Molten-Salt-Assisted Preparation of g-C3N4 for Photocatalytic Degradation of Tetracycline Hydrochloride: Degradation Mechanism, Pathway, and Toxicity Assessment
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of Photocatalysts
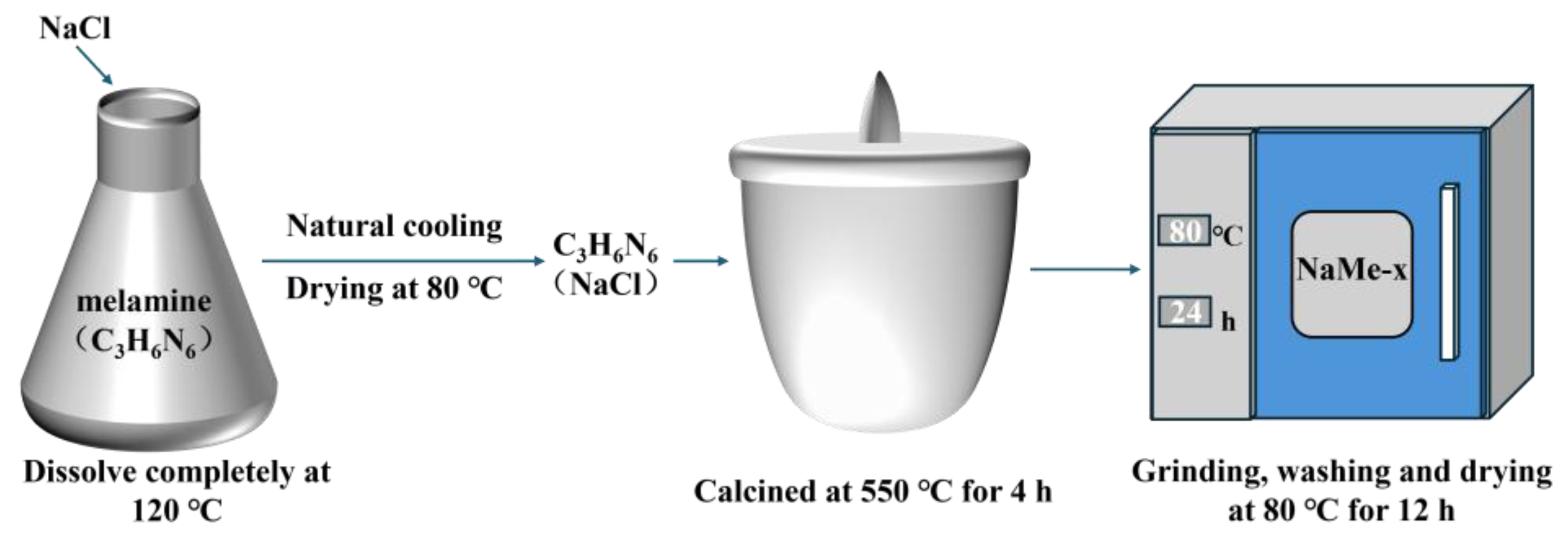
2.2.1. Preparation of Precursors
2.2.2. Synthesis of NaMe-x
2.3. Effect of Environmental Factors on the Degradation Efficiency of NaMe-x
2.4. Degradation Pathway and Degradation Mechanism Study of TC-HCl by NaMe-x
2.5. Toxicity Studies on Chlorella and Luminescent Bacteria
2.6. Sample Collection and Data Analysis
3. Results and Discussion
3.1. Characterization of the Materials
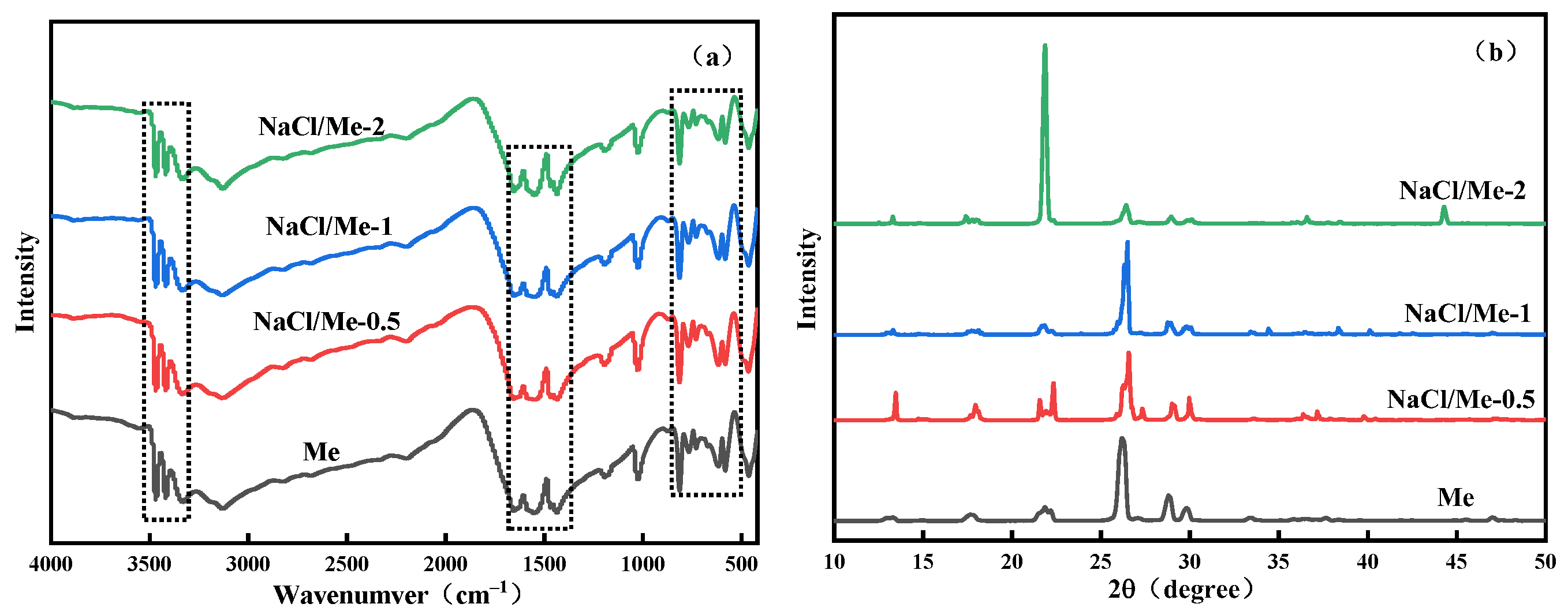
3.1.1. Characterization of Precursor Structure
3.1.2. SEM, FI-RT, and XRD Analysis of NaMe-x (Questions 1–4)
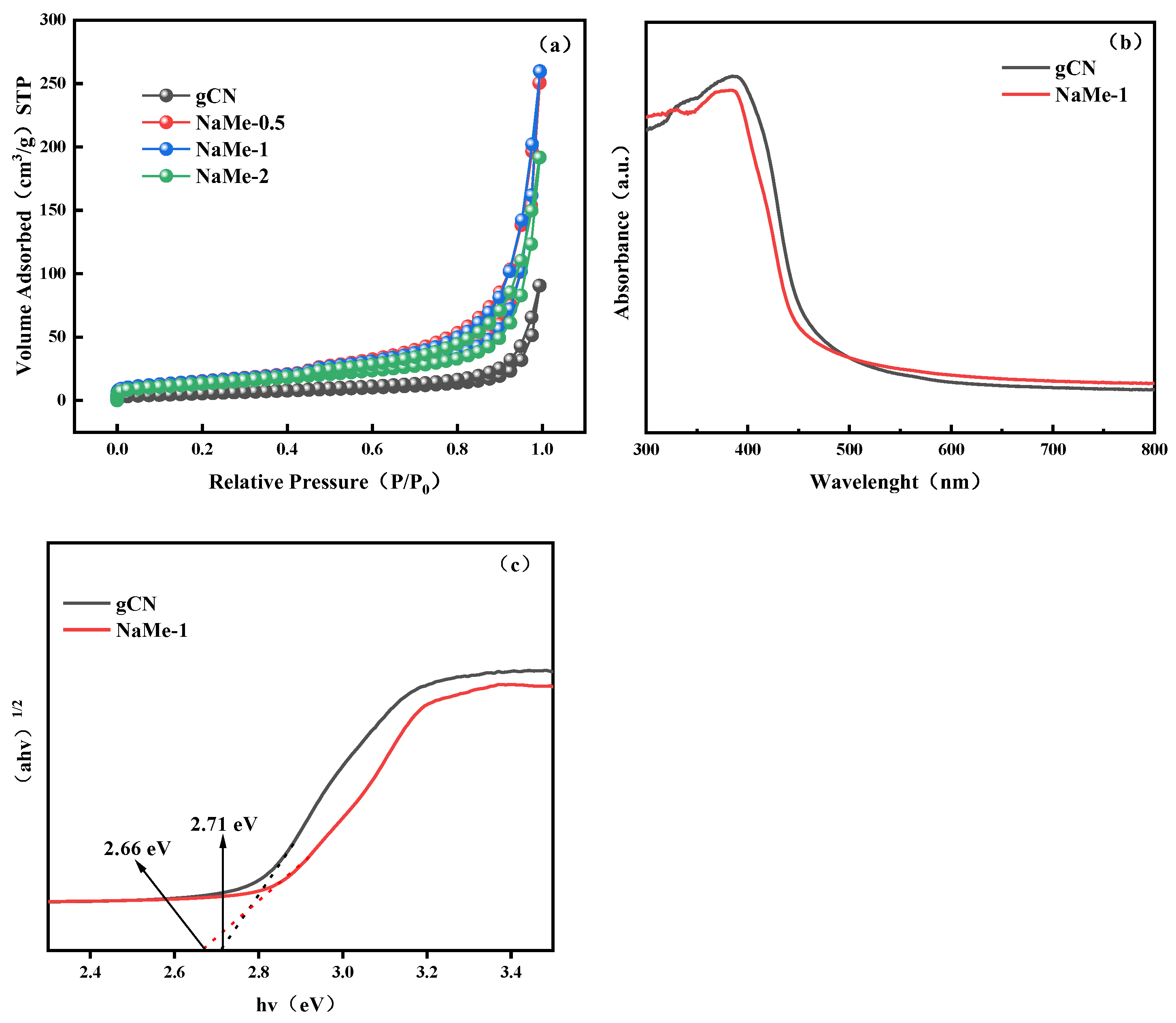
3.1.3. BET and UV-Vis-DRS Analysis
3.1.4. XPS Analysis
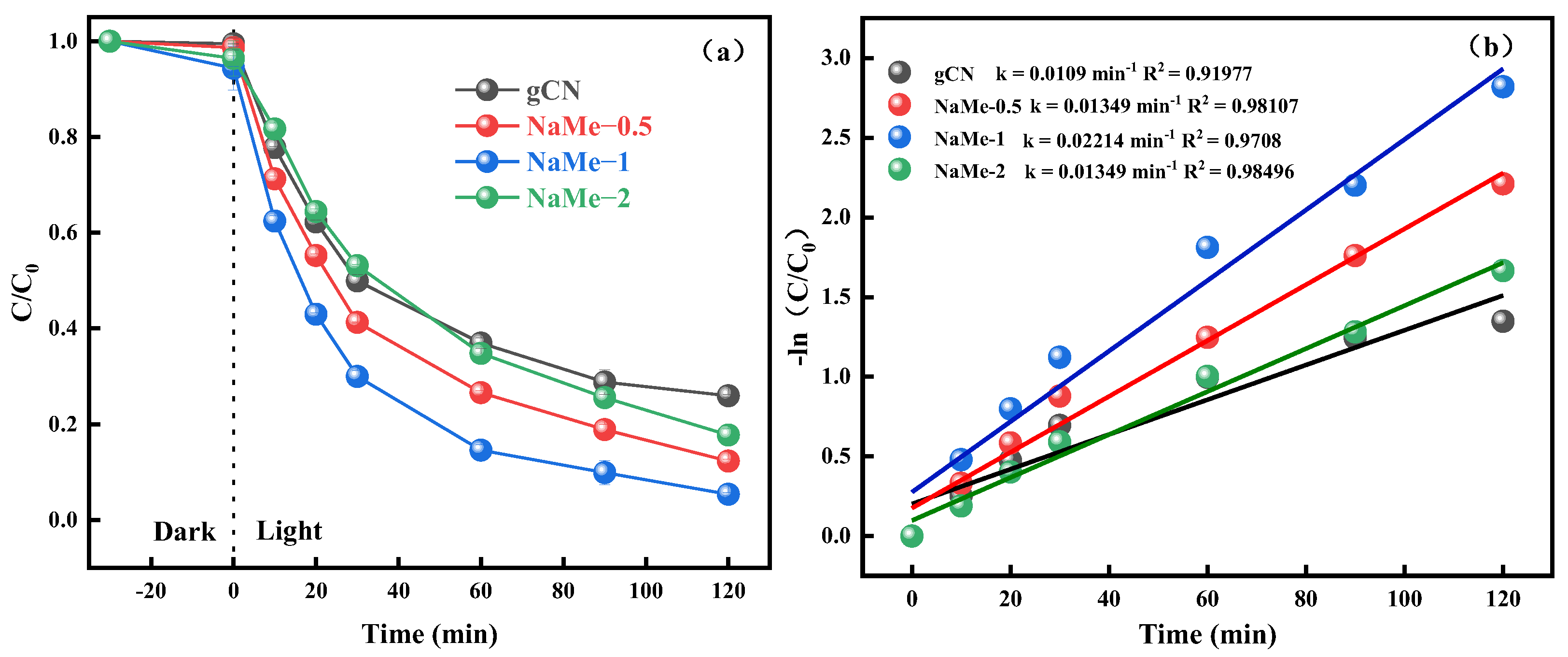
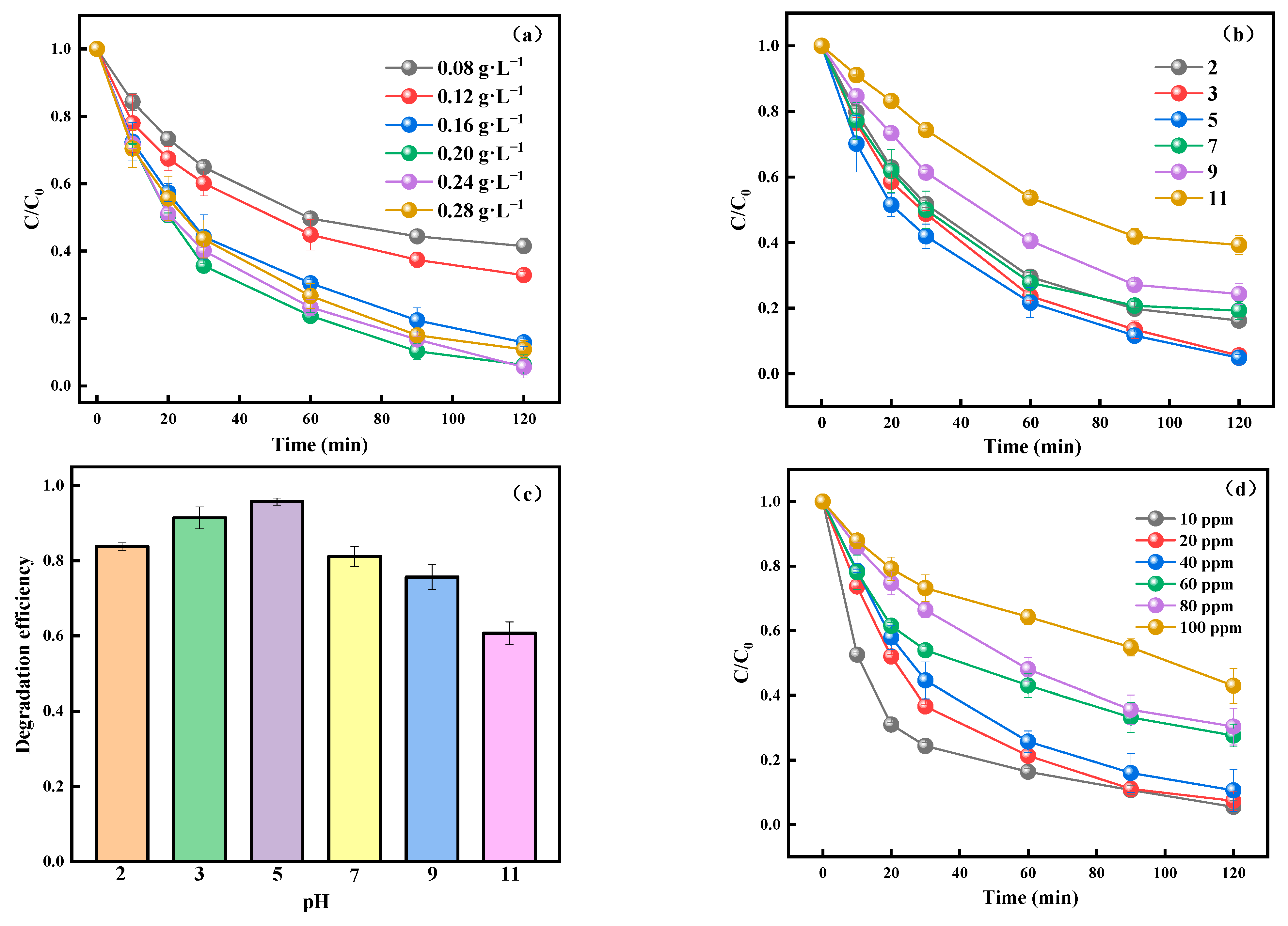
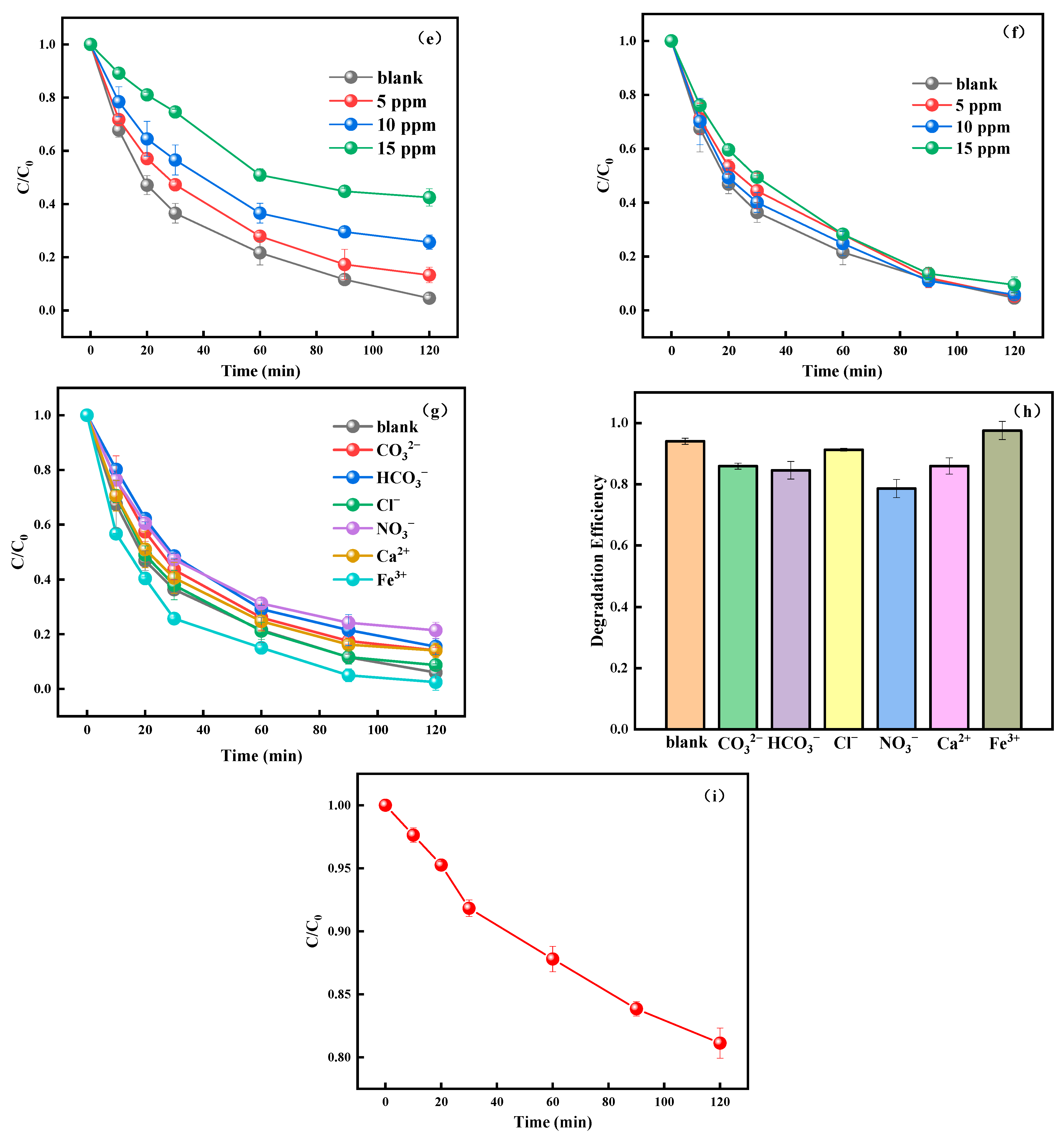
3.2. Photocatalytic Performance of TC-HCl Degradation by NaMe-x and the Effect of Environmental Factors
3.3. Investigation of Degradation Mechanism and Degradation Pathway of TC-HCl by NaMe-1
3.4. Toxicity Experiments with TC-HCl and Its Degradation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, N.; Kourampi, I.; Umar, T.P.; Almansoor, Z.R.; Anand, A.; Rehman, M.E.U.; Jain, S.; Reinis, A. Global population surpasses eight billion: Are we ready for the next billion? AIMS Public Health 2023, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pal, D. Antibiotic resistance and wastewater: Correlation, impact and critical human health challenges. J. Environ. Chem. Eng. 2018, 6, 52–58. [Google Scholar] [CrossRef]
- Sigiro, M. Natural biowaste of banana peel-derived porous carbon for in-vitro antibacterial activity toward Escherichia coli. Ain Shams Eng. J. 2021, 12, 4157–4165. [Google Scholar] [CrossRef]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Ding, C.; Zhu, Q.; Yang, B.; Petropoulos, E.; Xue, L.; Feng, Y.; He, S.; Yang, L. Efficient photocatalysis of tetracycline hydrochloride (TC-HCl) from pharmaceutical wastewater using AgCl/ZnO/g-C3N4 composite under visible light: Process and mechanisms. J. Environ. Sci. 2023, 126, 249–262. [Google Scholar] [CrossRef]
- Yang, L.; Wen, Q.; Zhao, Y.; Chen, Z.; Wang, Q.; Bürgmann, H. New insight into effect of antibiotics concentration and process configuration on the removal of antibiotics and relevant antibiotic resistance genes. J. Hazard. Mater. 2019, 373, 60–66. [Google Scholar] [CrossRef]
- Chenxi, Y.; Min, F.U.; Zhengbo, C. Treatment of tetracycline hydrochloride in wastewater by ultrasonic enhanced H2O2. Chin. J. Environ. Eng. 2021, 15, 1219–1226. [Google Scholar]
- Erim, B.; Ciğeroğlu, Z.; Şahin, S.; Yasser, Y. Photocatalytic degradation of cefixime in aqueous solutions using functionalized SWCNT/ZnO/Fe3O4 under UV-A irradiation. Chemosphere 2022, 291, 132929. [Google Scholar] [CrossRef]
