Drivers of Macroinvertebrate Communities in Mediterranean Rivers: A Mesohabitat Approach
Abstract
1. Introduction
2. Materials and Methods
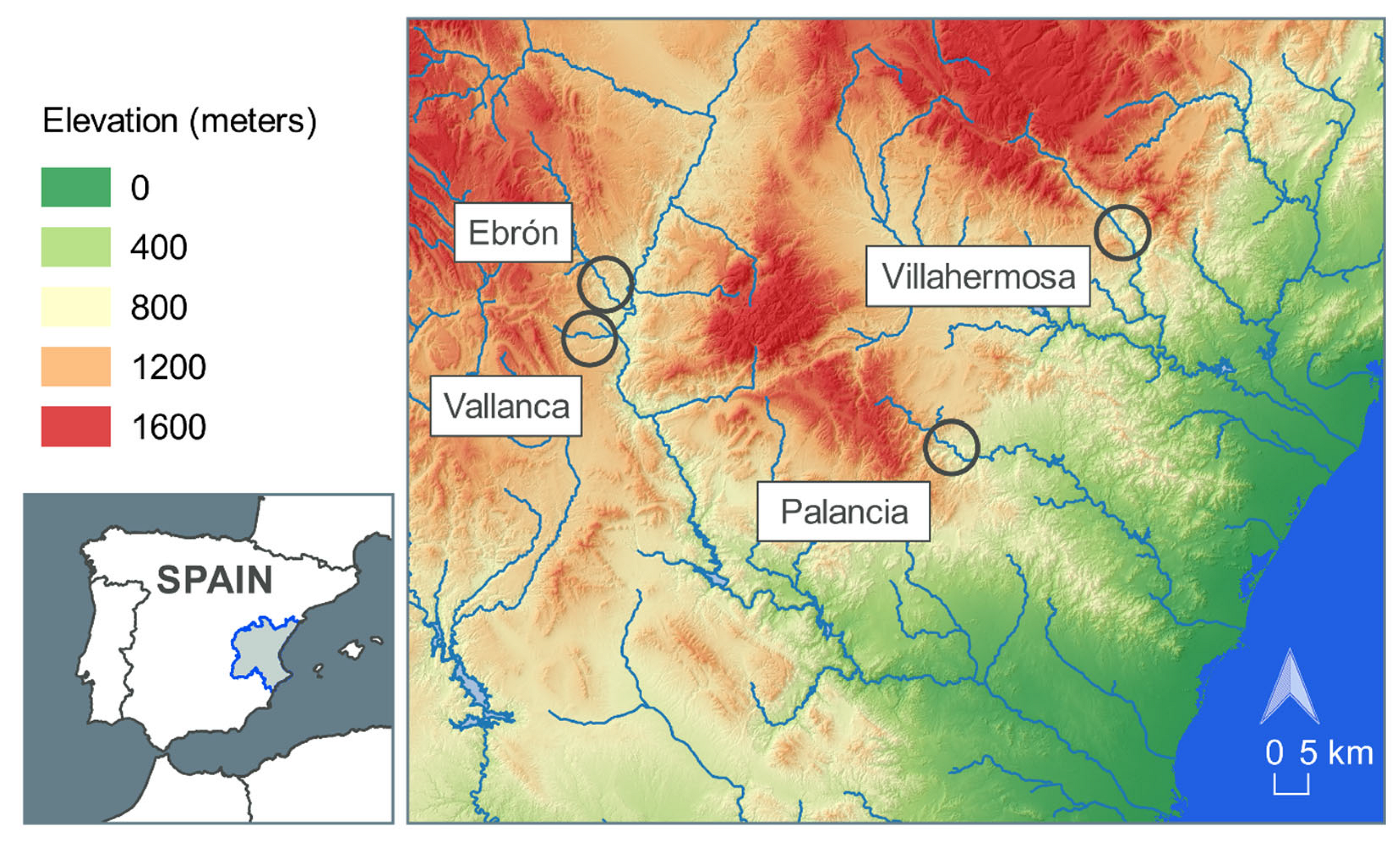
2.1. Study Sites
2.2. Data Collection
2.3. Mesohabitat Survey and Characterization
2.4. Macroinvertebrates Data Compilation
2.5. Statistical Analyses
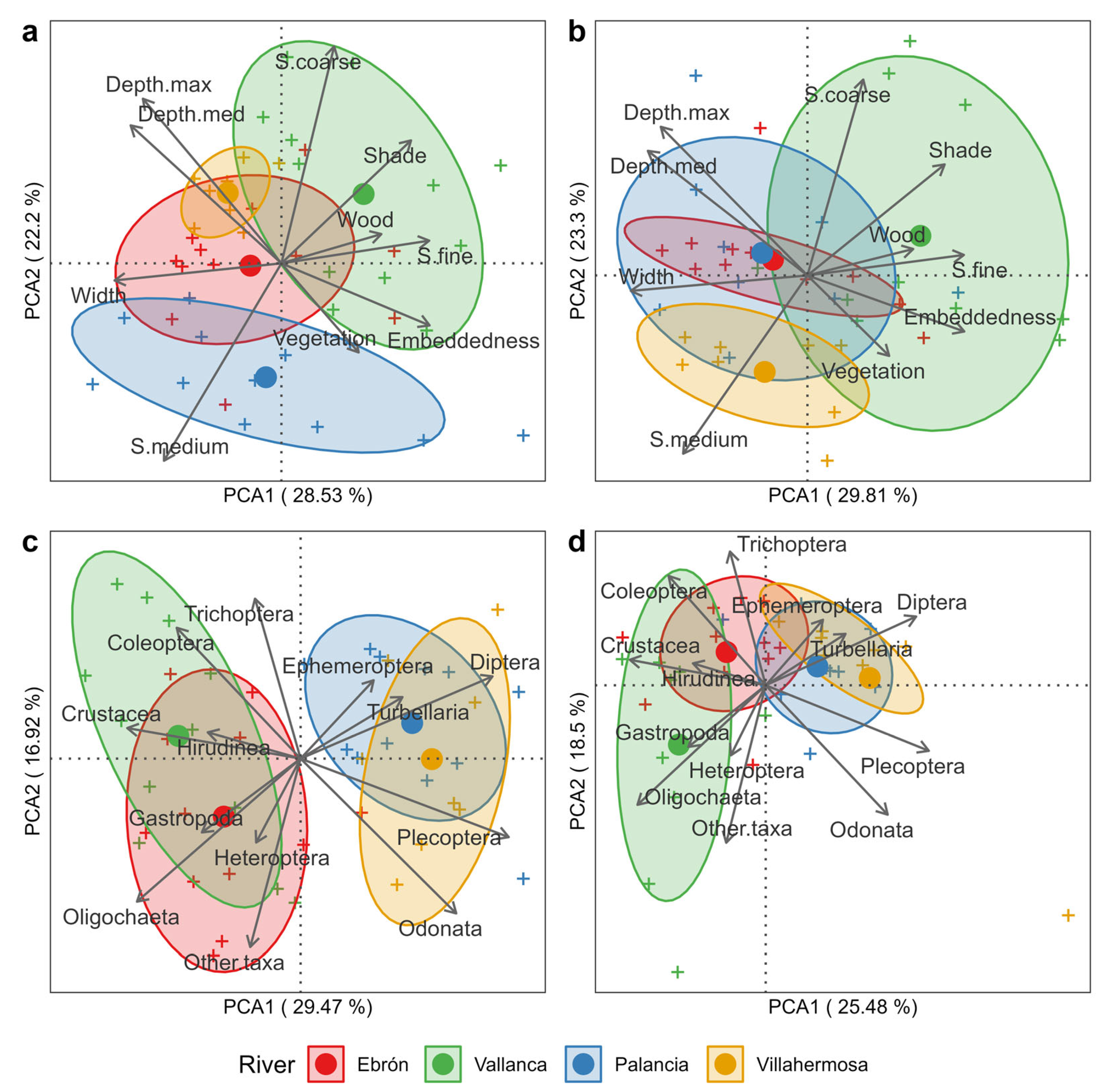
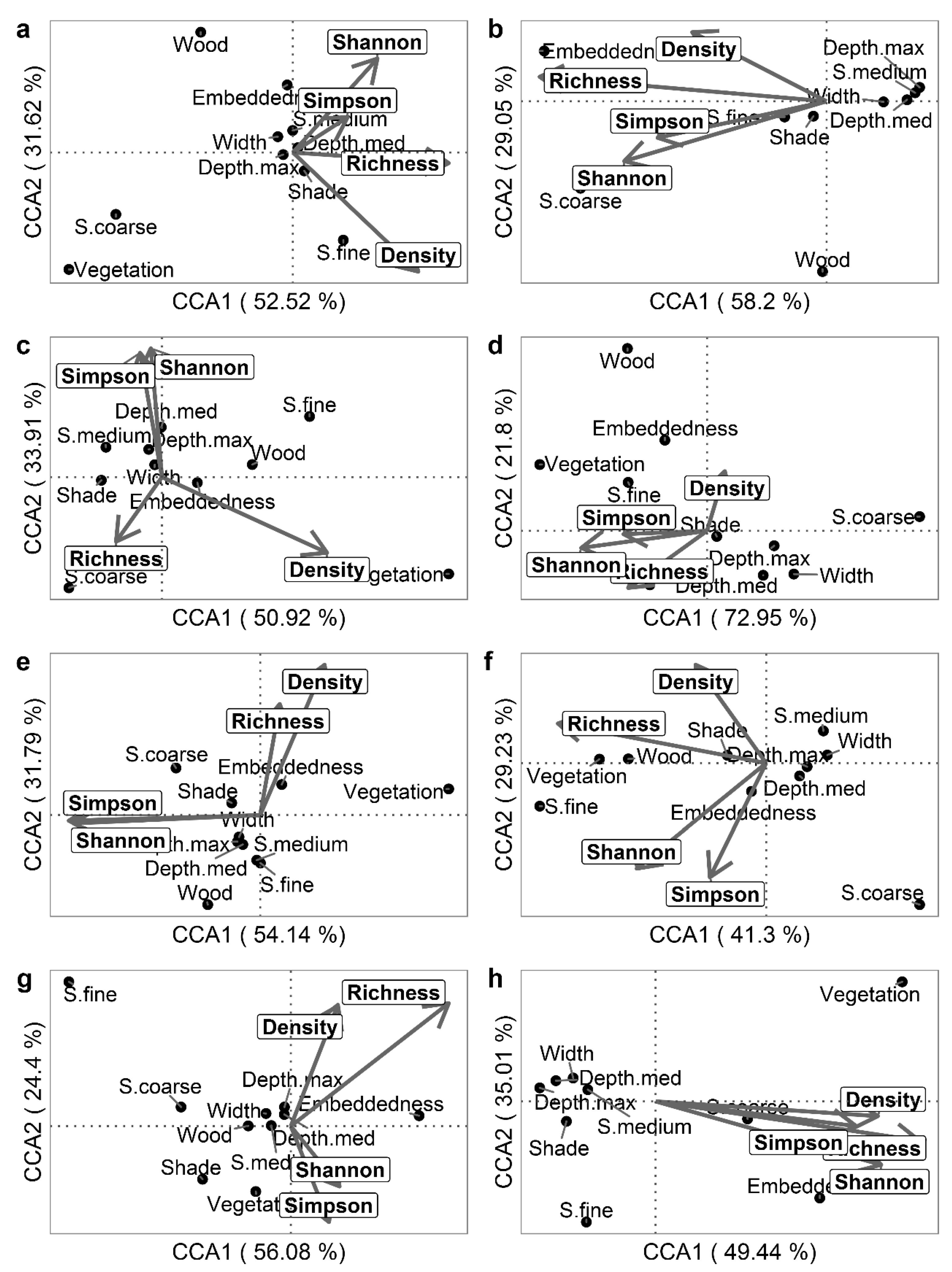
3. Results
3.1. Differences by River and HMU Type
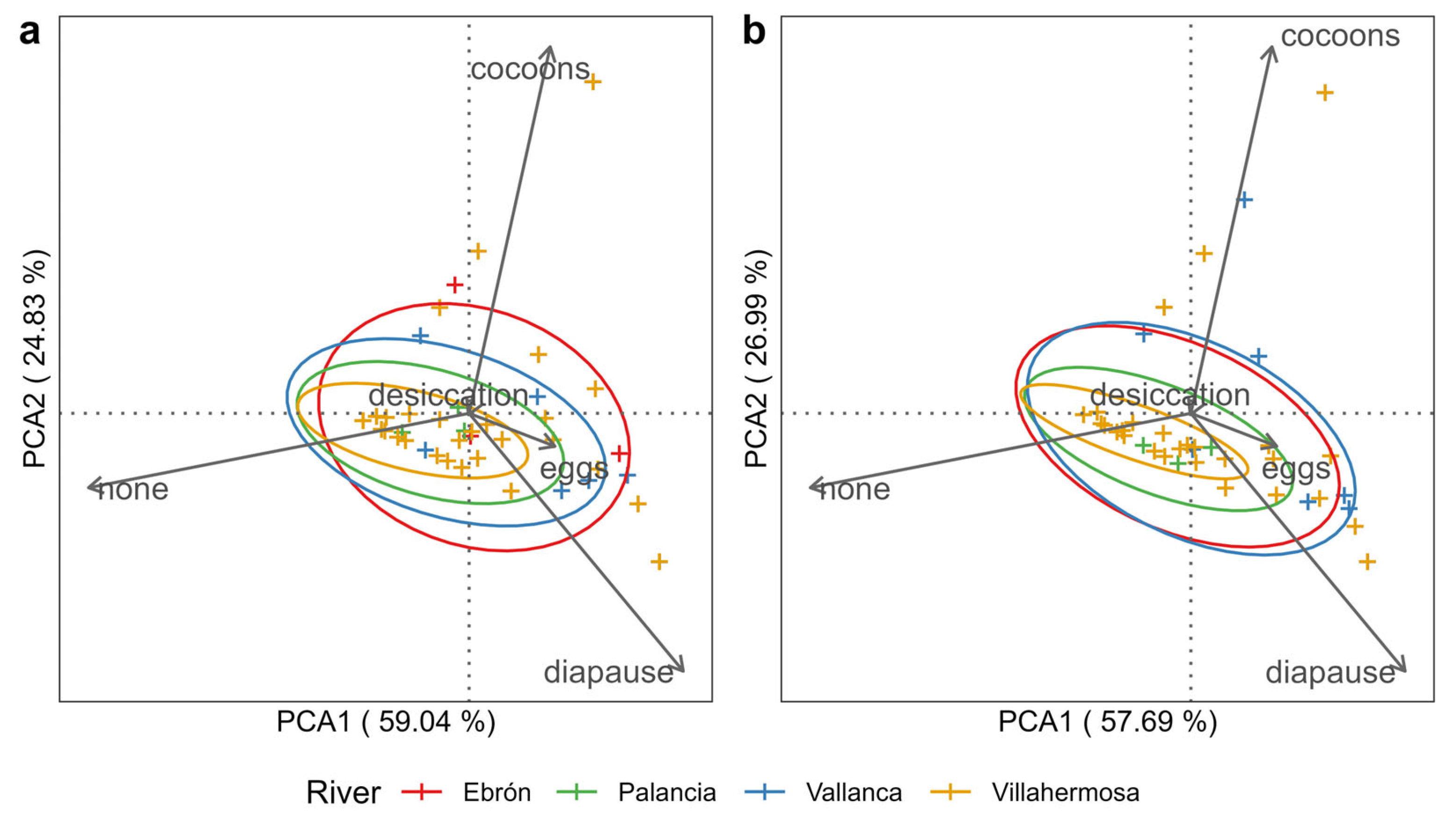
3.2. Analyses of Macroinvertebrate Traits (Resistant Forms)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radinger, J.; Alcaraz-Hernández, J.D.; García-Berthou, E. Environmental Filtering Governs the Spatial Distribution of Alien Fishes in a Large, Human-Impacted Mediterranean River. Divers. Distrib. 2019, 25, 701–714. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- Buss, D.F.; Carlisle, D.M.; Chon, T.S.; Culp, J.; Harding, J.S.; Keizer-Vlek, H.E.; Robinson, W.A.; Strachan, S.; Thirion, C.; Hughes, R.M. Stream Biomonitoring Using Macroinvertebrates around the Globe: A Comparison of Large-Scale Programs. Environ. Monit. Assess. 2015, 187, 4132. [Google Scholar] [CrossRef] [PubMed]
- Frainer, A.; McKie, B.G. Shifts in the Diversity and Composition of Consumer Traits Constrain the Effects of Land Use on Stream Ecosystem Functioning. In Advances in Ecological Research; Pawar, S., Woodward, G., Dell, A.I., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 52, pp. 169–200. ISBN 0065-2504. [Google Scholar]
- Soberón, J. Grinnellian and Eltonian Niches and Geographic Distributions of Species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, B.; Souchon, Y.; Usseglio-Polatera, P.; Ferréol, M.; Valette, L. Can We Predict Biological Condition of Stream Ecosystems? A Multi-Stressors Approach Linking Three Biological Indices to Physico-Chemistry, Hydromorphology and Land Use. Ecol. Indic. 2015, 48, 88–98. [Google Scholar] [CrossRef]
- Bruno, D.; Belmar, O.; Sánchez-Fernández, D.; Guareschi, S.; Millán, A.; Velasco, J. Responses of Mediterranean Aquatic and Riparian Communities to Human Pressures at Different Spatial Scales. Ecol. Indic. 2014, 45, 456–464. [Google Scholar] [CrossRef]
- Dolédec, S.; Simon, L.; Blemus, J.; Rigal, A.; Robin, J.; Mermillod-Blondin, F. Multiple Stressors Shape Invertebrate Assemblages and Reduce Their Trophic Niche: A Case Study in a Regulated Stream. Sci. Total Environ. 2021, 773, 145061. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.C.; Pinho, P.; Llop, E.; Branquinho, C.; Sousa, A.J.; Pereira, M.J. Multivariate Geostatistical Methods for Analysis of Relationships between Ecological Indicators and Environmental Factors at Multiple Spatial Scales. Ecol. Indic. 2013, 29, 339–347. [Google Scholar] [CrossRef]
