Effect of Inoculum Pretreatment and Substrate/Inoculum Ratio on Acidogenic Fermentation of Chemically Enhanced Primary Treatment Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate and Preparation Method
2.2. Inoculum Pretreatment Methods and Inoculum Activation
2.3. Effect of Inoculum Pretreatment Methods on VFA Production
2.4. Effect of Heat-Pretreated Inoculum and SIRs on the Production of VFAs
2.5. Analytical Methods
2.5.1. Chemical Indices Analysis
2.5.2. Calculations
2.5.3. Kinetic Analysis
2.6. Bacterial Community Analysis
3. Results and Discussion
3.1. Comparison of the Effects of Inoculum Pretreatment Methods and Activation on VFA Production
3.2. Effect of Different Inoculum Pretreatments on the Acidogenic Fermentation of CEPT Sludge
3.2.1. Acidogenic Fermentation Performance
3.2.2. VFA Composition
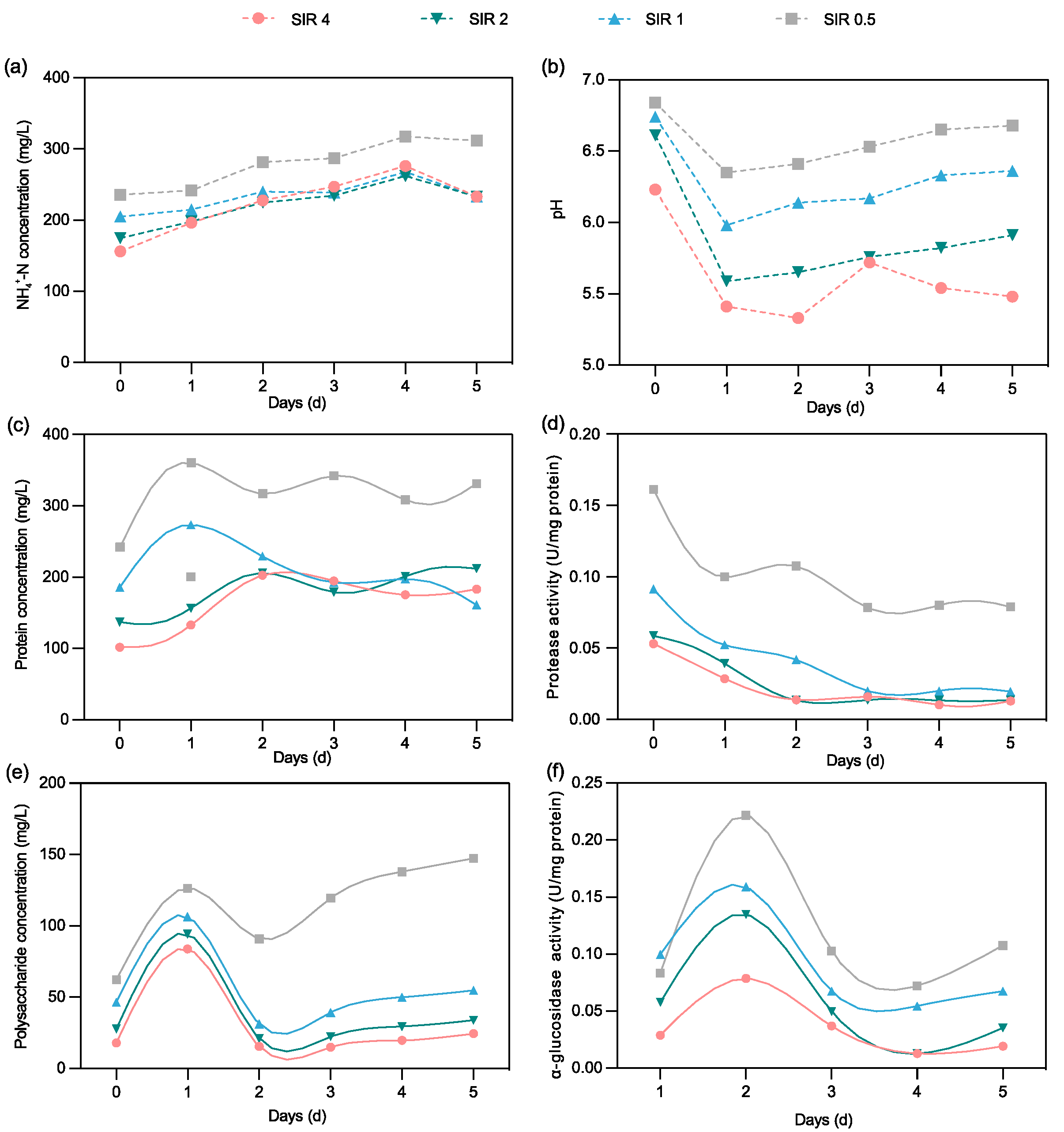
3.3. Effect of Different SIRs on the Acidogenic Fermentation of CEPT Sludge
3.3.1. SCOD Release and VFA Production
3.3.2. VFA Composition
3.3.3. Organics and Nutrients Release
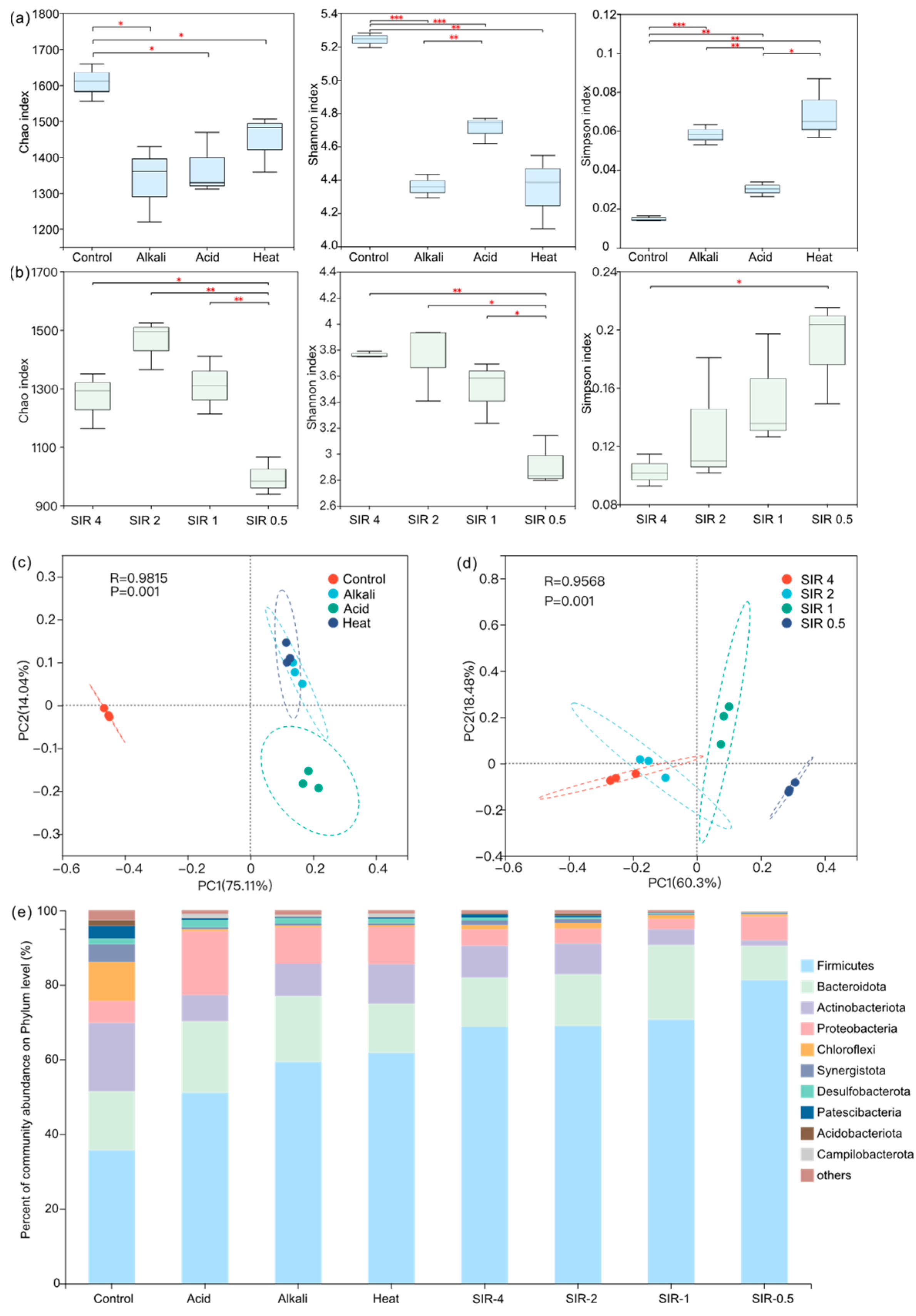
3.4. Microbial Community Analysis
3.4.1. Microbial Diversity
3.4.2. Microbial Community Analysis
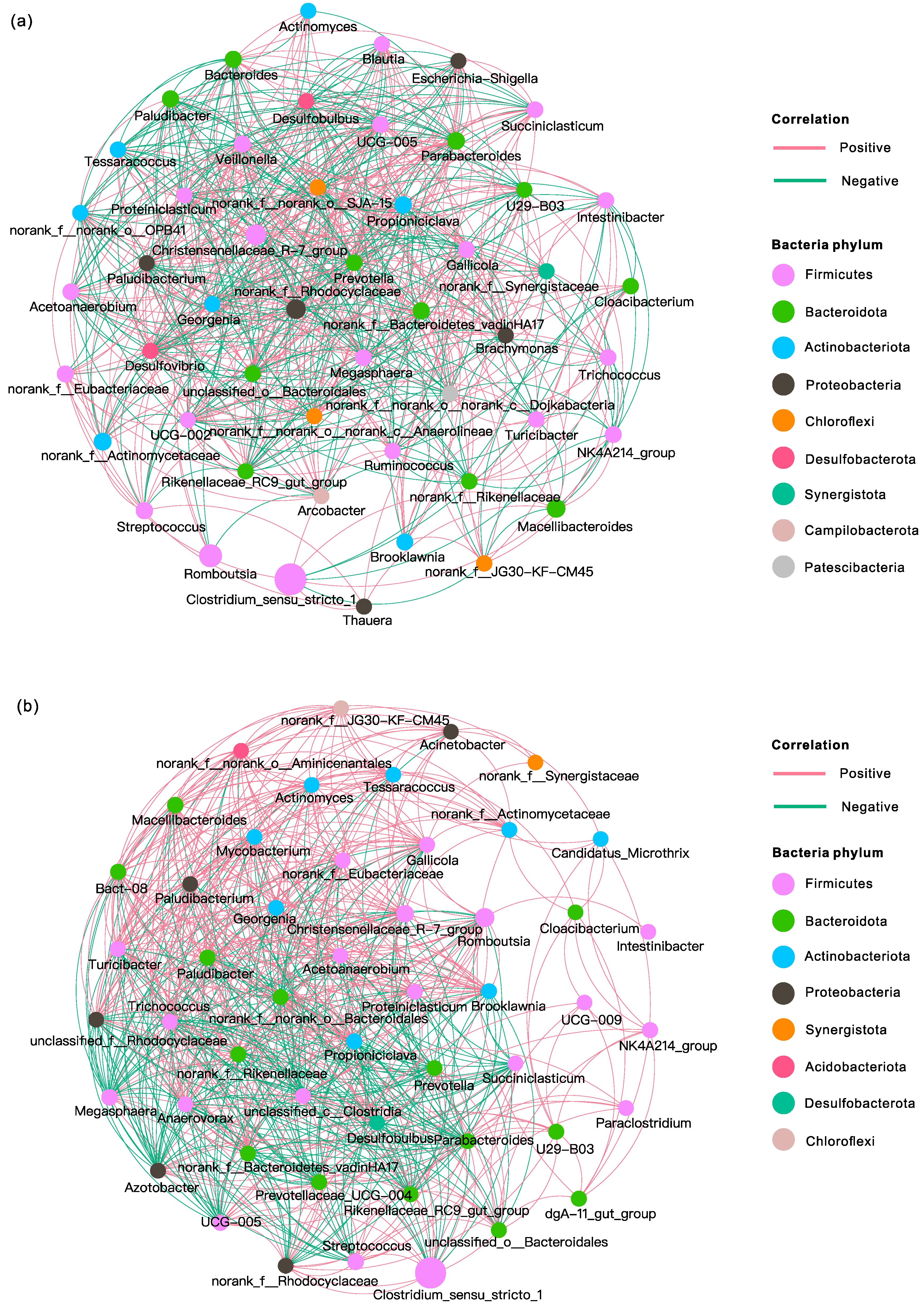
3.4.3. Microbial Correlation Network Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kacprzak, M.; Neczaj, E.; Fijalkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almas, A.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- Ignatowicz, K. The impact of sewage sludge treatment on the content of selected heavy metals and their fractions. Environ. Res. 2017, 156, 19–22. [Google Scholar] [CrossRef]
- Liu, H.; Han, P.; Liu, H.; Zhou, G.; Fu, B.; Zheng, Z. Full-scale production of VFAs from sewage sludge by anaerobic alkaline fermentation to improve biological nutrients removal in domestic wastewater. Bioresour. Technol. 2018, 260, 105–114. [Google Scholar] [CrossRef]
- Niero, L.; Norgren, R.; Kumpiene, J.; Jonsson, A. The effect of pH, temperature, and inoculum on the fermentation of pulp and paper biosludge: Increasing the nutrient availability for rearing of black soldier fly larvae. Biomass Convers. Biorefinery 2022, 13, 16685–16693. [Google Scholar] [CrossRef]
