Phytoplankton Communities’ Response to Thermal Stratification and Changing Environmental Conditions in a Deep-Water Reservoir: Stochastic and Deterministic Processes
Abstract
1. Introduction
2. Materials and Methods
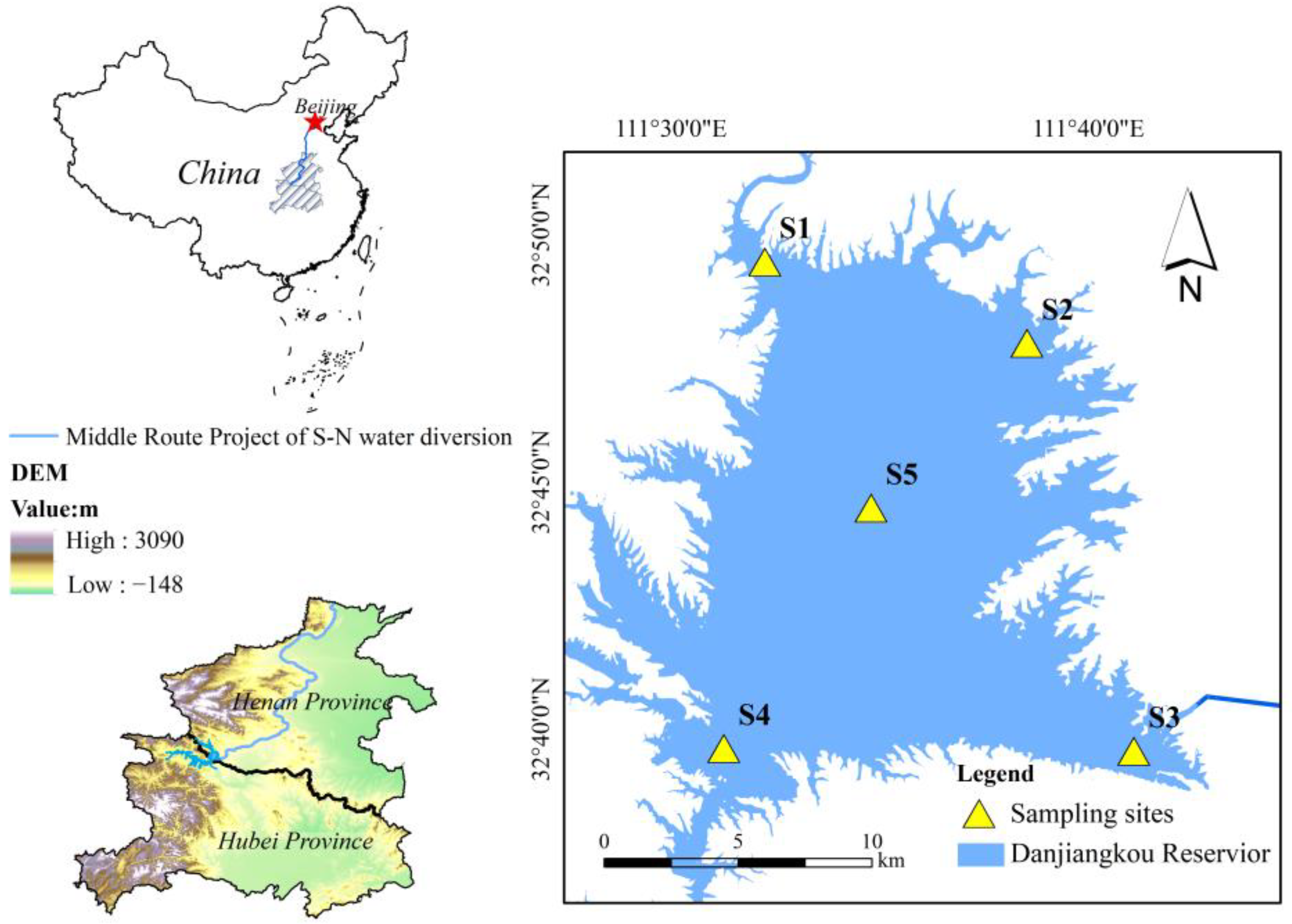
2.1. Study Area
2.2. Sample Collection and Determination
2.3. Data Processing
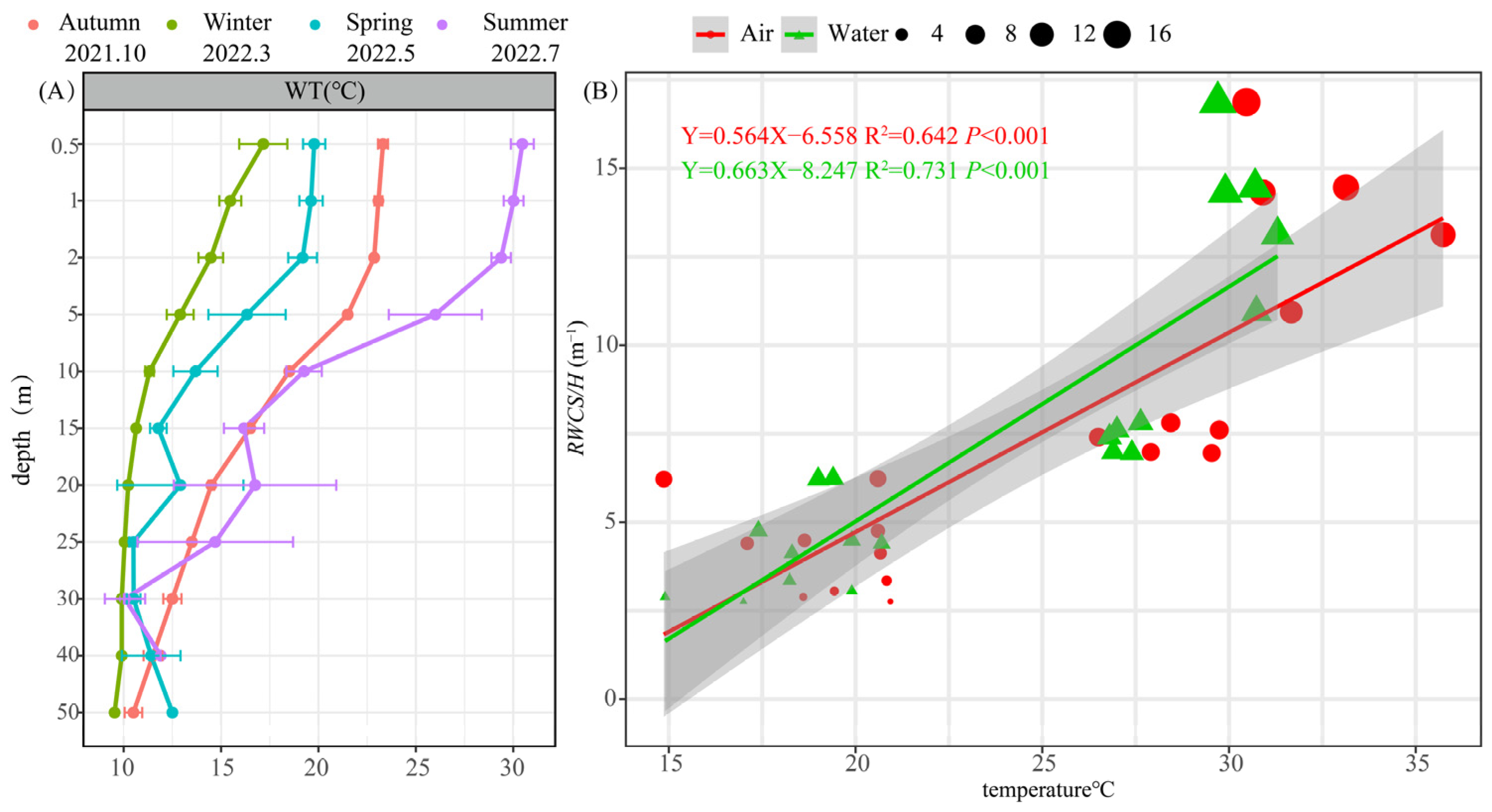
2.3.1. Relative Water Column Stability (RWCS) Calculation
2.3.2. Densities of Phytoplankton
2.3.3. Dominant Species and Niche Breadth of Phytoplankton
2.3.4. Phytoplankton Community Assembly
2.4. Data Analysis and Statistics
3. Results
3.1. Characterization of Water Temperature Stratification and Environmental Factors
3.1.1. Vertical Variation in Water Temperature and the Genesis of Thermal Stratification
3.1.2. Changes in Environmental Characteristics during Thermal Stratification Periods
3.2. Characteristics of Phytoplankton Community Structures during Thermal Stratification
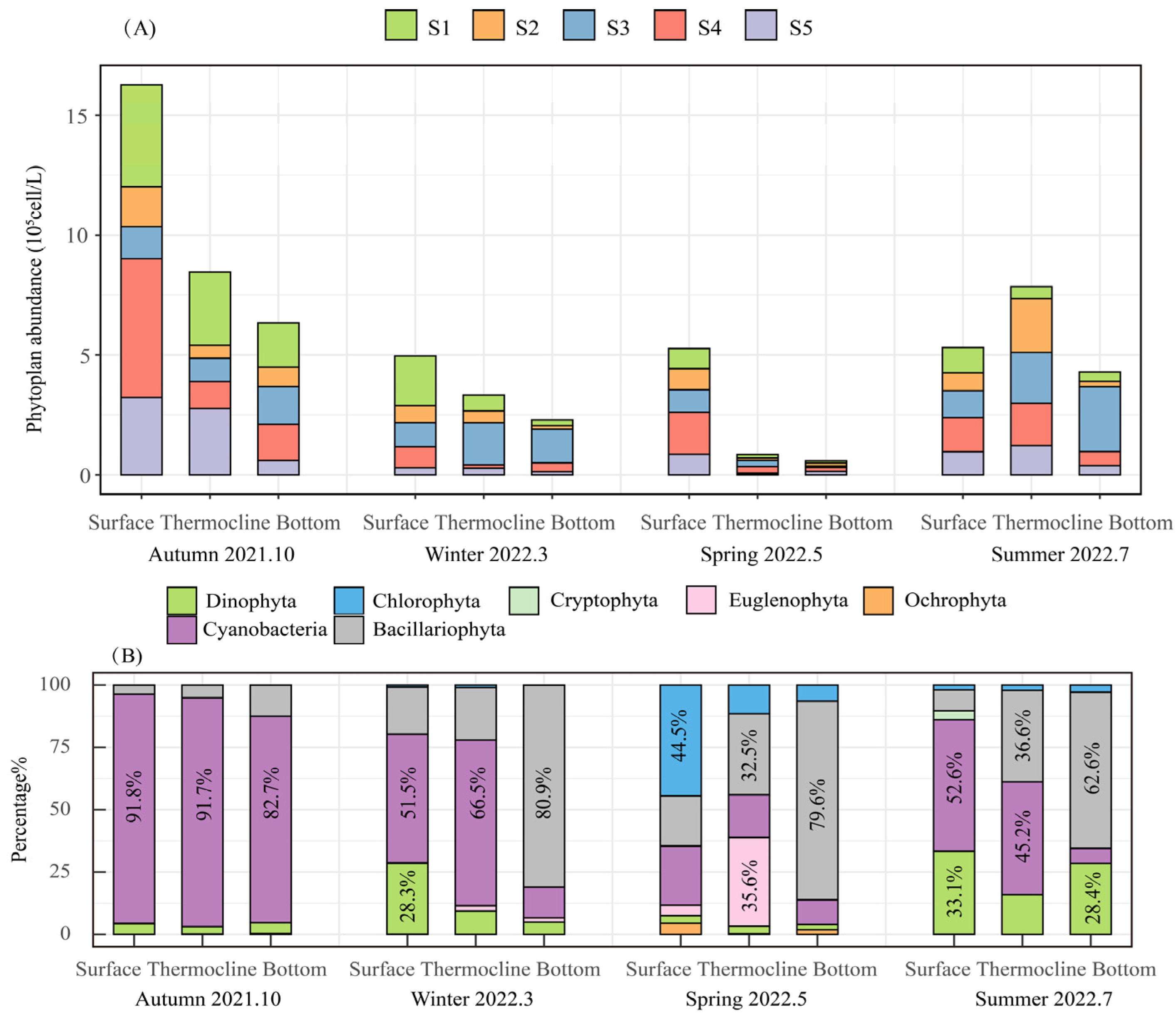
3.2.1. Phytoplankton Species Composition
3.2.2. Dominant Phytoplankton Species Composition
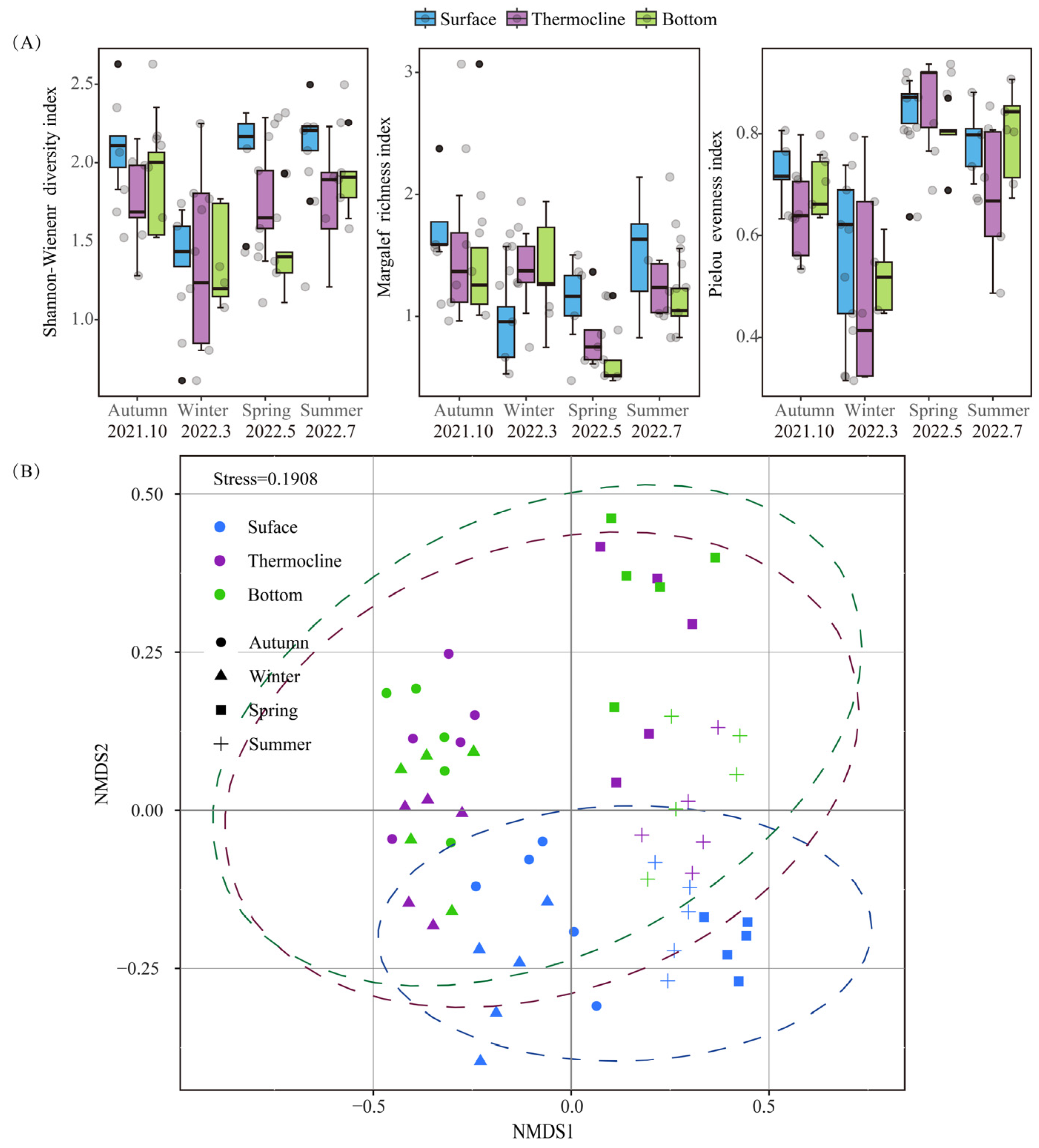
3.2.3. Changes in Phytoplankton Alpha Diversity Index
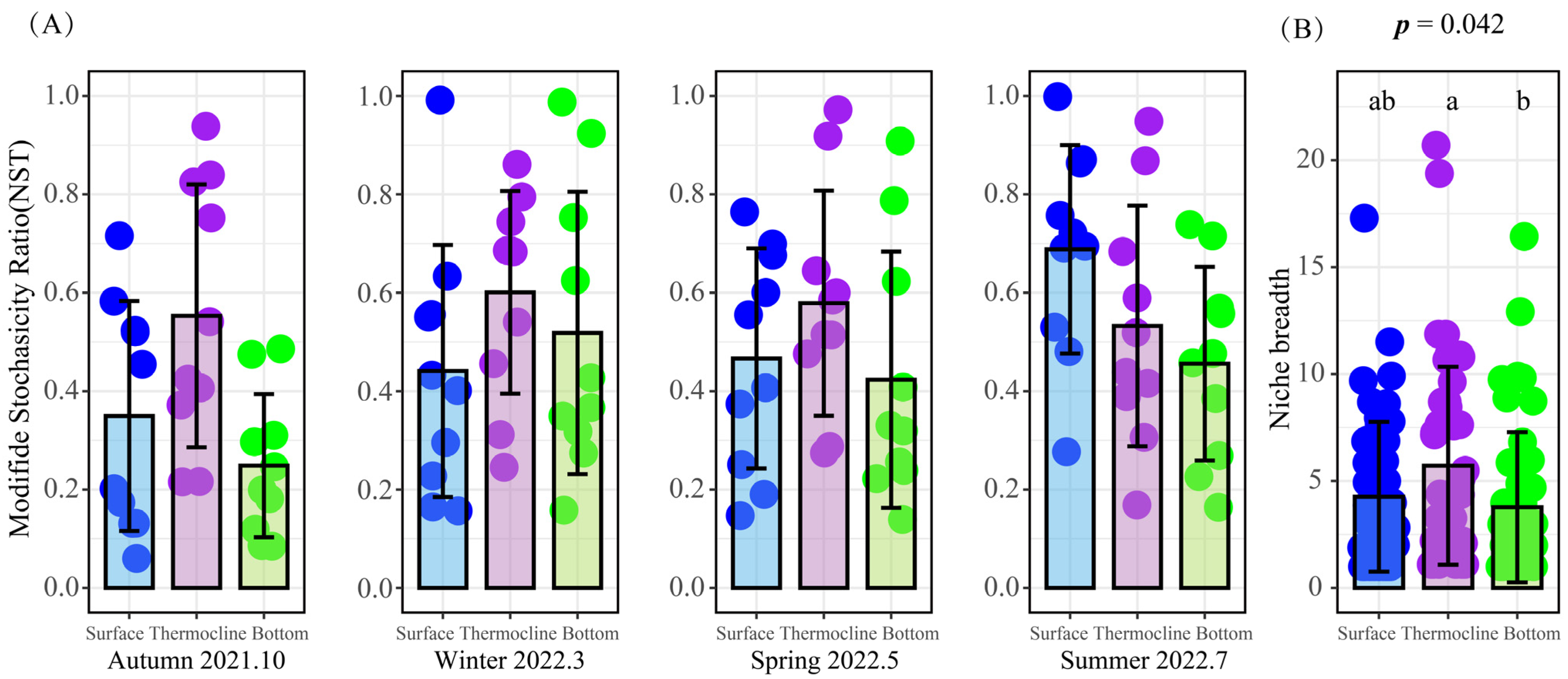
3.2.4. Vertical Distribution Pattern of Phytoplankton Communities and the Assembly Processes
3.3. Response of Phytoplankton to Environmental Factors during Thermal Stratification
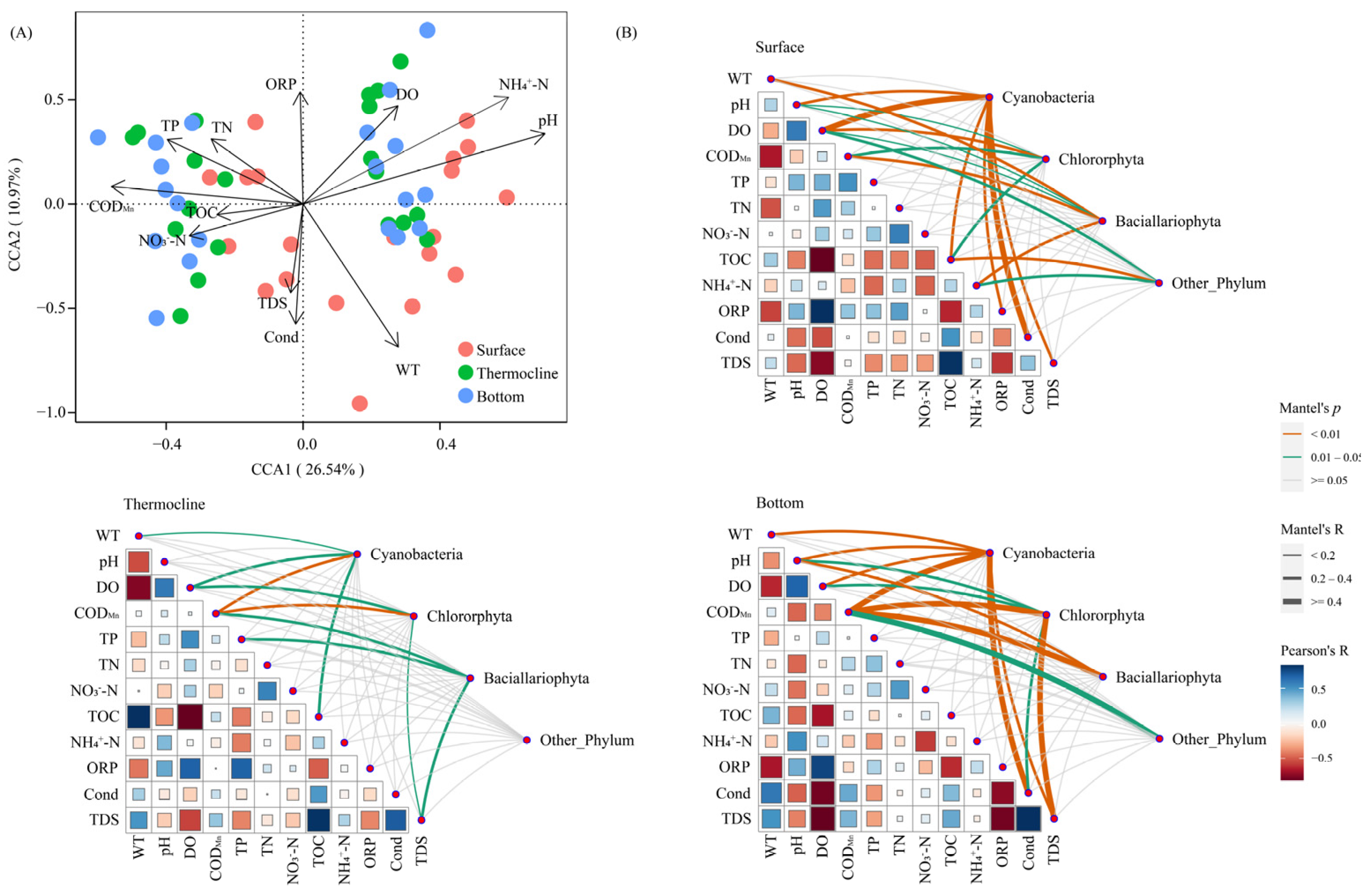
