Sustainable Processes and Physico-Chemical Characterization of Artisanal Spontaneous Gluten Free Sourdough (Quinoa, Amaranth and Brown Rice) Compared to Wheat Sourdough
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
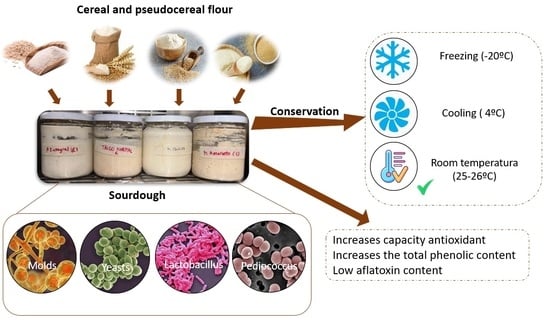
2.2.1. Sourdough Preparation
2.2.2. Physico-Chemical Parameters
2.2.3. Acetic Acid and Lactic Acid Content
2.2.4. Microbiological Analysis for Molds, Yeast and Lactic Acid Bacteria
2.2.5. Sample Extraction
2.2.6. Antioxidant Activity and Total Phenolic Content (TPC)
2.2.7. Content of Total Aflatoxins
2.2.8. Total Microbial Genomic DNA Extraction
2.2.9. Illumina Sequencing and Bioinformatic Analysis
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Parameters
3.2. Acetic Acid and Lactic Acid Content
3.3. Microbiological Analysis for Molds, Yeast and Lactic Acid Bacteria
3.4. Antioxidant Activity and Total Phenolic Content (TPC)
3.5. Content of Total Aflatoxins
3.6. Microbial Composition
3.6.1. Fungi



| Genus and Species | Type | Relationship with Food | Normally Found in | Pathogen | Metabolites and Biological Activity | References |
|---|---|---|---|---|---|---|
| Alternaria spp. A. destruens A. metachromatia A. rosae A. subcucurbitae | Filamentous fungi | Cereals, oilseeds, tomatoes, cucumbers, cauliflowers, peppers, apples, melons, tangerines, oranges, lemons and sunflower seeds | Soil and plants | Yes (Opportunistic) | Alternariol (AOH), altenariol monomethyl ether (AME), altenuene (ALT), tenuazonic acid (TeA), tentoxin (TEN), altertoxins I, II and III, dehydrocurvularin, pyrenochaetic acid, alternarienonic acid and altechromoneA | [67,68] |
| Aspergillus spp. A. intermedius A. penicillioides A. ruber | Filamentous fungi | Tea, coffee, rice and soybeans, meju (dried fermented soybeans), syrups, jams, jellies and salted meat products | Water and soil | Yes (Opportunistic) | Asperflavin, auroglaucin, dihydroauroglaucin, echinulins, epiheveadrides, flavoglaucin, isoechinulins, LL-S491β, neoechinulins, physcion, questin, tetrahydroauroglaucin, bisanthrons, catenarin, erythroglaucin, questin, questinol, tetracyclic | [69,70,71] |
| Bipolaris spp. B. oryzae B. yamadae | Filamentous fungi | Corn, rice and oatmeal | Soil | Yes (Opportunistic) | Bipolahidroquinonas A-C, coclioquinonas I–N, isococlioquinonas F y G. Anticancer activity | [72] |
| Bullera spp. B. alba | Fungi | n/a | n/a | No | n/a | |
| Cladosporium spp. C. basi-inflatum C. herbarum | Filamentous fungi | May cause food spoilage | Soil and air | No | Alkaloids, azaphilones, benzofluorantheneones, benzopyrones, binaphtopyrones, butanolides, butenolides, cinnamic acid, citrinin, coumarins, isocoumarins, diketopiperazines, flavonoids, gibberellins, fusicoccane, diterpene, glycosides, lactones, macrolides, naphthalene, naphthalenones, naphtoquinones, anthraquinones, perylenquinones, pyrones, sterols, tetramic acids, tropolones and xanthones. | [73,74] |
| Curvularia spp. C. lunata | Phytopathogenic fungus | Rice, sugarcane, rice, millet and maize (corn). | Plants and soil | Yes | Radicinin, radicinol and 3-epiradicinol (radicinol diastereomer). | [75,76] |
| Di oszegia spp. D. hungarica D. takashimae | Yeast | n/a | Plants and insects | No | Antimicrobial activity | [77] |
| Epicoccum spp. E. thailandicum | Fungi | Cheese | Air, soil and plant | No | Diketopiperazines, epicorazines, epicoccolides, epicocconigrones, epicocconones, epicolactone dimers, epipyrones, flavipins, triornicins epicoccamides, meroterpenoids and taxol. Antimicrobial activity and anticancer activity | [78] |
| Eremothecium spp. E. gossypii | Filamentous fungi | n/a | Cotton | n/a | Riboflavin (vitamin B2) quinones, flavins and melanin | [79] |
| Exserohilum spp. E. gedarefense E. monoceras | Fungi | n/a | Plant material like grasses, rotten wood and in the soil | n/a | n/a | [80] |
| Filobasidium spp. F. wieringae | Fungi | n/a | n/a | n/a | n/a | |
| Fusarium spp. F. equiseti F. graminearum F. tricinctum | Filamentous fungi | Cereals, fruits, nuts, spices, processed juices, grasses and vegetables | Soil, air and plants | Yes (Opportunistic) | Polyketides, alkaloids, terpenoids, peptides and steroids. antifungal activity | [81,82] |
| Hannaella spp. H. oryzae | Yeast | n/a | Soil and plants | No | n/a | [83] |
| Holleya spp. H. sinecauda | Fungi | Mustard seeds | n/a | Yes | n/a | [84] |
| Microdochium spp. M.Seminicola | Fungi | Cereals | Plants | Yes | n/a | [85] |
| Mucor spp. M. circinelloides | Filamentous fungi | n/a | Soil | No | Alkaloid, pigment, benzoic acid, terpenoid, cinnamic acid, benzopyran, aspalathin and phloretin, arachidonic acid and ecosanoic acid | [86] |
| Neocamarosporium spp. N. leipoldtiae | Fungi | n/a | n/a | n/a | n/a | |
| Nigrospora spp. N. hainanensis N. vesicularifera | Fungi | Fruits and oils | Soil and sea | No | Polyketides, terpenoids, steroids, N-containing compounds and fatty acids. | [87] |
| Ophiosphaerella spp. O. aquatica | Fungi | n/a | n/a | Yes | n/a | |
| Papiliotrema spp. P. rajasthanensis | Yeast | n/a | Soil and plants | n/a | n/a | |
| Parastagonospora spp. P. nodorum | Fungi | Wheat and cereals | n/a | Yes | n/a | [88] |
| Penicillium spp. P. citrinum | Fungi | Tea | Soil and sea | No | Citrinin and tanzawaic acid. Antimicrobial and antioxidant acitivity | [89,90,91] |
| Periconia spp. P. echinochloae | Fungi | Rice | Soil, detoriating or dead herbaceous stems, leaves, grasses, rushes and sedges | Yes | Diterpenes, sesquiterpenes, sesterterpenes and steroids | [92,93,94] |
| Phaeosphaeria spp. P. oryzae | Fungi | n/a | n/a | Yes | n/a | |
| Plenodomus spp. P. fallaciosus | Fungi | Grape | n/a | n/a | n/a | [95] |
| Pleospora spp. P. bjoerlingii | Fungi | Garlic | Air | n/a | n/a | [96] |
| Ramichloridium spp. R. cucurbitae | Fungi | n/a | n/a | No | n/a | |
| Rhizopus spp. R. arrhizus | Fungi | Vegetables and fruits | Soil | Yes | Fumaric acid | [97] |
| Saccharomycopsis spp. S. fibuligera | Yeast | Cereal-based fermented foods and beverages | Plants | No | Ethanol, carbon dioxide and diverse compounds including fusel alcohols and esters | [98,99,100] |
