Potential Bioactivities of Tamarind Seed Jellose at the Cellular Level for Cosmetic Product Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Jellose
2.3. Quantitative Analysis of Sugars Using High-Performance Liquid Chromatography (HPLC)
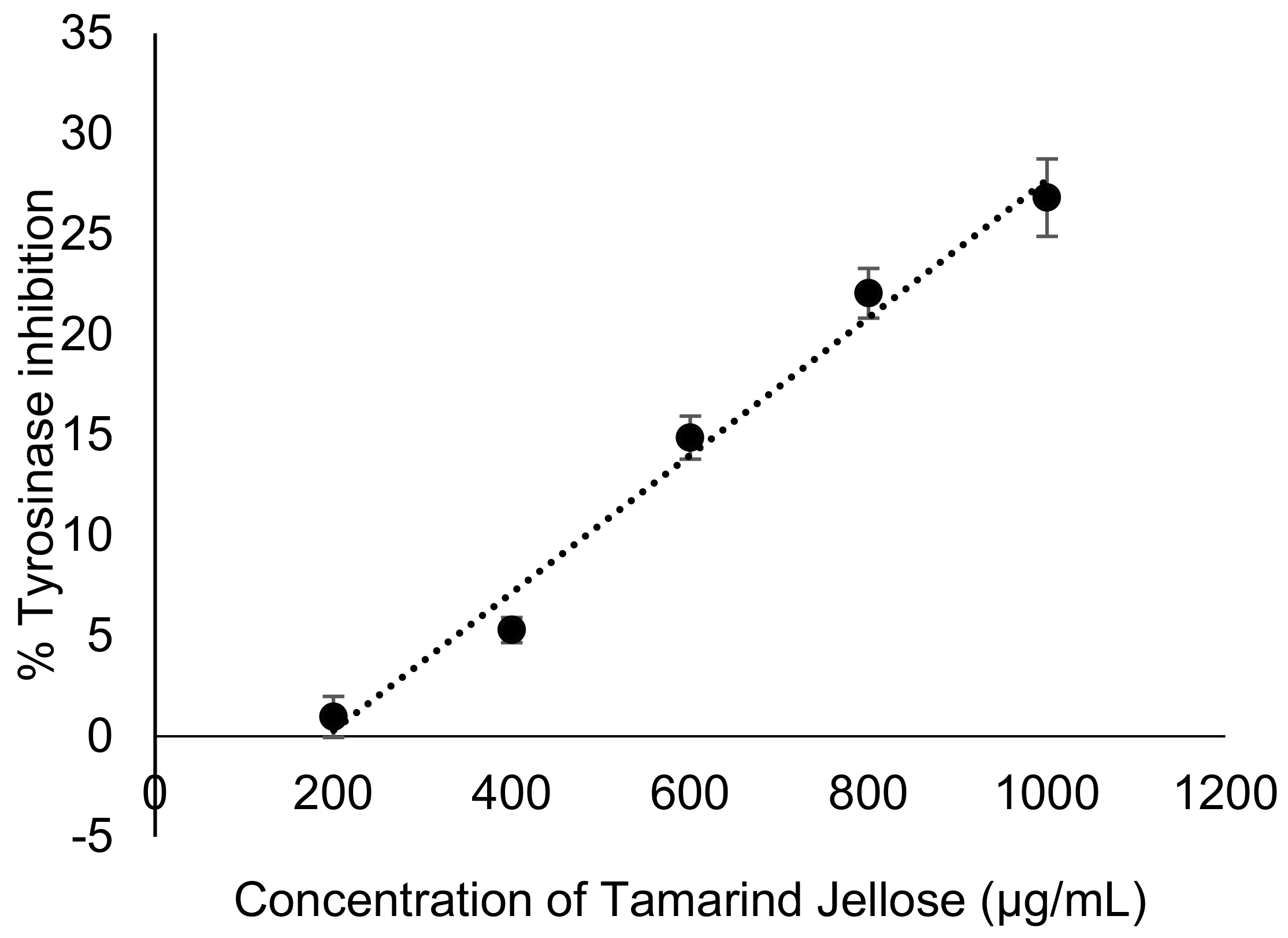
2.4. Mushroom Tyrosinase Assay
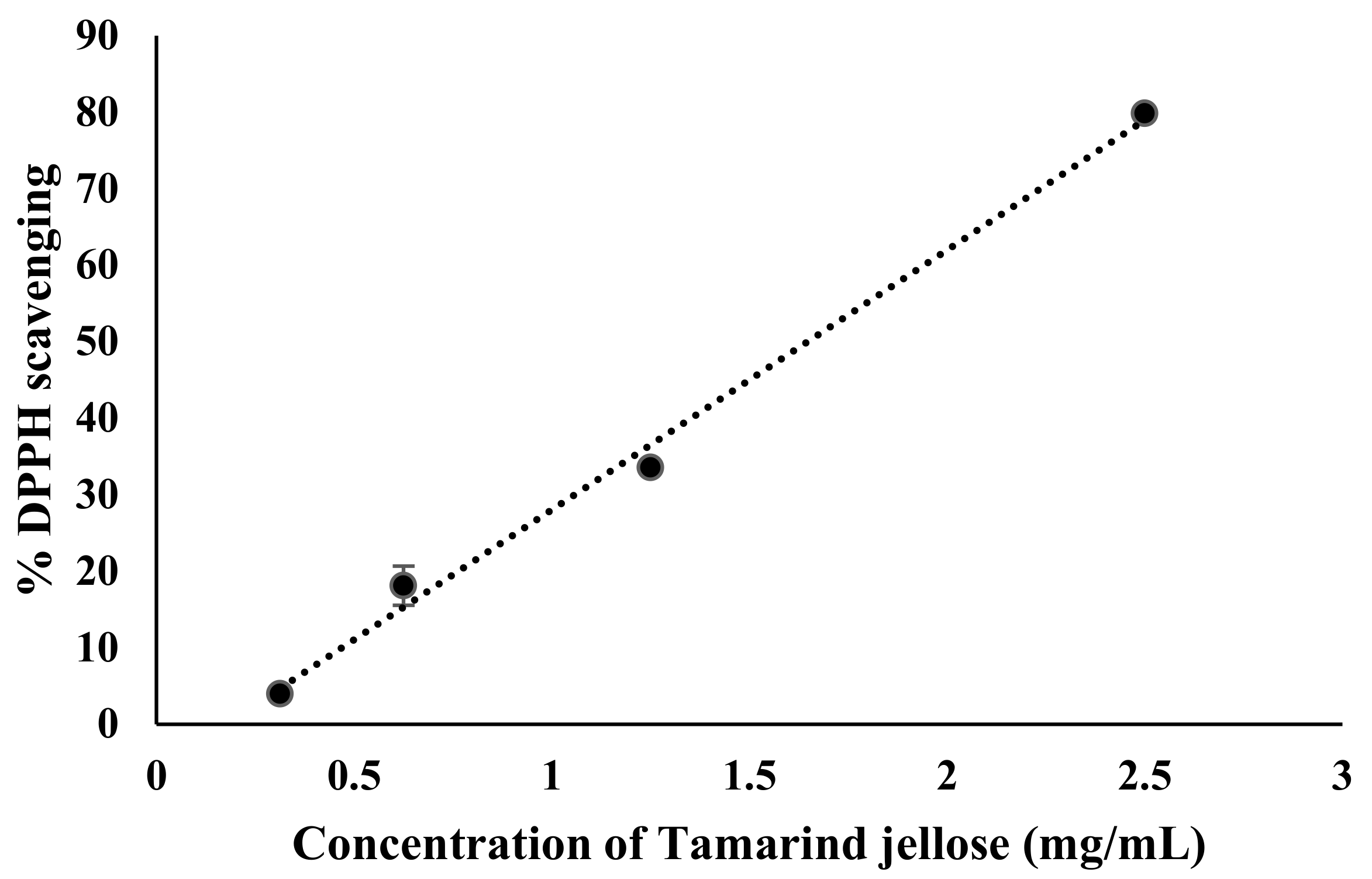
2.5. Determination of Antioxidant Activity
2.6. Cell Cultures
2.7. SRB Cell Viability Assay
2.8. Clonogenic Assay
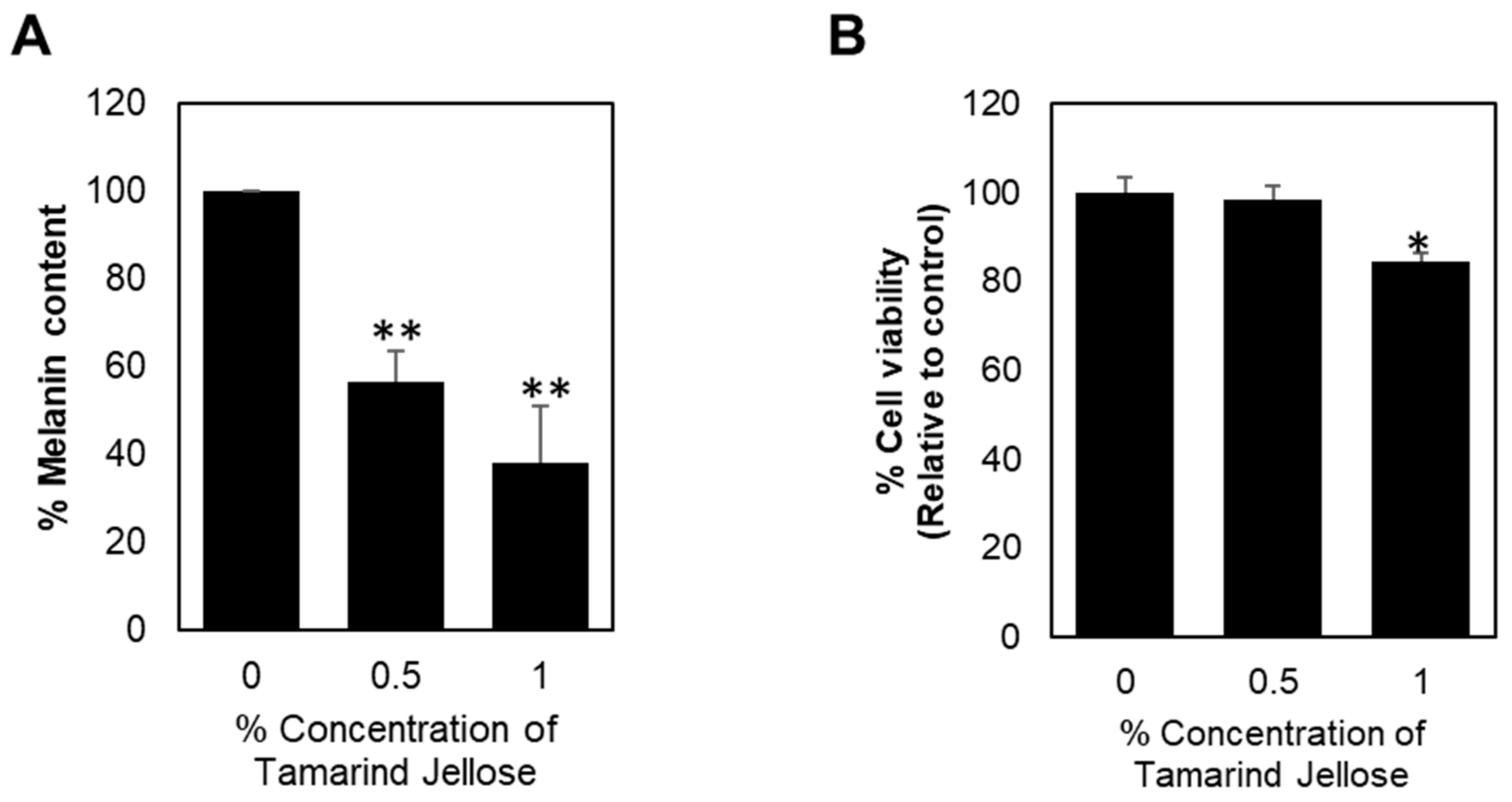
2.9. Quantitative Assay of Melanin Content in B16 Melanoma Cells
2.10. Quantitative Real-Time PCR (qRT-PCR)
2.11. Statistics
3. Results
3.1. Quantitative Determination of Sugars and Physicochemical Properties of Tamarind Jellose
3.2. Cytotoxicity Effect of Tamarind Jellose (TJ) on Skin Fibroblast Cells
3.3. Long-Term Cytotoxicity Effect of Tamarind Jellose (TJ) on Skin Fibroblast Cells
3.4. Inhibitory Effect of Tamarind Jellose on the Production of Melanin in B16 Melanoma Cells
3.5. Tamarind Jellose Inhibits Mushroom Tyrosinase Activity (Cell-Free Methods)
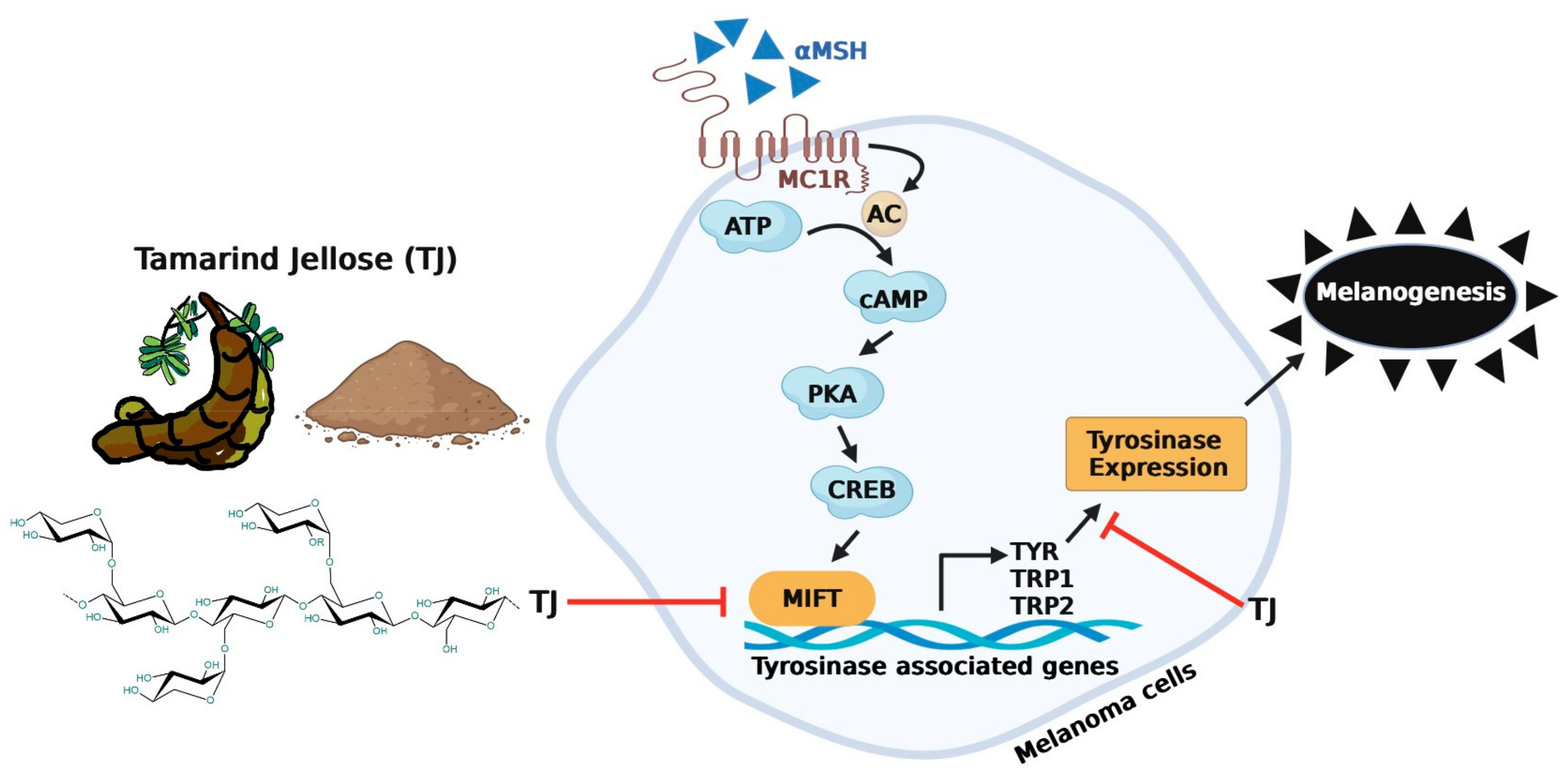
3.6. Effects of Tamarind Jellose on the Transcriptional Expression of Melanogenesis-Related Genes, Including Tyrosinase (TYR) and Microphthalmia-Associated Transcription Factor (MITF) in B16 Melanoma Cells
3.7. Antioxidant Activity of Tamarind Jellose
