Abstract
Amid the pressing challenge of global climate change, biogas (marsh gas) has garnered recognition as a clean and renewable energy source with significant potential to reduce greenhouse gas emissions and support sustainable energy production. Composed primarily of methane (CH4) and carbon dioxide (CO2), enhancing the CH4 content in biogas is essential for improving its quality and expanding its high-value applications. This review examines the mechanisms underlying CH4 and CO2 production in anaerobic digestion (AD) processes; investigates the effects of raw material types, process routes, and fermentation conditions on biogas production and CH4 content; and proposes feasible technical pathways for producing CH4-rich biogas. Research indicates that CH4-rich biogas can be produced through various strategies. Raw material pretreatment technologies and co-digestion strategies can enhance substrate performance, stabilize the AD process, and boost CH4 production. Process optimizations, such as multiphase AD and CH4 co-production techniques, significantly improve carbon utilization efficiency. Introducing exogenous reinforcement materials, including biochar and zero-valent iron nanoparticles, fosters microbial interactions and facilitates direct interspecies electron transfer (DIET). Furthermore, microbial regulation through genetic engineering and microbial community design presents promising prospects. By reviewing the mechanisms of gas production, influencing factors, and feasible pathways, this work aims to provide valuable insights for the technical research of AD to produce CH4-rich biogas.
1. Introduction
Anaerobic digestion (AD) is the process of using facultative and obligate anaerobic bacteria to degrade organic matter into biogas under anaerobic conditions [1]. Producing biogas (biogas specifically refers to marsh gas in this article) through AD not only reduces reliance on fossil fuels but also significantly curtails greenhouse gas emissions, including CH4 and CO2, delivering both economic and environmental benefits [2]. In the context of carbon neutrality, the biogas industry has garnered increased attention and support as an important tool for carbon reduction and renewable energy development [3]. For instance, the European Union and the United States have introduced biogas subsidy policies and green tax incentives to promote the development of biogas power generation projects [4]. Similarly, China has been actively promoting the goals of “carbon peak and carbon neutrality”. The government’s support for biogas production and utilization has been continuously strengthened, providing policy and financial support for rural livestock waste treatment, urban sewage treatment, and kitchen waste treatment projects [5,6]. The biogas industry is scalable and multifunctional in treating various organic wastes and has the dual advantages of waste management and sustainable energy production. It has become one of the cornerstones of renewable energy strategy.
Current research primarily focuses on mixed AD, raw material pretreatment, multiphase AD, and process condition optimization. Studies reveal that the co-digestion of diverse wastes with different organic substrates improves nutrient balance, enhances microbial activity, and increases biogas yield. For refractory organic solid wastes, such as straw, garden waste, and other lignocellulosic biomass, pretreatment enhances the biodegradability of complex organic materials, thereby boosting biogas production efficiency [7]. By separating hydrolysis and methanogenesis into distinct stages, researchers have optimized conditions for each phase, further improving biogas yield and stability. Through the optimization of AD conditions, such as temperature, organic loading rate (OLR), and hydraulic retention time, the biogas yield and digestion rate can be improved. In addition, many new application modes have emerged in the biogas industry, such as using biogas to produce biological natural gas, green hydrogen, and green methanol [8]. However, critical challenges remain, such as the unstable composition of biogas, the poor economy of the biogas system, and the difficult disposal of fermentation residue, which need further innovation and research.
Biogas is increasingly recognized as a versatile energy source suitable for power generation and heating, and as a precursor for bio-based chemical production. However, the efficiency and economic viability of biogas applications largely depend on the CH4 content, which directly determines the energy density and applicability of biogas. Biogas is a mixed gas, primarily composed of 50–70% CH4 and 30–50% CO2, with small amounts (usually less than 1%) of H2, N2, O2, and H2S. CH4 and CO2 are the key components. Their content and proportion determine the applicability and utilization value of biogas [9]. CH4 is the primary combustible component, which has a high calorific value of approximately 35.8 MJ/m3 and excellent combustion properties [10]. Increasing the proportion of CH4 in biogas can increase its total calorific value and reduce the amount of biogas required for the same energy output. When preparing bio-natural gas from biogas, the bio-natural gas going into the pipeline usually requires a CH4 content of over 95%. The initial CH4 content in biogas directly impacts purification efficiency. High-CH4-content biogas reduces the gas separation and purification costs, enhancing its economic value and applicability. Similarly, when using biogas for green hydrogen production, CO2 and trace gases lower utilization efficiency, hinder the main reaction, and increase system energy consumption [11]. The CH4 content of biogas produced by ordinary biogas engineering is generally 50–65%, which can only meet conventional combustion needs. For high-purity applications, further purification and refinement are required. Current purification methods, such as chemical absorption, physical separation, and membrane separation, face the problems of high energy consumption and equipment costs. Consequently, there is a growing need to develop strategies for enhancing CH4 content at the production stage, minimizing the reliance on costly downstream purification processes.
Effective management during AD can optimize CH4 and CO2 content to produce “CH4-rich biogas” in situ. Recent research on AD increasingly emphasizes optimizing biogas composition rather than merely enhancing total production, with a particular focus on improving CH4 content. The reinforcement strategy includes adjusting raw material nutrient profiles, optimizing key process parameters, incorporating exogenous promoters, and regulating microbial activity during the AD process. The most effective approach involves using biochar or conductive materials to facilitate direct interspecies electron transfer (DIET) among microorganisms. While these methods demonstrate significant potential in laboratory-scale studies, their practical applicability, economic feasibility, and long-term stability remain underexplored and require systematic investigation. This study summarizes the mechanisms underlying CH4 and CO2 production during AD, examines key factors influencing methane content in biogas, and evaluates the methods for enhancing CH4 content in situ. The aim is to provide a comprehensive framework for producing CH4-rich biogas. This research aligns with global carbon neutrality goals and offers new perspectives on sustainable energy development and waste resource utilization.
2. The Mechanism of CH4 and CO2 Generation in AD Process
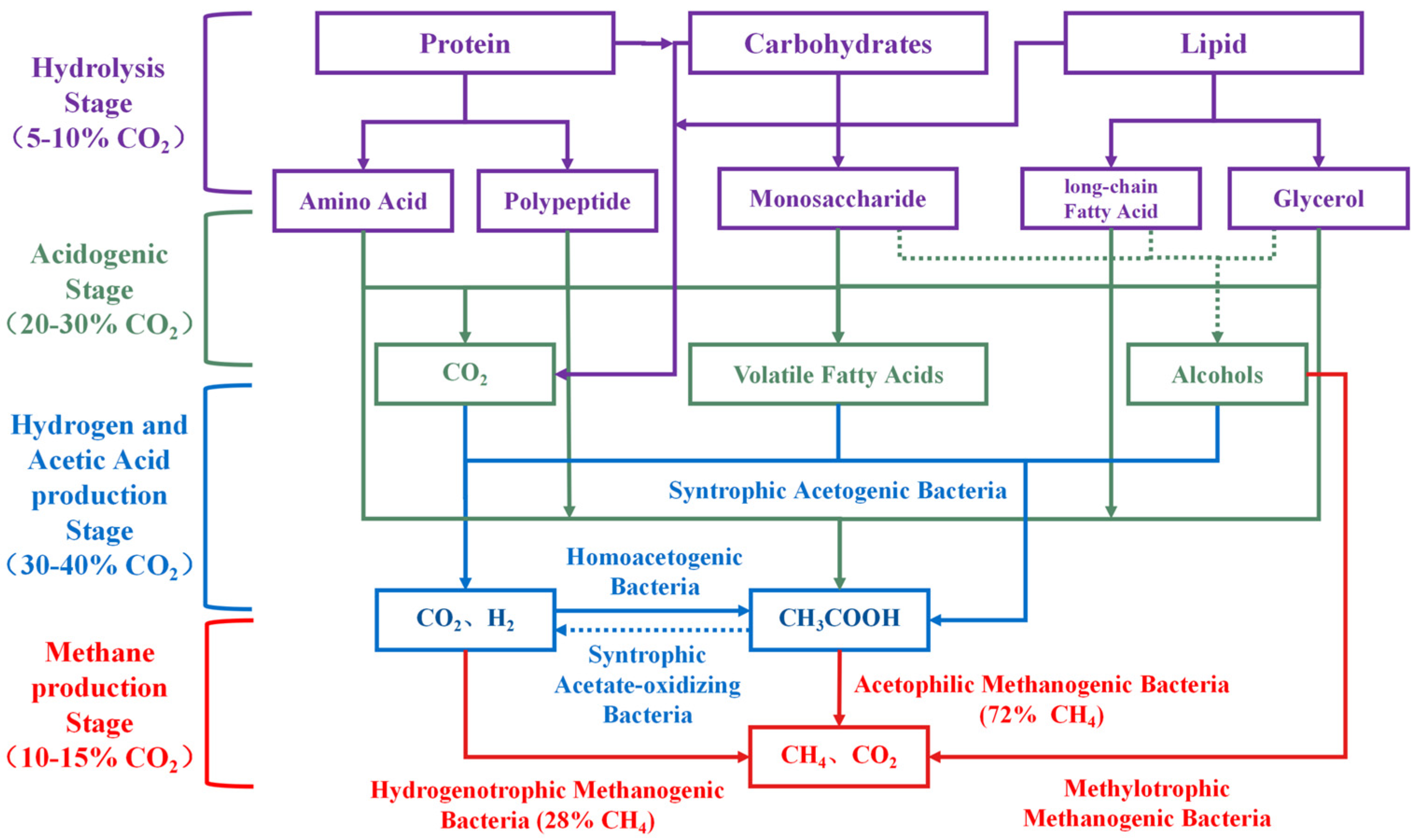
The AD process is a complex microbial metabolic process comprising four main stages: hydrolysis, acidification, acetic acid production, and CH4 generation [12]. Each stage is carried out by distinct microbial communities with specialized functions. The metabolic pathways of complex organic matter during AD are illustrated in Figure 1.
