Effects of Land-Use Type and Salinity on Soil Carbon Mineralization in Coastal Areas of Northern Jiangsu Province
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.2. Laboratory Incubation
2.3. Gas Sampling and Analysis
2.4. Soil Physical and Chemical Properties
2.5. Soil Microbial Biomass and Extracellular Enzyme Activity
2.6. Calculations
2.6.1. CO2 Emission Rate and Cumulative Release
2.6.2. First-Order Kinetic Model for Soil C Mineralization
2.6.3. Microbial Respiratory Quotient (qCO2)
2.6.4. Enzyme Stoichiometry
2.7. Data Analysis
3. Results
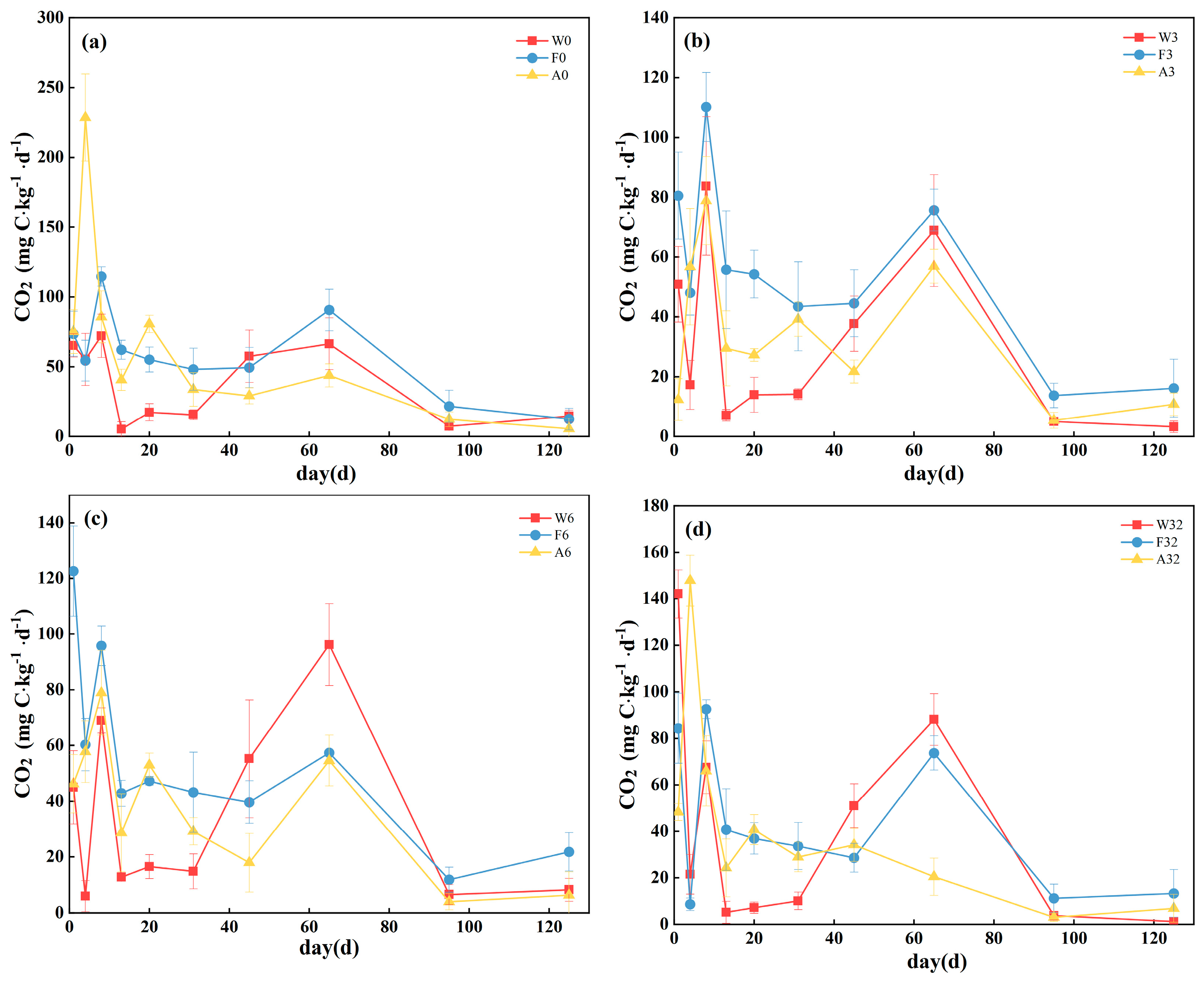
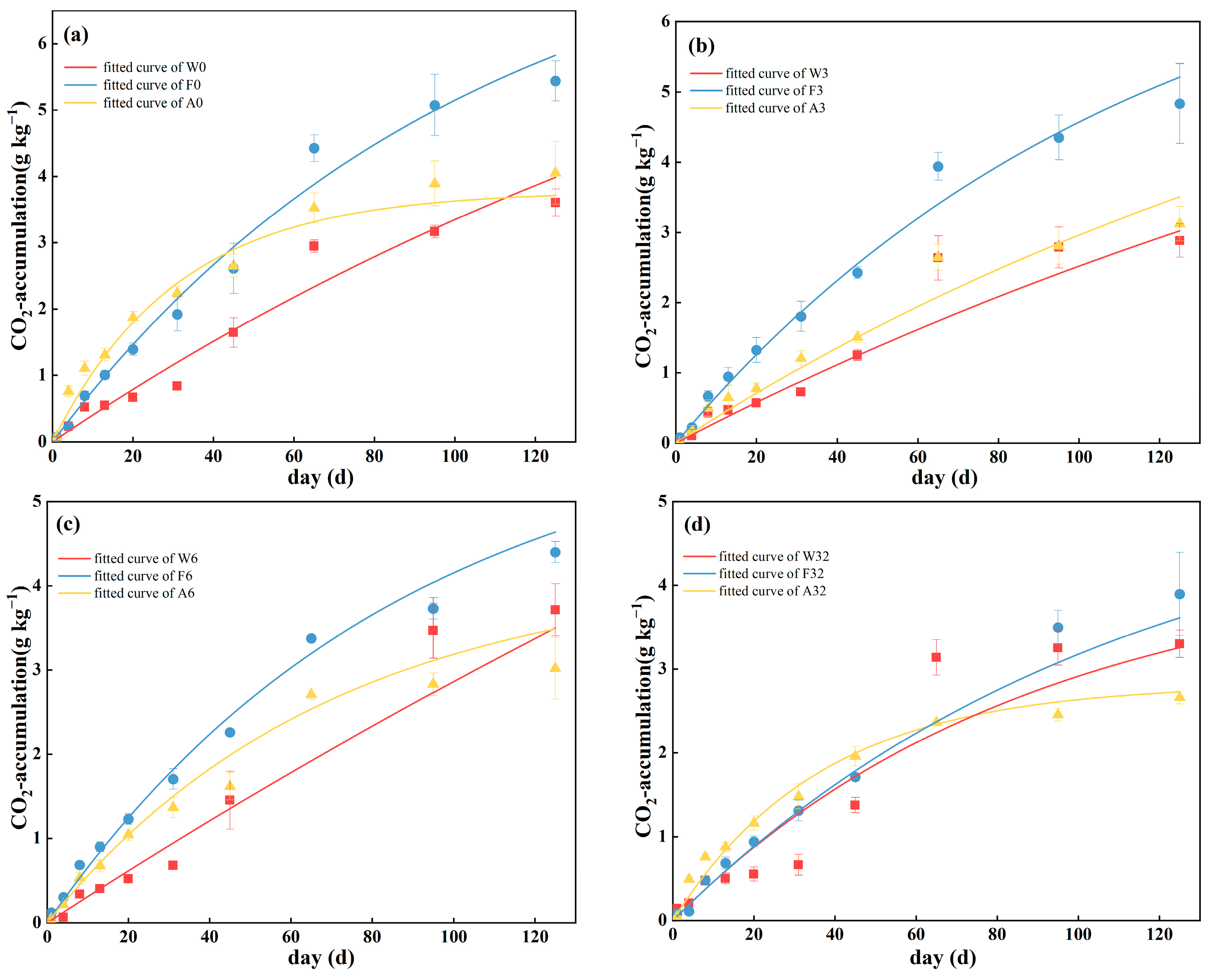
3.1. Mineralization Rate and First-Order Kinetic Model of Cumulative Mineralization
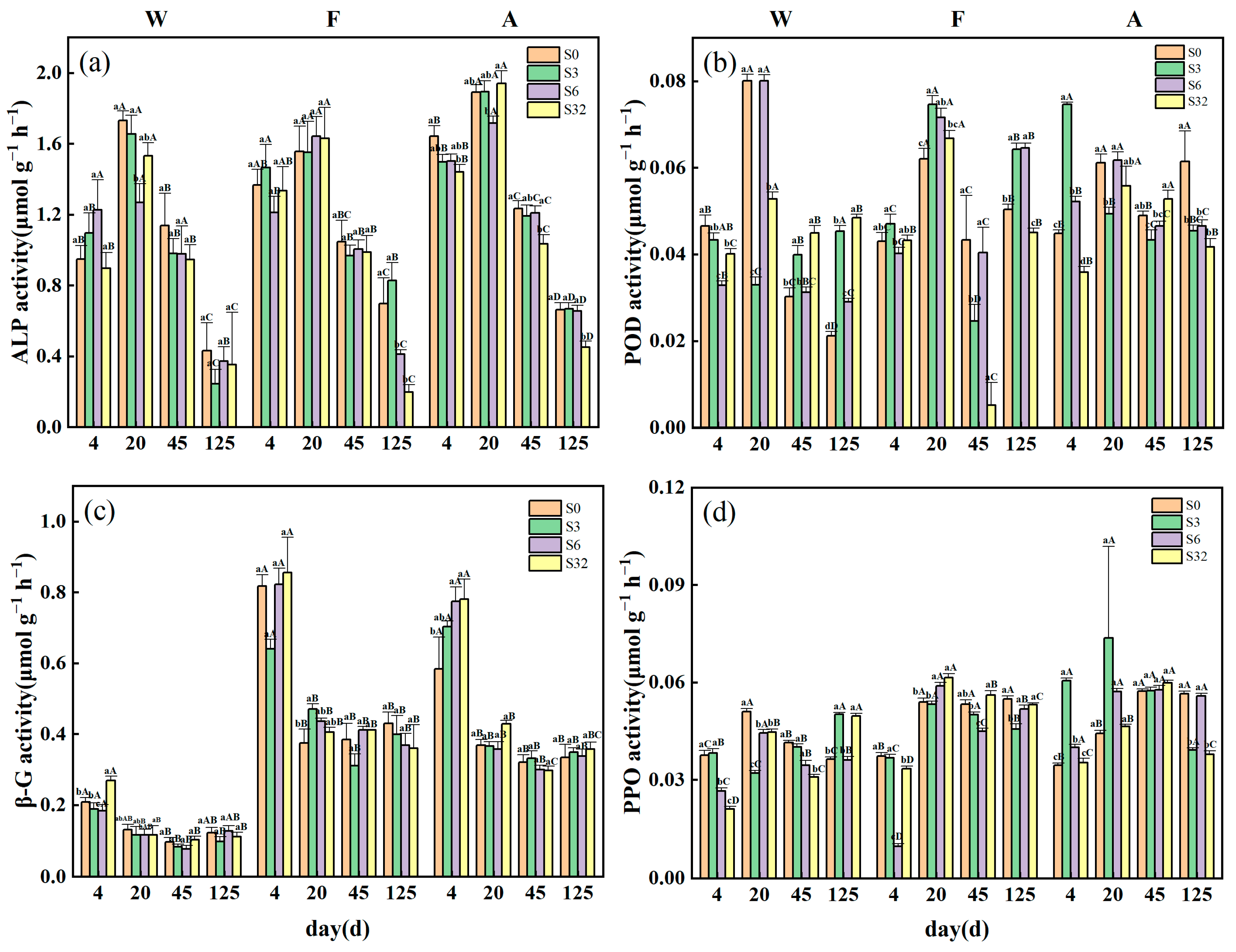
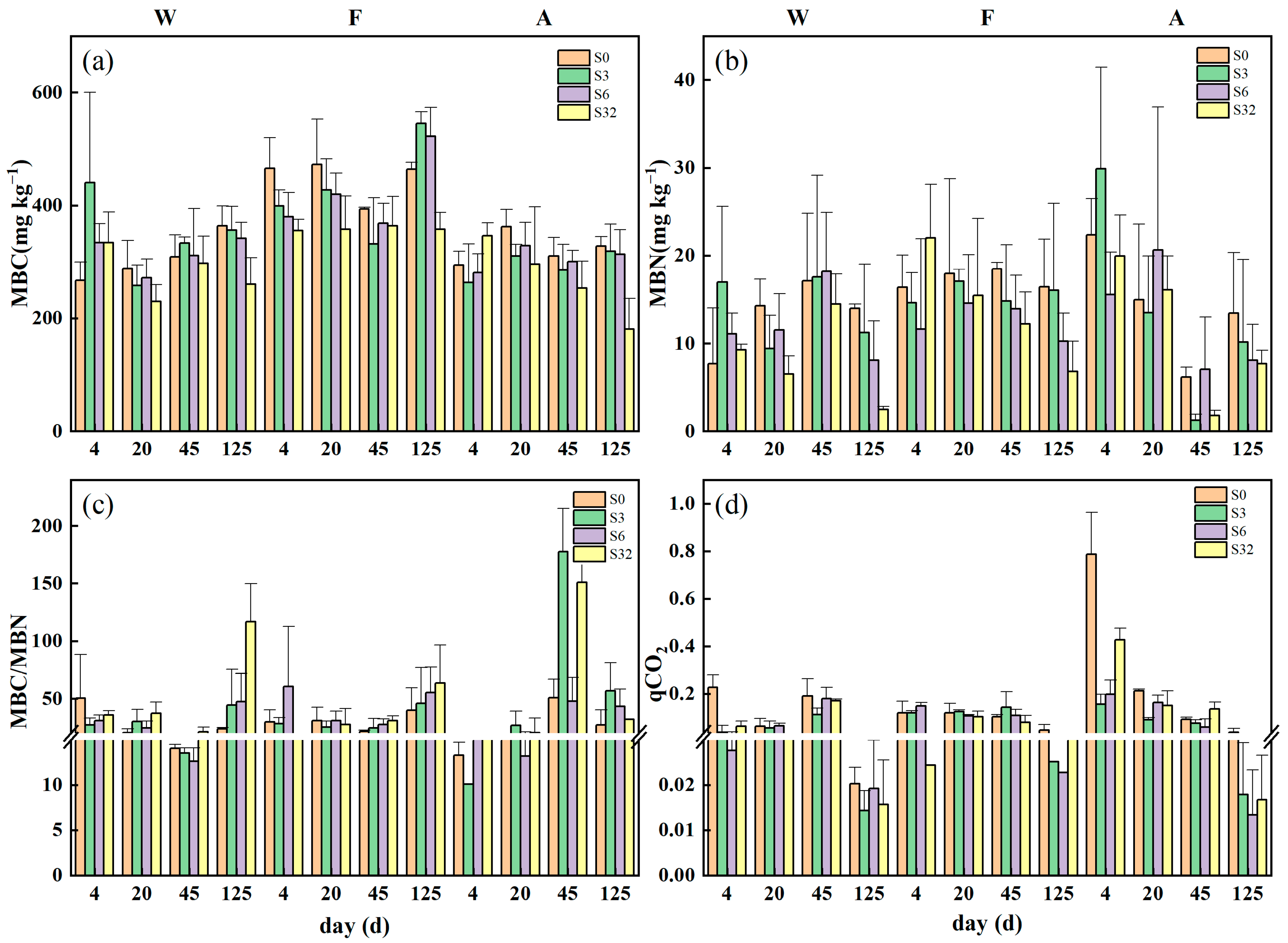
3.2. Changes in Soil Extracellular Enzyme Activities, Microbial Biomass, and qCO2 during Incubation
3.3. Relationships between Soil C Mineralization and Soil Physical and Chemical Properties, Extracellular Enzymes, and Microbial Biomass in Different Land-Use Types
3.4. Possible Pathways Driving Soil C Mineralization
4. Discussion
4.1. Effect of Salinity on Soil C Mineralization
4.2. Differences in Factors Influencing Soil C Mineralization for Different Land-Use Types
4.3. Factors Influencing Soil C Mineralization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, J.; Song, Y.; Zhu, B. Ecosystem-dependent responses of soil carbon storage to phosphorus enrichment. New Phytol. 2023, 238, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Elbasiouny, H.; El-Ramady, H.; Elbehiry, F.; Rajput, V.D.; Minkina, T.; Mandzhieva, S. Plant Nutrition under Climate Change and Soil Carbon Sequestration. Sustainability 2022, 14, 914. [Google Scholar] [CrossRef]