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Videira, I.F.d.S.; Moura, D.F.L.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Napolitano, A. Natural and Bioinspired Phenolic Compounds as Tyrosinase Inhibitors for the Treatment of Skin Hyperpigmentation: Recent Advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef]
- Liu, J.-K. Natural products in cosmetics. Nat. Products Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Takemasa, M.; Yamatoya, K.; Shirakawa, M. 19—Xyloglucan. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2009; pp. 535–566. Available online: http://www.sciencedirect.com/science/article/pii/B9781845694142500199 (accessed on 25 December 2022).
- Semenzato, A.; Costantini, A.; Baratto, G. Green Polymers in Personal Care Products: Rheological Properties of Tamarind Seed Polysaccharide. Cosmetics 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Freitas, R.A.; Martin, S.; Santos, G.L.; Valenga, F.; Buckeridge, M.S.; Reicher, F.; Sierakowski, M.-R. Physico-chemical properties of seed xyloglucans from different sources. Carbohydr. Polym. 2005, 60, 507–514. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Joshi, A.A.; Patil, C.L.; Amale, P.D.; Patel, H.M.; Surana, S.J.; Belgamwar, V.S.; Chaudhari, K.S.; Pardeshi, C.V. Xyloglucan: A functional biomacromolecule for drug delivery applications. Int. J. Biol. Macromol. 2017, 104, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Luzia, D.M.M.; Jorge, N. Antioxidant activity, fatty acid profile and tocopherols of Tamarindus indica L. seeds. Food Sci. Technol. 2011, 31, 497–501. [Google Scholar] [CrossRef]
