Study on the Sustainability of Carbon Emission Reduction in China’s Cement Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
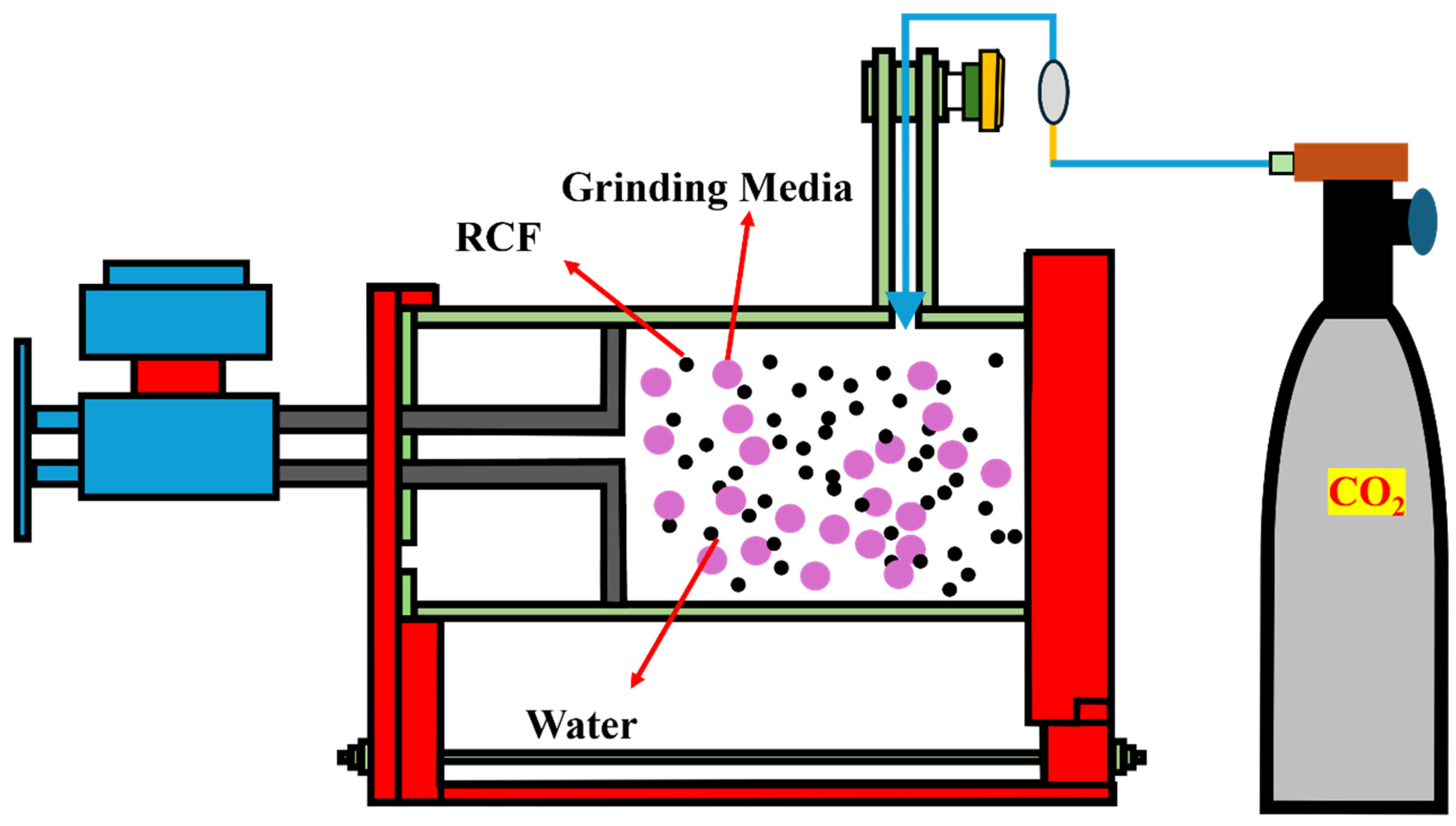
2.2. Sample Preparation
2.3. Characterizations
2.3.1. Compressive Strength
2.3.2. Particle Size Distribution and Specific Surface Area
2.3.3. Heat of Hydration
2.3.4. Phase Evolution
2.3.5. Microstructure Evolution
3. Results and Analysis
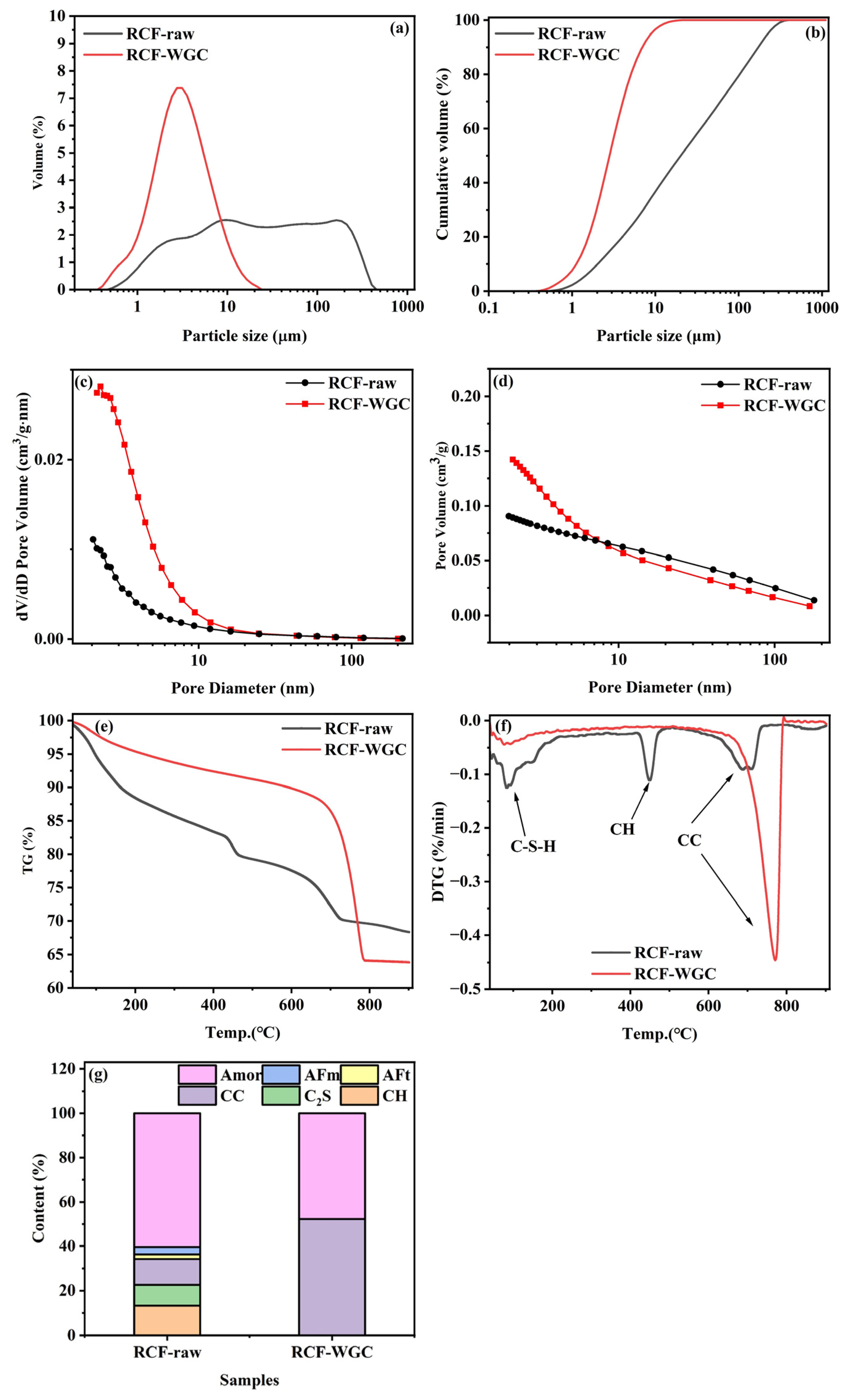
3.1. Characterizations of RCFs After WGC
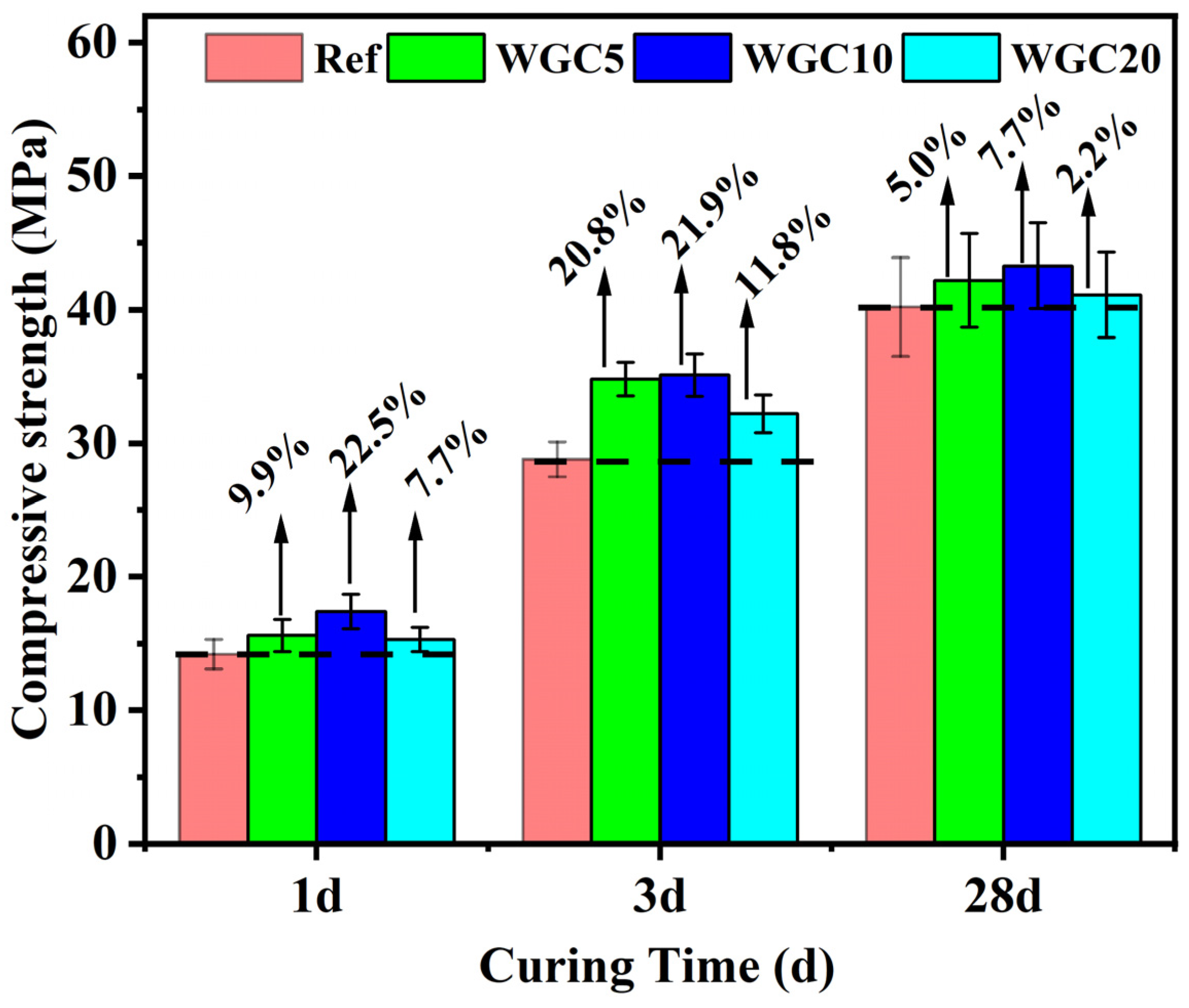
3.2. Compressive Strength
3.3. Hydration Heat
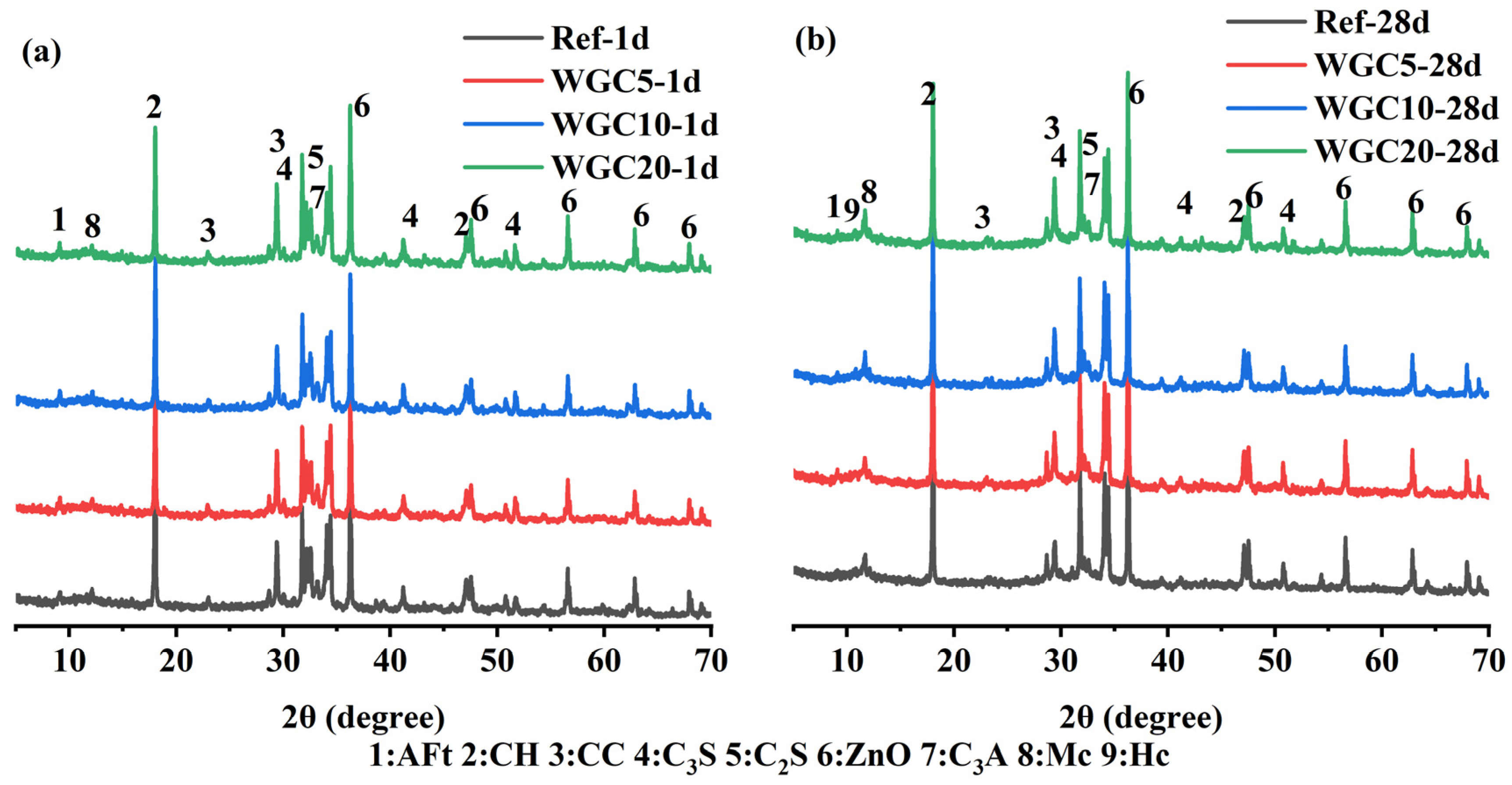
3.4. Phase Evolution of Hydration Samples
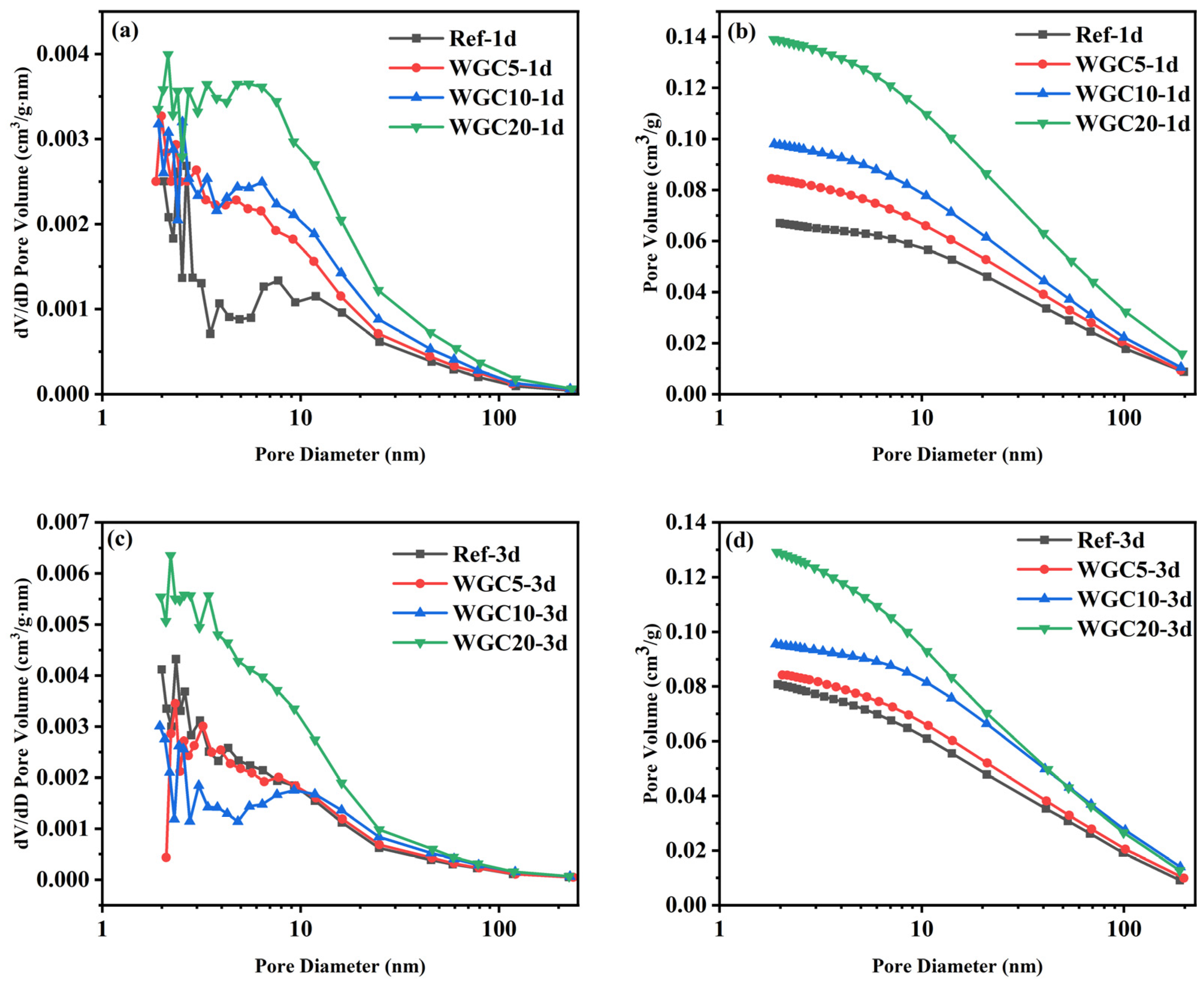
3.5. Pore Structure
3.6. Morphology
3.7. Sustainability Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, T.; Zhang, T.; Li, J.; Cui, K.; Chang, J. Microstructure and Properties of Ettringite with Different Al/(Fe + Al) Ratios in the C4A3-xFx$-2C$·H2 Binary Hydration System. ACS Sustain. Chem. Eng. 2025, 13, 8671–8686. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhong, H.; Sun, K.; Shen, B.; Cui, K.; Zhao, Y.; Xiong, G.; Qin, Q. Effect of composite activator on hydration kinetics and micromechanical properties of alkali-activated slag. Cem. Concr. Compos. 2025, 160, 106047. [Google Scholar] [CrossRef]
