Current Strategies in Controlling Aspergillus flavus and Aflatoxins in Grains during Storage: A Review
Abstract
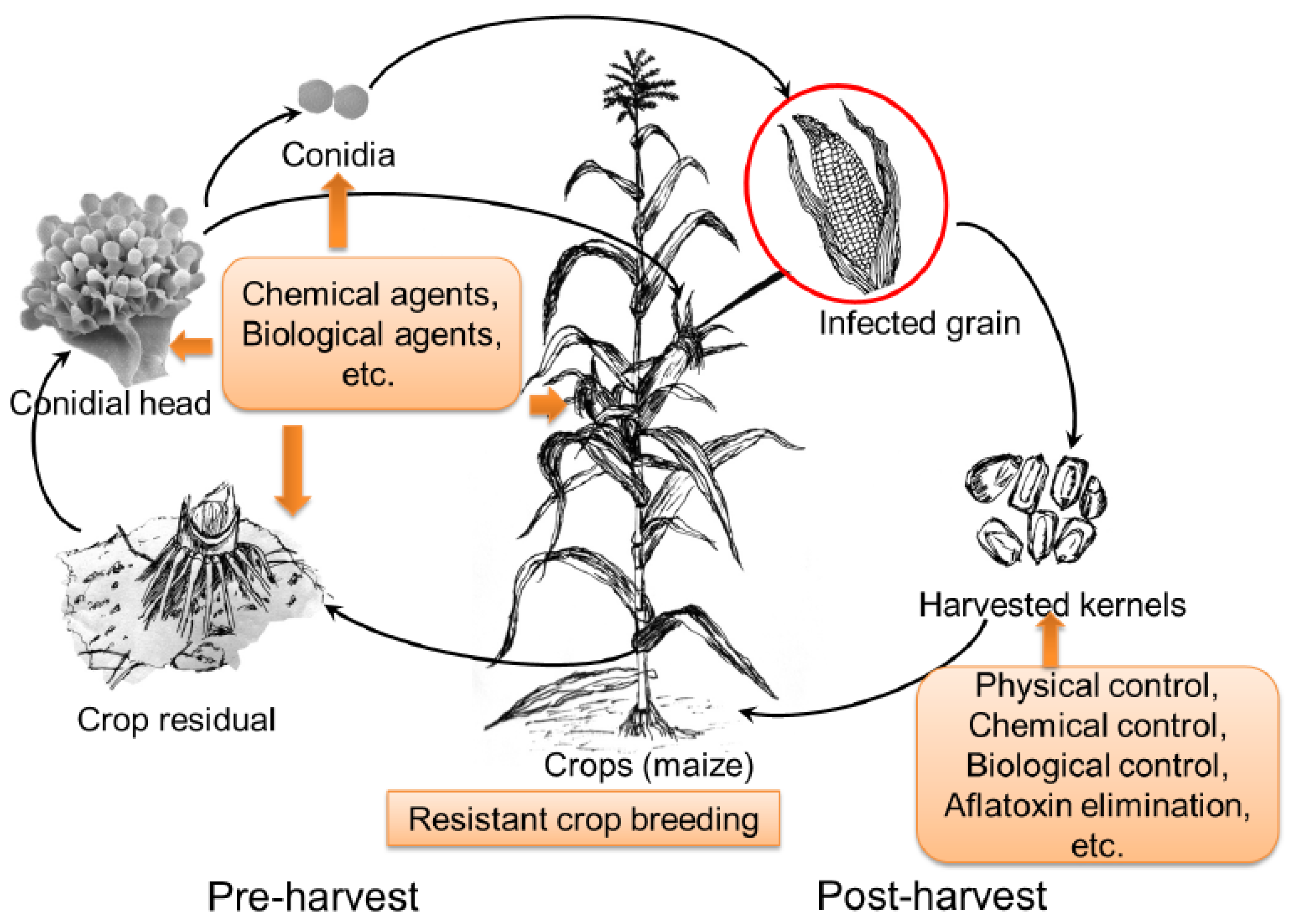
:1. Introduction
2. Physical Agents for the Control of A. flavus and Aflatoxins
2.1. Irradiation Methods
2.2. Low Oxygen Atmosphere Used in Controlling A. flavus and Aflatoxins
3. Chemical Agents for the Control of A. flavus and Aflatoxins
4. Biological Agents Used in Controlling A. flavus and Aflatoxins
4.1. Biological Agents from Plants
4.2. Biological Agents from Animals
4.3. Biological Agents from Micro-Organisms
| Micro-Organisms | Habitat | Antifungal Effects | Reference |
|---|---|---|---|
| Lactobacillus plantarum | Fermented Kenyan milk and maize products, etc. | (a). Produces antifungal biomolecules and other metabolites, inhibits fungal growth. (b). Adheres to the olive surface, produces a biofilm, competes for oxygen with A. flavus, and finally inhibits growth. | [82,83] |
| Bacillus subtilis fmbJ | Unknown | Produces bacillomycin D, injures the cell wall and cell membrane, prevents mycelial growth, sporulation, and spore germination. | [84] |
| Leuconostoc mesenteroides DU15 | Unknown | Produces peptides due to fungal cell lysis. | [85] |
| Bacillus subtilis UTBSP1 | Unknown | Produces fengycin and surfactin, which can reduce A. flavus growth and aflatoxin B1 content in pistachio nuts. | [86] |
| Pseudomonas sp. 4B | Effluent pond of a bovine abattoir located in southern Brazil. | Reduced fungal growth by 53.8–69%. The aflatoxin concentration reduced from 1472 ng/mL to 42.3 ng/mL. | [87] |
| Zygosaccharomyces rouxii | Unknown | Degraded AFB1 to new products; the detoxification rate reached 97%. | [88] |
| Hanseniaspora opuntiae L479 and H. uvarum L793 | Unknown | L479 produced a lot of acetic acid compounds, while L793 produced a lot of esters and alcohols compounds. These compounds could inhibit the growth of A. flavus. | [89] |
| Wickerhamomyces anomalus and Metschnikowia pulcherrima | Unknown | W. anomalus inhibits the growth of A. flavus through the production of volatiles and lytic enzymes, while M. pulcherrima performs biological control through competition for iron. | [90] |
| H. uvarum and H. opuntiae | Unknown | H. uvarum and H. opuntiae inhibit the growth of A. flavus by producing three volatiles, namely octanoic acid, 2-phenethyl acetate, and furfuryl acetate. | [91] |
| Saccharomyces cerevisiae | The Western and Eastern Ghats of India | Produces ethyl acetate, hexanal, 1-propanol, 1-heptanol, 1-butanol, benzothiazole, and other volatiles to inhibit the growth of A. flavus mycelia and AFB1 production. | [92] |
| Bacillus megaterium BM344-1 | Strawberry jam (imported from Turkey) marketed in Qatar | Produces hexadecanoic acid methyl ester (palmitic acid) and tetracosane to inhibit the growth of A. flavus. | [93] |
| B. megaterium and Pseudomonas protegens | Stored rice grains in Korea | Produces volatile organic compounds to inhibit the growth of A. flavus and aflatoxins production. | [94] |
| B. subtilis SV36-2 | Different cooked food (meat and vegetables) | Produces high quantities of carbon disulfide and 1,3-pentadiene to reduce mycelia and conidiation in A. flavus MG09. | [95] |
| Pichia kudriavzevii and Lachansea thermotolerans | Soil and pistachio nuts | Prevents A. flavus growth in dual culture, volatile, and non-volatile compounds reached 32–60%, 13–31% and 40–61%, respectively, while the inhibition rate of AFB1 production was 90.6–98.3%. | [96] |
| Pichia anomala WRL076 | Unknown | Produces the volatile compound 2-PE to inhibit the growth of A. flavus. | [97] |
| Candida nivariensis DMKU-CE18 | Leaves of rice, sugarcane, and corn in Thailand | Produces the volatile compound 1-pentanol to inhibit mycelial growth (64.9% inhibition) and conidial germination (49.3% inhibition) of A. flavus. | [98] |
| Streptomyces philanthi RL-1-178 | Chili pepper rhizosphere soil in southern Thailand | Produces the volatile compounds geosmin (13.75%), L-linalool (13.55%), 2-mercaptoethanol (9.71%), and heneicosane (5.96%) to inhibit the growth of A. parasiticus and A. flavus. | [99] |
| Streptomyces yanglinensis 3-10 | Rice (Oryza sativa), Huazhong Agricultural University, Wuhan, China | Produced 19 volatiles, including methyl 2-methylbutyrate, 2-phenylethanol, and β-caryophyllene, which can inhibit mycelial growth, sporulation, conidial germination, and expression of aflatoxin biosynthesis genes in A. flavus and A. parasiticus in vitro. | [100] |
| Alcaligenes faecalis N1-4 | Rhizosphere of tea plants | Produces dimethyl disulfide (DMDS) and methyl isovalerate (MI) to prevent conidial germination and mycelial growth of A. flavus. | [101] |
| Pseudomonas stutzeri YM6 | Sea sediment in the Yellow Sea of China | The main volatile organic compound dimethyl trisulfide (DMTS) at 200 μL/L can completely inhibit the growth of A. flavus. | [102] |
| Serratia marcescens Pt-3 | Rhizosphere of tea plants | Produces dimethyl disulfide (DMDS) to inhibit the growth of A. flavus. | [103] |
| Enterobacter asburiae Vt-7 | Rhizosphere of tea plants (North: 32°11′56.03″, East: 113°46′36.95″) | Produces 1-pentanol and phenylethyl alcohol to inhibit the growth of A. flavus. | [104] |
| Staphylococcus saprophyticus L-38 | Yellow Sea marine sediment | Produces 3,3-dimethyl-1,2-epoxybutane (3-DE) to inhibit the growth of A. flavus. | [105] |
| Shewanella algae strain YM8 | Yellow Sea marine sediment | Produces volatile organic compounds such as dimethyl trisulfide (DMTS), 2,4-bis(1,1-dimethylethyl)-phenol to reduce mycelial growth and conidial germination in A. flavus. | [106] |
5. Aflatoxin Elimination Methods
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Mellon, J.E.; Cotty, P.J.; Dowd, M.K. Aspergillus flavus hydrolases: Their roles in pathogenesis and substrate utilization. Appl. Microbiol. Biotechnol. 2007, 77, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.J.; Jiang, K.L.; Cheng, W.T.; Wang, Y.Y.; Pang, M.M.; Luo, H.; Lu, L.; Gao, K.; Tu, Y.X. Biocontrol of volatile organic compounds obtained from Bacillus subtilis CL2 against Aspergillus flavus in peanuts during storage. Biol. Control 2022, 176, 105094. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Wu, F. Global impacts of aflatoxin in maize: Trade and human health. World Mycotoxin J. 2015, 8, 137–142. [Google Scholar] [CrossRef]