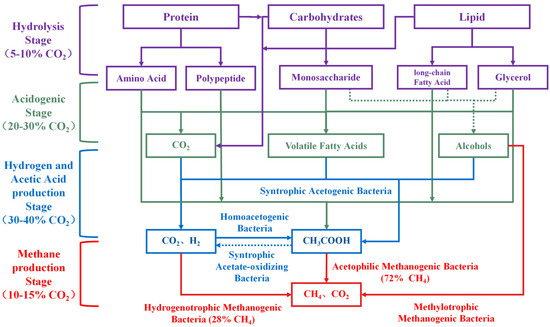
Figure 1.
The mechanism of CH4 and CO2 generation in AD process.
Hydrolysis is the initial stage of AD, during which complex large organic compounds (carbohydrates, proteins, lipids, etc.) are hydrolyzed into soluble organic small molecules by hydrolysis bacteria and enzymes. These smaller molecules can permeate cell membranes and be utilized by microorganisms in subsequent reactions [13]. The hydrolysis rate depends on the composition of the raw material as well as the activity of microorganisms in the AD system. CO2 produced during hydrolysis accounts for approximately 5–10% of total CO2, primarily from the partial oxidation of organic matter and microbial metabolic activities. During this process, chemical bonds between carbohydrates and lipids are broken, with some undergoing oxidation reactions, generating CO2 and other small-molecule intermediates.
Acidification is the second stage of AD, where acidogenic bacteria metabolize soluble small molecules (glucose, amino acids, long-chain fatty acids, glycerol, etc.) produced during hydrolysis to generate short-chain volatile fatty acids (VFAs) (such as acetic acid, propionic acid, and butyric acid), ethanol, hydrogen gas (H2), and CO2 as intermediate products [14]. The CO2 produced during this stage is significantly higher than in hydrolysis, accounting for approximately 20–30% of the total CO2. Its sources mainly include sugar fermentation metabolism, amino acid decarboxylation, lipid decomposition, and carbonate decomposition reactions. Monosaccharides (such as glucose and fructose) undergo fermentation by bacteria, producing VFAs, H2, and CO2 through pathways such as acetic acid, butyric acid, and mixed acid fermentation. The fermentation of amino acids also releases CO2 through decarboxylation and deamination reactions. Lipids are broken down into fatty acids and glycerol, with glycerol further fermenting to generate VFAs and CO2. The metabolism of fermentation bacteria during acidification alters environmental pH, particularly under VFAs accumulation, resulting in lower pH and increased acidity. These conditions promote the decomposition of carbonates and bicarbonates, releasing additional CO2.
The hydrogen and acetic acid production stage is the third stage of AD. The VFAs (such as propionic acid and butyric acid) and ethanol produced in the acidification stage are converted into acetic acid, H2, and CO2 by specific acetic-acid-producing bacteria [15]. Additionally, homologous acetogenic bacteria can reduce CO2 and H2 to produce acetic acid. The acetic acid production stage is an important step in the generation of CO2, accounting for approximately 30–40% of the total CO2, mainly from the oxidation reactions of intermediate products such as propionic acid, butyric acid, and ethanol. These reactions supply acetic acid, a key substrate for methane production, while generating significant amounts of H2 and CO2.
The CH4 production stage is the fourth stage of AD, in which substrates such as acetic acid, hydrogen, methanol, and methylamine are utilized by methanogenic archaea and converted into CH4 and CO2. Methanogenic archaea have multiple metabolic pathways involved in CH4 production. The first type is the acetic acid pathway, where methanogens utilize acetic acid or acetate salts to generate CH4 and CO2. The final ratio of CH4 to CO2 in this pathway is 1:1, and CH4 production accounts for approximately 72% of the total CH4 production. The second type is the hydrogenotrophic pathway, where methanogens utilize hydrogen and CO2 to produce CH4. The final ratio of CH4 to CO2 in this pathway is 1:0, and CH4 production accounts for approximately 28% of the total CH4 production. The third is the methylotrophic pathway, where methylated compounds (e.g., methanol, methylamine) are metabolized to produce CH4 and CO2, yielding a CH4-to-CO2 ratio of 3:1. CO2 production in this stage arises mainly from the decomposition of acetic acid and the metabolism of methylated compounds. Although the hydrogenotrophic pathway consumes CO2, its efficiency depends on hydrogen availability and microbial activity. CO2 generated during the CH4 production stage accounts for 10–15% of the total CO2.
Theoretically, when 72% of CH4 is produced via the acetic acid pathway and 28% via the hydrogenotrophic pathway, the final CH4-to-CO2 ratio is 25:11, and the CH4 content in biogas is 69.44%. Considering that the hydrolysis stage, acidification stage, and acetic acid production stage will also produce a large amount of CO2, and the CO2 produced by anaerobic microbial respiration and metabolism activities will also contribute to the total CO2 production, the CH4 content in biogas in actual production is much lower than the theoretical value. Therefore, we can clearly define “CH4-rich biogas”. When the CH4 content in biogas is 70% or higher, it can be called “CH4-rich biogas”.
3. Factors Affecting CH4 Content in Biogas During AD Process
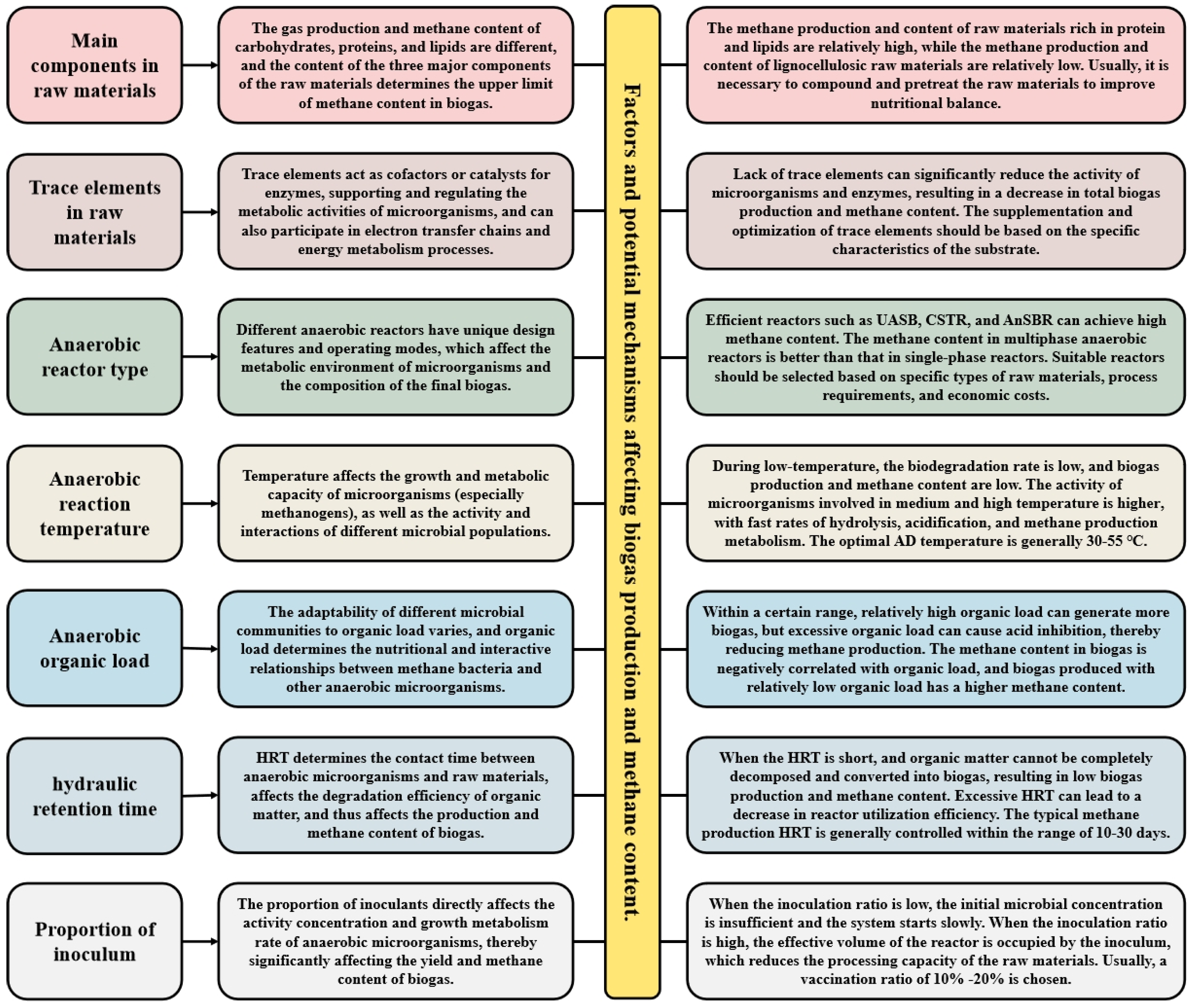
The production and composition of biogas during AD are influenced by multiple interrelated factors, ranging from the properties of the raw materials to specific process parameters. This section explores these critical factors, including the chemical composition of substrates, the roles of trace nutrients, and key process variables such as temperature, reactor type, and OLR (Figure 2). Understanding these elements is essential for optimizing CH4 yield and achieving high CH4 content in biogas. The discussion will provide a detailed analysis of how each factor affects microbial activity, metabolic pathways, and the resultant gas composition, offering insights into the strategies for enhancing the efficiency of biogas production.
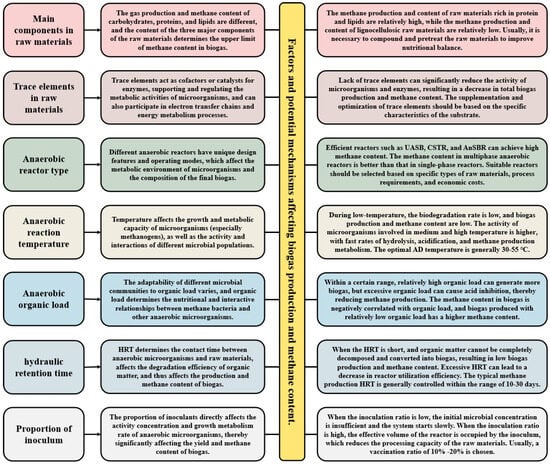
Figure 2.
The factors affecting CH4 content in biogas during AD process.
