Neonicotinoid Effects on Soil Microorganisms: Responses and Mitigation Strategies
Abstract
:1. Introduction
2. Neonicotinoids and Their Use
| NNIs | Chemical Structure | Target Pests | Application Mode | Formulation and Application Details |
|---|---|---|---|---|
| Acetamiprid (ACE) |  | Aphids; Thrips; Mirids; Spider mites | Foliar spray or used as a soil drench | Formulated as soluble granules for spray application |
| Clothianidin (CLO) | 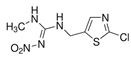 | Corn rootworm, Southern corn billbug, Chinch flea beetle, corn leaf aphid | Seed treatment use on corn and canola | Flowable concentrate prepared for use as a seed treatment |
| Dinotefuran (DNF) | 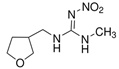 | Whiteflies, Mealybugs, Thrips, Aphids | Soil incorporation, foliar application | Soluble concentrates, granules, soluble granules |
| Imidacloprid (IMI) | 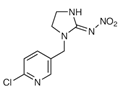 | Sucking and soil insects, Plant hoppers, Aphids, Termites | Seed treatment | Granules that are mixed with water and applied as a spray |
| Nitenpyram (NIT) | 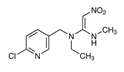 | Aphids; Thrips; Whitefly; Fleas; Ticks | Rice; Greenhouse crops; veterinary situations | Dusting powder, granules, drops |
| Thiacloprid (THA) | 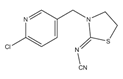 | Aphids; Pollen beetles; Blossom midge | Seed treatments | Oil dispersions, soluble concentrates, and granules |
| Thiamethoxam (THM) | 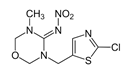 | Aphids; Whiteflies; Thrips; Lacewings; Leafhoppers; Mealybugs; Wireworms | Vegetables, including brassicas, cucurbits, fruiting vegetables | Flowable concentrates for seed treatments, water dispersible granules, and suspension concentrates |
3. Effect of Neonicotinoid Pesticides on Soil Microorganisms
3.1. Neonicotinoids’ Impact on Soil Microbial Activity
3.2. Neonicotinoids’ Impact on Soil Microbial Composition
3.3. Effects of Neonicotinoids on the Metabolic Process of Soil Microorganisms
4. Strategies for Mitigating Neonicotinoids Effects on Soil Health
5. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic Strategies for Improving Crop Yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Alsafran, M.; Rizwan, M.; Usman, K.; Saleem, M.H.; Jabri, H.A. Neonicotinoid Insecticides in the Environment: A Critical Review of Their Distribution, Transport, Fate, and Toxic Effects. J. Environ. Chem. Eng. 2022, 10, 108485. [Google Scholar] [CrossRef]
- Guerrieri, A.; Dong, L.; Bouwmeester, H.J. Role and Exploitation of Underground Chemical Signaling in Plants. Pest Manag. Sci. 2019, 75, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Wu, W. Soil and Crop Management Strategies to Ensure Higher Crop Productivity within Sustainable Environments. Sustainability 2019, 11, 1485. [Google Scholar] [CrossRef]
- Dilnashin, H.; Birla, H.; Hoat, T.X.; Singh, H.B.; Singh, S.P.; Keswani, C. Applications of Agriculturally Important Microorganisms for Sustainable Crop Production. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 403–415. ISBN 978-0-12-818469-1. [Google Scholar]
- Hermans, S.M.; Lear, G.; Case, B.S.; Buckley, H.L. The Soil Microbiome: An Essential, but Neglected, Component of Regenerative Agroecosystems. iScience 2023, 26, 106028. [Google Scholar] [CrossRef]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental Risks and Challenges Associated with Neonicotinoid Insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kania, J.; Kmiecik, E.; Malina, G.; Wątor, K. Fate of Selected Neonicotinoid Insecticides in Soil–Water Systems: Current State of the Art and Knowledge Gaps. Chemosphere 2020, 255, 126981. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, X.; Tu, C.; Long, T.; Bu, Y.; Wang, H.; Jeyakumar, P.; Jiang, J.; Deng, S. Remediation Technologies for Neonicotinoids in Contaminated Environments: Current State and Future Prospects. Environ. Int. 2023, 178, 108044. [Google Scholar] [CrossRef]