- Shewa, W.A.; Dagnew, M. Revisiting Chemically Enhanced Primary Treatment of Wastewater: A Review. Sustainability 2020, 12, 5928. [Google Scholar] [CrossRef]
- Lin, L.; Li, Y.-Y.; Li, X.-Y. Acidogenic sludge fermentation to recover soluble organics as the carbon source for denitrification in wastewater treatment: Comparison of sludge types. Front. Environ. Sci. Eng. 2018, 12, 3. [Google Scholar] [CrossRef]
- Jayakrishnan, U.; Deka, D.; Das, G. Regulation of volatile fatty acid accumulation from waste: Effect of inoculum pretreatment. Water Environ. Res. 2021, 93, 1019–1031. [Google Scholar] [CrossRef]
- Blasco, L.; Kahala, M.; Tampio, E.; Vainio, M.; Ervasti, S.; Rasi, S. Effect of Inoculum Pretreatment on the Composition of Microbial Communities in Anaerobic Digesters Producing Volatile Fatty Acids. Microorganisms 2020, 8, 581. [Google Scholar] [CrossRef]
- Montiel-Jarillo, G.; Gea, T.; Artola, A.; Fuentes, J.; Carrera, J.; Suárez-Ojeda, M.E. Towards PHA Production from Wastes: The Bioconversion Potential of Different Activated Sludge and Food Industry Wastes into VFAs Through Acidogenic Fermentation. Waste Biomass Valorization 2021, 12, 6861–6873. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Peng, Y.; Huang, W.; Liu, J.; Mironov, V.; Zhang, S. Deeper insights into the effects of substrate to inoculum ratio selection on the relationship of kinetic parameters, microbial communities, and key metabolic pathways during the anaerobic digestion of food waste. Water Res. 2022, 217, 118440. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, H.; Hua, D.; Zhao, B.; Mu, H.; Jin, F.; Meng, G.; Fang, X. Two-phase anaerobic digestion of lignocellulosic hydrolysate: Focusing on the acidification with different inoculum to substrate ratios and inoculum sources. Sci. Total Environ. 2020, 699, 134226. [Google Scholar] [CrossRef]
- Tawfik, A.; Ni, S.Q.; Awad, H.M.; Ismail, S.; Tyagi, V.K.; Khan, M.S.; Qyyum, M.A.; Lee, M. Recent Approaches for the Production of High Value-Added Biofuels from Gelatinous Wastewater. Energies 2021, 14, 4936. [Google Scholar] [CrossRef]
- Lin, L.; Li, X.Y. Acidogenic fermentation of iron-enhanced primary sedimentation sludge under different pH conditions for production of volatile fatty acids. Chemosphere 2018, 194, 692–700. [Google Scholar] [CrossRef]