| Saitozyma spp. S. paraflava | Yeast | n/a | n/a | n/a | n/a | |
| Sporobolomyces spp. S. roseus | Yeast | Smoked dried sausages, nectarine fruits, fermented tea, Chinese miscanthus, grapefruit, citrus fruits and apple must | Environment, tree leaves and soil | No | β-carotene, torulene and torularhodin | [101] |
| Stemphylium spp. S. vesicarium | Fungi | Cucumber, garlic, pear, parsley, asparagus, spinach and lettuce. | n/a | Yes | n/a | [102] |
| Vishniacozyma spp. V. tephrensis V. victoriae | Yeast | Grape and kiwi | n/a | No | n/a | [103] |
3.6.2. Bacterial Community Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferrari, A.; Vinderola, G.; Weill, R. Alimentos Fermentados Microbiología, Nutrición, Salud y Cultura; Instituto Danone del Cono Sur: Buenos Aires, Argentina, 2016; Volume 6, ISBN 9786021018187. [Google Scholar]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the Sourdough May Affect the Functional Features of Leavened Baked Goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Yeşil, S.; Levent, H. The Influence of Fermented Buckwheat, Quinoa and Amaranth Flour on Gluten-Free Bread Quality. LWT 2022, 160, 113301. [Google Scholar] [CrossRef]
- D’Amico, V.; Gänzle, M.; Call, L.; Zwirzitz, B.; Grausgruber, H.; D’Amico, S.; Brouns, F. Does Sourdough Bread Provide Clinically Relevant Health Benefits? Front. Nutr. 2023, 10, 1230043. [Google Scholar] [CrossRef]
- Trif, M.; Socol, C.T.; Bangar, S.P.; Rusu, A.V. Cereals and Cereal Sourdoughs as a Source of Functional and Bioactive Compounds. In Sourdough Innovations: Novel Uses of Metabolites, Enzymes, and Microbiota from Sourdough Processing; CRC Press: Boca Raton, FL, USA, 2023; pp. 31–62. ISBN 9781000899474. [Google Scholar]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health Benefits of Fermented Foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.; Gormley, T.R.; Arendt, E.K. Recent Advances in the Formulation of Gluten-Free Cereal-Based Products. Trends Food Sci. Technol. 2004, 15, 143–152. [Google Scholar] [CrossRef]
- Mariotti, M.; Lucisano, M.; Ambrogina Pagani, M.; Ng, P.K.W. The Role of Corn Starch, Amaranth Flour, Pea Isolate, and Psyllium Flour on the Rheological Properties and the Ultrastructure of Gluten-Free Doughs. Food Res. Int. 2009, 42, 963–975. [Google Scholar] [CrossRef]
- Cappa, C.; Lucisano, M.; Mariotti, M. Influence of Psyllium, Sugar Beet Fibre and Water on Gluten-Free Dough Properties and Bread Quality. Carbohydr. Polym. 2013, 98, 1657–1666. [Google Scholar] [CrossRef]
- Bender, D.; Schönlechner, R. Innovative Approaches towards Improved Gluten-Free Bread Properties. J. Cereal Sci. 2020, 91, 102904. [Google Scholar] [CrossRef]
- Moroni, A.V.; Dal Bello, F.; Arendt, E.K. Sourdough in Gluten-Free Bread-Making: An Ancient Technology to Solve a Novel Issue? Food Microbiol. 2009, 26, 676–684. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive Value of Pseudocereals and Their Increasing Use as Functional Gluten-Free Ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Pongrac, P.; Vogel-Mikuš, K.; Jeromel, L.; Vavpetič, P.; Pelicon, P.; Kaulich, B.; Gianoncelli, A.; Eichert, D.; Regvar, M.; Kreft, I. Spatially Resolved Distributions of the Mineral Elements in the Grain of Tartary Buckwheat (Fagopyrum Tataricum). Food Res. Int. 2013, 54, 125–131. [Google Scholar] [CrossRef]
- Abugoch James, L.E. Quinoa (Chenopodium quinoa Willd.): Composition, Chemistry, Nutritional, and Functional Properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Yaver, E.; Bilgiçli, N. Pseudocereals: Composition, Effect on Nutrition-Health and Usage in Cereal Products. Food Health 2020, 6, 41–56. [Google Scholar] [CrossRef]
- Malik, A.M.; Singh, A. Pseudocereals Proteins- A Comprehensive Review on Its Isolation, Composition and Quality Evaluation Techniques. Food Chem. Adv. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Brandt, M.J.; Loponen, J.; Cappelle, S. Technology of Sourdough Fermentation and Sourdough Application. In Handbook on Sourdough Biotechnology; Springer International Publishing: Cham, Switzerland, 2023; pp. 67–80. [Google Scholar]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty Years of Knowledge on Sourdough Fermentation: A Systematic Review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Van Kerrebroeck, S.; Maes, D.; De Vuyst, L. Sourdoughs as a Function of Their Species Diversity and Process Conditions, a Meta-Analysis. Trends Food Sci. Technol. 2017, 68, 152–159. [Google Scholar] [CrossRef]
- Lhomme, E.; Orain, S.; Courcoux, P.; Onno, B.; Dousset, X. The Predominance of Lactobacillus Sanfranciscensis in French Organic Sourdoughs and Its Impact on Related Bread Characteristics. Int. J. Food Microbiol. 2015, 213, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.T.M.; de Hosken, B.O.; De Dea Lindner, J.; Menezes, L.A.A.; Pirozi, M.R.; Martin, J.G.P. How to Deliver Sourdough with Appropriate Characteristics for the Bakery Industry? The Answer May Be Provided by Microbiota. Food Biosci. 2023, 56, 103072. [Google Scholar] [CrossRef]
- Katina, K.; Liukkonen, K.H.; Kaukovirta-Norja, A.; Adlercreutz, H.; Heinonen, S.M.; Lampi, A.M.; Pihlava, J.M.; Poutanen, K. Fermentation-Induced Changes in the Nutritional Value of Native or Germinated Rye. J. Cereal Sci. 2007, 46, 348–355. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry to Total Phenolics with Phosphomolybdic Acid Reagents. Am. J. Enol. Vinic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every Base Matters: Assessing Small Subunit RRNA Primers for Marine Microbiomes with Mock Communities, Time Series and Global Field Samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de la Presidencia, Relaciones con las Cortes e Igualdad. Real Decreto 308/2019, de 26 de Abril, Por El Que Se Aprueba La Norma de Calidad Para El Pan. 2019; Volume 113. Available online: https://gremipa.com/es/el-real-decreto-308-2019-of-26-dabril-by-which-saprova-the-norm-of-quality-for-the-pa-enter-in-force-the-proxim-1-of-july/ (accessed on 12 March 2024).