- Yang, M.; Hou, Z.; Guo, N.; Yang, E.; Sun, D.; Fang, Y. Effects of enhanced-efficiency nitrogen fertilizers on CH4 and CO2 emissions in a global perspective. Field Crops Res. 2022, 288, 108694. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Bossio, D.A.; Cook-Patton, S.C.; Ellis, P.W.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Zomer, R.J.; von Unger, M.; Emmer, I.M.; et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Yu, H.; Sui, Y.; Chen, Y.; Bao, T.; Jiao, X. Soil Organic Carbon Mineralization and Its Temperature Sensitivity under Different Substrate Levels in the Mollisols of Northeast China. Life 2022, 12, 712. [Google Scholar] [CrossRef] [PubMed]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; et al. Changes in Atmospheric Constituents and in Radiative Forcing. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the 4th Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Change Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef]
- Servais, S.; Kominoski, J.S.; Charles, S.P.; Gaiser, E.E.; Mazzei, V.; Troxler, T.G.; Wilson, B.J. Saltwater intrusion and soil carbon loss: Testing effects of salinity and phosphorus loading on microbial functions in experimental freshwater wetlands. Geoderma 2019, 337, 1291–1300. [Google Scholar] [CrossRef]
- Liáng, L.L.; Kirschbaum, M.U.F.; Arcus, V.L.; Schipper, L.A. The carbon-quality temperature hypothesis: Fact or artefact? Glob. Change Biol. 2023, 29, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Smith, P.; Moncrieff, J.B.; Smith, J.U. Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature 2005, 433, 57–59. [Google Scholar] [CrossRef]
- Chen, H.; Zou, J.; Cui, J.; Nie, M.; Fang, C. Wetland drying increases the temperature sensitivity of soil respiration. Soil Biol. Biochem. 2018, 120, 24–27. [Google Scholar] [CrossRef]
- Reynolds, L.L.; Lajtha, K.; Bowden, R.D.; Johnson, B.R.; Bridgham, S.D. The carbon quality-temperature hypothesis does not consistently predict temperature sensitivity of soil organic matter mineralization in soils from two manipulative ecosystem experiments. Biogeochemistry 2017, 136, 249–260. [Google Scholar] [CrossRef]
