Green Regenerative Bamboo Lignin-Based Epoxy Resin: Preparation, Curing Behavior, and Performance Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Purification of Bamboo Lignin
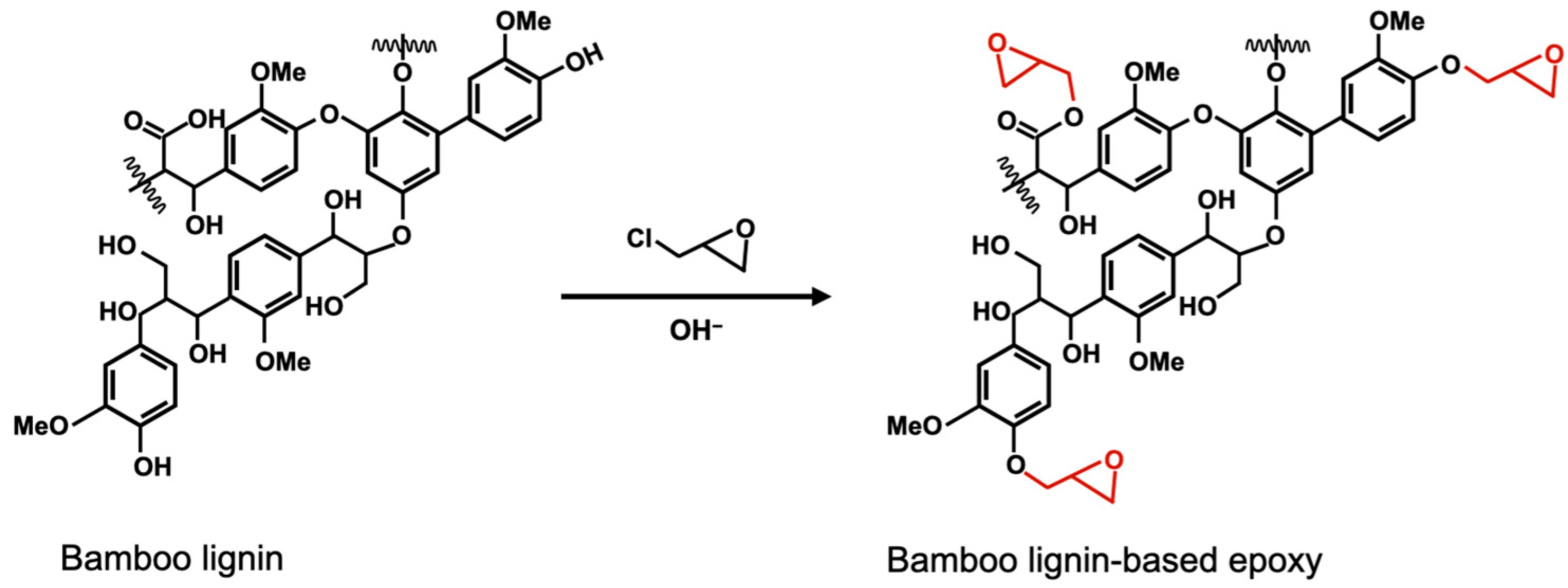
2.3. Epoxidation of Bamboo Lignin
2.4. Preparation of Bamboo Lignin-Based Epoxy Curing System
2.5. Fourier Transform Infrared Spectrometer (FTIR)
2.6. Molecular Weight Determination
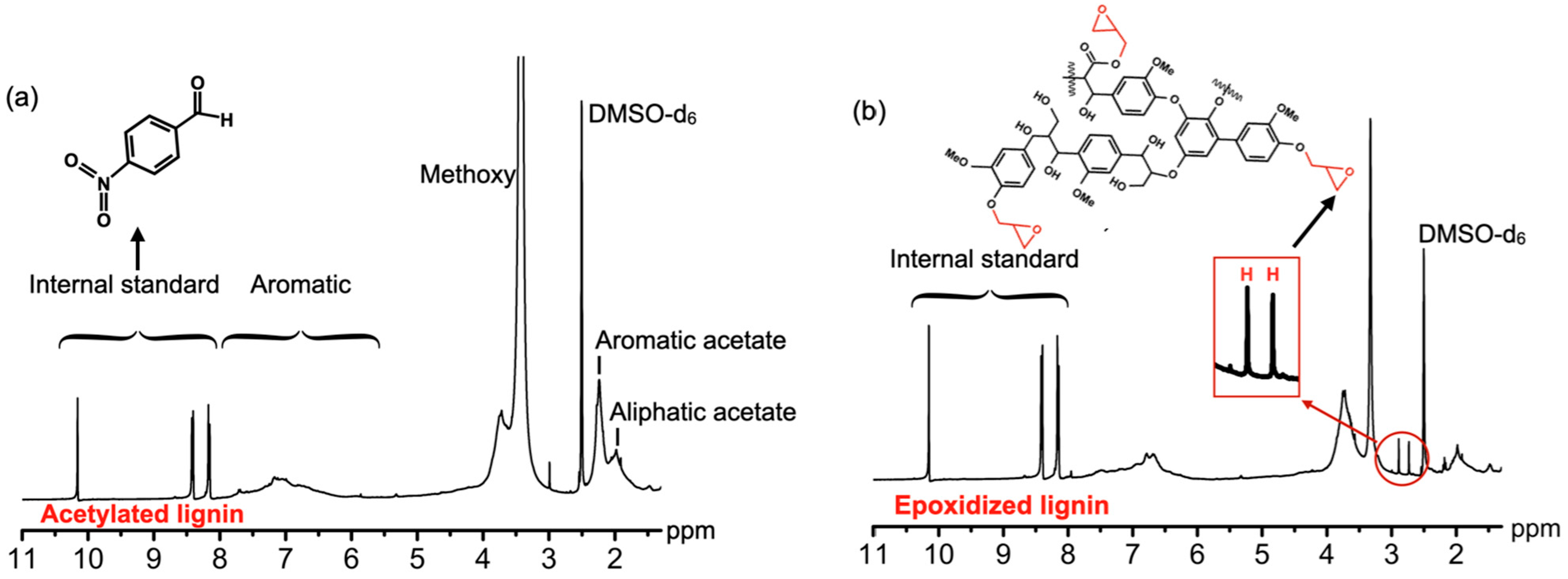
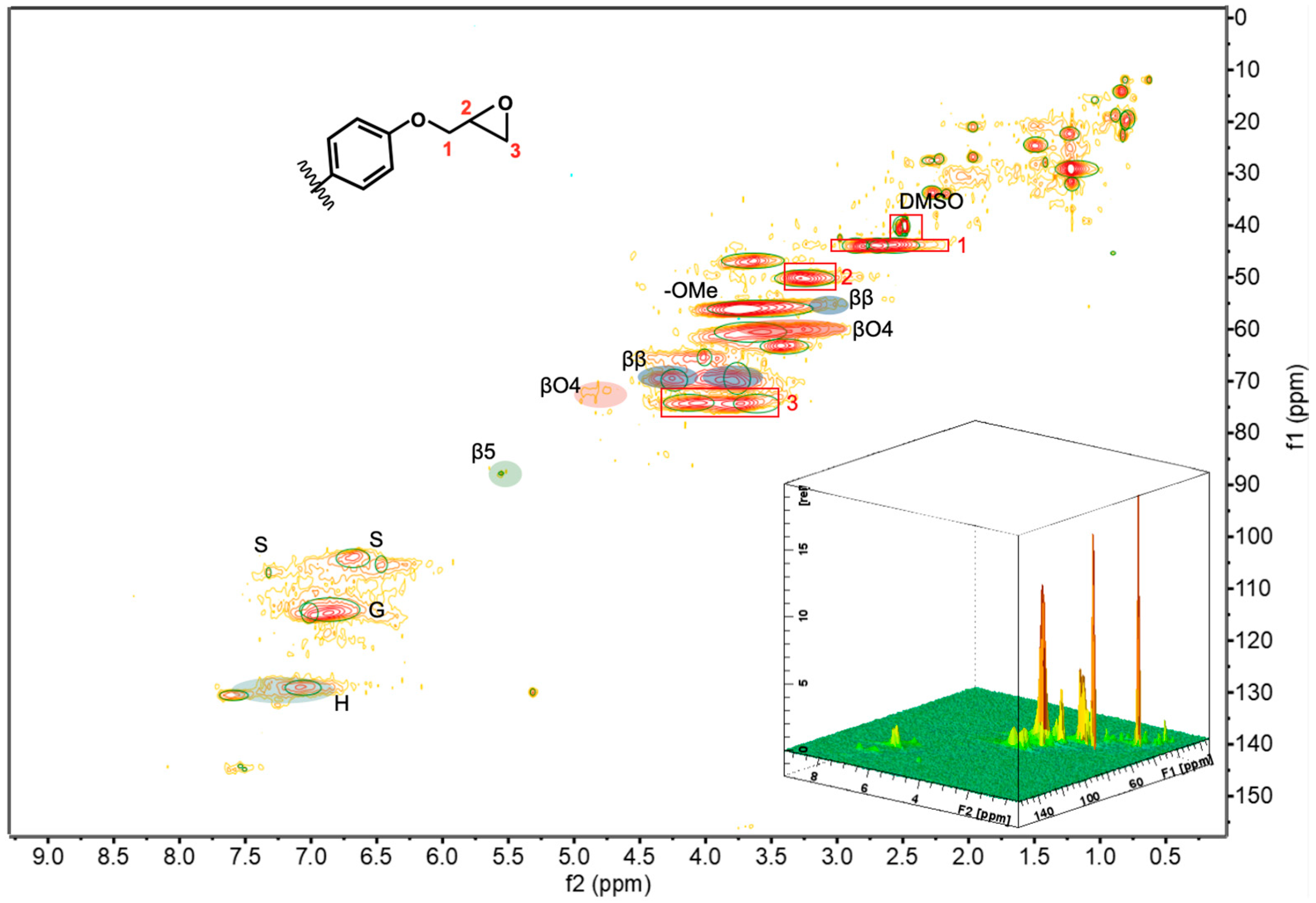
2.7. Nuclear Magnetic Resonance Spectra (NMR)
2.8. Determination of Epoxy Value
2.9. Thermogravimetric (TG) Analysis
2.10. Curing Thermal Behavior Test
2.11. Mechanical Property Test
3. Results and Discussion
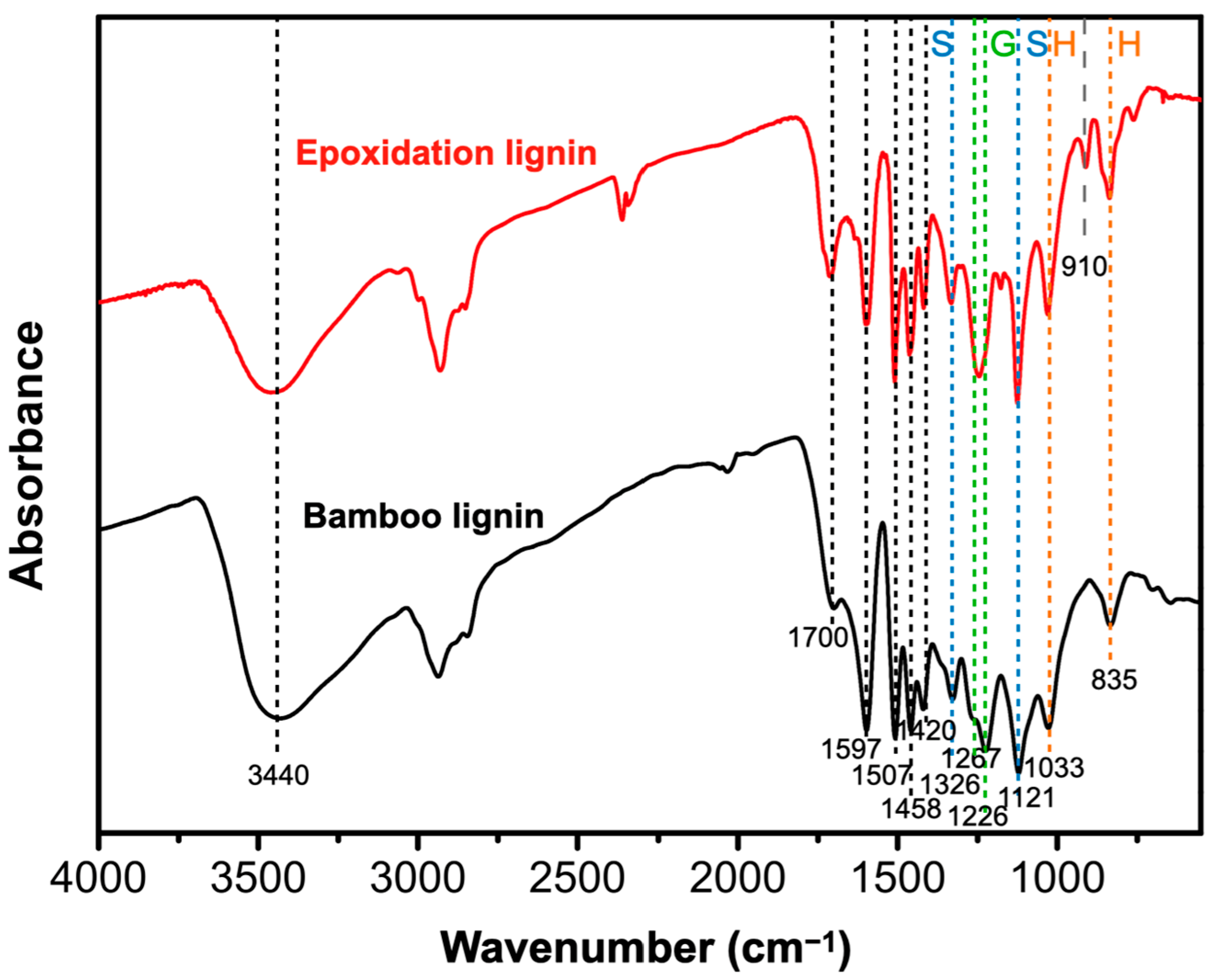
3.1. Infrared Spectral Analysis of Bamboo Lignin and Epoxidized Bamboo Lignin
3.2. Molecular Structure and Molecular Weight Analysis
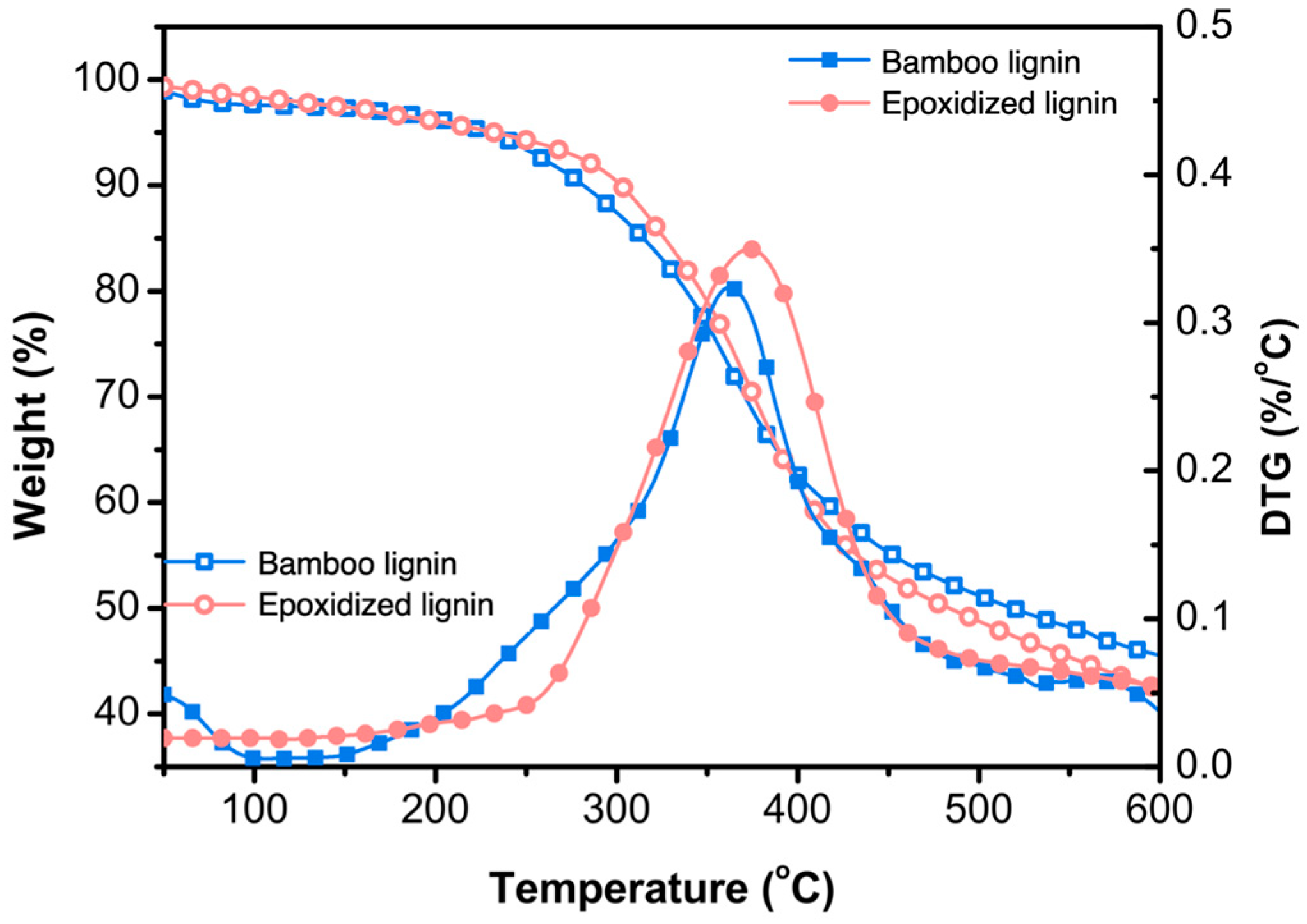
3.3. Thermal Stability Analysis of Bamboo Lignin
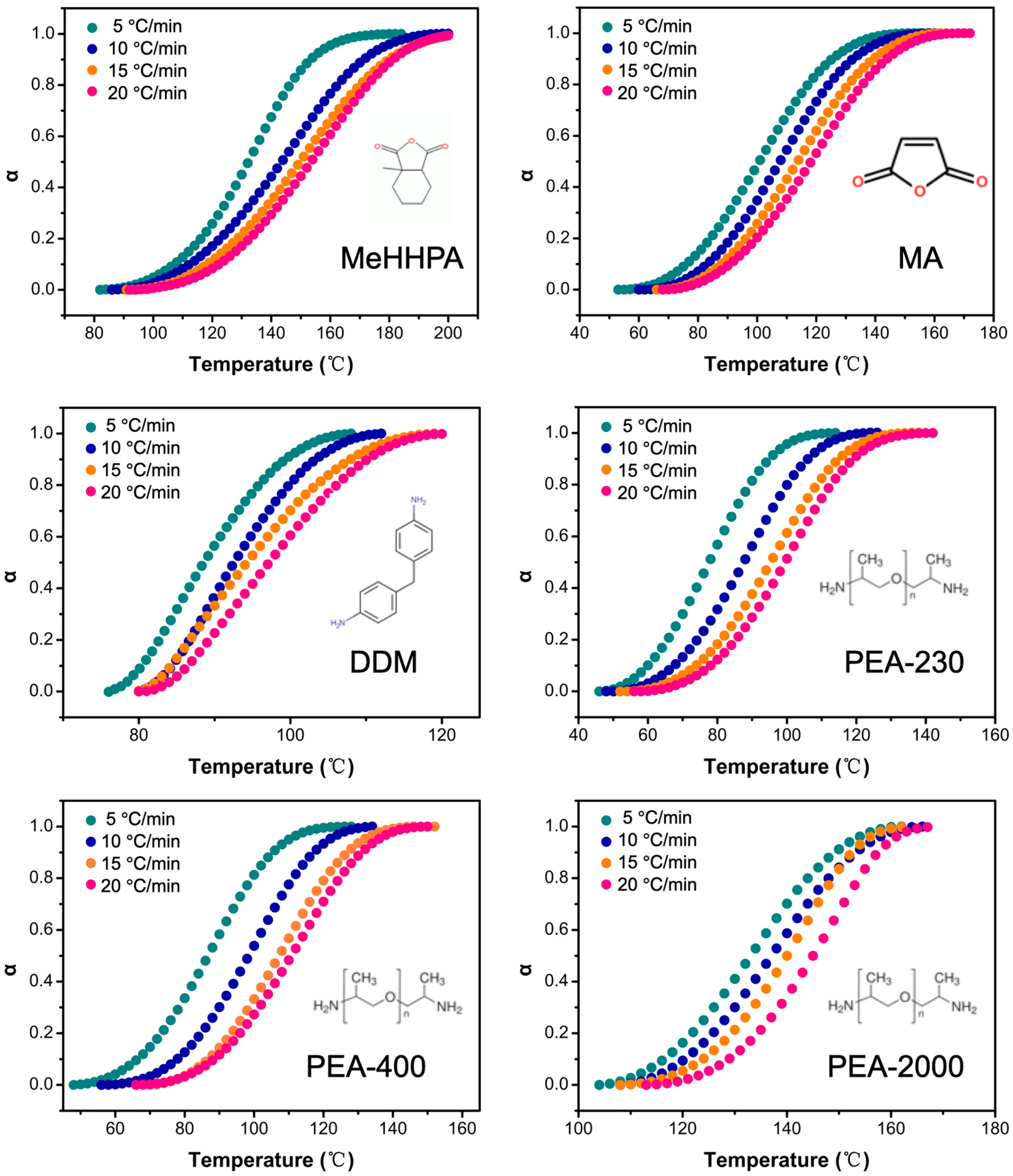
3.4. Curing Kinetics of Bamboo Lignin-Based Epoxy
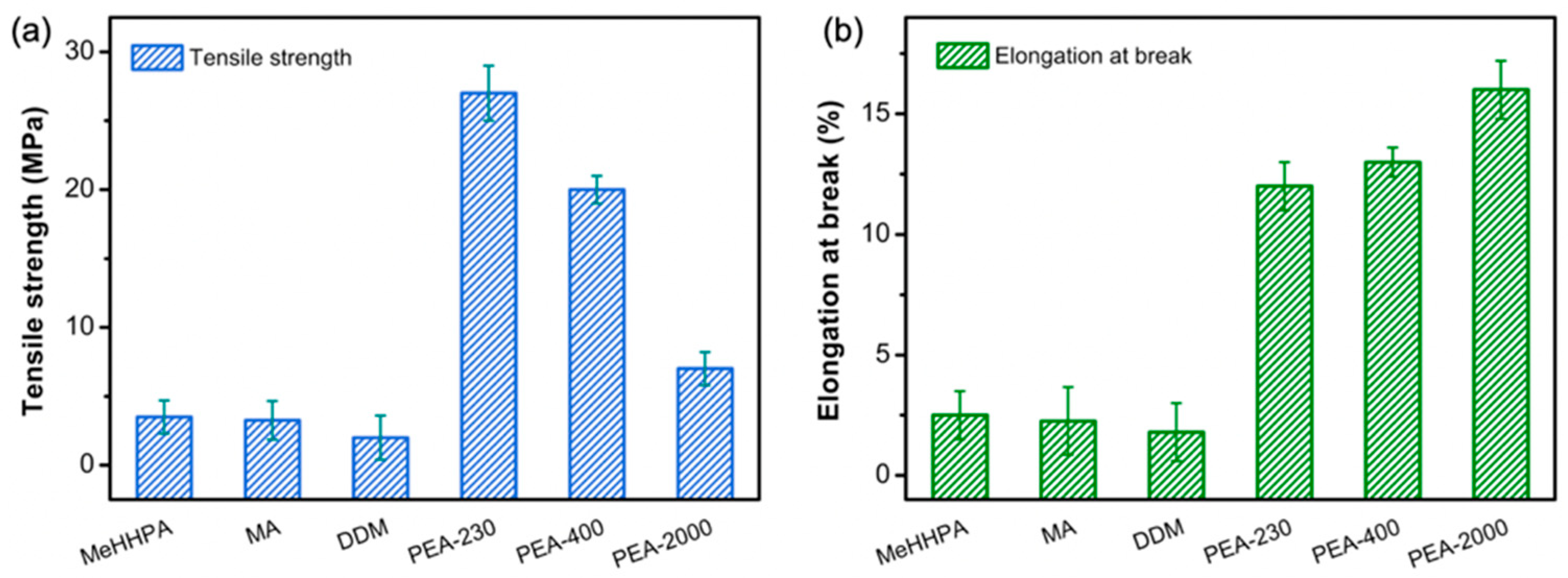
3.5. Mechanical Properties of Bamboo Lignin-Based Epoxy Curing Products
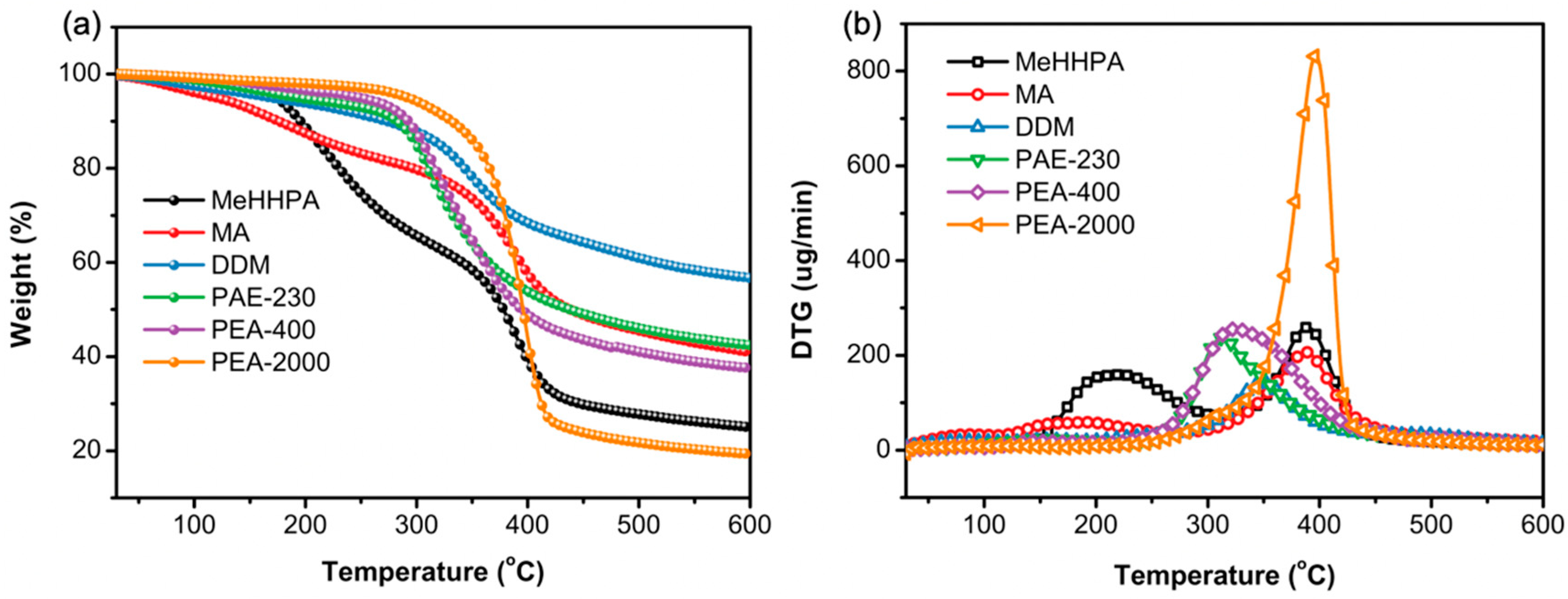
3.6. Thermal Stability of Bamboo Lignin-Based Epoxy Curing Products
