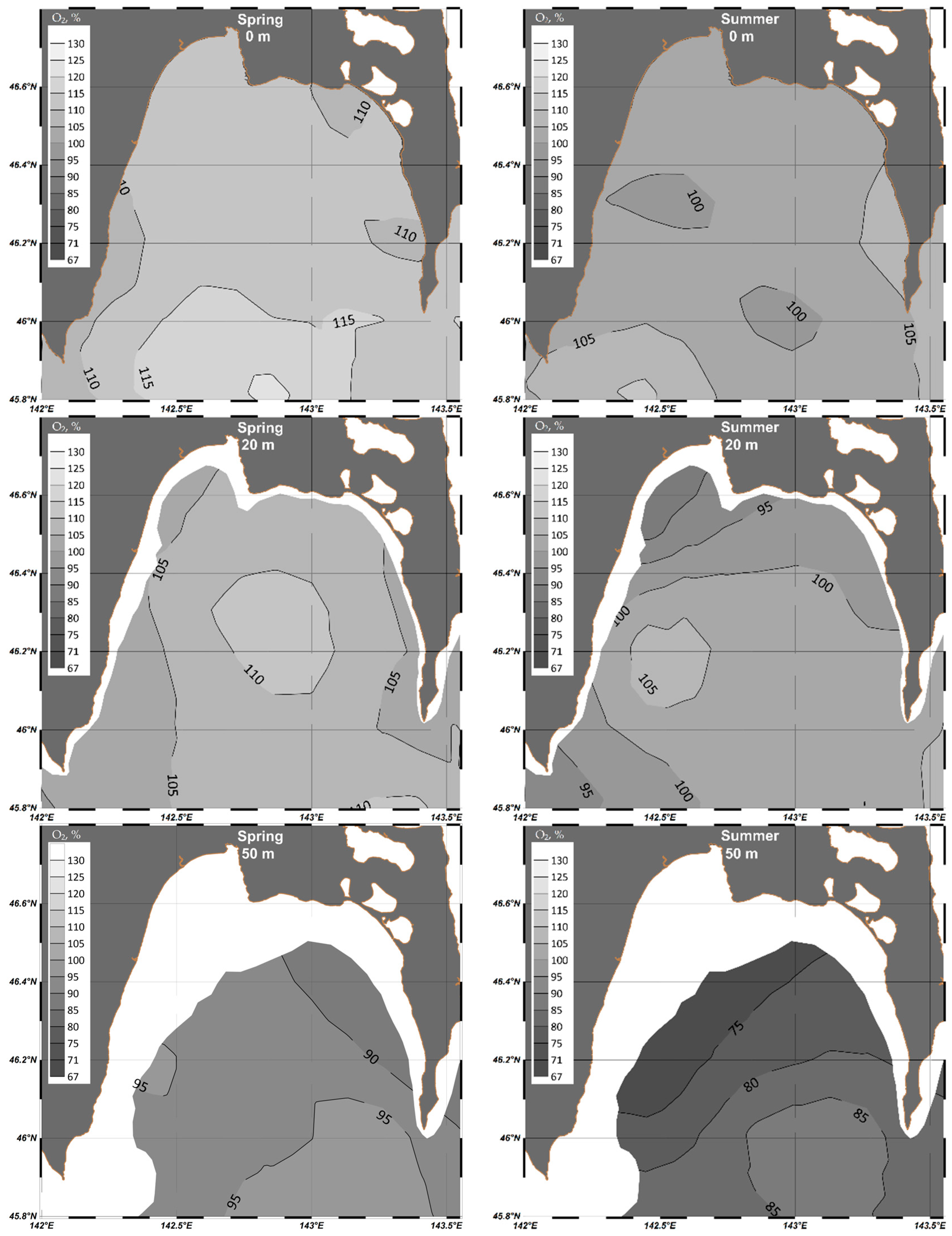
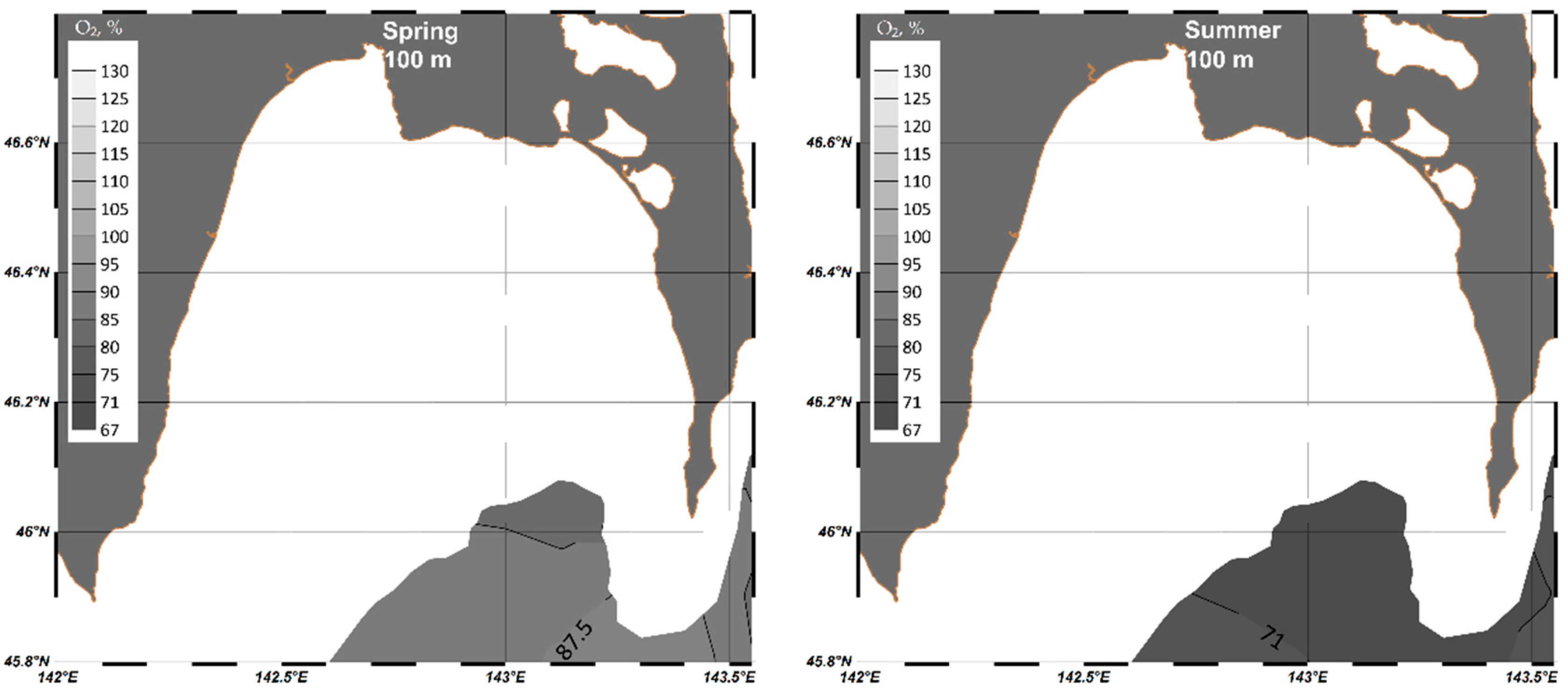


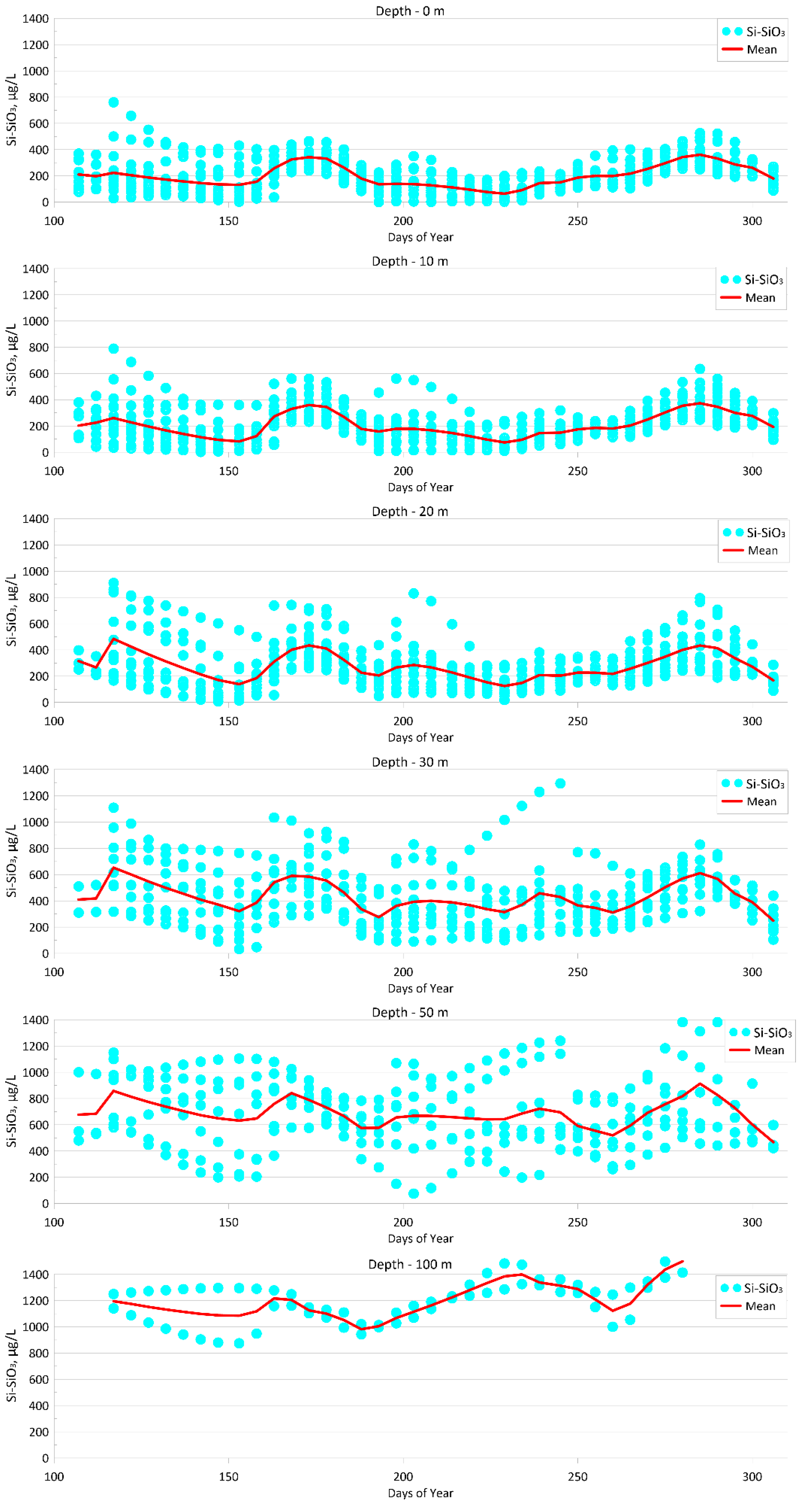
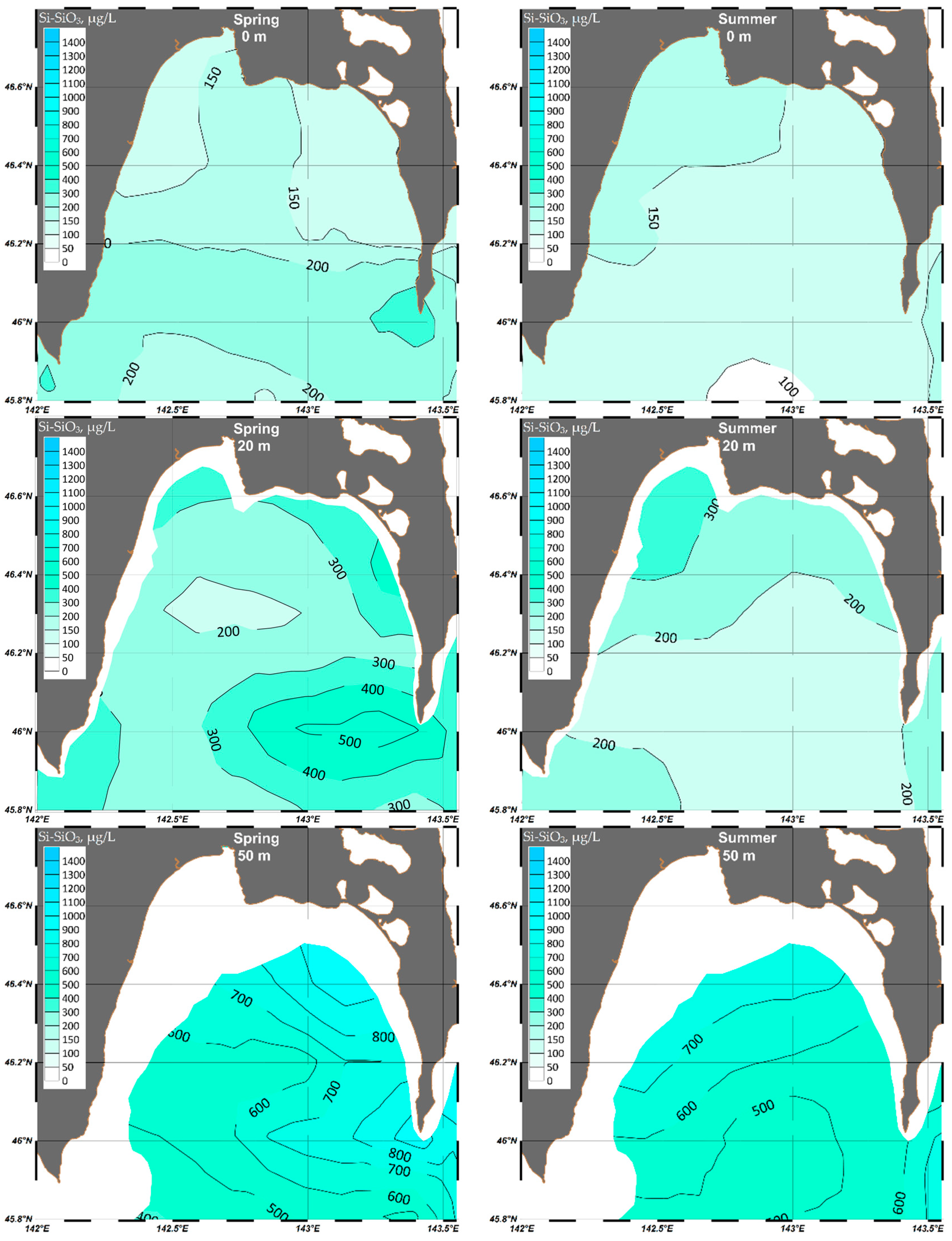
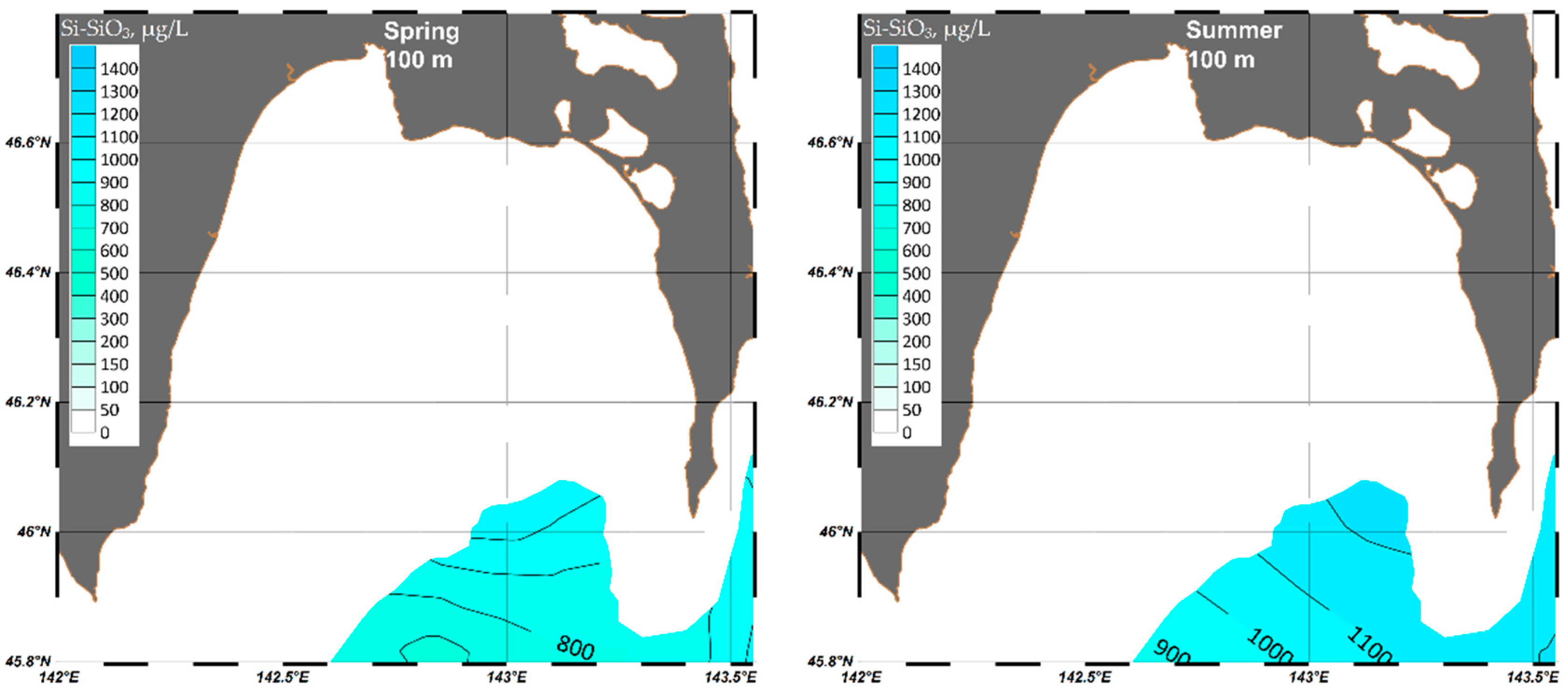