- Vryzas, Z. The Plant as Metaorganism and Research on Next-Generation Systemic Pesticides—Prospects and Challenges. Front. Microbiol. 2016, 7, 227404. [Google Scholar] [CrossRef]
- Radolinski, J.; Wu, J.; Xia, K.; Hession, W.C.; Stewart, R.D. Plants Mediate Precipitation-Driven Transport of a Neonicotinoid Pesticide. Chemosphere 2019, 222, 445–452. [Google Scholar] [CrossRef]
- Fischer, N.; Costa, C.P.; Hur, M.; Kirkwood, J.S.; Woodard, S.H. Impacts of Neonicotinoid Insecticides on Bumble Bee Energy Metabolism Are Revealed under Nectar Starvation. Sci. Total Environ. 2024, 912, 169388. [Google Scholar] [CrossRef] [PubMed]
- Wintermantel, D.; Odoux, J.-F.; Decourtye, A.; Henry, M.; Allier, F.; Bretagnolle, V. Neonicotinoid-Induced Mortality Risk for Bees Foraging on Oilseed Rape Nectar Persists despite EU Moratorium. Sci. Total Environ. 2020, 704, 135400. [Google Scholar] [CrossRef] [PubMed]
- Sponsler, D.B.; Grozinger, C.M.; Hitaj, C.; Rundlöf, M.; Botías, C.; Code, A.; Lonsdorf, E.V.; Melathopoulos, A.P.; Smith, D.J.; Suryanarayanan, S.; et al. Pesticides and Pollinators: A Socioecological Synthesis. Sci. Total Environ. 2019, 662, 1012–1027. [Google Scholar] [CrossRef]
- Zaller, J.G.; König, N.; Tiefenbacher, A.; Muraoka, Y.; Querner, P.; Ratzenböck, A.; Bonkowski, M.; Koller, R. Pesticide Seed Dressings Can Affect the Activity of Various Soil Organisms and Reduce Decomposition of Plant Material. BMC Ecol. 2016, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ren, C.; Sun, H.; Min, L. Sorption, Desorption and Degradation of Neonicotinoids in Four Agricultural Soils and Their Effects on Soil Microorganisms. Sci. Total Environ. 2018, 615, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Chen, Z.; Lu, X.; Huang, Y.; Zhou, Y.; Zhang, Q.; Wang, D.; Li, J. Effects on Soil Microbial Community after Exposure to Neonicotinoid Insecticides Thiamethoxam and Dinotefuran. Sci. Total Environ. 2020, 725, 138328. [Google Scholar] [CrossRef]
- Cycoń, M.; Markowicz, A.; Borymski, S.; Wójcik, M.; Piotrowska-Seget, Z. Imidacloprid Induces Changes in the Structure, Genetic Diversity and Catabolic Activity of Soil Microbial Communities. J. Environ. Manag. 2013, 131, 55–65. [Google Scholar] [CrossRef]
- Cai, Z.; Ma, J.; Wang, J.; Cai, J.; Yang, G.; Zhao, X. Impact of the Novel Neonicotinoid Insecticide Paichongding on Bacterial Communities in Yellow Loam and Huangshi Soils. Environ. Sci. Pollut. Res. 2016, 23, 5134–5142. [Google Scholar] [CrossRef]
- Cycoń, M.; Piotrowska-Seget, Z. Community Structure of Ammonia-Oxidizing Archaea and Ammonia-Oxidizing Bacteria in Soil Treated with the Insecticide Imidacloprid. BioMed Res. Int. 2015, 2015, 582938. [Google Scholar] [CrossRef]
- Zilli, J.É.; Alves, B.J.R.; Rouws, L.F.M.; Simões-Araujo, J.L.; De Barros Soares, L.H.; Cassán, F.; Castellanos, M.O.; O’Hara, G. The Importance of Denitrification Performed by Nitrogen-Fixing Bacteria Used as Inoculants in South America. Plant Soil. 2020, 451, 5–24. [Google Scholar] [CrossRef]
- Medo, J.; Maková, J.; Medová, J.; Lipková, N.; Cinkocki, R.; Omelka, R.; Javoreková, S. Changes in Soil Microbial Community and Activity Caused by Application of Dimethachlor and Linuron. Sci. Rep. 2021, 11, 12786. [Google Scholar] [CrossRef]
- Global Neonicotinoid Pesticides Market Size, Share & Industry Forecast 2020–2028.Pdf. Available online: https://www.adroitmarketresearch.com/industry-reports/neonicotinoid-pesticides-market (accessed on 12 December 2023).