3.3.1. Phytoplankton Community Structure in Relation to Environmental Factors
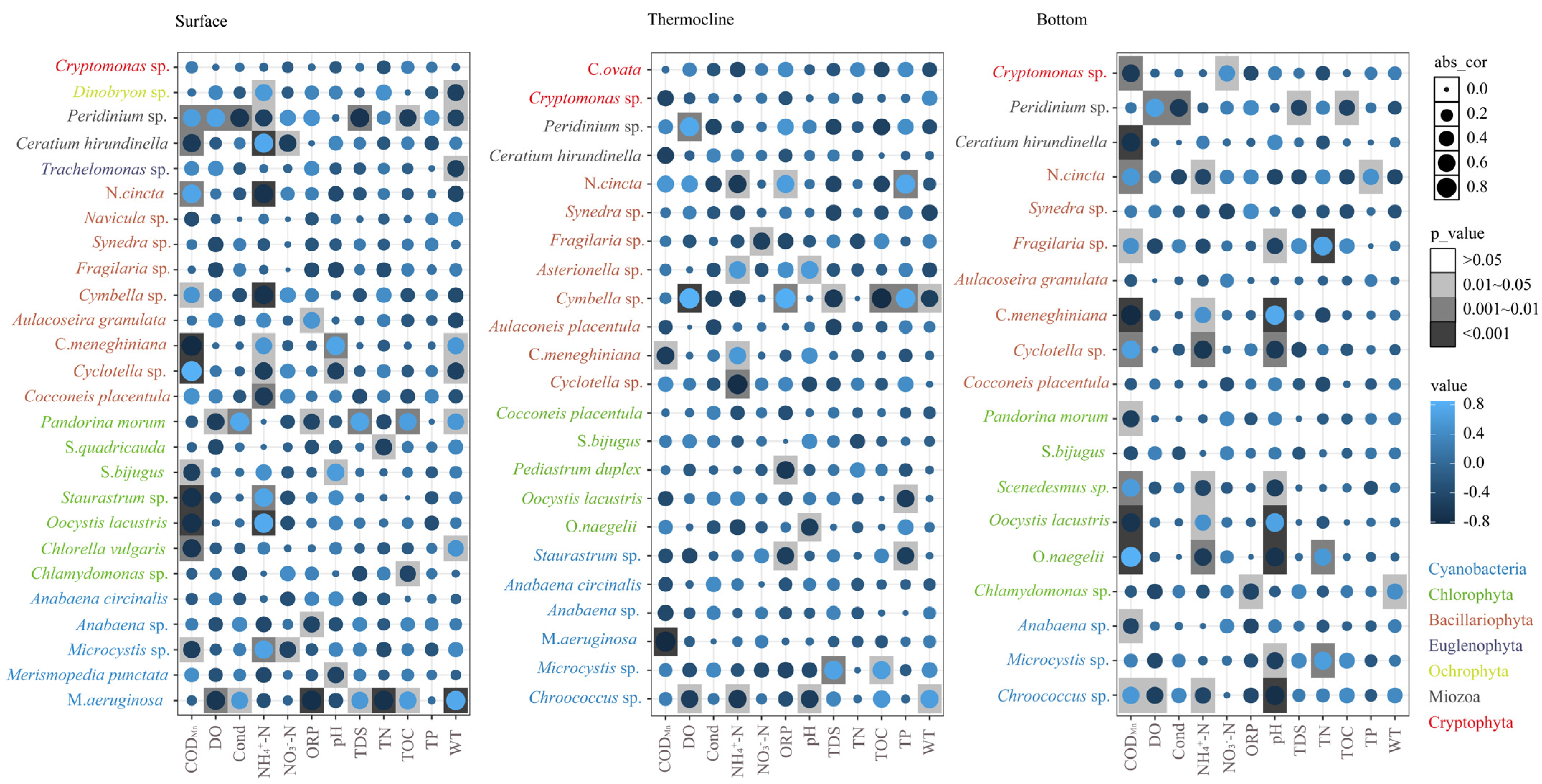
3.3.2. Relationships between Dominant Phytoplankton Species to Environmental Conditions
4. Discussion
4.1. The Characterization of Phytoplankton Assembly Patterns during Thermal Stratification
4.2. Relationships between Phytoplankton Community Structures and Environmental Factors with Thermal Stratification Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beaver, J.R.; Kirsch, J.E.; Tausz, C.E.; Samples, E.E.; Renicker, T.R.; Scotese, K.C.; McMaster, H.A.; Blasius-Wert, B.J.; Zimba, P.V.; Casamatta, D.A. Long-term trends in seasonal plankton dynamics in Lake Mead (Nevada-Arizona, USA) and implications for climate change. Hydrobiologia 2018, 822, 85–109. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, G.; Zhao, H.; Polk, J.; Liu, S. Physicochemical parameters and phytoplankton as indicators of the aquatic environment in karstic springs of South China. Sci. Total Environ. 2019, 659, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Tokatlı, C.; Varol, M.; Uğurluoğlu, A. Ecological risk assessment, source identification and spatial distribution of organic contaminants in terms of mucilage threat in streams of Çanakkale Strait Basin (Türkiye). Chemosphere 2024, 353, 141546. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tuo, Y.; Zhang, L.; Hu, X.; Huang, B.; Chen, M.; Li, Z. Vertical distribution rules and factors influencing phytoplankton in front of a drinking water reservoir outlet. Sci. Total Environ. 2023, 902, 166512. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Bao, Z.; Ma, X.; Shen, H.; Xie, P.; Chen, J. Thermocline stratification favors phytoplankton spatial overlap and species diversity in a subtropical deep reservoir. Sci. Total Environ. 2024, 913, 169712. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Joo, G.; Kim, H.G.; Kim, D.; Kim, H. Effects of Seasonal and Diel Variations in Thermal Stratification on Phytoplankton in a Regulated River. Sustainability 2023, 15, 16330. [Google Scholar] [CrossRef]
- Zhang, Z.; Meng, J.; Chen, Z.; Zhou, S.; Zhang, T.; Chen, Z.; Liu, Y.; Cui, J. Response of dissolved organic matter to thermal stratification and environmental indication: The case of Gangnan Reservoir. Sci. Total Environ. 2023, 868, 161615. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, A.B.; Rudolf, L.; Blasius, B. Vertical distribution and composition of phytoplankton under the influence of an upper mixed layer. J. Theor. Biol. 2010, 263, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Becker, V.; Caputo, L.; Ordóñez, J.; Marcé, R.; Armengol, J.; Crossetti, L.O.; Huszar, V.L.M. Driving factors of the phytoplankton functional groups in a deep Mediterranean reservoir. Water Res. 2010, 44, 3345–3354. [Google Scholar] [CrossRef]