- Bisson, P.A.; Montgomery, D.R.; Buffington, J.M. Valley Segments, Stream Reaches, and Channel Units. In Methods in Stream Ecology; Hauer, F.R., Lamberti, G.A., Eds.; Academic Press: San Diego, CA, USA, 2007; pp. 23–49. ISBN 9780123329080. [Google Scholar]
- Carbonneau, P.; Fonstad, M.A.; Marcus, W.A.; Dugdale, S.J. Making Riverscapes Real. Geomorphology 2012, 137, 74–86. [Google Scholar] [CrossRef]
- Rivas Casado, M.; Ballesteros González, R.; Ortega, J.F.; Leinster, P.; Wright, R. Towards a Transferable UAV-Based Framework for River Hydromorphological Characterization. Sensors 2017, 17, 2210. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.; Maddock, I.; Visser, F.; Acreman, M. A Framework for Evaluating the Spatial Configuration and Temporal Dynamics of Hydraulic Patches. River Res. Appl. 2012, 28, 585–593. [Google Scholar] [CrossRef]
- García-García, C.; Gilbert, J.D.; Guerrero, F. Macroinvertebrate Community in a Mediterranean Mountain River: Relationship with Environmental Factors Measured at Different Spatial and Temporal Scales. Sustainability 2024, 16, 1777. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and Riverscapes: The Influence of Land Use on Stream Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Townsend, C.R.; Doledec, S.; Norris, R.; Peacock, K.; Arbuckle, C. The Influence of Scale and Geography on Relationships between Stream Community Composition and Landscape Variables: Description and Prediction. Freshw. Biol. 2003, 48, 768–785. [Google Scholar] [CrossRef]
- Graça, M.A.S.; Pinto, P.; Cortes, R.; Coimbra, N.; Oliveira, S.; Morais, M.; Carvalho, M.J.; Malo, J. Factors Affecting Macroinvertebrate Richness and Diversity in Portuguese Streams: A Two-Scale Analysis. Int. Rev. Hydrobiol. 2004, 89, 151–164. [Google Scholar] [CrossRef]
- Mérigoux, S.; Dolédec, S. Hydraulic Requirements of Stream Communities: A Case Study on Invertebrates. Freshw. Biol. 2004, 49, 600–613. [Google Scholar] [CrossRef]
- Sandin, L.; Johnson, R.K. Local, Landscape and Regional Factors Structuring Benthic Macroinvertebrate Assemblages in Swedish Streams. Landsc. Ecol. 2004, 19, 501–515. [Google Scholar] [CrossRef]
- Brooks, A.J.; Haeusler, T.; Reinfelds, I.; Williams, S. Hydraulic Microhabitats and the Distribution of Macroinvertebrate Assemblages in Riffles. Freshw. Biol. 2005, 50, 331–344. [Google Scholar] [CrossRef]
- Burgazzi, G.; Vezza, P.; Negro, G.; Astegiano, L.; Pellicanó, R.; Pinna, B.; Viaroli, P.; Laini, A. Effect of Microhabitats, Mesohabitats and Spatial Position on Macroinvertebrate Communities of a Braided River. J. Ecohydraul. 2021, 6, 95–104. [Google Scholar] [CrossRef]
- González, M.; Graça, M.A.S. Influence of Detritus on the Structure of the Invertebrate Community in a Small Portuguese Stream. Int. Rev. Hydrobiol. 2005, 90, 534–545. [Google Scholar] [CrossRef]
- Buffagni, A. The Lentic and Lotic Characteristics of Habitats Determine the Distribution of Benthic Macroinvertebrates in Mediterranean Rivers. Freshw. Biol. 2021, 66, 13–34. [Google Scholar] [CrossRef]
