Impact of Climate Change on Culex pipiens Mosquito Distribution in the United States
Abstract
1. Introduction
- Evaluating C. pipiens present distribution in the US while taking ecological and climatic elements into account.
- Looking into how climate change can affect C. pipiens habitat suitability, paying special attention to how temperatures are rising and how precipitation patterns are changing.
- Analyzing the variations between scenarios and forecasting the future distribution range of C. pipiens in 2050 and 2070 under various climate impacts.
- Examining the main ecological and climatic parameters that contribute to the invasiveness or endemic patterns of C. pipiens and their capacity to change the dynamics of disease transmission.
2. Materials and Methods
2.1. Global Spatial Information
2.2. Environmental Variables and Multicollinearity
2.3. MaxEnt Model
3. Results and Potential Habitat Classification
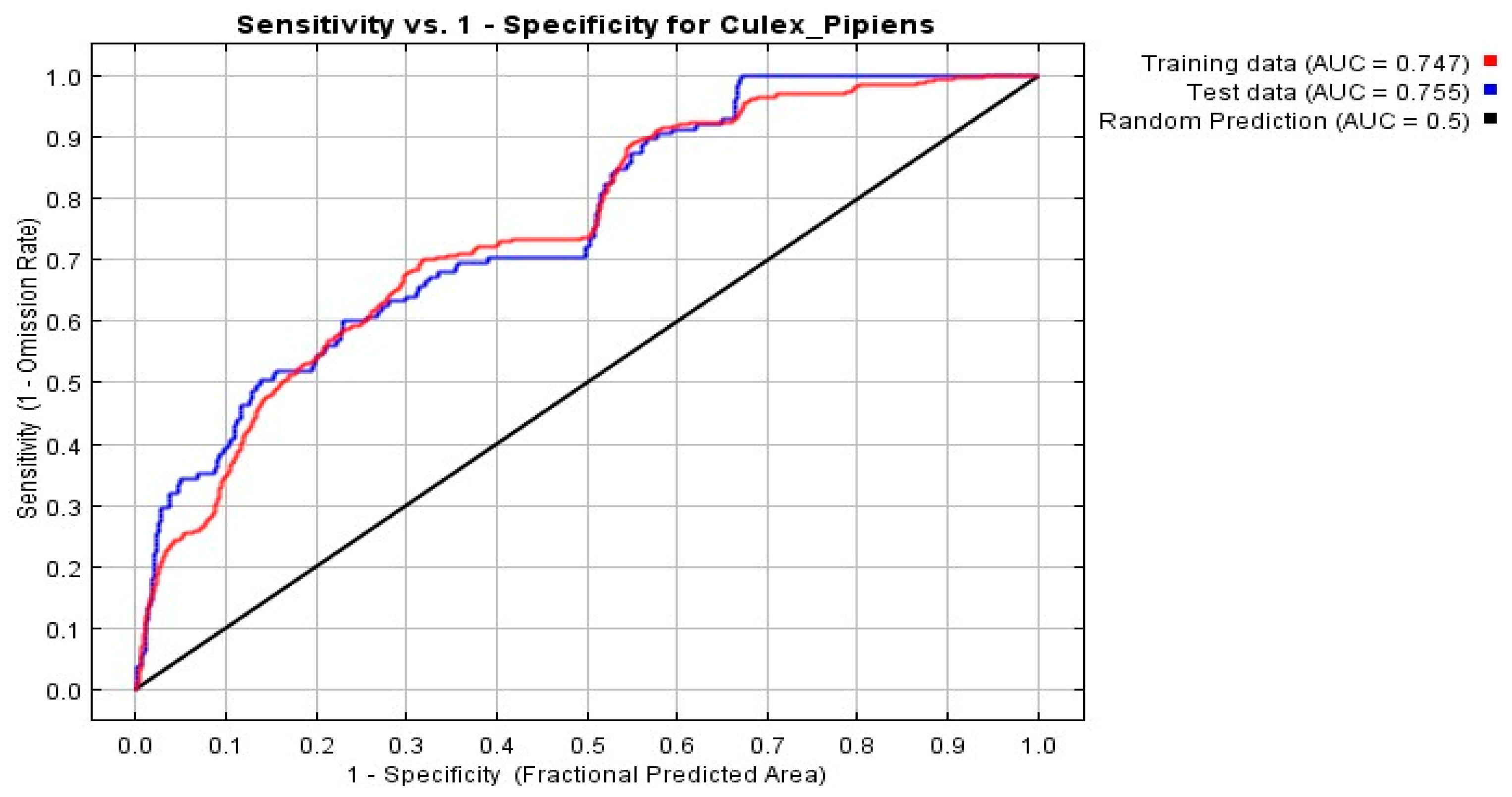
3.1. Evaluation of the Model
