Study on the Biofilm Kinetics in Micro-Electrolysis Biological Reactors
Abstract
:1. Introduction
2. Hypothesis of Biofilm Formation
- (1)
- The flow rate of the subject solution along the biofilm surface is high, and the liquid-film diffusion resistance is negligible.
- (2)
- The biofilm is a homogeneous membrane, and the membrane density remains constant.
- (3)
- The pollutants in the effluent are homogeneous, i.e., the ions have a uniform radius.
- (4)
- In the biofilm, the viscosity coefficient of the gelatinous viscous material produced by the microorganisms is a constant value.
- (5)
- The applied electric field is uniform.
- (6)
- The electrostatic force between ions is neglected.
- (7)
- The amount of electricity charged by the pollutants is the same.
3. Biofilm Kinetics Under the Action of an Electric Field
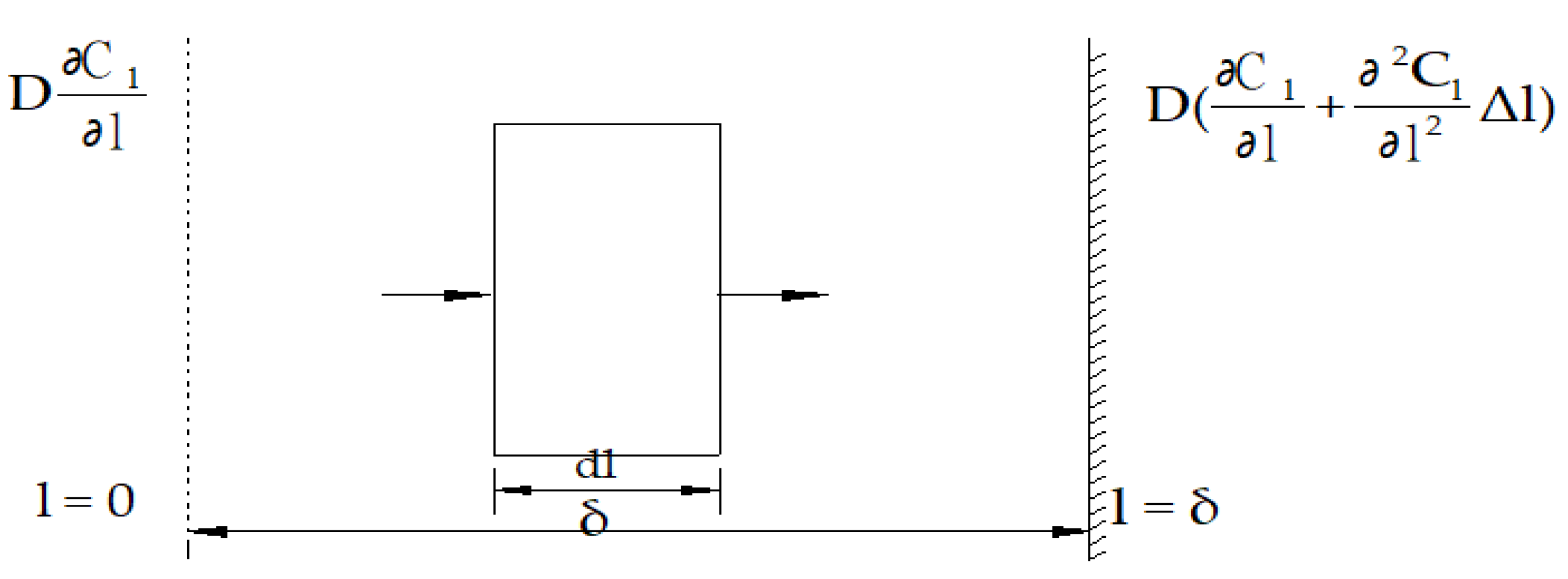
3.1. Determination of the Diffusion Coefficient D Under the Influence of an Electric Field
3.2. Dynamics Models
4. Discussion
4.1. Relationship Between Electric Field Strength and Reaction Rate
4.2. Relationship Between Electric Field Strength and Effluent Concentration
4.3. Relationship Between Electric Field Strength and Biofilm Thickness
5. Conclusions
- (1)
- Under the goal of realizing the efficient removal of pollutants, the relationship between the electric field strength and the pollutant reaction rate can be simplified as a linear equation in the appropriate voltage range.
- (2)
- In the case of a high concentration, the electric field strength is quadratic with the ratio of the difference between the concentrations of pollutants in and out of the water and the reaction time; in the case of a low concentration, the electric field strength is power-functional with the ratio of the difference between the concentrations of pollutants in and out of the water and the concentration of the water inlet and outlet.