- Sompong, O.T.; Prasertsan, P.; Birkeland, N.K. Evaluation of methods for preparing hydrogen-producing seed inocula under thermophilic condition by process performance and microbial community analysis. Bioresour. Technol. 2009, 100, 909–918. [Google Scholar]
- Gavala, H.N.; Yenal, U.; Skiadas, I.V.; Westermann, P.; Ahring, B.K. Mesophilic and thermophilic anaerobic digestion of primary and secondary sludge. Effect of pre-treatment at elevated temperature. Water Res. 2003, 37, 4561–4572. [Google Scholar] [CrossRef]
- Ministry of Construction of the People’s Republic of China. Determination Method for Municipal Sludge in Wastewater Treatment Plant; Ministry of Construction of the People’s Republic of China: Beijing, China, 2005. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005; p. 21. [Google Scholar]
- Yu, Y.; Ye, H.; Wu, D.; Shi, H.; Zhou, X. Chemoenzymatic quantification for monitoring unpurified polysaccharide in rich medium. Appl. Microbiol. Biotechnol. 2019, 103, 7635–7645. [Google Scholar] [CrossRef]
- Da Ros, C.; Conca, V.; Eusebi, A.L.; Frison, N.; Fatone, F. Sieving of municipal wastewater and recovery of bio-based volatile fatty acids at pilot scale. Water Res. 2020, 174, 115633. [Google Scholar] [CrossRef]
- Soomro, A.F.; Ni, Z.; Ying, L.; Liu, J. The effect of ISR on OFMSW during acidogenic fermentation for the production of AD precursor: Kinetics and synergies. RSC Adv. 2019, 9, 18147–18156. [Google Scholar] [CrossRef]
- Lin, L.; Li, R.-H.; Li, X.-Y. Recovery of organic resources from sewage sludge of Al-enhanced primary sedimentation by alkali pretreatment and acidogenic fermentation. J. Clean. Prod. 2018, 172, 3334–3341. [Google Scholar] [CrossRef]
- Ren, N.; Guo, W.; Wang, X.; Xiang, W.; Liu, B.; Wang, X.; Ding, J.; Chen, Z. Effects of different pretreatment methods on fermentation types and dominant bacteria for hydrogen production. Int. J. Hydrogen Energy 2008, 33, 4318–4324. [Google Scholar] [CrossRef]
- Toledo-Alarcón, J.; Cabrol, L.; Jeison, D.; Trably, E.; Steyer, J.P.; Tapia-Venegas, E. Impact of the microbial inoculum source on pre-treatment efficiency for fermentative H2 production from glycerol. Int. J. Hydrogen Energy 2020, 45, 1597–1607. [Google Scholar] [CrossRef]
