Abstract
Utilizing more bioresources exposes supply chains to agricultural risks. Therefore, sustainable agricultural practices are crucial for supporting a resilient transition. This requires tools for assessing the environmental performance of cropping systems. However, most existing tools are not adapted to the operational realities of, for example, cosmetics supply chains. The Field Cultivation Index (FCI) has been developed with a threefold aim: 1. assess cropping systems’ strengths and weaknesses and guide their improvement; 2. compare cropping systems to identify the most environmentally friendly; 3. promote initiatives and efforts made to improve sustainability. It also meets the requirements for tackling the specific operational challenges of global industries like cosmetics: it is (i) simple (data collection is easy and straightforward), (ii) deployable in all regions of the world (with a diversity of soils, climates, etc.), and (iii) adaptable to all cropping systems (industrial, family, etc.) and all plant species (perennials, annuals, etc.). Its methodology is based on scientifically recognized evaluation methods and economic operator initiatives. It is built around a theoretical framework embodying the concepts of regenerative agriculture, enabling the qualitative assessment of the impact of cropping systems on five main regenerative environmental outcomes: soil quality improvement water resource conservation biodiversity enhancement, pesticide reduction, and carbon emission mitigation. This article describes the FCI methodology and reveals initial results from the evaluation of about 40 diversified cropping systems. It demonstrates that the FCI is an operational, sensitive, and educational tool capable of determining the performance level of diversified cropping systems, while highlighting the need for a high level of regenerative practice deployment to achieve satisfactory scores. The tool supports farmers and supply chains in assessing and improving the environmental profile of agricultural production systems in line with sustainability goals. Finally, this paper discusses the benefits, limitations, and potential uses of this tool for monitoring the environmental impact of cropping systems within cosmetics supply chains.
1. Introduction
In the context of climate change, it becomes of the upmost importance to limit the impact of industrial activities on carbon emissions, water stress, biodiversity loss, and natural resource depletion [1]. Utilizing more bioresources exposes supply chains to agricultural risks. Therefore, sustainable biomass cultivation practices are crucial to support the transition towards resilience [2,3]. In this context, cosmetics manufacturers, among others, have the responsibility to set specific research programs and commit to operate within planetary boundaries to mitigate the impact of all its activities on climate, water, biodiversity, and natural resources [4]. The common climate change objective is to align greenhouse gas emissions to the +1.5 °C scenario within scopes 1, 2, and 3 [5]. Nowadays, an increasing number of cosmetic raw materials are derived from renewable sources and from many species of plants in all regions of the world. A significant amount of work has been conducted by many groups to develop formulas with bio-based ingredients. Cosmetic research has been working for many years to ensure high water quality and sustainable water quantity across all its value chains and throughout the watersheds it operates in. In a context where agriculture, in particular, is threatening planetary boundaries and resilience to climate change, sourcing ingredients in a sustainable and responsible manner is therefore a pre-requisite to effectively limit environmental impacts [6].
Many agricultural models aim to reduce their environmental impact (reasoned, extensive, precision, etc.) in order to promote sustainable development by mitigating the negative effects of the Technosphere on the Biosphere. These approaches are intended to reconcile human technological activities with ecological integrity, reducing resource depletion, pollution, and biodiversity loss, thereby contributing to long-term environmental resilience [7,8]. Some, often grouped under the heading of sustainable agriculture or agroecology, have proposed structural changes to production methods to maximize the ecosystem services provided by agroecosystems (soil conservation agriculture, organic farming, etc.) [8,9]. Within this landscape, regenerative agriculture is emerging as an innovative approach for restoring agroecosystems. Regenerative agriculture has recently undergone significant development, driven by industrials who see it as a promising and pragmatic approach [10,11]. Although there is no consensus on the definition of regenerative agriculture [10,11,12], various initiatives have a number of common points. In addition to ambitious social considerations in favor of farmers and landscapes, five environmental outcomes are regularly mentioned [13,14]: (i) Soil, with the aim of improving soil quality; (ii) Water, with the aim of managing water resources; (iii) Biodiversity, with the aim of preserving and developing it in agroecosystems; (iv) Pests and weeds, with the aim of reducing the use of chemicals; (v) Carbon, with the aim of sequestering carbon and limiting its emissions.
To respond to these environmental outcomes, regenerative agriculture proposes an approach to the conservation and rehabilitation of agricultural and food systems that aims first to protect and then enhance the agroecosystem by providing services in return [10].
These different outcomes are not independent of each other [13]. For example, soil quality influences its capacity to store carbon [15]. Similarly, reducing the use of pesticides is associated with a reduction in pollution, with positive effects on biodiversity and water resources [16]. Therefore, regenerative agriculture implies a holistic vision of agricultural activity [14] requiring the implementation of a set of specific agricultural practices [10,13,14].
In the field, professionals must be able to address these five environmental outcomes and propose improvements based on recognized sustainable practices. To achieve this, tools are required to assess the environmental performance of cropping systems (a set of agricultural practices used to produce a crop) [10,14]. However, these tools are generally not well-adapted to operational realities [17], particularly in supply chains that produce bioresources used in cosmetics [2,10,13,18]. Most existing tools focus on the farm level [19,20], whereas economic players mainly think in terms of supply chains (and therefore crops). In addition, they often focus on specific contexts, such as plant species (annuals, perennials, etc.) grown in specific regions of the world (temperate and tropical climates) [21], whereas cosmetic supplies involve a wide variety of plant species grown in many regions of the world [22]. Finally, data collection often requires considerable resources when supply chains are complex and have multiple intermediaries.
Therefore, Field Cultivation Index (FCI), a qualitative index evaluating cropping systems, has been developed. It is based on the five environmental outcomes identified in regenerative agriculture (see introduction): 1. soil (improving quality), 2. water (managing resources), 3. biodiversity (preservation and development in agroecosystems), 4. Pests and weeds (reducing the use of chemicals), 5. Carbon (sequestration and limitation of emissions). The aim of this tool is threefold: 1. Assess cropping systems’ strengths and weaknesses to guide possible improvement; 2. Compare cropping systems to identify the most environmentally friendly; 3. Promote initiatives and efforts made. It also meets the specific operational challenges of the cosmetics industry: (i) simple (data collection is easy and straightforward), (ii) deployable in all regions of the world (diversity of soils and climates, etc.), and (iii) adapted to all cropping systems (industrial, family, etc.) and all plant species (perennials, annuals, etc.).
FCI does not address livestock breeding and is not relevant to the supply chains studied here.
To date, FCI does not address social aspects, which are already managed by tools developed and implemented within organizations [23].
In this paper, FCI methodology is described in details. Initial results from the evaluation of approximately 40 cropping systems with diverse profiles in terms of crop type (annual plant, perennial plant), production method (more or less simplified, organic certified or not, etc.), location, and climate (temperate, Mediterranean, arid, and tropical) are presented. Finally, the benefits, limitations, and potential uses of this tool for monitoring the environmental impact of a cultivated plant biomass supply chain are discussed.
2. Materials and Methods
The “Field Cultivation Index” (FCI) is a easy-to-use tool based on scientifically recognized evaluation methods [24,25,26,27,28] and economic operator initiatives [29,30,31], and has been adapted to the specific operational context of the cosmetic-related supply chains presented in the introduction. Figure 1 summarizes the six phases of the FCI methodology.