3.1. The Impact of Raw Material Properties on the Composition of Biogas
3.1.1. Effects of Large Number of Nutritional Components on Gas Production
The chemical composition, surface structure, biodegradability, and nutrient elements in raw materials significantly influence the metabolic pathways and reaction rates of AD. Organic solid waste is primarily composed of organic components such as carbohydrates, proteins, fats, and lignin [16]. The content of carbohydrates (soluble sugars, cellulose, hemicellulose), proteins, and lipids can affect the activity and metabolic processes of microorganisms, thereby affecting CH4 content in biogas [17]. Carbohydrates are easily degraded into VFAs such as acetic, propionic, and butyric acid, and then converted into CH4 and CO2 during AD. While their fast degradation boosts short-term biogas production, excessive breakdown can cause acid accumulation, inhibiting methanogenic activity and reducing CH4 content. Theoretically, the CH4 content in the biogas produced by AD of carbohydrates is 50%. However, biogas from carbohydrate-rich materials like fruit waste and molasses contains 50–60% CH4 in actual production. Proteins, composed of amino acids, degrade into ammonia and VFAs, leading to CH4 and CO2 production. Moderate protein can help increase CH4 content, as ammonia can buffer acidity and stabilize anaerobic environments. However, excessive nitrogen content can lead to ammonia accumulation, reducing CH4 content. Biogas from protein-rich materials, such as manure or slaughterhouse waste, typically contains 55–65% CH4. Lipids are long-chain fatty acids with high carbon and hydrogen content. Their intermediate products are converted directly into acetic acid during AD, enabling rapid CH4 generation. For raw materials with high lipid content, such as kitchen oil and waste vegetable oil, the CH4 content is generally between 60% and 70%. However, the slow degradation of lipids and potential accumulation of long-chain fatty acids at high concentrations can inhibit microbial activity, posing challenges to the AD process. The properties of raw materials have a decisive impact on the CH4 content in biogas during AD. The composition of raw materials establishes the potential biogas yield and the maximum achievable CH4 content, while their structure influences the AD process stability [18]. To optimize the AD of organic solid waste, raw materials are typically pretreated and combined to enhance nutrient balance, improve biodegradability, and increase biogas production and CH4 content.
3.1.2. Effects of Trace Nutrient Components on Gas Production
The AD system requires multiple nutrients to participate in the coordinated development of different microbial populations. These include macronutrients such as carbon (C), nitrogen (N), and phosphorus (P), as well as trace elements like iron (Fe), nickel (Ni), cobalt (Co), and molybdenum (Mo) [19]. Trace elements function as cofactors or catalysts for enzymes, regulating microbial metabolic activities and influencing biogas production and CH4 content. They bind to key enzymes to maintain activity and enhance reaction rates. For example, iron is a component of hydrogenases, cobalt serves as a cofactor for methyl-coenzyme M reductase, molybdenum acts as the active center for nitrate reductase and formate dehydrogenase, and nickel is a core component of CH4 synthase. Meanwhile, trace elements participate in electron transfer chains and energy metabolism processes, promoting microbial energy generation and metabolic activity [20]. For instance, molybdenum facilitates the reduction of hydrogen sulfide and the oxidation of hydrogen and formic acid, enhancing hydrogen utilization and improving CH4 production efficiency. Zinc supports protein synthesis and microbial metabolism. Iron ions can combine with hydrogen sulfide to form insoluble iron sulfide, reducing the toxic effects of hydrogen sulfide on microorganisms. Trace elements are essential for the growth and metabolism of anaerobic microorganisms. Adequate amounts of trace elements can promote the activity of fermentation and methanogenic bacteria and increase biogas production. However, excessive concentrations may have toxic effects, inhibiting microbial metabolism [21]. In practical applications, the composition of trace elements in raw materials differs, and deficiencies of elements such as Fe, Ni, and Co are common in AD systems. Therefore, supplementation and optimization of trace elements should be tailored to the material’s specific characteristics. For raw materials with low trace element content, targeted additions of elements such as nickel, cobalt, and iron can optimize biogas production and CH4 content.
3.2. The Impact of Anaerobic Reaction Process on Biogas Composition
Different AD reaction processes can significantly affect the CH4 content in biogas, mainly because process conditions (such as reactor type, reaction temperature, organic load, reaction time, inoculation ratio, etc.) can affect microbial activity, degradation efficiency, and the generation of metabolites (Table 1).

Table 1.
The influence of reaction parameters in anaerobic digestion process.
3.2.1. Effects of Anaerobic Reactor Types
Selecting an appropriate reactor type is essential for increasing biogas production and optimizing CH4 content in AD processes. Different reactor designs and operating modes influence the metabolic environment of microorganisms and the composition of the final biogas [22]. Dry AD reactors are suitable for processing raw materials with high solid content (such as crop straw and forestry waste), typically ranging from 20% to 40%. These reactors feature long material residence times, high OLR, and minimal land usage [23]. However, dry reactors are difficult to stir and mix, often leading to material stratification and local acidification, with CH4 content generally ranging between 50% and 60% [24]. Continuously stirred tank reactor (CSTR) is a widely used AD system. It maintains the uniformity of the mixture through continuous stirring, ensuring thorough contact between materials and microorganisms [25]. This system provides consistent reactions, stable gas production, and CH4 content of 55–65%, making it suitable for various organic wastes. Up-flow anaerobic sludge beds (UASBs) can provide high sludge concentration and long residence time, promoting good contact between microorganisms and organic matter, and are suitable for treating high-concentration organic wastewater [26]. By using granular sludge as a microbial carrier, wastewater flows from bottom to top, which can significantly improve microbial activity and organic matter degradation efficiency, resulting in CH4 content of 60–70%. Anaerobic sequencing batch reactor (AnSBR) is an intermittent reactor that operates in multiple stages: feed, reaction, precipitation, discharge, and idle. This periodic process optimizes organic matter degradation and CH4 production by precisely controlling reaction conditions [27]. By precisely controlling the reaction conditions in stages, the AnSBR reactor can achieve high CH4 generation efficiency, with CH4 content typically ranging from 60% to 70%. Fixed membrane reactors contain carriers or fillers that help prevent sludge loss, support microbial growth, and improve system stability. Fixed membrane reactors can maintain high microbial concentrations and efficient CH4 generation, with CH4 content typically ranging from 65% to 75%. Overall, reactor types influence CH4 content by shaping the microbial growth environment and reaction conditions. Efficient systems like UASB, CSTR, and AnSBR yield higher CH4 content, whereas dry reactors generally produce lower CH4 levels. Selecting a suitable reactor based on specific raw material types, process requirements, and economic considerations can optimize the CH4 composition of biogas.
3.2.2. Effects of AD Temperature
The effect of AD temperature on CH4 content in biogas is a key factor, as temperature affects the metabolic rate of microorganisms and affects the activity and interactions of different microbial populations [28]. Specifically, temperature impacts the metabolic capacity of methanogenic bacteria by modulating enzyme activity in anaerobic microorganisms. The optimal temperature range for the growth and reproduction of methanogenic bacteria is 15–55 °C. AD can be divided into low temperature (20–30 °C), medium temperature (30–40 °C), and high temperature (50–60 °C) based on temperature classification. Under low-temperature conditions, methanogenic bacteria exhibit slow metabolic rates. Organic matter degradation is limited, and intermediates such as organic acids are not effectively converted into CH4 [29]. Consequently, CH4 content in biogas remains low, typically around 50–60%. In contrast, medium-temperature conditions enhance microbial activity, particularly accelerating the metabolism of mesophilic methanogens, leading to CH4 content of 70–75% in biogas [30]. High-temperature conditions improve mass and heat transfer, increasing the degradation and reaction rates of organic matter. Rapid hydrolysis and acidification provide ample substrates for CH4 production. Thermophilic methanogens exhibit robust metabolic activity, efficiently degrading intermediates and reducing digestion time. High-temperature AD typically produces biogas with CH4 content of 70–75%. Taking corn stover as an example, the CH4 content of biogas is about 55–60% during low-temperature AD at 30 °C, about 65–70% during medium-temperature AD at 35 °C, and about 70–75% during high-temperature AD at 55 °C. In practical applications, selecting and controlling the temperature of AD is the key to increasing the CH4 content in biogas. The temperature should be optimized based on the type of raw material being processed and the microbial community to achieve optimal biogas yield and CH4 concentration, thereby improving energy recovery efficiency.
3.2.3. Effects of Anaerobic Organic Load
Organic loading rate (OLR) refers to the mass of organic matter added per unit volume of the reactor, typically expressed as total solids (TS) or chemical oxygen demand (COD). OLR significantly affects biogas production and CH4 content in AD processes [31]. Within an optimal range, a higher organic load can increase biogas production. However, excessive organic load leads to acid inhibition, reducing CH4 production [32]. CH4 content in biogas is inversely related to organic load. Lower organic loads generally result in higher CH4 concentrations. Under low organic load (0–2 kg·TS/m3·d), the AD system operates stably, maintaining an appropriate pH range without acid accumulation. Microorganisms efficiently decompose organic matter, producing stable intermediates that are smoothly converted into CH4 [33]. Due to the lower organic input, CH4 content is relatively high, typically around 65–75%. At moderate organic loads (2–5 kg·TS/m3·d), the CH4 generation rate increases alongside microbial activity, with CH4 content ranging from 60% to 70%. In contrast, high organic loads (>5 kg·TS/m3·d) significantly reduce CH4 content, often dropping to 50–60% or lower in extreme cases. Excessive organic input causes the acid production rate to surpass CH4 generation. This imbalance results in the accumulation of VFAs, reduced pH levels, and suppression of methanogenic bacteria, ultimately decreasing CH4 production. In severe cases, it can cause the fermentation system to collapse and result in fermentation failure [34]. Taking kitchen waste as an example, the CH4 content of biogas produced under low organic load (1 kg·TS/m3·d) is about 65–70%; under moderate organic load (3 kg·TS/m3·d), it is about 60–65%; and under high organic load (5 kg·TS/m3·d), it is about 50–60% [35]. To balance biogas production and CH4 content, OLR must be carefully monitored and optimized. Dynamic adjustments based on raw material properties, reactor performance, and gas composition are essential to maintain process stability and efficiency.