- Chapman, S.K.; Hayes, M.A.; Kelly, B.; Langley, J.A. Exploring the oxygen sensitivity of wetland soil carbon mineralization. Biol. Lett. 2019, 15, 20180407. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Meng, J.; Lan, Y.; Chen, W.; Yang, T.; Yuan, J.; Liu, S.; Han, J. Effects of maize stover and its biochar on soil CO2 emissions and labile organic carbon fractions in Northeast China. Agric. Ecosyst. Environ. 2017, 240, 24–31. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, C.; Wei, L.; Wang, S.; Deng, Y.; Ling, W.; Xiang, W.; Kuzyakov, Y.; Zhu, Z.; Ge, T. Microbial mechanisms of organic matter mineralization induced by straw in biochar-amended paddy soil. Biochar 2024, 6, 1–13. [Google Scholar] [CrossRef]
- Morrissey, E.M.; Berrier, D.J.; Neubauer, S.C.; Franklin, R.B. Using microbial communities and extracellular enzymes to link soil organic matter characteristics to greenhouse gas production in a tidal freshwater wetland. Biogeochemistry 2014, 117, 473–490. [Google Scholar] [CrossRef]
- Soares, M.; Rousk, J. Microbial growth and carbon use efficiency in soil: Links to fungal-bacterial dominance, SOC-quality and stoichiometry. Soil Biol. Biochem. 2019, 131, 195–205. [Google Scholar] [CrossRef]
- Feng, S.; Huang, Y.; Ge, Y.; Su, Y.; Xu, X.; Wang, Y.; He, X. Variations in the patterns of soil organic carbon mineralization and microbial communities in response to exogenous application of rice straw and calcium carbonate. Sci. Total Environ. 2016, 571, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lv, D.; Jiang, S.; Lin, H.; Sun, J.; Li, K.; Sun, J. Soil salinity regulation of soil microbial carbon metabolic function in the Yellow River Delta, China. Sci. Total Environ. 2021, 790, 148258. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.S. Enzymic analysis of microbial pattern and process. Biol. Fertil. Soils 1994, 17, 69–74. [Google Scholar] [CrossRef]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Morrison, E.; Newman, S.; Bae, H.S.; He, Z.; Zhou, J.; Reddy, K.R.; Ogram, A. Microbial genetic and enzymatic responses to an anthropogenic phosphorus gradient within a subtropical peatland. Geoderma 2016, 268, 119–127. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, S.; Wang, X.; Xu, X.; Ai, C.; Liang, G.; Zhu, P.; Zhou, W. Enzymatic stoichiometry reveals phosphorus limitation-induced changes in the soil bacterial communities and element cycling: Evidence from a long-term field experiment. Geoderma 2022, 426, 116124. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Konvalina, P.; Moudry, J.; Kopecky, M.; Hoang, T.N. Carbon Pool Dynamic and Soil Microbial Respiration Affected by Land Use Alteration: A Case Study in Humid Subtropical Area. Land 2023, 12, 459. [Google Scholar] [CrossRef]
- Barto, E.K.; Alt, F.; Oelmann, Y.; Wilcke, W.; Rillig, M.C. Contributions of biotic and abiotic factors to soil aggregation across a land use gradient. Soil Biol. Biochem. 2010, 42, 2316–2324. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Tan, S.; Wu, X.; Song, X.; Gao, H.; Han, Z.; Jia, A.; Liang, G.; Li, S. Evaluation of carbon mineralization and its temperature sensitivity in different soil aggregates and moisture regimes: A 21-year tillage experiment. Sci. Total Environ. 2022, 837, 155566. [Google Scholar] [CrossRef] [PubMed]
- Mongil-Manso, J.; Navarro-Hevia, J.; San Martín, R. Impact of Land Use Change and Afforestation on Soil Properties in a Mediterranean Mountain Area of Central Spain. Land 2022, 11, 1043. [Google Scholar] [CrossRef]
- Kara, O.; Baykara, M. Changes in soil microbial biomass and aggregate stability under different land uses in the northeastern Turkey. Environ. Monit. Assess. 2014, 186, 3801–3808. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Dube, F.; Zagal, E.; Stolpe, N.; Espinosa, M. The influence of land-use change on the organic carbon distribution and microbial respiration in a volcanic soil of the Chilean Patagonia. For. Ecol. Manag. 2009, 257, 1695–1704. [Google Scholar] [CrossRef]
- Peng, L.; Tang, C.; Zhang, X.; Duan, J.; Yang, L.; Liu, S. Quantifying the Effects of Root and Soil Properties on Soil Detachment Capacity in Agricultural Land Use of Southern China. Forests 2022, 13, 1788. [Google Scholar] [CrossRef]