- Silva, D.R.O.; Ligeiro, R.; Hughes, R.M.; Callisto, M. Visually Determined Stream Mesohabitats Influence Benthic Macroinvertebrate Assessments in Headwater Streams. Environ. Monit. Assess. 2014, 186, 5479–5488. [Google Scholar] [CrossRef] [PubMed]
- Vimos-Lojano, D.J.; Martínez-Capel, F.; Hampel, H.; Vázquez, R.F. Hydrological Influences on Aquatic Communities at the Mesohabitat Scale in High Andean Streams of Southern Ecuador. Ecohydrology 2019, 12, e2033. [Google Scholar] [CrossRef]
- Belletti, B.; Rinaldi, M.; Bussettini, M.; Comiti, F.; Gurnell, A.M.; Mao, L.; Nardi, L.; Vezza, P. Characterising Physical Habitats and Fluvial Hydromorphology: A New System for the Survey and Classification of River Geomorphic Units. Geomorphology 2017, 283, 143–157. [Google Scholar] [CrossRef]
- Alcaraz-Hernández, J.D.; Martínez-Capel, F.; Peredo-Parada, M.; Hernández-Mascarell, A.B. Mesohabitat Heterogeneity in Four Mediterranean Streams of the Jucar River Basin (Eastern Spain). Limnetica 2011, 30, 363–378. [Google Scholar] [CrossRef]
- Nicola, G.G.; Almodóvar, A.; Elvira, B. Effects of Environmental Factors and Predation on Benthic Communities in Headwater Streams. Aquat. Sci. 2010, 72, 419–429. [Google Scholar] [CrossRef]
- García-Girón, J.; Heino, J.; García-Criado, F.; Fernández-Aláez, C.; Alahuhta, J. Biotic Interactions Hold the Key to Understanding Metacommunity Organisation. Ecography 2020, 43, 1180–1190. [Google Scholar] [CrossRef]
- Forcellini, M.; Plichard, L.; Dolédec, S.; Mérigoux, S.; Olivier, J.M.; Cauvy-Fraunié, S.; Lamouroux, N. Microhabitat Selection by Macroinvertebrates: Generality among Rivers and Functional Interpretation. J. Ecohydraul. 2022, 7, 28–41. [Google Scholar] [CrossRef]
- Gore, J.A.; Layzer, J.B.; Mead, J. Macroinvertebrate Instream Flow Studies after 20 Years: A Role in Stream Management and Restoration. Regul. Rivers Res. Manag. 2001, 17, 527–542. [Google Scholar] [CrossRef]
- Díez, J.R.; Larrańaga, S.; Elosegi, A.; Pozo, J. Effect of Removal of Wood on Streambed Stability and Retention of Organic Matter. J. N. Am. Benthol. Soc. 2000, 19, 621–632. [Google Scholar] [CrossRef]
- Enefalk, Å.; Bergman, E. Effects of Fine Wood on Macroinvertebrate Drift in Four Boreal Forest Streams. Hydrobiologia 2015, 765, 317–327. [Google Scholar] [CrossRef]
- Hoffmann, A.; Hering, D. Wood-Associated Macroinvertebrate Fauna in Central European Streams. Int. Rev. Hydrobiol. 2000, 85, 25–48. [Google Scholar] [CrossRef]
- Spänhoff, B.; Riss, W.; Jäkel, P.; Dakkak, N.; Meyer, E.I. Effects of an Experimental Enrichment of Instream Habitat Heterogeneity on the Stream Bed Morphology and Chironomid Community of a Straightened Section in a Sandy Lowland Stream. Environ. Manag. 2006, 37, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Mas, R.; Sánchez-Hernández, J.; McClain, M.E.; Tamatamah, R.; Mukama, S.C.; Martínez-Capel, F. Investigating the Influence of Habitat Structure and Hydraulics on Tropical Macroinvertebrate Communities. Ecohydrol. Hydrobiol. 2019, 19, 339–350. [Google Scholar] [CrossRef]