- Razzaghi-Abyaneh, M.; Chang, P.K.; Shams-Ghahfarokhi, M.; Rai, M. Global health issues of aflatoxins in food and agriculture: Challenges and opportunities. Front. Microbiol. 2014, 5, 420. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.N.; Farhana, N.I.; Salleh, B. Occurrence of Aspergillus spp. and aflatoxin B1 in Malaysian foods used for human consumption. J. Food Sci. 2011, 76, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Sumner, P.E.; Lee, R.D. Reducing Aflatoxin in Corn during Harvest and Storage. Bulletin, 1231, University of Georgia. Available online: https://hdl.handle.net/10724/12104 (accessed on 5 November 2009).
- Sharma, A.C.; Proshad, R.; Kormoker, T.; Islam, M.S.; Chandra, K. A review on aflatoxins in stored grain food, their sources, mechanisms and possible health hazard. Arch. Agric. Environ. Sci. 2018, 3, 416–423. [Google Scholar] [CrossRef]
- Maxwell, L.A.; Callicott, K.A.; Bandyopadhyay, R.; Mehl, H.L.; Orbach, M.J.; Cotty, P.J. Degradation of aflatoxins B1 by atoxigenic Aspergillus flavus biocontrol agents. Plant Dis. 2021, 105, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin contamination, its impact and management strategies: An updated review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Waliyar, F.; Umeh, V.C.; Traore, A.; Osiru, M.; Ntare, B.R.; Diarra, B.; Kodio, O.; Kumar, K.V.K.; Sudini, H. Prevalence and distribution of aflatoxin contamination in groundnut (Arachis hypogaea L.) in Mali, West Africa. Crop. Prot. 2015, 70, 1–7. [Google Scholar] [CrossRef]
- Jiang, Y.; Ogunade, I.M.; Vyas, D.; Adesogan, A.T. Aflatoxin in dairy cows: Toxicity, occurrence in feedstuffs and milk and dietary mitigation strategies. Toxins 2021, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Lee, S.Y.; Woo, S.Y.; Choi, H.Y.; Park, S.B.; Chun, H.S. Effect of plant-based compounds on the antifungal and antiaflatoxigenic efficiency of strobilurins against Aspergillus favus. J. Hazard. Mater. 2021, 415, 125663. [Google Scholar] [CrossRef] [PubMed]
- Peles, F.; Sipos, P.; Kovács, S.; Győri, Z.; Pócsi, I.; Pusztahelyi, T. Biological control and mitigation of aflatoxin contamination in commodities. Toxins 2021, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, N.; Oveisi, M.R.; Jannat, B.; Hajimahmoodi, M.; Bonyani, H.; Jannat, F. Incidence of aflatoxin M1 in human breast milk in Tehran, Iran. Food Control 2009, 20, 75–78. [Google Scholar] [CrossRef]
- Mahdavi, R.; Nikniaz, L.; Arefhosseini, S.R.; Vahed Jabbari, M. Determination of aflatoxin M1 in breast milk samples in Tabriz-Iran. Matern. Child Health J. 2010, 14, 141–145. [Google Scholar] [CrossRef] [PubMed]
- FAO. Worldwide Regulations for Mycotoxins in Food and Feed in 2003; Food and Nutrition Paper No. 81; FAO: Rome, Italy, 2004. [Google Scholar]
- Magan, N.; Medina, A.; Rodriguez, A. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 2014, 5, 348. [Google Scholar]
- Shephard, G.S. Impact of mycotoxins on human health in developing countries. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Khalil, O.A.A.; Hammad, A.A.; Sebaei, A.S. Aspergillus flavus and Aspergillus ochraceus inhibition and reduction of aflatoxins and ochratoxin A in maize by irradiation. Toxicon 2021, 198, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.H.; Cho, M.J.; Park, S.Y.; Chun, H.S.; Ha, S.D. Effects of gamma ray, electron beam, and X-ray on the reduction of Aspergillus flavus on red pepper powder (Capsicum annuum L.) and gochujang (red pepper paste). Food Sci. Technol. Int. 2019, 25, 649–658. [Google Scholar] [CrossRef]
- Frink, S.; Marjanovic, O.; Tran, P.; Wang, Y.; Guo, W.; Encarnacion, N.; Alcantara, D.; Moezzi, B.; Vrdoljak, G. Use of X-ray irradiation for inactivation of Aspergillus in cannabis flower. PLoS ONE 2022, 17, e0277649. [Google Scholar] [CrossRef]
- Ghisleni, D.D.M.; Braga, M.S.; Kikuchi, I.S.; Brașoveanu, M.; Nemțanu, M.R.; Dua, K.; Pinto, T.J.A. The microbial quality aspects and decontamination approaches for the herbal medicinal plants and products: An in-depth review. Curr. Pharm. Des. 2016, 22, 4264–4287. [Google Scholar] [CrossRef] [PubMed]
- Assunção, E.; Reis, T.A.; Baquião, A.C.; Corrêa, B. Effects of Gamma and Electron Beam Radiation on Brazil Nuts Artificially Inoculated with Aspergillus flavus. J. Food Prot. 2015, 78, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Nurtjahja, K.; Dharmaputra, O.S.; Rahayu, W.P.; Syarief, R. Gamma irradiation of Aspergillus flavus strains associated with Indonesian nutmeg (Myristica fragrans). Food Sci. Biotechnol. 2017, 26, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.H.; El-Zeany, S.A.; Moussa, L.A.A. Influence of γ-irradiation and maize lipids on the production of aflatoxin B1 by Aspergillus flavus. Nahrung 2002, 46, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.H.; Park, S.Y.; Lee, D.U.; Chun, H.S.; Ha, S.D. Effect of UV-C irradiation on inactivation of Aspergillus flavus and Aspergillus parasiticus and quality parameters of roasted coffee bean (Coffea arabica L.). Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2020, 37, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Al-Gabr, H.M.; Zheng, T.; Yu, X. Inactivation of Aspergillus flavus in drinking water after treatment with UV irradiation followed by chlorination. Sci. Total Environ. 2013, 463–464, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Villers, P.; Gummert, M. Seal of approval. Rice Today 2009, 8, 26–27. [Google Scholar]
- Lemos, J.G.; Stefanello, A.; Bernardi, A.O.; Garcia, M.V.; Magrini, L.N.; Cichoski, A.J.; Wagner, R.; Copetti, M.V. Antifungal efficacy of sanitizers and electrolyzed waters against toxigenic Aspergillus. Food Res. Int. 2020, 137, 109451. [Google Scholar] [CrossRef] [PubMed]
- Samapundo, S.; Devlieghere, F.; De Meulenaer, B.; Lamboni, Y.; Osei-Nimoh, D.; Debevere, J.M. Interaction of water activity and bicarbonate salts in the inhibition of growth and mycotoxin production by Fusarium and Aspergillus species of importance to corn. Int. J. Food Microbiol. 2007, 116, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Qin, Y.L.; Li, S.F.; Lv, Y.Y.; Zhai, H.C.; Hu, Y.S.; Cai, J.P. Antifungal mechanism of 1-nonanol against Aspergillus flavus growth revealed by metabolomic analyses. Appl. Microbiol. Biotechnol. 2021, 105, 7871–7888. [Google Scholar] [CrossRef] [PubMed]
- Samapundo, S.; De Meulenaer, B.; Osei-Nimoh, D.; Lamboni, Y.; Debevere, J.; Devlieghere, F. Can phenolic compounds be used for the protection of corn from fungal invasion and mycotoxin contamination during storage? Food Microbiol. 2007, 24, 465–473. [Google Scholar] [CrossRef]