- Ellis, J.B. Pharmaceutical and personal care products (PPCPs) in urban receiving waters. Environ. Pollut. 2006, 144, 184–189. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Sun, Y.; Qi, X.; Li, R.; Xie, Y.; Tang, Q.; Ren, B. Hydrothermal synthesis of 2D/2D BiOCl/g-C3N4 Z-scheme: For TC degradation and antimicrobial activity evaluation. Opt. Mater. 2020, 108, 110170. [Google Scholar] [CrossRef]
- Muaz, K.; Riaz, M.; Akhtar, S.; Park, S.; Ismail, A. Antibiotic residues in chicken meat: Global prevalence, threats, and decontamination strategies: A review. J. Food Prot. 2018, 81, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lian, Y.; Li, S.; Yang, M.; Xie, Q.; Qiu, L.; Liu, H.; Long, Y.; Hu, L.; Fang, C. The stress response of tetracycline resistance genes and bacterial communities under the existence of microplastics in typical leachate biological treatment system. J. Environ. Manag. 2024, 366, 121865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bian, Z.; Wang, F.; Zhang, Q. Fe reaction sites enhance the removal of tetracycline over Fe-Bi3O4Br with intensified adsorption and photocatalysis. Sep. Purif. Technol. 2025, 356, 129893. [Google Scholar] [CrossRef]
- da Rocha, L.R.; Segatelli, M.G.; Tarley, C.R.T. Preparation of novel semi-covalent molecularly imprinted polymer for highly improved adsorption performance of tetracycline in aqueous medium. J. Pharm. Biomed. Anal. Open 2024, 4, 100044. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, T.; Xu, Y.; Du, Y.; Ai, J.; Xue, N. Study on mechanism of removal of sudden Tetracycline by compound modified biological sand filtration process. J. Environ. Manag. 2024, 356, 120709. [Google Scholar] [CrossRef]
- Sun, Z.; Wei, Q.; Hua, W.; Chen, M.; Yuan, Q.; Yi, B.; Ok, Y.S. Engineered biochar for advanced oxidation process towards tetracycline degradation: Role of iron and graphitic structure. J. Environ. Chem. Eng. 2024, 12, 114290. [Google Scholar] [CrossRef]
- Koundle, P.; Nirmalkar, N.; Momotko, M.; Makowiec, S.; Boczkaj, G. Tetracycline degradation for wastewater treatment based on ozone nanobubbles advanced oxidation processes (AOPs)–Focus on nanobubbles formation, degradation kinetics, mechanism and effects of water composition. Chem. Eng. J. 2024, 501, 156236. [Google Scholar] [CrossRef]
- Zheng, X.; Zhai, Z.; Shi, H. Enhanced photo-Fenton catalytic activity over Cu-doped Bi12O17-xCl2 nanotube for tetracycline degradation: Oxygen vacancies-induced mixed valent Cu. Mol. Catal. 2023, 550, 113611. [Google Scholar] [CrossRef]
- He, X.; Kai, T.; Ding, P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, Y.; Bao, J.; Fang, J.; Zhao, S.; Zhang, Y.; Sheng, X.; Chen, W. Structure regulation of ZnS@ g-C3N4/TiO2 nanospheres for efficient photocatalytic H2 production under visible-light irradiation. Chem. Eng. J. 2018, 346, 226–237. [Google Scholar] [CrossRef]