- Carbó, R.; Gordún, E.; Fernández, A.; Ginovart, M. Elaboration of a Spontaneous Gluten-Free Sourdough with a Mixture of Amaranth, Buckwheat, and Quinoa Flours Analyzing Microbial Load, Acidity, and PH. Food Sci. Technol. Int. 2020, 26, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Harth, H.; Van Kerrebroeck, S.; De Vuyst, L. Community Dynamics and Metabolite Target Analysis of Spontaneous, Backslopped Barley Sourdough Fermentations under Laboratory and Bakery Conditions. Int. J. Food Microbiol. 2016, 228, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Sterr, Y.; Weiss, A.; Schmidt, H. Evaluation of Lactic Acid Bacteria for Sourdough Fermentation of Amaranth. Int. J. Food Microbiol. 2009, 136, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Vogelmann, S.A.; Seitter, M.; Singer, U.; Brandt, M.J.; Hertel, C. Adaptability of Lactic Acid Bacteria and Yeasts to Sourdoughs Prepared from Cereals, Pseudocereals and Cassava and Use of Competitive Strains as Starters. Int. J. Food Microbiol. 2009, 130, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hammes, W.P.; Brandt, M.J.; Francis, K.L.; Rosenheim, J.; Seitter, M.F.H.; Vogelmann, S.A. Microbial Ecology of Cereal Fermentations. Trends Food Sci. Technol. 2005, 16, 4–11. [Google Scholar] [CrossRef]
- Salovaara, H.; Valjakka, T. The Effect of Fermentation Temperature, Flour Type, and Starter on the Properties of Sour Wheat Bread. Int. J. Food Sci. Technol. 1987, 22, 591–597. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, K.; Dhaliwal, H.S. Nutritional Facts, Bio-Active Components and Processing Aspects of Pseudocereals: A Comprehensive Review. Food Biosci. 2021, 42, 101170. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.A.M.; Encina, C.R.; Binaghi, M.J.; Greco, C.B.; de Ferrer, P.A.R. Effects of Roasting and Boiling of Quinoa, Kiwicha and Kañiwa on Composition and Availability of Minerals in Vitro. J. Sci. Food Agric. 2010, 90, 2068–2073. [Google Scholar] [CrossRef] [PubMed]
- Fekri, A.; Abedinzadeh, S.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Considering Sourdough from a Biochemical, Organoleptic, and Nutritional Perspective. J. Food Compos. Anal. 2024, 125, 105853. [Google Scholar] [CrossRef]
- Zhang, C.; Brandt, M.J.; Schwab, C.; Gänzle, M.G. Propionic Acid Production by Cofermentation of Lactobacillus Buchneri and Lactobacillus Diolivorans in Sourdough. Food Microbiol. 2010, 27, 390–395. [Google Scholar] [CrossRef]
- Quattrini, M.; Liang, N.; Fortina, M.G.; Xiang, S.; Curtis, J.M.; Gänzle, M. Exploiting Synergies of Sourdough and Antifungal Organic Acids to Delay Fungal Spoilage of Bread. Int. J. Food Microbiol. 2019, 302, 8–14. [Google Scholar] [CrossRef]
- Roecken, W. Applied Aspects of Sourdough Fermentation. Adv. Food Sci. 1996, 18, 212–216. [Google Scholar]
- Hlebowicz, J.; Darwiche, G.; Björgell, O.; Almér, L.O. Effect of Cinnamon on Postprandial Blood Glucose, Gastric Emptying, and Satiety in Healthy Subjects. Am. J. Clin. Nutr. 2007, 85, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Spicher, G.; Brümmer, J.M. Baked Goods. In Biotechnology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; Volume 9, pp. 240–319. ISBN 9783527620920. [Google Scholar]
- Liljeberg, H.G.M.; Lonner, C.H.; Bjorck, I.M.E. Sourdough Fermentation or Addition of Organic Acids or Corresponding Salts to Bread Improves Nutritional Properties of Starch in Healthy Humans. J. Nutr. 1995, 125, 1503–1511. [Google Scholar]
- Le Lay, C.; Mounier, J.; Vasseur, V.; Weill, A.; Le Blay, G.; Barbier, G.; Coton, E. In Vitro and in Situ Screening of Lactic Acid Bacteria and Propionibacteria Antifungal Activities against Bakery Product Spoilage Molds. Food Control 2016, 60, 247–255. [Google Scholar] [CrossRef]
- Lazo-Vélez, M.A.; Garzon, R.; Guardado-Félix, D.; Serna-Saldivar, S.O.; Rosell, C.M. Selenized Chickpea Sourdoughs for the Enrichment of Breads. LWT 2021, 150, 112082. [Google Scholar] [CrossRef]