- Shao, M.; Guo, L.; She, Z.; Gao, M.; Zhao, Y.; Sun, M.; Guo, Y. Enhancing denitrification efficiency for nitrogen removal using waste sludge alkaline fermentation liquid as external carbon source. Environ. Sci. Pollut. Res. 2019, 26, 4633–4644. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Idris, A. Release and recovery of phosphorus from wastewater treatment sludge via struvite precipitation. Desalination Water Treat. 2014, 52, 5696–5703. [Google Scholar] [CrossRef]
- Yin, J.; Yu, X.; Wang, K.; Shen, D. Acidogenic fermentation of the main substrates of food waste to produce volatile fatty acids. Int. J. Hydrogen Energy 2016, 41, 21713–21720. [Google Scholar] [CrossRef]
- Tomei, M.C.; Braguglia, C.M.; Mininni, G. Anaerobic degradation kinetics of particulate organic matter in untreated and sonicated sewage sludge: Role of the inoculum. Bioresour. Technol. 2008, 99, 6119–6126. [Google Scholar] [CrossRef] [PubMed]
- Jianguo, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Elefsiniotis, P.; Li, D. The effect of temperature and carbon source on denitrification using volatile fatty acids. Biochem. Eng. J. 2006, 28, 148–155. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Y. Reduction of N2O and NO Generation in Anaerobic−Aerobic (Low Dissolved Oxygen) Biological Wastewater Treatment Process by Using Sludge Alkaline Fermentation Liquid. Environ. Sci. Technol. 2011, 45, 2137–2143. [Google Scholar] [CrossRef]
- Gao, S.; Huang, Y.; Yang, L.; Wang, H.; Zhao, M.; Xu, Z.; Huang, Z.; Ruan, W. Evaluation the anaerobic digestion performance of solid residual kitchen waste by NaHCO3 buffering. Energy Convers. Manag. 2015, 93, 166–174. [Google Scholar] [CrossRef]
- Habicher, T.; John, A.; Scholl, N.; Daub, A.; Klein, T.; Philip, P.; Büchs, J. Introducing substrate limitations to overcome catabolite repression in a protease producing Bacillus licheniformis strain using membrane-based fed-batch shake flasks. Biotechnol. Bioeng. 2019, 116, 1326–1340. [Google Scholar] [CrossRef]