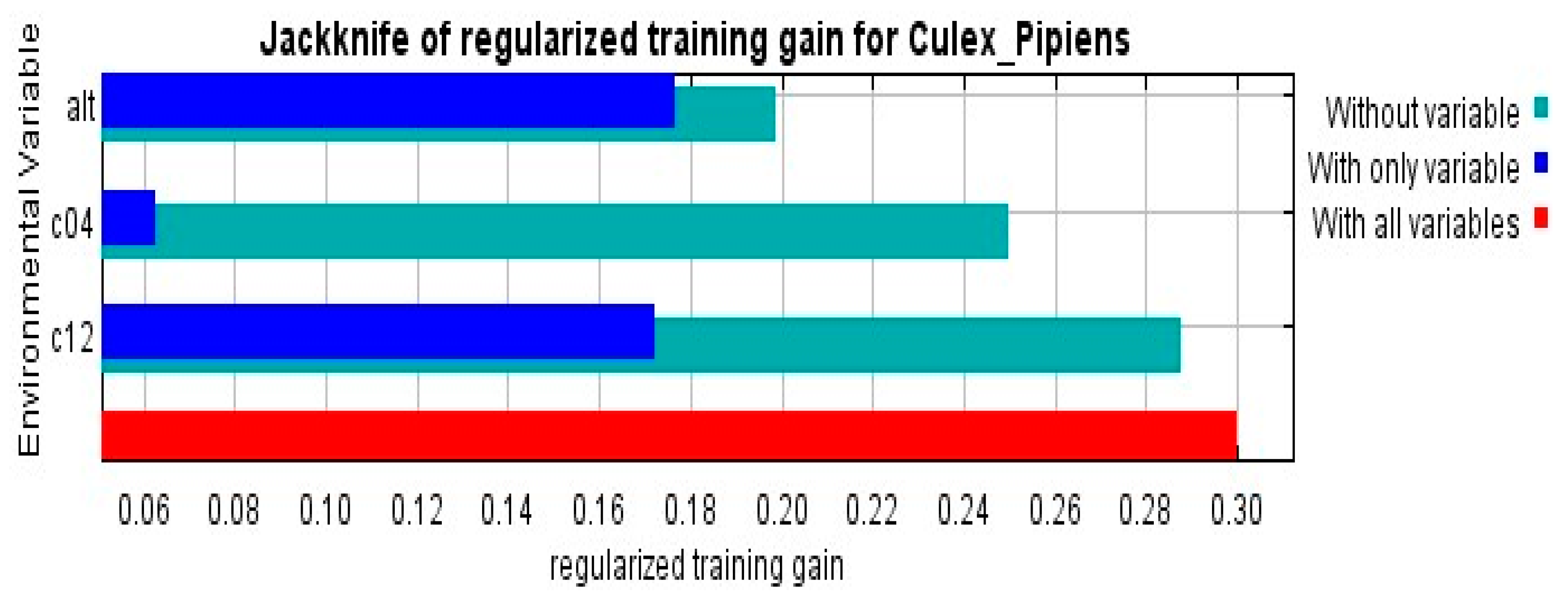
3.2. Climatic Variables Importance
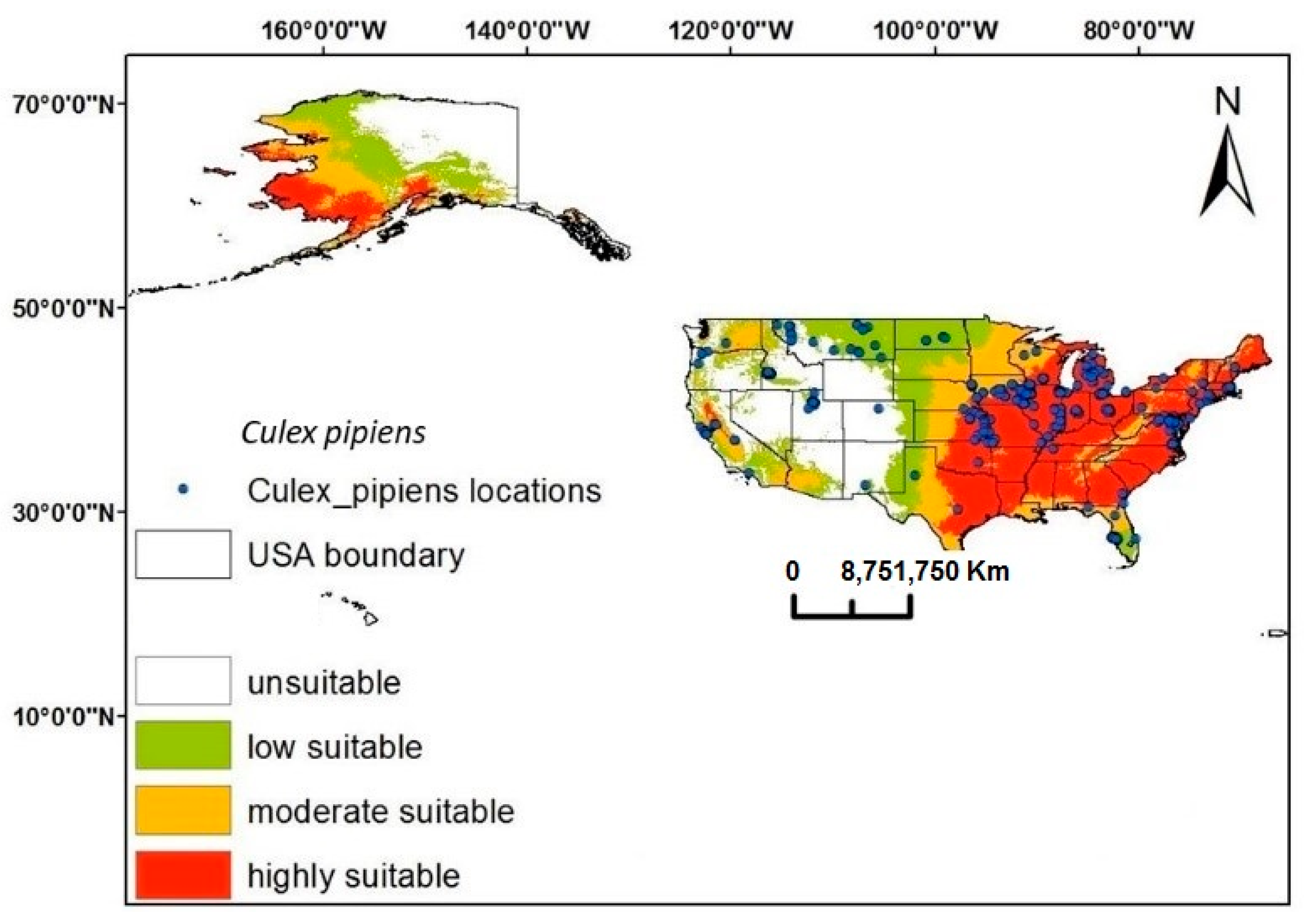
3.3. Spatial Prediction Model and Distribution Range of C. pipiens in the USA
3.4. Model Assessments
3.5. Climatic Suitability of C. pipiens Under Present and Future Climate Change with Impacts to Present Possiblel Distribution
3.6. The Predicted Future Potential Distribution Areas for 2050 and 2070
4. Discussion
Policy Implications from This Study
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rockl€ov, J.; Dubrow, R. Climate change: An enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 2020, 21, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, C.J.E.; Walter, K.S.; Wesolowski, A.; Buckee, C.O.; Shevliakova, E.; Tatem, A.J.; Boos, W.R.; Weinberger, D.M.; Pitzer, V.E. Identifying climate drivers of infectious disease dynamics: Recent advances and challenges ahead. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170901. [Google Scholar] [CrossRef] [PubMed]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N.Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef]
- Paz, S. Effects of climate change on vector-borne diseases: An updated focus on West Nile virus in humans. Emerg. Top. Life Sci. 2019, 3, 143–152. [Google Scholar] [PubMed]
- Ludwig, A.; Zheng, H.; Vrbova, L.; Drebot, M.A.; Iranpour, M.; Lindsay, L.R. Climate change and infectious diseases: The challenges: Increased risk of endemic mosquito-borne diseases in Canada due to climate change. Can. Commun. Dis. Rep. 2019, 45, 91. [Google Scholar] [CrossRef] [PubMed]
- U.S. CDC. The National Public Health Strategy to Prevent and Control Vector-Borne Diseases in People. U.S. DHHS, The U.S. Department of Health and Human Services and the U.S. Centers for Disease Control and Prevention. 2024. Available online: https://www.cdc.gov/vector-borne-diseases/media/pdfs/2024/05/VBD-National-Strategy-508.pdf (accessed on 22 November 2024).
