PM2.5 and O3 in an Enclosed Basin, the Guanzhong Basin of Northern China: Insights into Distributions, Appointment Sources, and Transport Pathways
Abstract
:1. Introduction
2. Materials and Methods
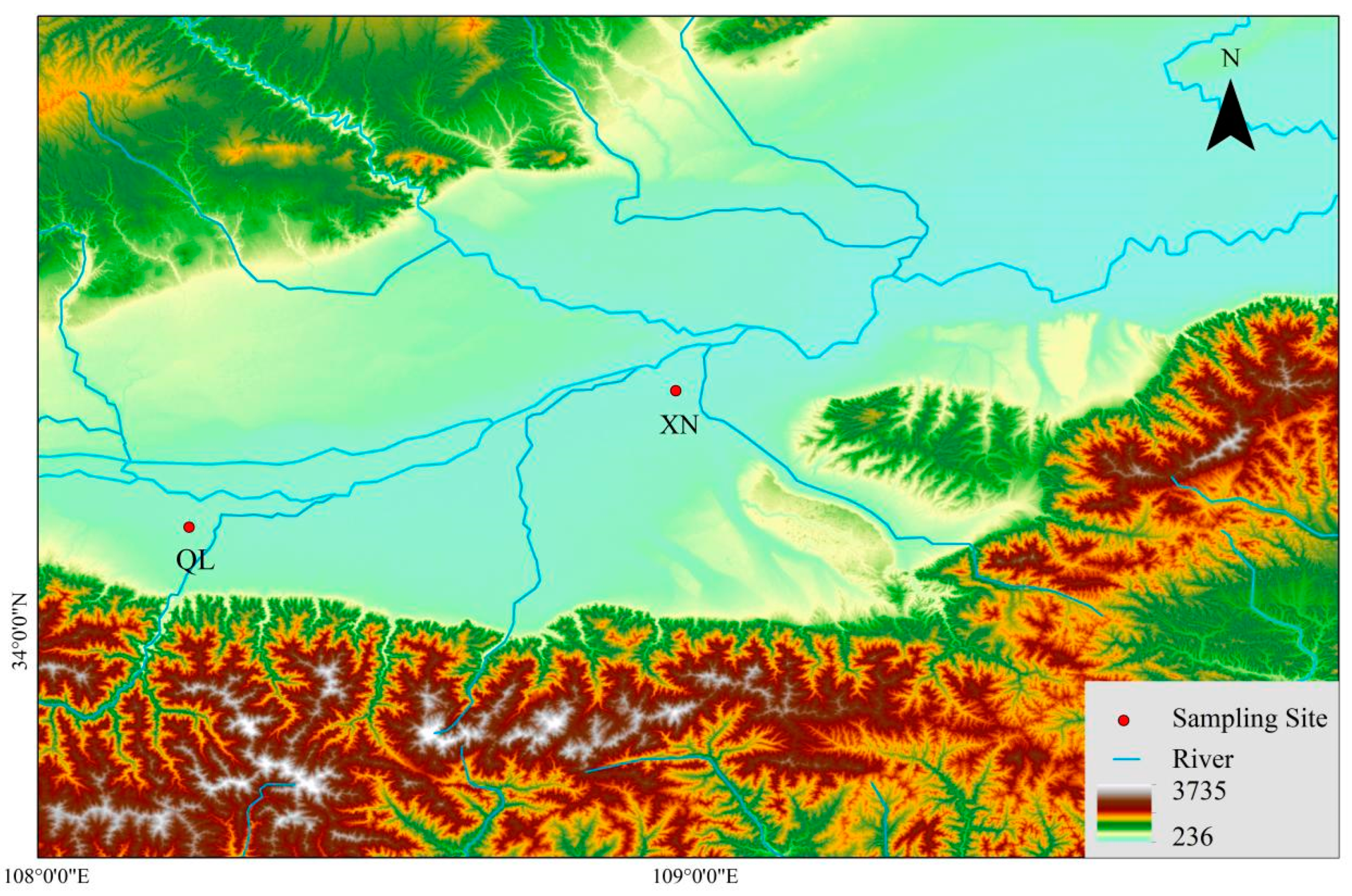
2.1. Sample Site
2.2. Sample Collection
2.3. Chemical Analyses
2.3.1. Carbon Matter
2.3.2. Water-Soluble Ions
2.4. Source Analysis
2.4.1. PMF Model
2.4.2. 48-H Backward Trajectory
3. Results and Discussion
3.1. Characteristics of PM2.5 and Major Components
3.1.1. PM2.5, OC, and EC
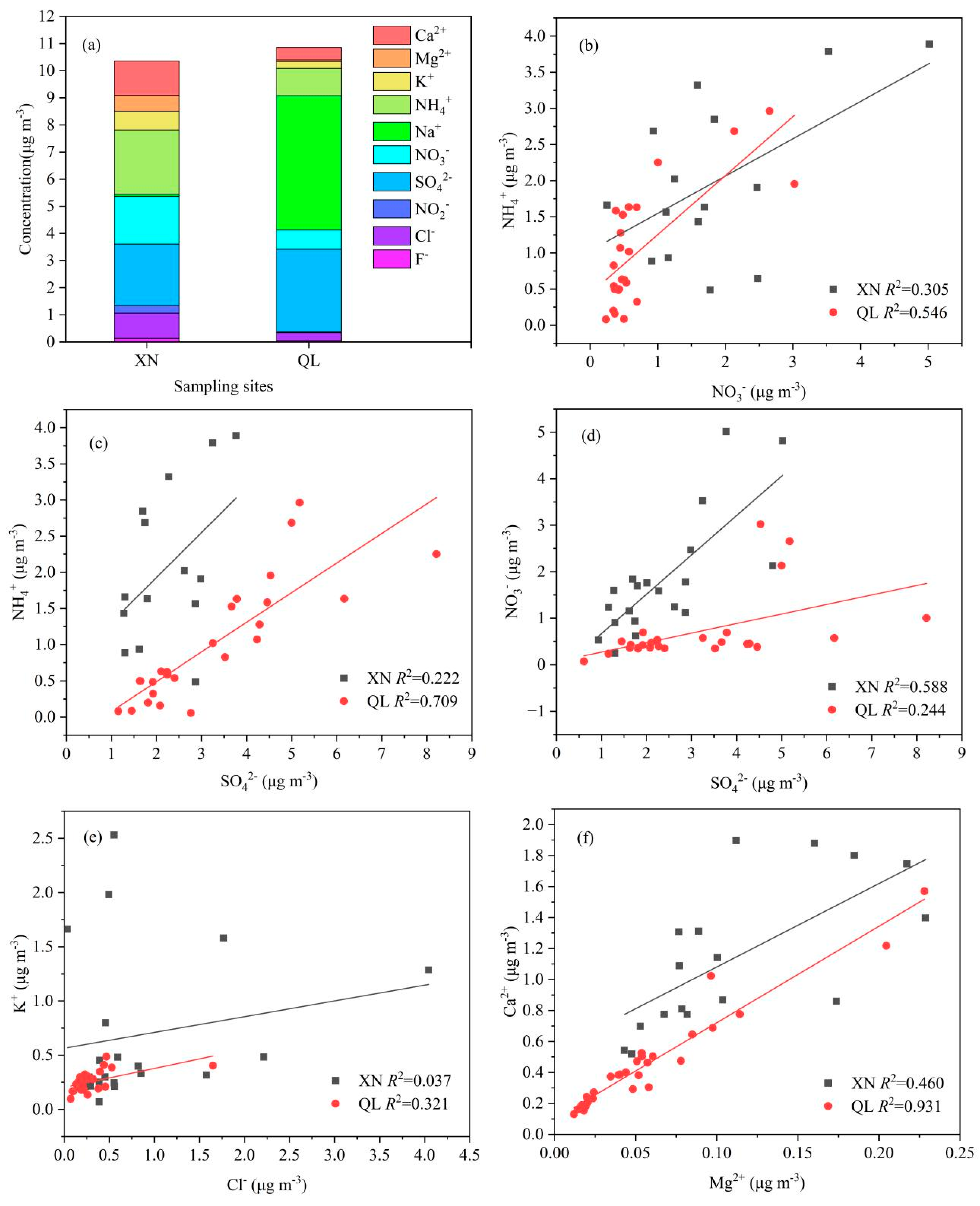
3.1.2. Water-Soluble Ions
3.2. Characteristics of O3
