Improving Physical and Chemical Properties of Saline Soils with Fly Ash Saline and Alkaline Amendment Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.2.1. The Effect of Fly Ash Saline and Alkaline Soil Amendment Materials on Soil Properties
2.2.2. The Effect of Fly Ash Saline and Alkaline Soil Amendment Materials on Plant Growth
2.2.3. Field Experiments in Zea mays L. Planting
2.3. Determination Method
2.3.1. Leaching Content of Heavy Metal Elements in Fly Ash Saline and Alkaline Soil Amendment Materials in Different pH Leaching Solutions
2.3.2. Hakanson Ecological Risk Assessment Method
2.3.3. Determination of Soil Physical and Chemical Properties
2.3.4. Determination of Soil Maximum Water-Holding Capacity and Soil Moisture Loss Rate
Determination of Soil Maximum Water-Holding Capacity
Determination of Soil Moisture Loss Rate
2.3.5. Determination of Soil Capacity, Porosity, Capillary Porosity, Non-Capillary Porosity and Three Comparisons
2.3.6. Measurement of Plant Agronomic Traits
2.4. Data Collection and Analysis
3. Results
3.1. Leaching Content of Heavy Metal Elements in Fly Ash Saline and Alkaline Soil Amendment Materials in Different pH Leaching Solutions
3.2. The Hakanson Potential Ecological Risk Index Evaluation
3.3. The Effect of Fly Ash Saline and Alkaline Soil Amendment Materials on Soil pH and EC
3.4. The Effect of Fly Ash Saline and Alkaline Soil Amendment Materials on Soil Organic Matter, Available Phosphorus and Available Potassium Content
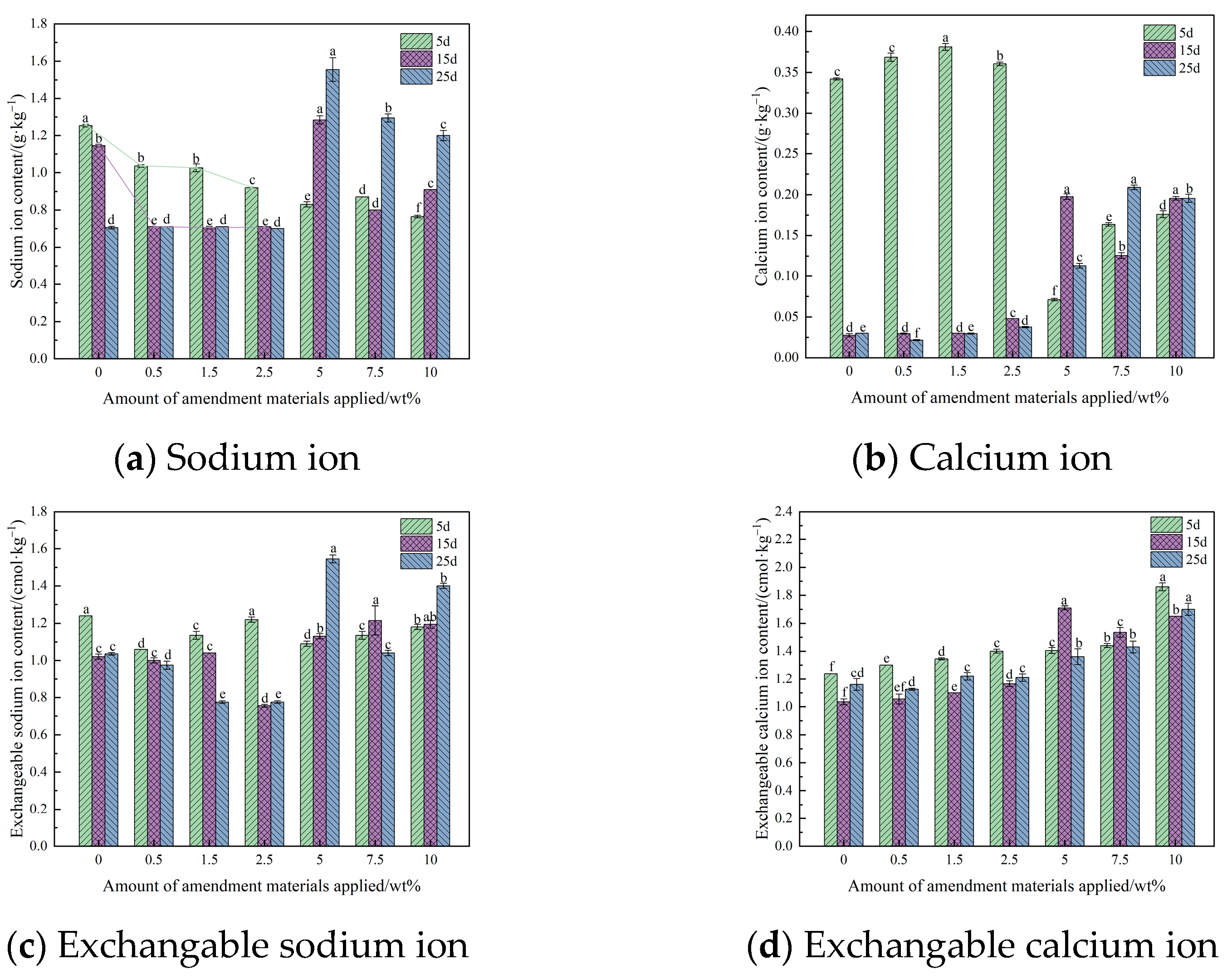
3.5. The Effect of Fly Ash Saline and Alkaline Soil Amendment Materials on Soil Sodium Ion, Calcium Ion, Exchangeable Sodium Ion, Exchangeable Calcium Ion Content
3.6. The Effect of Fly Ash Saline and Alkaline Soil Amendment Materials on Soil Maximum Soil Water-Holding Capacity and Moisture Loss Rate
3.7. The Effect of Fly Ash Saline and Alkaline Soil Amendment Materials on Soil Bulk Density, Total Porosity, Capillary Porosity, and Non-Capillary Porosity
3.8. The Effect of Fly Ash Saline and Alkaline Soil Amendment Materials on Soil Three Comparisons
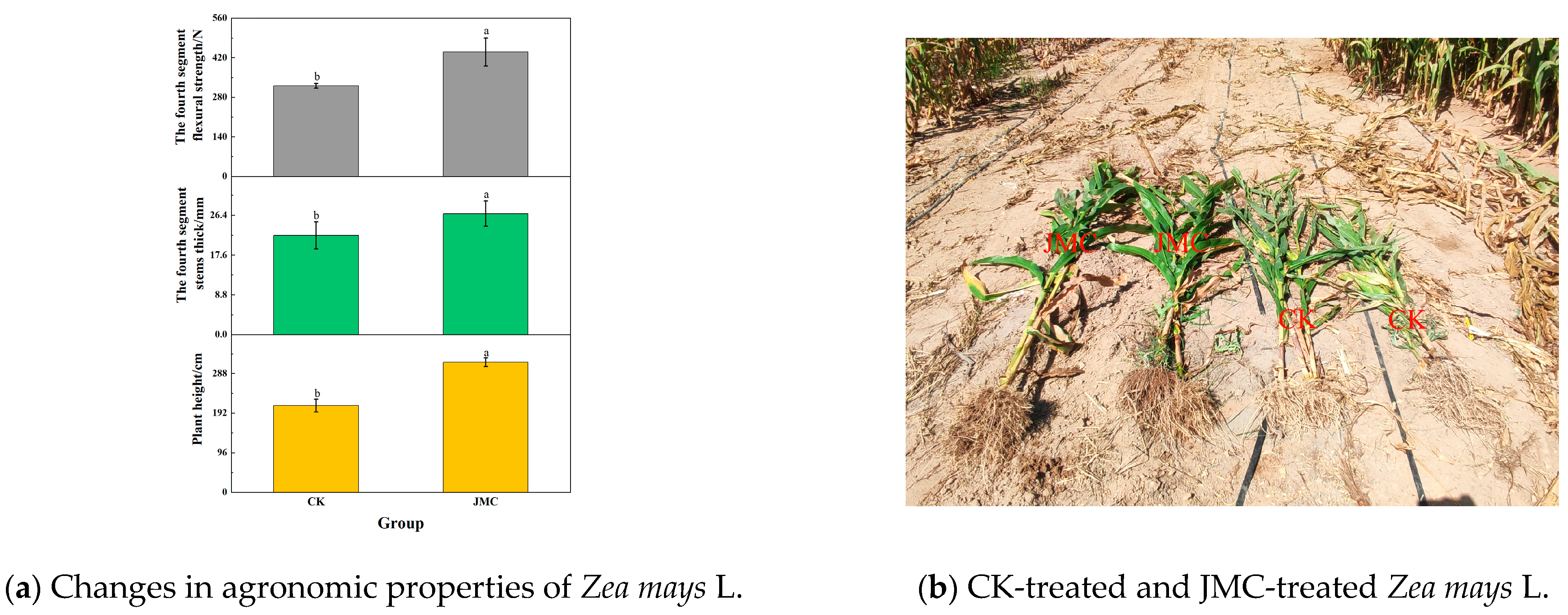
3.9. The Effect of Fly Ash Saline and Alkaline Soil Amendment Materials on Plant Agronomic Traits
3.10. Field Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Liu, H.; Gong, P.; Lin, E.; Bai, Z.; Li, P.; Wang, C.; Li, J. Multi-objective optimization of winter irrigation for cotton fields in salinized freeze-thaw areas. Eur. J. Agron. 2023, 143, 126715. [Google Scholar] [CrossRef]