- Pu, Y.; Tang, J.; Wang, X.C.; Hu, Y.; Huang, J.; Pan, S.; Li, Y. Enhancing nitrogen removal from wastewater in sequencing batch reactors (SBRs) using additional carbon source produced from food waste acidogenic fermentation at different temperatures. Environ. Sci. Pollut. Res. Int. 2019, 26, 34645–34657. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.; David, A.; Menges, E.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Carvalho, P.N.; Lv, T.; Arias, C.; Brix, H.; Chen, Z. Microbial density and diversity in constructed wetland systems and the relation to pollutant removal efficiency. Water Sci. Technol. 2015, 73, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Iglesias, R.; Campanaro, S.; Treu, L.; Kennes, C.; Veiga, M.C. Valorization of sewage sludge for volatile fatty acids production and role of microbiome on acidogenic fermentation. Bioresour. Technol. 2019, 291, 121817. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Du, Y.; Liu, D.; Luo, J.; Yan, H.; Li, P.; Chen, J.; Zhang, Y. Enhanced bioproduction of volatile fatty acids from excess sludge by sodium alcohol ether sulphate. Chem. Eng. J. 2022, 450, 138288. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Hu, P.; Zou, L.; Li, Y. Carbon source and phosphorus recovery from iron-enhanced primary sludge via anaerobic fermentation and sulfate reduction: Performance and future application. Bioresour. Technol. 2019, 294, 122174. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-H.; Guo, X.; Li, B.; Lin, L.; Li, P.; Wen, L.; Liang, J.; Li, X. Iron-enhanced primary sedimentation and acidogenic sludge fermentation to achieve self-sufficient organic carbon supply for enhanced nutrient removal in wastewater treatment. Resour. Conserv. Recycl. 2021, 164, 105220. [Google Scholar] [CrossRef]
- Joyce, A.; Ijaz, U.Z.; Nzeteu, C.; Vaughan, A.; Shirran, S.L.; Botting, C.H.; Quince, C.; Abram, F. Linking Microbial Community Structure and Function During the Acidified Anaerobic Digestion of Grass. Front. Microbiol. 2018, 9, 540. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ouyang, K.; Long, T.; Liu, Z.; Li, Y.; Qiu, Q. Dynamic Variations in Rumen Fermentation Characteristics and Bacterial Community Composition during In Vitro Fermentation. Fermentation 2022, 8, 276. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, T.; Yin, J. Impact of N-butyryl-l-homoserine lactone-mediated quorum sensing on acidogenic fermentation under saline conditions: Insights into volatile fatty acids production and microbial community. Bioresour. Technol. 2023, 368, 128354. [Google Scholar] [CrossRef]
