Nutrient Requirements in Diets: Fundamental Issues in Sustainable Aquaculture Development
Abstract
1. Introduction
2. How to Express Nutrient Requirements
2.1. Per Digestible Energy
2.2. Per Day
2.3. Per N Retention (Stoichiometric Ratio)
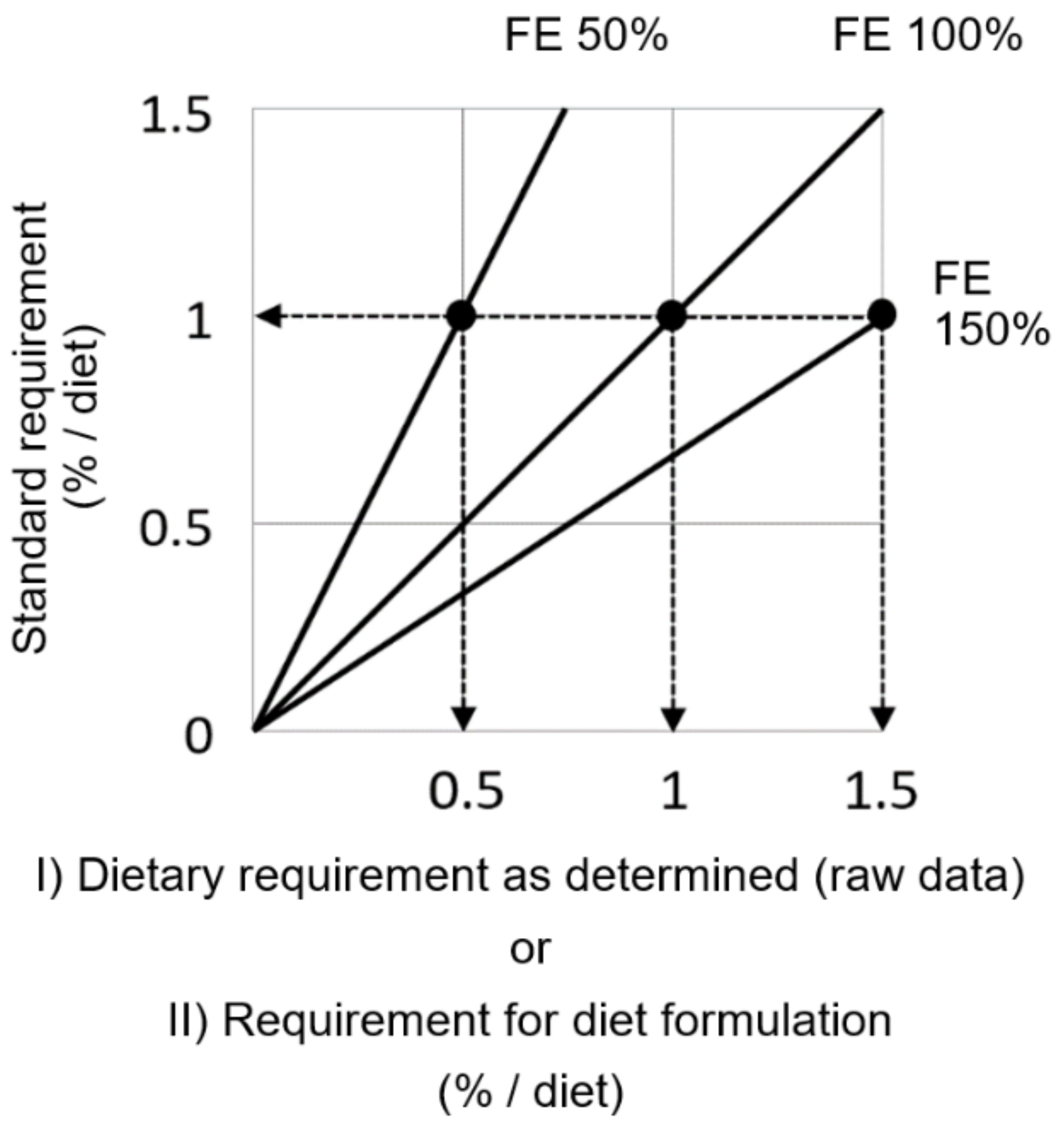
2.4. Per Feed Efficiency (FE: Fish Weight Gain/Feed Fed)
- (1)
- Standardized P requirement = (available P/feed)/FE.