- Negacz, K.; Malek, Z.; de Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Xia, J.; Ren, J.; Zhang, S.; Wang, Y.; Fang, Y. Forest and grass composite patterns improve the soil quality in the coastal saline-alkali land of the Yellow River Delta, China. Geoderma 2019, 349, 25–35. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rengel, Z. Environmental salinization processes: Detection, implications & solutions. Sci. Total Environ. 2021, 754, 142432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dai, S.; Finkelman, R.B.; French, D.; Graham, I.T.; Yang, Y.; Li, J.; Yang, P. Leaching behavior of trace elements from fly ashes of five Chinese coal power plants. Int. J. Coal Geol. 2020, 219, 103381. [Google Scholar] [CrossRef]
- Sakai, Y.; Shimizu, C.; Murata, H.; Seto, H.; Fukushima, R.; Koga, T.; Wang, C. Changes in Soil Physicochemical Properties and Maize Production Following Improvement of Salt-Affected Soils Using Coal Bio-Briquette Ash in Northeast China. Agronomy 2020, 10, 348. [Google Scholar] [CrossRef]
- Fan, Y.; Ge, T.; Zheng, Y.; Li, H.; Cheng, F. Use of mixed solid waste as a soil amendment for saline-sodic soil remediation and oat seedling growth improvement. Environ. Sci. Pollut. Res. 2016, 23, 21407–21415. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tashpolat, N. Current Status and Development Trend of Soil Salinity Monitoring Research in China. Sustainability 2023, 15, 5874. [Google Scholar] [CrossRef]
- Zhang, K.; Chang, L.; Li, G.; Li, Y. Advances and future research in ecological stoichiometry under saline-alkali stress. Environ. Sci. Pollut. Res. 2023, 30, 5475–5486. [Google Scholar] [CrossRef] [PubMed]
- Kaiwen, G.; Zisong, X.; Yuze, H.; Qi, S.; Yue, W.; Yanhui, C.; Jiechen, W.; Wei, L.; Huihui, Z. Effects of salt concentration, pH, and their interaction on plant growth, nutrient uptake, and photochemistry of alfalfa (Medicago sativa) leaves. Plant Signal. Behav. 2020, 15, 1832373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, K.; Qian, H.; Gao, Y.; Fang, Y.; Xiao, S.; Tang, S.; Zhang, Q.; Qu, W.; Ren, W. Characterization of soil salinization and its driving factors in a typical irrigation area of Northwest China. Sci. Total Environ. 2022, 837, 155808. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Liu, X. Reclamation effect of freezing saline water irrigation on heavy saline-alkali soil in the Hetao Irrigation District of North China. Catena 2021, 204, 105420. [Google Scholar] [CrossRef]
- Ors, S.; Sahin, U.; Khadra, R. Reclamation of Saline Sodic Soils with the Use of Mixed Fly Ash and Sewage Sludge. Arid Land Res. Manag. 2015, 29, 41–54. [Google Scholar] [CrossRef]
- Li, Y.; Li, G. Mechanisms of straw biochar’s improvement of phosphorus bioavailability in soda saline-alkali soil. Environ. Sci. Pollut. Res. 2022, 29, 47867–47872. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Hindawi, S.E.S.; Alghamdi, S.S.; Migdadi, H.H.; Khan, M.A.; Hasnain, M.U.; Arslan, M.; Habib Ur Rahman, M.; Sohaib, M. Potential Breeding Strategies for Improving Salt Tolerance in Crop Plants. J. Plant Growth Regul. 2023, 42, 3365–3387. [Google Scholar] [CrossRef]