3.7. Potential Curing Mechanism of Bamboo Lignin-Based Epoxy Resin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fache, M.; Boutevin, B.; Caillol, S. Epoxy thermosets from model mixtures of the lignin-to-vanillin process. Green Chem. 2016, 18, 712–725. [Google Scholar] [CrossRef]
- Anidha, S.; Latha, N.; Muthukkumar, M. Effect of polyaramid reinforced with sisal epoxy composites: Tensile, impact, flexural and morphological properties. J. Mater. Res. Technol. 2020, 9, 7947–7954. [Google Scholar] [CrossRef]
- El-Gawad, W.M.A.; Zaher, K.S.A.; Nawwar, G.A.M. Exploring the utilities of rice straw black liquor (part XI): Enhancing the UV resistance, color, antimicrobial, and mechanical characteristics of epoxy coatings using lignin-based hybrid nano-pigments. Prog. Org. Coat. 2024, 197, 108866. [Google Scholar] [CrossRef]
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. Control of reactions and network structures of epoxy thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef]
- Zou, T.; Sipponen, M.H.; Henn, A.; Osterberg, M. Solvent-resistant lignin-epoxy hybrid nanoparticles for covalent surface modification and high-strength particulate adhesives. ACS Nano 2021, 15, 4811–4823. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mao, Z.; Xu, H.; Wang, B.; Zhong, Y.; Ji, B.; Feng, X. Preparation of epoxy resin adhesives based on high phenolic nanomodified lignin particles. Int. J. Biol. Macromol. 2025, 309, 142886. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, J.; Zheng, S.; Song, M. Phenolic Hydroxyl-Activated Lignin for Preparation of Extreme Environments Adaptive Adhesives. J. Appl. Polym. Sci. 2025, 142, 56728. [Google Scholar] [CrossRef]
- Vasquez-Garay, F.; Carrillo-Varela, I.; Vidal, C.; Reyes-Contreras, P.; Faccini, M.; Teixeira Mendonca, R. A Review on the Lignin Biopolymer and Its Integration in the Elaboration of Sustainable Materials. Sustainability 2021, 13, 2697. [Google Scholar] [CrossRef]
- Song, X.; Lv, H.-B.; Jiang, Z.; Zhao, B.; Hu, W.; Zhang, K.; Shao, Z.-B. Sustainable lignin-based high-efficiency flame-retardant epoxy resins with excellent mechanical properties. Int. J. Biol. Macromol. 2024, 282, 136742. [Google Scholar] [CrossRef]
- Kaur, R.; Thakur, N.S.; Chandna, S.; Bhaumik, J. Sustainable Lignin-Based Coatings Doped with Titanium Dioxide Nanocomposites Exhibit Synergistic Microbicidal and UV-Blocking Performance toward Personal Protective Equipment. ACS Sustain. Chem. Eng. 2021, 9, 11223–11237. [Google Scholar] [CrossRef]