3.3. Source of O3, PM2.5, and Major Components
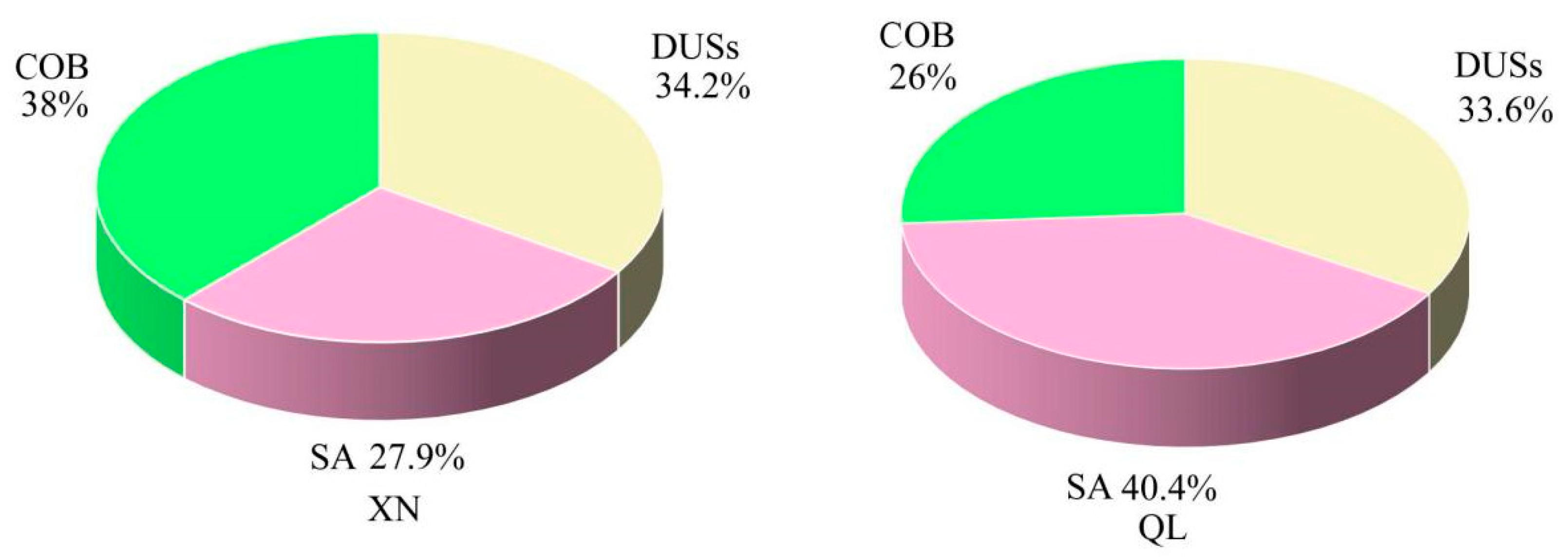
3.3.1. PMF Model
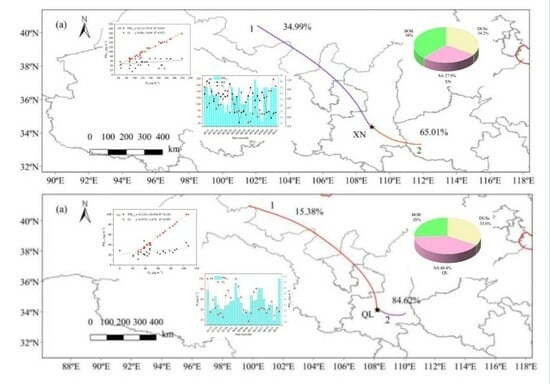
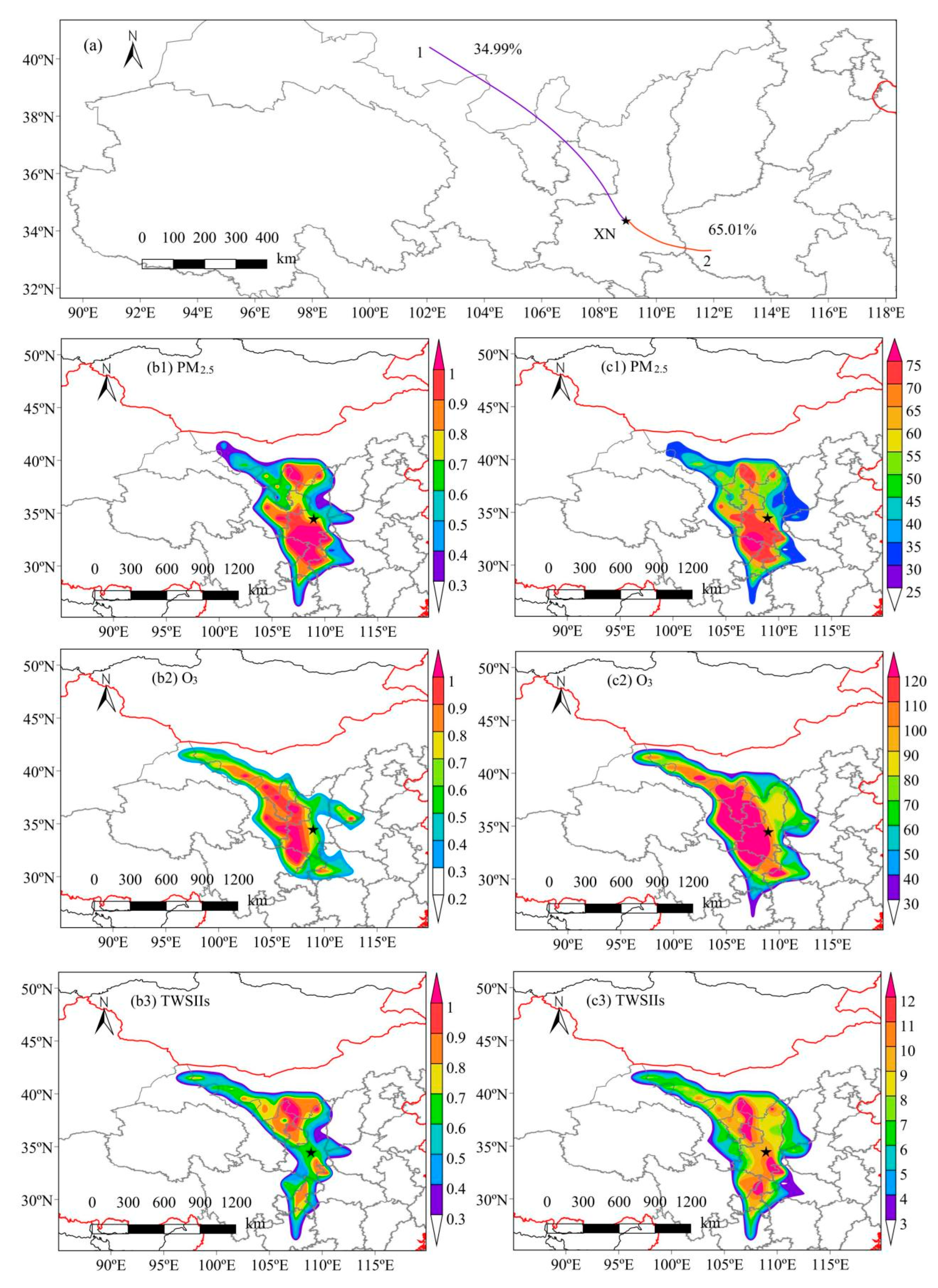
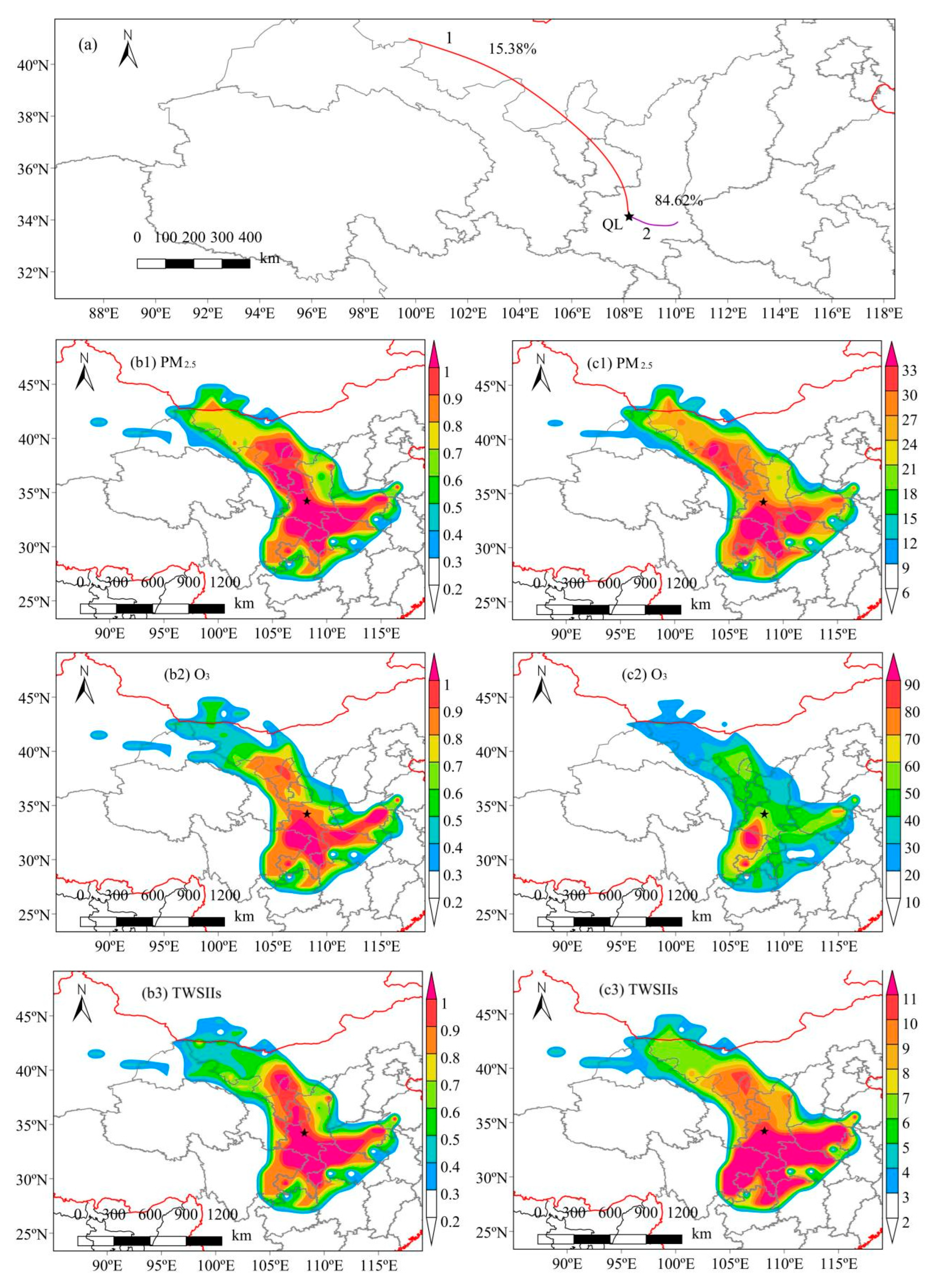
3.3.2. 48-h Backward Trajectory
3.4. Uncertainties
4. Conclusions
- The concentration of PM2.5 in XN (53.40 ± 17.42 μg m−3) was higher than that in QL (27.57 ± 8.27 μg m−3), but the concentrations of PM2.5 in XN and QL in summer were significantly lower than in winter. TWSIIs accounted for 19.40% and 39.37% of the PM2.5 mass in XN and QL, respectively. NH4+, SO42−, and NO3− were the most abundant in XN, accounting for 22.75%, 21.89%, and 17.00% of the TWSIIs mass, respectively; Na+, SO42−, and NH4+ were the most abundant in QL, accounting for 45.56%, 28.09% and 9.28% of the TWSIIs mass, respectively.
- The O3 concentrations in summer were 102.44 ± 35.08 μg m−3 and 47.95 ± 21.63 μg m−3 in XN and QL, respectively, while the O3 concentration and OX in summer showed a significant correlation in the GB. In the future, focus will continue to be placed on the joint prevention and control of PM2.5 and O3 pollution to control overall air pollution.
- The PMF model identified three sources (COB, SA, and DUSs) in XN and QL. Controlling COB may improve PM2.5 pollution in the GB. In addition, metal and water-insoluble components can be introduced in future research to provide more detailed and accurate sources of pollution.
- WPSCF and WCWT with the back-trajectory analysis showed that Inner Mongolia, the interior of Shaanxi, and nearby areas to the south were the sources and source areas of carbonaceous matter in XN and QL. Therefore, it is necessary to strengthen regional joint prevention and control efforts to effectively curb PM2.5 and O3 pollution.