- Yangjian, Z.; Juntao, Z.; Ruonan, S.; Li, W. Research progress on the effects of grazing on grassland ecosystem. Chin. J. Plant Ecol. 2020, 44, 553–564. [Google Scholar]
- Wang, B.; Liu, J.; Li, Z.; Morreale, S.J.; Schneider, R.L.; Xu, D.; Lin, X. The contributions of root morphological characteristics and soil property to soil infiltration in a reseeded desert steppe. Catena 2023, 225, 107020. [Google Scholar] [CrossRef]
- Neubauer, S.C.; Franklin, R.B.; Berrier, D.J. Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeosciences 2013, 10, 8171–8183. [Google Scholar] [CrossRef]
- Rath, K.M.; Rousk, J. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: A review. Soil Biol. Biochem. 2015, 81, 108–123. [Google Scholar] [CrossRef]
- Kempf, B.; Bremer, E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Mary, B.; Valé, M.; Justes, E. The sensitivity of C and N mineralization to soil water potential varies with soil characteristics: Experimental evidences to fine-tune models. Geoderma 2022, 409, 115644. [Google Scholar] [CrossRef]
- Xu, C.; Ruan, H.; Wu, X.; Xie, Y.; Yang, Y. Progress in drought stress on the accumulation and turnover of soil organic carbon in forests. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2022, 46, 195–206. [Google Scholar]
- Yang, J.; Zhan, C.; Li, Y.; Zhou, D.; Yu, Y.; Yu, J. Effect of salinity on soil respiration in relation to dissolved organic carbon and microbial characteristics of a wetland in the Liaohe River estuary, Northeast China. Sci. Total Environ. 2018, 642, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Kittredge, H.A.; Cannone, T.; Funk, J.; Chapman, S.K. Soil respiration and extracellular enzyme production respond differently across seasons to elevated temperatures. Plant Soil 2018, 425, 351–361. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Jia, J.; Wang, W.; Wang, D.; Zhao, Q.; Wang, C.; Chen, G. Soil microbial communities regulate the threshold effect of salinity stress on SOM decomposition in coastal salt marshes. Fundam. Res. 2023, 3, 868–879. [Google Scholar] [CrossRef]