3.2.4. Effects of Hydraulic Retention Time
In AD, hydraulic retention time (HRT) refers to the average duration of raw materials remaining in the reactor. HRT directly influences the degradation efficiency of anaerobic microorganisms on organic matter, thereby affecting biogas production and CH4 content [36]. Under shorter HRT conditions (HRT < 10 days), raw materials do not reside in the reactor long enough for microorganisms to fully decompose organic matter and convert it into biogas, reducing biogas production. The rapid loss and incomplete degradation of organic matter leads to the production of more CO2, resulting in a decrease in CH4 content in biogas, generally between 50% and 60%. At moderate HRT (10–20 days), microorganisms have sufficient time to decompose organic matter, and VFAs are efficiently converted into CH4. The biogas production and CH4 content are both at optimal levels, with CH4 content typically ranging from 60% to 70%. Further extending the HRT (HRT > 20 days) can enhance organic matter degradation, and biogas production gains diminish and eventually stabilize [37]. Most organic matter is decomposed, and methanogens effectively convert metabolites in the digestate to CH4, with CH4 content stabilizing at 65–70% or higher. However, excessively long HRT may reduce reactor utilization efficiency, increase operating costs, and lead to nutrient depletion, gradually decreasing microbial activity. For example, in sludge digestion, short HRT (10 days) results in incomplete organic matter decomposition and CH4 content of approximately 50–55%. At moderate HRT (20 days), CH4 content rises to 60–65%. At long HRT (30 days), organic matter is fully decomposed, methanogenic activity peaks, and CH4 content reaches 65–70%. Different raw materials and processes have varying HRT requirements. Lignocellulose-rich materials (such as crop straw, garden waste, etc.) require longer HRT, while easily degradable materials (such as kitchen waste, sugary wastewater, etc.) can utilize shorter HRT [38]. CSTR typically requires longer HRT to ensure efficient degradation, while UASB can operate at shorter HRT. The typical HRT for CH4 production ranges from 10 to 30 days. Moderate HRT can balance gas production efficiency, system stability, and cost-effectiveness.
3.2.5. Effects of the Proportion of Inoculum
In AD, the proportion of inoculum refers to the proportion of anaerobic activated sludge or other microbial communities added relative to the mass of the raw material. This ratio directly impacts the activity, growth, and metabolism of anaerobic microorganisms, significantly influencing biogas yield and CH4 content [39]. At low inoculum ratios (<10%), the initial microbial concentration is insufficient, leading to slow system start-up, difficulty in establishing a dominant microbial community, and low methanogenic activity. Consequently, biogas production is delayed, with CH4 content typically between 50% and 60% [40]. Insufficient inoculum also results in poor system stability, making it more vulnerable to environmental fluctuations such as pH and temperature changes. A moderate inoculation ratio (10–30%) can provide sufficient active microorganisms to enable rapid system start-up. Methanogens efficiently convert intermediate metabolites (such as VFAs and hydrogen) into CH4. This improves AD efficiency, significantly increases biogas production, and stabilizes CH4 content at 60–70%. High inoculum ratios (>30%) introduce larger quantities of active microorganisms, accelerating organic matter degradation. As a result, biogas production stabilizes more quickly, while high methanogenic activity reduces CO2 content and increases CH4 content. However, further increasing the inoculum ratio offers limited benefits. Excess inoculum occupies reactor volume, reducing raw material processing capacity and significantly increasing operating costs. For example, studies on sludge AD have shown that increasing the inoculum ratio from 5% to 20% raises CH4 content from 55% to 68%, with significant biogas production improvements. When further increased to 30%, the change in CH4 content tends to stabilize. Similarly, in kitchen waste AD, an inoculum ratio of 15% enables rapid system stabilization, with CH4 content reaching 65%. Conversely, at inoculum ratios below 5%, start-up time is prolonged, and CH4 content remains below 60%. In practical applications, an inoculum ratio of 10–20% is often selected to balance start-up speed, system stability, and economic feasibility. This range ensures rapid system start-up, higher CH4 content, and sustained efficient gas production.
4. Methods for Increasing CH4 Content in Biogas During AD Process
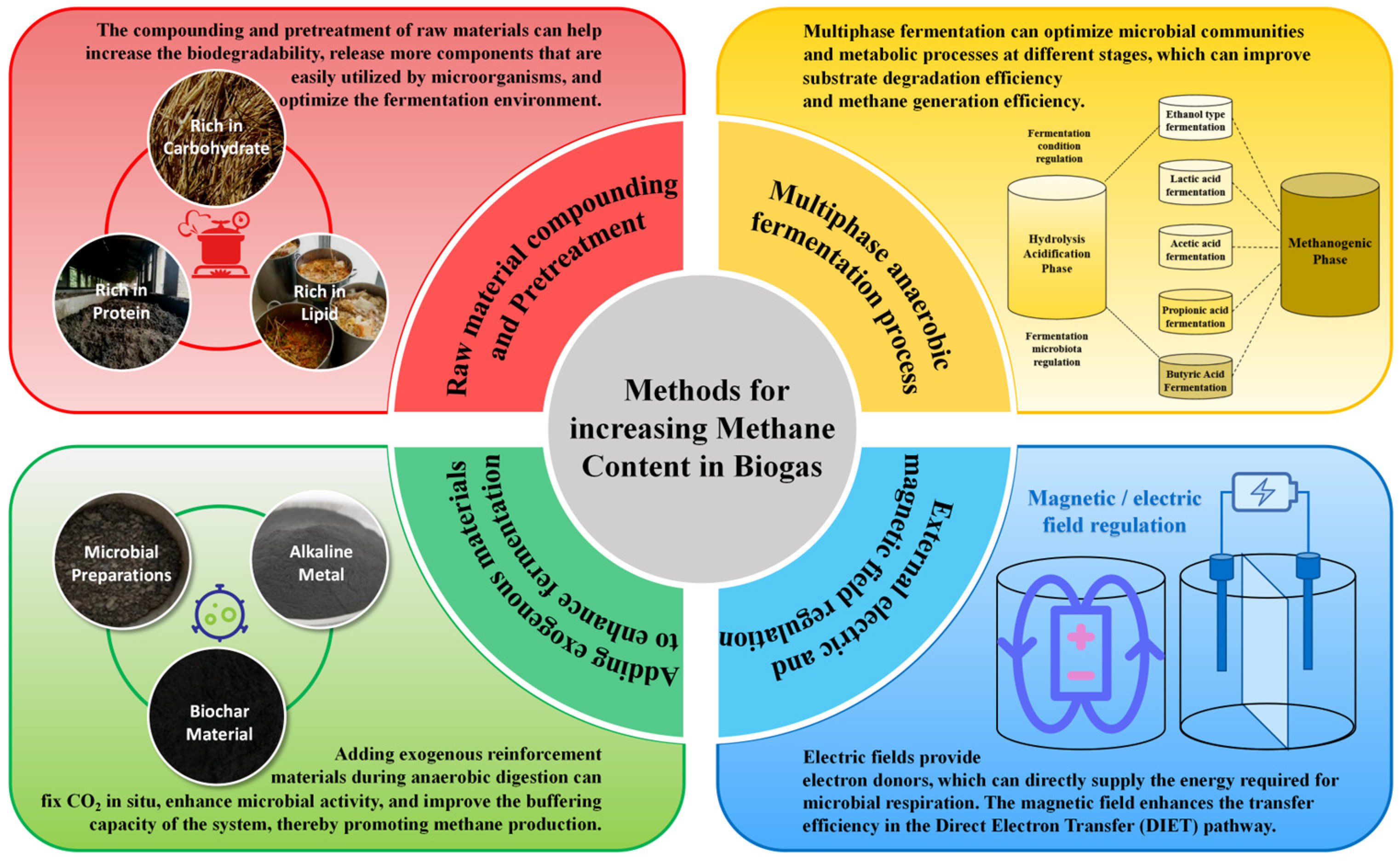
To increase methane content and improve the quality of biogas, various technological and methodological techniques have been developed. This section reviews methods for increasing CH4 production during the AD process, focusing on raw material pretreatment, co-digestion strategies, and process optimization techniques. It also reviews commonly used reinforcement measures, such as adding biochar, conductive materials, and microbial agents, as well as external regulatory methods like using electric and magnetic fields (Figure 3). By integrating these methods, biogas systems can achieve higher CH4 concentrations, better process stability, and reduced operational costs, thereby paving the way for their broader application in sustainable energy production and carbon neutrality efforts.
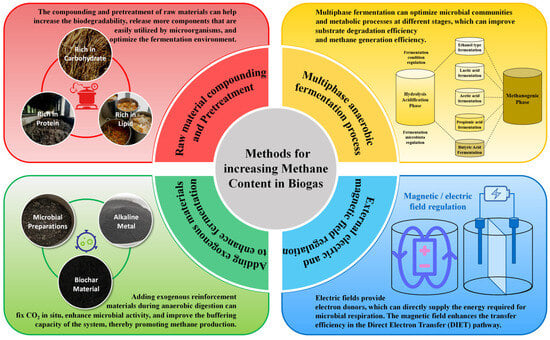
Figure 3.
The methods for increasing CH4 content in biogas during AD process.