- (2)
- Standardized P requirement = available P/body weight gain.
3. Additional Issues
3.1. Growth Magnification
3.2. Rational Criteria
3.3. Factorial Approach for Comparison
- Pfeffer and Pieper [76] used the following formula to derive the requirement value:
- Shearer [77] refined the dietary requirement calculation as follows:
- Nakashima and Leggett [78] provided the following formula:
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hepher, B. Nutrition of Pond Fishes; Cambridge University Press: Cambridge, UK, 1988; p. 388. [Google Scholar]
- NRC (National Research Council). Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011; p. 376. [Google Scholar]
- Adolph, E.F. Urges to eat and drink in rats. Am. J. Physiol. 1947, 151, 110–125. [Google Scholar] [CrossRef]
- Forbes, J.M. The Voluntary Food Intake of Farm Animals; Butterworths & Co.: London, UK, 1986. [Google Scholar]
- Kleiber, M. The Fire of Life, 2nd ed.; Robert, E., Ed.; Krieger Publishing Co.: Malabar, FL, USA, 1975; p. 453. [Google Scholar]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D. Animal Nutrition, 4th ed.; Longman Scientific & Technical, John Wiley & Sons, Inc.: New York, NY, USA, 1988. [Google Scholar]
- NRC (National Research Council). Predicting Feed Intake of Food-Producing Animals; National Academy Press: Washington, DC, USA, 1987; p. 85. [Google Scholar]
- Rozin, P.; Mayer, J. Regulation of food intake in the goldfish. Am. J. Physiol. 1961, 201, 968–974. [Google Scholar] [CrossRef]
- Bromley, P.J.; Adkins, T.C. The influence of cellulose filler on feeding, growth and utilization of protein and energy in rainbow trout, Salmo gairdnerii Richardson. J. Fish Biol. 1984, 24, 235–244. [Google Scholar] [CrossRef]
- Grove, D.J.; Loizides, L.G.; Nott, J. Satiation amount, frequency of feeding and gastric emptying rate in Salmo gairdneri. J. Fish Biol. 1978, 12, 507–516. [Google Scholar] [CrossRef]
- Hilton, J.W.; Atkinson, J.L.; Slinger, S.J. Effect of increased dietary fiber on the growth of rainbow trout (Salmo gairdneri). Can. J. Fish. Aquat. Sci. 1983, 40, 81–85. [Google Scholar] [CrossRef]
- Lee, D.J.; Putnam, G.B. The response of rainbow trout to varying protein/energy ratios in a test diet. J. Nutr. 1973, 103, 916–922. [Google Scholar] [CrossRef]
- Gatlin, D.M., III; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Herman, E.; Hu, G.; Krogdahl, A.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Hardy, R.W. Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- De Silva, S.S.; Ingram, B.A.; Nguyen, P.T.; Bui, T.M.; Gooley, G.J.; Turchini, G.M. Estimation of nitrogen and phosphorus in effluent from the striped catfish farming sector in the Mekong Delta, Vietnam. Ambio 2010, 39, 504–514. [Google Scholar] [CrossRef]
- Brett, J.R.; Groves, T.D.D. Physiological energetics. Fish Physiol. 1979, 8, 279–352. [Google Scholar]
- Lovell, R.T. Nutrition and feeding (Ch. 6). In Fish Farming Handbook; Brown, E.E., Gratzek, J.B., Eds.; AVI Publishing Co., Inc.: Westport, CT, USA, 1980; pp. 207–236. [Google Scholar]
- Cho, C.Y.; Cowey, C.B.; Watanabe, T. Finfish Nutrition in Asia: Methodological Approaches to Research and Development; IDRC: Ottawa, ON, Canada, 1985; p. 154. [Google Scholar]