- Goliszek, M.; Podkoscielna, B.; Rybinski, P.; Klapiszewska, I.; Klepka, T.; Masek, A.; Klapiszewski, L. Toward a green economy: Lignin-based hybrid materials as functional additives in flame-retardant polymer coatings. J. Polym. Res. 2024, 31, 316. [Google Scholar] [CrossRef]
- Costa, D.d.S.; de Novais, L.M.R.; D’Oca, C.D.R.M.; Marques, J.F.; Ferreira, C.A.; Mazzetto, S.E.; Lomonaco, D.; Avelino, F. Towards phosphorylated lignin-based epoxy resins: An integrated technological route to obtain a macromonomer with enhanced thermal and potential flame-retardant properties. Int. J. Biol. Macromol. 2025, 304, 140821. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Smith, R.C. Valorization of Lignin as a Sustainable Component of Structural Materials and Composites: Advances from 2011 to 2019. Sustainability 2020, 12, 734. [Google Scholar] [CrossRef]
- Rajagopalan, N.; Winberg, I.; Fearon, O.; Cardellini, G.; Liitia, T.; Kalliola, A. Environmental Performance of Oxidized Kraft Lignin-Based Products. Sustainability 2022, 14, 10897. [Google Scholar] [CrossRef]
- Adamcyk, J.; Beisl, S.; Friedl, A. High Temperature Lignin Separation for Improved Yields in Ethanol Organosolv Pre-Treatment. Sustainability 2023, 15, 3006. [Google Scholar] [CrossRef]