- Siddhuraju, P. Antioxidant activity of polyphenolic compounds extracted from defatted raw and dry heated Tamarindus indica seed coat. LWT—Food Sci. Technol. 2007, 40, 982–990. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Obiiyeke, G.E.; Chigor, V.N.; Okoh, A.I. Assessment of Tamarindus indica extracts for antibacterial activity. Int. J. Mol. Sci. 2011, 12, 6385–6396. [Google Scholar] [CrossRef] [PubMed]
- Phetdee, K.; Rattanamanee, K.; Teaktong, T.; Viyoch, J. Tamarind seed coat extract reduces melanin production via tyrosinase in melanocyte. J. Biol. Sci. 2012, 12, 239–245. [Google Scholar] [CrossRef][Green Version]
- Bonin, E.; Avila, V.D.; Carvalho, V.M.; Cardoso, M.A.P.; Matos, A.M.; Ramos, A.V.G.; Cabral, M.R.P.; Baldoqui, D.C.; Sarragiotto, M.H.; Filho, B.A.d.A.; et al. Study of chemical constituents, antioxidants and antimicrobial activities of Tamarindus indica L. seed. J. Food Sci. 2023, 88, 4639–4652. [Google Scholar] [CrossRef] [PubMed]
- Avachat, A.M.; Gujar, K.N.; Wagh, K. V Development and evaluation of tamarind seed xyloglucan-based mucoadhesive buccal films of rizatriptan benzoate. Carbohydr. Polym. 2013, 91, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Nayak, A.K. Novel tamarind seed polysaccharide-alginate mucoadhesive microspheres for oral gliclazide delivery: In vitro–in vivo evaluation. Drug Deliv. 