Approaches for Sampling and Sample Preparation for Microplastic Analysis in Laundry Effluents
Abstract
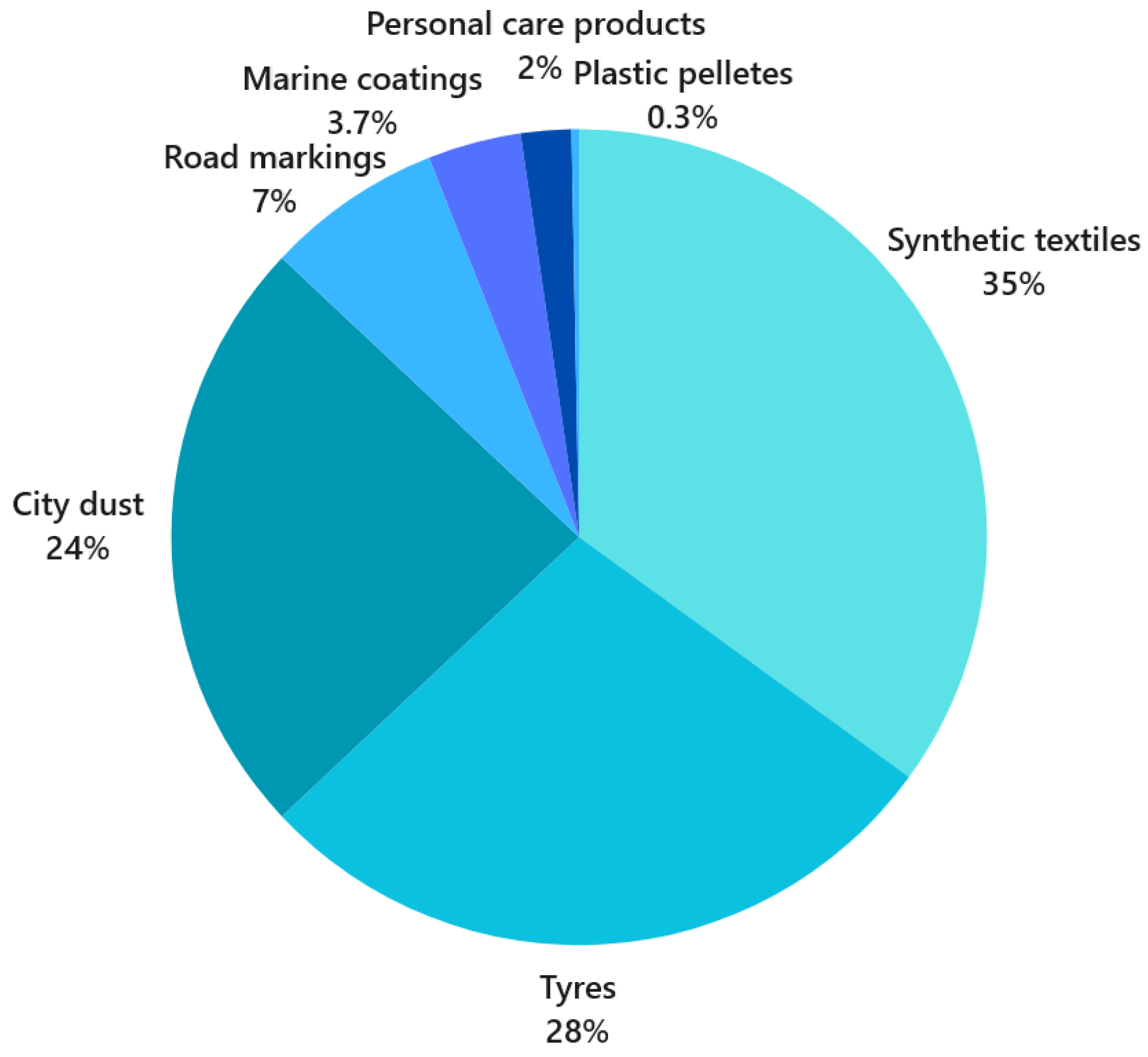
1. Introduction
- (1)
- Plastics are characterised by a complex chemical structure and high molecular mass, which makes them less susceptible to degradation [4];
- (2)
- Atmospheric conditions (solar radiation, water temperature, and abrasion processes) lead to photo-induced cleavage and cross-linking of the polymer chain as well as to thermally induced degradation of the polymer chain [5], releasing potentially toxic additives (brominated flame retardants, antioxidants, light stabilisers, plasticisers, and pigments) [6,7];
- (3)
- Fragmentation leads to an increase in the specific surface area and hydrophobicity of MPs [8], making them a good medium for various types of pollutants, such as persistent, bio-accumulative, and toxic chemicals (PBTCs) [9,10,11]. These particles absorb not only persistent organic pollutants (POPs) such as polychlorinated biphenyls (PCBs) and polycyclic aromatic hydrocarbons (PAHs) [12] but also heavy metals [13,14], pharmaceuticals [15], and pathogenic organisms [16].