- Menezes, S.; Baird, D.J.; Soares, A.M.V.M. Beyond Taxonomy: A Review of Macroinvertebrate Trait-Based Community Descriptors as Tools for Freshwater Biomonitoring. J. Appl. Ecol. 2010, 47, 711–719. [Google Scholar] [CrossRef]
- Lamouroux, N.; Dolédec, S.; Gayraud, S. Biological Traits of Stream Macroinvertebrate Communities: Effects of Microhabitat, Reach, and Basin Filters. J. N. Am. Benthol. Soc. 2004, 23, 449–466. [Google Scholar] [CrossRef]
- Demars, B.O.L.; Kemp, J.L.; Friberg, N.; Usseglio-Polatera, P.; Harper, D.M. Linking Biotopes to Invertebrates in Rivers: Biological Traits, Taxonomic Composition and Diversity. Ecol. Indic. 2012, 23, 301–311. [Google Scholar] [CrossRef]
- Messager, M.L.; Lehner, B.; Cockburn, C.; Lamouroux, N.; Pella, H.; Snelder, T.; Tockner, K.; Trautmann, T.; Watt, C.; Datry, T. Global Prevalence of Non-Perennial Rivers and Streams. Nature 2021, 594, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Belmar, O.; Velasco, J.; Gutiérrez-Cánovas, C.; Mellado-Díaz, A.; Millán, A.; Wood, P.J. The Influence of Natural Flow Regimes on Macroinvertebrate Assemblages in a Semiarid Mediterranean Basin. Ecohydrology 2013, 6, 363–379. [Google Scholar] [CrossRef]
- Mellado Díaz, A.; Suárez Alonso, M.L.; Vidal-Abarca Gutiérrez, M.R. Biological Traits of Stream Macroinvertebrates from a Semi-Arid Catchment: Patterns along Complex Environmental Gradients. Freshw. Biol. 2008, 53, 1–21. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and Future Köppen-Geiger Climate Classification Maps at 1-Km Resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Gasith, A.; Resh, V.H. Streams in Mediterranean Climate Regions: Abiotic Influences and Biotic Responses to Predictable Seasonal Events. Annu. Rev. Ecol. Syst. 1999, 30, 51–81. [Google Scholar] [CrossRef]
- Martínez-Fernández, J.; Sánchez, N.; Herrero-Jiménez, C.M. Recent Trends in Rivers with Near-Natural Flow Regime: The Case of the River Headwaters in Spain. Prog. Phys. Geogr. 2013, 37, 685–700. [Google Scholar] [CrossRef]
- Mouton, A.M.; Alcaraz-Hernández, J.D.; De Baets, B.; Goethals, P.L.M.; Martínez-Capel, F. Data-Driven Fuzzy Habitat Suitability Models for Brown Trout in Spanish Mediterranean Rivers. Environ. Model. Softw. 2011, 26, 615–622. [Google Scholar] [CrossRef]
- Alcaraz-Hernández, J.D.; Muñoz-Mas, R.; Martínez-Capel, F.; Garófano-Gómez, V.; Vezza, P. Generalized Additive Models to Predict Adult and Young Brown Trout (Salmo Trutta Linnaeus, 1758) Densities in Mediterranean Rivers. J. Appl. Ichthyol. 2016, 32, 217–228. [Google Scholar] [CrossRef]
- Shuman, T.C.; Smiley, P.C.; Gillespie, R.B.; Gonzalez, J.M. Influence of Physical and Chemical Characteristics of Sediment on Macroinvertebrate Communities in Agricultural Headwater Streams. Water 2020, 12, 2976. [Google Scholar] [CrossRef]