- Ye, L.; Wu, D.; Chu, K.H.; Wang, B.; Xie, H.; Yip, H.Y.; Wong, P.K. Phosphorylation of g-C3N4 for enhanced photocatalytic CO2 reduction. Chem. Eng. J. 2016, 304, 376–383. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, X.; Zheng, S.; Zhang, J.; Sheng, J. Effect of calcination on structure and photocatalytic property of N-TiO2/g-C3N4@ diatomite hybrid photocatalyst for improving reduction of Cr (Ⅵ). Environ. Pollut. 2019, 245, 53–62. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Al-Muhtaseb, A.; Naushad, M.; Ghfar, A.A.; Guo, C.; Stadler, F.J. Biochar-templated g-C3N4/Bi2O2CO3/CoFe2O4 nano-assembly for visible and solar assisted photo-degradation of paraquat, nitrophenol reduction and CO2 conversion. Chem. Eng. J. 2018, 339, 393–410. [Google Scholar] [CrossRef]
- Gu, L.; Wang, J.; Zou, Z.; Han, X. Graphitic-C3N4-hybridized TiO2 nanosheets with reactive {0 0 1} facets to enhance the UV-and visible-light photocatalytic activity. J. Hazard. Mater. 2014, 268, 216–223. [Google Scholar] [CrossRef]
- Zhai, H.S.; Cao, L.; Xia, X.H. Synthesis of graphitic carbon nitride through pyrolysis of melamine and its electrocatalysis for oxygen reduction reaction. Chin. Chem. Lett. 2013, 24, 103–106. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A review. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Li, J.; Shen, B.; Hong, Z.; Lin, B.; Gao, B.; Chen, Y. A facile approach to synthesize novel oxygen-doped g-C3N4 with superior visible-light photoreactivity. Chem. Commun. 2012, 48, 12017–12019. [Google Scholar] [CrossRef]
- Shi, L.; Chang, K.; Zhang, H.; Hai, X.; Yang, L.; Wang, T.; Ye, J. Drastic enhancement of photocatalytic activities over phosphoric acid protonated porous g-C3N4 nanosheets under visible light. Small 2016, 12, 4431–4439. [Google Scholar] [CrossRef]
- Chen, W.; Liu, T.Y.; Huang, T.; Liu, X.; Yang, X. Novel mesoporous P-doped graphitic carbon nitride nanosheets coupled with ZnIn2S4 nanosheets as efficient visible light driven heterostructures with remarkably enhanced photo-reduction activity. Nanoscale 2016, 8, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kou, X.; Zhao, R.; Su, Y. Molten salt derived crystalline graphitic carbon nitride to enable selective photo-oxidation of benzyl alcohol. J. Nanoparticle Res. 2021, 23, 88. [Google Scholar] [CrossRef]
- Song, H.; Liu, X.; Wang, Y.; Chen, L.; Zhang, J.; Zhao, C.; He, F.; Dong, P.; Li, B.; Wang, S.; et al. Synergy of intermolecular Donor-Acceptor and ultrathin structures in crystalline carbon nitride for efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2022, 607, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, M.; Luo, Z.; Zhang, J.; Wang, S.; Wang, X. Molten salt assisted assembly growth of atomically thin boron carbon nitride nanosheets for photocatalytic H2 evolution. Chem. Commun. 2020, 56, 2558–2561. [Google Scholar] [CrossRef]
- Yang, T.; Tang, Y.; Yang, F.; Qu, J.; Yang, X.; Cai, Y.; Du, F.; Li, C.; Hu, J. Molten salt-assisted anti-defect engineering to tailor ordered, highly crystalline g-C3N4 nanorods for efficient photocatalytic H2O2 production. Chem. Eng. J. 2023, 475, 146497. [Google Scholar] [CrossRef]