- Wudel, B.; Shadabi, E. Mosquito-Borne Disease in the Americas. Rapid Review|NCCID. 2016. Available online: https://centreinfection.ca/en/wp-content/uploads/sites/2/2016/07/RapidReviewClimateMosquito-EN.pdf (accessed on 2 December 2024).
- Nooten, S.S.; Andrew, N.R.; Hughes, L. Potential impacts of climate change on insect communities: A transplant experiment. PLoS ONE 2014, 9, e85987. [Google Scholar] [CrossRef]
- Turell, M.J. Members of the Culex pipiens complex as vectors of viruses. J. Am. Mosq. Control Assoc. 2012, 28 (Suppl. S4), 123–126. [Google Scholar] [CrossRef] [PubMed]
- Manimegalai, K.; Sukanya, S. Biology of the filarial vector, Culex quinquefasciatus (Diptera: Culicidae). Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 718–724. [Google Scholar]
- Abdallah, F.I.; Rady, M.H.; Merdan, B.A.; Shaarawi, F.A.; Mohammed, A.F.; Alshammery, K.A.; Al-Khalaf, A.A.; Selim, T.A.; Dahab, A.A. Effects of blood sources and artificial blood feeding membranes on the biological parameters and hepatitis C virus infectivity of Culex pipiens (Diptera: Culicidae). Afr. Entomol. 2021, 29, 262–273. [Google Scholar] [CrossRef]
- Abdallah, F.I.; Merdan, B.A.; Shaarawi, F.A.; Mohamed, A.F.; Selim, T.A.; Dahesh, S.M.; Rady, M.H. The potentiality of Culex pipiens (Diptera: Culicidae) complex holobiont in transmitting the hepatitis C virus (HCV) with the aid of bacterial microbiota in the midgut. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 119. [Google Scholar] [CrossRef]
- Mencattelli, G.; Ndione, M.H.D.; Silverj, A.; Diagne, M.M.; Curini, V.; Teodori, L.; Di Domenico, M.; Mbaye, R.; Leone, A.; Marcacci, M.; et al. Spatial and temporal dynamics of West Nile virus between Africa and Europe. Nat. Commun. 2023, 14, 6440. [Google Scholar] [CrossRef] [PubMed]
- Gould, C.V.; Staples, J.E.; Huang, C.Y.; Brault, A.C.; Nett, R.J. Combating West Nile Virus Disease—Time to Revisit Vaccination. N. Engl. J. Med. 2023, 388, 1633–1636. [Google Scholar] [CrossRef]
- Reiter, P. Climate change and mosquito-borne disease. Environ. Health Perspect. 2001, 109, 141–161. [Google Scholar]
- Hales, S.; de Wet, N.; Maindonald, J.; Woodward, A. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet 2002, 360, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Hasaballah, A.I.; El-Naggar, H.A.; Abdelbary, S.; Bashar, M.A.; Selim, T.A. Eco-friendly synthesis of zinc oxide nanoparticles by marine sponge, Spongia officinalis: Antimicrobial and insecticidal activities against the mosquito vectors, Culex pipiens and Anopheles pharoensis. BioNanoScience 2022, 12, 89–104. [Google Scholar] [CrossRef]
- Ahmed, J.; Bouloy, M.; Ergonul, O.; Fooks, A.; Paweska, J.; Chevalier, V.; Drosten, C.; Moormann, R.; Tordo, N.; Vatansever, Z.; et al. International network for capacity building for the control of emerging viral vector-borne zoonotic diseases: ARBO-ZOONET. Euro Surveill. 2009, 14, 19160. [Google Scholar] [CrossRef] [PubMed]
- Gabarty, A.; Selim, T.A.; Hasaballah, A.I. Effect of gamma irradiation on protease and nuclease enzymes activity and egg oviposition of Culex pipiens mosquito engorged with Hepatitis C Virus (HCV). J. Radiat. Res. Appl. Sci. 2022, 15, 1–6. [Google Scholar] [CrossRef]
- Hashem, A.H.; Selim, T.A.; Alruhaili, M.H.; Selim, S.; Alkhalifah, D.H.M.; Al Jaouni, S.K.; Salem, S.S. Unveiling antimicrobial and insecticidal activities of biosynthesized selenium nanoparticles using prickly pear peel waste. J. Funct. Biomater. 2022, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Selim, T.A.; Abd-El Rahman, I.E.; Mahran, H.A.; Adam, H.A.; Imieje, V.; Zaki, A.A.; Hasaballah, A.I.; Bashar, M.A.; Hwihy, H.; Hamed, A. Mosquitocidal Activity of the Methanolic Extract of Annickia chlorantha and Its Isolated Compounds against Culex pipiens, and Their Impact on the Non-Target Organism Zebrafish, Danio Rerio. Insects 2022, 13, 676. [Google Scholar] [CrossRef]