4.1. Raw Material Mixing and Pretreatment for Quality Improvement
4.1.1. Mixing and Compounding of Multiple Raw Materials
Mixed AD, or co-digestion, involves processing two or more types of organic waste in the same reactor to enhance AD efficiency and increase CH4 content. By combining substrates with complementary characteristics, the carbon-to-nitrogen (C/N) ratio of the feedstock can be optimized, diverse nutritional elements can be provided, and the biodegradability of substrates can be improved [41]. Mixed AD can better optimize fermentation conditions and fully utilize the advantages of various raw materials, thereby achieving higher energy and resource utilization efficiency. A single substrate may lack certain essential elements for microorganisms or chemical components that contribute to degradation, thereby limiting the degradation of organic matter. Different substrates usually have complementary nutrients, and mixing substrates can provide more comprehensive nutrients, improve the efficiency of microorganisms in decomposing organic matter, and increase the potential for CH4 generation. Compared to single-substrate AD, co-digestion of diverse organic wastes (such as agricultural waste, kitchen waste, livestock manure, etc.) for AD can increase CH4 content. Co-digestion supplies richer fermentable organic matter, such as volatile fatty acids (VFAs), amino acids, and simple sugars, which promote CH4 production [42]. The ideal C/N ratio for AD is typically 20:1 to 30:1; deviations from this range can impair microbial activity and reduce CH4 production. Co-digestion helps adjust the C/N ratio to the optimal range, preventing acidification or ammonia inhibition. Protein-rich materials provide nitrogen, while carbohydrate-based materials supply carbon, balancing microbial metabolism and promoting CH4 production. For example, co-digestion of carbon-rich plant waste and nitrogen-rich livestock manure creates a balanced nutritional environment, stimulating microbial growth and metabolism [43]. Mixing multiple raw materials also supplements trace elements and buffering substances, enhancing methanogenic bacteria activity. Some substrates release acidic intermediates during fermentation, risking system acidification and microbial inhibition. Mixing with buffering materials neutralizes excess acids, stabilizing pH and protecting CH4 bacteria (Table 2). For instance, co-digestion lipids and cellulose combine high-energy VFAs from lipids with a high buffer of cellulose, achieving nutritional complementarity and prolonging fermentation time, which enhances CH4 production. Vats demonstrated that a 35:65 mixture of sugarcane bagasse and food waste yielded the highest biogas production and CH4 content [44].
4.1.2. Raw Material Pretreatment for Quality Improvement
Pretreatment enhances the biodegradability of organic matter, releases easily utilizable components for microorganisms, and optimizes the fermentation environment, thereby promoting bacterial activity [45]. The primary mechanism for improving CH4 content through raw material pretreatment is to release or utilize CO2 from hydrolysis and acidification processes outside the anaerobic reaction device in advance, providing more suitable substrates for CH4 production metabolism. Physical pretreatment alters the physical structure of raw materials, making it easier for microorganisms to decompose and degrade organic matter [46]. Common methods include ultrasound, electrolysis, and heat treatment, with the effectiveness largely dependent on process parameters. Chemical pretreatment utilizes chemical agents (acids, bases, organic solvents, etc.) to decompose the structure of organic matter and improve the bioavailability of substrates [47]. Using alkali (such as sodium hydroxide and calcium hydroxide) to treat cellulose-rich raw materials can decompose lignin structure, making cellulose and hemicellulose more easily degradable [48]. Acid treatments are commonly used for raw materials rich in proteins or lipids to hydrolyze large organic molecules. Biological pretreatment uses specific microorganisms or enzymatic techniques to decompose recalcitrant organic matter in advance, improving the bioavailability of substrates. Using biological enzymes such as cellulase and pectinase to hydrolyze cellulose, hemicellulose, and pectin components in organic waste can increase the content of soluble organic matter, improve microbial utilization efficiency, and promote CH4 generation [49]. Fungal pretreatment suits waste materials with high lignocellulosic content [50]. Specific white rot fungi or brown rot fungi are used to decompose lignin components, making cellulose easier to degrade and improving bioavailability. In practical applications, a combination of physical, chemical, and biological pretreatments is often employed based on the raw materials’ characteristics to maximize CH4 production and content [51]. For example, thermal-alkali pretreatment combines high-temperature steaming with alkali treatment to maximize lignocellulose breakdown and enhance organic matter solubility. Ultrasound-enzymatic pretreatment combines ultrasound, disrupting cell structures and releasing soluble substances, with enzymatic hydrolysis to further degrade cellulose, promoting hydrolysis and CH4 generation.

Table 2.
Effects of co-digestion and two-phase AD on methane content in biogas.
Table 2.
Effects of co-digestion and two-phase AD on methane content in biogas.
| Feedstock | Type of Enhance Method | Strengthening Conditions | Results of the Study | Methane Content | References |
|---|---|---|---|---|---|
| Cocoa waste, cow manure | Co-digestion | Investigated anaerobic digestion of cocoa waste in four different treatments: mono-digestion, addition of synthetic nutrients, co-digestion with sterile cow manure, and co-digestion with raw cow manure. | Cow manure has several long-term positive effects on cocoa waste digestion. Buffering provision is the key contribution of manure to co-digestion with cocoa. Compared with the anaerobic digestion of cocoa waste alone, the methane content of biogas co-digested with cow dung increased by 18%. | 53% (35%) | [52] |
| Giant reed, pig slurry | Co-digestion | The experiments were performed according to a completely randomized experimental design with 3 treatments (GR, KOHw, and KOH) and 2 recipes (mono-digestion or co-digestion with PS), with 3 replicates. | Co-digestion increased CH4 production 2.4 folds, referring to unit volume. Pre-treatment and co-digestion enhanced CH4 yield up 59% vs. untreated giant reed. Pretreatment and co-digestion increased the methane concentration in biogas. | 54% (48%) | [53] |
| Food waste, algae | Co-digestion | Established an optimal mixture ratio for efficient co-digestion of FW and AL by testing the biochemical methane potential. Furthermore, process stability in accordance with step-increased OLR is examined. | Anaerobic co-digestion of food waste and algae showed a higher CH4 production and process stability. A lower OLR of 0.8–1.7 kg VS/m3∙d attained higher CH4 production with enriched CH4, whereas a higher OLR of 2.5 kg VS/m3∙d resulted in lower CH4 production and H2 generation. | 56% (52%) | [54] |
| Food waste | Pretreatment | AFW with a total solids (TS) content of 200 g/L was used for pretreatment. The 100 g TS of saccharized AFW was then fermented by adding 1.9 g of yeast and constantly stirring at 27 °C for 44 h. | Microbial community acclimatization has been proposed to be effective for increasing the CH4/CO2 ratio in biogas, and increased methane content (to 65–76%) compared with unacclimated samples (26–73%). | 76% (26%) | [55] |
| Secondary sludge | Pretreatment | A stock ammonium chloride solution (NH4Cl, 3 M) was added to the pretreatment reactor. The FA pretreatment lasted for 24 h at room temperature (22 ± 1 °C); then, the pre-treated sludge was pumped into the experiment reactor. | Pretreatment of secondary sludge for 24 h at an FA concentration of 560 mg NH3-N/L improved VS destruction by 26.4%, supported by a similar increase of 28.6% in methane production. The higher CH4 content was due to the higher pH in the experimental system than that in the control system, leading to maintenance of a good buffer in the experimental system. | 67.5% (62.0%) | [56] |
| Areca catechu husk | Pretreatment | AH was pretreated for 24 h at two different temperatures (25 °C and 90 °C) with four different chemicals. AD experiments were conducted under mesophilic conditions maintained at 35 ± 2 °C. | Alkaline pretreatment of AH at 90 °C resulted in the maximum biogas yield of 683.89 mL/g. Methane content of biogas produced using AH pretreated with 2–10% of NaOH was found to be between 71.53% and 75.06%; methane content of biogas using raw AH was 62.31%. | 75.06% (62.31%) | [57] |
| Swine manure | Pretreatment | Analyzed the influence of thermo-alkaline pretreatment (3% NaOH at 121 degrees C for 30 min) and the substrate/inoculum ratio (SI) on AD at 10 and 15% total solids. The experiments were conducted in batch mode at 37 degrees C with orbital shaking at 150 rpm for 90 days. | SM pretreatment increased cumulative methane yield from 30 to 205 mL/gVS. Increasing the SI ratio from 1 to 3 gVSsubstrate/gVSinoculum enhanced this yield from 205 to 268 mL/gVS. The methane content in biogas produced by pretreated pig manure was significantly higher than that of raw pig manure. | 68% (43%) | [58] |
| Whiskey by-product mixture | Two-phase AD | The first system consisted of a single reactor. The second system consisted of two reactors in series; one served as the acidogenic reactor, and the other reactor served as the methanogenesis reactor. | Three digester configurations produced similar methane yield (269–283 L CH4.kg−1 VS). Two-phase systems can produce 11.2 mg VFA. g−1 ww of distillery by-products. Two-phase systems had higher methane content (75%) than the traditional system (54%). | 75% (54%) | [59] |
| Food waste, spent mushroom substance | Two-phase AD | Compared the co-digestion performance of an ethanologenic-methanogenic two-phase system and an acidogenic-methanogenic system using food waste and spent mushroom substance as substrates. | The ethanologenic-methanogenic system increased the abundance of enzyme-encoding genes and promoted the degradation of acetate and CO2/H2, thereby enhancing methanogenic metabolic pathways, compared to the acidogenic-methanogenic system. | 69% (62%) | [60] |
| Food waste | Two-phase AD, Pretreatment | Using a semi-continuous two-phase system (hydrolyzed acidified phase and methanogenic phase), the partial food waste used in this study was pretreated. The yeast activation solution was added to the substrate of food waste for 24 h of ethanol pre-fermentation. | The inoculation of yeast in the hydrolyzed acidified phase of ethanol-type fermentation groups increased the relative concentrations of Clostridium (syntrophic acetate oxidising bacteria) and Methanobacterium (hydrogenotrophic methanogens) in the methanogenic phase and ultimately enhanced the hydrogen and CO2 methanogenesis pathway. | 67.8% (57.3%) | [61] |
4.2. Multiphase Anaerobic Digestion Process
The multiphase AD process divides the traditional single-phase AD process into multiple stages, each optimized for specific microbial populations and metabolic processes under controlled conditions. This approach enhances substrate degradation efficiency, improves CH4 generation, and increases CH4 content in biogas.
4.2.1. Dual-Phase Anaerobic Digestion Process
The most common configuration for multiphase AD is the two-phase AD process [62]. The first phase involves hydrolysis and acidification, where hydrolytic and acidogenic bacteria degrade complex macromolecular organic compounds (such as cellulose, proteins, and lipids) into simpler soluble compounds such as VFAs, alcohols, and H2. The second phase is methanogenic, in which acetogenic and methanogenic bacteria convert VFAs and H2 into CH4 and CO2 under neutral or slightly alkaline pH. Methanogenic bacteria, being sensitive to pH and temperature fluctuations, are generally regulated in the hydrolysis and acidification phases. The acidification products serve as substrates for methanogenesis, directly influencing the CH4 and CO2 content in biogas [63]. Conditions such as temperature, pH, redox potential, and residence time in the acidification phase can be adjusted to optimize the production of specific VFAs for subsequent CH4 production. This regulation promotes the conversion of complex organic compounds into VFAs and shifts pathways like propionic acid fermentation toward alcohol or butyric acid fermentation, producing high-quality substrates for methanogenesis. The dual-phase AD process enhances organic matter degradation and CH4 generation by separating stages and optimizing process conditions. It mitigates issues like acidification and inhibition while enabling precise control over biogas composition.