- Xu, T.; Hur, J.; Niu, P.; Wang, S.; Lee, S.; Chun, S.; Li, L. Synthesis of crystalline g-C3N4 with rock/molten salts for efficient photocatalysis and piezocatalysis. Green Energy Environ. 2024, 9, 890–898. [Google Scholar] [CrossRef]
- An, Y.; He, Y.; Li, M.; Yu, W.; Tian, N.; Zhang, Y.; Huang, H. Molten salt-assisted precursor regulation of crystalline g-C3N4 for high-efficient photocatalytic H2O2 production. Appl. Surf. Sci. 2024, 670, 160634. [Google Scholar] [CrossRef]
- Dou, Q.; Hou, J.; Hussain, A.; Zhang, G.; Zhang, Y.; Luo, M.; Wang, X.; Cao, C. One-pot synthesis of sodium-doped willow-shaped graphitic carbon nitride for improved photocatalytic activity under visible-light irradiation. J. Colloid Interface Sci. 2022, 624, 79–87. [Google Scholar] [CrossRef]
- Yu, Q.; Ren, X.; Pan, J.; Wang, Q.; Li, Y.; Shi, N. Chemical bonds in precursors regulate g-C3N4 structure and its photocatalytic performance. J. Alloys Compd. 2022, 910, 164953. [Google Scholar] [CrossRef]
- Cao, J.; Fan, H.; Wang, C.; Ma, J.; Dong, G.; Zhang, M. Facile synthesis of carbon self-doped g-C3N4 for enhanced photocatalytic hydrogen evolution. Ceram. Int. 2020, 46, 7888–7895. [Google Scholar] [CrossRef]
- Bao, N.; Hu, X.; Zhang, Q.; Miao, X.; Jie, X.; Zhou, S. Synthesis of porous carbon-doped g-C3N4 nanosheets with enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2017, 403, 682–690. [Google Scholar] [CrossRef]
- Yazdankish, E.; Foroughi, M.; Azqhandi MH, A. Capture of I131 from medical-based wastewater using the highly effective and recyclable adsorbent of g-C3N4 assembled with Mg-Co-Al-layered double hydroxide. J. Hazard. Mater. 2020, 389, 122151. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, E.; Velempini, T.; Molefe, M.; Pillay, K. Comparative study of KF, KCl and KBr doped with graphitic carbon nitride for superior photocatalytic degradation of methylene blue under visible light. J. Mater. Res. Technol. 2021, 15, 6340–6355. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, J.; Cai, J.; Liu, Q.; Zhang, X. Visible-light-driven photocatalytic degradation of dye and antibiotics by activated biochar composited with K+ doped g-C3N4: Effects, mechanisms, actual wastewater treatment and disinfection. Sci. Total Environ. 2022, 839, 155955. [Google Scholar] [CrossRef]
- Shu, Z.; Wang, Y.; Wang, W.; Zhou, J.; Li, T.; Liu, X.; Tan, Y.; Zhao, Z. A green one-pot approach for mesoporous g-C3N4 nanosheets with in situ sodium doping for enhanced photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2019, 44, 748–756. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Q.; Wang, Y.; Li, Z.; Li, Z.; Yuan, Q. New insights into the structure–performance relationships of mesoporous materials in analytical science. Chem. Soc. Rev. 2018, 47, 8766–8803. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Z.; Zhang, Z.; Niu, W.; Li, C.; Yang, N.; Chen, B.; Zhang, H. Two-dimensional metal nanomaterials: Synthesis, properties, and applications. Chem. Rev. 2018, 118, 6409–6455. [Google Scholar] [CrossRef]