- Wang, J.; Jing, H.; Xu, T.; Li, C.; Zhao, C.; Feng, W. Application Effect of Straw Returning and Biochar in the Improvement of Saline-Alkali Land in Northeast China. Biol. Bull. 2023, 50, 825–836. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Wang, X.; Shao, H.; Yang, J.; Wang, X. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar] [CrossRef]
- Jayaranjan, M.L.D.; van Hullebusch, E.D.; Annachhatre, A.P. Reuse options for coal fired power plant bottom ash and fly ash. Rev. Environ. Sci. Bio-Technol. 2014, 13, 467–486. [Google Scholar] [CrossRef]
- Marinina, O.; Nevskaya, M.; Jonek-Kowalska, I.; Wolniak, R.; Marinin, M. Recycling of Coal Fly Ash as an Example of an Efficient Circular Economy: A Stakeholder Approach. Energies 2021, 14, 3597. [Google Scholar] [CrossRef]
- Yin, C.; Zhao, J.; Liu, X.; Yu, Z.; Liu, H. Effect of Coal Water Slurry Gasification Slag on Soil Water Physical Characteristics and Properties in Saline-Alkali Soil Improvement. J. Sens. 2022, 2022, 1114343. [Google Scholar] [CrossRef]
- Cho, Y.K.; Jung, S.H.; Choi, Y.C. Effects of chemical composition of fly ash on compressive strength of fly ash cement mortar. Constr. Build. Mater. 2019, 204, 255–264. [Google Scholar] [CrossRef]
- Sun, L.; Luo, K.; Fan, J.; Lu, H. Experimental study of extracting alumina from coal fly ash using fluidized beds at high temperature. Fuel 2017, 199, 22–27. [Google Scholar] [CrossRef]
- Jambhulkar, H.P.; Shaikh, S.M.S.; Kumar, M.S. Fly ash toxicity, emerging issues and possible implications for its exploitation in agriculture; Indian scenario: A review. Chemosphere 2018, 213, 333–344. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Hooda, P.S.; Tsadilas, C.D. Opportunities and challenges in the use of coal fly ash for soil improvements—A review. J. Environ. Manag. 2014, 145, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, A.; Sasaoka, T.; Shimada, H.; Matsumoto, S. Amelioration of acidic soil using fly Ash for Mine Revegetation in Post-Mining Land. Int. J. Coal Sci. Technol. 2022, 9, 33. [Google Scholar] [CrossRef]
- Ou, Y.; Ma, S.; Zhou, X.; Wang, X.; Shi, J.; Zhang, Y. The Effect of a Fly Ash-Based Soil Conditioner on Corn and Wheat Yield and Risk Analysis of Heavy Metal Contamination. Sustainability 2020, 12, 7281. [Google Scholar] [CrossRef]
- Kim, D.; Kim, T.; Jeon, J.; Son, Y. Development of soil conditioner for reclaimed land desalinization based on high-iron fly ash. Paddy Water Environ. 2022, 20, 277–286. [Google Scholar] [CrossRef]
- Singh, R.P.; Gupta, A.K.; Ibrahim, M.H.; Mittal, A.K. Coal fly ash utilization in agriculture: Its potential benefits and risks. Rev. Environ. Sci. Bio-Technol. 2010, 9, 345–358. [Google Scholar] [CrossRef]
- Xu, J.-W.; Abbas, S.; Xiu, H.-F.; Ma, K.; Pan, Y.-T.; Lan, W.-K.-N.; Mao, Z.-S.; Liu, D. Effects of Different Materials on Desalting and Fertility of Coastal Saline Soil in Zhejiang Province, China. Water Air Soil Pollut. 2023, 234, 407. [Google Scholar] [CrossRef]