2012, 19, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Guo, P.; Chen, S.; Cao, Y.; Ji, W.; Lei, X.; Liu, L.; Zhao, P.; Wang, R.; Qi, C.; et al. Properties of xyloglucan hydrogel as the biomedical sustained-release carriers. J. Mater. Sci. Mater. Med. 2012, 23, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Uccello-Barretta, G.; Nazzi, S.; Zambito, Y.; Di Colo, G.; Balzano, F.; Sansò, M. Synergistic interaction between TS-polysaccharide and hyaluronic acid: Implications in the formulation of eye drops. Int. J. Pharm. 2010, 395, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Darvill, A.; Eberhard, S.; Pauly, M.; Albersheim, P.; Strickland, F.M.; Sun, Y. Preservation of the Delayed-Type Hypersensitivity Response to Alloantigen by Xyloglucans or Oligogalacturonide does not Correlate with the Capacity to Reject Ultraviolet-Induced Skin Tumors in Mice. J. Investig. Dermatol. 2001, 116, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Aravind, S.R.; Joseph, M.M.; Varghese, S.; Balaram, P.; Sreelekha, T.T. Antitumor and immunopotentiating activity of polysaccharide PST001 isolated from the seed kernel of Tamarindus indica: An in vivo study in mice. Sci. World J. 2012, 2012, 361382. [Google Scholar] [CrossRef] [PubMed]
- Burgalassi, S.; Raimondi, L.; Pirisino, R.; Banchelli, G.; Boldrini, E.; Saettone, M.F. Effect of Xyfoglucan (Tamarind Seed Polysaccharide) on Conjunctival Cell Adhesion to Laminin and on Corneal Epithelium Wound Healing. Eur. J. Ophthalmol. 2000, 10, 71–76. [Google Scholar] [CrossRef]
- Do Rosário, M.M.T.; Kangussu-Marcolino, M.M.; do Amaral, A.E.; Noleto, G.R.; de Oliveira Petkowicz, C.L. Storage xyloglucans: Potent macrophages activators. Chem. Biol. Interact. 2011, 189, 127–133. [Google Scholar] [CrossRef]