- Martens, W.J.M.; Jetten, T.H.; Rotmans, J.; Niessen, L.W. Climate change and vector-borne diseases: A global modelling perspective. Glob. Environ. Change 1995, 5, 195–209. [Google Scholar] [CrossRef]
- Ogden, N.; St-Onge, L.; Barker, I.; Brazeau, S.; Bigras-Poulin, M.; Charron, D.; Sompson, R. Risk maps for range expansion of the Lyme disease vector, Ixodes scapularis, in Canada now and with climate change. Int. J. Health Geogr. 2008, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Bouchard, C.; Kurtenbach, K.; Margos, G.; Lindsay, L.R.; Trudel, L.; Nguon, S.; Milord, F. Active and passive surveillance and phylogenetic analysis of Borrelia burgdorferi elucidate the process of Lyme disease risk emergence in Canada. Environ. Health Perspect. 2010, 118, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, J.S.; Holford, T.R.; Fish, D. Effect of climate change on Lyme disease risk in North America. EcoHealth 2005, 2, 38–46. [Google Scholar] [CrossRef]
- Gonzalez, C.; Wang, O.; Strutz, S.E.; Gonzalez-Salazar, C.; Sanchez-Cordero, V.; Sarkar, S. Climate change and risk of Leishmaniasis in North America: Predictions from ecological niche models of vector and reservoir species. PLoS Neglected Trop. Dis. 2010, 4, e585. [Google Scholar] [CrossRef] [PubMed]
- McKenney, D.M.; Pedlar, J.H.; Lawrence, K.; Campbell, K.L.; Hutchinson, M.F. Potential impacts of climate change on the distribution of North American trees. BioScience 2007, 57, 939–948. [Google Scholar] [CrossRef]
- Shelton, R.M. The effect of temperatures on development of eight mosquito species. Mosq. News 1973, 33, 1–12. [Google Scholar]
- Loetti, V.; Schweigmann, N.J.; Burroni, N.E. Temperature effects on the immature development time of Culex eduardoi Casal and Garcia (Diptera: Culicidae). Neotrop. Entomol. 2011, 40, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Mulla, M.S. Effects of temperature on development, mortality, mating and blood feeding behavior of Culiseta incidens (Diptera: Culicidae). J. Vector Ecol. 2001, 26, 83–92. [Google Scholar]
- Debat, V.; Begin, M.; Legout, H.; David, J.R. Allometric and nonallometric components of Drosophila wing shape respond differently to developmental temperature. Evolution 2003, 57, 2773–2784. [Google Scholar] [PubMed]
- Gunay, F.; Alten, B.; Ozsoy, E.D. Narrowsense heritability of body size and its response to different developmental temperatures in Culex quinquefasciatus (Say 1923). J. Vector Ecol. 2011, 36, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Beck, J. Predicting climate change effects on agriculture from ecological niche modeling: Who profits, who loses? Clim. Change 2013, 116, 177–189. [Google Scholar] [CrossRef]
- Bosso, L.; Rebelo, H.; Garonna, A.P.; Russo, D. Modelling geographic distribution and detecting conservation gaps in Italy for the threatened beetle Rosalia alpina. J. Nat. Conserv. 2013, 21, 72–80. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, G.; Bu, W.; Lis, J.A. Geographic distribution and niche divergence of two stinkbugs, Parastrachia japonensis and Parastrachia nagaensis. J. Insect Sci. 2013, 13, 102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Araujo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of species–climate impact models under climate change. Glob. Change Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef]
- ESRI (Environmental Systems Research Institute). ArcGIS®Desktop Help 10.3 Geostatistical Analyst. 2014. Available online: https://desktop.arcgis.com/en/arcmap/10.3 (accessed on 1 November 2020).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Yi, Y.J.; Zhou, Y.; Cai, Y.P.; Yang, W.; Li, Z.W.; Zhao, X. The influence of climate change on an endangered riparian plant species: The root of riparian Homonoia. Ecol. Indic. 2018, 92, 40–50. [Google Scholar] [CrossRef]
- Clements, A.N. The Biology of Mosquitoes. In Development, Nutrition and Reproduction; CABI Publishing: Wallingford, UK, 1992; Volume 1, 532p, Available online: https://www.cabi.org/bookshop/book/9780851993744 (accessed on 1 November 2024).