- Smith, R.R. Nutritional energetics. In Fish Nutrition, 2nd ed.; Halver, J.E., Ed.; Academic Press: San Diego, CA, USA, 1989; pp. 1–29. [Google Scholar]
- McCay, C.M.; Phillips, A.M. Feeds for the fish hatcheries. Prog. Fish-Cult. 1940, 7, 18–21. [Google Scholar] [CrossRef]
- Baker, D.H. Problems and pitfalls in animal experiments designed to establish dietary requirements for essential nutrients. J. Nutr. 1986, 116, 2339–2349. [Google Scholar] [CrossRef]
- McIntyre, P.B.; Flecker, A.S. Ecological stoichiometry as an integrative framework in stream fish ecology. In Community Ecology of Stream Fishes: Concepts, Approaches, and Techniques; American Fisheries Society, Symposium: Bethesda, MD, USA, 2010; Volume 73, pp. 539–558. [Google Scholar]
- Grisdale-Helland, B.; Shearer, K.D.; Helland, S.J. Energy and nutrient utilization of Atlantic cod, Atlantic salmon and rainbow trout fed diets differing in energy content. Aquac. Nutr. 2007, 13, 321–334. [Google Scholar] [CrossRef]
- Boersma, M.; Elser, J.J. Too much of a good thing: On stoichiometrically balanced diets and maximal growth. Ecology 2006, 87, 1325–1330. [Google Scholar] [CrossRef]
- Benstead, J.P.; Hood, J.M.; Whelan, N.V.; Kendrick, M.R.; Nelson, D.; Demi, L.M. Coupling of dietary phosphorus and growth across diverse fish taxa: A meta-analysis of experimental aquaculture studies. Ecology 2014, 95, 2768–2777. [Google Scholar] [CrossRef]
- IOM: Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academy Press: Washington, DC, USA, 1997; p. 432. [Google Scholar]
- Gevaerts, J. Diète sous phosphore. La Cell. 1901, 18, 7–33, (cited in Forbes, E.B.; Keith, M.H. Phosphorus Compounds in Animal Metabolism; Technical Series Bulletin No. 5; Ohio Agricultural Experiment Station: Wooster, OH, USA, 1914; p. 430). [Google Scholar]
- Gregersen, J.P. Untersuchungen über den phosphorstoffwechsel. Zeit. Physiol. Chem. 1911, 71, 49–99, (cited in Forbes, E.B.; Keith, M.H. Phosphorus Compounds in Animal Metabolism; Technical Series Bulletin No. 5; Ohio Agricultural Experiment Station: Wooster, OH, USA, 1914; pp. 207, 220, 428). [Google Scholar] [CrossRef][Green Version]
- Wolf, C.G.L.; Oesterberg, E. Eiweissstoffwechsel beim Hunde. II. Stickstoff und Schwefelstoffwechsel während des Hungers und bei Unternährung mit Eiweiss, Kohlenhydraten und Fetten. Biochem. Zeit. 1911, 35, 329–362, (cited in Forbes, E.B.; Keith, M.H. Phosphorus Compounds in Animal Metabolism; Technical Series Bulletin No. 5; Ohio Agricultural Experiment Station: Wooster, OH, USA, 1914; p. 445). [Google Scholar]
- Kleiber, M.; Goss, H.; Guilbert, H.R. Phosphorus deficiency metabolism and food utilization in beef heifers. J. Nutr. 1936, 12, 121–153. [Google Scholar] [CrossRef]
- Rudman, D.; Millikan, W.J.; Richardson, T.J.; Bixler, T.J.; Stackhouse, W.J.; McGarrity, W.C. Elemental balances during intravenous hyperalimentation of underweight adult subjects. J. Clin. Investig. 1975, 55, 94–104. [Google Scholar] [CrossRef]
- Bondi, A.A. Animal Nutrition, English ed.; John Wiley & Sons: New York, NY, USA, 1987; p. 540. [Google Scholar]
- Pilati, A.; Vanni, M.J. Ontogeny, diet shifts, and nutrient stoichiometry in fish. Oikos 2007, 116, 1663–1674. [Google Scholar] [CrossRef]