- Jia, G.; Wang, Y.; Yang, F. A Review on the Application of Circulating Fluidized Bed Fly Ash in Building Materials. Adv. Mater. Sci. Eng. 2022, 2022, 7099430. [Google Scholar] [CrossRef]
- Lu, X.; Liu, B.; Zhang, Q.; Wen, Q.; Wang, S.; Xiao, K.; Zhang, S. Recycling of Coal Fly Ash in Building Materials: A Review. Minerals 2023, 13, 25. [Google Scholar] [CrossRef]
- Vilakazi, A.Q.; Ndlovu, S.; Chipise, L.; Shemi, A. The Recycling of Coal Fly Ash: A Review on Sustainable Developments and Economic Considerations. Sustainability 2022, 14, 1958. [Google Scholar] [CrossRef]
- Hao, J.; Dou, Z.-H.; Zhang, T.A.; Wang, K.; Wan, X.-Y.; Qi, S. A clean and efficient utilization of fly ash with a focus on the strengthening decomposition mechanism of mullite. Fuel 2023, 333, 126473. [Google Scholar] [CrossRef]
- Su, H.; Lin, J.; Chen, H.; Wang, Q. Production of a novel slow-release coal fly ash microbial fertilizer for restoration of mine vegetation. Waste Manag. 2021, 124, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Anastopoulos, I.; Hamid, Y.; Wakeel, A. Recent trends in the use of fly ash for the adsorption of pollutants in contaminated wastewater and soils: Effects on soil quality and plant growth. Environ. Sci. Pollut. Res. 2022, 30, 124427–124446. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L. Aquatic contamination and ecological risk. An attempt to a conceptual framework. Water Res. 1984, 18, 1107–1108. [Google Scholar] [CrossRef]
- Liu, T.; Han, F.; Xing, Z.; Wang, J.; Dong, X.; An, C. Effects of different factors on fly ash-based functional soil and its oat grass cultivation. Front. Plant Sci. 2022, 13, 1048101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, M.; Na, K.; Hwang, J.; Shin, S.; Yin, L.; Deng, X.; Wang, S. The New Soil Conditioner DewEco Could Improve Sandy Soil’s Properties for Efficient Maize Growth. Agronomy 2022, 12, 1124. [Google Scholar] [CrossRef]
- Yang, P.; Zhai, X.; Huang, H.; Zhang, Y.; Zhu, Y.; Shi, X.; Zhou, L.; Fu, C. Association and driving factors of meteorological drought and agricultural drought in Ningxia, Northwest China. Atmos. Res. 2023, 289, 106753. [Google Scholar] [CrossRef]
- Foronda, D.A.; Colinet, G. Combined Application of Organic Amendments and Gypsum to Reclaim Saline-Alkali Soil. Agriculture 2022, 12, 1049. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, A.; Ma, F.; Liu, J.; Xiao, G.; Xu, X. Amendment of Saline-Alkaline Soil with Flue-Gas Desulfurization Gypsum in the Yinchuan Plain, Northwest China. Sustainability 2023, 15, 8658. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Wang, G.; Bi, X.; Sun, G.; Wu, X.; Wang, Q.; Li, Z. Concentrations, Speciation, and Potential Release of Hazardous Heavy Metals from the Solid Combustion Residues of Coal-Fired Power Plants. Int. J. Environ. Res. Public Health 2022, 19, 12617. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, Y.; Li, Y.; Gao, B.; Tang, Y.; Xie, J.; Zhao, H. Remediation of saline-sodic soil using organic and inorganic amendments: Physical, chemical, and enzyme activity properties. J. Soils Sediments 2020, 20, 1454–1467. [Google Scholar] [CrossRef]