2. Textiles as a Main Source of Fibre Fragments
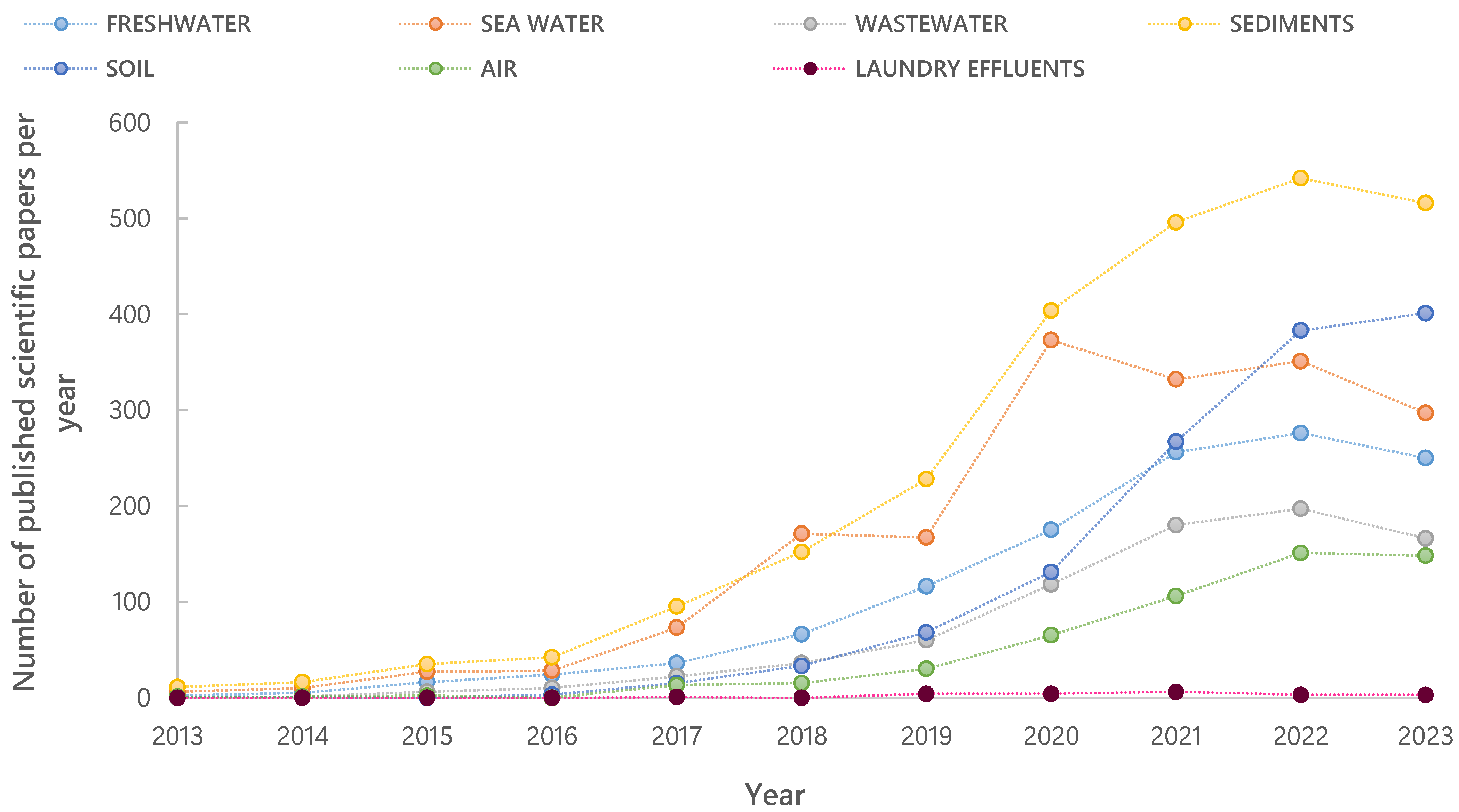
3. Challenges in the Analysis of Microplastic Particles in Environmental Samples
4. Sampling Strategies for Wastewater Samples
Laundry Effluents Sampling
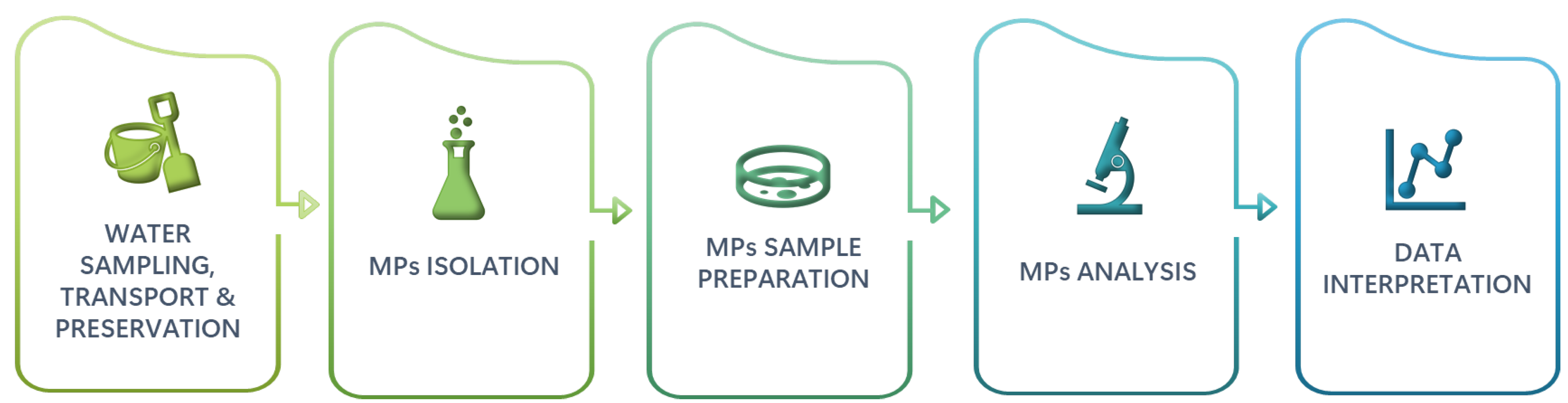
5. Sample Preparation and Isolation of Microplastic Particles
- (1)
- Particle size » size-based approach;
- (2)
- Density of the particles » density-based approach;
- (3)
- Chemical composition of the particles » chemical composition-based approach.
5.1. Size-Based Approach
5.2. Density-Based Approach
- (1)
- (2)
- Some fractions of microplastic particles, such as PET/PVC, LDPE/PP, and HDPE/PP, cannot be separated and require additional processing, as their density ranges overlap;
- (3)
- The efficiency of MPs isolation is inversely proportional to particle size; the smallest proportion of isolated particles are those with smaller dimensions [135];
- (4)
- Due to their hydrophobic nature, the particles often coalesce into agglomerates to which various pollutants and microorganisms are adsorbed and form biofilms [41]. Agglomerates exhibit altered specific density, which in turn alters their distribution along the water column. As a result, incomplete isolation of the particles occurs, leading to an underestimation of the amount of MP particles present in the sample [136]. The separation is then carried out over several cycles and with an extended flotation time [116] but also in combination with peroxide digestion [133].
5.3. Chemical Composition-Based Approach
- (1)
- Type of reagent (acids, bases, and oxidising agents or enzymes);
- (2)
- Reagent concentration;
- (3)
- Processing temperature (room temperature or elevated temperature);
- (4)
- Duration of digestion (from a few hours to days);
- (5)
- Successive treatments.
5.4. Advanced Techniques for the Isolation of Microplastic Particles
- (1)
- Individual particles with a partially hydrophilic character may remain in the aqueous phase;
- (2)
- For samples with a high content of organic substances, it is necessary to carry out a digestion beforehand;
- (3)
- When mixing a sample containing surface-active substances (e.g., wastewater from laundry), partial emulsification of the oil may occur;
- (4)
- Some particles may remain in the oil during the washing process.