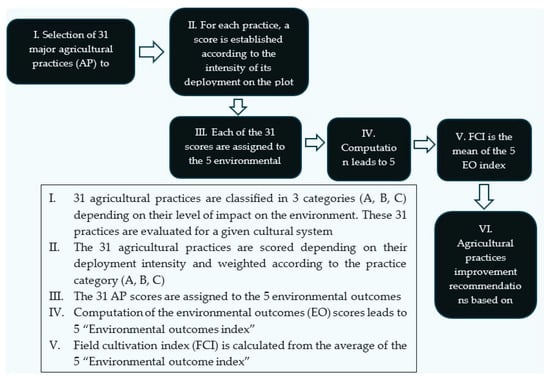
Figure 1.
Schematic summary of the FCI calculation method.
2.1. Constitution of Agricultural Practices List
The FCI methodology is based on the evaluation of 31 agricultural practices (listed in Table 1) frequently mentioned in the technical and scientific literature on regenerative agriculture and agroecology, and requires few resources for evaluation (qualitative data are sufficient). They cover both recognized sustainable agronomic techniques (the use of cover crops, rotation diversity, etc.) and “AgTechs” (innovative agricultural technologies such as connected tools and use of robots).

Table 1.
Agricultural practices designation, categories and their impact on the 5 environmental outcomes.
The 31 practices were characterized according to two key criteria: (i) their global impact on the environment and (ii) their specific impact on each of the five environmental outcomes identified in regenerative agriculture.
2.1.1. Global Impact on the Environment
The practices were classified (Table 1) into three categories (A, B, and C). This classification is based on criteria found in other evaluation methods (mentioned above) and technical and scientific literature (references summarized in Table 1). The practices referenced as most important in regenerative agriculture are included in category A, while those described as less regenerative are included in category C. Category B includes practices with intermediate effects. The most regenerative practices were weighted by a factor of four for cat A, two for cat B, and one for cat C (see phase II and Table 2 below).

Table 2.
Example of 3 Agricultural practices categories (and weight), assessment pre-established entries, grades and corresponding scores.
2.1.2. Specific Impact on the 5 Environmental Outcomes
The impact of the 31 practices was characterized in relation to the five environmental outcomes identified in regenerative agriculture (summarized in Table 1). The direct impact is noted as 1, while the indirect impact is 0.5. The absence of a coefficient indicates no significant impact. As before, this classification is based on technical and scientific literature (references summarized in Table 1) and expert opinion.
For example, practice #3 “Associated crop production” has a direct impact on environmental outcome III “Biodiversity,” an indirect impact on outcome IV “Pest management” and no significant impact on the three others.
2.2. Agricultural Practices Assessment and Scoring
The 31 agricultural practices (Table 1) were assessed through questions allowing a maximum of three pre-established entries (e.g., Yes/No/Partially) from which a grade out of 100 was attributed (0 for the least and 100 for the most regenerative configuration) (Table 2). The “agricultural practice score” is the grade weighted according to the agricultural practice category (Table 1). The maximum “agricultural practice score” for a practice is therefore 400 for a category “A” (100 × 4), 200 for a “B” (100 × 2) and 100 for a “C” (100 × 1) (Table 2).
2.3. Assignment of the 31 Agricultural Practices Scores to the 5 Environmental Outcomes
The agricultural practices scores are assigned to each of the 5 environmental outcomes according to the relationship presented in Table 1. For example, the agricultural practice #16 “Use of precision weeding” scored 100 (answer “yes” weight C/1) has an indirect impact on water and biodiversity, and therefore assigned 50 (100 × 0.5), while having a direct impact on pest & weed management assigned 100 (100 × 1). The other environmental outcomes were not considered related to this practice and were thus not assigned (Table 3).

Table 3.
Examples of agricultural practices scores assigned to the 5 environmental outcomes.
2.4. Environmental Outcomes Index Calculation
The sum of all the scores assigned to an environmental outcome according to the methodology described above (Table 3), divided by the sum of all the potential maximum corresponding scores (most regenerative configuration), results in a “outcome index” (out of 100). The five outcome indices are represented in a radar diagram, providing an initial level of analysis (Figure 2).
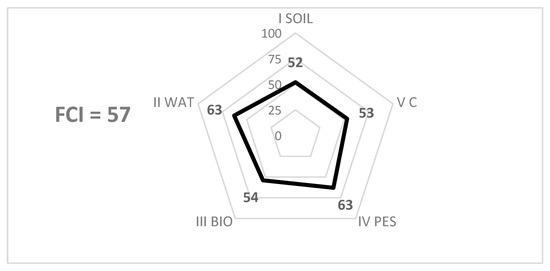
Figure 2.
Example of a FCI assessment for a crop—FCI, 5 environmental outcomes index and diagram (radar). SOIL: Soil outcome; WAT: Water outcome; BIO: Biodiversity outcome; PES: Pest management outcome; C: Carbon outcome.
2.5. Field Cultivation Index Calculation
The average of the five environmental outcome indices corresponds to the Field Cultivation Index (FCI), which provides information on the overall level of environmental performance of the cultural system. For example, for the crop shown in Figure 2, the FCI is 57 (out of 100), which corresponds to the average of the five environmental outcome values presented.
To date, an agricultural system is considered as “satisfying” when a threshold of 70 or more is reached. This threshold remains arbitrary at this stage, and is based on the results obtained from the 40 agricultural systems studied and completed by our agronomic expertise.
2.6. Practices Improvement Recommendations
The FCI index is accompanied by a list of “suggested” improvement practices drawn from the assessment. Implementing such practices in the cultural system increases its regenerative nature and, therefore, increases the FCI score.
2.7. Methodology Adaptation for Annual and Perennial Crops
Because perennial and annual crops have specific agronomic characteristics, the methodology is adapted as follows:
Practice #1 “Duration of rotation” assesses the diversity of the agricultural system. This will be done by evaluating the duration of rotations for annual crops, and the duration of intercropping for perennial crops.
Some practices have also been addressed only for perennial crops, and others only for annuals. For example, practice #4 “Proportion of soil surface covered during cultivation” only concerns perennials. It is assumed that only perennial plants are grown in rows, in which case inter-row coverage is a stake. Practice #5 “Incorporating intermediate cover crops into rotation for agronomic purposes” only concerns annuals, for which the frequency of intercropping is much greater.
Finally, the global impact of some practices on the environment is not the same for perennial or annual cropping systems. Therefore, the agricultural practice classification (categories A, B, or C) varies according to crop type. For instance, Practice #1 “Duration of the rotation” will be classified as A for annual systems because its impact is referenced as significant in the literature, whereas it will be B for perennial systems as the assessment is based on the duration of intercropping. Similarly, practice #25 “tillage” is described as having stronger impact on the soil for annual crops system (therefore classified A) than for perennial crops system grown over a decade (classified in category B).
3. Results
3.1. Polyvalence and Discriminating Character of FCI
The FCI assessment was conducted on about 40 cropping systems of 12 different crops, with diverse characteristics: four major climatic regions (Temperate, Mediterranean, Arid, Tropical), perennial and annual crops, and certified organic or not. Figure 3 presents the results for 12 different crops produced using different cropping systems. The FCI scores ranged from 16 to 79, showing clear discrimination between the systems, and 10 cropping systems reached an acceptable threshold of 70.