- Van Kerrebroeck, S.; Bastos, F.C.C.; Harth, H.; De Vuyst, L. A Low PH Does Not Determine the Community Dynamics of Spontaneously Developed Backslopped Liquid Wheat Sourdoughs but Does Influence Their Metabolite Kinetics. Int. J. Food Microbiol. 2016, 239, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of Sourdough Made with Quinoa (Chenopodium quinoa) Flour and Autochthonous Selected Lactic Acid Bacteria for Enhancing the Nutritional, Textural and Sensory Features of White Bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Rühmkorf, C.; Jungkunz, S.; Wagner, M.; Vogel, R.F. Optimization of Homoexopolysaccharide Formation by Lactobacilli in Gluten-Free Sourdoughs. Food Microbiol. 2012, 32, 286–294. [Google Scholar] [CrossRef]
- Liukkonen, K.-H.; Katina, K.; Wilhelmsson, A.; Myllymaki, O.; Lampi, A.-M.; Kariluoto, S.; Piironen, V.; Heinonen, S.-M.; Nurmi, T.; Adlercreutz, H.; et al. Process-Induced Changes on Bioactive Compounds in Whole Grain Rye. Proc. Nutr. Soc. 2003, 62, 117–122. [Google Scholar] [CrossRef]
- Colosimo, R.; Gabriele, M.; Cifelli, M.; Longo, V.; Domenici, V.; Pucci, L. The Effect of Sourdough Fermentation on Triticum Dicoccum from Garfagnana: 1H NMR Characterization and Analysis of the Antioxidant Activity. Food Chem. 2020, 305, 125510. [Google Scholar] [CrossRef] [PubMed]
- Drakula, S.; Novotni, D.; Čukelj Mustač, N.; Voučko, B.; Krpan, M.; Hruškar, M.; Ćurić, D. Alteration of Phenolics and Antioxidant Capacity of Gluten-Free Bread by Yellow Pea Flour Addition and Sourdough Fermentation. Food Biosci. 2021, 44, 101424. [Google Scholar] [CrossRef]
- Nieto, G.; Bañón, S.; Garrido, M. Administration of distillate thyme leaves into the diet of Segureña ewes: Effect on lamb meat quality. Animal 2012, 6, 2048–2056. [Google Scholar] [CrossRef]
- Han, Y.; Chi, J.; Zhang, M.; Zhang, R.; Fan, S.; Huang, F.; Xue, K.; Liu, L. Characterization of Saponins and Phenolic Compounds: Antioxidant Activity and Inhibitory Effects on α-Glucosidase in Different Varieties of Colored Quinoa (Chenopodium quinoa Willd). Biosci. Biotechnol. Biochem. 2019, 83, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Lancetti, R.; Salvucci, E.; Moiraghi, M.; Pérez, G.T.; Sciarini, L.S. Gluten-Free Flour Fermented with Autochthonous Starters for Sourdough Production: Effect of the Fermentation Process. Food Biosci. 2022, 47, 101723. [Google Scholar] [CrossRef]
- Nionelli, L.; Curri, N.; Curiel, J.A.; Di Cagno, R.; Pontonio, E.; Cavoski, I.; Gobbetti, M.; Rizzello, C.G. Exploitation of Albanian Wheat Cultivars: Characterization of the Flours and Lactic Acid Bacteria Microbiota, and Selection of Starters for Sourdough Fermentation. Food Microbiol. 2014, 44, 96–107. [Google Scholar] [CrossRef]
- Martínez, J.; Nieto, G.; Castillo, J.; Ros, G. Influence of in vitro gastrointestinal digestion and/or grape seed extract addition on antioxidant capacity of meat emulsions. LWT Food Sci. Technol. 2014, 59, 834–840. [Google Scholar] [CrossRef]
- Nieto, G. Incorporation of by-products of rosemary and thyme in the diet of ewes: Effect on the fatty acid profile of lamb. Eur. Food Res. Technol. 2013, 236, 379–389. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Raeisi, M.; Nematollahi, Z. Biological Control of Foodborne Pathogens and Aflatoxins by Selected Probiotic LAB Isolated from Rice Bran Sourdough. Biol. Control 2019, 130, 70–79. [Google Scholar] [CrossRef]
- Gerbaldo, G.A.; Barberis, C.; Pascual, L.; Dalcero, A.; Barberis, L. Antifungal Activity of Two Lactobacillus Strains with Potential Probiotic Properties. FEMS Microbiol. Lett. 2012, 332, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A.; Njobeh, P.B.; Gbashi, S.; Nwinyi, O.C.; Mavumengwana, V. Review on Microbial Degradation of Aflatoxins. Crit. Rev. Food Sci. Nutr. 2017, 57, 3208–3217. [Google Scholar] [CrossRef] [PubMed]
- EU. Reglamento (CE) 1881/2006 de La Comisión, de 19 de Diciembre de 2006 Por El Que Se Fija El Contenido Máximo de Determinados Contaminantes En Los Productos Alimenticios. Diario Oficial de la Unión Europea. 2006, Volume 2010, pp. 5–24. Available online: https://eur-lex.europa.eu/legal-content/ES/ALL/?uri=CELEX%3A32006R1881 (accessed on 12 March 2024).