- Kamel, M.; Bream, A.S.; Moursy, M.M.; Ragab, S.H. Predicting the geographic distribution habitats of Schizomyia buboniae (Diptera: Cecidomyiidae) and its host plant Deverra tortuosa (Apiaceae) in Egypt by using MaxEnt modeling. J. Basic Appl. Zool. 2021, 82, 27. [Google Scholar] [CrossRef]
- Alto, B.W.; Juliano, S.A. Temperature effects on the dynamics of Aedes albopictus (Diptera: Culicidae) populations in the laboratory. J. Med. Entomol. 2001, 38, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Ragab, S.H.; Alqurashi, S.I.; Aljameeli, M.M.; Tyshenko, M.G.; Abdelwahab, A.H.; Selim, T.A. Predicting the Global Distribution of Gryllus bimaculatus Under Climate Change: Implications for Biodiversity and Animal Feed Production. Sustainability 2024, 16, 10278. [Google Scholar] [CrossRef]
- Orabi, G.M.; Semida, F.M.; Medany, D.M.; Issa, M.A.; Ragab, S.H.; Kamel, M. Predicting the Invasion Range of the Common Myna, Acridotheres tristis Linnaeus, 1766 in Egypt under Climate Change. Sustainability 2024, 16, 6495. [Google Scholar] [CrossRef]
- Kamel, M.; Ragab, S.H. Ecological-niche modeling of the gall midge Psectrosema tamaricum and its host plant Tamarix nilotica in Egypt. Int. J. Trop. Insect Sci. 2024, 44, 885–900. [Google Scholar] [CrossRef]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Naimi, B.; Arau’jo, M.B. sdm: A reproducible and extensible R platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017; ISBN 0521765137. [Google Scholar]
- Naimi, B. usdm: Uncertainty Analysis for Species Distribution Models. R Package Version 1.1–15. R Doc. 2015. Available online: http://www.rdocumentation.org/packages/usdm (accessed on 12 December 2020).
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; pp. 655–662. [Google Scholar]
- Phillips, S. A brief tutorial on Maxent, versions: 3.3.1. Lessons Conserv. 2016, 3, 108–135. [Google Scholar]
- Semenza, J.C.; Suk, J.E.; Estevez, V.; Ebi, K.L.; Lindgren, E. Mapping climate change vulnerabilities to infectious diseases in Europe. Environ. Health Perspect. 2012, 120, 385–392. [Google Scholar] [CrossRef]
- Mallya, S.; Sander, B.; Roy-Gagnon, M.H.; Taljaard, M.; Jolly, A.; Kulkarni, M.A. Factors associated with human West Nile virus infection in Ontario: A generalized linear mixed modelling approach. BMC Infect. Dis. Mar. 2018, 18, 141. [Google Scholar] [CrossRef]
- Lee, S.H.; Nam, K.W.; Jeong, J.Y.; Yoo, S.J.; Koh, Y.S.; Lee, S.; Heo, S.T.; Seong, S.Y.; Lee, K.H. The effects of climate change and globalization on mosquito vectors: Evidence from Jeju Island, South Korea on the potential for Asian tiger mosquito (Aedes albopictus) influxes and survival from Vietnam rather than Japan. PLoS ONE 2013, 8, e68512. [Google Scholar] [CrossRef]
- Wang, J.; Ogden, N.H.; Zhu, H. The impact of weather conditions on Culex pipiens and Culex restuans (Diptera: Culicidae) abundance: A case study in Peel Region. J. Med. Entomol. 2011, 48, 468–475. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.; Mathis, S.; Martin, S.W.; Erin Staples, J.; Fischer, M.; Lindsey, N.P. Surveillance for West Nile virus disease—United States, 2009–2018. Am. J. Transplant. 2021, 21, 1959–1974. [Google Scholar] [CrossRef] [PubMed]