- De Lucca, A.J.; Carter-Wientjes, C.H.; Boué, S.; Bhatnagar, D. Volatile trans-2-hexenal, a soybean aldehyde, inhibits Aspergillus flavus growth and aflatoxin production in corn. J. Food Sci. 2011, 76, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Hareyama, Y.; Tarao, M.; Toyota, K.; Furukawa, T.; Fujii, Y.; Kushiro, M. Effects of four isothiocyanates in dissolved and gaseous states on the growth and aflatoxin production of Aspergillus flavus in vitro. Toxins 2022, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Mateo, E.M.; Gómez, J.V.; Gimeno-Adelantado, J.V.; Romera, D.; Mateo-Castro, R.; Jiménez, M. Assessment of azole fungicides as a tool to control growth of Aspergillus flavus and aflatoxin B1 and B2 production in maize. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Wagacha, J.M.; Muthomi, J.W. Mycotoxin problem in Africa: Current status, implications to food safety and health and possible management strategies. Int. J. Food Microbiol. 2008, 124, 1–12. [Google Scholar] [CrossRef]
- Ma, J.; Mo, H.; Chen, Y.; Ding, D.; Hu, L. Inhibition of aflatoxin synthesis in Aspergillus flavus by three structurally modified Lentinans. Int. J. Mol. Sci. 2014, 15, 3860–3870. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhuang, Z.; Zhang, F.; Song, F.; Zhong, H.; Ran, F.; Yu, S.; Xu, G.; Lan, F.; Wang, S. Inhibition of aflatoxin metabolism and growth of Aspergillus flavus in liquid culture by a DNA methylation inhibitor. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 32, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Jose, M.A.I.; Teves, F.G. Sclerotial formation inhibition by vitamin A, C, and E in Aspergillus flavus. Adv. Environ. Biol. 2015, 9, 37–40. [Google Scholar]
- Hu, C.H.; Li, L.L.; Liu, K.; Zhou, L.; Xiao, Y.H. Study the inhibition of food preservatives on spore germination and mycelium growth of poisonous Aspergillus flavus. Sci. Technol. Food Ind. 2014, 35, 282–285. [Google Scholar]
- Li, J.; Li, J.; Lu, Z.; Liu, Y.; Li, C.M. Transient transmembrane secretion of H2O2: A mechanism for the citral-caused inhibition of aflatoxin production from Aspergillus flavus. Chem. Commun. 2015, 51, 17424–17427. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Qiu, L.; Deng, Q.; Fang, Z.; Sun, L.; Wang, Y.; Gooneratne, R.; Zhao, J. L-Cysteine hydrochloride inhibits Aspergillus flavus growth and AFB1 synthesis by disrupting cell structure and antioxidant system balance. J. Hazard. Mater. 2023, 459, 132218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lv, Y.; Lv, A.; Wei, S.; Zhang, S.; Li, C.; Hu, Y. Sub3 inhibits Aspergillus flavus growth by disrupting mitochondrial energy metabolism, and has potential biocontrol during peanut storage. J. Sci. Food Agric. 2021, 101, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.B.; Ban, F.F.; Li, H.B.; Qian, P.P.; Shen, Q.S.; Zhao, Y.Y.; Mo, H.Z.; Zhou, X. Thymol induces conidial apoptosis in Aspergillus flavus via stimulating K+ eruption. J. Agric. Food Chem. 2018, 66, 8530–8536. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.L.; Zhang, S.B.; Lv, Y.Y.; Zhai, H.C.; Hu, Y.S.; Cai, J.P. The antifungal mechanisms of plant volatile compound 1-octanol against Aspergillus flavus growth. Appl. Microbiol. Biotechnol. 2022, 106, 5179–5196. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, Z.; Wang, X. UHPLC-HRMS-based untargeted lipidomics reveal mechanism of antifungal activity of carvacrol against Aspergillus flavus. Foods 2021, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Passone, M.A.; Etcheverry, M. Antifungal impact of volatile fractions of Peumus boldus and Lippia turbinata on Aspergillus section Flavi and residual levels of these oils in irradiated peanut. Int. J. Food Microbiol. 2014, 168–169, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Thobunluepop, P. The inhibitory effect of the various seed coating substances against rice seed borne fungi and their shelf-life during storage. Pak. J. Biol. Sci. 2009, 12, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Dubey, N.K. Antifungal and antiaflatoxigenic properties of Cuminum cyminum (L.) seed essential oil and its efficacy as a preservative in stored commodities. Int. J. Food Microbiol. 2014, 168–169, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Paranagama, P.A.; Abeysekera, K.H.T.; Abeywickrama, K.; Nugaliyadde, L. Fungicidal and anti-aflatoxigenic effects of the essential oil of Cymbopogon citratus (DC.) Stapf. (lemongrass) against Aspergillus flavus Link. isolated from stored rice. Lett. Appl. Microbiol. 2003, 37, 86–90. [Google Scholar] [CrossRef]
- Camiletti, B.X.; Asensio, C.M.; Pecci, M.P.G.; Lucini, E.I. Natural control of corn postharvest fungi Aspergillus flavus and Penicillium sp. using essential oils from plants grown in Argentina. J. Food Sci. 2014, 79, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Rehman, A.; Ahmad, I. Antibacterial, antifungal, and insecticidal potentials of Oxalis corniculata and its isolated compounds. Int. J. Anal. Chem. 2015, 2015, 842468. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, T.E.; Carter-Wientjes, C.H.; De Lucca, A.J.; Boué, S.M. Effect of soybean volatile compounds on Aspergillus flavus growth and aflatoxin production. J. Food Sci. 2009, 74, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Zhao, Q.; Zhao, K.; Pei, H.; Tao, F. The efficacy of composite essential oils against aflatoxigenic fungus Aspergillus flavus in maize. Toxins 2020, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.C.; Poitevin, C.G.; da Luz, T.S.; Mazarotto, E.J.; Furuie, J.L.; Martins, C.E.N.; do Amaral, W.; Cipriano, R.R.; da Rosa, J.M.; Pimentel, I.C.; et al. Antifungal activity of essential oils and their combinations against storage fungi. Environ. Sci. Pollut. Res. 2023, 30, 48559–48570. [Google Scholar] [CrossRef] [PubMed]
- Tøndervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Powell, L.C.; Pritchard, M.F.; Khan, S.; Craine, K.M.; Onsøyen, E.; Rye, P.D.; et al. Alginate oligosaccharides inhibit fungal cell growth and potentiate the activity of antifungals against Candida and Aspergillus spp. PLoS ONE 2014, 9, e112518. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, W.; Li, M.; Liu, H.; Zhao, X.; Yang, S.; Yang, M. Litsea cubeba essential oil as the potential natural fumigant: Inhibition of Aspergillus flavus and AFB1 production in licorice. Ind. Crops Prod. 2016, 80, 186–193. [Google Scholar] [CrossRef]