6. Internal Quality Control
Mitigation of Cross-Contamination
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- ISO 24187:2023; Principles for the Analysis of Microplastics Present in the Environment. ISO (International Organization for Standardization): Geneva, Switzerland, 2023.
- Carpenter, E.J.; Smith, K.L., Jr. Plastics on the Sargasso Sea Surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. B 2009, 364, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the degradation of (micro)plastics: Degradation methods, influencing factors, environmental impacts. Sci. Total Environ. 2022, 806, 151312. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.; René Mikkelsen, R.; Kristiansen, S.; Hinge, M. Real-time ageing of polyesters with varying diols. Mater. Chem. Phys. 2021, 261, 124240. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, H.; Peter, K.T.; Gonzalez, M.; Wetzel, J.; Wu, C.; Hu, X.; Prat, J.; Mudrock, E.; Hettinger, R.; et al. A ubiquitous tire rubber-derived chemical induces acute mortality in coho salmon. Science 2021, 371, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Kim, S.W.; Kim, T.Y.; Waldman, W.R. The Global Plastic Toxicity Debt. Environ. Sci. Technol. 2021, 55, 2717–2719. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wanga, J. The chemical behaviors of microplastics in marine environment: A review. Mar. Pollut. Bull. 2019, 142, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gouin, T.; Roche, N.; Lohmann, R.; Hodges, G. A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic. Environ. Sci. Technol. 2011, 45, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in Aquatic Ecosystems. Angew. Chem. Int. Ed. Engl. 2017, 56, 1720–1739. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Hollman, P.C.; Peters, R.J. Potential Health Impact of Environmentally Released Micro- and Nanoplastics in the Human Food Production Chain: Experiences from Nanotoxicology. Environ. Sci. Technol. 2015, 49, 8932–8947. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf. Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Guo, X.; Hu, G.; Fan, X.; Jia, H. Sorption properties of cadmium on microplastics: The common practice experiment and A two-dimensional correlation spectroscopic study. Ecotoxicol. Environ. Saf. 2020, 190, 110118. [Google Scholar] [CrossRef] [PubMed]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, N.; Sarmah, A.K.; Bank, M.S.; You, S.; Ok, Y.S. Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport. Environ. Int. 2021, 149, 106367. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in surface waters of urban rivers: Concentration, sources, and associated bacterial assemblages. Ecosphere 2016, 7, 01556. [Google Scholar] [CrossRef]
- Aves, A.R.; Revell, L.E.; Gaw, S.; Ruffell, H.; Schuddeboom, A.; Wotherspoon, N.E.; LaRue, M.; McDonald, A.J. First evidence of microplastics in Antarctic snow. Cryosphere 2022, 16, 2127–2145. [Google Scholar] [CrossRef]
- Xiao, S.; Cui, Y.; Brahney, J.; Mahowald, N.M.; Li, Q. Long-distance atmospheric transport of microplastic fibers influenced by their shapes. Nat. Geosci. 2023, 16, 863–870. [Google Scholar] [CrossRef]
- Jiang, J. Occurrence of microplastics and its pollution in the environment: A review. Sustain. Prod. Consum. 2018, 13, 16–23. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Raveendran, S.; Singh, S.; Pillai, S. Environmental Impacts of Microplastics and Nanoplastics: A Current Overview. Front. Microbiol. 2021, 12, 768297. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) 2023/2055 of 25 September 2023 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Synthetic Polymer Microparticles. Available online: https://eur-lex.europa.eu/eli/reg/2023/2055/oj (accessed on 12 February 2024).
- Ng, K.L.; Obbard, J.P. Prevalence of microplastics in Singapore’s coastal marine environment. Mar. Pollut. Bull. 2016, 52, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhang, K.; Chen, X.; Shi, H.; Luo, Z.; Wu, C. Sources and distribution of microplastics in China’s largest inland lake—Qinghai Lake. Environ. Pollut. 2018, 235, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf. Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Levermore, J.M.; Smith, T.E.L.; Kelly, F.J.; Wright, S.L. Detection of Microplastics in Ambient Particulate Matter Using Raman Spectral Imaging and Chemometric Analysis. Anal. Chem. 2020, 92, 8732–8740. [Google Scholar] [CrossRef] [PubMed]
- Samandra, S.; Johnston, J.M.; Jaeger, J.E.; Symons, B.; Xie, S.; Currell, M.; Ellis, A.V.; Clarke, B.O. Microplastic contamination of an unconfined groundwater aquifer in Victoria, Australia. Sci. Total Environ. 2022, 802, 149727. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, E.; Twiddy, M.; Rotchell, J.M. Microplastic contamination of drinking water: A systematic review. PLoS ONE 2020, 15, 236838. [Google Scholar] [CrossRef] [PubMed]
- Anbumani, S.; Kakkar, P. Ecotoxicological effects of microplastics on biota: A review. Environ. Sci. Pollut. Res. Int. 2018, 25, 14373–14396. [Google Scholar] [CrossRef]
- Schymanski, D.; Oßmann, B.E.; Benismail, N.; Boukerma, K.; Dallmann, G.; von der Esch, E.; Fischer, D.; Fischer, F.; Gilliland, D.; Glas, K.; et al. Analysis of microplastics in drinking water and other clean water samples with micro-Raman and micro-infrared spectroscopy: Minimum requirements and best practice guidelines. Anal. Bioanal. Chem. 2021, 413, 5969–5994. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, A.P.; Tehrani-Bagha, A. A review on microplastic emission from textile materials and its reduction techniques. Polym. Degrad. Stab. 2022, 199, 109901. [Google Scholar] [CrossRef]
- Herzke, D.; Ghaffari, P.; Sundet, J.H.; Tranang, C.A.; Halsband, C. Microplastic Fiber Emissions From Wastewater Effluents: Abundance, Transport Behavior and Exposure Risk for Biota in an Arctic Fjord. Front. Environ. Sci. 2021, 9, 662168. [Google Scholar] [CrossRef]
- Hosseini Ravandi, S.A.; Valizadeh, M. Properties of Fibers and Fabrics That Contribute to Human comfort. In Improving Comfort in Clothing; Song, G., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 61–78. ISBN 978-1-84569-539-2. [Google Scholar]
- AATCC TM212-2021; Test Method for Fiber Fragment Release During Home Laundering. American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 2021.