- Sanchez-Bayo, F.; Tennekes, H.A.; Gok, K. Impact of Systemic Insecticides on Organisms and Ecosystems. In Insecticides—Development of Safer and More Effective Technologies; Trdan, S., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-0958-7. [Google Scholar]
- Mörtl, M.; Vehovszky, Á.; Klátyik, S.; Takács, E.; Győri, J.; Székács, A. Neonicotinoids: Spreading, Translocation and Aquatic Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 2006. [Google Scholar] [CrossRef]
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental Fate and Exposure; Neonicotinoids and Fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An International Database for Pesticide Risk Assessments and Management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Bi, G.; Ward, T.J.; Li, L. Adsorption and Degradation of Neonicotinoid Insecticides in Agricultural Soils. Environ. Sci. Pollut. Res. 2023, 30, 47516–47526. [Google Scholar] [CrossRef] [PubMed]
- Karpouzas, D.G.; Tsiamis, G.; Trevisan, M.; Ferrari, F.; Malandain, C.; Sibourg, O.; Martin-Laurent, F. ”LOVE TO HATE” Pesticides: Felicity or Curse for the Soil Microbial Community? An FP7 IAPP Marie Curie Project Aiming to Establish Tools for the Assessment of the Mechanisms Controlling the Interactions of Pesticides with Soil Microorganisms. Environ. Sci. Pollut. Res. 2016, 23, 18947–18951. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.C.D.; De Paula, D.F.; Mendes, K.F.; De Sousa, R.N.; Araújo, G.R.; Inoue, M.H.; Tornisielo, V.L. Can Soil Type Interfere in Sorption-Desorption, Mobility, Leaching, Degradation, and Microbial Activity of the 14C-Tebuthiuron Herbicide? J. Hazard. Mater. Adv. 2022, 6, 100074. [Google Scholar] [CrossRef]
- Hussain, S.; Hartley, C.J.; Shettigar, M.; Pandey, G. Bacterial Biodegradation of Neonicotinoid Pesticides in Soil and Water Systems. FEMS Microbiol. Lett. 2016, 363, fnw252. [Google Scholar] [CrossRef]
- Fang, W.; Yan, D.; Wang, Q.; Huang, B.; Ren, Z.; Wang, X.; Wang, X.; Li, Y.; Ouyang, C.; Migheli, Q.; et al. Changes in the Abundance and Community Composition of Different Nitrogen Cycling Groups in Response to Fumigation with 1,3-Dichloropropene. Sci. Total Environ. 2019, 650, 44–55. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Fan, Z.; Liu, F.; Liu, H.; Wang, L.; Wu, H. Soil Bacterial Community Dynamics Following Bioaugmentation with Paenarthrobacter Sp. W11 in Atrazine-Contaminated Soil. Chemosphere 2021, 282, 130976. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, S.; Rong, Y.; Zhu, X.; Zhao, X.; Cai, Z. Pyrosequencing Reveals Bacterial Communities and Enzyme Activities Differences after Application of Novel Chiral Insecticide Paichongding in Aerobic Soils. Appl. Soil. Ecol. 2017, 112, 18–27. [Google Scholar] [CrossRef]
- Parizadeh, M.; Mimee, B.; Kembel, S.W. Neonicotinoid Seed Treatments Have Significant Non-Target Effects on Phyllosphere and Soil Bacterial Communities. Front. Microbiol. 2021, 11, 619827. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Hulugalle, N.R.; Jasonsmith, J.; Strong, C.L. Changes in Soil Microbial Communities after Exposure to Neonicotinoids: A Systematic Review. Environ. Microbiol. Rep. 2023, 15, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Ufarté, L.; Laville, É.; Duquesne, S.; Potocki-Veronese, G. Metagenomics for the Discovery of Pollutant Degrading Enzymes. Biotechnol. Adv. 2015, 33, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil Biological Activity as an Indicator of Soil Pollution with Pesticides—A Review. Appl. Soil. Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Wang, F.; Yao, J.; Chen, H.; Yi, Z.; Choi, M.M.F. Influence of Short-Time Imidacloprid and Acetamiprid Application on Soil Microbial Metabolic Activity and Enzymatic Activity. Environ. Sci. Pollut. Res. 2014, 21, 10129–10138. [Google Scholar] [CrossRef]
- Castillo Diaz, J.M.; Martin-Laurent, F.; Beguet, J.; Nogales, R.; Romero, E. Fate and Effect of Imidacloprid on Vermicompost-Amended Soils under Dissimilar Conditions: Risk for Soil Functions, Structure, and Bacterial Abundance. Sci. Total Environ. 2017, 579, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Wolińska, A.; Stępniewska, S. Dehydrogenase Activity in the Soil Environment. Chapter 8. In Dehydrogenases; Intech: London, UK, 2012. [Google Scholar] [CrossRef]
- Moorman, T.B. A Review of Pesticide Effects on Microorganisms and Microbial Processes Related to Soil Fertility. J. Prod. Agric. 1989, 2, 14–23. [Google Scholar] [CrossRef]
- Sim, J.X.F.; Doolette, C.L.; Vasileiadis, S.; Drigo, B.; Wyrsch, E.R.; Djordjevic, S.P.; Donner, E.; Karpouzas, D.G.; Lombi, E. Pesticide Effects on Nitrogen Cycle Related Microbial Functions and Community Composition. Sci. Total Environ. 2022, 807, 150734. [Google Scholar] [CrossRef]
- Onwona-Kwakye, M.; Plants-Paris, K.; Keita, K.; Lee, J.; Brink, P.J.V.D.; Hogarh, J.N.; Darkoh, C. Pesticides Decrease Bacterial Diversity and Abundance of Irrigated Rice Fields. Microorganisms 2020, 8, 318. [Google Scholar] [CrossRef]
- Sim, J.X.F.; Drigo, B.; Doolette, C.L.; Vasileiadis, S.; Donner, E.; Karpouzas, D.G.; Lombi, E. Repeated Applications of Fipronil, Propyzamide and Flutriafol Affect Soil Microbial Functions and Community Composition: A Laboratory-to-Field Assessment. Chemosphere 2023, 331, 138850. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Bhattacherjee, A.K.; Shukla, P.K.; Singh, B. Influence of Imidacloprid on Bacterial Community Diversity of Mango Orchard Soil Assessed through 16S rRNA Sequencing-Based Metagenomic Analysis. Environ. Monit. Assess. 2021, 193, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z.; Song, J.; Mei, J.; Fang, H.; Gui, W. Reduced Bacterial Network Complexity in Agricultural Soils after Application of the Neonicotinoid Insecticide Thiamethoxam. Environ. Pollut. 2021, 274, 116540. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, Z.; Ma, Y.; Luo, J.; Gao, X.; Ning, J.; Mei, X.; She, D. Influence of the Neonicotinoid Insecticide Thiamethoxam on Soil Bacterial Community Composition and Metabolic Function. J. Hazard. Mater. 2021, 405, 124275. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; An, J.; Dang, Z.; Lv, H.; Pan, W.; Gao, Z. Treating Wheat Seeds with Neonicotinoid Insecticides Does Not Harm the Rhizosphere Microbial Community. PLoS ONE 2018, 13, e0205200. [Google Scholar] [CrossRef] [PubMed]
- Parizadeh, M.; Mimee, B.; Kembel, S.W. Soil Microbial Gene Expression in an Agricultural Ecosystem Varies with Time and Neonicotinoid Seed Treatments. Microbiology 2023, 169, 1318. [Google Scholar] [CrossRef] [PubMed]