4.2.2. Biogas Polygeneration Process
Biogas co-production technologies, such as ethanol–biogas co-production, hydrogen–biogas co-production, and lactic acid–biogas co-production, integrate multiple biological conversion pathways to achieve efficient resource utilization of organic waste. The essence of these multi-generation technologies lies in the full utilization of the carbon elements in waste, thereby significantly improving energy and product conversion efficiency. The ethanol–biogas co-production technology utilizes yeast to convert sugars in organic waste into ethanol, with fermentation residues and waste liquids subsequently entering the AD system to produce biogas. This process not only effectively utilizes carbohydrates to generate ethanol, but also converts residual organic matter into biogas, achieving multi-stage conversion and efficient carbon utilization [64]. The hydrogen–biogas co-production begins with the fermentation of organic matter into H2 and organic acids, followed by the conversion of these intermediates into CH4 in the AD system [65]. Biogas produced through this process typically contains over 70% CH4. This technology optimizes the hierarchical conversion pathway of organic matter, achieves synchronous production of hydrogen and CH4, and effectively improves the utilization efficiency of carbon elements [66]. If H2 generated during the hydrogen production stage is fed into the CH4 production phase, H2 can reduce CO2 to CH4, and the final CH4 content in the biogas can increase to 90%. Binghua Yan demonstrated that recycling acidification phase gas into the CH4 production phase during AD maximizes CH4 content [67]. This co-production technology is particularly suited for treating industrial wastewater and food processing wastewater to achieve efficient energy recovery. The lactic acid–biogas co-production involves fermenting carbohydrates in organic waste into lactic acid using lactic acid bacteria, with the fermentation by-products and waste liquids subsequently anaerobically digested to produce biogas. This technology is particularly effective for processing food and kitchen waste, providing raw materials for the bio-based chemical industry while recovering energy [68]. In summary, these co-production technologies optimize the graded bio-transformation pathway of organic waste, maximizing carbon utilization and achieving multiple outputs of energy and high-value-added chemicals. This biogas co-production model reduces carbon emissions during waste treatment and supports the sustainable economy.
4.3. Addition of Exogenous Strengthening Materials
Due to differences in digestion systems, influencing factors such as substrate characteristics, key anaerobic parameters, and inoculum properties are often different in AD. It is difficult to achieve a universal strengthening strategy by adjusting a single parameter. Adding external additives to the reactor is an effective and universal strategy to enhance AD efficiency. Common additives include biochar, alkaline metals, conductive materials, external gases, and biological agents (Table 3). They can effectively increase the methane content in biogas in various fermentation environments.

Table 3.
Effect of exogenous strengthening materials’ addition on methane content in biogas.
4.3.1. Addition of Biochar Materials
Biochar is a porous, carbon-rich material typically produced through pyrolysis or hydrothermal treatment of raw materials such as straw, livestock manure, and garden waste. The properties of biochar, including its surface functional group and porous structure, vary depending on the preparation methods and feedstocks used [79]. Biochar can increase biogas production and CH4 content during AD, and its mechanism of action involves multiple aspects. Biochar serves as a microbial carrier, providing a favorable environment for the attachment and growth of anaerobic bacterial communities, and stabilizing microbial community structure. Its porous structure and surface functional groups enable excellent adsorption capabilities, allowing it to remove inhibitory intermediates such as ammonia and VFAs, and help maintain neutral or slightly alkaline pH conditions through buffering. Biochar is rich in nutrients such as nitrogen, phosphorus, and potassium, as well as trace elements such as iron, calcium, and magnesium. It can provide important nutrients for microbial metabolism, activate the activity of key enzymes, and accelerate the degradation of organic matter and CH4 production [80]. Furthermore, biochar’s catalytic properties enhance the metabolism of hydrolytic and acidogenic bacteria, increasing the degradation of organic matter and the generation of CH4 precursors [81]. Its conductive groups facilitate DIET, further improving substrate conversion efficiency to CH4. Collectively, these mechanisms make biochar an effective additive for enhancing CH4 content in AD.
4.3.2. Addition of Alkaline Metal Materials
Alkaline metal materials can significantly enhance biogas production and CH4 content by fixing CO2 in situ, enhancing microbial activity, and improving buffering capacity. Existing methods for regulating biogas components require the addition of a new process module after AD, which first separates and purifies CO2 and CH4, then mixes them in a fixed ratio to achieve the regulation of the component content in biogas. However, there is growing recognition that simply capturing and storing CO2 does not address the root issue. Instead, reducing CO2 emissions in situ at the source is the most effective approach [82]. The mineral carbonation of alkaline metals can permanently convert CO2 into stable carbonate minerals, which is an important technical approach for CO2 sequestration. The in situ adsorption of CO2 by alkaline metals has been considered an effective technique for increasing CH4 content in biogas. Alkaline metal materials include natural metal minerals, industrial by-products (such as alkaline metal solid waste and ash), and artificially prepared metal salts. Alkaline metals are mainly composed of metal oxides and hydroxides, which contain a large amount of calcium, magnesium, phosphorus, hydroxide ions, etc. They react with the CO2 and H2S in biogas to form precipitates, thereby increasing CH4 content. They can combine with the CO2 and H2S in biogas to form precipitates, reducing the CO2 content in biogas. For example, adding 16.25 g/L of wollastonite during simulated sludge AD effectively fixed CO2 and significantly increased CH4 content. Alkaline metals contain various essential elements for microorganisms to maintain their life activities, enhancing the activity of microorganisms and metabolism-related enzymes. In addition, the addition of alkaline metals can regulate pH and buffering capacity, creating an optimal environment for methanogenic bacteria. Qing Wang demonstrated that adding 2.0 g/L of tourmaline increased cumulative CH4 production from corn stover and cow manure by 22.76%. Microbial diversity analysis revealed that low-dose tourmaline improved the microbial distribution, making methanogens the dominant archaea in the fermentation broth [83]. Compared to other additives, alkaline metals have the advantages of cost-effectiveness, wide sources, and stable properties, making them suitable for large-scale applications.
4.3.3. Addition of Conductive Materials
Conductive material fortifiers primarily include carbon-based materials (such as conductive carbon powder, graphite carbon, and carbon nanotubes), metal-based materials (such as zero-valent iron, iron oxide, nickel powder), and composite materials (such as metal carbon composite materials) [84]. These materials function as electronic mediators between hydrogen-producing bacteria and methane-producing bacteria, facilitating DIET. DIET improves electron exchange efficiency, mitigates the inhibitory effects of hydrogen accumulation, accelerates substrate conversion, and enhances CH4 production and content. Conductive materials have high catalytic activity and can promote substrate degradation during hydrolysis and acidification stages, accelerating the generation of CH4 precursors such as acetic acid and hydrogen. They also reduce energy loss during organic matter decomposition, optimize reaction kinetics, and improve CH4 generation efficiency. For instance, Tugui Yuan demonstrated that zero-valent iron significantly enhanced hydrogen and CH4 production during AD of food waste, improved bioenergy recovery efficiency, and stimulated the growth of syntrophic bacteria, Pseudomonas, and methanogenic archaea [85]. Similarly, Xiaohu Dai found that magnetite enhanced DIET, effectively resolving electron transfer limitations in high-solid sludge AD and increasing CH4 production [86]. The porous structure of metal-carbon composites provides an ideal attachment site for methanogens and other anaerobic bacteria and increases microbial density. The porous structure of metal-carbon composite materials provides a good attachment site for methanogens and other anaerobic bacteria, stabilizes microbial communities, and increases microbial density. These materials also regulate the microbial community composition in AD systems, giving methanogens a competitive advantage. Additionally, metal-based conductive materials, such as nano iron and nickel, release trace elements during reactions, which serve as enzyme cofactors, further promoting microbial metabolism and CH4 production. In summary, conductive materials enhance CH4 generation in AD through improved electron transfer, optimized microbial community structures, and the provision of essential nutrients [87].
4.3.4. Addition of New Materials
Carbon quantum dots (CQDs) and metal-organic frameworks (MOFs) have shown exceptional potential as novel reinforcement materials in AD. These materials improve CH4 production efficiency and biogas yield by promoting microbial metabolic activity, optimizing electron transfer pathways, and regulating the redox environment in anaerobic systems. CQDs are nanomaterials with excellent electron transfer properties. Their unique electrochemical characteristics and high conductivity facilitate microbial metabolism in AD. CQDs enhance DIET between microorganisms, particularly between methanogens and fermenting microbes [88]. The surface of CQDs can be enriched with electron donors such as hydrogen and acetic acid. By increasing these substances’ local concentrations, CQDs promote CH4-producing metabolic pathways. CQDs can regulate the microbial cell membrane potential, further improving the activity and tolerance of microorganisms. Acting as redox regulators, CQDs optimize the redox environment, adjust redox potential, and inhibit competitive metabolic pathways of non-CH4-producing microbial communities. MOFs are porous materials with high specific surface areas, adjustable structures, and strong catalytic activity, making them particularly advantageous for the AD process. Their high surface area and porosity enable effective adsorption of organic substrates, increasing substrate concentrations locally and facilitating faster utilization by fermentation microorganisms, thereby enhancing AD efficiency. MOFs also possess catalytic activity, serving as electron donors or catalysts to accelerate reduction reactions in AD [89]. The conductivity of MOFs allows them to function as electronic bridges in the DIET pathway, promoting electron transfer between fermentation microorganisms [90]. CQDs and MOFs exhibit synergistic effects in AD, significantly enhancing substrate utilization and CH4 production. CQDs provide rapid electron transfer pathways, while MOFs offer ample adsorption sites and catalytic activity. Overall, the application of nanomaterials represented by CQDs and MOFs in AD provides a new approach to enhancing CH4 production and content.