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Madhumitha, C.T.; Karmegam, N.; Biruntha, M.; Arun, A.; Al Kheraif, A.A.; Kim, W.; Kumar, P. Extraction, identification, and environmental risk assessment of microplastics in commercial toothpaste Chemosphere 2022, 296, 133976. 296. [CrossRef]
- Gregory, M.R. Plastic ‘Scrubbers’ in Hand Cleansers: A Further (and Minor) Source for Marine Pollution Identified. Mar. Pollut. Bull. 1996, 32, 867–871. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; pp. 12–16. ISBN 978-2-8317-1827-9. [Google Scholar]
- Talvitie, J.; Mikola, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater?—A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Bretas Alvim, C.; Bes-Piá, M.A.; Mendoza-Roca, J.A. Separation and identification of microplastics from primary and secondary effluents and activated sludge from wastewater treatment plants. Chem. Eng. J. 2020, 402, 126293. [Google Scholar] [CrossRef]
- Suaria, G.; Achtypi, A.; Perold, V.; Lee, J.R.; Pierucci, A.; Bornman, T.G.; Aliani, S.; Ryan, P.G. Microfibers in oceanic surface waters: A global characterization. Sci. Adv. 2020, 6, eaay8493. [Google Scholar] [CrossRef] [PubMed]
- Athey, S.N.; Adams, J.K.; Erdle, L.M.; Jantunen, L.M.; Helm, P.A.; Finkelstein, S.A.; Diamond, M.L. The Widespread Environmental Footprint of Indigo Denim Microfibers from Blue Jeans. Environ. Sci. Technol. Lett. 2020, 7, 840–847. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J.; et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Gullo, M.P.; Gentile, G.; Di Pace, E.; Cocca, M.; Gelabert, L.; Brouta-Agnésa, M.; Rovira, A.; Escudero, R.; Villalba, R.E.; et al. Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ. Pollut. 2017, 236, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Sinner, H. Über das Waschen mit Haushaltswaschmaschinen, 2nd ed.; Auflage Haus und Heim Verlag: Hamburg, Germany, 1960; pp. 9–10. [Google Scholar]
- Pušić, T.; Vojnović, B.; Flinčec Grgac, S.; Čurlin, M.; Malinar, R. Particle Shedding from Cotton and Cotton-Polyester Fabrics in the Dry State and in Washes. Polymers 2023, 15, 3201. [Google Scholar] [CrossRef] [PubMed]
- Hartline, N.L.; Bruce, N.J.; Karba, S.N.; Ruff, E.O.; Sonar, S.U.; Holden, P.A. Microfiber Masses Recovered from Conventional Machine Washing of New or Aged Garments. Environ. Sci. Technol. 2016, 50, 11532–11538. [Google Scholar] [CrossRef] [PubMed]
- Šaravanja, A.; Pušić, T.; Dekanić, T. Microplastics in Wastewater by Washing Polyester Fabrics. Materials 2022, 15, 2683. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.K.; Uddin, M.E.; Jamal, M. Detection and removal of microplastics in wastewater: Evolution and impact. Environ. Sci. Pollut. Res. Int. 2021, 28, 16925–16947. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Arias, C.A.; Nguyen, L.X.; Salvadó, V.; Brix, H. Occurrence and behavior of emerging contaminants in surface water and a restored wetland. Chemosphere 2012, 88, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; Brandsma, S.H.; van Velzen, M.J.; Vethaak, A.D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Antune, J.; Pais, J.; Pequeno, J.; Caetano, P.S.; Rocha, F.; Sobral, P.; Costa, M.H. Distribution patterns of microplastics in subtidal sediments from the Sado river estuary and the Arrábida marine park, Portugal. Front. Environ. Sci. 2022, 10, 998513. [Google Scholar] [CrossRef]
- Kaile, N.; Lindivat, M.; Elío, J.; Thuestad, G.; Crowley, Q.; Hoell, I.A. Preliminary Results From Detection of Microplastics in Liquid Samples Using Flow Cytometry. Front. Mar. Sci. 2020, 7, 552688. [Google Scholar] [CrossRef]
- Frias, J.P.; Otero, V.; Sobral, P. Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Mar. Environ. Res. 2014, 95, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Sinclair, C.J.; Bradley, E.; Ju-Nam, Y.; Ojeda, J.J. Microplastic Monitoring at Different Stages in a Wastewater Treatment Plant Using Reflectance Micro-FTIR Imaging. Front. Environ. Sci. 2020, 8, 145. [Google Scholar] [CrossRef]
- Luo, Y.; Gibson, C.T.; Tang, Y.; Naidu, R.; Fang, C. Characterising microplastics in shower wastewater with Raman imaging. Sci. Total Environ. 2022, 811, 152409. [Google Scholar] [CrossRef] [PubMed]
- Okutan, H.M.; Sağir, Ç.; Fontaine, C.; Nauleau, B.; Kurtulus, B.; Le Coustumer, P.; Razack, M. One-Dimensional Experimental Investigation of Polyethylene Microplastic Transport in a Homogeneous Saturated Medium. Front. Environ. Sci. 2022, 10, 885875. [Google Scholar] [CrossRef]
- Sharaf Din, K.; Khokhar, M.F.; Butt, S.I.; Qadir, A.; Younas, F. Exploration of microplastic concentration in indoor and outdoor air samples: Morphological, polymeric, and elemental analysis. Sci. Total Environ. 2024, 908, 168398. [Google Scholar] [CrossRef] [PubMed]
- Čurlin, M.; Pušić, T.; Vojnović, B.; Dimitrov, N. Particle Characterization of Washing Process Effluents by Laser Diffraction Technique. Materials 2021, 14, 7781. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, N.; Čurlin, M.; Pušić, T.; Vojnović, B. Application of GC/MS Pyrolysis for Assessment Residues of Textile Composites after Filtration of Washing and Rinsing Effluents. Separations 2022, 9, 292. [Google Scholar] [CrossRef]
- Majewsky, M.; Bitter, H.; Eiche, E.; Horn, H. Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC). Sci. Total Environ. 2016, 568, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef] [PubMed]
- Hanvey, J.S.; Lewis, P.J.; Lavers, J.L.; Crosbie, N.D.; Pozode, K.; Clarke, B.O. A review of analytical techniques for quantifying microplastics in sediments. Anal. Methods 2017, 9, 1369–1383. [Google Scholar] [CrossRef]
- Derraik, J.G. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Serra, T.; Colomer, J.; Solari, L. Suspended sediments mediate microplastic sedimentation in unidirectional flows. Sci. Total Environ. 2023, 890, 164363. [Google Scholar] [CrossRef] [PubMed]