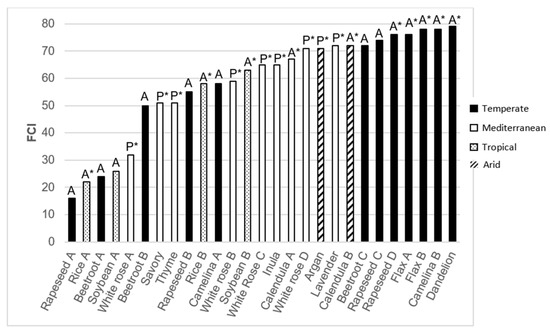
Figure 3.
Main results of FCI assessments carried out. A: Annual crop; P: Perennial crop; *: organic certification. For the same crop evaluated in different agricultural systems, the letter A, B or C is mentioned in order to differentiate between them.
In these analyses, annual crops grown in temperate environments had the best FCI scores (74–79) because of the implementation of significant numbers of A practices. In average, organic farming systems also received the highest scores, but it is to be noted that “Rice A” and “White rose A” are among the lowest-scoring systems (22 and 32 respectively), even though they are organic. Reversely, “Rapeseed C” run conventionally, has one of the highest scores (74). These results, which are specific to each farming system, will be refined with a larger number of future assessments.
3.2. FCI Is a Simple and Easy-to-Use Tool
The FCI tool was tested on approximately 40 producers (farmers, technicians, and production site managers). Data were collected either by direct interviews or by sending out the questionnaire after a preliminary phase of the methodology explanation.
At the operational level, it is demonstrated that it is easy to gather information. The interviews lasted for an average of 30 min. The precision of the questions and the simple pre-established entries (answers) facilitate interviewee responses based on their knowledge, without having to refer to documents or other cultural notebooks. In addition, apart from a few occasional requests for clarification, the questionnaire was filled immediately. Feedback from interviewees was very positive: questions were relevant, clear, and straight to the point with the right level of detail.
3.3. Use Cases
3.3.1. Production Systems Comparison and Regeneration Process Initiation
The FCI was tested on four white roses production sites (W, X, Y, and Z; Figure 4). All are organic-certified, but with contrasting agricultural practices. Site W adopts a “simplified” approach with little regenerative potential: intensive soil preparation, few soil coverage and limited integration of agroecological infrastructures. In contrast, Site Z, which is a demonstration plot for stakeholders, is the most advanced in terms of regenerative practices. Sites X and Y are intermediaries between sites W and Z.
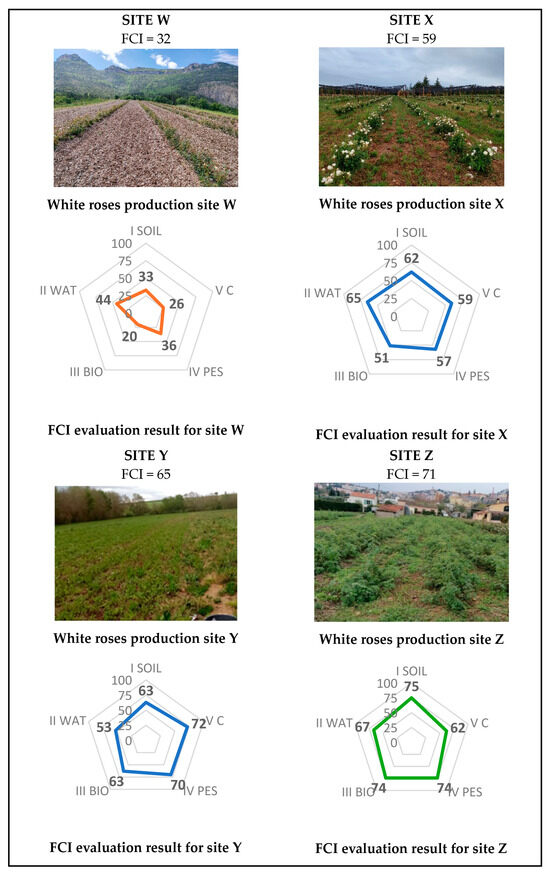
Figure 4.
FCI evaluation results for 4 white roses production sites (W, X, Y, Z).
The FCI assessment confirmed the exemplary nature of Site Z (FCI = 71). The organic-certified site W (FCI = 32) appears to have considerable scope for improvement as its 5 environmental outcomes index are low, especially the “Biodiversity” (20) and “Carbon” (26) ones. Site X (FCI = 59) would require improvement, especially on “biodiversity outcome” (Index = 51) to reach more satisfactory score. Site Y (FCI = 65) still needs improvement, particularly on the “Water management” outcome (53). With the best performing “Carbon outcome” (72), it can also inspire all other sites, even the Z site.
The FCI tool suggests several practices to be deployed at different sites to engage in the regeneration process. Table 4 summarizes, per site, the most impactful and easily deployable (non-exhaustive list) because they are implemented on at least one of the sites.

Table 4.
Suggested most impactful agricultural practices to implement on white roses production sites W, X, Y, Z.
3.3.2. Production Systems Comparison in One Cooperative
The FCI was tested at 21 rapeseed production sites (Figure 5). With many ‘A,’ ‘B’ and ‘C’ category practices, the S21 system scored FCI = 74, demonstrates it is possible to achieve a satisfactory score above 70 while other systems are scored between 19 to 49. For example, there are no ‘A’ category practices deployed in systems scored < 30, whereas several were implemented in systems scored > 40. This may provide inspiration for the less advanced systems, which, by introducing a few ‘A’ practices, could increase their environmental performance without involving radical changes (e.g., including additional species and cover crops in the rotation, plant hedges around plots, etc.). Although this would not lead to a satisfactory score of 70, it would be the first step towards improving the performance of the given cultural system.
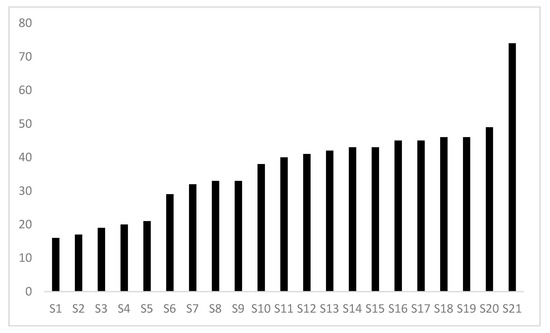
Figure 5.
FCI scores from the evaluation of 21 different production systems for an annual crop in a temperate climate.
3.3.3. Comparison of Production Systems for a Crop Sourced from Two Different Cooperatives
The FCI assessment was carried out for crops produced in different French farms (similar sizes and characteristics) belonging to cooperatives A (n = 11) or B (n = 18) (Figure 6). As the contexts studied were similar, the average FCI score was calculated per cooperative. The aim was to compare the crop production systems within the two cooperatives, not the cooperatives in a broad sense. The crops produced in cooperative A had a significantly higher average score (58) than those in cooperative B (43) (Student p < 0.05).