4.4. Adding Exogenous Gas or Biological Agent
4.4.1. External Gas Addition
In addition to adding fortifiers to the liquid phase of AD, introducing exogenous gases such as H2, CO2, and CO into the gas phase can effectively regulate the CH4 production pathway, enhancing both CH4 yield and purity. Adding H2 provides an additional hydrogen source for hydrogenotrophic methanogens, as H2 acts as an electron donor in methanogenic metabolism. Methanogenic archaea reduce CO2 to CH4 through the hydrogenotrophic pathway, increasing CH4 production [91]. This approach not only enhances CH4 yield but also lowers CO2 content in biogas, improving CH4 purity. In sludge AD, increasing reaction pressure via external hydrogen supply can improve the transfer rates of H2 and CO2. For instance, at 300 kPa, the average CH4 concentration in the digester exhaust gas reaches 92.9% [92]. Introducing CO2 supplies additional carbon sources for methanogenic bacteria, promoting their growth. Moderate CO2 supplementation can also buffer system acidity, preventing the inhibition of microbial activity due to low pH. Combining H2 and CO2 in controlled ratios further enhances CH4 production and biogas yield. Under carefully regulated conditions, small amounts of CO can serve as electron donors for CH4-producing bacteria to boost CH4 production. However, due to its potential toxicity to microorganisms, CO application requires caution. Maintaining optimal gas concentrations, temperature, and pH is crucial for preserving the activity of methanogens. To maximize the benefits, intermittent or continuous aeration methods are employed with controlled gas flow rates to minimize disturbance to the anaerobic microbial community. In summary, the strategic introduction of exogenous gases can significantly enhance the CH4 production and purity of biogas systems, while improving the economic and sustainable performance of AD.
4.4.2. Biological Agent Addition
Adding biological agents is an effective strategy to enhance AD performance, increasing both CH4 yield and content. Commonly used biological agents include microbial and enzymatic preparations. Microbial agents optimize the microbial community structure during AD by introducing specific strains or microbial communities [93]. Adding hydrolytic and acid-producing bacteria can accelerate the decomposition process of complex organic matter, rapidly converting it into CH4 precursor substances, providing more substrates for methanogenesis, and significantly reducing the digestion cycle [94]. Adding efficient CH4-producing microbial agents, such as methanogens acidophilus and methanogens hydrogenophilus, helps balance acid generation and CH4 conversion rates and enhances CH4 yield and purity in biogas [95]. The synergistic interactions between microorganisms are essential for gas production during AD. By introducing screened composite microbial agents and optimizing the ratio and activity of different microorganisms, the stability and CH4 production efficiency of the system can be further improved [96]. Compound microbial agents balance the activities of acidogenic and methanogenic bacteria, preventing excessive acid accumulation during the early stages of digestion and mitigating inhibition of CH4 production. Optimized microbial communities exhibit higher tolerance, substrate utilization, and gas production efficiency. In addition to microbial agents, hydrolytic enzymes are commonly used as anaerobic biological enhancers. Hydrolases directly act on substrates, which helps accelerate the hydrolysis process of substrates [97]. For instance, cellulase and xylanase break down cellulose and hemicellulose in plant waste into fermentable small molecules, improving substrate availability. Other enzymes facilitate volatile fatty acid (VFA) production and conversion, further boosting CH4 precursor formation and CH4 content. Enzymes also reduce the accumulation of inhibitory by-products, preventing adverse effects on methanogens. Methyl coenzyme M reductase can reduce the accumulation of inhibitory by-products and improve the overall CH4 conversion rate. Incorporating biological agents improves the performance of AD systems by increasing CH4 yield and content, shortening digestion cycles, and enhancing the system’s resilience to disturbances. The effectiveness of biologics is influenced by factors such as dosage, method of addition, and environmental conditions. Maintaining optimal temperature and pH ensures the activity of biological agents. Addition dosage should be tailored to substrate composition and organic load, as excessive amounts may disrupt the microbial balance or deactivate enzymes. Application methods include direct dosing and carrier immobilization, with the latter extending formulation stability.
4.5. Introduction of External Electric and Magnetic Field Regulation
4.5.1. Introduction of External Electric Field Regulation
The application of electrochemical technology in AD primarily involves microbial electrolysis cells (MEC) and electro-chemically anaerobic digestion (EAD) technology. MEC is an emerging approach that uses an external electric field to lower the cathode potential, promoting electron transfer and thereby accelerating CH4 production [98]. In MEC, electroactive microorganisms act as biocatalysts, integrating electrochemical stimulation with microbial metabolism. This enables the self-proliferation of microorganisms, stabilizes the AD system over extended periods, enhances CH4 production, and improves energy recovery efficiency [99]. Zhe Yu demonstrated that MECs significantly improved biodegradation compared to traditional AD, achieving a chemical oxygen demand (COD) removal of 40.33% [100]. EAD technology combines electrochemistry with AD systems, applying voltage or potential to electrodes to facilitate electron generation and transfer [101]. The anode oxidizes organic matter into CO2, protons, and electrons under an applied voltage. These electrons are transferred to the cathode through an external circuit, where electroactive methanogens utilize them to reduce CO2 to CH4. EAD also enriches specific electroactive microorganisms, which significantly proliferate under the action of an electric field, further boosting CH4 production [102]. External electric fields optimize redox conditions and enhance electron transfer pathways between microorganisms, accelerating CH4 production. The electric field promotes DIET, supplying electrons as donors for CH4-producing bacteria, which reduces CO2 and increases CH4 content. Moreover, electric field regulation maintains the reducibility of anaerobic systems while inhibiting the activity of other bacteria. In practical applications, using pulsed electric fields can intermittently stimulate microorganisms to reduce excessive stimulation. Additionally, selecting appropriate electrode materials and voltages can further optimize CH4 production.
4.5.2. Introduction of External Magnetic Field Regulation
Magnetic anaerobic digestion (AD) technology enhances microbial metabolic activity and electron transfer efficiency through the application of a magnetic field, leading to increased biogas production and CH4 content. Magnetic fields optimize the synergistic interactions among microorganisms and improve material transfer pathways within and between cells, making AD processes more efficient [103]. This approach stimulates the activity of enzymes involved in microbial metabolism, accelerates electron transfer, and promotes microbial community synergy, thereby enhancing CH4 production. External magnetic fields influence microbial cell membranes and metabolic processes through magnetic induction, boosting metabolic activity and enzymatic reaction efficiency. They also increase membrane permeability, facilitate substance exchange among microorganisms, and strengthen metabolic coupling. Like electric fields, magnetic fields enhance the DIET pathway, accelerating electron transfer and reducing CH4 generation time. Magnetic fields can be applied in either constant or alternating modes. Constant magnetic fields facilitate steady-state reactions, while alternating magnetic fields provide periodic stimulation to enhance short-term CH4 production. Reasonable control of magnetic field strength and frequency is necessary to avoid excessive stimulation and inhibition of microbial activity. With proper implementation, external magnetic fields significantly improve CH4 yield and content in AD systems.
The combined application of electric and magnetic enhancement technologies in AD can significantly boost CH4 production efficiency [104]. These two physical reinforcement methods operate synergistically through distinct mechanisms [105]. Electric fields stabilize the oxidation-reduction potential of the system, while magnetic fields enhance microbial synergistic metabolism. Electromagnetic coupling strengthens the metabolic interactions between microbial communities, thereby improving metabolic efficiency and increasing CH4 production rates. Moreover, this technology accelerates the decomposition and transformation of inhibitors, such as ammonia nitrogen and VFAs, reducing their inhibitory effects on the system. Overall, the integration of electric and magnetic enhancement technologies optimizes microbial activity, electron transfer efficiency, and the system environment in AD. This combined approach has significant value in enhancing CH4 production, stabilizing fermentation processes, and utilizing organic waste resources (Table 4).

Table 4.
Effect of exogenous regulation on methane content in biogas.
4.6. Comparison of Different Methanogenic Enhancement Technologies
Methane enhancement technologies in AD vary widely in mechanism, cost, and applicability (Table 5). While each offers unique advantages, their selection depends on specific operational goals, scale, and budget constraints. Co-digestion and raw material pretreatment are cost-effective and straightforward, making them ideal for diverse waste streams. Alkaline metal addition is particularly suited for scalable applications due to its economic feasibility, ease of integration, and reliable methane content improvements. Biochar and conductive materials enhance microbial stability and methane yield, though costs may vary depending on sourcing. Advanced materials, such as CQDs and MOFs, can significantly boost methane production but require higher investment and technical expertise, limiting their large-scale adoption. Similarly, external gas addition and electric or magnetic field regulation achieve high methane content but incur substantial operational and infrastructure costs, reducing their viability for widespread use. Although promising, many advanced technologies still require further research and development to address technical and economic challenges. This evaluation provides a comprehensive framework for selecting methane enhancement technologies, supporting the advancement of sustainable energy and resource recovery systems.

Table 5.
The comparison of different methanogenic enhancement technologies.
5. Conclusions and Prospect
This review indicates that the production of CH4-rich biogas offers significant advantages in optimizing energy utilization and serves as a critical pathway toward achieving carbon neutrality in the renewable energy sector. Methane content increase involves optimizing various stages of AD, including hydrolysis, acidification, acetic acid production, and CH4 production. Factors such as raw material properties, fermentation conditions, and process design profoundly influence the production and composition of CH4 and CO2 in biogas. By optimizing process flows, compounding multiple raw materials, and adjusting fermentation parameters, the CH4 content in biogas can be effectively increased. In addition, enhancing microbial metabolism by adding materials such as biochar and conductive materials, as well as electromagnetic regulation such as electric and magnetic fields, can significantly increase the CH4 content in biogas. The comprehensive application of these technologies reduces strengthening costs, improves energy utilization efficiency, and enhances the economic value and market competitiveness of biogas.