- Ding, J.; Chen, L.; Zhang, B.; Liu, L.; Yang, G.; Fang, K.; Chen, Y.; Li, F.; Kou, D.; Ji, C.; et al. Linking temperature sensitivity of soil CO2 release to substrate, environmental, and microbial properties across alpine ecosystems: Microbial Decomposition in Alpine Soils. Glob. Biogeochem. Cycles 2016, 30, 1310–1323. [Google Scholar] [CrossRef]
- Chandrajith, R.; Chaturangani, D.; Abeykoon, S.; Barth, J.A.C.; Van Geldern, R.; Edirisinghe, E.A.N.V.; Dissanayake, C.B. Quantification of groundwater–seawater interaction in a coastal sandy aquifer system: A study from Panama, Sri Lanka. Environ. Earth Sci. 2013, 72, 867–877. [Google Scholar] [CrossRef]
- Wilmoth, J.L.; Schaefer, J.K.; Schlesinger, D.R.; Roth, S.W.; Hatcher, P.G.; Shoemaker, J.K.; Zhang, X. The role of oxygen in stimulating methane production in wetlands. Glob. Chang. Biol. 2021, 27, 5831–5847. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Willett, V. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- WU, J.; Joergensen, R.G.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C by fumigation-extraction—An automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEN value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Allison, S.D.; Chacon, S.S.; German, D.P. Substrate concentration constraints on microbial decomposition. Soil Biol. Biochem. 2014, 79, 43–49. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.; Sun, J.; Wang, X.; Zhou, W. Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 2012, 173–174, 330–338. [Google Scholar] [CrossRef]
- Yu, J.; Miao, S.; Qiao, Y.; Li, T.; Wang, X.; Zhao, Y. Effects of straw addition on organic carbon mineralization in different types of soils. J. Agro-Environ. Sci. 2024, 1–12. Available online: https://kns.cnki.net/kcms2/article/abstract?v=9CXCstbk-tsjb7FiwltxYDVFxafFzMaWBWenZO15U1RfeA2XEpSkhyL5smzbGbSwamEvEJsHarDbeiSveNVwIBOU_yhy-NYAN6sYLB0vr3ptmZmR7-mQWQ==&uniplatform=NZKPT&language=gb (accessed on 26 February 2024).