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alves, C.; Evtyugina, M.; Vicente, E.; Vicente, A.; Rienda, I.C.; de la Campa, A.S.; Tomé, M.; Duarte, I. PM2.5 chemical composition and health risks by inhalation near a chemical complex. J. Environ. Sci. 2023, 124, 860–874. [Google Scholar] [CrossRef]
- Jerrett, M. The death toll from air-pollution sources. Nature 2015, 525, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Yin, Y.; Peng, L.; Du, L.; Geng, F.; Zhu, L. Acute effects of black carbon and PM2.5 on children asthma admissions: A time-series study in a Chinese city. Sci. Total Environ. 2014, 481, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lee, S.; Chow, J.C.; Watson, J.G.; Ho, K.; Zhang, R.; Jin, Z.; Shen, Z.; Chen, G.; Kang, Y. Spatial and seasonal distributions of carbonaceous aerosols over China. J. Geophys. Res. Atmos. 2007, 112, 1–9. [Google Scholar] [CrossRef]
- Pachauri, T.; Singla, V.; Satsangi, A.; Lakhani, A.; Kumari, K.M. Characterization of carbonaceous aerosols with special reference to episodic events at Agra, India. Atmos. Res. 2013, 128, 98–110. [Google Scholar] [CrossRef]
- Feng, Y.; Ramanathan, V.; Kotamarthi, V. Brown carbon: A significant atmospheric absorber of solar radiation? Atmos. Chem. Phys. 2013, 13, 8607–8621. [Google Scholar] [CrossRef]
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Kärcher, B.; Koch, D. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Ramanathan, V.; Carmichael, G. Global and regional climate changes due to black carbon. Nat. Geosci. 2008, 1, 221–227. [Google Scholar] [CrossRef]
- Tian, M.; Wang, H.; Chen, Y.; Zhang, L.; Shi, G.; Liu, Y.; Yu, J.; Zhai, C.; Wang, J.; Yang, F. Highly time-resolved characterization of water-soluble inorganic ions in PM2.5 in a humid and acidic mega city in Sichuan Basin, China. Sci. Total Environ. 2017, 580, 224–234. [Google Scholar] [CrossRef]
- Zhang, Y.; Lang, J.; Cheng, S.; Li, S.; Zhou, Y.; Chen, D.; Zhang, H.; Wang, H. Chemical composition and sources of PM1 and PM2.5 in Beijing in autumn. Sci. Total Environ. 2018, 630, 72–82. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, R.; Gomez, M.E.; Yang, L.; Levy Zamora, M.; Hu, M.; Lin, Y.; Peng, J.; Guo, S.; Meng, J. Persistent sulfate formation from London Fog to Chinese haze. Proc. Natl. Acad. Sci. USA 2016, 113, 13630–13635. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xue, L.; Brimblecombe, P.; Lam, Y.F.; Li, L.; Zhang, L. Ozone pollution in China: A review of concentrations, meteorological influences, chemical precursors, and effects. Sci. Total Environ. 2017, 575, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Dimakopoulou, K.; Douros, J.; Samoli, E.; Karakatsani, A.; Rodopoulou, S.; Papakosta, D.; Grivas, G.; Tsilingiridis, G.; Mudway, I.; Moussiopoulos, N. Long-term exposure to ozone and children’s respiratory health: Results from the RESPOZE study. Environ. Res. 2020, 182, 109002. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Unger, N.; Harper, K.; Xia, X.; Liao, H.; Zhu, T.; Xiao, J.; Feng, Z.; Li, J. Ozone and haze pollution weakens net primary productivity in China. Atmos. Chem. Phys. 2017, 17, 6073–6089. [Google Scholar] [CrossRef]
- Ren, W.; Tian, H.; Tao, B.; Chappelka, A.; Sun, G.; Lu, C.; Liu, M.; Chen, G.; Xu, X. Impacts of tropospheric ozone and climate change on net primary productivity and net carbon exchange of China’s forest ecosystems. Glob. Ecol. Biogeogr. 2011, 20, 391–406. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J.; Guo, J.; Jiang, Z.; Chu, Y.; Chang, L.; Yang, Y.; Liao, H. The impact of synoptic patterns on summertime ozone pollution in the North China Plain. Sci. Total Environ. 2020, 735, 139559. [Google Scholar] [CrossRef]
- Guo, X.; Song, H.; Liang, L.; Liu, P. Spatial and temporal variations of ozone concentration in China during 2015–2017. Meteorol. Env. Sci 2020, 43, 41–50. [Google Scholar]
- Zhao, S.; Yin, D.; Yu, Y.; Kang, S.; Qin, D.; Dong, L. PM2.5 and O3 pollution during 2015–2019 over 367 Chinese cities: Spatiotemporal variations, meteorological and topographical impacts. Environ. Pollut. 2020, 264, 114694. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Li, J.; Wang, Z.; Chen, X.; Yang, W.; Sun, Y.; Xin, J.; Pan, X.; Wang, W.; Ye, Q. Assessment of the effect of meteorological and emission variations on winter PM2.5 over the North China Plain in the three-year action plan against air pollution in 2018–2020. Atmos. Res. 2022, 280, 106395. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, Y.; Tong, D.; Shao, M.; Wang, S.; Zhang, Y.; Xu, X.; Wang, J.; He, H.; Liu, W.; et al. Drivers of improved PM2.5 air quality in China from 2013 to 2017. Proc. Natl. Acad. Sci. USA 2019, 116, 24463–24469. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Li, M.; Liao, Z.; Sun, Y.; Song, T.; Gao, W.; Wang, Y.; Li, Y.; Ji, D. Quantifying the impact of synoptic circulation patterns on ozone variability in northern China from April to October 2013–2017. Atmos. Chem. Phys. 2019, 19, 14477–14492. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, C.; Liu, C.; Hu, Q. Variability of PM2.5 and O3 concentrations and their driving forces over Chinese megacities during 2018–2020. J. Environ. Sci. 2023, 124, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, B.; Zhang, Y.; Hu, H. Correlation between surface PM2.5 and O3 in eastern China during 2015–2019: Spatiotemporal variations and meteorological impacts. Atmos. Environ. 2023, 294, 119520. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, Y.; Li, C. Spatiotemporal distribution of PM2.5 and O3 and their interaction during the summer and winter seasons in Beijing, China. Sustainability 2018, 10, 4519. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Chen, L.-W.A.; Chang, M.O.; Robinson, N.F.; Trimble, D.; Kohl, S. The IMPROVE_A temperature protocol for thermal/optical carbon analysis: Maintaining consistency with a long-term database. J. Air Waste Manag. Assoc. 2007, 57, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Cheng, C.L.; Huang, Y.; Tao, J.; Ren, Y.Q.; Wu, F.; Meng, J.J.; Li, J.J.; Cheng, Y.T.; Cao, J.J. Evolution of aerosol chemistry in Xi’an, inland China, during the dust storm period of 2013–Part 1: Sources, chemical forms and formation mechanisms of nitrate and sulfate. Atmos. Chem. Phys. 2014, 14, 11571–11585. [Google Scholar] [CrossRef]
- Dai, W.-J.; Zhou, Y.; Wang, X.-Q.; Qi, P. Variation characteristics of PM2.5 pollution and transport in typical transport channel Cities in Winter. Huan Jing Ke Xue= Huanjing Kexue 2024, 45, 23–35. [Google Scholar] [PubMed]
- Niu, X.; Cao, J.; Shen, Z.; Ho, S.S.H.; Tie, X.; Zhao, S.; Xu, H.; Zhang, T.; Huang, R. PM2.5 from the Guanzhong Plain: Chemical composition and implications for emission reductions. Atmos. Environ. 2016, 147, 458–469. [Google Scholar] [CrossRef]
- Li, X.; Yu, F.; Song, Y.; Zhang, C.; Yan, F.; Hu, Z.; Lei, Y.; Tripathee, L.; Zhang, R.; Guo, J. Water-soluble brown carbon in PM2.5 at two typical sites in Guanzhong Basin: Optical properties, sources, and implications. Atmos. Res. 2023, 281, 106499. [Google Scholar] [CrossRef]
- Ji, D.; Gao, M.; Maenhaut, W.; He, J.; Wu, C.; Cheng, L.; Gao, W.; Sun, Y.; Sun, J.; Xin, J. The carbonaceous aerosol levels still remain a challenge in the Beijing-Tianjin-Hebei region of China: Insights from continuous high temporal resolution measurements in multiple cities. Environ. Int. 2019, 126, 171–183. [Google Scholar] [CrossRef]
- Wang, J.; Ho, S.S.H.; Ma, S.; Cao, J.; Dai, W.; Liu, S.; Shen, Z.; Huang, R.; Wang, G.; Han, Y. Characterization of PM2.5 in Guangzhou, China: Uses of organic markers for supporting source apportionment. Sci. Total Environ. 2016, 550, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cao, J.; Shen, Z.; Xu, B.; Zhu, C.; Chen, L.W.A.; Su, X.; Liu, S.; Han, Y.; Wang, G. Aerosol particles at a high-altitude site on the Southeast Tibetan Plateau, China: Implications for pollution transport from South Asia. J. Geophys. Res. Atmos. 2013, 118, 11,360–11,375. [Google Scholar] [CrossRef]
- Wang, M.; Xu, B.; Cao, J.; Tie, X.; Wang, H.; Zhang, R.; Qian, Y.; Rasch, P.J.; Zhao, S.; Wu, G. Carbonaceous aerosols recorded in a southeastern Tibetan glacier: Analysis of temporal variations and model estimates of sources and radiative forcing. Atmos. Chem. Phys. 2015, 15, 1191–1204. [Google Scholar] [CrossRef]
- Cong, Z.; Kang, S.; Kawamura, K.; Liu, B.; Wan, X.; Wang, Z.; Gao, S.; Fu, P. Carbonaceous aerosols on the south edge of the Tibetan Plateau: Concentrations, seasonality and sources. Atmos. Chem. Phys. 2015, 15, 1573–1584. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, H.; Chun, X.; Wan, Z.; Liu, T.; Sun, B.; Wang, J.; Zhang, W. Characteristics of fine carbonaceous aerosols in Wuhai, a resource-based city in Northern China: Insights from energy efficiency and population density. Environ. Pollut. 2022, 292, 118368. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Zhang, J.; He, J.; Wang, X.; Pang, B.; Liu, Z.; Wang, L.; Wang, Y. Characteristics of atmospheric organic and elemental carbon aerosols in urban Beijing, China. Atmos. Environ. 2016, 125, 293–306. [Google Scholar] [CrossRef]
- Pandis, S.N.; Harley, R.A.; Cass, G.R.; Seinfeld, J.H. Secondary organic aerosol formation and transport. Atmos. Environ. Part A Gen. Top. 1992, 26, 2269–2282. [Google Scholar] [CrossRef]
- Xu, H.; Cao, J.; Chow, J.C.; Huang, R.-J.; Shen, Z.; Chen, L.A.; Ho, K.F.; Watson, J.G. Inter-annual variability of wintertime PM2.5 chemical composition in Xi’an, China: Evidences of changing source emissions. Sci. Total Environ. 2016, 545, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; He, L.-Y.; Zhang, Y.-H.; Wang, M.; Kim, Y.P.; Moon, K. Seasonal variation of ionic species in fine particles at Qingdao, China. Atmos. Environ. 2002, 36, 5853–5859. [Google Scholar] [CrossRef]