- Sameza, M.L.; Tchameni, S.N.; Ekoué, J.D.A.; Jazet, P.M.D.; Tchoumbougnang, F. Growth inhibition of the stored fish (Ethmalosa fimbriata) fungus Aspergillus flavus, exposed to extracted essential oils from Callistemon citrinus and Ocimum canum. Afr. J. Microbiol. Res. 2016, 10, 1164–1172. [Google Scholar]
- Achugbu, A.N.; Amadi, J.E.; Ilodibia, C.V.; Ikegbunam, M.N. Effects of Garcinia kola and Azadirachta indica seeds in the inhibition of Aspergillus flavus and Aspergillus parasiticus isolated from Zea mays L. Awka, Nigeria. Am. J. Plant Sci. 2016, 7, 1555–1563. [Google Scholar] [CrossRef]
- Alizadeh-Salteh, S. Growth inhibition of Aspergillus flavus isolated from pistachio by secondary metabolites. J. Nuts 2015, 6, 85–94. [Google Scholar]
- Zhang, D.; Yang, Y.; Yao, B.; Hu, T.; Ma, Z.; Shi, W.; Ye, Y. Curcumin inhibits Aspergillus flavus infection and aflatoxin production possibly by inducing ROS burst. Food Res. Int. 2023, 167, 112646. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Zhang, S.B.; Lv, Y.Y.; Zhai, H.C.; Cai, J.P.; Hu, Y.S. Mechanisms underlying the inhibitory effects of linalool on Aspergillus flavus spore germination. Appl. Microbiol. Biotechnol. 2022, 106, 6625–6640. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.M.; Wang, Y.R.; Zhao, W.B.; Ding, Y.Y.; Wu, Z.R.; Wang, G.H.; Deng, P.; Zhang, S.Y.; An, J.X.; Zhang, Z.J.; et al. Efficacy of pterostilbene suppression on Aspergillus flavus growth, aflatoxin B1 biosynthesis and potential mechanisms. Int. J. Food Microbiol. 2023, 404, 110318. [Google Scholar] [CrossRef] [PubMed]
- Nobili, C.; de Acutis, A.; Reverberi, M.; Bello, C.; Leone, G.P.; Palumbo, D.; Natella, F.; Procacci, S.; Zjalic, S.; Brunori, A. Buckwheat hull extracts inhibit Aspergillus flavus growth and AFB1 biosynthesis. Front. Microbiol. 2019, 10, 1997. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Aguilar, R.I.; Gónzalez-Andrade, C.; Hernández-López, M.; Correa-Pacheco, Z.N.; Teksür, P.K.; Ramos-García, M.L.; Bautista-Baños, S. Effect of biodegradable coatings on the growth of Aspergillus flavus in vitro, on maize grains, and on the quality of tortillas during storage. Molecules 2022, 27, 4545. [Google Scholar] [CrossRef] [PubMed]
- Forma, E.; Bryś, M. Anticancer activity of propolis and its compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef] [PubMed]
- El-Guendouz, S.; Lyoussi, B.; Miguel, M.G. Insight on propolis from mediterranean countries: Chemical composition, biological activities and application fields. Chem. Biodivers. 2019, 16, e1900094. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Freitas, A.S.; Cunha, A.; Oliveira, R.; Almeida-Aguiar, C. Propolis antibacterial and antioxidant synergisms with gentamicin and honey. J. Appl. Microbiol. 2022, 132, 2733–2745. [Google Scholar] [PubMed]
- de Souza, E.L.; Sales, C.V.; de Oliveira, C.E.V.; Lopes, L.A.A.; da Conceição, M.L.; Berger, L.R.R.; Stamford, T.C.M. Efficacy of a coating composed of chitosan from Mucor circinelloides and carvacrol to control Aspergillus flavus and the quality of cherry tomato fruits. Front. Microbiol. 2015, 6, 732. [Google Scholar]
- Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. A study on antifungal activity of water-soluble chitosan against Macrophomina phaseolina. Int. J. Biol. Macromol. 2014, 67, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Ding, B.; Zhang, M.; Dong, T.; Fu, Y.; Lv, Q.; Ding, W.; Wang, X. Study on inhibitory activity and mechanism of chitosan oligosaccharides on Aspergillus Flavus and Aspergillus Fumigatus. Carbohydr. Polym. 2022, 275, 118673. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, A.A.; Shaker, E.M.; El-Sharkawy, E.E.; Elsherif, W.M. Antifungal and antitoxin effects of propolis and its nanoemulsion formulation against Aspergillus flavus isolated from human sputum and milk powder samples. Vet. World 2021, 14, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Higareda, M.; Ramos-García, M.L.; Correa-Pacheco, Z.N.; Del Río-García, J.C.; Bautista-Baños, S. Nanostructured chitosan/propolis formulations: Characterization and effect on the growth of Aspergillus flavus and production of aflatoxins. Heliyon 2019, 5, e01776. [Google Scholar] [CrossRef]
- Aparicio-García, P.F.; Ventura-Aguilar, R.I.; del Río-García, J.C.; Hernández-López, M.; Guillén-Sánchez, D.; Salazar-Piña, D.A.; Ramos-García, M.L.; Bautista-Baños, S. Edible chitosan/propolis coatings and their effect on ripening, development of Aspergillus flavus, and sensory quality in fig fruit, during controlled storage. Plants 2021, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.N.; Reddy, C.S.; Muralidharan, K. Potential of botanicals and biocontrol agents on growth and aflatoxin production by Aspergillus flavus infecting rice grains. Food Control 2009, 20, 173–178. [Google Scholar] [CrossRef]
- Cho, K.M.; Math, R.K.; Hong, S.Y.; Asraful Islam, S.M.; Mandanna, D.K.; Cho, J.J.; Yun, M.G.; Kim, J.M.; Yun, H.D. Iturin produced by Bacillus pumilus HY1 from Korean soybean sauce (kanjang) inhibits growth of aflatoxin producing fungi. Food Control 2009, 20, 402–406. [Google Scholar] [CrossRef]
- Gong, A.D.; Song, M.G.; Wang, H.L.; Wang, G.Z.; Wang, J.H.; Zhang, J.B. Inhibitory effect of volatile organic compounds from Bacillus flexus TR-1 against Aspergillus flavus and aflatoxins in grains during storage. Biocontrol 2023, 68, 181–190. [Google Scholar] [CrossRef]
- Kong, Q.; Shan, S.; Liu, Q.; Wang, X.; Yu, F. Biocontrol of Aspergillus flavus on peanut kernels by use of a strain of marine Bacillus megaterium. Int. J. Food Microbiol. 2010, 139, 31–35. [Google Scholar] [CrossRef]
- Kachouri, F.; Ksontini, H.; Hamdi, M. Removal of aflatoxin B1 and inhibition of Aspergillus flavus growth by the use of Lactobacillus plantarum on olives. J. Food Prot. 2014, 77, 1760–1767. [Google Scholar] [CrossRef]