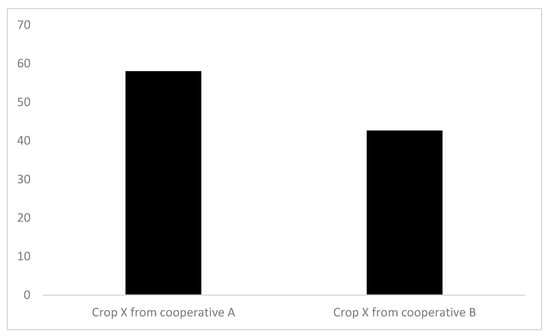
Figure 6.
Average FCI scores and standard deviation for crop X production systems sourced from 2 different cooperatives. Crop X is a major agricultural crop in temperate climate. Cooperative A: n = 11, Cooperative B: n = 18. The average FCI scores are significantly different (Student < 0.05).
This use case suggests that FCI can be used to evaluate crops from different suppliers. To ensure that the farms assessed are representative of the supplier, the sampling method is a key factor in this approach. In addition, each FCI score should be weighted based on certain criteria such as the surface area of the farm plots evaluated. Because of operational constraints in the data collection, this weighting was not performed in this example.
4. Discussion
The results of the testing phase confirmed objectives of FCI: 1. Perform a qualitative assessment of cropping systems and identify their strengths and weaknesses to guide regenerative practices; 2. compared the cropping systems of a given crop and identified the most environmentally friendly system. In particular, the FCI can discriminate cropping system performance based on the practices that are deployed, and the low scores obtained by some of the organic certified systems demonstrate a high level of ambition set by the FCI. The FCI’s ability to provide a list of recommended practices appears to be an asset for improving the cultural system. The case of white rose shows the enhanced value of these recommendations when comparing production systems for the same species.
The testing phase also confirmed that first users were predominantly interested in the FCI methodology and considered it easy to implement, collaborative, rewarding, and not intrusive. The information was collected by a limited number of interviewers with sufficient expertise in regenerative agriculture to guide the farmers’ interviews. This ensured consistency and coherence of the results. However, the simplicity of such approaches can lead to a certain bias linked to individual value judgments [89]. Before deploying the method more broadly, drawing up a user guide is a prerequisite to limiting the bias generated by the diversity and multiplicity of interviewers.
Finally the 70 satisfactory threshold value seems, to date, appropriate and in line with observations made in the field. Further evaluation in different contexts, for example, on sites with recognized certifications (organic, ROC, Regenagri, etc.) will enable us to refine the calibration.
Limitations of the method must also be acknowledged.
Although the FCI methodology is based on the holistic vision of a production system, it is limited to a single-plant production system. For example, livestock farming or the integration of the cultural system into the local area or landscape is not considered in the current FCI assessment. If the aim is to steer the development of the agricultural production system at this level, then such additional elements should be complemented using other tools.
The FCI does not integrate the social and economic aspects of farming practices. Integrating these dimensions into the FCI would risk increasing the complexity of data collection, particularly due to the potential unavailability or confidentiality of information. Such an extension could reduce the tool’s operational simplicity and its applicability across diverse farming systems. However, the inclusion of these considerations could be explored as a potential improvement in future developments of the tool, provided that its usability and accessibility for field stakeholders are preserved. Therefore, it is recommended to complement specific and adapted evaluation methods to offer a global vision of all aspects of sustainability. For instance, L’Oréal is implementing an internal methodology based on recognized standards, scoring the social aspects of a global supply chain from 1 to 4. Therefore, FCI is complemented by this social score.
FCI is a qualitative assessment tool based on resource indicators. Although this approach makes the tool highly operational, it has two important limitations. Firstly, a qualitative approach using a limited list of pre-established entries (for example: “presence”, “partial presence”, “absence”) can be imprecise for assessing the importance of some practices. As most of the practices studied were applied at the plot level, the loss of precision induced by this approach seems limited. Second, even if supported by a bibliography, the presence of resources does not necessarily imply the achievement of results. Therefore, the FCI was supplemented by (1) The deployment of a regenerative assessment grid requires a more detailed analysis to initiate a continuous improvement process, specifically geared towards regeneration; (2) Quantitative measurements to confirm the positive impact of the deployed resources. For example, specific soil, water, and biodiversity measurements can be used to correlate efforts made over time with quantitative results and impacts. However, such quantitative measurements provide only a snapshot of the system’s status at a given time and do not capture the trajectory of change. In this respect, the FCI offers a complementary perspective by evaluating the potential direction of system evolution based on the implementation of agricultural practices. The combination of both approaches enables a comprehensive assessment of current system performance and its expected development over time
Also, It would be valuable to test the FCI method across diverse production systems and compare it with other assessment tools to evaluate its robustness, highlight its strengths, and identify areas for improvement
Finally, the FCI evaluates 31 agricultural practices that are valid in all contexts. These practices were selected based on their relevance to regenerative agriculture, following a review of the literature. Further selection and addition of complementary practices could be considered to enhance the specificity and comprehensiveness of the evaluation. However, this would also risk increasing the complexity and reducing the operational functionality of the tool, potentially making it less accessible and user-friendly for field stakeholders. The strength of the FCI lies precisely in this balance: it provides sufficiently precise and relevant information to guide decision-making while remaining simple, practical, and applicable in diverse agricultural contexts.
Furthermore, in some specific contexts, the deployment of a recommended practice may not improve the performance of the cultural system, or may be practically unrealistic. The aim was not to systematically implement all 31 practices assessed, the reason why the satisfactory threshold was set at 70 and not 100. Rather, the approach consists of identifying the optimum combination that meets the sustainability challenges and the specific context (soil and climate, resources, etc.) of each specific cultural system, which should be considered for guidance and tested against the expertise of those working in the field.
5. Conclusions
The Field Cultivation Index (FCI) is a relevant method that stands out from existing tools because it responds to the specific context and variety of agricultural systems used for, for instance, cosmetic bioresource production. It is built around a theoretical framework embodying the concepts of regenerative agriculture, enabling the qualitative assessment of the impact of cropping systems on five main regenerative environmental outcomes: soil, water, biodiversity, pesticide reduction, and carbon emission mitigation Furthermore, it helps address the weakness levelled so far in regenerative agriculture, which has not yet been a reliable means of situating itself on progress trajectories depending on the production context [10].
Tests conducted on approximately 40 cropping systems in a variety of contexts have shown that the FCI is
Appropriate: In line with its ambition, it values the best performing cropping systems with regard to the five environmental outcomes.
Sensitive: capable of discriminating cropping systems according to their level of performance.
Demanding: reaching a satisfaction threshold of 70 requires a high level of regenerative practice deployment.
Operational: information is quick and easy to collect.
Educational: results are easy to understand and interpret, even by novice users.
FCI provides farmers in any country the opportunity to assess the environmental profile of their cropping systems based on a common benchmark, to identify ways of improving and promoting sustainable practices that have been deployed. For bioresource procurement, it offers a solution to compare suppliers and assess the overall impact of a supply chain.