- Chen, H.; Wang, A.; Yan, C.; Liu, S.; Li, L.; Wu, Q.; Liu, Y.; Liu, Y.; Nie, G.; Nie, S.; et al. Study on the Solubility of Industrial Lignin in Choline Chloride-Based Deep Eutectic Solvents. Sustainability 2023, 15, 7118. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C.C. Sustainable lignin-based epoxy resins cured with aromatic and aliphatic amine curing agents: Curing kinetics and thermal properties. Thermochim. Acta 2015, 618, 48–55. [Google Scholar] [CrossRef]
- Gouveia, J.R.; Garcia, G.E.; Antonino, L.D.; Tavares, L.B.; Dos Santos, D.J. Epoxidation of kraft lignin as a tool for improving the mechanical properties of epoxy adhesive. Molecules 2020, 25, 2513. [Google Scholar] [CrossRef]
- Guo, X.; Xin, J.; Huang, J.; Wolcott, M.P.; Zhang, J. Preparation and toughening of mechanochemically modified lignin-based epoxy. Polymer 2019, 183, 121859. [Google Scholar] [CrossRef]
- Kong, X.; Xu, Z.; Guan, L.; Di, M. Study on polyblending epoxy resin adhesive with lignin I-curing temperature. Int. J. Adhes. Adhes. 2014, 48, 75–79. [Google Scholar] [CrossRef]
- Yang, J.Y.; He, X.W.; Wang, H.X.; Liu, X.H.; Lin, P.; Yang, S.X.; Fu, S.Y. High-toughness, environment-friendly solid epoxy resins: Preparation, mechanical performance, curing behavior, and thermal properties. J. Appl. Polym. Sci. 2020, 137, 48596. [Google Scholar] [CrossRef]