- Parker, N. Exploring the role of temperature and other environmental factors in West Nile virus incidence and prediction in California counties from 2017–2022 using a zero-inflated model. PLOS Neglected Trop. Dis. 2024, 18, e0012051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, H.E.; Clifton, M.; Harbison, J.E.; Erkapic, A.; Barrett-Wilt, G.A.; Paskewitz, S.; Bartholomay, L. Assessment of Truck-Mounted Area-Wide S-methoprene Applications to Manage West Nile Virus Vector Species in the Suburbs of Chicago, IL, USA. J. Med. Entomol. 2023, 60, 384–391. [Google Scholar] [CrossRef] [PubMed]
- McMillan, J.R.; Harden, C.A.; Burtis, J.C.; Breban, M.I.; Shepard, J.J.; Petruff, T.A.; Misencik, M.J.; Bransfield, A.B.; Poggi, J.D.; Harrington, L.C.; et al. The community-wide effectiveness of municipal larval control programs for West Nile virus risk reduction in Connecticut, USA. Pest Manag. Sci. 2021, 77, 5186–5201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Floore, T.G. Mosquito larval control practices: Past and present. J. Am. Mosq. Control Assoc. 2006, 22, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Gómez, J.C.; Espinosa-Juárez, J.V.; Ovando-Zambrano, J.C.; Briones-Aranda, A.; Cruz-Salomón, A.; Esquinca-Avilés, H.A. A Review of Botanical Extracts with Repellent and Insecticidal Activity and Their Suitability for Managing Mosquito-Borne Disease Risk in Mexico. Pathogens 2024, 13, 737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zang, C.; Wang, X.; Liu, Y.; Wang, H.; Sun, Q.; Cheng, P.; Zhang, Y.; Gong, M.; Liu, H. Wolbachia and mosquitoes: Exploring transmission modes and coevolutionary dynamics in Shandong Province, China. PLOS Neglected Trop. Dis. 2024, 18, e0011944. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, X.; Hu, J.; Ding, C.; Portieles, R.; Xu, H.; Gao, J.; Du, L.; Gao, X.; Yue, Q.; Zhao, L.; et al. New native Bacillus thuringiensis strains induce high insecticidal action against Culex pipiens pallens larvae and adults. BMC Microbiol. 2023, 23, 100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaronski, S.T. Chapter 11—Mass Production of Entomopathogenic Fungi: State of the Art. In Mass Production of Beneficial Organisms; Morales-Ramos, J.A., Rojas, M.G., Shapiro-Ilan, D.I., Eds.; Academic Press: New York, NY, USA; Elsevier Inc.: Cambridge, MA, USA, 2014; pp. 357–413. ISBN 9780123914538. [Google Scholar]
- Agnew, P.; Bedhomme, S.; Haussy, C.; Michalakis, Y. Age and size at maturity of the mosquito Culex pipiens infected by the microsporidian parasite Vavraia culicis. Proc. Biol. Sci. 1999, 266, 947. [Google Scholar] [CrossRef] [PubMed Central]
- Benedict, M.Q.; Robinson, A.S. The first releases of transgenic mosquitoes: An argument for the sterile insect technique. Trends Parasitol. 2003, 19, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Ochomo, E.; Rund, S.S.C.; Mthawanji, R.S.; Antonio-Nkondjio, C.; Machani, M.; Samake, S.; Wolie, R.Z.; Nsango, S.; Lown, L.A.; Matoke-Muhia, D.; et al. Mosquito control by abatement programmes in the United States: Perspectives and lessons for countries in sub-Saharan Africa. Malar. J. 2024, 23, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, F.; Lavine, L.; O’Neal, S.; Lavine, M.; Foss, C.; Walsh, D. Insecticide Resistance and Management Strategies in Urban Ecosystems. Insects 2016, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.K.; Shugart, J.M. Zika virus in workers: Considerations for ongoing exposure prevention. Am. J. Ind. Med. 2019, 62, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.E.; Doggett, S.L. Exotic mosquito threats require strategic surveillance and response planning. Public. Health Res. Pract. 2016, 26, 2651656. [Google Scholar] [CrossRef] [PubMed]