- Chubarenko, I.; Bagaev, A.; Zobkov, M.; Esiukova, E. On some physical and dynamical properties of microplastic particles in marine environment. Mar. Pollut. Bull. 2016, 108, 105–112. [Google Scholar] [CrossRef]
- Montoto-Martínez, T.; Meléndez-Díez, C.; Melián-Ramírez, A.; Hernández-Brito, J.J.; Gelado-Caballero, M.D. Comparison between the traditional Manta net and an innovative device for microplastic sampling in surface marine waters. Mar. Pollut. Bull. 2022, 185, 114237. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowicz, A.; Zubrowska-Sudol, M.; Krasinski, A.; Sudol, M. Cross-Contamination as a Problem in Collection and Analysis of Environmental Samples Containing Microplastics—A Review. Sustainability 2021, 13, 12123. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hou, Q.; Xue, Y.; Jian, Y.; Wang, L. Pollution characteristics and fate of microfibers in the wastewater from textile dyeing wastewater treatment plant. Water Sci. Technol. 2018, 78, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Dubaish, F.; Liebezeit, G. Suspended Microplastics and Black Carbon Particles in the Jade System, Southern North Sea. Water Air Soil Pollut. 2013, 224, 1352. [Google Scholar] [CrossRef]
- Conley, K.; Clum, A.; Deepe, J.M.; Lane, H.; Beckingham, B.A. Wastewater treatment plants as a source of microplastics to an urban estuary: Removal efficiencies and loading per capita over one year. Water Res. X 2019, 3, 100030. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, E.A.; Habibi, M.; Haddad, E.; Hasanin, M.; Angel, D.L.; Booth, A.M.; Sabbah, I. Microplastic distributions in a domestic wastewater treatment plant: Removal efficiency, seasonal variation and influence of sampling technique. Sci. Total Environ. 2021, 752, 141880. [Google Scholar] [CrossRef] [PubMed]
- Alavian Petroody, S.S.; Hashemi, S.H.; van Gestel, C.A.M. Factors affecting microplastic retention and emission by a wastewater treatment plant on the southern coast of Caspian Sea. Chemosphere 2020, 261, 128179. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, A.; Mitchella, J.; Arsem, N. Extraction and identification of microplastic particles from secondary wastewater treatment plant (WWTP) effluent. Anal. Methods 2017, 9, 1412–1418. [Google Scholar] [CrossRef]
- Hoellein, T.J.; McCormick, A.R.; Hittie, J.; London, M.G.; Scott, J.W.; Kelly, J.J. Longitudinal patterns of microplastic concentration and bacterial assemblages in surface and benthic habitats of an urban river. Freshw. Sci. 2017, 36, 491–507. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, B.; Pileggi, V.; Chang, S. Methods to recover and characterize microplastics in wastewater treatment plants. Case Stud. Chem. Environ. Eng. 2022, 5, 100183. [Google Scholar] [CrossRef]
- Zeri, C.; Adamopoulou, A.; Koi, A.; Koutsikos, N.; Lytras, E.; Dimitriou, E. Rivers and Wastewater-Treatment Plants as Microplastic Pathways to Eastern Mediterranean Waters: First Records for the Aegean Sea, Greece. Sustainability 2021, 13, 5328. [Google Scholar] [CrossRef]
- Green, D.S.; Kregting, L.; Boots, B.; Blockley, D.J.; Brickle, P.; da Costa, M.; Crowley, Q. A comparison of sampling methods for seawater microplastics and a first report of the microplastic litter in coastal waters of Ascension and Falkland Islands. Mar. Pollut. Bull. 2018, 137, 695–701. [Google Scholar] [CrossRef]
- Barrows, A.P.; Neumann, C.A.; Berger, M.L.; Shaw, S.D. Grab vs. neuston tow net: A microplastic sampling performance comparison and possible advances in the field. Anal. Methods 2017, 9, 1446–1453. [Google Scholar] [CrossRef]
- Jemaa, S.; Mahfouz, C.; Kazour, M.; Lteif, M.; Hassoun, A.E.R.; Ghsoub, M.; Amara, R.; Khalaf, G.; Fakhri, M. Floating Marine Litter in Eastern Mediterranean From Macro to Microplastics: The Lebanese Coastal Area as a Case Study. Front. Environ. Sci. 2021, 9, 699343. [Google Scholar] [CrossRef]
- Vermaire, J.C.; Pomeroy, C.; Herczegh, S.M.; Haggart, O.; Murphy, M.T. Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. Facets 2017, 2, 301–314. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, K.; Xion, X. Microplastic Pollution in Inland Waters Focusing on Asia. In Freshwater Microplastics, 1st ed.; Wagner, M., Lambert, S., Eds.; Springer: Cham, Switzerland, 2017; Volume 58, pp. 85–99. ISBN 978-3-319-61614-8. [Google Scholar]
- Tamminga, M.; Stoewer, S.C.; Fischer, E.K. On the representativeness of pump water samples versus manta sampling in microplastic analysis. Environ. Pollut. 2019, 254, 112970. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Kärrman, A.; Rotander, A.; Hassellöv, M. Comparison between manta trawl and in situ pump filtration methods, and guidance for visual identification of microplastics in surface waters. Environ. Sci. Pollut. Res. Int. 2020, 27, 5559–5571. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Lenz, R.; Labrenz, M. Small Microplastic Sampling in Water: Development of an Encapsulated Filtration Device. Water 2018, 10, 1055. [Google Scholar] [CrossRef]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. Water Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Funck, M.; Yildirim, A.; Nickel, C.; Schram, J.; Schmidt, T.C.; Tuerk, J. Identification of microplastics in wastewater after cascade filtration using Pyrolysis-GC-MS. MethodsX 2019, 7, 100778. [Google Scholar] [CrossRef] [PubMed]