- Wang, L.; Zeraatpisheh, M.; Wei, Z.; Xu, M. Heavy metal pollution and risk assessment of farmland soil around abandoned domestic waste dump in Kaifeng City. Front. Environ. Sci. 2022, 10, 946298. [Google Scholar] [CrossRef]
- Chen, R.; Cai, X.; Ding, G.; Ren, F.; Wang, Q.; Cheng, N.; Liu, J.; Li, L.; Shi, R. Ecological risk assessment of heavy metals in farmland soils in Beijing by three improved risk assessment methods. Environ. Sci. Pollut. Res. 2021, 28, 57970–57982. [Google Scholar] [CrossRef] [PubMed]
- Koc, D.L.; Kanber, R. Change of Salinity and Sodicity Criteria of Saline-Sodic Helvaci Series Soils. Ksu Tarim Ve Doga Derg.-Ksu J. Agric. Nat. 2020, 23, 1064–1077. [Google Scholar] [CrossRef]
- Lakhdar, A.; Rabhi, M.; Ghnaya, T.; Montemurro, F.; Jedidi, N.; Abdelly, C. Effectiveness of compost use in salt-affected soil. J. Hazard. Mater. 2009, 171, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, B.; Siri, M.; Liu, C.; Feng, C.; Shao, X.; Liu, K. Calcium-modified biochar rather than original biochar decreases salinization indexes of saline-alkaline soil. Environ. Sci. Pollut. Res. 2023, 30, 74966–74976. [Google Scholar] [CrossRef]
- Zuo, W.; Xu, L.; Qiu, M.; Yi, S.; Wang, Y.; Shen, C.; Zhao, Y.; Li, Y.; Gu, C.; Shan, Y.; et al. Effects of Different Exogenous Organic Materials on Improving Soil Fertility in Coastal Saline-Alkali Soil. Agronomy 2023, 13, 61. [Google Scholar] [CrossRef]
- Li, K.; Li, Q.; Liu, C. Effect of freezing temperature and water content on pore structure characteristics of coastal saline-alkali soil under frost heave. J. Soils Sediments 2022, 22, 1819–1827. [Google Scholar] [CrossRef]
- An, X.; Liu, Q.; Pan, F.; Yao, Y.; Luo, X.; Chen, C.; Liu, T.; Zou, L.; Wang, W.; Wang, J.; et al. Research Advances in the Impacts of Biochar on the Physicochemical Properties and Microbial Communities of Saline Soils. Sustainability 2023, 15, 14439. [Google Scholar] [CrossRef]
- Che, W.; Piao, J.; Gao, Q.; Li, X.; Li, X.; Jin, F. Response of soil physicochemical properties, soil nutrients, enzyme activity and rice yield to rice straw returning in highly saline-alkali paddy soils. J. Soil Sci. Plant Nutr. 2023, 23, 4396–4411. [Google Scholar] [CrossRef]
- Bai, Y.; Tao, T.; Gu, C.; Wang, L.; Feng, K.; Shan, Y. Mudflat soil amendment by sewage sludge: Soil physicochemical properties, perennial ryegrass growth, and metal uptake. Soil Sci. Plant Nutr. 2013, 59, 942–952. [Google Scholar] [CrossRef]