- Sharples, J.; Ross, O.N.; Scott, B.E.; Greenstreet, S.P.R.; Fraser, H. Inter-annual variability in the timing of stratification and the spring bloom in the North-western North Sea. Cont. Shelf Res. 2006, 26, 733–751. [Google Scholar] [CrossRef]
- Gittings, J.A.; Raitsos, D.E.; Krokos, G.; Hoteit, I. Impacts of warming on phytoplankton abundance and phenology in a typical tropical marine ecosystem. Sci. Rep. 2018, 8, 2240. [Google Scholar] [CrossRef]
- Li, P.; Yao, Y.; Lian, J.; Ma, C. Effect of thermal stratified flow on algal blooms in a tributary bay of the Three Gorges reservoir. J. Hydrol. 2021, 601, 126648. [Google Scholar] [CrossRef]
- Wang, J.; Huo, D.; Guo, C.; Zhu, G.; Gong, Z.; Fan, Y.; Wang, J. Vertical Distribution Characteristics and Influencing Factors of Phytoplankton Community Structure in Qiandao Lake. Environ. Sci. 2022, 43, 3575–3586. [Google Scholar]
- Cui, Z.; Gao, W.; Li, Y.; Wang, W.; Wang, H.; Liu, H.; Fan, P.; Fohrer, N.; Wu, N. Dissolved oxygen and water temperature drive vertical spatiotemporal variation of phytoplankton community: Evidence from the largest diversion water source area. Int. J. Environ. Res. Public Health 2023, 20, 4307. [Google Scholar] [CrossRef]
- Zhang, C.; Nong, X.; Behzadian, K.; Campos, L.C.; Chen, L.; Shao, D. A new framework for water quality forecasting coupling causal inference, time-frequency analysis and uncertainty quantification. J. Environ. Manag. 2024, 350, 119613. [Google Scholar] [CrossRef]