- Tang, N.; Xing, X.; Suo, B.; Li, H.; Gou, Q.; Xu, T.; Ai, Z.; Yang, Y. Multi-Omics Analysis Reveals the Mechanism Underlying the Dimorphic Formation of Saccharomycopsis Fibuligera during Dough Fermentation. Food Biosci. 2024, 57, 103490. [Google Scholar] [CrossRef]
- Jin, J.; Nguyen, T.T.H.; Humayun, S.; Park, S.H.; Oh, H.; Lim, S.; Mok, I.K.; Li, Y.; Pal, K.; Kim, D. Characteristics of Sourdough Bread Fermented with Pediococcus Pentosaceus and Saccharomyces Cerevisiae and Its Bio-Preservative Effect against Aspergillus Flavus. Food Chem. 2021, 345, 128787. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, H.; Qi, H.; Zhang, D. Microbiota Diversity, Composition and Drivers in Waxy Proso Millet Sourdoughs of Niandoubao, a Traditional Fermented Cereal Food in Northeast China. LWT 2023, 180, 114699. [Google Scholar] [CrossRef]
- Munch-Andersen, C.B.; Porcellato, D.; Devold, T.G.; Østlie, H.M. Isolation, Identification, and Stability of Sourdough Microbiota from Spontaneously Fermented Norwegian Legumes. Int. J. Food Microbiol. 2024, 410, 110505. [Google Scholar] [CrossRef]
- Yan, B.; Sadiq, F.A.; Cai, Y.; Fan, D.; Chen, W.; Zhang, H.; Zhao, J. Microbial Diversity in Traditional Type I Sourdough and Jiaozi and Its Influence on Volatiles in Chinese Steamed Bread. LWT 2019, 101, 764–773. [Google Scholar] [CrossRef]
- Lee, H.B.; Patriarca, A.; Magan, N. Alternaria in Food: Ecophysiology, Mycotoxin Production and Toxicology. Mycobiology 2015, 43, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Spoilage of Stored, Processed and Preserved Foods. In Fungi and Food Spoilage; Springer: New York, NY, USA, 2009; pp. 401–421. [Google Scholar]
- Chen, A.J.; Hubka, V.; Frisvad, J.C.; Visagie, C.M.; Houbraken, J.; Meijer, M.; Varga, J.; Demirel, R.; Jurjević, Ž.; Kubátová, A.; et al. Polyphasic Taxonomy of Aspergillus Section Aspergillus (Formerly Eurotium), and Its Occurrence in Indoor Environments and Food. Stud. Mycol. 2017, 88, 37–135. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi, Fungal Biodiversity Centre Utrecht; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010. [Google Scholar]
- Taniwaki, M.H.; Pitt, J.I.; Magan, N. Aspergillus Species and Mycotoxins: Occurrence and Importance in Major Food Commodities. Curr. Opin. Food Sci. 2018, 23, 38–43. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Si, J.; Wu, L. Metabolites of Medicine Food Homology-Derived Endophytic Fungi and Their Activities. Curr. Res. Food Sci. 2022, 5, 1882–1896. [Google Scholar] [CrossRef] [PubMed]
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The Genus Cladosporium. Stud. Mycol. 2012, 72, 1–401. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. The Genus Cladosporium: A Rich Source of Diverse and Bioactive Natural Compounds. Molecules 2021, 26, 3959. [Google Scholar] [CrossRef] [PubMed]
- Venkateshbabu, G.; Prasannakumar, M.K.; Kamalraj, S.; Narayan, K.S.; Palani, P. Genetic Analysis, Purification and Docking Studies of Trihydroxynaphthalene Reductase Involved in Pathogenesis of Rice Pathogen, Curvularia Lunata. Process Biochem. 2023, 135, 61–74. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh Kapkoti, D.; Gupta, M.; Rout, P.K.; Singh Bhakuni, R.; Samad, A. Enhanced Production of Phytotoxic Polyketides Isolated from Curvularia Lunata by Applying Chemical Stresses. Ind. Crops Prod. 2021, 160, 113156. [Google Scholar] [CrossRef]
- Vergata, C.; Contaldi, F.; Baccelli, I.; Santini, A.; Pecori, F.; Buti, M.; Mengoni, A.; Vaccaro, F.; Moura, B.B.; Ferrini, F.; et al. How Does Particulate Matter Affect Plant Transcriptome and Microbiome? Environ. Exp. Bot. 2023, 209, 105313. [Google Scholar] [CrossRef]
- Rodríguez, J.; Vázquez, L.; Flórez, A.B.; Mayo, B. Epicoccum Sp. as the Causative Agent of a Reddish-Brown Spot Defect on the Surface of a Hard Cheese Made of Raw Ewe Milk. Int. J. Food Microbiol. 2023, 406, 110401. [Google Scholar] [CrossRef]
- Umar, A.; Darwish, D.B.E.; Alenezi, M.A. Fungal Pigments: Secondary Metabolites and Their Application. In Fungal Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2024; pp. 173–195. ISBN 9780323952415. [Google Scholar]
- Therese, K.L.; Madhavan, H.N. Molecular Detection of Human Fungal Pathogens; Routledge: London, UK, 2011. [Google Scholar]
- Ejaz, M.R.; Jaoua, S.; Ahmadi, M.; Shabani, F. An Examination of How Climate Change Could Affect the Future Spread of Fusarium Spp. around the World, Using Correlative Models to Model the Changes. Environ. Technol. Innov. 2023, 31, 103177. [Google Scholar] [CrossRef]
- Sun, J.; Yang, X.Q.; Wan, J.L.; Han, H.L.; Zhao, Y.D.; Cai, L.; Yang, Y.B.; Ding, Z.T. The Antifungal Metabolites Isolated from Maize Endophytic Fungus Fusarium Sp. Induced by OSMAC Strategy. Fitoterapia 2023, 171, 105710. [Google Scholar] [CrossRef] [PubMed]