- Wu, Z.; Prakash, B.; Shi, Y. Synthesizing lignin-based gelators to prepare oleogels used as green and fossil-free greases. Int. J. Biol. Macromol. 2025, 305, 141074. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Li, H.; Xu, Z.; Wang, Q.; Zhu, S.; Wang, Z.; Yuan, Z. Facile synthesis of lignin-based epoxy resins with excellent thermal-mechanical performance. Int. J. Biol. Macromol. 2021, 182, 276–285. [Google Scholar] [CrossRef]
- Gioia, C.; Lo Re, G.; Lawoko, M.; Berglund, L. Tunable thermosetting epoxies based on fractionated and well-characterized lignins. J. Am. Chem. Soc. 2018, 140, 4054–4061. [Google Scholar] [CrossRef]
- Gioia, C.; Colonna, M.; Tagami, A.; Medina, L.; Sevastyanova, O.; Berglund, L.A.; Lawoko, M. Lignin-Based Epoxy Resins: Unravelling the Relationship between Structure and Material Properties. Biomacromolecules 2020, 21, 1920–1928. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Liu, X.; Fu, S.; Song, P. A nano-TiO2/regenerated cellulose biohybrid enables simultaneously improved strength and toughness of solid epoxy resins. Compos. Sci. Technol. 2021, 212, 108884. [Google Scholar] [CrossRef]