- Ahlberg, S.; Joutsjoki, V.; Laurikkala, S.; Varmanen, P.; Korhonen, H. Aspergillus flavus growth inhibition by Lactobacillus strains isolated from traditional fermented Kenyan milk and maize products. Arch. Microbiol. 2016, 199, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Zhang, C.; Lu, F.; Zhao, H.; Bie, X.; Lu, Z. Identification of bacillomycin D from Bacillus subtilis fmbJ and its inhibition effects against Aspergillus flavus. Food Control 2014, 36, 8–14. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Hassan, Z.; Abu Bakar, F.; Algboory, H.L.; Saari, N. Novel antifungal peptides produced by Leuconostoc mesenteroides DU15 effectively inhibit growth of Aspergillus niger. J. Food Sci. 2015, 80, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, M.; Shi, Z.Q.; Ahmadzadeh, M.; Hu, L.B.; Ghassempour, A. Inhibition of the Aspergillus flavus growth and aflatoxin B1 contamination on pistachio nut by fengycin and surfactin-producing Bacillus subtilis UTBSP1. Plant Pathol. J. 2016, 32, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Veras, F.F.; Fontoura, R.; Correa, A.P.F.; Einloft, T.C.; Brandelli, A.; Welke, J.E. Inhibition of growth of Aspergillus flavus and aflatoxin production by Pseumononas sp. strain 4B. Blucher Food Sci. Proc. 2014, 1, 551–552. [Google Scholar]
- Zhou, G.; Chen, Y.; Kong, Q.; Ma, Y.; Liu, Y. Detoxification of aflatoxin B1 by Zygosaccharomyces rouxii with solid state fermentation in peanut meal. Toxins 2017, 9, 42. [Google Scholar] [CrossRef]
- Tejero, P.; Martín, A.; Rodríguez, A.; Galván, A.I.; Ruiz-Moyano, S.; Hernández, A. In vitro biological control of Aspergillus flavus by Hanseniaspora opuntiae L479 and Hanseniaspora uvarum L793, producers of antifungal volatile organic compounds. Toxins 2021, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Parafati, L.; Restuccia, C.; Cirvilleri, G. Efficacy and mechanism of action of food isolated yeasts in the control of Aspergillus flavus growth on pistachio nuts. Food Microbiol. 2022, 108, 104100. [Google Scholar] [CrossRef] [PubMed]
- Galván, A.I.; Hernández, A.; Córdoba, M.G.; Martín, A.; Serradilla, M.J.; López-Corrales, M.; Rodríguez, A. Control of toxigenic Aspergillus spp. in dried figs by volatile organic compounds (VOCs) from antagonistic yeasts. Int. J. Food Microbiol. 2022, 376, 109772. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Balachandar, D.; Senthil, N.; Velazhahan, R.; Paranidharan, V. Volatiles of antagonistic soil yeasts inhibit growth and aflatoxin production of Aspergillus flavus. Microbiol. Res. 2022, 263, 127150. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.E.; Ul-Hassan, Z.; Zeidan, R.; Al-Shamary, N.; Al-Yafei, T.; Alnaimi, H.; Higazy, N.S.; Migheli, Q.; Jaoua, S. Biocontrol activity of Bacillus megaterium BM344-1 against toxigenic fungi. ACS Omega 2021, 6, 10984–10990. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, M.; Oh, J.Y.; Kim, K.D. Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus flavus and aflatoxin production on stored rice grains. Mycobiology 2017, 45, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Chaves-lópez, C.; Serio, A.; Gianotti, A.; Sacchetti, G.; Ndagijimana, M.; Ciccarone, C.; Stellarini, A.; Corsetti, A.; Paparella, A. Diversity of food-borne Bacillus volatile compounds and influence on fungal growth. J. Appl. Microbiol. 2015, 119, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Rohani, M.; Fani, S.R.; Mosavian, M.T.H.; Probst, C.; Khodaygan, P. Biocontrol potential of native yeast strains against Aspergillus flavus and aflatoxin production in pistachio. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2020, 37, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.S.T.; Beck, J.J.; Sarreal, S.B.L.; Gee, W. The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res. 2014, 30, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jaibangyang, S.; Nasanit, R.; Limtong, S. Biological control of aflatoxin-producing Aspergillus flavus by volatile organic compound-producing antagonistic yeasts. Biocontrol 2020, 65, 377–386. [Google Scholar] [CrossRef]
- Boukaew, S.; Prasertsan, P. Efficacy of volatile compounds from Streptomyces philanthi RL-1-178 as a biofumigant for controlling growth and aflatoxin production of the two aflatoxin-producing fungi on stored soybean seeds. J. Appl. Microbiol. 2020, 129, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Lyu, A.; Yang, L.; Wu, M.; Zhang, J.; Li, G. High efficacy of the volatile organic compounds of Streptomyces yanglinensis 3-10 in suppression of Aspergillus contamination on peanut kernels. Front. Microbiol. 2020, 11, 142. [Google Scholar] [CrossRef]
- Gong, A.D.; Wu, N.N.; Kong, X.W.; Zhang, Y.M.; Hu, M.J.; Gong, S.J.; Dong, F.Y.; Wang, J.H.; Zhao, Z.Y.; Liao, Y.C. Inhibitory effect of volatiles emitted from Alcaligenes faecalis N1-4 on Aspergillus flavus and aflatoxins in storage. Front. Microbiol. 2019, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.D.; Lei, Y.Y.; He, W.J.; Liao, Y.C.; Ma, L.; Zhang, T.T.; Zhang, J.B. The inhibitory effect of Pseudomonas stutzeri YM6 on Aspergillus flavus growth and aflatoxins production by the production of volatile Dimethyl Trisulfide. Toxins 2022, 14, 788. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.D.; Wang, G.Z.; Sun, Y.K.; Song, M.G.; Dimuna, C.; Gao, Z.; Wang, H.L.; Yang, P. Dual activity of Serratia marcescens Pt-3 in phosphate-solubilizing and production of antifungal volatiles. BMC Microbiol. 2022, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.D.; Dong, F.Y.; Hu, M.J.; Kong, X.W.; Wei, F.F.; Gong, S.J.; Zhang, Y.M.; Zhang, J.B.; Wu, A.B.; Liao, Y.C. Antifungal activity of volatile emitted from Enterobacter asburiae Vt-7 against Aspergillus flavus and aflatoxins in peanuts during storage. Food Control 2019, 106, 106718. [Google Scholar] [CrossRef]
- Gong, A.D.; Sun, G.J.; Zhao, Z.Y.; Liao, Y.C.; Zhang, J.B. Staphylococcus saprophyticus L-38 produces volatile 3,3-dimethyl-1,2-epoxybutane with strong inhibitory activity against Aspergillus flavus germination and aflatoxin production. World Mycotoxin J. 2020, 13, 247–258. [Google Scholar] [CrossRef]
- Gong, A.D.; Li, H.P.; Shen, L.; Zhang, J.B.; Wu, A.B.; He, W.J.; Yuan, Q.S.; He, J.D.; Liao, Y.C. The Shewanella algae strain YM8 produces volatiles with strong inhibition activity against Aspergillus pathogens and aflatoxins. Front. Microbiol. 2015, 6, 1091. [Google Scholar] [CrossRef] [PubMed]
- Zeinvand-Lorestani, H.; Sabzevari, O.; Setayesh, N.; Amini, M.; Nili-Ahmadabadi, A.; Faramarzi, M.A. Comparative study of in vitro prooxidative properties and genotoxicity induced by aflatoxin B1 and its laccase-mediated detoxification products. Chemosphere 2015, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mukendi, N.; Rollmann, B.; de Meester, C. Detoxification of aflatoxin B1 by different chemical methods and evaluation of the effectiveness of the treatments applied. J. Pharm. Belg. 1991, 46, 182–188. [Google Scholar]