- Sharuddin, S.S.; Ramli, N.; Yusoff, M.Z.M.; Muhammad, N.A.N.; Ho, L.S.; Maeda, T. Advancement of Metatranscriptomics towards Productive Agriculture and Sustainable Environment: A Review. Int. J. Mol. Sci. 2022, 23, 3737. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, R.; Kumari, M.; Sharma, S. Nontarget Effects of Chemical Pesticides and Biological Pesticide on Rhizospheric Microbial Community Structure and Function in Vigna Radiata. Environ. Sci. Pollut. Res. 2015, 22, 11290–11300. [Google Scholar] [CrossRef]
- Fang, W.; Yan, D.; Wang, X.; Huang, B.; Wang, X.; Liu, J.; Liu, X.; Li, Y.; Ouyang, C.; Wang, Q.; et al. Responses of Nitrogen-Cycling Microorganisms to Dazomet Fumigation. Front. Microbiol. 2018, 9, 2529. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Z.; Teng, Y.; Christie, P.; Wang, J.; Ren, W.; Luo, Y.; Li, Z. Non-Target Effects of Repeated Chlorothalonil Application on Soil Nitrogen Cycling: The Key Functional Gene Study. Sci. Total Environ. 2016, 543, 636–643. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A.N.; Saxena, R.; Paul, D.; Tomar, R.S. Biodiversity of Pesticides Degrading Microbial Communities and Their Environmental Impact. Biocatal. Agric. Biotechnol. 2021, 31, 101883. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, Z.; Wang, X.; Zhou, Z.; Chen, D.; Zeng, H.; Zhao, S.; Chen, L.; Hu, Y.; Zhang, C.; et al. Diversity and Contributions to Nitrogen Cycling and Carbon Fixation of Soil Salinity Shaped Microbial Communities in Tarim Basin. Front. Microbiol. 2018, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Cui, D.; Zhong, G.; Liu, J. Microbial Technologies Employed for Biodegradation of Neonicotinoids in the Agroecosystem. Front. Microbiol. 2021, 12, 759439. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Li, W.; Zhang, X.; Shang, C.; Luo, S.; Cao, R.; Jin, D. Biodegradation and Detoxification of Neonicotinoid Insecticide Thiamethoxam by White-Rot Fungus Phanerochaete chrysosporium. J. Hazard. Mater. 2021, 417, 126017. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Fang, W.-W.; Guo, L.-L.; Yao, C.-F.; Zhao, Y.-X.; Ge, F.; Dai, Y.-J. Biodegradation of the Neonicotinoid Insecticide Acetamiprid by Actinomycetes Streptomyces Canus CGMCC 13662 and Characterization of the Novel Nitrile Hydratase Involved. J. Agric. Food Chem. 2019, 67, 5922–5931. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-L.; Yang, W.-L.; Guo, J.-J.; Zhou, Y.-N.; Rui, X.; Chen, C.; Ge, F.; Dai, Y.-J. Biodegradation of the Neonicotinoid Insecticide Acetamiprid in Surface Water by the Bacterium Variovorax Boronicumulans CGMCC 4969 and Its Enzymatic Mechanism. RSC Adv. 2017, 7, 25387–25397. [Google Scholar] [CrossRef]
- Yang, H.; Hu, S.; Wang, X.; Chuang, S.; Jia, W.; Jiang, J. Pigmentiphaga Sp. Strain D-2 Uses a Novel Amidase To Initiate the Catabolism of the Neonicotinoid Insecticide Acetamiprid. Appl. Environ. Microbiol. 2020, 86, e02425-19. [Google Scholar] [CrossRef] [PubMed]
- Boufercha, O.; Monforte, A.R.; Boudemagh, A.; Ferreira, A.C.; Castro, P.M.L.; Moreira, I.S. Biodegradation and Metabolic Pathway of the Neonicotinoid Insecticide Thiamethoxam by Labrys Portucalensis F11. Int. J. Mol. Sci. 2022, 23, 14326. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Sun, S.; Li, P.; Zhou, X.; Wang, J. Neonicotinoid Insecticide-Degrading Bacteria and Their Application Potential in Contaminated Agricultural Soil Remediation. Agrochemicals 2024, 3, 29–41. [Google Scholar] [CrossRef]