Author Contributions
Conceptualization, D.B., M.B. and A.D.; Methodology, D.B., M.B., B.L. and A.D.; Validation, D.B., M.B., B.L., M.C. and D.H.; Formal analysis, D.B., M.B., S.G. and M.C.; Investigation, M.B., B.L. and E.O.; Resources, B.L. and A.D.; Data curation, B.L. and E.O.; Writing—review & editing, D.B., M.B. and S.G.; Visualization, A.D.; Supervision, D.B. and M.C.; Project administration, D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted under the guidelines of L’Oréal Ethics Committee. L’Oréal R&I conducted scientific research aimed at diagnosing and assessing environmental state of the sourcing of ingredients. Field Cultivation Index study enabled collecting information related to agricultural practices within L’Oréal supply chain and assessing possible improvement towards more regenerative and sustainable ones. This study required technical Interviews from agricultural practitioners that where run under the code of ethics of L’Oréal (https://www.loreal.com/-/media/project/loreal/brand-sites/corp/master/lcorp/2-group/governance-and-ethics/ethics-rework/code-of-ethics-2023/codeofethicseng.pdf?rev=765a100d8f904e0bbcf015ba95c1bdbe, accessed on 2 June 2024, L’Oréal Code of Ethics). In the specific case of the work done for FCI publication, L’Oréal involved suppliers and partners in the supply chains, following ethical rules that are: (1) Conduction research and innovation with integrity, handling personal data with care. In our case, a questionnaire (see interview script attached) has been utilized following prior consent from the interviewees. The interviewees have been informed of our study and its objectives The interviewees answered on voluntary basis, and from existing data. All data collected during the surveys have been kept anonymous, (2) Building strong relationships with our business partners. In our case, the study was conducted in alignment with the partners of our supply chains and within the group commitment to have transparency on the work done with their involvement. In that context, feedback has been given to each of them once the study was terminated.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to thank all the farmers and supply chain stakeholders, as well as the internal staff who took part in constructing and testing the FCI.
Conflicts of Interest
Delphine Bouvier, Aurore Dieu, Magda Carrasco and David Hazoumé were employed by L’Oréal Research and Innovation (Aulnay-sous-Bois). Mathieu Bayot was employed by Soliance Alimentaire. Bertrand Lacroix and Elsa Ogé were were employed by L’Oréal Research and Innovation (Chevillt-Larue). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviation
The following abbreviation is used in this manuscript:
| FCI | Field Cultivation Index |
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Réchauffement planétaire de 1,5 °C, Résumé à l’intention des décideurs, résumé technique et foire aux questions. In Groupe D’experts Intergouvernemental sur L’évolution du Climat; GIEC, Ed.; Organisation Météorologique Mondiale: Geneva, Switzerland, 2019; ISBN 92-9169-253-8. [Google Scholar]
- Bom, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A Step Forward on Sustainability in the Cosmetics Industry: A Review. J. Clean. Prod. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- Jaffee, S.; Siegel, P.; Andrews, C. Rapid Agricultural Supply Chain Risk Assessment: A Conceptual Framework. In Agriculture and Rural Development Discussion Paper; The World Bank: Washington, DC, USA, 2010; Volume 47, pp. 1–64. [Google Scholar]
- Mondello, A.; Salomone, R.; Mondello, G. Exploring Circular Economy in the Cosmetic Industry: Insights from a Literature Review. Environ. Impact Assess. Rev. 2024, 105, 107443. [Google Scholar] [CrossRef]
- L’Oréal. L’Oréal for the Future, Our Sustainability Commitments for 2030; L’Oréal: Paris, France, 2020; p. 17. [Google Scholar]
- OCDE; Organisation des Nations Unies pour L’alimentation et L’agriculture. Guide OCDE-FAO Pour des Filières Agricoles Responsables; OECD: Paris, France, 2016; ISBN 978-92-64-26402-1. [Google Scholar]
- Vermeire, M.-L.; Belmin, R. Les Sept Familles de L’agriculture Durable. Available online: http://theconversation.com/les-sept-familles-de-lagriculture-durable-227407 (accessed on 2 June 2024).
- Steffen, W.; Broadgate, W.; Deutsch, L.; Gaffney, O.; Ludwig, C. The Trajectory of the Anthropocene: The Great Acceleration. Anthr. Rev. 2015, 2, 81–98. [Google Scholar] [CrossRef]
- Husson, O.; Sarthou, J.-P.; Duru, M. Référentiels et nouveaux indicateurs pour fonder une agriculture régénératrice. Agron. Environ. Soc. 2023, 13, 1–18. [Google Scholar] [CrossRef]
- Duru, M.; Sarthou, J.-P.; Therond, O. L’agriculture régénératrice: Summum de l’agroécologie ou greenwashing? Cah. Agric. 2022, 31, 17. [Google Scholar] [CrossRef]
- Gordon, E.; Davila, F.; Riedy, C. Regenerative Agriculture: A Potentially Transformative Storyline Shared by Nine Discourses. Sustain. Sci. 2023, 18, 1833–1849. [Google Scholar] [CrossRef]
- Tittonell, P.; El Mujtar, V.; Felix, G.; Kebede, Y.; Laborda, L.; Luján Soto, R.; De Vente, J. Regenerative Agriculture—Agroecology without Politics? Front. Sustain. Food Syst. 2022, 6, 844261. [Google Scholar] [CrossRef]
- FAIRR Initiative. The Four Labours of Regenerative Agriculture. Paving the Way towards Meaningful Commitments; FAIRR Initiative: London, UK, 2023; p. 40. [Google Scholar]
- Kelley, S.; Jensen, B.; Shepard, S.; Denes, H.; Kniestedt, Z. Regenerative Agriculture Landscape Analysis; Textile Exchange Burbank: California, CA, USA, 2022. [Google Scholar]
- Lal, R.; Follett, R.F.; Stewart, B.A.; Kimble, J.M. Soil Carbon Sequestration to Mitigate Climate Change and Advance Food Security. Soil Sci. 2007, 172, 943–956. [Google Scholar] [CrossRef]
- Mougin, C.; Caquet, T. Plan Écophyto. Pesticides et biodiversité: Premiers enseignements. Sci. Pseudo-Sci. Hors-Série 2016, 316, 81–85. Available online: https://www.afis.org/IMG/pdf/sps315-316-hs_pesticides.pdf#page=83 (accessed on 2 June 2024).
- Coteur, I.; Marchand, F.; Debruyne, L.; Dalemans, F.; Lauwers, L. Participatory Tuning Agricultural Sustainability Assessment Tools to Flemish Farmer and Sector Needs. Environ. Impact Assess. Rev. 2018, 69, 70–81. [Google Scholar] [CrossRef]
- Trabelsi, M. Comment mesurer la performance agroécologique d’une exploitation agricole pour l’accompagner dans son processus de transition? Ph.D. Thesis, Université Paul Valéry-Montpellier III, Montpellier, France, 2017. [Google Scholar]
- Girardin, P.; Bockstaller, C.; Van der Werf, H. Assessment of Potential Impacts of Agricultural Practices on the Environment: The AGRO* ECO Method. Environ. Impact Assess. Rev. 2000, 20, 227–239. [Google Scholar] [CrossRef]
- Binder, C.R.; Feola, G.; Steinberger, J.K. Considering the Normative, Systemic and Procedural Dimensions in Indicator-Based Sustainability Assessments in Agriculture. Environ. Impact Assess. Rev. 2010, 30, 71–81. [Google Scholar] [CrossRef]
- Jayasiri, M.M.J.G.C.N.; Dayawansa, N.D.K.; Ingold, K.; Yadav, S. Are Rice Systems Sustainable in Sri Lanka?—A Case of Deduru Oya Reservoir Irrigation Scheme. Environ. Impact Assess. Rev. 2024, 106, 107503. [Google Scholar] [CrossRef]
- Soulé, E.; Michonneau, P.; Michel, N.; Bockstaller, C. Environmental Sustainability Assessment in Agricultural Systems: A Conceptual and Methodological Review. J. Clean. Prod. 2021, 325, 129291. [Google Scholar] [CrossRef]
- L’Oréal 4—Rapport de Durabilité 2024. In L’Oréal—Document D’enregistrement Universel 2024; L’Oréal: Paris, France, 2024; p. 98.