- Pušić, T.; Vojnović, B.; Čurlin, M.; Bekavac, I.; Kaurin, T.; Grgić, K.; Šimić, K.; Kovačević, Z. Assessment of Polyester Fabrics, Effluents and Filtrates after Standard and Innovative Washing Processes. Microplastics 2022, 1, 494–504. [Google Scholar] [CrossRef]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Cheng, J.J.; Venditti, R.A. Microfibers generated from the laundering of cotton, rayon and polyester based fabrics and their aquatic biodegradation. Mar. Pollut. Bull. 2019, 142, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qiao, F.; Lei, K.; Li, H.; Kang, Y.; Cui, S.; An, L. Microfiber release from different fabrics during washing. Environ. Pollut. 2019, 249, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibers from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Pirc, U.; Vidmar, M.; Mozer, A.; Kržan, A. Emissions of microplastic fibers from microfiber fleece during domestic washing. Environ. Sci. Pollut. Res. Int. 2016, 23, 22206–22211. [Google Scholar] [CrossRef] [PubMed]
- Varg, J.E.; Svanbäck, R. Multi stress system: Microplastics in freshwater and their effects on host microbiota. Sci. Total Environ. 2023, 856 Pt 2, 159106. [Google Scholar] [CrossRef] [PubMed]
- Smulders, E. Laundry Detergents; Wiley-VCH: Weinheim, Germany, 2002; pp. 38–98. ISBN 3-527-30520-3. [Google Scholar]
- Hu, L.; Chernick, M.; Hinton, D.E.; Shi, H. Microplastics in Small Waterbodies and Tadpoles from Yangtze River Delta, China. Environ. Sci. Technol. 2018, 52, 8885–8893. [Google Scholar] [CrossRef] [PubMed]
- Barrows, A.P.W.; Christiansen, K.S.; Bode, E.T.; Hoellein, T.J. A watershed-scale, citizen science approach to quantifying microplastic concentration in a mixed land-use river. Water Res. 2018, 147, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Lombardini, E.; Martellini, T.; Katsoyiannis, A.; Fossi, M.C.; Corsolini, S. Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR. Chemosphere 2017, 175, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Huppertsberg, S.; Knepper, T.P. Validation of an FT-IR microscopy method for the determination of microplastic particles in surface waters. MethodsX 2020, 7, 100874. [Google Scholar] [CrossRef]
- Cai, H.; Chen, M.; Chen, Q.; Du, F.; Liu, J.; Shi, H. Microplastic quantification affected by structure and pore size of filters. Chemosphere 2020, 257, 127198. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are There Nanoplastics in Your Personal Care Products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef]
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Peter Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Oßmann, B.E.; Sarau, G.; Schmitt, S.W.; Holtmannspötter, H.; Christiansen, S.H.; Dicke, W. Development of an optimal filter substrate for the identification of small microplastic particles in food by micro-Raman spectroscopy. Anal. Bioanal. Chem. 2017, 409, 4099–4109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Kanaya, Y.; Nakajima, R.; Tsuchiya, M.; Nomaki, H.; Kitahashi, T.; Fujikura, K. Characterization of microplastics on filter substrates based on hyperspectral imaging: Laboratory assessments. Environ. Pollut. 2020, 263, 114296. [Google Scholar] [CrossRef] [PubMed]
- Cabernard, L.; Roscher, L.; Lorenz, C.; Gerdts, G.; Primpke, S. Comparison of Raman and Fourier Transform Infrared Spectroscopy for the Quantification of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2018, 52, 13279–13288. [Google Scholar] [CrossRef] [PubMed]
- Käppler, A.; Windrich, F.; Löder, M.G.; Malanin, M.; Fischer, D.; Labrenz, M.; Eichhorn, K.J.; Voit, B. Identification of microplastics by FTIR and Raman microscopy: A novel silicon filter substrate opens the important spectral range below 1300 cm(-1) for FTIR transmission measurements. Anal. Bioanal. Chem. 2015, 407, 6791–6801. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef] [PubMed]
- Simon-Sánchez, L.; Grelaud, M.; Garcia-Orellana, J.; Ziveri, P. River Deltas as hotspots of microplastic accumulation: The case study of the Ebro River (NW Mediterranean). Sci. Total Environ. 2019, 687, 1186–1196. [Google Scholar] [CrossRef]
- Zhao, S.; Danley, M.; Ward, J.E.; Li, D.; Mincer, T.J. An approach for extraction, characterization and quantitation of microplastic in natural marine snow using Raman microscopy. Anal. Methods 2017, 9, 1470–1478. [Google Scholar] [CrossRef]
- Peng, G.; Zhu, B.; Yang, D.; Su, L.; Shi, H.; Li, D. Microplastics in sediments of the Changjiang Estuary, China. Environ. Pollut. 2017, 225, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Nieva, J.; Perales, J.A.; Gonzales-Leal, J.M.; Rojo-Nieto, R. A new analytical technique for extraction and quantification of microplastics in marine sediments focused on the easy implementation and repeatability. Anal. Methods 2017, 9, 6371–6378. [Google Scholar] [CrossRef]
- Pagter, E.; Frias, J.; Nash, R. Microplastics in Galway Bay: A comparison of sampling and separation methods. Mar. Pollut. Bull. 2018, 135, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.; Murphy, F.; Ewins, C. Validation of density separation for the rapid recovery of microplastics from sediment. Anal. Methods 2017, 9, 1491–1498. [Google Scholar] [CrossRef]
- Corcoran, P.L.; Biesinger, M.C.; Grifi, M. Plastics and beaches: A degrading relationship. Mar. Pollut. Bull. 2009, 58, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Crichton, E.M.; Noël, M.; Gies, E.A.; Ross, P.S. A novel, density-independent and FTIR-compatible approach for the rapid extraction of microplastics from aquatic sediments. Anal. Methods 2017, 9, 1419–1428. [Google Scholar] [CrossRef]
- Nuelle, M.T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Coppock, R.L.; Cole, M.; Lindeque, P.K.; Queirós, A.M.; Galloway, T.S. A small-scale, portable method for extracting microplastics from marine sediments. Environ. Pollut. 2017, 230, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Imhof, H.K.; Laforsch, C.; Wiesheu, A.C.; Schmid, J.; Anger, P.M.; Niessner, R.; Ivleva, N.P. Pigments and plastic in limnetic ecosystems: A qualitative and quantitative study on microparticles of different size classes. Water Res. 2016, 98, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Kedzierski, M.; Le Tilly, V.; César, G.; Sire, O.; Bruzaud, S. Efficient microplastics extraction from sand. A cost effective methodology based on sodium iodide recycling. Mar. Pollut. Bull. 2017, 115, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and identification of microplastics from soil and sewage sludge. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.Q.; Fu, J.G.; Gu, G.H. Flotability and flotation separation of polymer materials modulated by wetting agents. Waste Manag. 2014, 34, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Nava, V.; Leoni, B. Comparison of Different Procedures for Separating Microplastics from Sediments. Water 2021, 13, 2854. [Google Scholar] [CrossRef]