- Pang, S.; Lin, Z.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Insights into the Microbial Degradation and Biochemical Mechanisms of Neonicotinoids. Front. Microbiol. 2020, 11, 868. [Google Scholar] [CrossRef]
- Gautam, P.; Dubey, S.K. Biodegradation of Neonicotinoids: Current Trends and Future Prospects. Curr. Pollut. Rep. 2023, 9, 410–432. [Google Scholar] [CrossRef]
- Xu, B.; Xue, R.; Zhou, J.; Wen, X.; Shi, Z.; Chen, M.; Xin, F.; Zhang, W.; Dong, W.; Jiang, M. Characterization of Acetamiprid Biodegradation by the Microbial Consortium ACE-3 Enriched from Contaminated Soil. Front. Microbiol. 2020, 11, 1429. [Google Scholar] [CrossRef]
- Wang, X.; Xue, L.; Chang, S.; He, X.; Fan, T.; Wu, J.; Niu, J.; Emaneghemi, B. Bioremediation and Metabolism of Clothianidin by Mixed Bacterial Consortia Enriched from Contaminated Soils in Chinese Greenhouse. Process Biochem. 2019, 78, 114–122. [Google Scholar] [CrossRef]
- Cai, X.-Y.; Xu, M.; Zhu, Y.-X.; Shi, Y.; Wang, H.-W. Removal of Dinotefuran, Thiacloprid, and Imidaclothiz Neonicotinoids in Water Using a Novel Pseudomonas monteilii FC02–Duckweed (Lemna aequinoctialis) Partnership. Front. Microbiol. 2022, 13, 906026. [Google Scholar] [CrossRef]
- Erguven, G.O.; Demirci, U. Using Ochrobactrum thiophenivorans and Sphingomonas melonis for Bioremediation of Imidacloprid. Environ. Technol. Innov. 2021, 21, 101236. [Google Scholar] [CrossRef]
- Wang, G.; Chen, M.; Jiang, L.; Zhang, Y. Nitenpyram Biodegradation by a Novel Nitenpyram-Degrading Bacterium, Ochrobactrum Sp. Strain DF-1, and Its Novel Degradation Pathway. Front. Microbiol. 2023, 14, 1209322. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Jiang, H.-Y.; Cheng, X.; Zhu, Y.-X.; Fan, Z.-X.; Dai, Z.-L.; Guo, L.; Liu, Z.-H.; Dai, Y.-J. Neonicotinoid Thiacloprid Transformation by the N2-Fixing Bacterium Microvirga flocculans CGMCC 1.16731 and Toxicity of the Amide Metabolite. Int. Biodeterior. Biodegrad. 2019, 145, 104806. [Google Scholar] [CrossRef]
- Rana, S.; Jindal, V.; Mandal, K.; Kaur, G.; Gupta, V.K. Thiamethoxam Degradation by Pseudomonas and Bacillus Strains Isolated from Agricultural Soils. Environ. Monit. Assess. 2015, 187, 300. [Google Scholar] [CrossRef]

| Pesticide | Molecular Mass (g mol−1) | Water Solubility (mg L−1) | Koc | Soil Degradation 2 (d) | Soil Metabolite |
|---|---|---|---|---|---|
| ACE | 222.67 | 2950 | 200 | 3 | 6-chloronicotinic acid |
| CLO | 249.7 | 327 | 123 | 121 | N-methyl-N-nitroguanidine |
| DNF | 202.21 | 39,830 | 26 | 75 | None |
| IMI | 255.66 | 610 | ND | 174 | 6-chloronicotinic acid |
| NIT | 270.72 | 570,000 | 60 | 8 | None |
| THA | 252.72 | 184 | ND | 8 | Thiacloprid-amide |
| THM | 291.71 | 4100 | 56.2 | 39 | Clothianidin |
| Pesticide | Application Rate | Condition | Microbiological Response | Reference |
|---|---|---|---|---|
| IMI | 1 and 10 mg kg−1 | Laboratory | The total biomass was reduced on days 1 and 14 with the low dose of IMI, and on days 1, 14, and 28 with the high dose. In addition, the higher dosage induced changes in the composition of microbial communities and their metabolic activity. | [18] |