- Feghali, E.; van de Pas, D.J.; Parrott, A.J.; Torr, K.M. Biobased epoxy thermoset polymers from depolymerized native hardwood lignin. ACS Macro Lett. 2020, 9, 1155–1160. [Google Scholar] [CrossRef]
- Thanki, J.D.; Parsania, P.H. Dynamic DSC curing kinetics and thermogravimetric study of epoxy resin of 9,9′-bis(4-hydroxyphenyl)anthrone-10. J. Therm. Anal. Calorim. 2017, 130, 2145–2156. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Zhang, Y.; Jin, Z. Kinetics of partially depolymerized lignin as co-curing agent for epoxy resin. Int. J. Biol. Macromol. 2020, 150, 786–792. [Google Scholar] [CrossRef]
- Ferdosian, F.; Zhang, Y.; Yuan, Z.; Anderson, M.; Xu, C.C. Curing kinetics and mechanical properties of bio-based epoxy composites comprising lignin-based epoxy resins. Eur. Polym. J. 2016, 82, 153–165. [Google Scholar] [CrossRef]



| Curing System | Bamboo Lignin-Based Epoxy/g | Curing Agent/g |
|---|---|---|
| MeHHPA | 10 | 4.2 |
| MA | 10 | 2.5 |
| DDM | 10 | 1.5 |
| PEA-230 | 10 | 1.7 |
| PEA-400 | 10 | 3 |
| PEA-2000 | 10 | 15 |
| Sample | Hydroxyl Groups (mmol/g) | Carboxyl Groups (mmol/g) | |
|---|---|---|---|
| Aliphatic Hydroxyls | Phenolic Hydroxyls | ||
| Bamboo lignin | 2.01 | 2.89 | 0.31 |
| Sample | Molecular Weight (g/mol) | PDI | |