- Shearer, K.D. Changes in elemental composition of hatchery-reared rainbow trout, Salmo gairdneri, associated with growth and reproduction. Can. J. Fish. Aquat. Sci. 1984, 41, 1592–1600. [Google Scholar] [CrossRef]
- NRC (National Research Council). Nutrient Requirements of Swine, 11th rev. ed.; National Academies Press: Washington, DC, USA, 2012; p. 400. [Google Scholar]
- Lanari, D.; d’Agaro, E.; Ballestrazzi, R. Effect of dietary DP/DE ratio on apparent digestibility, growth and nitrogen and phosphorus retention in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Nutr. 1995, 1, 105–110. [Google Scholar] [CrossRef]
- Sugiura, S.H. Development of Low-Pollution Feeds for Sustainable Aquaculture. Ph.D. Dissertation, University of Washington, Seattle, WA, USA, 1998; p. 252. [Google Scholar]
- Sugiura, S.H. Phosphorus in Fish Nutrition; Bookway Academic Publishing: Hyogo, Japan, 2018; p. 420. [Google Scholar]
- Schindler, D.E.; Eby, L.A. Stoichiometry of fishes and their prey: Implications for nutrient recycling. Ecology 1997, 78, 1816–1831. [Google Scholar] [CrossRef]
- Jahan, P.; Watanabe, T.; Kiron, V.; Satoh, S. Phosphorus and nitrogen excretion during growth span of carp kept under two rearing systems. Fish. Sci. 2003, 69, 431–437. [Google Scholar] [CrossRef]
- Shearer, K.D. Dietary potassium requirement of juvenile chinook salmon. Aquaculture 1988, 73, 119–129. [Google Scholar] [CrossRef]
- Asgard, T.; Shearer, K.D. Dietary phosphorus requirement of juvenile Atlantic salmon, Salmo salar L. Aquac. Nutr. 1997, 3, 17–23. [Google Scholar] [CrossRef]
- Shearer, K.D. Factors affecting the proximate composition of cultured fishes with emphasis on salmonids. Aquaculture 1994, 119, 63–88. [Google Scholar] [CrossRef]
- Breck, J.E. Body composition in fishes: Body size matters. Aquaculture 2014, 433, 40–49. [Google Scholar] [CrossRef]
- Papoutsoglou, S.E.; Papoutsoglou, E.G.P. Comparative studies on body composition of rainbow trout (Salmo gairdneri R.) in relation to type of diet and growth rate. Aquaculture 1978, 13, 235–243. [Google Scholar] [CrossRef]
- Shimma, H.; Sato, R. Comparison of proximate composition among the five races of common carp, Cyprinus carpio. Bull. Natl. Res. Inst. Aquac. 1985, 7, 37–43, (In Japanese with English abstract). [Google Scholar]
- Sugiura, S.H.; Dong, F.M.; Hardy, R.W. A new approach to estimating the minimum dietary requirement of phosphorus based on non-fecal excretions of phosphorus and nitrogen. J. Nutr. 2000, 130, 865–872. [Google Scholar] [CrossRef]
- Jongbloed, A.W.; Everts, H.; Kemme, P.A. Phosphorus availability and requirements in pigs. In Recent Advances in Animal Nutrition 1991; Haresign, W., Cole, D.J.A., Eds.; Butterworth-Heinemann Ltd.: Oxford, UK, 1991; pp. 65–80. [Google Scholar]
- Miñoso, M.G.G.; Borlongan, I.G.; Satoh, S. Essentiality of phosphorus, magnesium, iron, zinc, and manganese in milkfish diet. Fish. Sci. 1999, 65, 721–725. [Google Scholar]
- Mwangamilo, J.J.; Jiddawi, N.S. Nutritional studies and development of a practical feed for milkfish (Chanos chanos) culture in Zanzibar, Tanzania. West. Indian Ocean. J. Mar. Sci. 2003, 2, 137–146. [Google Scholar]
- Borlongan, I.G.; Satoh, S. Dietary phosphorus requirement of juvenile milkfish, Chanos chanos (Forsskal). Aquac. Res. 2001, 32 (Suppl. S1), 26–32. [Google Scholar] [CrossRef]