- Carvalho, I.C.; Andrade Neto, J.S.; Matos, P.R.; Lothenbach, B.; Kirchheim, A.P. The role of foreign ions in Portland cement production and properties: A state-of-the-art review on phase formation, polymorphism and hydration. Cem. Concr. Compos. 2025, 159, 105989. [Google Scholar] [CrossRef]
- Cui, K.; Liang, K.; Jiang, T.; Zhang, J.; Lau, D.; Chang, J. Understanding the role of carbon nanotubes in low-carbon concrete: From experiment to molecular dynamics. Cem. Concr. Compos. 2023, 142, 105189. [Google Scholar] [CrossRef]
- Zheng, Y.; Cui, K.; Zhao, Y.; Wu, W.; Shen, P.; Poon, C.S. Development of high-performance phosphogypsum-based cementitious materials through CO2-assisted alkali activation. Cem. Concr. Compos. 2025, 162, 106144. [Google Scholar] [CrossRef]
- Mache, E.; Rajczakowska, M.; Cwirzen, A. Process Residues in Cement Clinker Production: A Review. Waste Manag. Bull. 2025, 3, 100205. [Google Scholar] [CrossRef]
- Luo, W.; Liu, L.; Hu, J.; Hou, H.; Hu, H.; Yang, J. Optimization of industrial waste composition for eco-cement production using machine learning with meta-heuristic method. Resour. Conserv. Recycl. 2025, 220, 108328. [Google Scholar] [CrossRef]