- Yang, X.; Wu, F.; Xu, R.; Li, N.; Zhang, Z.; Xue, P.; Wang, W.; Zhao, X. Concentrations, sources, and influential factors of water-soluble ions of atmospheric particles in Dunhuang Mogao Grottoes, a world heritage site in China. J. Arid Land 2022, 14, 1395–1412. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; He, C.; Zhang, Z.; Chen, N.; Hu, J.; Liu, H.; Wang, X. Analysis of the diurnal changes in the water-soluble ion concentration in Wuhan between 2016 and 2019. Atmosphere 2022, 13, 582. [Google Scholar] [CrossRef]
- Zhou, J.; Xing, Z.; Deng, J.; Du, K. Characterizing and sourcing ambient PM2.5 over key emission regions in China I: Water-soluble ions and carbonaceous fractions. Atmos. Environ. 2016, 135, 20–30. [Google Scholar] [CrossRef]
- Wang, H.; Miao, Q.; Shen, L.; Yang, Q.; Wu, Y.; Wei, H. Air pollutant variations in Suzhou during the 2019 novel corona virus (COVID-19) lockdown of 2020: High time-resolution measurements of aerosol chemical compositions and source apportionment. Environ. Pollut. 2021, 271, 116298. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Peng, C.; Chen, Y.; Li, T.; Yang, F. Pollution characteristics of water-soluble inorganic ions in PM2.5 from a mountainous city in southwest China. Atmosphere 2022, 13, 1713. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, F.; Xu, Y.; Chen, J. Characterization of water-soluble inorganic ions in size-segregated aerosols in coastal city, Xiamen. Atmos. Res. 2011, 99, 546–562. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, Y.; Qin, D.; Yin, D.; Dong, L.; He, J. Analyses of regional pollution and transportation of PM2.5 and ozone in the city clusters of Sichuan Basin, China. Atmos. Pollut. Res. 2019, 10, 374–385. [Google Scholar] [CrossRef]
- Gao, M.; Gao, J.; Zhu, B.; Kumar, R.; Lu, X.; Song, S.; Zhang, Y.; Jia, B.; Wang, P.; Beig, G. Ozone pollution over China and India: Seasonality and sources. Atmos. Chem. Phys. 2020, 20, 4399–4414. [Google Scholar] [CrossRef]
- Li, N.; He, Q.; Greenberg, J.; Guenther, A.; Li, J.; Cao, J.; Wang, J.; Liao, H.; Wang, Q.; Zhang, Q. Impacts of biogenic and anthropogenic emissions on summertime ozone formation in the Guanzhong Basin, China. Atmos. Chem. Phys. 2018, 18, 7489–7507. [Google Scholar] [CrossRef]
- Zou, Y.; Charlesworth, E.; Yin, C.; Yan, X.; Deng, X.; Li, F. The weekday/weekend ozone differences induced by the emissions change during summer and autumn in Guangzhou, China. Atmos. Environ. 2019, 199, 114–126. [Google Scholar] [CrossRef]
- Tyagi, B.; Singh, J.; Beig, G. Seasonal progression of surface ozone and NOx concentrations over three tropical stations in North-East India. Environ. Pollut. 2020, 258, 113662. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, W.; Zhang, T.; Qiu, Y.; Ma, P.; Li, L.; Wang, L.; Wang, M.; Zheng, D.; Zhao, W. Analysis of the characteristics of ozone pollution in the North China Plain from 2016 to 2020. Atmosphere 2022, 13, 715. [Google Scholar] [CrossRef]
- Qin, M.; Hu, A.; Mao, J.; Li, X.; Sheng, L.; Sun, J.; Li, J.; Wang, X.; Zhang, Y.; Hu, J. PM2.5 and O3 relationships affected by the atmospheric oxidizing capacity in the Yangtze River Delta, China. Sci. Total Environ. 2022, 810, 152268. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, L.; Liao, H.; Dang, R. Correlations between PM2.5 and ozone over China and associated underlying reasons. Atmosphere 2019, 10, 352. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Liu, C.; Wu, B.; Shi, K. A study of cross-correlations between PM2.5 and O3 based on Copula and Multifractal methods. Phys. A Stat. Mech. Its Appl. 2022, 589, 126651. [Google Scholar] [CrossRef]
- Wang, B.; Wu, R.; Lau, K. Interannual variability of the Asian summer monsoon: Contrasts between the Indian and the western North Pacific–East Asian monsoons. J. Clim. 2001, 14, 4073–4090. [Google Scholar] [CrossRef]
- Song, X.; Hao, Y. Analysis of ozone pollution characteristics and transport paths in Xi’an City. Sustainability 2022, 14, 16146. [Google Scholar] [CrossRef]
- Masiol, M.; Squizzato, S.; Rampazzo, G.; Pavoni, B. Source apportionment of PM2.5 at multiple sites in Venice (Italy): Spatial variability and the role of weather. Atmos. Environ. 2014, 98, 78–88. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, L.T.; Chen, M.Z.; Zheng, Y. The 2013 severe haze over the Southern Hebei, China: PM2.5 composition and source apportionment. Atmos. Pollut. Res. 2014, 5, 759–768. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Zhang, J.; Gui, H.; Du, P.; Yu, T.; Wang, J.; Lu, Y.; Liu, W.; Cheng, Y. Identification of long-range transport pathways and potential sources of PM2.5 and PM10 in Beijing from 2014 to 2015. J. Environ. Sci. 2017, 56, 214–229. [Google Scholar] [CrossRef]