- Wang, S.; Tang, J.; Li, Z.; Liu, Y.; Zhou, Z.; Wang, J.; Qu, Y.; Dai, Z. Carbon Mineralization under Different Saline—Alkali Stress Conditions in Paddy Fields of Northeast China. Sustainability 2020, 12, 2921. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as ph, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Tapia-Torres, Y.; Elser, J.J.; Souza, V.; García-Oliva, F. Ecoenzymatic stoichiometry at the extremes: How microbes cope in an ultra-oligotrophic desert soil. Soil Biol. Biochem. 2015, 87, 34–42. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Li, Q.; Song, Z.; Xia, S.; Guo, L.; Singh, B.P.; Shi, Y.; Wang, W.; Luo, Y.; Li, Y.; Chen, J.; et al. Substrate quality overrides soil salinity in mediating microbial respiration in coastal wetlands. Land Degrad. Dev. 2023, 34, 4546–4560. [Google Scholar] [CrossRef]
- Cui, Y.; Moorhead, D.L.; Wang, X.; Xu, M.; Wang, X.; Wei, X.; Zhu, Z.; Ge, T.; Peng, S.; Zhu, B.; et al. Decreasing microbial phosphorus limitation increases soil carbon release. Geoderma 2022, 419, 115868. [Google Scholar] [CrossRef]
- Moreno-de las Heras, M. Development of soil physical structure and biological functionality in mining spoils affected by soil erosion in a Mediterranean-Continental environment. Geoderma 2009, 149, 249–256. [Google Scholar] [CrossRef]
- Liu, Y. Effects of Salt on the Temperature Sensitivity of Soil Organic Carbon to Anaerobic Mineralization in Wetland of the Yellow River Estuary. Master’s Thesis, Ludong University, Yantai, China, 2021. [Google Scholar]
- Yang, S. Temperature Sensitivity of Soil Organic Carbon Mineralization in Temperate Forests in Northeast China. Ph.D. Dissertation, Shenyang Agricultural University, Shenyang, China, 2019. [Google Scholar]
- Yan, N.; Marschner, P. Response of microbial activity and biomass to increasing salinity depends on the final salinity, not the original salinity. Soil Biol. Biochem. 2012, 53, 50–55. [Google Scholar] [CrossRef]
- Rath, K.M.; Fierer, N.; Murphy, D.V.; Rousk, J. Linking bacterial community composition to soil salinity along environmental gradients. ISME J. 2019, 13, 836–846. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Zechmeister-Boltenstern, S.; Richter, A. Stoichiometric imbalances between terrestrial decomposer communities and their resources: Mechanisms and implications of microbial adaptations to their resources. Front. Microbiol. 2014, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Funakawa, S.; Kosaki, T. Different effects of pH on microbial biomass carbon and metabolic quotients by fumigation–extraction and substrate-induced respiration methods in soils under different climatic conditions. Soil Sci. Plant Nutr. 2009, 55, 363–374. [Google Scholar] [CrossRef]
- Yang, Y.; Moorhead, D.L.; Craig, H.; Luo, M.; Chen, X.; Huang, J.; Olesen, J.E.; Chen, J. Differential Responses of Soil Extracellular Enzyme Activities to Salinization: Implications for Soil Carbon Cycling in Tidal Wetlands. Glob. Biogeochem. Cycles 2022, 36, e2021GB007285. [Google Scholar] [CrossRef]