- Safara, M.; Zaini, F.; Hashemi, S.J.; Mahmoudi, M.; Khosravi, A.R.; Shojai-Aliabadi, F. Aflatoxin detoxification in rice using citric acid. Iran. J. Public Health 2010, 39, 24–29. [Google Scholar]
- Aiko, V.; Edamana, P.; Mehta, A. Decomposition and detoxification of aflatoxin B1 by lactic acid. J. Sci. Food Agric. 2016, 96, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Diao, E.; Li, X.; Zhang, Z.; Dong, H. Detoxification and safety evaluation of aflatoxin B1 in peanut oil using alkali refining. J. Sci. Food Agric. 2015, 96, 4009–4014. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wang, R.; Wang, L.; Li, Y.; Wang, Y.; Chen, Z. Detoxification of aflatoxin in corn flour by ozone. J. Sci. Food Agric. 2014, 94, 2253–2258. [Google Scholar] [CrossRef] [PubMed]
- Jardon-Xicotencatl, S.; Díaz-Torres, R.; Marroquín-Cardona, A.; Villarreal-Barajas, T.; Méndez-Albores, A. Detoxification of aflatoxin-contaminated maize by neutral electrolyzed oxidizing water. Toxins 2015, 7, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Vijayanandraj, S.; Brinda, R.; Kannan, K.; Adhithya, R.; Vinothini, S.; Senthil, K.; Chinta, R.R.; Paranidharan, V.; Velazhahan, R. Detoxification of aflatoxin B1 by an aqueous extract from leaves of Adhatoda vasica Nees. Microbiol. Res. 2014, 169, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Yehia, R.S. Aflatoxin detoxification by manganese peroxidase purified from Pleurotus ostreatus. Braz. J. Microbiol. 2014, 45, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ma, F.; Li, P.W.; Zhang, W.; Ding, X.X.; Zhang, Q.; Li, M.; Wang, Y.R.; Xu, B.C. Effect of ozone on aflatoxins detoxification and nutritional quality of peanuts. Food Chem. 2014, 146, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Diao, E.; Shen, X.; Zhang, Z.; Ji, N.; Ma, W.; Dong, H. Safety evaluation of aflatoxin B1 in peanut oil after ultraviolet irradiation detoxification in a photodegradation reactor. Int. J. Food Sci. Technol. 2015, 50, 41–47. [Google Scholar] [CrossRef]
- Wang, B.; Mahoney, N.E.; Pan, Z.; Khir, R.; Wu, B.; Ma, H.; Zhao, L. Effectiveness of pulsed light treatment for degradation and detoxification of aflatoxin B1 and B2 in rough rice and rice bran. Food Control 2016, 59, 461–467. [Google Scholar] [CrossRef]
- Shao, S.; Cai, J.; Du, X.; Wang, C.G.; Lin, J.G.; Dai, J. Biotransformation and detoxification of aflatoxin B1 by extracellular extract of Cladosporium uredinicola. Food Sci. Biotechnol. 2016, 25, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Sakudo, A.; Toyokawa, Y.; Misawa, T.; Imanishi, Y. Degradation and detoxification of aflatoxin B1 using nitrogen gas plasma generated by a static induction thyristor as a pulsed power supply. Food Control 2016, 73, 619–626. [Google Scholar] [CrossRef]
- Kamber, U.; Gülbaz, G.; Aksu, P.; Doğan, A. Detoxification of aflatoxin B1 in red pepper (Capsicum annuum L.) by ozone treatment and its effect on microbiological and sensory quality. J. Food Process. Preserv. 2017, 41, e13102. [Google Scholar] [CrossRef]
- Zahoor, M.; Khan, F.A. Aflatoxin B1 detoxification by magnetic carbon nanostructures prepared from maize straw. Desalination Water Treat. 2015, 57, 11893–11903. [Google Scholar] [CrossRef]
- Campos-Avelar, I.; de la Noue, A.C.; Durand, N.; Cazals, G.; Martinez, V.; Strub, C.; Fontana, A.; Schorr-Galindo, S. Aspergillus flavus growth inhibition and Aflatoxin B1 decontamination by Streptomyces isolates and their metabolites. Toxins 2021, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.F.; Gibbons, J.G.; Lee, M.K.; Han, K.H.; Hong, S.B.; Yu, J.H. Controlling aflatoxin contamination and propagation of Aspergillus flavus by a soy-fermenting Aspergillus oryzae strain. Sci. Rep. 2018, 8, 16871. [Google Scholar] [CrossRef] [PubMed]
- Ciegler, A.; Lillehoj, E.B.; Peterson, R.E.; Hall, H.H. Microbial detoxification of aflatoxin. Appl. Microbiol. 1966, 14, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; Ma, L.; Gu, L.Q.; Liang, R.; Yao, D.S.; Chen, W.Q. Armillariella tabescen enzymatic detoxification of aflatoxin B1: Part III. Immobilized enzymatic detoxification. Ann. N. Y. Acad. Sci. 1998, 864, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.J.; Erickson, K.E.; Sengstag, C.; Eaton, D.L. Expression of human microsomal epoxide hydrolase in Saccharomyces cerevisiae reveals a functional role in aflatoxin B1 detoxification. Toxicol. Sci. 2002, 65, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Petchkongkaew, A.; Taillandier, P.; Gasaluck, P.; Lebrihi, A. Isolation of Bacillus spp. from Thai fermented soybean (Thua-nao): Screening for aflatoxin B1 and ochratoxin A detoxification. J. Appl. Microbiol. 2008, 104, 1495–1502. [Google Scholar] [CrossRef]
- Topcu, A.; Bulat, T.; Wishah, R.; Boyaci, I.H. Detoxification of aflatoxin B1 and patulin by Enterococcus faecium strains. Int. J. Food Microbiol. 2010, 139, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yan, P.S.; Cao, L.X.; Ding, Q.L.; Shao, C.; Zhao, T.F. Potential of chitinolytic Serratia marcescens strain JPP1 for biological control of Aspergillus parasiticus and aflatoxin. BioMed Res. Int. 2013, 2013, 397142. [Google Scholar]
- Verheecke, C.; Liboz, T.; Anson, P.; Diaz, R.; Mathieu, F. Reduction of aflatoxin production by Aspergillus flavus and Aspergillus parasiticus in interaction with Streptomyces. Microbiology 2015, 161, 967–972. [Google Scholar] [CrossRef]
- Zuo, R.Y.; Chang, J.; Yin, Q.Q.; Wang, P.; Yang, Y.R.; Wang, X.; Wang, G.Q.; Zheng, Q.H. Effect of the combined probiotics with aflatoxin B1-degrading enzyme on aflatoxin detoxification, broiler production performance and hepatic enzyme gene expression. Food Chem. Toxicol. 2013, 59, 470–475. [Google Scholar] [CrossRef]
- Scarpari, M.; Bello, C.; Pietricola, C.; Zaccaria, M.; Bertocchi, L.; Angelucci, A.; Ricciardi, M.R.; Scala, V.; Parroni, A.; Fabbri, A.A.; et al. Aflatoxin control in maize by Trametes versicolor. Toxins 2014, 6, 3426–3437. [Google Scholar] [CrossRef]
- Zeng, R.S.; Wen, Z.; Niu, G.; Berenbaum, M.R. Aflatoxin B1: Toxicity, bioactivation and detoxification in the polyphagous caterpillar Trichoplusia ni. Insect Sci. 2013, 20, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Lu, F.P.; Tan, C.P.; Yao, D.S.; Chu, M.Q.; Xie, C.F.; Liu, D.L. The furofuran-ring selectivity, hydrogen peroxide-production and low Km value are the three elements for highly effective detoxification of aflatoxin oxidase. Food Chem. Toxicol. 2014, 76, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Chen, X.Y.; Zhu, R.Z.; Choi, B.M.; Kim, B.R. Sulforaphane induces glutathione S-transferase isozymes which detoxify aflatoxin B1-8,9-epoxide in AML 12 cells. Biofactors 2010, 36, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Moreno, M. Metabolic changes of aflatoxin B1 to become an active carcinogen and the control of this toxin. Immune Res. 2015, 11, 104. [Google Scholar] [CrossRef]