- Cui, K.; Zheng, Y.; Zhao, Y.; Qin, Q.; Liang, K.; Chang, J.; Liu, F.; Shen, P.; Poon, C.S. Development of in-situ highly active calcium carbonate through anhydrous carbonation of OPC: Effect on hydration and properties of cement composites. Cem. Concr. Res. 2025, 197, 107980. [Google Scholar] [CrossRef]
- Gao, P.; Zha, W.; Chu, Y.; Yu, Y.; Zhan, B.; Yu, Q.; Xie, F.; Lu, H. Calculation model for CO2 emissions of blended cement production. J. Clean. Prod. 2025, 489, 144646. [Google Scholar] [CrossRef]
- Cui, K.; Zheng, Y.; Zhao, Y.; He, J.; Wu, W.; Du, X.; Chang, J.; Jin, H.; Shen, P.; Poon, C.S. Promoting the simultaneous reaction of carbonate and aluminate phases through anhydrous carbonation: Improving the properties of sulfoaluminate cement and stabilizing ettringite. Cem. Concr. Compos. 2025, 163, 106209. [Google Scholar] [CrossRef]
- Lippiatt, N.; Ling, T.-C.; Pan, S.-Y. Towards carbon-neutral construction materials: Carbonation of cement-based materials and the future perspective. J. Build. Eng. 2020, 28, 101062. [Google Scholar] [CrossRef]
- Feng, W.; Yu, Z.; Bao, R.; Xiong, J.; Yan, K.; Liu, R.; Zhang, R.; Lu, X. From waste to resource: Advanced activation techniques for tailings in sustainable cement production. J. Build. Eng. 2024, 97, 110780. [Google Scholar] [CrossRef]