- He, Z.; Zou, Z.; Sun, Q.; Jin, H.; Yao, X.; Yang, W.; Tang, C.; Zhou, A.; Liu, W.; Ren, Y.; et al. Freezing-low temperature treatment facilitates short-chain fatty acids production from waste activated sludge with short-term fermentation. Bioresour. Technol. 2022, 347, 126337. [Google Scholar] [CrossRef] [PubMed]
- Rudakiya, D.M.; Narra, M. Microbial Community Dynamics in Anaerobic Digesters for Biogas Production. In Microbial Rejuvenation of Polluted Environment; Springer Singapore: Singapore, 2021; Volume 3, pp. 143–159. [Google Scholar]
- Guo, Y.; Hassan, F.U.; Li, M.; Tang, Z.; Peng, L.; Peng, K.; Yang, C. Effect of Hydrogen-Consuming Compounds on In Vitro Ruminal Fermentation, Fatty Acids Profile, and Microbial Community in Water Buffalo. Curr. Microbiol. 2022, 79, 220. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Unit | Mean Value |
|---|---|---|
| pH | 7.06 ± 0.03 * | |
| TS | g/L | 9.44 ± 0.14 |
| VS | g/L | 6.65 ± 0.22 |
| TCOD | mgCOD/L | 11,727.27 ± 29.02 |
| SCOD | mgCOD/L | 479.74 ± 32.40 |
| VFAs | mgCOD/L | 339.58 ± 48.95 |
| TN | mg/L | 529.97 ± 20.64 |
| TP | mg/L | 250.02 ± 10.03 |
| Proteins | mg/L | 421.17 ± 29.18 |
| Polysaccharide | mg/L | 503.72 ± 7.33 |
| Step | Parameters | Inoculum Pretreatments | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Alkali | Acid | Heat | 4 | 2 | 1 | 0.5 | ||
| Hydrolysis | R2 | 0.9325 | 0.9156 | 0.9170 | 0.9389 | 0.9684 | 0.9824 | 0.9835 | 0.9655 |
| α | 0.2728 ± 0.1768 | 0.3709 ± 0.1960 | 0.1896 ± 0.0401 | 0.2655 ± 0.0214 | 0.2242 ± 0.0104 | 0.1970 ± 0.0065 | 0.0965 ± 0.0033 | 0.0632 ± 0.0137 | |
| kH | 0.0941 ± 0.0738 | 0.1241 ± 0.0843 | 0.2564 ± 0.0897 | 0.4888 ± 0.0954 | 0.9195 ± 0.1430 | 0.9442 ± 0.1059 | 0.7176 ± 0.0784 | 0.2668 ± 0.0891 | |
| α•kH | 0.0257 | 0.0460 | 0.0486 | 0.1298 | 0.2062 | 0.1860 | 0.0692 | 0.0169 | |
| Acidogenesis | R2 | 0.9209 | 0.9545 | 0.9651 | 0.9354 | 0.9782 | 0.9858 | 0.9196 | 0.9371 |
| kVFAs | 1.074 ± 0.0939 | 1.185 ± 0.0595 | 1.021 ± 0.0496 | 1.798 ± 0.1595 | 2.214 ± 0.1197 | 3.094 ± 0.1435 | 1.437 ± 0.1388 | 0.6387 ± 0.0461 | |