- Gamero, A.; Quintilla, R.; Groenewald, M.; Alkema, W.; Boekhout, T.; Hazelwood, L. High-Throughput Screening of a Large Collection of Non-Conventional Yeasts Reveals Their Potential for Aroma Formation in Food Fermentation. Food Microbiol. 2016, 60, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Schade, D.; Walther, A.; Wendland, J. The Development of a Transformation System for the Dimorphic Plant Pathogen Holleya Sinecauda Based on Ashbya Gossypii DNA Elements. Fungal Genet. Biol. 2003, 40, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Gagkaeva, T.Y.; Orina, A.S.; Gavrilova, O.P.; Gogina, N.N. Evidence of Microdochium Fungi Associated with Cereal Grains in Russia. Microorganisms 2020, 8, 340. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, S.A.; Ijaz, M.U.; Ullah, S.; Muhammad, Z.; Suleria, H.A.R.; Song, Y. Antioxidant Activity of Polyphenolic Extracts of Filamentous Fungus Mucor Circinelloides (WJ11): Extraction, Characterization and Storage Stability of Food Emulsions. Food Biosci. 2020, 34, 100525. [Google Scholar] [CrossRef]
- Xu, T.; Song, Z.; Hou, Y.; Liu, S.; Li, X.; Yang, Q.; Wu, S. Secondary Metabolites of the Genus Nigrospora from Terrestrial and Marine Habitats: Chemical Diversity and Biological Activity. Fitoterapia 2022, 161, 105254. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A.; Borde, C.; Genta-Jouve, G.; Escargueil, A.; Prado, S. Cytotoxic Constituents from the Wheat Plant Pathogen Parastagonospora Nodorum SN15. Nat. Prod. Res. 2022, 36, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Malmstrøm, J.; Christophersen, C.; Frisvad, J.C. Secondary Metabolites Characteristic of Penicillium Citrinum, Penicillium Steckii and Related Species. Phytochemistry 2000, 54, 301–309. [Google Scholar] [CrossRef]
- Qin, J.; Teng, J.; Li, Z.; Xia, N.; Wei, B.; Huang, L. Expression of Citrinin Biosynthesis Gene in Liupao Tea and Effect of Penicillium Citrinum on Tea Quality. Fungal Genet. Biol. 2022, 163, 103742. [Google Scholar] [CrossRef]
- Kumari, P.; Singh, A.; Singh, D.K.; Sharma, V.K.; Kumar, J.; Gupta, V.K.; Bhattacharya, S.; Kharwar, R.N. Isolation and Purification of Bioactive Metabolites from an Endophytic Fungus Penicillium Citrinum of Azadirachta Indica. S. Afr. J. Bot. 2021, 139, 449–457. [Google Scholar] [CrossRef]
- Li, B.X.; Shu, Y.; Zhang, S.Q.; Yang, R.D.; Yao, L.L.; Liu, J.Q.; Liu, S.X.; Wang, J.P.; Cai, L. Macrostines A and B: Tetracyclic Fisicoccane from the Fungus Periconia Macrospinosa WTG-10. Fitoterapia 2023, 165, 105429. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, F.; Lin, S.; Yang, B.; Wang, J.; Cao, J.; Hu, Z.; Zhang, Y. Two New Lanostane-Type Triterpenoids from the Fungus Periconia Sp. TJ403-Rc01. Nat. Prod. Res. 2023, 37, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, R.; Janakiraman, D.; Rajapandian, S.G.K.; Appavu, S.P.; Namperumalsamy Venkatesh, P.; Prajna, L. Periconia Species—An Unusual Fungal Pathogen Causing Mycotic Keratitis. Indian J. Med. Microbiol. 2021, 39, 36–40. [Google Scholar] [CrossRef]
- Milanović, V.; Cardinali, F.; Ferrocino, I.; Boban, A.; Franciosa, I.; Gajdoš Kljusurić, J.; Mucalo, A.; Osimani, A.; Aquilanti, L.; Garofalo, C.; et al. Croatian White Grape Variety Maraština: First Taste of Its Indigenous Mycobiota. Food Res. Int. 2022, 162, 111917. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.A.; Lee, J.E.; Tsujimoto, M.; Imura, S.; Bergstrom, D.M.; Ware, C.; Lebouvier, M.; Huiskes, A.H.L.; Gremmen, N.J.M.; Frenot, Y.; et al. Food for Thought: Risks of Non-Native Species Transfer to the Antarctic Region with Fresh Produce. Biol. Conserv. 2011, 144, 1682–1689. [Google Scholar] [CrossRef]
- Corzo-León, D.E.; Uehling, J.K.; Ballou, E.R. Rhizopus Arrhizus. Trends Microbiol. 2023, 31, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Smith, M.T. Saccharomycopsis Schiönning (1903). Yeasts 2011, 2, 751–763. [Google Scholar] [CrossRef]
- Chi, Z.; Chi, Z.; Liu, G.; Wang, F.; Ju, L.; Zhang, T. Saccharomycopsis Fibuligera and Its Applications in Biotechnology. Biotechnol. Adv. 2009, 27, 423–431. [Google Scholar] [CrossRef]
- Son, E.Y.; Lee, S.M.; Kim, M.; Seo, J.A.; Kim, Y.S. Comparison of Volatile and Non-Volatile Metabolites in Rice Wine Fermented by Koji Inoculated with Saccharomycopsis Fibuligera and Aspergillus Oryzae. Food Res. Int. 2018, 109, 596–605. [Google Scholar] [CrossRef]
- Kot, A.M.; Kieliszek, M.; Piwowarek, K.; Błażejak, S.; Mussagy, C.U. Sporobolomyces and Sporidiobolus—Non-Conventional Yeasts for Use in Industries. Fungal Biol. Rev. 2021, 37, 41–58. [Google Scholar] [CrossRef]
- Yu, X.; Han, R.; Zhang, W.; Li, Z.; Zhang, X.; Wang, X.; Xiang, W.; Zhao, J. Leaf Spot on Cucumber Caused by Stemphylium Vesicarium Newly Reported in China. Crop Prot. 2023, 174, 106415. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Y.; Xiao, J.; Li, D.; Wang, B. Effects of Harvest Dates on Microbial Communities of Ice Grape Skins from Xinjiang of China. Process Biochem. 2020, 98, 202–210. [Google Scholar] [CrossRef]