- Hossain, M.U.; Ng, S.T.; Antwi-Afari, P.; Amor, B. Circular economy and the construction industry: Existing trends, challenges and prospective framework for sustainable construction. Renew. Sustain. Energy Rev. 2020, 130, 109948. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Cui, K.; Shen, P.; Poon, C.S.; Peng, G.; Guo, R.; Xia, D. Development of ultrafine and highly reactive SCMs via combined CO2 and mechanical activation of steel slag. Chem. Eng. J. 2025, 516, 163999. [Google Scholar] [CrossRef]
- Wang, B.; Yan, L.; Fu, Q.; Kasal, B. A Comprehensive Review on Recycled Aggregate and Recycled Aggregate Concrete. Resour. Conserv. Recycl. 2021, 171, 105565. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, Y.; Zhang, H. Flexural behavior and durability properties of recycled aggregate concrete (RAC) beams subjected to long-term loading and chloride attacks. Constr. Build. Mater. 2021, 277, 122277. [Google Scholar] [CrossRef]
- Tang, Q.; Ma, Z.; Wu, H.; Wang, W. The utilization of eco-friendly recycled powder from concrete and brick waste in new concrete: A critical review. Cem. Concr. Compos. 2020, 114, 103807. [Google Scholar] [CrossRef]
- Zajac, M.; Skibsted, J.; Skocek, J.; Durdzinski, P.; Bullerjahn, F.; Ben Haha, M. Phase assemblage and microstructure of cement paste subjected to enforced, wet carbonation. Cem. Concr. Res. 2020, 130, 105990. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, Y.; Jiang, Y.; Zhan, B.; Lu, J.; Zhang, S.; Xuan, D.; Poon, C.S. Phase assemblance evolution during wet carbonation of recycled concrete fines. Cem. Concr. Res. 2022, 154, 106733. [Google Scholar] [CrossRef]
- Shen, P.; Sun, Y.; Liu, S.; Jiang, Y.; Zheng, H.; Xuan, D.; Lu, J.; Poon, C.S. Synthesis of amorphous nano-silica from recycled concrete fines by two-step wet carbonation. Cem. Concr. Res. 2021, 147, 106526. [Google Scholar] [CrossRef]
- Yuan, C.; Yong, L.; Ziao, C.; Tianyi, Y.; Rui, Y. Development of Ultra-High Performance Concrete (UHPC) matrix based on recycled concrete fines subjected to coupling curing of microwave and wet carbonation. J. Build. Eng. 2024, 95, 110038. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Ma, Z.; Shen, P.; Poon, C.S.; Peng, G.; Guo, R.; Xia, D. Mechanochemical carbonation of recycled concrete fines: Towards a high-efficiency recycling and CO2 sequestration. Cem. Concr. Res. 2024, 185, 107654. [Google Scholar] [CrossRef]