- Rolando, M.; Valente, C. Establishing the tolerability and performance of tamarind seed polysaccharide (TSP) in treating dry eye syndrome: Results of a clinical study. BMC Ophthalmol. 2007, 7, 5. [Google Scholar] [CrossRef]
- Amnuaikit, T.; Khakhong, S.; Khongkow, P. Formulation Development and Facial Skin Evaluation of Serum Containing Jellose from Tamarind Seeds. J. Pharm. Res. Int. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Sukhawanli, S.; Thamakorn, P. Extraction of tamarind seed jellose under different conditions and their rheological properties. Food Appl. Biosci. J. 1970, 2, 61–68. [Google Scholar] [CrossRef]
- González-Hernández, J.C.; Farías Rosales, L.; Zamudio Jaramillo, M.Á.; Álvarez-Navarrete, M.; Vera Villa, J.C.; Martínez Corona, R.; Chávez-Parga, M.; Peña, A. Chemical Hydrolysis of the Polysaccharides of the Tamarind Seed. J. Mex. Chem. Soc. 2012, 56, 395–401. [Google Scholar] [CrossRef]
- Chan, G.; Kalaitzidis, D.; Neel, B.G. The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis Rev. 2008, 27, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gidley, M.J.; Lillford, P.J.; Rowlands, D.W.; Lang, P.; Dentini, M.; Crescenzi, V.; Edwards, M.; Fanutti, C.; Grant Reid, J.S. Structure and solution properties of tamarind-seed polysaccharide. Carbohydr. Res. 1991, 214, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.R.; Morris, G.A.; Ebringerová, A.; Vodeničarová, M.; Velebný, V.; Ortega, A.; Garcia de la Torre, J.; Harding, S.E. Global conformation analysis of irradiated xyloglucans. Carbohydr. Polym. 2008, 74, 845–851. [Google Scholar] [CrossRef]