- Shu, Z.; Xie, C.; Zhou, J.; Li, T.; Chen, Y.; Wang, W.; Tan, Y.; Zhao, Z. Nanoporous g-C3N4 nanosheets: Facile synthesis and excellent visible-light photocatalytic H2 evolution performance. J. Alloys Compd. 2018, 747, 140–148. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Huang, C.P.; Doong, R.; Chen, C.; Dong, C. CoO-3D ordered mesoporous carbon nitride (CoO@ mpgCN) composite as peroxymonosulfate activator for the degradation of sulfamethoxazole in water. J. Hazard. Mater. 2021, 401, 123326. [Google Scholar] [CrossRef]
- Huang, J.; Feng, X.; Bi, F.; Huang, G.; Rao, R.; Qiao, R.; Zhang, X. Strategic defect engineering in TiO2 catalysts through electron beam irradiation: Unraveling enhanced photocatalytic pathways for multicomponent VOCs degradation. Sep. Purif. Technol. 2025, 359, 130804. [Google Scholar] [CrossRef]
- Gao, B.; Bi, F.; Zhou, Z.; Zhang, Y.; Wei, J.; Lv, X.; Liu, B.; Huang, Y.; Zhang, X. A bimetallic MOF-derived MnCo spinel oxide catalyst to enhance toluene catalytic degradation. Chem. Commun. 2024, 60, 7455–7458. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, T.; Hou, J.; Zhang, T.; Zhang, G.; Zhang, Y.; Wang, X. Oxygen vacancies induced narrow band gap of BiOCl for efficient visible-light catalytic performance from double radicals. J. Mater. Sci. Technol. 2022, 114, 240–248. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Huo, Y.; Dai, K.; Li, S.; Chen, S. Two-dimensional sulfur-and chlorine-codoped g-C3N4/CdSe-amine heterostructures nanocomposite with effective interfacial charge transfer and mechanism insight. Appl. Catal. B Environ. 2021, 280, 119452. [Google Scholar] [CrossRef]
- Zhou, S.; Hou, M.; Sun, Y.; Zhao, W.; Wang, H.; Guo, Q.; Yu, X.; Ma, X.; Zhao, J. Ultrahigh-performance visible-light photodegradation enabled by direct Z-scheme AgI/(Na, F)–C3N4 composites. Compos. Part B Eng. 2021, 224, 109200. [Google Scholar] [CrossRef]
- Jing, H.; Chen, L.; Yi, S.; Li, T.; Sun, J.; Chen, D. Comparative insight into effect of hybridizing potassium and hydrogen ions on photocatalytic Reduction/Oxidization behavior of g-C3N4 nanocrystals. Chem. Eng. J. 2021, 417, 129187. [Google Scholar] [CrossRef]
- Wei, M.; Gao, L.; Li, J.; Fang, J.; Cai, W.; Li, X.; Xu, A. Activation of peroxymonosulfate by graphitic carbon nitride loaded on activated carbon for organic pollutants degradation. J. Hazard. Mater. 2016, 316, 60–68. [Google Scholar] [CrossRef]
- Chen, L.; Ning, S.; Liang, R.; Xia, Y.; Huang, R.; Yan, G.; Wang, X. Potassium doped and nitrogen defect modified graphitic carbon nitride for boosted photocatalytic hydrogen production. Int. J. Hydrog. Energy 2022, 47, 14044–14052. [Google Scholar] [CrossRef]
- Yuan, B.; Zou, X.; Yan, T.; Fei, J.; Chu, Z. Green synthesis of graphitic carbon nitride nanodots using sodium chloride template. J. Nanoparticle Res. 2016, 18, 110. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Nan, Z. Effects of adsorption capacity and activity site on Fenton-like catalytic performance for Na and Fe co-doped g-C3N4. Sep. Purif. Technol. 2021, 256, 117765. [Google Scholar] [CrossRef]