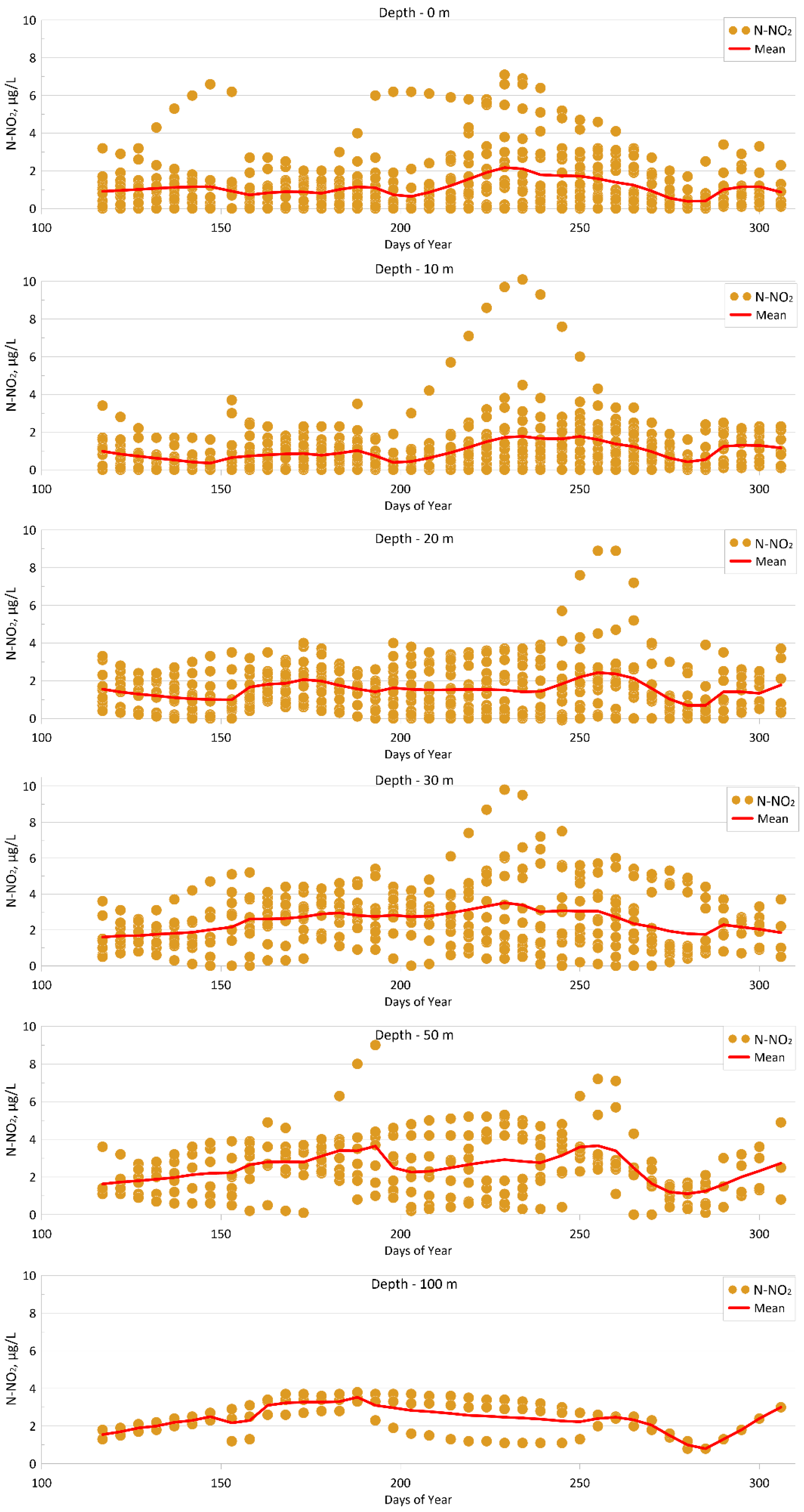
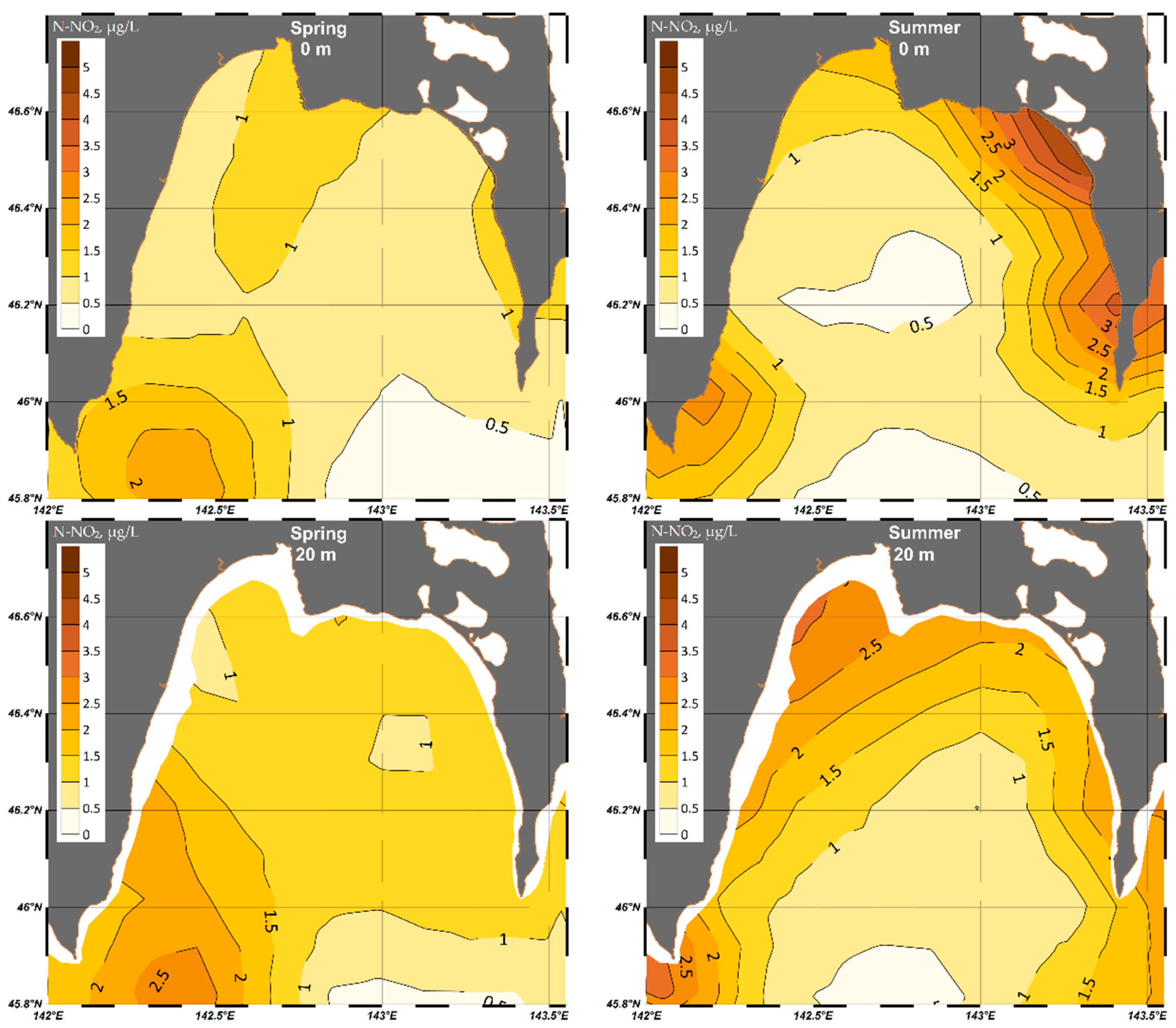

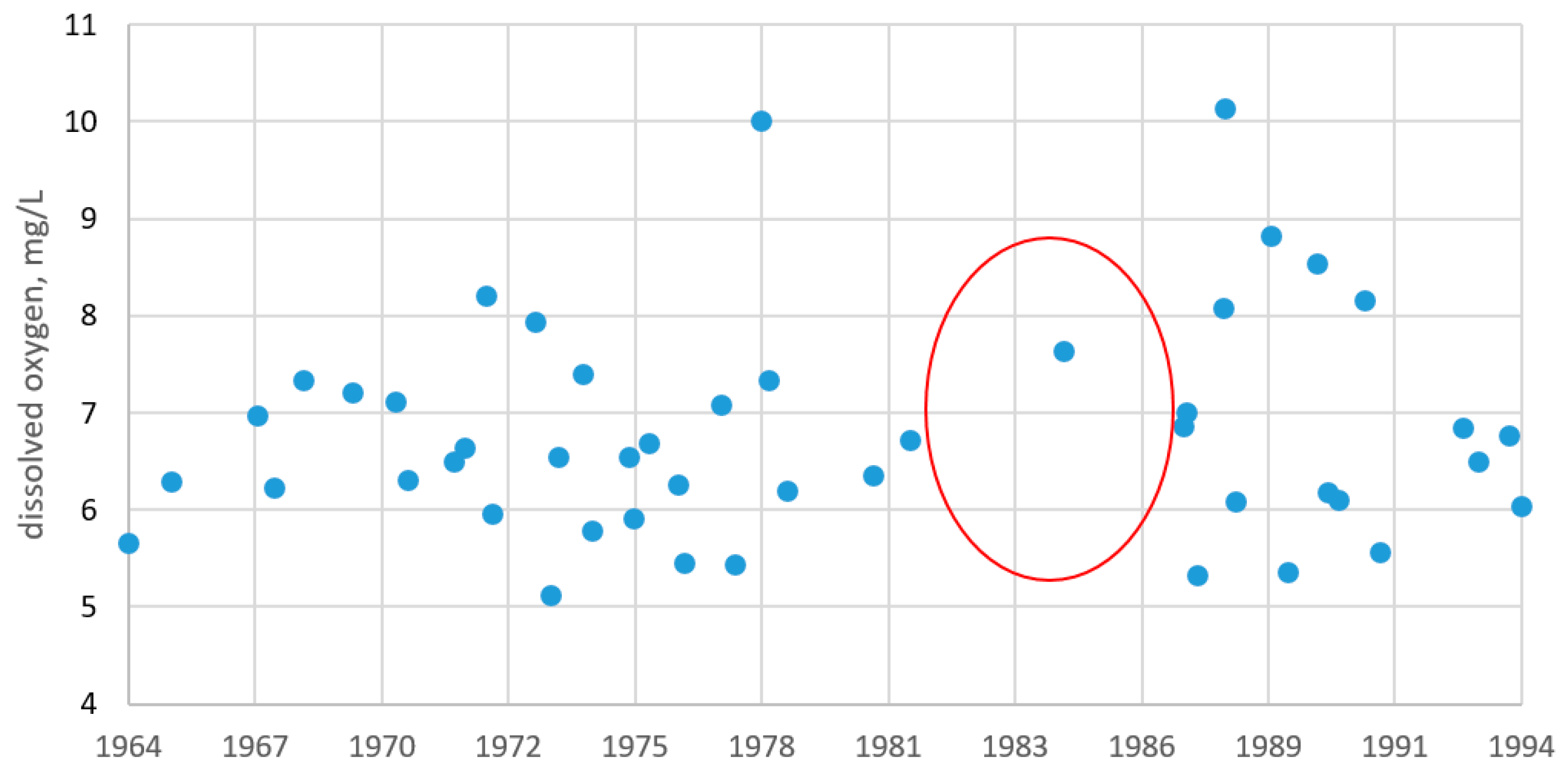
| Parameter | O2 | pH | P-PO4 | N-NO2 | Si-SiO3 |
|---|---|---|---|---|---|