- Lin, L.; Pan, X.; Zhang, S.; Li, D.; Zhai, W.; Wang, Z.; Tao, J.; Mi, C.; Li, Q.; Crittenden, J.C. Distribution and source of microplastics in China’s second largest reservoir—Danjiangkou Reservoir. J. Environ. Sci. 2021, 102, 74–84. [Google Scholar] [CrossRef]
- Gao, X.; Chen, H.; Gu, B.; Jeppesen, E.; Xue, Y.; Yang, J. Particulate organic matter as causative factor to eutrophication of subtropical deep freshwater: Role of typhoon (tropical cyclone) in the nutrient cycling. Water Res. 2021, 188, 116470. [Google Scholar] [CrossRef] [PubMed]
- Jargal, N.; An, K.G. Seasonal and interannual responses of blue-green algal taxa and chlorophyll to a monsoon climate, flow regimes, and N:P ratios in a temperate drinking-water reservoir. Sci. Total Environ. 2023, 896, 165306. [Google Scholar] [CrossRef]
- Cui, G.; Li, X.; Li, S.; Ding, S.; Li, Q.; Yang, M.; Lv, H.; Wang, Y. Varying water column stability controls the denitrification process in a subtropical reservoir, Southwest China. J. Environ. Sci. 2022, 111, 208–219. [Google Scholar] [CrossRef]
- Shi, J.; Wang, L.; Yang, Y.; Huang, T. A case study of thermal and chemical stratification in a drinking water reservoir. Sci. Total Environ. 2022, 848, 157787. [Google Scholar] [CrossRef]
- SL733-2016; Technical Code of Phytoplankton Monitoring in Inland Waters. Ministry of Water Resources of the People’s Republic of China: Beijing, China, 2016.