- Cooper, J.A.; Drijber, R.A.; Malakar, A.; Jin, V.L.; Miller, D.N.; Kaiser, M. Evaluating coal char as an alternative to biochar for mitigating nutrient and carbon loss from manure-amended soils: Insights from a greenhouse experiment. J. Environ. Qual. 2022, 51, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Mtolera, I.; She, D.; Ma, T.; Chen, K.; Yu, S.E. Effects of Saline Water Irrigation and Biochar Amendment on Okra Growth and Nutrient Leaching in Coastal Saline Soils. Commun. Soil Sci. Plant Anal. 2021, 52, 651–665. [Google Scholar] [CrossRef]
- Xiao, M.; Liu, G.; Jiang, S.; Guan, X.; Chen, J.; Yao, R.; Wang, X. Bio-Organic Fertilizer Combined with Different Amendments Improves Nutrient Enhancement and Salt Leaching in Saline Soil: A Soil Column Experiment. Water 2022, 14, 4084. [Google Scholar] [CrossRef]
- Sastre-Conde, I.; Carmen Lobo, M.; Icela Beltran-Hernandez, R.; Poggi-Varaldo, H.M. Remediation of saline soils by a two-step process: Washing and amendment with sludge. Geoderma 2015, 247, 140–150. [Google Scholar] [CrossRef]
- Niamat, B.; Naveed, M.; Ahmad, Z.; Yaseen, M.; Ditta, A.; Mustafa, A.; Rafique, M.; Bibi, R.; Sun, N.; Xu, M. Calcium-Enriched Animal Manure Alleviates the Adverse Effects of Salt Stress on Growth, Physiology and Nutrients Homeostasis of Zea mays L. Plants 2019, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Bello, S.K.; Alayafi, A.H.; Al-Solaimani, S.G.; Abo-Elyousr, K.A.M. Mitigating Soil Salinity Stress with Gypsum and Bio-Organic Amendments: A Review. Agronomy 2021, 11, 1735. [Google Scholar] [CrossRef]
- Bettani, S.R.; Ragazzo, G.d.O.; Santos, N.L.; Kieckbusch, T.G.; Bastos, R.G.; Soares, M.R.; da Silva, M.A. Sugarcane vinasse and microalgal biomass in the production of pectin particles as an alternative soil fertilizer. Carbohydr. Polym. 2019, 203, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Ghiberto, P.; Imhoff, S.; Genero, F.; Heymo, A. Soil chemical changes and nutrient leaching losses following application of a farm-dairy effluent. Agrochimica 2020, 64, 331–346. [Google Scholar] [CrossRef]
- Jordan, M.M.; Almendro-Candel, M.B.; Navarro-Pedreno, J.; Pardo, F.; Garcia-Sanchez, E.; Bech, J. Bioavailability, mobility and leaching of phosphorus in a Mediterranean agricultural soil (ne Spain) amended with different doses of biosolids. Environ. Geochem. Health 2022, 44, 7–14. [Google Scholar] [CrossRef]
- Li, J.; Gao, J.-M.; Guo, Y.; Cheng, F. Energy-efficient leaching process for preparation of aluminum sulfate and synergistic extraction of Li and Ga from circulating fluidized bed fly ash. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 4398–4410. [Google Scholar] [CrossRef]