- Sugiura, S.H. Improvement study on the domestic diets VI. In JOCV Technical Report; General Establishment of Fisheries: Jableh, Syria, 1991; p. 8. [Google Scholar]
- Hardy, R.W.; Gatlin, D.M. Nutritional strategies to reduce nutrient losses in intensive aquaculture. In Avances en Nutrición Acuícola VI; Memorias del VI Simposium Internacional de Nutrición Acuícola: San Nicolás de los Garza, Mexico, 2002. [Google Scholar]
- Miyashita, S. Yellowtail. In Aquaculture of Seawater Fishes; Kumai, H., Ed.; Sobunsha: Tokyo, Japan, 2005; pp. 52–77. (In Japanese) [Google Scholar]
- Watanabe, M. Development of compound feed for amberjack aquaculture and the effects on the aquatic environment. In Kochi Prefectural Fisheries Experiment Station, Annual Report for the Fiscal Year of 2005; Kochi Prefectural Fisheries Experiment Station: Susaki, Japan, 2006; pp. 94–117. (In Japanese) [Google Scholar]
- Takii, K.; Akira, T.; Seoka, M.; Kitamura, S.; Kurifuji, K. Feeding protocols with artificial diet affect on growth performance and energy partition of red sea bream, Pagrus major. Aquac. Sci. 2008, 56, 237–243. [Google Scholar]
- Roloff, F. Ueber osteomalacie und rachitis. Arch. Prakt. Tierheilk. 1875, 1, 189–220, (cited in McCay, C.M. Nutrition of the Dog, 2nd ed.; Comstock Publishing Company, Inc.: Ithaca, NY, USA, 1949; p. 80). [Google Scholar]
- Voit, E. Ueber die bedeutung des kalkes für den thierischen organismus. Zeit. Biol. 1880, 16, 55–118, (cited in Hess, A.F. Rickets Including Osteomalacia and Tetany; Lea & Febiger: Philadelphia, PA, USA, 1929; p. 63; McCay, C.M. Nutrition of the Dog, 2nd ed.; Comstock Publishing Company, Inc.: Ithaca, NY, USA, 1949; p. 80; McCay, C.M. Notes on the History of Nutrition Research; Hans Huber Publishers: Bern, Switzerland, 1973; pp. 181–186). [Google Scholar]
- Embody, G.C.; Gordon, M. A comparative study of natural and artificial foods of brook trout. Trans. Am. Fish. Soc. 1924, 54, 185–200. [Google Scholar] [CrossRef]
- Murakami, Y. Research on cranial deformity prevalent in common carp fingerlings-I. Bull. North. Reg. Freshw. Fish Advis. Cent. Hiroshima Prefect. 1967, 6, 60–67. (In Japanese) [Google Scholar]
- Murakami, Y. Research on cranial deformity prevalent in common carp fingerlings. Fish Pathol. 1967, 2, 1–10. (In Japanese) [Google Scholar] [CrossRef]
- Aubel, C.E.; Hughes, J.S.; Lienhardt, H.F. The effects of low-phosphorus rations on growing pigs. J. Agric. Res. 1936, 52, 149–159. [Google Scholar]
- Day, H.G.; McCollum, E.V. Mineral metabolism, growth, and symptomology of rats on a diet extremely deficient in phosphorus. J. Biol. Chem. 1939, 130, 269–283. [Google Scholar] [CrossRef]
- Gillis, M.B.; Norris, L.C.; Heuser, G.F. The utilization by the chick of phosphorus from different sources. J. Nutr. 1948, 35, 195–207. [Google Scholar] [CrossRef]
- Nose, T.; Arai, S. Recent advances in studies on mineral nutrition of fish in Japan. In Advances in Aquaculture; Pillay, T.V.R., Dill, W.A., Eds.; FAO/Fishing News Books Ltd.: Farnam, England, 1979; pp. 584–590. [Google Scholar]