- Wei, Y.; Hu, H. The Freshwater Algae of China Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006. [Google Scholar]
- Liu, Q.; Zhang, H.; Zhang, Y.; Li, D.; Gao, Y.; Li, H.; Duan, L.; Zhang, X.; Liu, F.; Xu, J.; et al. Heterogeneous bacterial communities affected by phytoplankton community turnover and microcystins in plateau lakes of Southwestern China. Sci. Total Environ. 2023, 903, 166303. [Google Scholar] [CrossRef] [PubMed]
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Zhang, J.; Zhang, B.; Liu, Y.; Guo, Y.; Shi, P.; Wei, G. Distinct large-scale biogeographic patterns of fungal communities in bulk soil and soybean rhizosphere in China. Sci. Total Environ. 2018, 644, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.N.; Kolasa, J.; Cottenie, K. Contrasts between habitat generalists and specialists: An empirical extension to the basic metacommunity framework. Ecology 2009, 90, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Isabwe, A.; Yang, J.R.; Wang, Y.; Liu, L.; Chen, H.; Yang, J. Community assembly processes underlying phytoplankton and bacterioplankton across a hydrologic change in a human-impacted river. Sci. Total Environ. 2018, 630, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Skouroliakou, D.I.; Breton, E.; Irion, S.; Artigas, L.F.; Christaki, U. Stochastic and Deterministic Processes Regulate Phytoplankton Assemblages in a Temperate Coastal Ecosystem. Microbiol. Spectr. 2022, 10, e242722. [Google Scholar] [CrossRef] [PubMed]
- Istvánovics, V.; Honti, M. Stochastic simulation of phytoplankton biomass using eighteen years of daily data—Predictability of phytoplankton growth in a large, shallow lake. Sci. Total Environ. 2021, 764, 143636. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Ning, D.; Deng, Y.; Tiedje, J.M.; Zhou, J. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Fan, P.; Li, Y.; Wang, H.; Wang, W.; Liu, H.; Messyasz, B.; Goldyn, R.; Li, B. Assessing the Ecosystem Health of Large Drinking-Water Reservoirs Based on the Phytoplankton Index of Biotic Integrity (P-IBI): A Case Study of Danjiangkou Reservoir. Sustainability 2023, 15, 5282. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Lin, Z.; Zheng, N.; Chen, Y. Identification of water pollution sources and analysis of pollution trigger conditions in Jiuqu River, Luxian County, China. Environ. Sci. Pollut. Res. 2024, 31, 19815–19830. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Higher Education Press: Beijing, China, 2020. [Google Scholar]
- Ji, F.; Han, D.; Yan, L.; Yan, S.; Zha, J.; Shen, J. Assessment of benthic invertebrate diversity and river ecological status along an urbanized gradient using environmental DNA metabarcoding and a traditional survey method. Sci. Total Environ. 2022, 806, 150587. [Google Scholar] [CrossRef]
- Basu, S.; Gogoi, P.; Bhattacharyya, S.; Lohith, K.K.; Das, S.K.; Das, B.K. Variability in the zooplankton assemblages in relation to environmental variables in the tidal creeks of Sundarbans estuarine system, India. Environ. Sci. Pollut. Res. 2022, 29, 45981–46002. [Google Scholar] [CrossRef] [PubMed]
- Yongo, E.; Mutethya, E.; Jin, F.; Zhang, P.; Lek, S.; Mo, L.; Li, J.; Guo, Z. Spatio-temporal variation in water quality and phytoplankton community structure in Changwang, Meishe, and Wuyuan Rivers in Hainan Island, China. Environ. Monit. Assess. 2023, 195, 905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Y.; Liu, X.; Huang, T.; Ma, B.; Li, N.; Yang, W.; Li, H.; Zhao, K. Novel insights in seasonal dynamics and co-existence patterns of phytoplankton and micro-eukaryotes in drinking water reservoir, Northwest China: DNA data and ecological model. Sci. Total Environ. 2023, 857, 159160. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Meng, Q.; Wang, L.; Yu, J.; Chen, X.; Liu, D.; Li, D.; Wang, C.; Liang, F.; Ma, W.; et al. Identification of odor-causing compounds in six species of odor-producing microalgae separated from drinking water source with distinct fishy odor: Insight into microalgae growth and odor characteristics. Chemosphere 2024, 350, 141043. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Li, K.; Huang, T.; Wang, S.; Tang, Y.; Wen, G.; Zhang, H.; Li, X.; Cai, X. Extending improvements of eutrophication and water quality via induced natural mixing after artificial mixing in a stratified reservoir. J. Environ. Manag. 2022, 322, 116048. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Bramburger, A.J.; Reavie, E.D. A comparison of phytoplankton communities of the deep chlorophyll layers and epilimnia of the Laurentian Great Lakes. J. Great Lakes Res. 2016, 42, 1016–1025. [Google Scholar] [CrossRef]
- Sheng, H.; Xu, Y.; Wang, L.; Zhang, M.; Kong, L.; Cai, Q. Spatial and Temporal Variations of Phytoplankton in Danjiangkou Reservoir and its Affecting Factors. Plant Sci. J. 2011, 29, 683–690. [Google Scholar]
- Tan, X.; Xia, X.; Cheng, X.; Zhang, Q. Temporal and Spatial Pattern of Phytoplankton Community and Its Biodiversity Indices in the Danjiangkou Reservoir. Chin. J. Environ. Sci. 2011, 32, 2875–2882. [Google Scholar]