- (3)
- There is a fold increase in microbial film thickness in a micro-electrolysis bioreactor compared to the thickness of a microbial film formed in the absence of an electric field, and it becomes thicker with increases in electric field strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, R.; Hong, Y. Research on the application of new sewage treatment technologies in water environment protection. Hydropower Sci. Technol. 2024, 7, 122–124. [Google Scholar]
- Zou, Z.; He, S.; Han, C.; Zhang, L.; Luo, Y. Research progress on heavy metal wastewater treatment technologies. Ind. Water Treat. 2010, 30, 9–12. [Google Scholar]
- Zhang, Z. Technological innovation and energy-saving and consumption-reduction research in sewage treatment. Eng. Constr. Manag. 2023, 1, 4–6. [Google Scholar]
- Ren, J.; Wu, C.; Yang, Z.; Wang, R.; Yang, L.; Tao, L. Study on the treatment of domestic wastewater using iron-carbon fillers coupled with biofilm method. Water Treat. Technol. 2025, 51, 90–96+113. [Google Scholar]
- Xu, G.Y. Study on the Performance of Iron-Carbon Micro-Electrolysis and Iron Oxide Coupled Biological Denitrification. Master’s Thesis, Chang’an University, Xi’an, China, 2023. [Google Scholar]
- Hu, B. Study on Treatment of Pharmaceutical Wastewater by Combined Process of Ferric-Carbon Micro Electrolysis-Biochemical Technology. Master’s Thesis, Chongqing University, Chongqing, China, 2008. [Google Scholar]
- Zhao, M.; Fan, J.; Ma, L. Mechanism and application of electro-biological technology for enhanced wastewater treatment. Sichuan Environ. 2010, 29, 55–60. [Google Scholar]
- Qi, Y. Study on the Process and Equipment of Iron-Carbon Micro-Electrolysis-Biofilm Method-Advanced Oxidation for Textile Wastewater Treatment. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2018. [Google Scholar]
- Li, L.; Chen, J.; Liao, M.; Su, D.; Zhu, J.; Li, X. Application and research of iron-carbon micro-electrolysis technology. Environ. Sci. Manag. 2017, 42, 98–101. [Google Scholar]
- Zhang, J. Treatment of Plating Wastewater with Heavy Metal Ions by Micro-Electrolysis and Biofilm Process. Master’s Thesis, Tianjin University, Tianjin, China, 2005. [Google Scholar]
- Sun, Q.; Yin, Y.; He, Y.; Yu, L.; Liang, X.; Wang, G.; Zhao, B. Study on the purification effect of electrolysis-enhanced constructed wetlands under low-temperature conditions for simulated low-pollution water. Front. Environ. Prot. 2021, 11, 938–945. [Google Scholar]
- Ling, H. Study on the Electrochemical-Biological Method for Treating Typical PPCPs. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2011. [Google Scholar]
- Ma, W. Enhanced Treatment of Phenolic Compounds in Coal Gasification Wastewater by Micro-Electrolysis Coupled Biological Process. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2020. [Google Scholar]
- Liu, X.; Huang, X.; Jin, W. Study on Simultaneous Nitrification and Denitrification in Electrode Biofilm Reactors. Ind. Water Wastewater 2009, 40, 42–45. [Google Scholar]
- Cong, L.; Zheng, T.; Xie, Z. Micro-Electrolysis/Coagulation/Anaerobic Membrane Bio-Reactor Combined Process for High-Concentration Landfill Leachate Treatment. J. Xiamen Univ. (Nat. Sci.) 2006, 45, 824–827. [Google Scholar]
- Cao, L.; Zhang, S.; Zhang, Y.; Deng, X.; Lu, Y. Research Progress of Micro-Electrolysis Materials. Mod. Chem. Ind. 2015, 35, 13–17. [Google Scholar]
- Wang, S.; Zhou, P. Kinetic Mechanism of Organic Matter Degradation in Biological Fluidized Beds. J. Southeast Univ. (Nat. Sci. Ed.) 1993, 23, 62–68. [Google Scholar]
- Liu, Y.; Wang, Q. Biofilm Growth Kinetic Model. J. Beijing Technol. Univ. (Nat. Sci. Ed.) 1997, 2, 28–31. [Google Scholar]
- Zhou, Z.; Lu, R.; Ye, C.; Yue, S.; Li, J.; Li, Y. Application of PLC Control in Treatment of Rubber Wastewater by D-A2O Combination Technology. China Water Wastewater 2018, 34, 5. [Google Scholar]
- Beshkov, V.; Velizarov, S.; Agathos, S.N.; Lukova, V. Bacterial denitrification of wastewater stimulated by constant electric field. Biochem. Eng. J. 2004, 17, 141–145. [Google Scholar] [CrossRef]