- Zeller, H.; Marrama, L.; Sudre, B.; Van Bortel, W.; Warns-Petit, E. Mosquito-borne disease surveillance by the European Centre for Disease Prevention and Control. Clin. Microbiol. Infect. 2013, 19, 693–698. [Google Scholar] [CrossRef]
- Semwal, A.; Melvin, L.M.J.; Mohan, R.E.; Ramalingam, B.; Pathmakumar, T. AI-Enabled Mosquito Surveillance and Population Mapping Using Dragonfly Robot. Sensors 2022, 22, 4921. [Google Scholar] [CrossRef]
- Liu, W.L.; Wang, Y.; Chen, Y.X.; Chen, B.Y.; Lin, A.Y.; Dai, S.T.; Chen, C.H.; Liao, L.D. An IoT-based smart mosquito trap system embedded with real-time mosquito image processing by neural networks for mosquito surveillance. Front. Bioeng. Biotechnol. 2023, 11, 1100968. [Google Scholar] [CrossRef] [PubMed]
- Averett, E.; Neuberger, J.S.; Hansen, G.; Fox, M.H. Evaluation of West Nile virus education campaign. Emerg. Infect. Dis. 2005, 11, 1751–1753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kraftcheck, D.J.; Paterson, J.M.; Alton, B.; Cheung, W.; Goebel, C.; Kennedy, W.; Kraftcheck, D.; Levy, R.; Neimanis, I.; Ohayon, J.; et al. Ontario’s 2003 West Nile virus public education campaign: Was anybody listening? Can. Commun. Dis. Rep. 2003, 29, 189–194. [Google Scholar] [PubMed]



| Code | Variables | Units | Percent Contribution (%) | VIF |
|---|---|---|---|---|
| Alt | Altitude | m | 60.3 | 1.26 |
| bio_04 | Temperature Seasonality (standard deviation × 100) | °C | 31 | 2.61 |
| bio_12 | Annual precipitation (mm)) | mm | 8.7 | 2.36 |
| Climatic Scenario | AUC Value |
|---|---|
| Current Climate | 0.747 |
| BCC-CSM1_ssp126_2041-2060 | 0.749 |
| BCC-CSM1_ssp126_2061-2080 | 0.770 |
| BCC-CSM1_ssp585_2041-2060 | 0.745 |
| BCC-CSM1_ssp585_2061-2080 | 0.733 |
| Variable | Alt | bio4 | bio12 |
|---|---|---|---|
| Current Climate (%) | 60.3 | 31 | 8.7 |
| BCC-CSM1_ssp126_2041-2060 (%) | 63.7 | 18.9 | 17.3 |
| BCC-CSM1_ssp126_2061-2080 (%) | 59.2 | 30.7 | 10.2 |
| BCC-CSM1_ssp585_2041-2060 (%) | 61.6 | 28.5 | 9.9 |
| BCC-CSM1_ssp585_2061-2080 (%) | 65.6 | 26.2 | 8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragab, S.H.; Alkhaibari, A.M.; Alharbi, J.; Areshi, S.M.; Mashlawi, A.M.; Embaby, D.M.; Tyshenko, M.G.; Selim, T.A.; Kamel, M. Impact of Climate Change on Culex pipiens Mosquito Distribution in the United States. Sustainability 2025, 17, 102. https://doi.org/10.3390/su17010102
Ragab SH, Alkhaibari AM, Alharbi J, Areshi SM, Mashlawi AM, Embaby DM, Tyshenko MG, Selim TA, Kamel M. Impact of Climate Change on Culex pipiens Mosquito Distribution in the United States. Sustainability. 2025; 17(1):102. https://doi.org/10.3390/su17010102
Chicago/Turabian StyleRagab, Sanad H., Abeer Mousa Alkhaibari, Jalal Alharbi, Sultan Mohammed Areshi, Abadi M. Mashlawi, Doaa M. Embaby, Michael G. Tyshenko, Tharwat A. Selim, and Mohamed Kamel. 2025. "Impact of Climate Change on Culex pipiens Mosquito Distribution in the United States" Sustainability 17, no. 1: 102. https://doi.org/10.3390/su17010102
APA StyleRagab, S. H., Alkhaibari, A. M., Alharbi, J., Areshi, S. M., Mashlawi, A. M., Embaby, D. M., Tyshenko, M. G., Selim, T. A., & Kamel, M. (2025). Impact of Climate Change on Culex pipiens Mosquito Distribution in the United States. Sustainability, 17(1), 102. https://doi.org/10.3390/su17010102