- Quan, Y.; Li, J.; Li, X.; Chen, R.; Zhang, Y.; Huang, J.; Hu, J.; Lai, Y. Molten salt-assisted synthesis of carbon nitride with defective sites as visible-light photocatalyst for highly efficient hydrogen evolution. Appl. Catal. B Environ. Energy 2025, 362, 124711. [Google Scholar] [CrossRef]
- Fernandes, E.; Mazierski, P.; Miodyńska, M.; Klimczuk, T.; Medynska, A.Z.; Oliveira, J.; Matos, A.M.; Martins, R.C.; Gomes, J. Emerging contaminants and pathogenic microorganisms elimination in secondary effluent by graphitic carbon nitride photocatalytic ozonation processes. Catal. Today 2024, 432, 114624. [Google Scholar] [CrossRef]
- Song, H.; Liu, L.; Wang, H.; Feng, B.; Xiao, M.; Tang, Y.; Qu, X.; Gai, H.; Huang, T. Adjustment of the band gap of co-doped KCl/NH4Cl/g-C3N4 for enhanced photocatalytic performance under visible light. Mater. Sci. Semicond. Process. 2021, 128, 105757. [Google Scholar] [CrossRef]
- Miao, S.; Zhang, Y.; Xia, H.; Gao, H.; Tao, W.; Zhang, Z.; Huang, C.; Huang, L. Preparation of highly N-doped CoFeN by pyrolysis g-C3N4 for markedly enhanced Fenton degradation of MO and TC-HCl under visible illumination. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131192. [Google Scholar] [CrossRef]
- Lian, X.; Li, Y.; Zou, Y.; An, D.; Wang, Q.; Zhou, Q.; Li, X. High vis-light photocatalytic property of g-C3N4 on four pollutants (RhB, MB, TC-HCl and P-Nitrophenol). Curr. Appl. Phys. 2022, 39, 196–204. [Google Scholar] [CrossRef]
- Zhu, C.; Li, Y.; Li, Y.; Yang, N.; Wang, K.; Guo, X. NaBiO3/g-C3N4 Z-type heterojunction modified by Ag-anchorage to enhance the photocatalytic performance. J. Alloys Compd. 2024, 1010, 177944. [Google Scholar] [CrossRef]
- Liang, H.; Wang, A.; Cheng, R.; Chen, F.; Jing, S.; Brouzgou, A.; Tsiakaras, P. Graphitic carbon nitride coupled with molybdenum selenide composite photocatalyst for tetracycline degradation. J. Environ. Chem. Eng. 2024, 12, 111809. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.; Xu, R.; Wu, J.; Li, S.; Song, H. One-step preparation of hierarchical porous graphitic carbon nitride photocatalyst for wastewater treatment under real sunlight. Colloids Surf. A Physicochem. Eng. Asp. 2024, 699, 134718. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Li, J.; Luo, R.; Hu, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. In-situ incorporation of iron-copper bimetallic particles in electrospun carbon nanofibers as an efficient Fenton catalyst. Appl. Catal. B Environ. 2017, 207, 316–325. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Z.; Xiong, W.; Zhou, Y.; Peng, Y.; Li, X.; Zhou, C.; Xu, R.; Zhang, Y. One-step synthesis of Co-doped UiO-66 nanoparticle with enhanced removal efficiency of tetracycline: Simultaneous adsorption and photocatalysis. Chem. Eng. J. 2018, 353, 126–137. [Google Scholar] [CrossRef]