| Parameters | Unit | Inoculum Pretreatments | SIRs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Alkali | Acid | Heat | 4 | 2 | 1 | 0.5 | ||
| SCOD | mgCOD/L | 1867.0 ± 94.5 | 2946.8 ± 45.2 | 2630.5 ± 61.2 | 3764.5 ± 350.2 | 2957.1 ± 9.7 | 2697.6 ± 144.9 | 1632.2 ± 9.7 | 860.5 ± 38.6 |
| VFAs | mgCOD/L | 1330.4 ± 68.2 | 2888.6 ± 61.3 | 2557.8 ± 45.5 | 3215.9 ± 367.9 | 2723.0 ± 234.2 | 2265.5 ± 27.6 | 1453.4 ± 24.5 | 827.0 ± 3.6 |
| NH4-N | mgN/L | 262.1 ± 19.7 | 157.3 ± 20.1 | 147.5 ± 12.6 | 168.0 ± 14.3 | 262.1 ± 0.1 | 216.2 ± 0.3 | 187.1 ± 0.8 | 187.1 ± 0.8 |
| PO4-P | mgP/L | 7.4 ± 1.0 | 34.4 ± 7.3 | 35.2 ± 3.4 | 36.8 ± 6.7 | 26.2 ± 2.4 | 28.0 ± 1.4 | 23.2 ± 0.4 | 26.5 ± 0.7 |
| VFAs yield | mgCOD/gVS | 131.4 ± 6.5 | 372.6 ± 6.5 | 282.4 ± 4.8 | 401.9 ± 56.4 | 331.4 ± 35.2 | 266.9 ± 6.9 | 147.6 ± 3.7 | 84.7 ± 0.5 |
| Hydrolysis efficiency | % | 16.3 ± 0.9 | 26.2 ± 0.4 | 23.1 ± 0.5 | 32.7 ± 3.6 | 24.6 ± 0.1 | 22.4 ± 1.2 | 13.6 ± 0.1 | 7.2 ± 0.3 |
| Acidogenesis efficiency | % | 11.4 ± 0.6 | 26.6 ± 1.4 | 21.4 ± 1.7 | 27.1 ± 3.0 | 22.7 ± 0.4 | 17.3 ± 0.4 | 11.5 ± 1.2 | 6.2 ± 0.03 |
| N release | % | 47.2 ± 2.6 | 29.7 ± 3.8 | 33.7 ± 7.6 | 29.6 ± 0.5 | 49.5 ± 0.02 | 40.8 ± 0.05 | 35.3 ± 0.2 | 37.2 ± 0.1 |
| P release | % | 2.9 ± 0.4 | 13.8 ± 2.9 | 14.1 ± 1.4 | 14.7 ± 2.7 | 10.5 ± 0.9 | 11.2 ± 0.6 | 9.3 ± 0.1 | 0.6 ± 0.3 |
| VFAs/NH4-N | mgCOD/mgN | 5.1 ± 0.3 | 18.6 ± 3.05 | 17.7 ± 1.1 | 19.3 ± 3.8 | 11.3 ± 0.04 | 12.5 ± 0.7 | 8.7 ± 0.1 | 4.4 ± 0.2 |
| VFAs/PO4-P | mgCOD/mgP | 224.3 ± 35.7 | 74.0 ± 2.8 | 74.6 ± 9.4 | 85.7 ± 4.9 | 113.5 ± 10.7 | 96.3 ± 0.4 | 70.2 ± 0.6 | 32.5 ± 2.3 |
| Class | Oder (%) | Inoculum Pretreatments | SIRs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Alkali | Acid | Heat | 4 | 2 | 1 | 0.5 | ||
| Clostridia | Clostridia | 3.66 | 23.03 | 13.85 | 29.13 | 33.38 | 39.79 | 42.95 | 49.54 |
| Bacteroidia | Bacteroidia | 15.30 | 16.35 | 18.20 | 11.41 | 12.86 | 13.21 | 19.44 | 9.16 |
| Clostridia | Clostridia | 15.87 | 13.20 | 8.75 | 10.08 | 17.16 | 13.09 | 10.25 | 8.89 |
| Clostridia | Clostridia | 6.21 | 5.42 | 3.64 | 4.95 | 9.75 | 6.23 | 2.72 | 0.26 |
| Gammaproteobacteria | Gammaproteobacteria | 1.73 | 6.96 | 13.55 | 6.62 | 2.41 | 1.81 | 1.43 | 1.00 |
| Clostridia | Clostridia | 1.42 | 6.51 | 7.12 | 5.69 | 1.71 | 3.05 | 5.77 | 2.00 |