| IMI-ACE | 0 to 80 mg kg−1 | Laboratory | ACE showed higher toxicity than IMI with a dose–response relationship. Microbial activity was reduced over a short period of time. ACE and IMI reduced dehydrogenase activity by 40% and 30%, respectively. Urease activity declined by 21% and 30% with IMI and ACE-treated soil, respectively, after two days. | [39] |
| IPP | 10 mg kg−1 | Laboratory | Protease activity increased two times after IPP application at 20, 30, and 45 days. Catalase activity increased 133–155% at day 100. Dehydrogenase activity was decreased, and urease was increased. | [34] |
| IMI | 3 mg kg−1 | Laboratory | Vermicompost-amended soil increased dehydrogenase activity 2 and 4-fold after 30 days of pesticide application, while urease decreased. IMI induces changes in abundance, structure, and activity with a better tolerance in amended soil. | [40] |
| Pesticide | Application Rate | Condition | Microbiological Response | Reference |
|---|---|---|---|---|
| IPP | 10 mg kg−1 | Laboratory | The phyla Pseudomonadota, Bacillota, Planctomycetota, Chloroflexota, Armatimonadota, and Chlorobiota were stimulated. Phyla Bacteroidota, Actinomycetota, and Acidobacteriota were inhibited. | [19] |
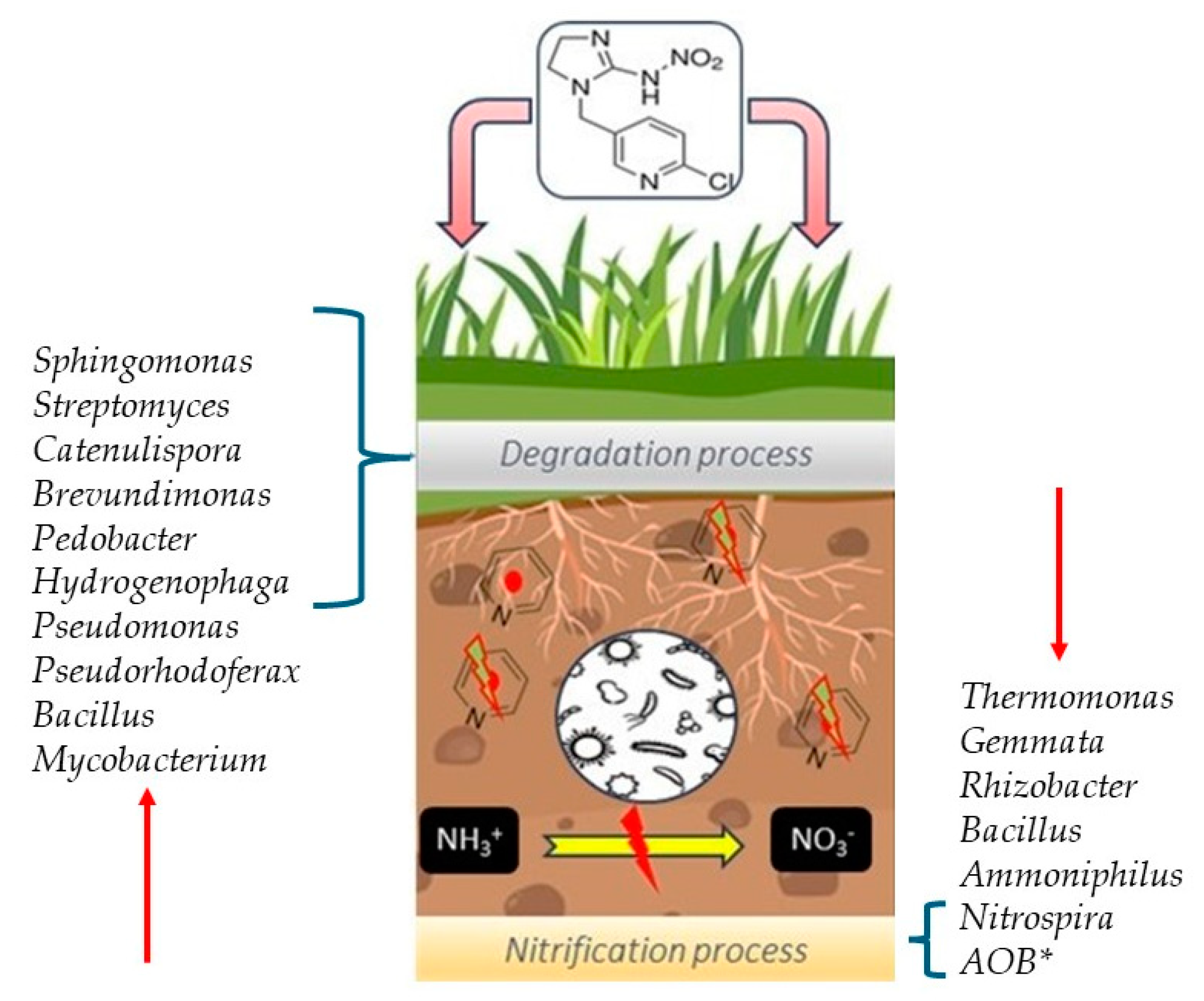
| IPP | 10 mg kg−1 | Laboratory | The genera Pseudomonas and Pseudorhodoferax increased from 0.3% to 21.4% and 0.1% to 14.3%, respectively, while Thermomonas decreased from 2.7% to 0.6%, after 60 days. In other soils, Pseudomonas, Mycrovirga, and Brevundimonas were stimulated to increase. | [34] |
| IMI-THA-CLO | 5 mg kg−1 | Laboratory | Representative families of the phylum Pseudomonadota and Bacteroidota increased by at least 50% at days 20 and 60 after NNI application. | [16] |
| IMI | 0.005% | Field | Phylum Pseudomonadota, Planctomycetota, Chloroflexota, and Verrucomicrobiota decreased, while Actinomycetota increased. The genus Gemmata totally disappeared in IMI treated soil, and microorganisms belonging to the genus Prevotella were present. | [46] |
| IMI-CLO | 240 a.i. g/100 kg−1 seed | Field | The species richness of the bacterial and fungal communities was suppressed in the wheat seedling stage, but during the reviving period, stimulation of soil microorganisms was observed. | [49] |
| THM | 1.5 to 4 mg kg−1 | Laboratory | The richness of the soil bacterial community in treated soils was reduced by about 20%. The plyla Pseudomonadota and Verrucomicrobiota increased, while Phyla Chloroflexota, Acidobacteriota, and Nitrospirota decreased after 60 days of THM application. | [47] |
| THM | 0.25 mg seed−1 | Field | Pesticides affected the bacterial community structure (2.6%) and over time (2.4%). The phyla Actinomycetota and Chloroflexota were more abundant while Pseudomonadota were less abundant in THM-treated soil. More than 60 genera of soil bacteria were impacted, i.e., Ammoniphilus, Bacillus, and Rhizobacter. | [35] |
| THM | 1.8 to 180 mg kg−1 | Laboratory | THM increased the bacterial abundance by 0.09 to 0.72 fold in one soil, but in another it was reduced. THM reduced the abundance of Actinomycetota and Chloroflexota. Bacteroidota and Bacillota increased in the basic soil, and Patescibacteria and Acidobacteriota increased in the acidic soil. | [48] |
| THM-DNF | 0.2 to 2 mg kg−1 | Laboratory | The phyla Bacteroidota, Gemmatimonadota, and Candidatus Paceibacterota decreased at a rate > 10%. Chloroflexota and Nitrospirota increased at a rate > 10%. Pseudomonadota and Acidobacteriota also change (increased or decreased) at a rate < 10%. | [17] |