|---|---|---|---|
| Mn | Mw | ||
| Bamboo lignin | 1138 | 4197 | 3.68 |
| Epoxidized bamboo lignin | 1231 | 4853 | 3.94 |
| Curing System | A | n | Dynamic Model |
|---|---|---|---|
| MeHHPA | 0.99 | ||
| MA | 0.99 | ||
| DDM | 0.99 | ||
| PEA-230 | 0.99 | ||
| PEA-400 | 0.99 | ||
| PEA-2000 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Fei, J.; Wang, X. Green Regenerative Bamboo Lignin-Based Epoxy Resin: Preparation, Curing Behavior, and Performance Characterization. Sustainability 2025, 17, 6201. https://doi.org/10.3390/su17136201
Yang J, Fei J, Wang X. Green Regenerative Bamboo Lignin-Based Epoxy Resin: Preparation, Curing Behavior, and Performance Characterization. Sustainability. 2025; 17(13):6201. https://doi.org/10.3390/su17136201
Chicago/Turabian StyleYang, Jiayao, Jie Fei, and Xingxing Wang. 2025. "Green Regenerative Bamboo Lignin-Based Epoxy Resin: Preparation, Curing Behavior, and Performance Characterization" Sustainability 17, no. 13: 6201. https://doi.org/10.3390/su17136201
APA StyleYang, J., Fei, J., & Wang, X. (2025). Green Regenerative Bamboo Lignin-Based Epoxy Resin: Preparation, Curing Behavior, and Performance Characterization. Sustainability, 17(13), 6201. https://doi.org/10.3390/su17136201