- Jiang, B. Study on the Efficiency and Mechanism for Phenolic Wastewater Treatment by Coupled System of Biochar-Electric Field Attached Membrane Bioreactor. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2020. [Google Scholar]
- Zhong, F.; Cao, H.; Li, X. Biochemical mass transfer model of biofilm under external electric field. J. Jilin Chem. Eng. Univ. 2003, 4, 59–61. [Google Scholar]
- Jia, H.; Yan, L.; Li, X.L. Application of electrostatic technology in cell engineering. Electrostatics 1988, 3, 23–26. [Google Scholar]
- Kong, Q. Research and application of advanced treatment technology for domestic wastewater. Mar. Eng. Equip. Technol. 2019, 6, 409–413. [Google Scholar]
- Li, J. Study on the Enhancement of Tetrabromobisphenol A Degradation by Three-Dimensional Electrode Biofilm Reactor. Master’s Thesis, East China Normal University, Shanghai, China, 2024. [Google Scholar]
- Kang, M. Study on the Combined Treatment of Oily Ballast Water from Ships by Iron-Carbon Micro-Electrolysis and Biofilm Method. Master’s Thesis, Zhejiang Ocean University, Zhoushan, China, 2019. [Google Scholar]
- Xiong, C. Mathematical Model Establishment and Study on Algae-Bacteria Symbiotic System for Treatment of Livestock and Poultry Breeding Wastewater. Master’s Thesis, Nanchang University, Nanchang, China, 2023. [Google Scholar]
- Deng, Q. Study on optimization and application of biofilm reactor in specific industrial wastewater treatment. Heilongjiang Environ. Bull. 2024, 37, 12–14. [Google Scholar]
- Dou, H. Study on Dynamics and Thermodynamics of Sulfate-Reducing Bacteria Biofilm. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2023. [Google Scholar]
- Yang, P.; Guo, Y.; Shi, Y.F. Study on settling performance of porous biological particles and bed expansion characteristics in a biofilm fluidized bed. Chem. Eng. Process. 2003, 19, 8. [Google Scholar]
- Pau, P.C.F.; Berg, J.O.; McMillan, W.G. Application of Stokes’ law to ions in aqueous solution. J. Phys. Chem. 1990, 94, 2671–2679. [Google Scholar] [CrossRef]
- Shearer, S.A.; Hudson, J.R. Fluid mechanics: Stokes’ law and viscosity. Meas. Lab. 2008, 3, EGR 101. [Google Scholar]
- Chen, B.; Wang, J.; Zhang, R.J. How is the Lorentz force formula given. College Phys. 2008, 27, 42. [Google Scholar]
- Pan, J.; Tong, J.M.; Tian, M.B. Fundamentals of Materials Science (Revised Edition); Tsinghua University Press: Beijing, China, 2011. [Google Scholar]
- He, L.; Lin, J.; Shi, B. Fick’s law and the thermodynamic theory of diffusion. J. Anqing Teachers Coll. Nat. Sci. Ed. 2006, 4, 38–39. [Google Scholar]
- Zhou, P.; Wang, S. Effect of biofilm thickness on the performance of fluidized bed reactors. Environ. Sci. 1994, 2, 1–5+92. [Google Scholar]
- Qiu, L.; Ma, J. Biofilm and microbial community characteristics in an aerated biofilter. Chin. Environ. Sci. 2005, 25, 4. [Google Scholar]
- Zhao, Y. Growth and Variation Characteristics of Biofilm on Semi-Suspended Carriers. Master’s Thesis, Guangdong University of Technology, Guangzhou, China, 2017. [Google Scholar]
- Wang, Z. Study on Plastic Optical Fiber Sensor for Accurate Online Measurement of Microalgae Biofilm Thickness. Master’s Thesis, Chongqing University of Technology, Chongqing, China, 2019. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, Z.; Xu, J.; Pei, L.; Han, T.; Zhao, J. Study on the Biofilm Kinetics in Micro-Electrolysis Biological Reactors. Sustainability 2025, 17, 1105. https://doi.org/10.3390/su17031105
Zhang X, Zhang Z, Xu J, Pei L, Han T, Zhao J. Study on the Biofilm Kinetics in Micro-Electrolysis Biological Reactors. Sustainability. 2025; 17(3):1105. https://doi.org/10.3390/su17031105
Chicago/Turabian StyleZhang, Xiaohui, Zeya Zhang, Jingyi Xu, Liang Pei, Tongshun Han, and Jianguo Zhao. 2025. "Study on the Biofilm Kinetics in Micro-Electrolysis Biological Reactors" Sustainability 17, no. 3: 1105. https://doi.org/10.3390/su17031105
APA StyleZhang, X., Zhang, Z., Xu, J., Pei, L., Han, T., & Zhao, J. (2025). Study on the Biofilm Kinetics in Micro-Electrolysis Biological Reactors. Sustainability, 17(3), 1105. https://doi.org/10.3390/su17031105