- Zahm, F.; Girard, S.; Alonso Ugaglia, A.; Barbier, J.-M.; Boureau, H.; Carayon, D.; Cohen, S.; Del’homme, B.; Gafsi, M.; Gasselin, P.; et al. La Méthode IDEA4—Indicateurs de Durabilité des Exploitations Agricoles. Principes et Guide D’utilisation; Educagri: Dijon, France, 2023; ISBN 979-10-275-0544-9. [Google Scholar]
- Mottet, A.; Bicksler, A.; Lucantoni, D.; De Rosa, F.; Scherf, B.; Scopel, E.; López-Ridaura, S.; Gemmil-Herren, B.; Bezner Kerr, R.; Sourisseau, J.-M. Assessing Transitions to Sustainable Agricultural and Food Systems: A Tool for Agroecology Performance Evaluation (TAPE). Front. Sustain. Food Syst. 2020, 4, 579154. [Google Scholar] [CrossRef]
- Magnard, A. Mesurez le “score agroécologique” de Votre Ferme. Pleinchamp. Available online: https://www.pleinchamp.com/actualite/mesurez-le-score-agroecologique-de-votre-ferme (accessed on 29 February 2024).
- Générations Futures. HVE: Quelles Différences Avec L’agriculture Bio? Générations Futures: Paris, France, 2023; p. 26. [Google Scholar]
- ACTA DiaAgroEco, Votre Outil de Diagnostic Agro-Ecologique. Diagnostic de L’engagement D’une Exploitation Dans une Démarche Agro-Ecologique. Available online: https://diagagroeco.org/ (accessed on 29 February 2024).
- Regenerative Organic Alliance Framework for Regenerative Organic Certified. Version 4.1. 2023. Available online: https://regenorganic.org/wp-content/uploads/2023/03/Regenerative-Organic-Certified-Framework.pdf (accessed on 2 June 2024).
- Regenagri. Regenagri Standard Criteria. Version 2.1. 2022. Available online: https://regenagri.org/wp-content/uploads/2022/02/regenagri-standard-criteria-v2.1.pdf (accessed on 2 June 2024).
- Danone. Danone Regenerative Agriculture Scorecard; Danone: Paris, France, 2021; p. 25. [Google Scholar]
- Viaux, P. Systèmes Intégrés: Une Troisième Voie En Grande Culture, 2nd ed.; Agri Production; La France Agricole: Paris, France, 2013. [Google Scholar]
- Karlen, D.L.; Varvel, G.E.; Bullock, D.G.; Cruse, R.M. Crop Rotations for the 21st Century. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1994; Volume 53, pp. 1–45. ISBN 978-0-12-000753-0. [Google Scholar]
- Meynard, J.-M.; Messéan, A.; Charlier, A.; Charrier, F.; Fares, M.; Le Bail, M.; Magrini, M.-B. Freins et Leviers à la Diversification des Cultures. Étude au Niveau des Exploitations Agricoles et des Filières; Auto-saisine: Synthèse du rapport d’étude; INRA: Paris, France, 2013; p. 52. [Google Scholar]
- Bennett, A.J.; Bending, G.D.; Chandler, D.; Hilton, S.; Mills, P. Meeting the Demand for Crop Production: The Challenge of Yield Decline in Crops Grown in Short Rotations. Biol. Rev. 2012, 87, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Balue, M. Court-Noué Attention au Repos du sol. 2013. AREDVI, IFV, Chambre D’agriculture. Available online: https://www.vignevin.com/wp-content/uploads/2019/01/Court_noue_repos_sol_AREDVI.pdf (accessed on 2 June 2024).
- Laget, E.; Guadagnini, M.; Plénet, D.; Simon, S.S.; Assie, G.; Billotte, B.; Borioli, P.; Bourguoin, B.; Fratantuono, M.; Guérin, A. Guide Ecophyto Fruits-Guide Pour la Conception de Systèmes de Production Fruitière Economes en Produits Phytopharmaceutiques. 2015. Available online: https://www.ecophyto-pro.fr/documents/view/875/guide_ecophyto_fruits_guide_pour_la_conception_de_systemes_de_production_fruitiere_economes_en_produits_phytopharmaceutiques (accessed on 2 June 2024).
- Beillouin, D.; Ben-Ari, T.; Malézieux, E.; Seufert, V.; Makowski, D. Positive but Variable Effects of Crop Diversification on Biodiversity and Ecosystem Services. Glob. Change Biol. 2021, 27, 4697–4710. [Google Scholar] [CrossRef]
- Tamburini, G.; Bommarco, R.; Wanger, T.C.; Kremen, C.; Van Der Heijden, M.G.A.; Liebman, M.; Hallin, S. Agricultural Diversification Promotes Multiple Ecosystem Services without Compromising Yield. Sci. Adv. 2020, 6, eaba1715. [Google Scholar] [CrossRef]
- Le Roux, X.; Barbault, R.; Baudry, J.; Burel, F.; Chauvel, B.; Couvet, D.; Deverre, C.; Doussan, I.; Farrugia, A.; Fleury, P. Agriculture et Biodiversité: Valoriser Les Synergies: Expertise Scientifique Collective INRA Juillet 2008; INRA: Paris, France, 2009; ISBN 2-7592-0309-3. [Google Scholar]
- Blanco-Canqui, H.; Shaver, T.M.; Lindquist, J.L.; Shapiro, C.A.; Elmore, R.W.; Francis, C.A.; Hergert, G.W. Cover Crops and Ecosystem Services: Insights from Studies in Temperate Soils. Agron. J. 2015, 107, 2449–2474. [Google Scholar] [CrossRef]
- Arrouays, D.; Balesdent, J.; Germon, J.C.; Jayet, P.-A.P.-A.; Soussana, J.-F.; Stengel, P. Stocker du Carbone Dans les Sols Agricoles de France? Synthèse du Rapport D’expertise; INRA: Paris, France, 2002; p. 32. [Google Scholar]
- Garcia, L.; Celette, F.; Gary, C.; Metral, R.; Metay, A. Vers des systèmes de culture agroécologiques—Usage des couverts végétaux semés ou spontanés comme cultures de services dans les vignobles—Comment raisonner leur pilotage en viticulture? La Rev. Anol. Des Tech. Vitivinic. Anol. 2020, 177, 28–31. [Google Scholar]
- Tibi, A.; Martinet, V.; Vialatte, A.; Alignier, A.; Angeon, V.; Bohan, D.; Bougherara, D.; Cordeau, S.; Courtois, P.; Deguine, J.-P.; et al. Protéger les cultures en augmentant la diversité végétale des espaces agricoles. In Synthèse de L’expertise Scientifique Collective; INRAE: Paris, France, 2022; 86p. [Google Scholar] [CrossRef]
- Chambre d’agriculture Bourgogne. Cultures intermédiaires—Fiches de conseil collectif 2015. Available online: https://saoneetloire.chambres-agriculture.fr/fileadmin/user_upload/232_chambre_dagriculture_de_saone-et-loire/6-Documents/2015_Cultures_intermediaires_VFinale.pdf (accessed on 2 June 2024).