- Shi, K.X.; Qiu, F.; Wang, P.; Li, H.; Wang, C. Magnetic MgFe2O4/MIL-88A catalyst for photo-Fenton sulfamethoxazole decomposition under visible light. Sep. Purif. Technol. 2022, 301, 121965. [Google Scholar] [CrossRef]
- Sun, B.; Li, H.; Li, X.; Liu, X.; Zhang, C.; Xu, H.; Zhao, X.S. Degradation of organic dyes over fenton-like Cu2O–Cu/C catalysts. Ind. Eng. Chem. Res. 2018, 57, 14011–14021. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; Kazan-Kaya, E.S.; El Messaoudi, N.; Fernine, Y.; Américo-Pinheiro, J.H.P.; Jada, A. Remediation of tetracycline from aqueous solution through adsorption on g-C3N4-ZnO-BaTiO3 nanocomposite: Optimization, modeling, and theoretical calculation. J. Mol. Liq. 2023, 369, 120866. [Google Scholar] [CrossRef]
- Ni, S.; Fu, Z.; Li, L.; Ma, M.; Liu, Y. Step-scheme heterojunction g-C3N4/TiO2 for efficient photocatalytic degradation of tetracycline hydrochloride under UV light. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129475. [Google Scholar] [CrossRef]
- Wen, X.J.; Niu, C.G.; Zhang, L.; Liang, C.; Guo, H.; Zeng, G. Photocatalytic degradation of ciprofloxacin by a novel Z-scheme CeO2–Ag/AgBr photocatalyst: Influencing factors, possible degradation pathways, and mechanism insight. J. Catal. 2018, 358, 141–154. [Google Scholar] [CrossRef]
- Sarafraz, M.; Sadeghi, M.; Yazdanbakhsh, A.; Amini, M.M.; Sadani, M.; Eslami, A. Enhanced photocatalytic degradation of ciprofloxacin by black Ti3+/N-TiO2 under visible LED light irradiation: Kinetic, energy consumption, degradation pathway, and toxicity assessment. Process Saf. Environ. Prot. 2020, 137, 261–272. [Google Scholar] [CrossRef]
- Yali, L.I.U.; Xian, L.U.; Zhang, Y.; Sun, D.; Gao, N. UV/chlorine as an advanced oxidation process for the degradation of ciprofloxacin: Degradation efficiency, mechanism and toxicity evaluation. Environ. Chem. 2022, 41, 3766–3777. [Google Scholar]
- Ji, Y.; Shi, Y.; Dong, W.; Wen, X.; Jiang, M.; Lu, J. Thermo-activated persulfate oxidation system for tetracycline antibiotics degradation in aqueous solution. Chem. Eng. J. 2016, 298, 225–233. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Sengeløv, G.; Tjørnelund, J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch. Environ. Contam. Toxicol. 2002, 42, 263–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Chen, X.; Wang, L.; Cai, W. Coupling of heterogeneous advanced oxidation processes and photocatalysis in efficient degradation of tetracycline hydrochloride by Fe-based MOFs: Synergistic effect and degradation pathway. Chem. Eng. J. 2019, 369, 745–757. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, X.; Sun, H.; Zhang, H.; Zhao, X.; Wang, L. Construction of a 0D/3D AgI/MOF-808 photocatalyst with a one-photon excitation pathway for enhancing the degradation of tetracycline hydrochloride: Mechanism, degradation pathway and DFT calculations. Chem. Eng. J. 2023, 460, 141842. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, F.; Xiao, Y.; Fang, J.; Zhao, C.; Zhao, N.; Zhao, C.; Zhang, W.; Zhang, W.; Qiu, Q. High Fe utilization efficiency and low toxicity of Fe3C@ Fe0 loaded biochar for removing of tetracycline hydrochloride in wastewater. J. Clean. Prod. 2022, 353, 131630. [Google Scholar] [CrossRef]