- Ukwattage, N.L.; Ranjith, P.G.; Bouazza, M. The use of coal combustion fly ash as a soil amendment in agricultural lands (with comments on its potential to improve food security and sequester carbon). Fuel 2013, 109, 400–408. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Gupta, M.; Shikha; Singh, N.; Tewari, S.K. Amelioration of sodic soil for wheat cultivation using bioaugmented organic soil amendment. Land Degrad. Dev. 2016, 27, 1245–1254. [Google Scholar] [CrossRef]
- Yunusa, I.A.M.; Manoharan, V.; Odeh, I.O.A.; Shrestha, S.; Skilbeck, C.G.; Eamus, D. Structural and hydrological alterations of soil due to addition of coal fly ash. J. Soils Sediments 2011, 11, 423–431. [Google Scholar] [CrossRef]
- Chengfeng, Z.; Qiang, Y.; Junming, S. Characteristics of particulate matter from emissions of four typical coal-fired power plants in China. Fuel Process. Technol. 2005, 86, 757–768. [Google Scholar] [CrossRef]
- Talebnejad, R.; Sepaskhah, A.R. Modification of transient state analytical model under different saline groundwater depths, irrigation water salinities and deficit irrigation for quinoa. Int. J. Plant Prod. 2016, 10, 365–389. [Google Scholar]
- Dai, L.; Yuan, Y.; Guo, X.; Du, Y.; Ke, X.; Zhang, F.; Li, Y.; Li, Q.; Lin, L.; Zhou, H.; et al. Soil water retention in alpine meadows under different degradation stages on the northeastern Qinghai-Tibet Plateau. J. Hydrol. 2020, 590, 125397. [Google Scholar] [CrossRef]
- Garg, R.N.; Pathak, H.; Das, D.K.; Tomar, R.K. Use of flyash and biogas slurry for improving wheat yield and physical properties of soil. Environ. Monit. Assess. 2005, 107, 1–9. [Google Scholar] [CrossRef]
- Shakeel, A.; Khan, A.A.; Ahmad, G. The potential of thermal power plant fly ash to promote the growth of Indian mustard (Brassica juncea) in agricultural soils. SN Appl. Sci. 2019, 1, 375. [Google Scholar] [CrossRef]
- Shakeel, A.; Bhat, A.H.; Bhat, A.A.; Khan, A.A. Interactive effect of Meloidogyne incognita and fly ash on the growth, physiology, and antioxidant properties of carrot (Daucus carota L.). Environ. Sci. Pollut. Res. 2022, 29, 7661–7677. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Rai, U.N.; Tripathi, R.D.; Inouhe, M. Impacts of fly-ash on soil and plant responses. J. Plant Res. 2002, 115, 401–409. [Google Scholar] [CrossRef]
- Ahmad, G.; Khan, A.A.; Mohamed, H.I. Impact of the low and high concentrations of fly ash amended soil on growth, physiological response, and yield of pumpkin (Cucurbita moschata Duch. Ex Poiret L.). Environ. Sci. Pollut. Res. 2021, 28, 17068–17083. [Google Scholar] [CrossRef] [PubMed]







| Properties | pH | EC/(μs·cm−1) | Ca% | Mg% | Si% | P% | K% |
|---|---|---|---|---|---|---|---|
| Content | 8.9 | 900.00 | 2.77 | 0.41 | 0.36 | 0.35 | 0.35 |
| Properties | Cu (mg·kg−1) | Ni (mg·kg−1) | Cr (mg·kg−1) | Cd (mg·kg−1) | Pb (mg·kg−1) |
|---|---|---|---|---|---|
| Content | 42.00 | 28.00 | 48.00 | 0.50 | 63.00 |