- Dolloff, C.A.; Hankin, D.G.; Reeves, G.H. Basinwide Estimation of Habit at and Fish Populations in St Reams; General Technical Report SE-83; US Department of Agriculture, Forest Service, Southeastern Forest Experiment Station: Asheville, NC, USA, 1993. [Google Scholar]
- Oscoz, J.; Galicia, D.; Miranda, R. (Eds.) Identification Guide of Freshwater Macroinvertebrates of Spain; Springer: Dordrecht, The Netherlands, 2011; Volume 53, ISBN 978-94-007-1553-0. [Google Scholar]
- Pires, M.M.; Grech, M.G.; Stenert, C.; Maltchik, L.; Epele, L.B.; McLean, K.I.; Kneitel, J.M.; Bell, D.A.; Greig, H.S.; Gagne, C.R.; et al. Does Taxonomic and Numerical Resolution Affect the Assessment of Invertebrate Community Structure in New World Freshwater Wetlands? Ecol. Indic. 2021, 125, 107437. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Thioulouse, J.; Prin, Y.; Duponnois, R. Multivariate Analyses in Soil Microbial Ecology: A New Paradigm. Environ. Ecol. Stat. 2012, 19, 499–520. [Google Scholar] [CrossRef]
- Cundari, T.R.; Sarbu, C.; Pop, H.F. Robust Fuzzy Principal Component Analysis (FPCA). A Comparative Study Concerning Interaction of Carbon-Hydrogen Bonds with Molybdenum-Oxo Bonds. J. Chem. Inf. Comput. Sci. 2002, 42, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Tachet, H.; Richoux, P.; Bournaud, M.; Usseglio-Polatera, P. Invertébrés d’eau Douce, 2nd ed.; CNRS Editions: Paris, France, 2002; ISBN 2-271 057450. [Google Scholar]
- Leigh, C.; Bonada, N.; Boulton, A.J.; Hugueny, B.; Larned, S.T.; Vander Vorste, R.; Datry, T. Invertebrate Assemblage Responses and the Dual Roles of Resistance and Resilience to Drying in Intermittent Rivers. Aquat. Sci. 2015, 78, 291–301. [Google Scholar] [CrossRef]
- Chevene, F.; Doleadec, S.; Chessel, D. A Fuzzy Coding Approach for the Analysis of Long-Term Ecological Data. Freshw. Biol. 1994, 31, 295–309. [Google Scholar] [CrossRef]
- Statzner, B.; Bonada, N.; Dolédec, S. Predicting the Abundance of European Stream Macroinvertebrates Using Biological Attributes. Oecologia 2008, 156, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Dray, S.; Dufour, A.B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, H.M.S.; Wagner, H. Vegan: Community Ecology Package; R Package Version 1.17-6; R Core Team: Vienna, Austria, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R. Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Buffagni, A.; Erba, S.; Cazzola, M.; Barca, E.; Belfiore, C. The Ratio of Lentic to Lotic Habitat Features Strongly Affects Macroinvertebrate Metrics Used in Southern Europe for Ecological Status Classification. Ecol. Indic. 2020, 117, 106563. [Google Scholar] [CrossRef]
- Cesarini, G.; Gallitelli, L.; Traversetti, L.; Bandini, T.; Scalici, M. Hydromorphological Discontinuities Deeply Modify the Benthic Multi-Species Assemblage Diversity in a Mediterranean Running River. Rend. Lincei 2023, 34, 257–266. [Google Scholar] [CrossRef]