- Goyal, P.; Kumar, V.; Sharma, P. Graft copolymerization of acrylamide onto tamarind kernel powder in the presence of ceric ion. J. Appl. Polym. Sci. 2008, 108, 3696–3701. [Google Scholar] [CrossRef]
- Chawananorasest, K.; Saengtongdee, P.; Kaemchantuek, P. Extraction and Characterization of Tamarind (Tamarind indica L.) Seed Polysaccharides (TSP) from Three Difference Sources. Molecules 2016, 21, 775. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Deters, A.M. Tamarind Seed Xyloglucans Promote Proliferation and Migration of Human Skin Cells through Internalization via Stimulation of Proproliferative Signal Transduction Pathways. Dermatol. Res. Pract. 2013, 2013, 359756. [Google Scholar] [CrossRef] [PubMed]
- Funasaka, Y.; Komoto, M.; Ichihashi, M. Depigmenting Effect of α-Tocopheryl Ferulate on Normal Human Melanocytes. Pigment Cell Res. 2000, 13, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.; Arora, S.C.; Manchanda, R. Tamarind seed polysaccharide and its modifications-versatile pharmaceutical excipients—A review. Int. J. Pharm. Technol. Res. 2014, 6, 412–420. [Google Scholar]
- Wexler, A.; Hasegawa, S. Relative humidity-temperature relationships of some saturated salt solutions in the temperature range 0 to 50 °C. J. Res. Natl. Bur. Stand. 1954, 53, 19–26. [Google Scholar] [CrossRef]