- Fangio, M.F.; Roura, S.I.; Fritz, R. Isolation and Identification of Bacillus Spp. and Related Genera from Different Starchy Foods. J. Food Sci. 2010, 75, M218–M221. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Celano, G.; Lattanzi, A.; Tedone, L.; de Mastro, G.; Gobbetti, M.; de Angelis, M. Lactic Acid Bacteria in Durum Wheat Flour Are Endophytic Components of the Plant during Its Entire Life Cycle. Appl. Environ. Microbiol. 2015, 81, 6736–6748. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Carafa, I.; Fava, F.; Tuohy, K.M.; Nikoloudaki, O.; Gobbetti, M.; Cagno, R. Di Sourdough Performances of the Golden Cereal Tritordeum: Dynamics of Microbial Ecology, Biochemical and Nutritional Features. Int. J. Food Microbiol. 2022, 374, 109725. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Siepmann, F.B.; Rojas Tovar, L.E.; Chen, X.; Gänzle, M.G. Effect of Copy Number of the SpoVA2mob Operon, Sourdough and Reutericyclin on Ropy Bread Spoilage Caused by Bacillus spp. Food Microbiol. 2020, 91, 103507. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, M.; Jiaxin, C.; Luo, Y.; Ye, F.; Jiao, S.; Hu, X.; Zhang, J.; Lü, X. Bacterial Diversity in Traditional Sourdough from Different Regions in China. LWT 2018, 96, 251–259. [Google Scholar] [CrossRef]
- Kam, W.Y.; Wan Aida, W.M.; Sahilah, A.M.; Maskat, M.Y. Volatile Compounds and Lactic Acid Bacteria in Spontaneous Fermented Sourdough. Sains Malays. 2011, 40, 135–138. [Google Scholar]
- Brummer, J.; Lorenz, K. European Developments in Wheat Sourdoughs. CFW Rev. 1991, 36, 310–314. [Google Scholar]
- Fujimoto, A.; Ito, K.; Itou, M.; Narushima, N.; Ito, T.; Yamamoto, A.; Hirayama, S.; Furukawa, S.; Morinaga, Y.; Miyamoto, T. Microbial Behavior and Changes in Food Constituents during Fermentation of Japanese Sourdoughs with Different Rye and Wheat Starting Materials. J. Biosci. Bioeng. 2018, 125, 97–104. [Google Scholar] [CrossRef] [PubMed]
- González-Alonso, V.; Pradal, I.; Wardhana, Y.R.; Cnockaert, M.; Wieme, A.D.; Vandamme, P.; De Vuyst, L. Microbial Ecology and Metabolite Dynamics of Backslopped Triticale Sourdough Productions and the Impact of Scale. Int. J. Food Microbiol. 2024, 408, 110445. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W.; Sadiq, F.A.; Arbab, S.H.; He, G. Microbiota Succession and Metabolite Changes during the Traditional Sourdough Fermentation of Chinese Steamed Bread. CYTA-J. Food 2019, 17, 172–179. [Google Scholar] [CrossRef]
- Montemurro, M.; Celano, G.; De Angelis, M.; Gobbetti, M.; Rizzello, C.G.; Pontonio, E. Selection of Non-Lactobacillus Strains to Be Used as Starters for Sourdough Fermentation. Food Microbiol. 2020, 90, 103491. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Lattanzi, A.; De Angelis, M.; Celano, G.; Gobbetti, M. House Microbiotas as Sources of Lactic Acid Bacteria and Yeasts in Traditional Italian Sourdoughs. Food Microbiol. 2015, 52, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, S.; Van Beeck, W.; Allonsius, C.N.; van den Broek, M.F.; Lebeer, S. Applications of Plant-Based Fermented Foods and Their Microbes. Curr. Opin. Biotechnol. 2020, 61, 45–52. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Georgescu, L.A.; Vasile, M.A.; Rocha, J.M.; Bahrim, G.E. Selection of Wild Lactic Acid Bacteria Strains as Promoters of Postbiotics in Gluten-Free Sourdoughs. Microorganisms 2020, 8, 643. [Google Scholar] [CrossRef]
- Novotni, D.; Gänzle, M.; Rocha, J.M. Composition and Activity of Microbiota in Sourdough and Their Effect on Bread Quality and Safety. In Trends in Wheat and Bread Making; Elsevier: Amsterdam, The Netherlands, 2020; pp. 129–172. ISBN 9780128210482. [Google Scholar]




| Processing Days | |||||
|---|---|---|---|---|---|
| Parameters | Sample | 0 | 1 | 2 | 3 |
| pH | Quinoa | 6.55 ± 0.46 a,1 | 5.91 ± 0.09 a,1 | 4.39 ± 0.08 b,1 | 4.11 ± 0.12 b,1 |
| Amaranth | 6.72 ± 0.25 a,1 | 5.90 ± 0.35 b,1 | 4.27 ± 0.15 c,1 | 4.09 ± 0.08 c,1 | |
| Brown rice | 6.64 ± 0.25 a,1 | 5.70 ± 0.26 b,1,2 | 4.06 ± 0.20 c,1 | 3.97 ± 0.18 c,1 | |
| Wheat | 5.89 ± 0.14 a,2 | 5.24 ± 0.07 b,2 | 4.42 ±0.07 c,1 | 3.65 ± 0.17 d,1 | |
| TTA (ml NaOH/10 g) | Quinoa | 7.33 ± 0.29 a,1 | 6.98 ± 0.75 a,1 | 17.47 ± 1.82 b,2 | 28.25 ± 2.96 a,1 |
| Amaranth | 4.67 ± 1.26 a,1 | 10.83 ± 0.76 a,3 | 25.08 ± 1.48 a,2 | 29.50 ± 6.50 c,a,2 | |
| Brown rice | 3.93 ± 1.40 a,1 | 7.67 ± 2.25 a,3 | 10.73 ± 2.81 b,3 | 23.57 ± 4.01 a,b,2 | |
| Wheat | 2.70 ± 0.39 b,1 | 4.45 ± 0.31 b,1 | 11.41 ± 0.78 b,2 | 16.84 ± 1.31 b,2 | |
| L* | Quinoa | 62.44 ± 1.35 a,1 | 65.05 ± 0.47 a,1 | 63.64 ± 1.43 a,1 | 72.71 ± 3.81 b,2 |
| Amaranth | 80.09 ± 0.17 b,2 | 69.01 ± 0.61 b,1,3 | 72.63 ± 0.42 b,1 | 66.47 ± 1.06 c,3 | |