- Ding, T.; Xiao, J. Estimation of building-related construction and demolition waste in Shanghai. Waste Manag. 2014, 34, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Prentice, D.; Collin, M.; Balonis, M.; La Plante, E.; Torabzadegan, M.; Gadt, T.; Sant, G. Calcium nitrate effectively mitigates alkali–silica reaction by surface passivation of reactive aggregates. J. Am. Ceram. Soc. 2024, 107, 7513–7527. [Google Scholar] [CrossRef]
- Liang, C.; Pan, B.; Ma, Z.; He, Z.; Duan, Z. Utilization of CO2 curing to enhance the properties of recycled aggregate and prepared concrete: A review. Cem. Concr. Compos. 2020, 105, 103446. [Google Scholar] [CrossRef]
- Liu, S.; Shen, P.; Xuan, D.; Li, L.; Sojobi, A.; Zhan, B.; Poon, C.S. A comparison of liquid-solid and gas-solid accelerated carbonation for enhancement of recycled concrete aggregate. Cem. Concr. Compos. 2021, 118, 103988. [Google Scholar] [CrossRef]
- Nedunuri, A.S.S.S.; Mohammed, A.y.; Muhammad, S. Carbonation potential of concrete debris fines and its valorisation through mineral carbonation. Constr. Build. Mater. 2021, 310, 125162. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, K.; He, J.; Zheng, Y.; Shen, P.; Poon, C.S.; Peng, G.; Guo, R.; Xia, D. An innovative strategy for maximizing CO2 reduction in concrete through preparing carbon sequestration precursors by accelerated carbonation. Cem. Concr. Compos. 2024, 152, 105618. [Google Scholar] [CrossRef]
- He, X.; Zeng, J.; Yang, J.; Su, Y.; Wang, Y.; Jin, Z.; Zheng, Z.; Tian, C. Wet grinding carbonation technique: Achieving rapid carbon mineralization of concrete slurry waste under low CO2 flow rate. Chem. Eng. J. 2024, 493, 152836. [Google Scholar] [CrossRef]
- Xing, T.; Mateti, S.; Li, L.H.; Ma, F.; Du, A.; Gogotsi, Y.; Chen, Y. Gas Protection of Two-Dimensional Nanomaterials from High-Energy Impacts. Sci. Rep. 2016, 6, 35532. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, K.; He, J.; Zheng, Y.; Shen, P.; Poon, C.S.; Peng, G.; Guo, R.; Xia, D. Highly reactive carbonated recycled concrete fines prepared via mechanochemical carbonation: Influence on the early performance of cement composites. Cem. Concr. Compos. 2024, 152, 105636. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Standardization Administration of China. Method of Testing Cement: Determination of Compres-sive Strength. China Standards Press: Beijing, China, 2021.