- Kaur, L.; Singh, J.; Singh, H.; McCarthy, O.J. Starch–cassia gum interactions: A microstructure—Rheology study. Food Chem. 2008, 111, 1–10. [Google Scholar] [CrossRef]




| Parameters | Results |
|---|---|
| Organoleptic properties | |
| Color | Sand brown |
| Odor | Roasted bean |
| Taste | Bean |
| Texture | Rough powder |
| pH | 6.30–7.65 |
| Dispersion (% w/v) | 2.5 |
| Angle of repose (◦) | 35.42 ± 0.73 |
| Moisture sorption (% w/w) | 6.47 |
| Rheology | Pseudoplastic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khongkow, P.; Khakhong, S.; Thammarat, C.; Amnuaikit, T. Potential Bioactivities of Tamarind Seed Jellose at the Cellular Level for Cosmetic Product Development. Sustainability 2024, 16, 3114. https://doi.org/10.3390/su16083114
Khongkow P, Khakhong S, Thammarat C, Amnuaikit T. Potential Bioactivities of Tamarind Seed Jellose at the Cellular Level for Cosmetic Product Development. Sustainability. 2024; 16(8):3114. https://doi.org/10.3390/su16083114
Chicago/Turabian StyleKhongkow, Pasarat, Suphatsa Khakhong, Chayanee Thammarat, and Thanaporn Amnuaikit. 2024. "Potential Bioactivities of Tamarind Seed Jellose at the Cellular Level for Cosmetic Product Development" Sustainability 16, no. 8: 3114. https://doi.org/10.3390/su16083114
APA StyleKhongkow, P., Khakhong, S., Thammarat, C., & Amnuaikit, T. (2024). Potential Bioactivities of Tamarind Seed Jellose at the Cellular Level for Cosmetic Product Development. Sustainability, 16(8), 3114. https://doi.org/10.3390/su16083114