| Actinobacteria | Actinobacteria | 5.81 | 3.88 | 3.37 | 4.45 | 4.39 | 2.95 | 1.36 | 0.32 |
| Bacilli | Bacilli | 3.61 | 2.44 | 2.67 | 3.43 | 2.52 | 2.88 | 4.60 | 3.16 |
| Negativicutes | Negativicutes | 0.49 | 2.11 | 6.80 | 1.55 | 0.16 | 0.27 | 0.55 | 12.86 |
| Actinobacteria | Actinobacteria | 3.02 | 2.60 | 1.88 | 3.25 | 1.18 | 1.73 | 0.75 | 0.23 |
| Synergistia | Synergistia | 4.77 | 0.55 | 0.55 | 0.49 | 1.19 | 1.05 | 0.35 | 0.40 |
| Bacilli | Bacilli | 1.34 | 1.21 | 1.35 | 1.28 | 1.46 | 1.05 | 0.95 | 0.79 |
| Actinobacteria | Actinobacteria | 2.79 | 0.87 | 0.67 | 1.27 | 1.08 | 1.20 | 0.46 | 0.14 |
| Negativicutes | Negativicutes | 0.14 | 1.80 | 2.56 | 1.41 | 0.06 | 0.30 | 0.36 | 2.08 |
| Clostridia | Clostridia | 0.77 | 1.23 | 1.70 | 1.54 | 0.75 | 0.67 | 1.10 | 0.53 |
| Gammaproteobacteria | Gammaproteobacteria | 0.22 | 0.44 | 0.69 | 0.55 | 0.31 | 0.45 | 0.24 | 4.87 |
| Clostridia | Clostridia | 0.71 | 1.28 | 1.61 | 1.85 | 0.74 | 0.74 | 0.50 | 0.28 |
| Alphaproteobacteria | Alphaproteobacteria | 1.53 | 0.77 | 0.88 | 0.98 | 0.71 | 0.70 | 0.42 | 0.17 |
| Bacteroidia | Bacteroidia | 0.28 | 1.17 | 0.83 | 1.56 | 0.22 | 0.43 | 0.50 | 0.05 |
| Chloroflexia | Chloroflexia | 2.05 | 0.30 | 0.38 | 0.25 | 0.55 | 0.60 | 0.43 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Pan, L.; Wang, L. Effect of Inoculum Pretreatment and Substrate/Inoculum Ratio on Acidogenic Fermentation of Chemically Enhanced Primary Treatment Sludge. Sustainability 2024, 16, 3347. https://doi.org/10.3390/su16083347
Tian L, Pan L, Wang L. Effect of Inoculum Pretreatment and Substrate/Inoculum Ratio on Acidogenic Fermentation of Chemically Enhanced Primary Treatment Sludge. Sustainability. 2024; 16(8):3347. https://doi.org/10.3390/su16083347
Chicago/Turabian StyleTian, Lu, Lei Pan, and Lin Wang. 2024. "Effect of Inoculum Pretreatment and Substrate/Inoculum Ratio on Acidogenic Fermentation of Chemically Enhanced Primary Treatment Sludge" Sustainability 16, no. 8: 3347. https://doi.org/10.3390/su16083347
APA StyleTian, L., Pan, L., & Wang, L. (2024). Effect of Inoculum Pretreatment and Substrate/Inoculum Ratio on Acidogenic Fermentation of Chemically Enhanced Primary Treatment Sludge. Sustainability, 16(8), 3347. https://doi.org/10.3390/su16083347