- Hardy, R.W.; Fairgrieve, W.T.; Scott, T.M. Periodic feeding of low-phosphorus diet and phosphorus retention in rainbow trout (Oncorhynchus mykiss). In Fish Nutrition in Practice; Kanshik, S.J., Luquet, P., Eds.; INRA: Biarritz, France, 1993; pp. 403–412. [Google Scholar]
- Uyan, O.; Koshio, S.; Ishikawa, M.; Uyan, S.; Ren, T.; Yokoyama, S.; Michael, F.R. Effects of dietary phosphorus and phospholipid level on growth, and phosphorus deficiency signs in juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 2007, 267, 44–54. [Google Scholar] [CrossRef]
- Dupree, H.K. Vitamins essential for growth of channel catfish. In Technical Papers 7; Bureau of Sport Fisheries and Wildlife: Washington, DC, USA, 1966; p. 11. [Google Scholar]
- Sugiura, S.H.; Kelsey, K.; Ferraris, R.P. Molecular and conventional responses of large rainbow trout to dietary phosphorus restriction. J. Comp. Physiol. B 2007, 177, 461–472. [Google Scholar] [CrossRef]
- Rodehutscord, M. Response of rainbow trout (Oncorhynchus mykiss) growing from 50 to 200g to supplements of dibasic sodium phosphate in a semipurified diet. J. Nutr. 1996, 126, 324–331. [Google Scholar] [CrossRef]
- Sugiura, S.H. Phosphorus, aquaculture, and the environment. Rev. Fish. Sci. Aquac. 2018, 26, 515–521. [Google Scholar] [CrossRef]
- NRC (National Research Council). Nutrient Requirements of Swine, 10th ed.; National Academy Press: Washington, DC, USA, 1998; p. 189. [Google Scholar]
- Sauveur, B.; Perez, J.M. Mineral nutrition of non-ruminants. In Feeding of Non-Ruminant Livestock; Wiseman, J., Ed. and Translator; Butterworth & Co., Ltd.: London, UK, 1987; pp. 19–25. [Google Scholar]
- Ogino, C.; Takeda, H. Mineral requirements in fish-III. Calcium and phosphorus requirements of carp. Bull. Jpn. Soc. Sci. Fish. 1976, 42, 793–799. [Google Scholar] [CrossRef]
- Bureau, D.P.; Cho, C.Y. Phosphorus utilization by rainbow trout (Oncorhynchus mykiss): Estimation of dissolved phosphorus waste output. Aquaculture 1999, 179, 127–140. [Google Scholar] [CrossRef]
- Pfeffer, E.; Pieper, A. Application of the factorial approach for deriving nutrient requirements of growing fish. In Finfish Nutrition and Fishfeed Technology; Halver, J.E., Tiews, K., Eds.; Heenemann Verlagsgesellschaft mbH: Berlin, Gremany, 1979; Volume II, pp. 545–553. [Google Scholar]
- Shearer, K.D. The use of factorial modeling to determine the dietary requirements for essential elements in fishes. Aquaculture 1995, 133, 57–72. [Google Scholar] [CrossRef]
- Nakashima, B.S.; Leggett, W.C. Natural sources and requirements of phosphorus for fishes. Can. J. Fish. Aquat. Sci. 1980, 37, 679–686. [Google Scholar] [CrossRef]
- Ogino, C. Proteins and amino acids. In Fish Nutrition and Diets; Yone, Y., Ed.; Koseisha-Koseikaku, Inc.: Tokyo, Japan, 1985; pp. 9–19. (In Japanese) [Google Scholar]
- Schwarz, F.J. Determination of mineral requirements of fish. J. Appl. Ichthyol. 1995, 11, 164–174. [Google Scholar] [CrossRef]
| Fish 1 (Body wt: Initial–Final) | Diet Type | FE (%) 2 | Ref. 3 |
|---|---|---|---|
| Mirror carp (28–106 g) | Economical feed (locally made) | 65 | 1 |
| Mirror carp (28–202 g) | Commercial feed (for carp) | 105 | 1 |
| Mirror carp (28–248 g) | Commercial feed (high protein) | 130 | 1 |
| Atlantic salmon | Commercial feeds of the 1970s | 42 | 2 |
| Atlantic salmon | Commercial feeds of the 2000s | 118 | 2 |