| Brown rice | 57.04 ± 0.50 c,3 | 62.27 ± 1.33 a,1 | 66.98 ± 0.76 a,2 | 65.39 ± 1.12 c,1,2 | |
| Wheat | 71.95 ± 0.35 1 | 73.71 ± 0.81 c,1 | 79.16 ± 0.72 c,3 | 81.59 ± 0.16 a,3 | |
| C* | Quinoa | 17.43 ± 0.26 a,1 | 17.59 ± 0.17 a,b,1 | 14.51 ± 2.38 a,1 | 13.23 ± 0.91 a,1 |
| Amaranth | 39.50 ± 0.49 b,2 | 29.48 ± 0.80 b,1,2 | 18.85 ± 0.79 a,1 | 18.36 ± 0.34 a,1 | |
| Brown rice | 13.09 ± 0.21 a,1 | 14.17 ± 0.42 a,1 | 15.12 ± 1.25 a,1 | 16.53 ± 0.24 a,1 | |
| Wheat | 11.67 ± 0.57 a,1 | 13.77 ± 0.22 a,1 | 13.77 ± 0.23 a,1 | 14.32 ± 0.08 a,1 | |
| h | Quinoa | 84.42 ± 0.09 a,1 | 85.50 ± 0.14 a,1,2 | 87.30 ± 0.07 b,2,3 | 88.13 ± 0.72 c,3 |
| Amaranth | 80.12 ± 0.06 b,2 | 82.85 ± 2.06 b,1 | 80.88 ± 0.09 a,2 | 79.09 ± 0.20 a,2 | |
| Brown rice | 79.85 ± 0.07 b,2 | 81.58 ± 0.28 b,2 | 87.76 ± 1.07 b,1 | 80.99 ± 0.13 a b,3,2 | |
| Wheat | 80.97 ± 0.32 b,1.2 | 82.77 ± 0.50 b,1 | 80.61 ± 0.09 a,2 | 82.20 ± 0.10 b,1,2 | |
| a* | Quinoa | 1.17 ± 0.02 a,1 | 0.91 ± 0.04 a,1,2 | 0.61 ± 0.09 b,2 3 | 0.43 ± 0.14 c,3 |
| Amaranth | 1.18 ± 0.03 a,1 | 4.41 ± 0.38 b,2 | 2.99 ± 0.15 c,3 | 3.47 ± 0.08 d,4 | |
| Brown rice | 2.31 ± 0.03 b,2 | 2.07 ± 0.07 c,2 | 2.54 ± 0.17 a,1 | 2.59 ± 0.02 a,1 | |
| Wheat | 2.57 ± 0.04 b,2 | 2.43 ± 0.07 c,2 | 2.24 ± 0.02 a,2,1 | 1.95 ± 0.03 b,1 | |
| b* | Quinoa | 12.04 ± 0.34 a,1,2 | 11.55 ± 0.17 a,2 | 11.45 ± 0.27 a,2 | 13.22 ± 0.92 b,1 |
| Amaranth | 25.24 ± 0.59 b,1 | 29.40 ± 0.79 b,2 | 18.58 ± 0.82 c,3 | 18.02 ± 0.33 c,3 | |
| Brown rice | 12.88 ± 0.21 a,1 | 14.02 ± 0.42 c,1 | 16.25 ± 0.61 b,2 | 16.33 ± 0.25 a,2 | |
| Wheat | 10.90 ± 0.77 c,1 | 12.49 ± 0.66 a,c,1 | 13.59 ± 0.23 d,2 | 14.19 ± 0.08 b,2 | |
| Sample | Acetic Acid (g/L) | Lactic Acid (g/L) |
|---|---|---|
| Quinoa | 3.92 ± 0.07 a | 0.33 ± 0.01 a |
| Amaranth | 3.90 ± 0.03 a | 0.32 ± 0.01 a |
| Brown rice | 4.45 ± 0.02 b | 0.28 ± 0.01 b |
| Wheat | 2.88 ± 0.05 c | 0.42 ± 0.01 c |
| Type of Sample | Sample | TYMC | NSY | LAB |
|---|---|---|---|---|
| Sourdough | Quinoa | 6.75 ± 0.08 a | 7.39 ± 0.05 a | 7.86 ± 0.10 a,b |
| Amaranth | 7.97 ± 0.08 b | 7.78 ± 0.10 b | 8.10 ± 0.06 a | |
| Brown rice | 7.84 ± 0.04 b | 7.67 ± 0.12 a,b | 8.01 ± 0.02 a,b | |
| Wheat | 3.94 ± 0.04 c | 6.41 ± 0.03 c | 7.40 ± 0.03 b | |
| Flour | Quinoa | <10 | 3.25 ± 0.22 d | 5.03 ± 0.08 c |
| Amaranth | 4.58 ± 0.06 d | 3.11 ± 0.10 d | <10 | |
| Brown rice | 3.39 ± 0.35 e | 3.66 ± 0.07 e | 5.37 ± 0.08 c | |
| Wheat | 3.98 ± 0.02 c | 3.92 ± 0.05 e | 3.79 ± 0.57 d | |
| Whole wheat | 2.66 ± 0.03 f | 2.69 ± 0.04 f | 2.69 ± 2.71 e |
| Type of Sample | Sample | TPC | Antioxidant Capacity | ||
|---|---|---|---|---|---|
| ABTS | ORAC | FRAP | |||
| Sourdough | Quinoa | 23.45 ± 0.61 a | 12.00 ± 0.31 a | 269.48 ± 0.58 c | 8.52 ± 0.31 a |
| Amaranth | 18.16 ± 0.34 b | 11.60 ± 0.39 a | 188.67 ± 0.55 d | 7.35 ± 0.41 b | |
| Brown rice | 9.77 ± 0.12 c | 10.28 ± 0.40 a,b | 125.00 ± 0.12 b,f | 6.05 ± 0.32 c,f | |
| Wheat | 33.03 ± 0.41 d | 31.84 ± 2.45 c | 142.93 ± 0.68 a | 8.65 ± 0.36 a | |
| Flour | Quinoa | 10.30 ± 0.22 c | 12.15 ± 1.18 a | 161.33 ± 5.98 a | 7.36 ± 0.43 b |
| Amaranth | 5.28 ± 0.97 e | 8.57 ± 0.26 b d | 120.68 ± 15.42 b,e | 4.40 ± 0.19 d,e | |
| Brown rice | 2.75 ± 0.10 f | 9.47 ± 0.15 a,d | 142.91 ± 18.73 a | 5.30 ± 0.36 c,d | |
| Wheat | 3.78 ± 0.20 g | 6.85 ± 0.11 d | 100.58 ± 0.25 e | 3.48 ± 0.34 e | |
| Whole wheat | 5.33 ± 0.21 e | 8.60 ± 0.32 b,d | 104.94 ± 1.42 e,f | 6.76 ± 0.59 b,f | |
| Sample | Total Aflatoxins |
|---|---|
| Quinoa | <LoQ b |
| Amaranth | 0.66 ± 0.23 a |
| Brown rice | <LoQ b |
| Wheat | <LoQ b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñalver, R.; Díaz-Vásquez, W.; Maulén, M.; Nieto, G. Sustainable Processes and Physico-Chemical Characterization of Artisanal Spontaneous Gluten Free Sourdough (Quinoa, Amaranth and Brown Rice) Compared to Wheat Sourdough. Sustainability 2024, 16, 3297. https://doi.org/10.3390/su16083297
Peñalver R, Díaz-Vásquez W, Maulén M, Nieto G. Sustainable Processes and Physico-Chemical Characterization of Artisanal Spontaneous Gluten Free Sourdough (Quinoa, Amaranth and Brown Rice) Compared to Wheat Sourdough. Sustainability. 2024; 16(8):3297. https://doi.org/10.3390/su16083297
Chicago/Turabian StylePeñalver, Rocío, Waldo Díaz-Vásquez, Mario Maulén, and Gema Nieto. 2024. "Sustainable Processes and Physico-Chemical Characterization of Artisanal Spontaneous Gluten Free Sourdough (Quinoa, Amaranth and Brown Rice) Compared to Wheat Sourdough" Sustainability 16, no. 8: 3297. https://doi.org/10.3390/su16083297
APA StylePeñalver, R., Díaz-Vásquez, W., Maulén, M., & Nieto, G. (2024). Sustainable Processes and Physico-Chemical Characterization of Artisanal Spontaneous Gluten Free Sourdough (Quinoa, Amaranth and Brown Rice) Compared to Wheat Sourdough. Sustainability, 16(8), 3297. https://doi.org/10.3390/su16083297