- Charles, R.; Montfort, F.; Sarthou, J.-P. Effets biotiques des cultures intermédiaires sur les adventices, la microflore et la faune. In Réduire les Fuites de Nitrate au Moyen de Cultures Intermédiaires: Conséquences sur Les Bilans D’eau et D’azote, Autres Services Ecosystémiques; Ministère de l’Ecologie, du Développement Durable et de l’Energie: Paris, France, 2012; pp. 193–261. [Google Scholar]
- Chambre d’agriculture Bretagne. Référentiel Agronomique Régional. Guide Cultures Intermédiaires Pièges A Nitrates (CIPAN); 2009; p. 16, Chambre d’agriculture Bretagne; Available online: https://geco.ecophytopic.fr/documents/20182/21720/pdf_Implanter_des_cultures_interm_diaires_pi_ges___nitrates_4.pdf (accessed on 2 June 2024).
- Escudier, J.-L.; de Cortazar Atauri, I.G.; Giraud-Heraud, E.E.; Le Roux, R.; Ollat, N.; Quénol, H.; Touzard, J.-M. Le vignoble français à l’épreuve du changement climatique. La Rech. 2016, 513–514, 60–63. [Google Scholar]
- Jeuffroy, M.-H.; Meynard, J.-M.; de Vallavieille-Pope, C.; Fraj, M.B.; Saulas, P. Les associations de variétés de blé: Performances et maîtrise des maladies. Le Sélectionneur Français 2010, 61, 75–84. [Google Scholar]
- Zomer, R.J.; Neufeldt, H.; Xu, J.; Ahrends, A.; Bossio, D.; Trabucco, A.; Van Noordwijk, M.; Wang, M. Global Tree Cover and Biomass Carbon on Agricultural Land: The Contribution of Agroforestry to Global and National Carbon Budgets. Sci. Rep. 2016, 6, 29987. [Google Scholar] [CrossRef] [PubMed]
- Foucaud-Scheunemann, C. Agroforesterie: Des Arbres Pour une Agriculture Durable. Available online: https://www.inrae.fr/actualites/agroforesterie-arbres-agriculture-durable (accessed on 2 June 2024).
- Verboom, B.; Spoelstra, K. Effects of Food Abundance and Wind on the Use of Tree Lines by an Insectivorous Bat, Pipistrellus pipistrellus. Can. J. Zool. 1999, 77, 1393–1401. [Google Scholar] [CrossRef]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; De Hollander, M.; Soto, R.L.; Bouffaud, M.-L.; Buée, M.; Dimmers, W.; et al. Soil Networks Become More Connected and Take up More Carbon as Nature Restoration Progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, C.; Georges, R.; Aviron, S.; Baudry, J.; Burel, F. Projet FarmLand: Quel est le rôle de l’hétérogénéité spatiale de la mosaïque des cultures sur la biodiversité et les services écosystémiques? In Proceedings of the IVe Journées IALE France, Rennes, France, 11–14 June 2013; p. 1. [Google Scholar]
- Siriwardena, G.M.; Cooke, I.R.; Sutherland, W.J. Landscape, Cropping and Field Boundary Influences on Bird Abundance. Ecography 2012, 35, 162–173. [Google Scholar] [CrossRef]
- Pellerin, S.; Bamière, L.; Angers, D.; Béline, F.; Benoît, M.; Butault, J.-P.; Chenu, C.; Colnenne-David, C.; de Cara, S.; Delame, N.; et al. Quelle Contribution de L’agriculture Française à la Réduction des Emissions de gaz à Effet de Serre? Potentiel D’atténuation et Coût de dix Actions Techniques; INRA: Paris, France, 2013; p. 92. [Google Scholar]
- Kleijn, D.; Winfree, R.; Bartomeus, I.; Carvalheiro, L.G.; Henry, M.; Isaacs, R.; Klein, A.-M.; Kremen, C.; M’gonigle, L.K.; Rader, R. Delivery of Crop Pollination Services Is an Insufficient Argument for Wild Pollinator Conservation. Nat. Commun. 2015, 6, 7414. [Google Scholar] [CrossRef]
- Perrot, T.; Bretagnolle, V.; Gaba, S. Environmentally Friendly Landscape Management Improves Oilseed Rape Yields by Increasing Pollinators and Reducing Pests. J. Appl. Ecol. 2022, 59, 1825–1836. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Carvalheiro, L.G.; Vaissière, B.E.; Gemmill-Herren, B.; Hipólito, J.; Freitas, B.M.; Ngo, H.T.; Azzu, N.; Sáez, A.; Åström, J.; et al. Mutually Beneficial Pollinator Diversity and Crop Yield Outcomes in Small and Large Farms. Science 2016, 351, 388–391. [Google Scholar] [CrossRef]
- Villenave, C.; Chauvin, C.; Puissant, J.; Henaux, M.; Trap, J. Impact des pratiques agricoles sur l’état biologique du sol: SIPANEMA, un outil d’aide à la décision basé sur les nématodes. Etude Gest. Sols 2022, 29, 199–209. [Google Scholar]
- Bodin, E.; Bodin, D.; Chaigne, C.; Desmoulin, F.; Gnagni, R.; Jame, A.; Mouclier, F. La Lutte Biologique, Une Solution Viable Pour L’avenir? Barot, S., Ed.; Société Française d’Écologie et d’Évolution, 2021; Available online: https://sfecologie.org/regard/re3-mars-2021-bodin-et-al-lutte-biologique/ (accessed on 2 June 2024).
- Attoumani-Ronceaux, A.; Jouy, L.; Mischler, P.; Omon, B.; Petit, M.-S.; Pleyber, E.; Reau, R.; Seiler, A.; Guichard, L.; Aubertot, J.-N. Guide Pratique Pour la Conception de Systèmes de Culture Plus Economes en Produits Phytosanitaires—Application Aux Systèmes de Polyculture; Ecophyto 2018; MEDDTL, MAAPRAT, RMT Système de Culture Innovants: Paris, France, 2018; p. 116. [Google Scholar]
- Eckert, C.; Rougier, M.; Chartier, N.; Houdin, A. Le Réseau DEPHY EXPE en Cultures Légumières—Un Premier Bilan à Mi-Parcours; Info CTIFL: Paris, France, 2017; p. 10. [Google Scholar]
- Pradel, M.; de Fays, M.; Seguineau, C. Analyse du Cycle de Vie des Pratiques de Désherbage Intra-Rang et Inter-Rang Avec des Systèmes Robotisés Autonomes Dans Trois Vignobles Français; INRAE—TSCF, NAÏO Technologies: Escalquens, France, 2022; p. 91. [Google Scholar]
- Maillot, T.; Jones, G.; Vioix, J.-B.; Colbach, N. Des technologiques innovantes pour optimiser le désherbage de précision. Innov. Agron. 2020, 81, 101–116. [Google Scholar] [CrossRef]
- AGRESTE. Enquête Pratiques Culturales 2011—Principaux Résultats; AGRESTE: Paris, France, 2014; p. 70. [Google Scholar]
- Schneider, A.; Huyghe, C. Les Légumineuses Pour Des Systèmes Agricoles et Alimentaires Durables; Editions Quae: Versailles, France, 2015; ISBN 978-2-7592-2335-0. [Google Scholar]
- ARVALIS—Institut du végétal. Les Légumineuses, Comment ça Marche? 2010; ISBN 978-2-8179-0037-7; Available online: https://agriculture-de-conservation.com/sites/agriculture-de-conservation.com/IMG/pdf/legumineuses-elevage.pdf (accessed on 2 June 2024).