- Yu, C.; Pang, H.; Wang, J.H.; Chi, Z.Y.; Zhang, Q.; Kong, F.T.; Xu, Y.P.; Li, S.Y.; Che, J. Occurrence of antibiotics in waters, removal by microalgae-based systems, and their toxicological effects: A review. Sci. Total Environ. 2022, 813, 151891. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Hu, C.; Hu, X.; Wei, D.; Chen, Y.; Qu, J. Photodegradation and toxicity changes of antibiotics in UV and UV/H2O2 process. J. Hazard. Mater. 2011, 185, 1256–1263. [Google Scholar] [CrossRef] [PubMed]






| Samples | SBET(m2·g−1) | Vtotal (cc·g−1) | DBJH (nm) |
|---|---|---|---|
| gCN | 19.410 | 0.14 | 3.70 |
| NaMe-0.5 | 55.153 | 0.39 | 3.71 |
| NaMe-1 | 55.209 | 0.41 | 3.71 |
| NaMe-2 | 45.754 | 0.30 | 3.71 |
| Acute Toxicity (ppm) | Chronic Toxicity (ppm) | |||||
|---|---|---|---|---|---|---|
| Intermediate Products | Fish (LC50) | Daphnid (LC50) | Green Algae (EC50) | Fish (ChV) | Daphnid (ChV) | Green Algae (ChV) |
| TC-HCl | 13,100 | 1060 | 1890 | 2490 | 59.90 | 474 |
| P1 m/z = 495.16 | 434 | 46.10 | 47.90 | 36.10 | 3.38 | 14.60 |
| P2 m/z = 427.15 | 1210 | 118 | 146 | 133 | 7.90 | 41.80 |
| P3 m/z = 410.12 | 926 | 91.40 | 110 | 96.10 | 6.24 | 31.80 |
| P4 m/z = 459.14 | 332,000 | 21,000 | 60,700 | 133,000 | 934 | 12,800 |
| P5 m/z = 431.14 | 17,500 | 1380 | 2570 | 3570 | 76.10 | 635 |
| P6 m/z = 364.14 | 5110 | 438 | 693 | 812 | 26.10 | 181 |
| P7 m/z = 461.15 | 28,100 | 2150 | 4270 | 6310 | 115 | 1030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Mao, Y.; Liu, Q.; Ma, Y.; Fu, F.; Jian, S.; Liu, Y.; Lu, S. Molten-Salt-Assisted Preparation of g-C3N4 for Photocatalytic Degradation of Tetracycline Hydrochloride: Degradation Mechanism, Pathway, and Toxicity Assessment. Sustainability 2025, 17, 1166. https://doi.org/10.3390/su17031166
Jiao Y, Mao Y, Liu Q, Ma Y, Fu F, Jian S, Liu Y, Lu S. Molten-Salt-Assisted Preparation of g-C3N4 for Photocatalytic Degradation of Tetracycline Hydrochloride: Degradation Mechanism, Pathway, and Toxicity Assessment. Sustainability. 2025; 17(3):1166. https://doi.org/10.3390/su17031166
Chicago/Turabian StyleJiao, Yujie, Yaqi Mao, Qikai Liu, Yongxia Ma, Fei Fu, Shenglong Jian, Yang Liu, and Sujin Lu. 2025. "Molten-Salt-Assisted Preparation of g-C3N4 for Photocatalytic Degradation of Tetracycline Hydrochloride: Degradation Mechanism, Pathway, and Toxicity Assessment" Sustainability 17, no. 3: 1166. https://doi.org/10.3390/su17031166
APA StyleJiao, Y., Mao, Y., Liu, Q., Ma, Y., Fu, F., Jian, S., Liu, Y., & Lu, S. (2025). Molten-Salt-Assisted Preparation of g-C3N4 for Photocatalytic Degradation of Tetracycline Hydrochloride: Degradation Mechanism, Pathway, and Toxicity Assessment. Sustainability, 17(3), 1166. https://doi.org/10.3390/su17031166