- Boyero, L. Multiscale Patterns of Spatial Variation in Stream Macroinvertebrate Communities. Ecol. Res. 2003, 18, 365–379. [Google Scholar] [CrossRef]
- Carter, J.L.; Fend, S.V. Inter-Annual Changes in the Benthic Community Structure of Riffles and Pools in Reaches of Contrasting Gradient. Hydrobiologia 2001, 459, 187–200. [Google Scholar] [CrossRef]
- Costa, S.S.; Melo, A.S. Beta Diversity in Stream Macroinvertebrate Assemblages: Among-Site and among-Microhabitat Components. Hydrobiologia 2008, 598, 131–138. [Google Scholar] [CrossRef]
- Pace, G.; Andreani, P.; Barile, M.; Buffagni, A.; Erba, S.; Mancini, L.; Belfiore, C. Macroinvertebrate Assemblages at Mesohabitat Scale in Small Sized Volcanic Siliceous Streams of Central Italy (Mediterranean Ecoregion). Ecol. Indic. 2011, 11, 688–696. [Google Scholar] [CrossRef]
- Seger, K.R.; Smiley, P.C.; King, K.W.; Fausey, N.R. Influence of Riparian Habitat on Aquatic Macroinvertebrate Community Colonization within Riparian Zones of Agricultural Headwater Streams. J. Freshw. Ecol. 2012, 27, 393–407. [Google Scholar] [CrossRef]
- Shearer, K.; Hayes, J.; Jowett, I.; Olsen, D. Habitat Suitability Curves for Benthic Macroinvertebrates from a Small New Zealand River. N. Z. J. Mar. Freshw. Res. 2015, 49, 178–191. [Google Scholar] [CrossRef]
- Vimos-Lojano, D.J.; Martínez-Capel, F.; Hampel, H. Riparian and Microhabitat Factors Determine the Structure of the EPT Community in Andean Headwater Rivers of Ecuador. Ecohydrology 2017, 10, e1894. [Google Scholar] [CrossRef]
- Vimos-Lojano, D.; Hampel, H.; Vázquez, R.F.; Martínez-Capel, F. Community Structure and Functional Feeding Groups of Macroinvertebrates in Pristine Andean Streams under Different Vegetation Cover. Ecohydrol. Hydrobiol. 2020, 20, 357–368. [Google Scholar] [CrossRef]
- Jowett, I.G. Hydraulic Constraints on Habitat Suitability for Benthic Invertebrates in Gravel-Bed Rivers. River Res. Appl. 2003, 19, 495–507. [Google Scholar] [CrossRef]
- Death, R.G.; Winterbourn, M.J. Diversity Patterns in Stream Benthic Invertebrate Communities: The Influence of Habitat Stability. Ecology 1995, 76, 1446–1460. [Google Scholar] [CrossRef]
- Rice, S.P.; Greenwood, M.T.; Joyce, C.B. Macroinvertebrate Community Changes at Coarse Sediment Recruitment Points along Two Gravel Bed Rivers. Water Resour. Res. 2001, 37, 2793–2803. [Google Scholar] [CrossRef]
- McKenzie, M.; England, J.; Foster, I.; Wilkes, M. Evaluating the Performance of Taxonomic and Trait-Based Biomonitoring Approaches for Fine Sediment in the UK. Ecol. Indic. 2022, 134, 108502. [Google Scholar] [CrossRef]
- Jowett, I.G.; Davey, A.J.H. A Comparison of Composite Habitat Suitability Indices and Generalized Additive Models of Invertebrate Abundance and Fish Presence–Habitat Availability. Trans. Am. Fish. Soc. 2007, 136, 428–444. [Google Scholar] [CrossRef]