- Keene, J.; Turner, A. Microplastics in coastal urban sediments: Discrepancies in concentration and character revealed by different approaches to sample processing. Sci. Total Environ. 2023, 865, 161140. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzawi, M.S.M.; Kefer, S.; Weißer, J.; Reichel, J.; Schwaller, C.; Glas, K.; Knoop, O.; Drewes, J.E. Validation of Sample Preparation Methods for Microplastic Analysis in Wastewater Matrices—Reproducibility and Standardization. Water 2020, 12, 2445. [Google Scholar] [CrossRef]
- Enders, K.; Tagg, A.S.; Labrenz, M. Evaluation of Electrostatic Separation of Microplastics From Mineral-Rich Environmental Samples. Front. Environ. Sci. 2020, 8, 112. [Google Scholar] [CrossRef]
- Enders, K.; Lenz, R.; Beer, S.; Stedmon, C.A. Extraction of microplastic from biota: Recommended acidic digestion destroys common plastic polymers. ICES J. Mar. Sci. 2017, 74, 326–331. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, D.; Munoz, M.; Nieto-Sandoval, J.; Romera-Castillo, C.; de Pedro, Z.M.; Casas, J.A. Insights into the degradation of microplastics by Fenton oxidation: From surface modification to mineralization. Chemosphere 2022, 309, 136809. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.S.; Rocha-Santos, T.; Prata, J.C.; Duarte, A.C.; Girão, A.V.; Lopes, P.; Cristovão, T.; da Costa, J.P. A straightforward method for microplastic extraction from organic-rich freshwater samples. Sci. Total Environ. 2022, 815, 152941. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, T.G. A novel method for extraction, quantification, and identification of microplastics in CreamType of cosmetic products. Sci. Rep. 2021, 11, 18074. [Google Scholar] [CrossRef] [PubMed]
- Schrank, I.; Möller, J.N.; Imhof, H.K.; Hauenstein, O.; Zielke, F.; Agarwal, S.; Löder, M.G.J.; Greiner, A.; Laforsch, C. Microplastic sample purification methods—Assessing detrimental effects of purification procedures on specific plastic types. Sci. Total Environ. 2022, 833, 154824. [Google Scholar] [CrossRef] [PubMed]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, F.; Fischer, E.K. Various Digestion Protocols Within Microplastic Sample Processing—Evaluating the Resistance of Different Synthetic Polymers and the Efficiency of Biogenic Organic Matter Destruction. Front. Environ. Sci. 2020, 8, 572424. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 2015, 111, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Dehaut, A.; Cassone, A.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef]
- Cole, M.; Webb, H.; Lindeque, P.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 4258. [Google Scholar] [CrossRef] [PubMed]
- Süssmann, J.; Krause, T.; Martin, D.; Walz, E.; Greiner, R.; Rohn, S.; erstin Fischer, E.K.; Fritsche, J. Evaluation and optimisation of sample preparation protocols suitable for the analysis of plastic particles present in seafood. Food Control 2021, 125, 107969. [Google Scholar] [CrossRef]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, C. Recent advances in the analysis methodologies for microplastics in aquatic organisms: Current knowledge and research challenges. Anal. Methods 2020, 12, 2944–2957. [Google Scholar] [CrossRef]
- Maw, M.M.; Boontanon, N.; Fujii, S.; Boontanon, S.K. Rapid and efficient removal of organic matter from sewage sludge for extraction of microplastics. Sci. Total Environ. 2022, 853, 158642. [Google Scholar] [CrossRef] [PubMed]
- Sujathan, S.; Kniggendorf, A.K.; Kumar, A.; Roth, B.; Rosenwinkel, K.H.; Nogueira, R. Heat and Bleach: A Cost-Efficient Method for Extracting Microplastics from Return Activated Sludge. Arch. Environ. Contam. Toxicol. 2017, 73, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Han, J.; Zhou, H.; Lau, Y.L.; An, W.; Wie, P.; Cheung, S.G.; Yang, Y.; Tam, N.F. Development of a digestion method for determining microplastic pollution in vegetal-rich clayey mangrove sediments. Sci. Total Environ. 2020, 707, 136030. [Google Scholar] [CrossRef] [PubMed]
- Tagg, A.S.; Harrison, J.P.; Ju-Nam, Y.; Sapp, M.; Bradley, E.L.; Sinclair, C.J.; Ojeda, J.J. Fenton’s reagent for the rapid and efficient isolation of microplastics from wastewater. Chem. Commun. 2017, 53, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Mbachu, O.; Jenkins, G.; Pratt, C.; Kaparaju, P. Enzymatic purification of microplastics in soil. MethodsX 2021, 8, 101254. [Google Scholar] [CrossRef] [PubMed]
- Courtene-Jones, W.; Quinn, B.; Murphy, F.; Gary, S.F.; Narayanaswamy, B.E. Optimisation of enzymatic digestion and validation of specimen preservation methods for the analysis of ingested microplastics. Anal. Methods 2017, 9, 1437–1445. [Google Scholar] [CrossRef]