- Wang, Y.; Chen, L.; Niu, Y.; Yu, H.; Luo, M. Spatio-temporal variation in phytoplankton community and its influencing factors in Danjiangkou Reservoir. J. Lake Sci. 2016, 28, 1057–1065. [Google Scholar]
- Mantang, X.; Puze, W.; Shaowen, Y.E.; Gongliang, Y.U.; Jing, Y.; Jiashou, L.; Tanglin, Z. Spatio-temporal characteristics of the phytoplankton community and assessment of fish productivity in the Danjiangkou Reservoir, the wa-ter source for the South-to-North Water Diversion Project, China. J. Fish. Sci. China 2021, 28, 715–727. [Google Scholar]
- Wu, N.; Guo, K.; Suren, A.M.; Riis, T. Lake morphological characteristics and climatic factors affect long-term trends of phytoplankton community in the Rotorua Te Arawa lakes, New Zealand during 23 years observation. Water Res. 2023, 229, 119469. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Pan, B.; Zhao, G.; Sun, C.; Han, X.; Li, M. Geo-climatic factors weaken the effectiveness of phytoplankton diversity as a water quality indicator in a large sediment-laden river. Sci. Total Environ. 2021, 792, 148346. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Xiao, P.; Heino, J.; Tan, F.; Soininen, J.; Chen, H.; Yang, J. Eutrophication increases the similarity of cyanobacterial community features in lakes and reservoirs. Water Res. 2023, 250, 120977. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Peng, F.; Gao, X.; Xiao, P.; Logares, R.; Jeppesen, E.; Ren, K.; Xue, Y.; Yang, J. Low shifts in salinity determined assembly processes and network stability of microeukaryotic plankton communities in a subtropical urban reservoir. Microbiome 2021, 9, 128. [Google Scholar] [CrossRef]
- He, L.; Sun, X.; Li, S.; Zhou, W.; Yu, J.; Zhao, G.; Chen, Z.; Bai, X.; Zhang, J. Depth effects on bacterial community altitudinal patterns and assembly processes in the warm-temperate montane forests of China. Sci. Total Environ. 2024, 914, 169905. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Zhao, D.; Zhou, T.; Wu, Q.L.; Zeng, J. Habitat-specific regulation of bacterial community dynamics during phytoplankton bloom succession in a subtropical eutrophic lake. Water Res. 2023, 242, 120252. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, W.; Wei, C.; Huang, H.; Nan, F.; Liu, X.; Liu, Q.; Lv, J.; Feng, J.; Xie, S. Rainfall-induced changes in aquatic microbial communities and stability of dissolved organic matter: Insight from a Fen river analysis. Environ. Res. 2024, 246, 118107. [Google Scholar] [CrossRef] [PubMed]
- Vikram, S.; Ramond, J.B.; Ortiz, M.; Maggs-Kölling, G.; Pelser, K.; Cowan, D.A. Soil fungal diversity and assembly along a xeric stress gradient in the central Namib Desert. Fungal Biol. 2023, 127, 997–1003. [Google Scholar] [CrossRef]
- Su, W.; Han, Q.; Yang, J.; Yu, Q.; Wang, S.; Wang, X.; Qu, J.; Li, H. Heavy rainfall accelerates the temporal turnover but decreases the deterministic processes of buried gravesoil bacterial communities. Sci. Total Environ. 2022, 836, 155732. [Google Scholar] [CrossRef]
- Wu, B.; Wang, P.; Ding, M.; Huang, G.; Zhang, H.; Yan, C.; Nie, M. Effects of anthropogenic intensity on bacterioplankton community structure in Jinjiang River. Acta Sci. Circumstantiae 2022, 42, 459–473. [Google Scholar]
- Yang, S.; Yang, Q.; Li, X.; Chao, X.; Liu, H.; Wei, L.; Ba, S. Deterministic processes dominate the geographic distribution pattern and community assembly of phytoplankton in typical plateau rivers. Biodivers. Sci. 2023, 31, 23092. [Google Scholar] [CrossRef]
- Muhid, P.; Davis, T.W.; Bunn, S.E.; Burford, M.A. Effects of inorganic nutrients in recycled water on freshwater phytoplankton biomass and composition. Water Res. 2013, 47, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Cui, Z.; Fan, P.; Lan, P.; Wang, X.; Wu, D.; Liu, H.; Chen, Z.; Li, Y.; Li, B. Spatial-temporal characteristics of inorganic anions in the Middle Route of South-North Water Diversion project in Henan province (China). Phys. Chem. Earth Parts A/B/C 2023, 132, 103450. [Google Scholar] [CrossRef]
- Vascotto, I.; Bernardi, A.F.; Bastianini, M.; Mozetič, P.; Finotto, S.; Francé, J. Exploring the mesoscale connectivity of phytoplankton periodic assemblages’ succession in northern Adriatic pelagic habitats. Sci. Total Environ. 2024, 913, 169814. [Google Scholar] [CrossRef] [PubMed]
- Noskov, Y.A.; Manasypov, R.M.; Ermolaeva, N.I.; Antonets, D.V.; Shirokova, L.S.; Pokrovsky, O.S. Environmental factors controlling seasonal and spatial variability of zooplankton in thermokarst lakes along a permafrost gradient of Western Siberia. Sci. Total Environ. 2024, 922, 171284. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Liu, D.; Yang, Z.; Tian, Z. Effects of water temperature on the phytoplankton community structure. Environ. Sci. Technol. 2014, 37, 45–50. [Google Scholar]
- Yu, Y.; Liu, D.; Yang, Z.; Zhang, J.; Xu, Y.; Liu, J.; Yan, G. Vertical Stratification Characteristics of Dissolved Oxygen and Phytoplankton in Thousand-Island Lake and Their Influencing Factors. Environ. Sci. 2017, 38, 1393–1402. [Google Scholar]