- Wang, J.; Ogata, M.; Hirai, H.; Kawagishi, H. Detoxification of aflatoxin B1 by manganese peroxidase from the white-rot fungus Phanerochaete sordida YK-624. FEMS Microbiol. Lett. 2011, 314, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Simula, T.P.; Glancey, M.J.; Wolf, C.R. Human glutathione S-transferase-expressing Salmonella typhimurium tester strains to study the activation/detoxification of mutagenic compounds: Studies with halogenated compounds, aromatic amines and aflatoxin B1. Carcinogenesis 1993, 14, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; Yao, D.S.; Huang, B.H.; Xie, C.F.; Liang, Y.Q.; Ma, L. Characterization of immobilized aflatoxin-detoxizyme. Chin. J. Biotechnol. 2003, 19, 603–607. [Google Scholar]
- Liu, R.; Yang, Q.; Thanaboripat, D.; Thansukon, P. Biocontrol of Aspergillus flavus and aflatoxin production. In Proceedings of the 2nd International Symposium on Bio-Control and Biotechnology, Bangkok, Thailand, 6 January 2004. [Google Scholar]
- Yin, Y.N.; Yan, L.Y.; Jiang, J.H.; Ma, Z.H. Biological control of aflatoxin contamination of crops. J. Zhejiang Univ. Sci. B 2008, 9, 787–792. [Google Scholar] [CrossRef] [PubMed]
- González-Aguilar, G.A.; Ayala-Zavala, J.F.; Olivas, G.I.; de la Rosa, L.A.; Álvarez-Parrilla, E. Preserving quality of fresh-cut products using safe technologies. J. Verbrauch. Lebensm. 2010, 5, 65–72. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Hocking, A.D.; Pitt, J.I.; Fleet, G.H. Growth and mycotoxin production by food spoilage fungi under high carbon dioxide and low oxygen atmospheres. Int. J. Food Microbiol. 2009, 132, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Khalek, H.H.; Hammad, A.A.; El-Kader, R.M.A.; Youssef, K.A.; Abdou, D.A. Combinational inhibitory action of essential oils and gamma irradiation for controlling Aspergillus flavus and Aspergillus parasiticus growth and their aflatoxins biosynthesis in vitro and in situ conditions. Food Sci. Technol. Int. 2022, 28, 703–715. [Google Scholar] [CrossRef]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Park, S.B.; Zheng, Y.; Chun, H.S. Antifungal activity of essential oil and plant-derived natural compounds against Aspergillus flavus. Antibiotics 2022, 11, 1727. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.; Cristiana, S.; Gabriella, P.; Rita, B.A. Antifungal activity of different essential oils against Malassezia pathogenic species. J. Ethnopharmacol. 2019, 249, 112376. [Google Scholar]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Siahmoshteh, F.; Siciliano, I.; Banani, H.; Hamidi-Esfahani, Z.; Razzaghi-Abyaneh, M.; Gullino, M.L.; Spadaro, D. Efficacy of Bacillus subtilis and Bacillus amyloliquefaciens in the control of Aspergillus parasiticus growth and aflatoxins production on pistachio. Int. J. Food Microbiol. 2017, 254, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Gibellato, S.L.; Dalsóquio, L.F.; do Nascimento, I.C.A.; Alvarez, T.M. Current and promising strategies to prevent and reduce aflatoxin contamination in grains and food matrices. World Mycotoxin J. 2020, 14, 293–304. [Google Scholar] [CrossRef]
| Compounds | Effects | Reference |
|---|---|---|
| Carboxymethylation, sulfation and phosphorylation of lentinan derivatives | Lentinan at 200 μg/mL completely inhibits aflatoxin production. Sulfated derivatives led to reduced inhibition compared to lentinan. The phosphorylated derivatives showed complete inhibition of aflatoxins biosynthesis at 50 μg/mL. | [39] |
| 5-azacytidine (5-AC) | 5-AC, a DNA methylation inhibitor, decreased aflatoxin production and changed fungal cell morphology. | [40] |
| Vitamins A, C, and E | Vitamins A, C, and E can prevent sclerotium formation in A. flavus. High concentrations of these vitamins in the medium resulted in a small number of sclerotia. | [41] |
| Potassium sorbate and sodium benzoate | Inhibited A. flavus growth and its infection of peanut and other crops. | [42] |
| Citral | Citral caused transient transmembrane secretion of H2O2 and led to the inhibition of aflatoxin production. | [43] |
| L-Cysteine hydrochloride (L-CH) | L-CH induced glutathione (GSH) synthesis to clear intracellular reactive oxygen species (ROS), leading to hyphal dwarfing. L-CH inhibited hyphal branching by preventing the expression of cell wall and spore development-related genes. | [44] |
| Sub3 | Sub3, over 0.15 g/L, prevented the germination of A. flavus spores in a potato dextrose broth medium. | [45] |
| Thymol | 200 μg/mL thymol induced conidial apoptosis in A. flavus. | [46] |
| 1-Octanol | 1-Octanol can inhibit A. flavus spore germination in a dose-dependent manner, and 300 μL/L 1-octanol vapor could completely inhibit the growth of A. flavus in wheat, maize, and rice with a 20% moisture content. | [47] |
| Carvacrol (CV) | The spore germination rates of A. flavus at 50 µg/mL, 100 µg/mL, and 200 µg/mL CV treatments were reduced to 84.0%, 26.7%, and 11.3%, respectively. | [48] |
| Plant | Products | Mechanism | Reference |
|---|---|---|---|
| Seaweed | Alginate oligomer | Could disrupt fungal biofilm formation, increase cell surface roughness to disrupt fungal growth. | [58] |
| Litsea cubeba | Essential oil containing (Z)-limonene oxide (30.14%), (E)-limonene oxide (27.92%) and D-limonene (11.86%) | Controlled A. flavus growth and aflatoxin B1 production in licorice. | [59] |
| Callistemon citrinus and Ocimum canum | Major components in C. citrinus are 1,8-cineole (60.6%), α-pinene (18.5%). O. canum containing 1.8-cineole (20.8%), linalol (14.3%), and eugenol (11.9%) | Used as a fumigant in Ethmalosa fimbriata preservation against A. flavus. | [60] |
| Neem and bitter kola seeds | Methanolic and ethanolic extracts | Inhibited the growth of A. flavus with antifungal compounds in the extraction. | [61] |
| Pistachio nut | Carvacrol and allyl isothiocyanate | Controlled conidia germination and mycelial growth of A. flavus. | [62] |