- Rozenberg, G.S.; Zinchenko, T.D. The Sustainability of Aquatic Ecosystems: An Overview of the Problem. Arid Ecosyst. 2014, 4, 234–243. [Google Scholar] [CrossRef]
- Bonada, N.; Dolédec, S. Do Mediterranean Genera Not Included in Tachet et al. 2002 Have Mediterranean Trait Characteristics? Limnetica 2011, 30, 129–142. [Google Scholar] [CrossRef]
- Bonada, N.; Resh, V.H. Mediterranean-Climate Streams and Rivers: Geographically Separated but Ecologically Comparable Freshwater Systems. Hydrobiologia 2013, 719, 1–29. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Coscieme, L.; Cristiano, G. No Post-Drought Recovery of the Macroinvertebrate Community after Five Months upon Rewetting of an Irregularly Intermittent Apennine River (Aterno River). Ecohydrol. Hydrobiol. 2023, 23, 141–151. [Google Scholar] [CrossRef]
- Bonada, N.; Rieradevall, M.; Prat, N. Macroinvertebrate Community Structure and Biological Traits Related to Flow Permanence in a Mediterranean River Network. Hydrobiologia 2007, 589, 91–106. [Google Scholar] [CrossRef]
- Bonada, N.; Cañedo-Argüelles, M.; Gallart, F.; von Schiller, D.; Fortuño, P.; Latron, J.; Llorens, P.; Múrria, C.; Soria, M.; Vinyoles, D.; et al. Conservation and Management of Isolated Pools in Temporary Rivers. Water 2020, 12, 2870. [Google Scholar] [CrossRef]
- Estrela-Segrelles, C.; Gómez-Martínez, G.; Pérez-Martín, M.Á. Climate Change Risks on Mediterranean River Ecosystems and Adaptation Measures (Spain). Water Resour. Manag. 2023, 37, 2757–2770. [Google Scholar] [CrossRef]
- Buckley, L.B. Temperature-Sensitive Development Shapes Insect Phenological Responses to Climate Change. Curr. Opin. Insect Sci. 2022, 52, 100897. [Google Scholar] [CrossRef] [PubMed]
- Shipley, J.R.; Twining, C.W.; Mathieu-Resuge, M.; Parmar, T.P.; Kainz, M.; Martin-Creuzburg, D.; Weber, C.; Winkler, D.W.; Graham, C.H.; Matthews, B. Climate Change Shifts the Timing of Nutritional Flux from Aquatic Insects. Curr. Biol. 2022, 32, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Belmar, O.; Bruno, D.; Guareschi, S.; Mellado-Díaz, A.; Millán, A.; Velasco, J. Functional Responses of Aquatic Macroinvertebrates to Flow Regulation Are Shaped by Natural Flow Intermittence in Mediterranean Streams. Freshw. Biol. 2019, 64, 1064–1077. [Google Scholar] [CrossRef]
- Bruno, D.; Belmar, O.; Maire, A.; Morel, A.; Dumont, B.; Datry, T. Structural and Functional Responses of Invertebrate Communities to Climate Change and Flow Regulation in Alpine Catchments. Glob. Chang. Biol. 2019, 25, 1612–1628. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulos, C.; Karaouzas, I. Climate Change and the Future of Mediterranean Freshwater Macroinvertebrates: A Model-Based Assessment. Hydrobiologia 2021, 848, 5033–5050. [Google Scholar] [CrossRef]