| Properties | Organic Matter /% | Available Phosphorus /(mg·kg−1) | Available Potassium /(mg·kg−1) | Exchangeable Sodium /(cmol·kg−1) | Exchangeable Calcium /(cmol·kg−1) |
|---|---|---|---|---|---|
| Content | 7.10 | 6.74 | 173.00 | 1.32 | 1.03 |
| Properties | pH | EC /(ms·cm−1) | Bulk Density /(g·cm−3) | Total Soil Porosity /% | Sodium Ion /(cmol·kg−1) | Calcium Ion /(cmol·kg−1) |
|---|---|---|---|---|---|---|
| Content | 10.51 | 2.31 | 1.49 | 40.29 | 2.93 | 0.53 |
| Hazard Level | Eri | RI |
|---|---|---|
| Low ecological hazards I | Eri < 40 | RI < 150 |
| Medium ecological hazards II | 40 ≤ Eri < 80 | 150 ≤ RI < 300 |
| High ecological hazards III | 80 ≤ Eri < 160 | 300 ≤ RI < 600 |
| High ecological hazards IV | 160 ≤ Eri < 320 | |
| Extremely high ecological hazard V | Eri ≥ 320 | RI ≥ 600 |
| pH | Cu/(μg·L−1) | Pb/(μg·L−1) | Ni/(μg·L−1) | Cd/(μg·L−1) | Cr/(μg·L−1) |
|---|---|---|---|---|---|
| 8.5 | 6.56 ± 0.014 c | <0.09 | 6.27 ± 0.007 d | 0.21 ± 0.014 a | 0.40 ± 0.007 b |
| 9.3 | 6.25 ± 0.021 d | <0.09 | 6.42 ± 0.085 c | 0.18 ± 0.014 a | 0.45 ± 0.000 a |
| 10.3 | 7.15 ± 0.028 b | <0.09 | 6.60 ± 0.050 b | 0.20 ± 0.000 a | 0.45 ± 0.014 a |
| 11.3 | 8.67 ± 0.000 a | <0.09 | 6.80 ± 0.042 a | 0.20 ± 0.007 a | 0.44 ± 0.026 a |
| pH | Metals | Csi | Cni | Cfi | Tri | Eri | RI |
|---|---|---|---|---|---|---|---|
| 8.5 | Cu | 6.56 | 20.9 | 0.3139 | 5 | 1.5694 | 2.0568 |
| Cr | 0.4 | 61.3 | 0.0065 | 2 | 0.0131 | ||
| Cd | 0.21 | 92 | 0.0023 | 20 | 0.0457 | ||
| Ni | 6.26 | 29.2 | 0.2144 | 2 | 0.4288 | ||
| 9.3 | Cu | 6.24 | 20.9 | 0.2986 | 5 | 1.4928 | 1.9864 |
| Cr | 0.45 | 61.3 | 0.0073 | 2 | 0.0147 | ||
| Cd | 0.18 | 92 | 0.0020 | 20 | 0.0391 | ||
| Ni | 6.42 | 29.2 | 0.2199 | 2 | 0.4397 | ||
| 10.3 | Cu | 7.15 | 20.9 | 0.3421 | 5 | 1.7105 | 2.2207 |
| Cr | 0.45 | 61.3 | 0.0073 | 2 | 0.0147 | ||
| Cd | 0.2 | 92 | 0.0022 | 20 | 0.0435 | ||
| Ni | 6.6 | 29.2 | 0.2260 | 2 | 0.4521 | ||
| 11.3 | Cu | 8.67 | 20.9 | 0.4148 | 5 | 2.0742 | 2.5977 |
| Cr | 0.44 | 61.3 | 0.0072 | 2 | 0.0144 | ||
| Cd | 0.2 | 92 | 0.0022 | 20 | 0.0435 | ||
| Ni | 6.8 | 29.2 | 0.2329 | 2 | 0.4658 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, C.; Han, F.; Li, N.; Zheng, J.; Li, M.; Liu, Y.; Liu, H. Improving Physical and Chemical Properties of Saline Soils with Fly Ash Saline and Alkaline Amendment Materials. Sustainability 2024, 16, 3216. https://doi.org/10.3390/su16083216
An C, Han F, Li N, Zheng J, Li M, Liu Y, Liu H. Improving Physical and Chemical Properties of Saline Soils with Fly Ash Saline and Alkaline Amendment Materials. Sustainability. 2024; 16(8):3216. https://doi.org/10.3390/su16083216
Chicago/Turabian StyleAn, Changcong, Fenglan Han, Ning Li, Jintao Zheng, Maohui Li, Yanan Liu, and Haipeng Liu. 2024. "Improving Physical and Chemical Properties of Saline Soils with Fly Ash Saline and Alkaline Amendment Materials" Sustainability 16, no. 8: 3216. https://doi.org/10.3390/su16083216