- Mueller, P.; Granse, D.; Nolte, S.; Weingartner, M.; Hoth, S.; Jensen, K. Unrecognized controls on microbial functioning in Blue Carbon ecosystems: The role of mineral enzyme stabilization and allochthonous substrate supply. Ecol. Evol. 2020, 10, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Tziperman, E.; Gildor, H. On the mid-Pleistocene transition to 100-kyr glacial cycles and the asymmetry between glaciation and deglaciation times. Paleoceanography 2003, 18, 1-1–1-8. [Google Scholar] [CrossRef]



| Parameters | W | F | A |
|---|---|---|---|
| EC (μS cm−1) | 2133 ± 287.24 a | 117.72 ± 25.39 b | 116.23 ± 2.53 b |
| pH | 9.21 ± 0.12 a | 8.74 ± 0.03 b | 8.62 ± 0.03 b |
| ROC (mg kg−1) | 1.75 ± 0.24 a | 1.89 ± 0.18 a | 1.41 ± 0.32 a |
| SOC (g kg−1) | 6.0 ± 0.5 a | 5.0 ± 1.3 a | 4.4 ± 0.2 a |
| TN (g kg−1) | 0.36 ± 0.1 a | 0.34 ± 0.1 a | 0.41 ± 0.01 a |
| C/N | 17.22 ± 1.25 a | 15.6 ± 1.39 a | 10.83 ± 0.71 b |
| TC (g kg−1) | 17.7 ± 0.7 a | 16.3 ± 1.4 a | 16.3 ± 0.2 a |
| Salinity (‰) | 5.57 ± 0.7 a | 0.6 ± 0.26 b | 0.3 ± 0.06 b |
| DOC (mg kg−1) | 129.12 ± 1.55 a | 105.36 ± 5.68 ab | 118.12 ± 4.81 b |
| SWC (%) | 20.32 ± 0.12 c | 24.1 ± 0.54 a | 22.56 ± 0.13 b |
| BD (g cm−3) | 1.54 ± 0.06 a | 1.36 ± 0.01 b | 1.51 ± 0.01 a |
| Site | Sample | C0 | k | R2 |
|---|---|---|---|---|
| W | W0 | 6.21171 ± 2.06664 | 0.00741 ± 0.00348 | 0.96105 |
| W3 | 4.77821 ± 1.74486 | 0.00845 ± 0.00455 | 0.93871 | |
| W6 | 8.77711 ± 6.81797 | 0.00490 ± 0.00281 | 0.92998 | |
| W32 | 6.01388 ± 3.13204 | 0.00738 ± 0.00542 | 0.91233 | |
| F | F0 | 8.28744 ± 1.32245 | 0.00972 ± 0.00199 | 0.98852 |
| F3 | 7.82512 ± 2.14833 | 0.00878 ± 0.00301 | 0.98874 | |
| F6 | 6.10313 ± 1.24228 | 0.01142 ± 0.0033 | 0.99130 | |
| F32 | 5.33381 ± 2.65549 | 0.00905 ± 0.00544 | 0.97176 | |
| A | A0 | 3.78004 ± 0.49681 | 0.03226 ± 0.00701 | 0.97868 |
| A3 | 7.79639 ± 5.95586 | 0.00478 ± 0.00422 | 0.95960 | |
| A6 | 4.18488 ± 0.58664 | 0.01433 ± 0.00307 | 0.99102 | |
| A32 | 2.81944 ± 0.21986 | 0.02734 ± 0.00531 | 0.99174 |
| B | Beta | t | p | VIF | F | R2 | ||
|---|---|---|---|---|---|---|---|---|
| Site W | (constant) | 244.09 | 1.845 | 0.092 | 19.647 *** | 0.877 | ||
| pH | −49.438 | −0.476 | −3.806 | 0.003 | 1.404 | |||
| MBN | 3.232 | 0.765 | 5.37 | <0.001 | 1.818 | |||
| MBC | −0.255 | 0.67 | −3.578 | 0.004 | 3.144 | |||
| TC | 150.394 | 0.378 | 2.33 | 0.04 | 2.356 | |||
| Site F | (constant) | 48.487 | 12.307 | <0.001 | 19.522 *** | 0.75 | ||
| ROC | −3.173 | −0.843 | −6.03 | <0.001 | 1.016 | |||
| salinity | −2.02 | −0.333 | −2.381 | 0.033 | 1.016 | |||
| Site A | (constant) | 6.191 | 0.513 | 0.616 | ||||
| ALP | 38.846 | 0.778 | 4.771 | <0.001 | 1.119 | 14.534 *** | 0.691 | |
| βG | −0.586 | −0.641 | −3.929 | 0.002 | 1.119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Chu, D.; Hu, H.; Deng, W.; Chen, J.; Guo, S. Effects of Land-Use Type and Salinity on Soil Carbon Mineralization in Coastal Areas of Northern Jiangsu Province. Sustainability 2024, 16, 3285. https://doi.org/10.3390/su16083285
Yang X, Chu D, Hu H, Deng W, Chen J, Guo S. Effects of Land-Use Type and Salinity on Soil Carbon Mineralization in Coastal Areas of Northern Jiangsu Province. Sustainability. 2024; 16(8):3285. https://doi.org/10.3390/su16083285
Chicago/Turabian StyleYang, Xu, Dongsheng Chu, Haibo Hu, Wenbin Deng, Jianyu Chen, and Shaojun Guo. 2024. "Effects of Land-Use Type and Salinity on Soil Carbon Mineralization in Coastal Areas of Northern Jiangsu Province" Sustainability 16, no. 8: 3285. https://doi.org/10.3390/su16083285