- Peng, L.; Jiang, Y.; Ban, J.; Shen, Y.; Ma, Z.; Zhao, Y.; Shen, P.; Poon, C.-S. Mechanism underlying early hydration kinetics of carbonated recycled concrete fines-ordinary portland cement (CRCF-OPC) paste. Cem. Concr. Compos. 2023, 144, 105275. [Google Scholar] [CrossRef]
- Wu, Z.; Khayat, K.H.; Shi, C.; Tutikian, B.F.; Chen, Q. Mechanisms underlying the strength enhancement of UHPC modified with nano-SiO2 and nano-CaCO3. Cem. Concr. Compos. 2021, 119, 103992. [Google Scholar] [CrossRef]
- Thomas, J.J.; Jennings, H.M.; Chen, J.J. Influence of Nucleation Seeding on the Hydration Mechanisms of Tricalcium Silicate and Cement. J. Phys. Chem. C 2009, 113, 4327–4334. [Google Scholar] [CrossRef]
- Wu, Y.; Mehdizadeh, H.; Mo, K.H.; Ling, T.-C. High-temperature CO2 for accelerating the carbonation of recycled concrete fines. J. Build. Eng. 2022, 52, 104526. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Durdzinski, P.; Bullerjahn, F.; Skibsted, J.; Ben Haha, M. Effect of carbonated cement paste on composite cement hydration and performance. Cem. Concr. Res. 2020, 134, 106090. [Google Scholar] [CrossRef]





| Materials | CaO | Al2O3 | SiO2 | SO3 | Fe2O3 | MgO | K2O | Others |
|---|---|---|---|---|---|---|---|---|
| OPC | 62.32 | 5.24 | 18.76 | 4.67 | 3.32 | 1.21 | 0.76 | 3.72 |
| RCF | 65.43 | 5.56 | 17.95 | 3.28 | 3.71 | 1.34 | 0.57 | 2.16 |
| Mixture | OPC | RCF-WGC | Water |
|---|---|---|---|
| Ref | 120 | / | 60 |
| WGC5 | 114 | 6 | 60 |
| WGC10 | 108 | 12 | 60 |
| WGC20 | 96 | 24 | 60 |
| Sample | C2S | CH | CC | AFm | AFt | Amor | Crystal Size of Calcite |
|---|---|---|---|---|---|---|---|
| RCF-raw | 8.2 | 14.3 | 12.5 | 3.9 | 2.9 | 58.3 | 259 |
| RCF-WGC | / | / | 51.2 | / | / | 48.8 | 249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Bao, C.; Zhang, B. Study on the Sustainability of Carbon Emission Reduction in China’s Cement Industry. Sustainability 2025, 17, 6349. https://doi.org/10.3390/su17146349
Zhao K, Bao C, Zhang B. Study on the Sustainability of Carbon Emission Reduction in China’s Cement Industry. Sustainability. 2025; 17(14):6349. https://doi.org/10.3390/su17146349
Chicago/Turabian StyleZhao, Kui, Congling Bao, and Bingxin Zhang. 2025. "Study on the Sustainability of Carbon Emission Reduction in China’s Cement Industry" Sustainability 17, no. 14: 6349. https://doi.org/10.3390/su17146349
APA StyleZhao, K., Bao, C., & Zhang, B. (2025). Study on the Sustainability of Carbon Emission Reduction in China’s Cement Industry. Sustainability, 17(14), 6349. https://doi.org/10.3390/su17146349