- Lawrence, J.E.; Lunde, K.B.; Mazor, R.D.; Bêche, L.A.; McElravy, E.P.; Resh, V.H. Long-Term Macroinvertebrate Responses to Climate Change: Implications for Biological Assessment in Mediterranean-Climate Streams. J. N. Am. Benthol. Soc. 2010, 29, 1424–1440. [Google Scholar] [CrossRef]
| Attribute | Unit | Slow HMU | Fast HMU | Statistical Test |
|---|---|---|---|---|
| Width | m | 4.69 (1.84–8.54) | 4.84 (1.26–8.80) | ANOVA tests; p = 0.685 |
| Mean depth | m | 0.43 (0.11–0.79) | 0.25 (0.04–0.53) | ANOVA tests; p < 0.001 |
| Maximum depth | m | 0.78 (0.32–1.20) | 0.44 (0.15–0.83) | ANOVA tests; p < 0.001 |
| Shade | % | 56.70 (0–100) | 49.47 (0–100) | Kruskal–Wallis test; p = 0.341 |
| Embeddedness | % | 39.09 (0–100) | 21.84 (0–100) | Kruskal–Wallis test; p = 0.013 |
| Aquatic vegetation | % | 8.64 (0–60) | 8.42 (0–95) | Kruskal–Wallis test; p = 0.862 |
| Substrate coarse | % | 14.09 (0–90) | 12.63 (0–100) | Kruskal–Wallis test; p = 0.183 |
| Substrate medium | % | 67.16 (0–100) | 77.76 (0–100) | Kruskal–Wallis test; p = 0.072 |
| Substrate fine | % | 18.98 (0–85) | 9.61 (0–60) | Kruskal–Wallis test; p = 0.034 |
| Woody debris | pieces/m2 | 0.009 (0.000–0.142) | 0.003 (0.000–0.051) | Kruskal–Wallis test; p = 0.038 |
| Ebrón | Palancia | Vallanca | Villahermosa | Statistical Test | ||
|---|---|---|---|---|---|---|
| Slow water | Abundance | 4503.3 | 7276.2 | 10,258.2 | 11,545 | Kruskal–Wallis test; p = 0.830 |
| Simpson | 0.8 | 0.7 | 0.7 | 0.6 | Kruskal–Wallis test; p = 0.615 | |
| Shannon | 0.8 | 0.8 | 0.7 | 0.7 | ANOVA tests; p = 0.415 | |
| Richness | 17.9 | 21.2 | 18 | 18.9 | ANOVA tests; p = 0.464 | |
| Fast water | Abundance | 4621.8 | 16,314.4 | 4475 | 8782.3 | Kruskal–Wallis test; p = 0.008 |
| Simpson | 0.8 | 0.7 | 0.6 | 0.7 | ANOVA tests; p = 0.029 | |
| Shannon | 0.9 | 0.8 | 0.7 | 0.7 | ANOVA tests; p = 0.055 | |
| Richness | 18.4 | 23.7 | 14.7 | 16.3 | Kruskal–Wallis test; p = 0.063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcaraz-Hernández, J.D.; Sánchez-Hernández, J.; Muñoz-Mas, R.; Martínez-Capel, F. Drivers of Macroinvertebrate Communities in Mediterranean Rivers: A Mesohabitat Approach. Sustainability 2024, 16, 3075. https://doi.org/10.3390/su16073075
Alcaraz-Hernández JD, Sánchez-Hernández J, Muñoz-Mas R, Martínez-Capel F. Drivers of Macroinvertebrate Communities in Mediterranean Rivers: A Mesohabitat Approach. Sustainability. 2024; 16(7):3075. https://doi.org/10.3390/su16073075
Chicago/Turabian StyleAlcaraz-Hernández, Juan Diego, Javier Sánchez-Hernández, Rafael Muñoz-Mas, and Francisco Martínez-Capel. 2024. "Drivers of Macroinvertebrate Communities in Mediterranean Rivers: A Mesohabitat Approach" Sustainability 16, no. 7: 3075. https://doi.org/10.3390/su16073075
APA StyleAlcaraz-Hernández, J. D., Sánchez-Hernández, J., Muñoz-Mas, R., & Martínez-Capel, F. (2024). Drivers of Macroinvertebrate Communities in Mediterranean Rivers: A Mesohabitat Approach. Sustainability, 16(7), 3075. https://doi.org/10.3390/su16073075