| Number of stations in the “ATLAS” database | 603 | 581 | 257 | 263 | 295 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pishchalnik, V.; Myslenkov, S.; Latkovskaya, E.; Arkhipkin, V. Assessment of the Hydrochemical Characteristics of the Carbon Observational Site ‘Carbon-Sakhalin’ (Aniva Bay, Sea of Okhotsk). Sustainability 2024, 16, 3031. https://doi.org/10.3390/su16073031
Pishchalnik V, Myslenkov S, Latkovskaya E, Arkhipkin V. Assessment of the Hydrochemical Characteristics of the Carbon Observational Site ‘Carbon-Sakhalin’ (Aniva Bay, Sea of Okhotsk). Sustainability. 2024; 16(7):3031. https://doi.org/10.3390/su16073031
Chicago/Turabian StylePishchalnik, Vladimir, Stanislav Myslenkov, Elena Latkovskaya, and Victor Arkhipkin. 2024. "Assessment of the Hydrochemical Characteristics of the Carbon Observational Site ‘Carbon-Sakhalin’ (Aniva Bay, Sea of Okhotsk)" Sustainability 16, no. 7: 3031. https://doi.org/10.3390/su16073031
APA StylePishchalnik, V., Myslenkov, S., Latkovskaya, E., & Arkhipkin, V. (2024). Assessment of the Hydrochemical Characteristics of the Carbon Observational Site ‘Carbon-Sakhalin’ (Aniva Bay, Sea of Okhotsk). Sustainability, 16(7), 3031. https://doi.org/10.3390/su16073031