| Rainbow trout | Commercial feeds of the 1970s | 51 | 2 |
| Rainbow trout | Commercial feeds of the 2000s | 91 | 2 |
| Yellowtail (40–1244 g) | Commercial feed | 56–67 | 3 |
| Yellowtail (900–4600 g) | ibid. | 45–48 | 3 |
| Amberjack (100–210 g) | Commercial feed (CP 54%, fat 15%) | 105 | 4 |
| Amberjack (1500–2100 g) | ibid. | 54 | 4 |
| Atlantic salmon (486–962 g) | Commercial feed (CP 53%, fat 28%) | 103 | 5 |
| Rainbow trout (415–859 g) | ibid. | 90 | 5 |
| Atlantic cod (439–682 g) | ibid. | 143 | 5 |
| Red seabream (10–15 g) | Commercial feed (CP 52%, fat 15%) | 77–91 | 6 |
| Red seabream (80–110 g) | ibid. | 67 | 6 |
| 1. Factors that can be normalized by feed efficiency (FE) |
| ► Feed composition, including protein and energy density, nutrient balance, deficiencies in essential nutrients, palatability, anti-nutritional factors, digestibility of macronutrients, indigestible matter content, immunomodulators, feed processing-storage conditions, etc. |
| ► Growth rate that is influenced by fish size or age, species or strain, feeding rate and frequency, water temperature, water quality, various stressors, and other culture conditions. |
| ► Physiological state that is influenced by maturation, reproduction, smoltification, and disease infection, among others. |
| 2. Factors independent of feed efficiency (FE): technical factors |
| ► Feeding duration, i.e., growth magnification to offset initial body reserves; thus, also diet history (described in the text below). |
| ► Nutrient supply from non-dietary sources, e.g., natural organisms, bioflocs, soil, and water (branchial and surface absorption of various ions and drinking water containing minerals). When these factors are significant, dietary requirements will be water-dependent. |
| ► Leaching loss of soluble nutrients (significant in microparticulate diets and loose pellets) and uneaten feed due to excess feeding, poor feeding practices, and bad pellets (inadequate sizes, fines, and loose pellets). |
| ► Response criteria used, e.g., growth, mortality, bone P content, gene expression, immune function, enzyme activity, blood levels, and other indicators (described in the text below). |
| ► Methodology including dose–response, factorial estimates, balance method, statistical methods (e.g., logistic regression, polynomial, and broken-line), and the level of plateau chosen (e.g., 90%, 95%, and 100%) (described in the text below). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugiura, S.H. Nutrient Requirements in Diets: Fundamental Issues in Sustainable Aquaculture Development. Sustainability 2025, 17, 1289. https://doi.org/10.3390/su17031289
Sugiura SH. Nutrient Requirements in Diets: Fundamental Issues in Sustainable Aquaculture Development. Sustainability. 2025; 17(3):1289. https://doi.org/10.3390/su17031289
Chicago/Turabian StyleSugiura, Shozo H. 2025. "Nutrient Requirements in Diets: Fundamental Issues in Sustainable Aquaculture Development" Sustainability 17, no. 3: 1289. https://doi.org/10.3390/su17031289
APA StyleSugiura, S. H. (2025). Nutrient Requirements in Diets: Fundamental Issues in Sustainable Aquaculture Development. Sustainability, 17(3), 1289. https://doi.org/10.3390/su17031289