| Curcuma longa | Curcumin | Curcumin inhibited the mycelial growth and sporulation of A. flavus, inhibited the biosynthesis of ergosterol, and enhanced the permeability of cell membranes. | [63] |
| Oregano variety Mendocino (OMen), Cordobes (OCor), and Compacto (OCom) | Essential oils | The compounds of thymol in OCor (18.66%), OMen (12.18%), and OCom (9.44%) showed the best antifungal activity. | [53] |
| Zanthoxylum schinifolium pericarp | Linalool | Linalool vapor at 800 μL/L prevented A. flavus growth, and linalool at 10 μL/mL caused A. flavus spore death. | [64] |
| Pterocarpus indicus Willd., Vaccinium spp. and Vitis vinifera L. | Pterostilbene | Pterostilbene inhibited mycelial growth of A. flavus with EC50 (the concentration that causes inhibition by 50%) at 15.94 μg/mL. Pterostilbene at 250 and 500 μg/mL effectively inhibited A. flavus infection in peanuts. | [65] |
| Buckwheat hull | Polyphenols, tocopherols, phytosterols, and fatty acids | Lipophilic extract at 10 μg/mL and polyphenol extract at 100 ng/mL inhibited the growth of A. flavus by 74% and 38%, respectively. A mixture of the two inhibited the growth of A. flavus by 86%. | [66] |
| Aflatoxin Detoxification Agents | Detoxification Effects | Reference |
|---|---|---|
| Vasaka leaf extract (Adhatoda vasica Nees) | Alkaloid extracted from leaves showed strong aflatoxin B1 (AFB1) detoxification activity. The degradation rate was ≥98%. | [114] |
| Manganese peroxidase from white rot edible mushrooms Pleurotus ostreatus | The degradation efficiency of AFB1 was the highest (90%) when incubated under 1.5 U/mL enzyme activity for 48 h. | [115] |
| Ozonation | The detoxification rates of ozone (6 mg/L applied for 30 min at room temperature) to the total aflatoxins and AFB1 were 65.8% and 65.9%, respectively. | [116] |
| Ultraviolet irradiation | The optimal enzymatic reaction occurred in 0.1 M of citrate buffer containing 20% dimethyl sulfoxide at 35 °C, a pH of 4.5, and a laccase activity of 30 U/mL. | [107] |
| Ultraviolet irradiation | AFB1 was decreased from 51.96 to 7.23 μg/kg in 10 min and reduced by 86.08% in peanut oil. | [117] |
| Pulsed light (PL) | PL treatment (80 s) reduced AFB1 and aflatoxin B2 (AFB2) in rough rice by 75.0% and 39.2%, respectively; treatment for 15 s reduced AFB1 and AFB2 in rice bran by 90.3% and 86.7%, respectively. | [118] |
| Extracellular extract of Cladosporium uredinicola | Thermostable enzyme in the extract of C. uredinicola can eliminate AFB1 by 84.5% at 37 °C. | [119] |
| Nitrogen gas plasma | Nitrogen gas plasma degrades AFB1 (200 ppb) by 90% within 15 min. | [120] |
| Ozone | In red pepper samples containing AFB1 treated with ozone 80 mg/L for 40 min, the reduction in AFB1 was 74.1%. Additionally, the mesophilic bacteria and mold/yeast counts decreased by 7–22.1% and 27.2–33.7%, respectively. | [121] |
| Magnetic carbon nanocomposites | The equilibrium times at pHs 7 and 3 were 96 and 180 min, respectively, and nearly 90% of AFB1 was removed in both adsorbents. | [122] |
| Fifty-nine Streptomyces isolates and Mycostop®’s Streptomyces griseoviridis K61 | After 10 days of culture, most strains in 59 Streptomyces isolates were able to degrade AFB1 on solid medium (mean = 33%, median = 32%), while the Streptomyces griseoviridis strain degraded it to undetectable levels. | [123] |
| Aspergillus oryzae M2040 strain | In peanuts, the 1% inoculation level of A. oryzae M2040 could secrete inhibitory compounds and effectively inhibit AFB1 production and A. flavus growth. | [124] |
| Approach | Advantages | Disadvantages |
|---|---|---|
| Irradiation | It leaves no residue, has no legal restrictions, is easy to use, and is lethal to a wide range of hazardous micro-organisms [143]. | The application of irradiation in long-term storage is laborious and uneconomic. The effect is not obvious in dry crops [21]. |
| Low oxygen atmosphere | Minimizes the use of chemical preservatives and integrated control of both microbial growth and insect infestation [144]. | May not control or prevent fungal growth and possible production of mycotoxins because some fungi can grow under facultatively anaerobic conditions [144]. |
| Chemical agents | It has great antifungal efficiency [145]. | Some of these agents can adversely affect the nutritional, sensory, and functional properties of foods, produce harmful toxic residues, contaminate the environment, and create resistant fungal pathogens [146,147]. |
| Phyto materials | A variety of compounds are present in essential oils, and their antibacterial activities may be due to the interaction of several mechanisms of action in different parts of microbial cells, which may result in the bacteria not developing resistance [148]. | The application of these essential oils from plants is always dose-dependent [149]. These substances are difficult to produce in a short period of time owing to large planting areas needed, long growth cycles, daily management, etc. [149]. |
| Animal derivatives | Cheap and natural origins [75]. | Often results in a strong taste that can change the character of the food [75]. These substances are also difficult to produce in a short period of time owing to livestock scales, standardized management, and long breeding periods. [75]. |
| Microbial agents | Low in toxicity, biodegradable, and environmentally friendly [150]; they also have high efficiency and specificity [151]. | The method is in the research and experimentation stage; there are still many questions to answer, and currently few microbe strains can commercially be used in practice for aflatoxin degradation [11]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, A.; Song, M.; Zhang, J. Current Strategies in Controlling Aspergillus flavus and Aflatoxins in Grains during Storage: A Review. Sustainability 2024, 16, 3171. https://doi.org/10.3390/su16083171
Gong A, Song M, Zhang J. Current Strategies in Controlling Aspergillus flavus and Aflatoxins in Grains during Storage: A Review. Sustainability. 2024; 16(8):3171. https://doi.org/10.3390/su16083171
Chicago/Turabian StyleGong, Andong, Mengge Song, and Jingbo Zhang. 2024. "Current Strategies in Controlling Aspergillus flavus and Aflatoxins in Grains during Storage: A Review" Sustainability 16, no. 8: 3171. https://doi.org/10.3390/su16083171
APA StyleGong, A., Song, M., & Zhang, J. (2024). Current Strategies in Controlling Aspergillus flavus and Aflatoxins in Grains during Storage: A Review. Sustainability, 16(8), 3171. https://doi.org/10.3390/su16083171