- Catarino, A.I.; Macchia, V.; Sanderson, W.G.; Thompson, R.C.; Henry, T.B. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibers fallout during a meal. Environ. Pollut. 2018, 237, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.; Vethaak, A.D.; Carney Almroth, B.; Ariese, F.; van Velzen, M.J.M.; Hassellöv, M.; Leslie, H.A. Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar. Pollut. Bull. 2017, 122, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Vroom, R.J.E.; Koelmans, A.A.; Besseling, E.; Halsband, C. Aging of microplastics promotes their ingestion by marine zooplankton. Environ. Pollut. 2017, 231, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gaspéri, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Seghers, J.; Stefaniak, E.A.; La Spina, R.; Cella, C.; Mehn, D.; Gilliland, D.; Held, A.; Jacobsson, U.; Emteborg, H. Preparation of a reference material for microplastics in water—Evaluation of homogeneity. Anal. Bioanal. Chem. 2022, 414, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Lievens, S.; Vervoort, E.; Poma, G.; Covaci, A.; Van Der Borght, M. A Production and Fractionation Protocol for Polyvinyl Chloride Microplastics. Methods Protoc. 2023, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Stéphane Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Turner, S.; Horton, A.A.; Rose, N.L.; Hall, C. A temporal sediment record of microplastics in an urban lake, London, UK. J. Paleolimnol. 2019, 61, 449–462. [Google Scholar] [CrossRef]
- Wiesheu, A.C.; Anger, P.M.; Baumann, T.; Niessnera, R.; Ivleva, N.P. Raman microspectroscopic analysis of fibers in beverages. Anal. Methods 2016, 8, 5722–5725. [Google Scholar] [CrossRef]
- Harris, L.S.T.; La Beur, L.; Olsen, A.Y.; Smith, A.; Eggers, L.; Pedersen, E.; Van Brocklin, J.; Brander, S.M.; Larson, S. Temporal Variability of Microparticles Under the Seattle Aquarium, Washington State: Documenting the Global COVID-19 Pandemic. Environ. Toxicol. Chem. 2022, 41, 917–930. [Google Scholar] [CrossRef]
- Miller, E.; Sedlak, M.; Lin, D.; Box, C.; Holleman, C.; Rochman, C.M.; Sutton, R. Recommended Best Practices for Collecting, Analyzing, and Reporting Microplastics in Environmental Media: Lessons Learned from Comprehensive Monitoring of San Francisco Bay. J. Hazard. Mater. 2020, 409, 124770. [Google Scholar] [CrossRef] [PubMed]
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.J.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.E.; Thompson, R.C. The deep sea is a major sinkfor microplastic debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef] [PubMed]
- Remy, F.; Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Lepoint, G. When Microplastic Is Not Plastic: The Ingestion of Artificial Cellulose Fibers by Macrofauna Living in Seagrass Macrophytodetritus. Environ. Sci. Technol. 2015, 49, 11158–11166. [Google Scholar] [CrossRef]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Wiggin, K.J.; Holland, E.B. Validation and application of cost and time effective methods for the detection of 3-500 μm sized microplastics in the urban marine and estuarine environments surrounding Long Beach, California. Mar. Pollut. Bull. 2019, 143, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Löder, M.G.J.; Gerdts, G. Methodology Used for the Detection and Identification of Microplastics—A Critical Appraisal. In Marine Anthropogenic Litter, 1st ed.; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 201–227. ISBN 978-3-319-16509-7. [Google Scholar]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Kankanige, D.; Babel, S. Smaller-sized micro-plastics (MPs) contamination in single-use PET-bottled water in Thailand. Sci. Total Environ. 2020, 717, 137232. [Google Scholar] [CrossRef] [PubMed]
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.J.; Koelmans, A.A. Plastic in north sea fish. Environ. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef] [PubMed]
- Torre, M.; Digka, N.; Anastasopoulou, A.; Tsangaris, C.; Mytilineou, C. Anthropogenic microfibers pollution in marine biota. A new and simple methodology to minimize airborne contamination. Mar. Pollut. Bull. 2016, 113, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Foekema, E.M.; Van Franeker, J.A.; Leopold, M.F.; Kühn, S.; Bravo Rebolledo, E.L.; Heße, E.; Mielke, L.; IJzer, J.; Kamminga, P.; et al. Microplastic in a macro filter feeder: Humpback whale Megaptera novaeangliae. Mar. Pollut. Bull. 2015, 95, 248–252. [Google Scholar] [CrossRef] [PubMed]


| Polymer Type | Polymer Density (g cm−3) | Polymer Type | Polymer Density (g cm−3) |
|---|---|---|---|
| LDPE | 0.89–0.93 | PVAL | 1.19–1.31 |
| HDPE | 0.94–0.98 | PFTE | 2.10–2.30 |
| PP | 0.85–0.92 | PVC | 1.16–1.58 |
| PS | 1.04–1.10 | PMMA | 1.17–1.20 |
| PA | 1.12–1.15 | PET | 1.37–1.45 |
| PES | 1.24–2.30 | PC | 1.20–1.22 |
| PAN | 1.09–1.20 | PU | 1.20–1.26 |
| POM | 1.41–1.61 | CA | 1.22–1.24 |
| Water Solubility at 25 °C (g dm−3) | Density (g cm−3) | Toxicity | Price per 100 g (EUR) | References | |
|---|---|---|---|---|---|
| Sodium chloride, NaCl | 360 | 1.0–1.2 | LOW | 3.4 | [118,120,121,122] |
| Sodium bromide, NaBr | 943 | 1.37–1.4 | LOW | 18.77 | [123] |
| Sodium tungstate dihydrate, Na2WO4 x 2 H2O | 742 | 1.4 | LOW | 58.4 | [122] |
| Sodium polytungstate, 3 Na2WO4 x 9 WO3 x H2O | 3100 | 1.4 | LOW | 332 | [124] |
| Calcium chloride, CaCl2 | 811 | 1.30–1.35 | LOW | 43.2 | [28,125] |
| Zinc chloride, ZnCl2 | 4320 | 1.5–1.8 | HIGH | 17.24 | [126,127,128] |
| Zinc bromide, ZnBr2 | 4470 | 1.71 | HIGH | 34.4 | [122] |
| Sodium iodide, NaI | 1842 | 1.6–1.8 | HIGH | 108 | [118,125,126,129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojnović, B.; Mihovilović, P.; Dimitrov, N. Approaches for Sampling and Sample Preparation for Microplastic Analysis in Laundry Effluents. Sustainability 2024, 16, 3401. https://doi.org/10.3390/su16083401
Vojnović B, Mihovilović P, Dimitrov N. Approaches for Sampling and Sample Preparation for Microplastic Analysis in Laundry Effluents. Sustainability. 2024; 16(8):3401. https://doi.org/10.3390/su16083401
Chicago/Turabian StyleVojnović, Branka, Petra Mihovilović, and Nino Dimitrov. 2024. "Approaches for Sampling and Sample Preparation for Microplastic Analysis in Laundry Effluents" Sustainability 16, no. 8: 3401. https://doi.org/10.3390/su16083401
APA StyleVojnović, B., Mihovilović, P., & Dimitrov, N. (2024). Approaches for Sampling and Sample Preparation for Microplastic Analysis in Laundry Effluents. Sustainability, 16(8), 3401. https://doi.org/10.3390/su16083401