- Reff, A.; Eberly, S.I.; Bhave, P.V. Receptor modeling of ambient particulate matter data using positive matrix factorization: Review of existing methods. J. Air Waste Manag. Assoc. 2007, 57, 146–154. [Google Scholar] [CrossRef]
- Stohl, A. Computation, accuracy and applications of trajectories—A review and bibliography. Atmos. Environ. 1998, 32, 947–966. [Google Scholar] [CrossRef]
- Wu, C.; Wu, D.; Yu, J.Z. Estimation and uncertainty analysis of secondary organic carbon using 1 year of hourly organic and elemental carbon data. J. Geophys. Res. Atmos. 2019, 124, 2774–2795. [Google Scholar] [CrossRef]
- Wu, C.; Yu, J.Z. Determination of primary combustion source organic carbon-to-elemental carbon (OC/EC) ratio using ambient OC and EC measurements: Secondary OC-EC correlation minimization method. Atmos. Chem. Phys. 2016, 16, 5453–5465. [Google Scholar] [CrossRef]
- Xu, L.; Chen, X.; Chen, J.; Zhang, F.; He, C.; Zhao, J.; Yin, L. Seasonal variations and chemical compositions of PM2.5 aerosol in the urban area of Fuzhou, China. Atmos. Res. 2012, 104, 264–272. [Google Scholar] [CrossRef]
- Shon, Z.H.; Ghosh, S.; Kim, K.H.; Song, S.K.; Jung, K.; Kim, N.J. Analysis of water-soluble ions and their precursor gases over diurnal cycle. Atmos. Res. 2013, 132, 309–321. [Google Scholar] [CrossRef]
- Paatero, P. The multilinear engine—A table-driven, least squares program for solving multilinear problems, including the n-way parallel factor analysis model. J. Comput. Graph. Stat. 1999, 8, 854–888. [Google Scholar]
- Jaeckels, J.M.; Bae, M.S.; Schauer, J.J. Positive matrix factorization (PMF) analysis of molecular marker measurements to quantify the sources of organic aerosols. Environ. Sci. Technol. 2007, 41, 5763–5769. [Google Scholar] [CrossRef] [PubMed]
- Prendes, P.; Andrade, J.M.; López-Mahía, P.; Prada, D. Source apportionment of inorganic ions in airborne urban particles from Coruña city (N.W. of Spain) using positive matrix factorization. Talanta 1999, 49, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Wang, M.; Sun, H.; Mu, Z.; Zhang, L.; Li, Y.; Chen, Q. Source apportionment of environmentally persistent free radicals (EPFRs) in PM2.5 over Xi’an, China. Sci. Total Environ. 2019, 689, 193–202. [Google Scholar] [CrossRef]
- Paatero, P.; Hopke, P.K.; Song, X.H.; Ramadan, Z. Understanding and controlling rotations in factor analytic models. Chemom. Intell. Lab. Syst. 2002, 60, 253–264. [Google Scholar] [CrossRef]
- Polissar, A.V.; Hopke, P.K.; Harris, J.M. Source regions for atmospheric aerosol measured at Barrow, Alaska. Environ. Sci. Technol. 2001, 35, 4214–4226. [Google Scholar] [CrossRef] [PubMed]
- Sulaymon, I.D.; Mei, X.; Yang, S.; Chen, S.; Zhang, Y.; Hopke, P.K.; Schauer, J.J.; Zhang, Y. PM2.5 in Abuja, Nigeria: Chemical characterization, source apportionment, temporal variations, transport pathways and the health risks assessment. Atmos. Res. 2020, 237, 104833. [Google Scholar] [CrossRef]
- Hsu, Y.K.; Holsen, T.M.; Hopke, P.K. Comparison of hybrid receptor models to locate PCB sources in Chicago. Atmos. Environ. 2003, 37, 545–562. [Google Scholar] [CrossRef]

| XN | QL | |||||
|---|---|---|---|---|---|---|
| Avg | Max | Min | Avg | Max | Min | |
| PM2.5 (μg m−3) | 53.40 ± 17.42 | 82.64 | 13.89 | 27.57 ± 8.27 | 48.10 | 11.38 |
| OC (μg m−3) | 4.61 ± 2.41 | 9.94 | 0.90 | 4.23 ± 1.37 | 7.73 | 1.35 |
| EC (μg m−3) | 0.78 ± 0.60 | 2.15 | 0.10 | 0.67 ± 0.53 | 2.03 | 0.03 |
| OC/EC | 9.84 ± 6.95 | 24.53 | 2.02 | 10.00 ± 6.99 | 28.12 | 3.18 |
| POC (μg m−3) | 1.54 ± 1.23 | 4.32 | 0.16 | 2.19 ± 1.65 | 6.46 | 0.22 |
| SOC (μg m−3) | 3.32 ± 1.65 | 6.18 | 0.90 | 2.35 ± 1.05 | 4.72 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Guo, J.; Wan, X.; Yang, Z.; Tripathee, L.; Yu, F.; Zhang, R.; Yang, W.; Wang, Q. PM2.5 and O3 in an Enclosed Basin, the Guanzhong Basin of Northern China: Insights into Distributions, Appointment Sources, and Transport Pathways. Sustainability 2024, 16, 3074. https://doi.org/10.3390/su16073074
Li X, Guo J, Wan X, Yang Z, Tripathee L, Yu F, Zhang R, Yang W, Wang Q. PM2.5 and O3 in an Enclosed Basin, the Guanzhong Basin of Northern China: Insights into Distributions, Appointment Sources, and Transport Pathways. Sustainability. 2024; 16(7):3074. https://doi.org/10.3390/su16073074
Chicago/Turabian StyleLi, Xiaofei, Jingning Guo, Xuequan Wan, Zhen Yang, Lekhendra Tripathee, Feng Yu, Rui Zhang, Wen Yang, and Qiyuan Wang. 2024. "PM2.5 and O3 in an Enclosed Basin, the Guanzhong Basin of Northern China: Insights into Distributions, Appointment Sources, and Transport Pathways" Sustainability 16, no. 7: 3074. https://doi.org/10.3390/su16073074
APA StyleLi, X., Guo, J., Wan, X., Yang, Z., Tripathee, L., Yu, F., Zhang, R., Yang, W., & Wang, Q. (2024). PM2.5 and O3 in an Enclosed Basin, the Guanzhong Basin of Northern China: Insights into Distributions, Appointment Sources, and Transport Pathways. Sustainability, 16(7), 3074. https://doi.org/10.3390/su16073074