| Pesticide | Application Rate | Condition | Microbiological Response | Reference |
|---|---|---|---|---|
| IMI | 1 and 10 mg kg−1 | Laboratory | The nitrification rate was decreased by 25–65%, and the ammonification process was stimulated on days 14, 28, and 56. IMI applied at a dose of 10 mg kg−1 suppressed the AOA community members for 56 days. The diversity and richness of AOB decreased on days 1 and 14. | [20] |
| IMI-THA-CLO | 5 mg kg−1 | Laboratory | Family Nitrosomonadaceae, Nitrososphaeraceae, and Nitrospiraceae increased after pesticide application. | [16] |
| THM | 1.5 to 4 mg kg−1 | Laboratory | Bacterial genera Sphingomonas, Streptomyces, and Catenulispora were associated with biodegradation. | [47] |
| THM | 0.25 mg seed−1 | Field | Genera such as Ammoniphilus, Bacillus, Nitrospira, Nitrosospira, and Rhizobacter, among others, were affected. The genera Mycobacterium and Streptomyces were dominant. | [35] |
| IPP | 10 mg kg−1 | Laboratory | The genera Bacillus, Pseudomonas, Azohydromonas, and Paenibacillus increased with the pesticide. THe genera Brevundimonas, Pedobacter, and Hydrogenophaga were related to IPP degradation. | [19] |
| NNIs | Microorganisms | Response | Reference |
|---|---|---|---|
| ACE | Sphingobium, Acinetobacter, Afipia, Stenotrophomonas, and Microbacterium | Consortia was able to degrade completely 50 mg L−1 ACE in 144 h. | [66] |
| CLO | Ochrobactrum anthropi, Acinetobacter johnsonii, Pseudomonas sp., and Stenotrophomonas maltophilia | >79% of CLO (500 mg L−1) was degraded by bacterial consortia. | [67] |
| DNF | Pseudomonas monteilii FC02 | >92 DNF was removed after 14 days. | [68] |
| IMI | Sphingomonas melonis | Bioremediate the insecticide with an efficiency > 90%. | [69] |
| NIT | Ochrobactrum sp. strain DF-1 | > 90.9% NIT (10 mg kg−1) degradation was achieved, after two weeks. | [70] |
| THA | Microvirga flocculans CGMCC 1.16731 | In soil, the bacterium transformed >92% of 80 μmol kg −1 soil THA in 9 d. | [71] |
| THM | Bacillus aeromonas strain IMBL 4.1 and Pseudomonas putida strain IMBL 5.2 | >45 and 38% THM (50 μg mL−1) was removed in 15 days. | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briceño, G.; Diez, M.C.; Palma, G.; Jorquera, M.; Schalchli, H.; Saez, J.M.; Benimeli, C.S. Neonicotinoid Effects on Soil Microorganisms: Responses and Mitigation Strategies. Sustainability 2024, 16, 3769. https://doi.org/10.3390/su16093769
Briceño G, Diez MC, Palma G, Jorquera M, Schalchli H, Saez JM, Benimeli CS. Neonicotinoid Effects on Soil Microorganisms: Responses and Mitigation Strategies. Sustainability. 2024; 16(9):3769. https://doi.org/10.3390/su16093769
Chicago/Turabian StyleBriceño, Gabriela, Maria Cristina Diez, Graciela Palma, Milko Jorquera, Heidi Schalchli, Juliana María Saez, and Claudia Susana Benimeli. 2024. "Neonicotinoid Effects on Soil Microorganisms: Responses and Mitigation Strategies" Sustainability 16, no. 9: 3769. https://doi.org/10.3390/su16093769
APA StyleBriceño, G., Diez, M. C., Palma, G., Jorquera, M., Schalchli, H., Saez, J. M., & Benimeli, C. S. (2024). Neonicotinoid Effects on Soil Microorganisms: Responses and Mitigation Strategies. Sustainability, 16(9), 3769. https://doi.org/10.3390/su16093769