- ADEME. Fiche n°5—Cultiver des légumineuses pour réduire l’utilisation d’intrants de synthèse. In Agriculture & Environnement, des Pratique clefs Pour la Préservation du Climat, des sols et de l’air, et les Economies D’énergie; Références; ADEME: Angers, France, 2015; pp. 55–66. ISBN 978-2-35838-614-2. [Google Scholar]
- Dumont, B.; Basso, B.; Destain, J.-P.; Meza Morales, W.; Bodson, B. Développement d’un système d’aide à la décision multicritère pour l’optimisation de la fertilisation azotée. In Proceedings of the Phloeme 2018-Premieres Biennales de l’innovation céréalière, 2018; p. 9. Available online: https://hdl.handle.net/2268/220356 (accessed on 2 June 2024).
- Hocdé, A.; Joly, P. Fertilisation Azotée et Outils D’aide à la Décision—Executive Summary; ASIRPA Analyse Socio-économique des Impacts de la Recherche Publique Agricole; INRA: Paris, France, 2013; p. 9. [Google Scholar]
- Recous, S.; Jeuffroy, M.-H.; Hénault, C.; Bamière, L. Réduire le recours aux engrais azotés de synthèse: Quel potentiel et quel impact sur les émissions de N2O à l’échelle France ? Innov. Agron. 2014, 37, 11–22. [Google Scholar] [CrossRef]
- Chambre d’agriculture Normandie. Guide de Calcul des Doses D’azote—Références Normandie; Fertilisation azotée en Zone Vulnérable; Chambre d’agriculture Normandie: Bois Guillaume, France, 2021; p. 51. [Google Scholar]
- Peyraud, J.-L.; Cellier, P.; Donnars, C.; Réchauchère, O. Les flux d’azote liés aux élevages: Réduire les pertes, rétablir les équilibres. In Synthèse du Rapport D’expertise Scientifique Collective; Expertises collectives; INRA: Paris, France, 2012; p. 68. [Google Scholar]
- Hagemann, N.; Joseph, S.; Schmidt, H.-P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic Coating on Biochar Explains Its Nutrient Retention and Stimulation of Soil Fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef] [PubMed]
- Agence de l’environnement et de la maîtrise de l’énergie; CITEPA. Guide des Bonnes Pratiques Agricoles Pour L’amélioration de la Qualité de l’air; Clés pour agir; ADEME: Montrouge, France, 2020; p. 84. [Google Scholar]
- CITEPA. Organisation et Méthodes des Inventaires Nationaux des Emissions Atmosphériques en France—OMINEA—18ème Edition; Ministère de la transition écologique, CITEPA: Paris, France, 2021; p. 1044. [Google Scholar]
- Saliu, F.; Luqman, M.; Alkhaz’leh, H.S. A Review on the Impact of Sustainable Agriculture Practices on Crop Yields and Soil Health. Int. J. Res. Adv. Agric. Sci. 2023, 2, 1–13. [Google Scholar]
- Gloria, C.; Nicolas, D.; Baratte, E.; Vincent, M.-H. Les vertus du non-labour. Réussir Céréales Gd. Cult. 2007, 26–39. [Google Scholar]
- Uri, N.D.; Atwood, J.D.; Sanabria, J. An Evaluation of the Environmental Costs and Benefits of Conservation Tillage. Environ. Impact Assess. Rev. 1998, 18, 521–550. [Google Scholar] [CrossRef]
- Solagro; Oréade Brèche; Cereg. Etude Pour le Renforcement des Actions D’économies d’eau en Irrigation Dans le Bassin Adour-Garonne; Agence de l’eau Adour-Garonne: Toulouse, France, 2017; p. 20. [Google Scholar]
- Barta, R.; Broner, I.; Schneekloth, J.; Waskom, R. Colorado High Plains Irrigation Practices Guide. Water Saving Options for Irrigators in Eastern Colorado; Colorado Water Resources Research Institute: Fort Collins, CO, USA, 2004; p. 80. [Google Scholar]
- Chambre d’agriculture de la Mayenne, Infiltr’eau 53, Phyt’eau Propre 53, Département de la Mayenne. Guide Agricole Pour la Préservation des Ressources en eau, 2020. p. 90. Available online: https://www.calameo.com/read/0020054844e05850edecf (accessed on 2 June 2024).
- Ayphassorho, H.; Bertrand, N.; Mitteault, F.; Pujos, C.; Rollin, D.; Sallenave, M. Changement Climatique, eau, Agriculture. Quelles Trajectoires d’ici 2050? In Rapport CGEDD; Ministère de la Transition Ecologique, Ministère de L’Agriculture et de l’Alimentation: Paris, France, 2020; p. 333, n° 012819-01, CGAAER n° 19056. [Google Scholar]
- Pellerin, S.; Bamière, L. Stocker du Carbone dans les Sols Français. Quel Potentiel au Regard de L’objectif 4 Pour 1000 et à Quel Coût? INRA: Paris, France, 2019; p. 114. [Google Scholar]
- Gendre, S.; Tscheiller, R.; Moynier, J.-L.; Deschamps, T. Teneur en eau des sols: Quel est l’effet des couverts d’interculture? Perspectives Agricoles 2021, N°489. Available online: https://www.perspectives-agricoles.com/conduite-de-cultures/teneur-en-eau-des-sols-quel-est-leffet-des-couverts-dinterculture (accessed on 2 June 2024).
- Amigues, J.-P.; Debaeke, P.P.; Itier, B.B.; Lemaire, G.G.; Seguin, B.; Tardieu, F.F.; Thomas, A.; Uesc, E.S.C.; Pêche, M.d.L.E.d.L. Sécheresse et Agriculture. Réduire la Vulnérabilité de L’agriculture à un Risque Accru de Manque d’eau. Expertise Scientifique Collective. Synthèse du Rapport; INRA: Paris, France, 2006; p. 77. [Google Scholar]
- Boé, J.; Terray, L.; Martin, E.; Habets, F. Projected Changes in Components of the Hydrological Cycle in French River Basins during the 21st Century. Water Resour. Res. 2009, 45, W0842. [Google Scholar] [CrossRef]
- Rodrigues, G.S.; Campanhola, C.; Kitamura, P.C. An Environmental Impact Assessment System for Agricultural R&D. Environ. Impact Assess. Rev. 2003, 23, 219–244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).