- Koblížek, M.; Ferrera, I.; Kolářová, E.; Duhamel, S.; Popendorf, K.J.; Gasol, J.M.; Van Mooy, B. Growth and mortality of aerobic anoxygenic phototrophs in the North Pacific Subtropical Gyre. Appl. Environ. Microb. 2024, e3224. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, T.R.; Tiwari, N.K.; Das, B.K.; Swain, H.S.; Jhonson, C.; Banerjee, T. Riverine connectivity influences the phytoplankton ecology in the open floodplain wetland of the lower river Ganga. Environ. Monit. Assess. 2023, 195, 1403. [Google Scholar] [CrossRef] [PubMed]
- Reinl, K.L.; Harris, T.D.; Elfferich, I.; Coker, A.; Zhan, Q.; De Senerpont, D.L.; Morales-Williams, A.M.; Bhattacharya, R.; Grossart, H.P.; North, R.L.; et al. The role of organic nutrients in structuring freshwater phytoplankton communities in a rapidly changing world. Water Res. 2022, 219, 118573. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Chen, X.; Huang, Y.; Zhang, Y.; Wang, P.; Duan, X.; Qin, X.; Zou, Y.; Deng, Z.; Zhao, Q. Potential risk of eutrophication in the deepest lake of Southwest China: Insights from phosphorus enrichment in bottom water. J. Contam. Hydrol. 2023, 253, 104127. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, G.; Vardi, A. Algal blooms. Curr. Biol. 2020, 30, R1116–R1118. [Google Scholar] [CrossRef] [PubMed]
- Herndon, J.; Cochlan, W.P. Nitrogen utilization by the raphidophyte Heterosigma akashiwo: Growth and uptake kinetics in laboratory cultures. Harmful Algae 2007, 6, 260–270. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Dao, T.S.; Strady, E.; Nguyen, T.; Aimé, J.; Gratiot, N.; Némery, J. Phytoplankton characterization in a tropical tidal river impacted by a megacity: The case of the Saigon River (Southern Vietnam). Environ. Sci. Pollut. Res. 2022, 29, 4076–4092. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.M.; Hull, E.A.; King, C.E.; Burkart, K.; Ott, K.A.; Ryan, J.N.; Gawel, J.E.; Neumann, R.B. Increased exposure of plankton to arsenic in contaminated weakly-stratified lakes. Sci. Total Environ. 2018, 625, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, N.; Albanese, D.; Capelli, C.; Boscaini, A.; Pindo, M.; Donati, C. Diversity and Cyclical Seasonal Transitions in the Bacterial Community in a Large and Deep Perialpine Lake. Microb. Ecol. 2018, 76, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer-Natan, O.; Ofek-Lalzar, M.; Sher, D.; Sukenik, A. Particle-Associated Microbial Community in a Subtropical Lake During Thermal Mixing and Phytoplankton Succession. Front. Microbiol. 2019, 10, 2142. [Google Scholar] [CrossRef]
- Varol, M. Phytoplankton functional groups in a monomictic reservoir: Seasonal succession, ecological preferences, and relationships with environmental variables. Environ. Sci. Pollut. Res. 2019, 26, 20439–20453. [Google Scholar] [CrossRef]
| Parameters | Surface Layer | Thermocline | Bottom Layer |
|---|---|---|---|
| WT (°C) | 22.68 ± 5.18 | 15.58 ± 4.92 | 13.82 ± 5.20 |
| pH | 8.47 ± 0.41 | 8.23 ± 0.44 | 8.29 ± 0.52 |
| Cond (μS/cm) | 349.53 ± 21.69 | 284.63 ± 52.48 | 301.7 ± 29.49 |
| TDS (mg/L) | 187.75 ± 18.51 | 193.34 ± 18.58 | 193.08 ± 16.11 |
| ORP (mV) | 146.49 ± 95.52 | 99.37 ± 116.11 | 176.19 ± 81.11 |
| DO (mg/L) | 9.97 ± 1.63 | 7.14 ± 2.19 | 5.25 ± 3.55 |
| CODMn (mg/L) | 2.34 ± 0.78 | 2.50 ± 1.04 | 2.45 ± 1.16 |
| TOC (mg/L) | 0.54 ± 0.25 | 0.56 ± 0.34 | 0.83 ± 1.43 |
| TP (mg/L) | 0.022 ± 0.01 | 0.024 ± 0.01 | 0.030 ± 0.01 |
| TN (mg/L) | 1.64 ± 0.59 | 1.66 ± 0.54 | 1.86 ± 0.47 |
| NO3−-N (mg/L) | 0.922 ± 0.39 | 0.991 ± 0.45 | 1.141 ± 0.45 |
| NH4+-N (mg/L) | 0.174 ± 0.13 | 0.204 ± 0.14 | 0.219 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, Y.; Li, Y.; Liu, H.; Wang, W.; Zhang, P.; Fohrer, N.; Li, B.-L.; Zhang, Y. Phytoplankton Communities’ Response to Thermal Stratification and Changing Environmental Conditions in a Deep-Water Reservoir: Stochastic and Deterministic Processes. Sustainability 2024, 16, 3058. https://doi.org/10.3390/su16073058
Wang H, Li Y, Li Y, Liu H, Wang W, Zhang P, Fohrer N, Li B-L, Zhang Y. Phytoplankton Communities’ Response to Thermal Stratification and Changing Environmental Conditions in a Deep-Water Reservoir: Stochastic and Deterministic Processes. Sustainability. 2024; 16(7):3058. https://doi.org/10.3390/su16073058
Chicago/Turabian StyleWang, Hongtian, Yixuan Li, Yuying Li, Han Liu, Wanping Wang, Pengcheng Zhang, Nicola Fohrer, Bai-Lian Li, and Yixin Zhang. 2024. "Phytoplankton Communities’ Response to Thermal Stratification and Changing Environmental Conditions in a Deep-Water Reservoir: Stochastic and Deterministic Processes" Sustainability 16, no. 7: 3058. https://doi.org/10.3390/su16073058
APA StyleWang, H., Li, Y., Li, Y., Liu, H., Wang, W., Zhang, P., Fohrer, N., Li, B.-L., & Zhang, Y. (2024). Phytoplankton Communities’ Response to Thermal Stratification and Changing Environmental Conditions in a Deep-Water Reservoir: Stochastic and Deterministic Processes. Sustainability, 16(7), 3058. https://doi.org/10.3390/su16073058