Future research should focus on developing more efficient and cost-effective methods for CH4-rich biogas production. Key innovation areas include optimizing raw material quality, developing novel reinforcement materials, and advancing microbial regulation strategies. Appropriate pretreatment technologies and co-digestion strategies can improve substrate degradability and nutrient balance, thereby enhancing biogas production and CH4 content. Concurrently, developing advanced reinforcement materials, such as conductive biochar and zero-valent iron nanoparticles, presents opportunities to facilitate microbial interactions and electron transfer within the AD system. Microbial community design can enhance functionality by increasing the abundance of microbes capable of DIET. Gene editing can create efficient methanogens capable of digesting specific substrates, boosting biogas production and CH4 content. Future developments are likely to emphasize the integration of emerging technologies. For instance, direct interspecific electron transfer and microbial metabolism optimization can be achieved simultaneously by combining biological regulation with electrochemical enhancement. Integrating real-time monitoring technologies and artificial intelligence (AI) will enable precise control of AD processes. Dynamic process control systems can regulate biogas output and adapt to operational fluctuations, ensuring optimal CH4 content. Furthermore, cooperation among academia, industry, and government can promote the consistency of policy frameworks and market incentives with technological progress, which is essential to promote the development and application of CH4-rich biogas production technology.
Author Contributions
Conceptualization, S.Q. and L.C.; methodology, S.X.; validation, S.X. and C.Z.; formal analysis, X.L.; investigation, Z.X.; data curation, J.Z.; writing—original draft preparation, S.X.; writing—review and editing, J.Z.; supervision, S.Q. and L.C.; project administration, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Shiqing Qian and Luming Chen were employed by the company Guangdong Guoneng Longyuan New Energy Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kamani, M.H.; Es, I.; Lorenzo, J.M.; Remize, F.; Roselló-Soto, E.; Barba, F.J.; Clark, J.; Khaneghah, A.M. Advances in plant materials, food by-products, and algae conversion into biofuels: Use of environmentally friendly technologies. Green Chem. 2019, 21, 3213–3231. [Google Scholar] [CrossRef]
- Roy, U.K.; Radu, T.; Wagner, J.L. Carbon-negative biomethane fuel production: Integrating anaerobic digestion with algae-assisted biogas purification and hydrothermal carbonisation of digestate. Biomass Bioenergy 2021, 148, 106029. [Google Scholar] [CrossRef]
- Qi, M.; Liu, Y.; He, T.B.; Yin, L.; Shu, C.M.; Moon, I. System perspective on cleaner technologies for renewable methane production and utilisation towards carbon neutrality: Principles, techno-economics, and carbon footprints. Fuel 2022, 327, 125130. [Google Scholar] [CrossRef]
- He, M.Y.; Sun, Y.H.; Han, B.X. Green Carbon Science: Efficient Carbon Resource Processing, Utilization, and Recycling towards Carbon Neutrality. Angew. Chem. 2022, 61, e202112835. [Google Scholar] [CrossRef]
- Chen, M.P.; Cui, Y.R.; Jiang, S.; Forsell, N. Toward carbon neutrality before 2060: Trajectory and technical mitigation potential of non-CO greenhouse gas emissions from Chinese agriculture. J. Clean. Prod. 2022, 368, 133186. [Google Scholar] [CrossRef]
- Huang, X.L.; Wang, S.; Shi, Z.L.; Fang, L.N.; Yin, C.B. Challenges and strategies for biogas production in the circular agricultural waste utilization model: A case study in rural China. Energy 2022, 241, 122889. [Google Scholar] [CrossRef]
- Fackler, N.; Heijstra, B.D.; Rasor, B.J.; Brown, H.; Martin, J.; Ni, Z.F.; Shebek, K.M.; Rosin, R.R.; Simpson, S.D.; Tyo, K.E.; et al. Stepping on the Gas to a Circular Economy: Accelerating Development of Carbon-Negative Chemical Production from Gas Fermentation. Annu. Rev. Chem. Biomol. 2021, 12, 439–470. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New approach for biogas purification using cryogenic separation and distillation process for CO2 capture. Energy 2018, 156, 328–351. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.; Madi, H.; Rüsch-Pfund, F.; Gandiglio, M.; Biollaz, S. Biogas composition from agricultural sources and organic fraction of municipal solid waste. Renew. Energy 2022, 181, 1000–1007. [Google Scholar] [CrossRef]
- Barzegaravval, H.; Hosseini, S.E.; Wahid, M.A.; Saat, A. Effects of fuel composition on the economic performance of biogas-based power generation systems. Appl. Therm. Eng. 2018, 128, 1543–1554. [Google Scholar] [CrossRef]
- Capa, A.; García, R.; Chen, D.; Rubiera, F.; Pevida, C.; Gil, M.V. On the effect of biogas composition on the H2 production by sorption enhanced steam reforming (SESR). Renew. Energy 2020, 160, 575–583. [Google Scholar] [CrossRef]
- Chorukova, E.; Simeonov, I. Mathematical modeling of the anaerobic digestion in two-stage system with production of hydrogen and methane including three intermediate products. Int. J. Hydrogen Energy 2020, 45, 11550–11558. [Google Scholar] [CrossRef]
- Zhang, J.X.; Loh, K.C.; Li, W.L.; Lim, J.W.; Dai, Y.J.; Tong, Y.W. Three-stage anaerobic digester for food waste. Appl. Energy 2017, 194, 287–295. [Google Scholar] [CrossRef]
- Ma, S.J.; Ma, H.J.; Hu, H.D.; Ren, H.Q. Effect of mixing intensity on hydrolysis and acidification of sewage sludge in two-stage anaerobic digestion: Characteristics of dissolved organic matter and the key microorganisms. Water Res. 2019, 148, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.F.; Zhao, L.; Li, C.X.; Angelidaki, I.; Lv, N.; Ning, J.; Cai, G.J.; Zhu, G.F. Deep insights into the network of acetate metabolism in anaerobic digestion: Focusing on syntrophic acetate oxidation and homoacetogenesis. Water Res. 2021, 190, 116774. [Google Scholar] [CrossRef]
- Xue, S.R.; Wang, Y.B.; Lyu, X.G.; Zhao, N.; Song, J.H.; Wang, X.J.; Yang, G.H. Interactive effects of carbohydrate, lipid, protein composition and carbon/nitrogen ratio on biogas production of different food wastes. Bioresour. Technol. 2020, 312, 123566. [Google Scholar] [CrossRef]
- Dandikas, V.; Heuwinkel, H.; Lichti, F.; Drewes, J.E.; Koch, K. Correlation between biogas yield and chemical composition of energy crops. Bioresour. Technol. 2014, 174, 316–320. [Google Scholar] [CrossRef]
- Herrmann, C.; Idler, C.; Heiermann, M. Biogas crops grown in energy crop rotations: Linking chemical composition and methane production characteristics. Bioresour. Technol. 2016, 206, 23–35. [Google Scholar] [CrossRef]
- Wintsche, B.; Glaser, K.; Sträuber, H.; Centler, F.; Liebetrau, J.; Harms, H.; Kleinsteuber, S. Trace Elements Induce Predominance among Methanogenic Activity in Anaerobic Digestion. Front. Microbiol. 2016, 7, 2034. [Google Scholar] [CrossRef]
- Wintsche, B.; Jehmlich, N.; Popp, D.; Harms, H.; Kleinsteuber, S. Metabolic Adaptation of Methanogens in Anaerobic Digesters Upon Trace Element Limitation. Front. Microbiol. 2018, 9, 405. [Google Scholar] [CrossRef]
- Bougrier, C.; Dognin, D.; Laroche, C.; Gonzalez, V.; Benali-Raclot, D.; Rivero, J.A.C. Anaerobic digestion of Brewery Spent Grains: Trace elements addition requirement. Bioresour. Technol. 2018, 247, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Kariyama, I.D.; Zhai, X.D.; Wu, B.X. Influence of mixing on anaerobic digestion efficiency in stirred tank digesters: A review. Water Res. 2018, 143, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef] [PubMed]
- Momayez, F.; Karimi, K.; Taherzadeh, M.J. Energy recovery from industrial crop wastes by dry anaerobic digestion: A review. Ind. Crops Prod. 2019, 129, 673–687. [Google Scholar] [CrossRef]
- Shen, R.X.; Jing, Y.; Feng, J.; Zhao, L.X.; Yao, Z.L.; Yu, J.D.; Chen, J.K.; Chen, R.L. Simultaneous carbon dioxide reduction and enhancement of methane production in biogas via anaerobic digestion of cornstalk in continuous stirred-tank reactors: The influences of biochar, environmental parameters, and microorganisms. Bioresour. Technol. 2021, 319, 124146. [Google Scholar] [CrossRef]
- Jia, H.; Yang, G.; Ngo, H.H.; Guo, W.S.; Zhang, H.W.; Gao, F.; Wang, J. Enhancing simultaneous response and amplification of biosensor in microbial fuel cell-based upflow anaerobic sludge bed reactor supplemented with zero-valent iron. Chem. Eng. J. 2017, 327, 1117–1127. [Google Scholar] [CrossRef]
- Zhao, B.S.; Liu, J.; Frear, C.; Holtzapple, M.; Chen, S.L. Consolidated bioprocessing of microalgal biomass to carboxylates by a mixed culture of cow rumen bacteria using anaerobic sequencing batch reactor (ASBR). Bioresour. Technol. 2016, 222, 517–522. [Google Scholar] [CrossRef]
- Madigou, C.; Lê Cao, K.A.; Bureau, C.; Mazéas, L.; Déjean, S.; Chapleur, O. Ecological consequences of abrupt temperature changes in anaerobic digesters. Chem. Eng. J. 2019, 361, 266–277. [Google Scholar] [CrossRef]
- Liu, D.D.; Zhang, L.; Chen, S.; Buisman, C.; ter Heijne, A. Bioelectrochemical enhancement of methane production in low temperature anaerobic digestion at 10 °C. Water Res. 2016, 99, 281–287. [Google Scholar] [CrossRef]
- Lin, R.C.; Cheng, J.; Ding, L.K.; Murphy, J.D. Improved efficiency of anaerobic digestion through direct interspecies electron transfer at mesophilic and thermophilic temperature ranges. Chem. Eng. J. 2018, 350, 681–691. [Google Scholar] [CrossRef]
- Zhan, Y.H.; Cao, X.X.; Xiao, Y.T.; Wei, X.Y.; Wu, S.; Zhu, J. Start-up of co-digestion of poultry litter and wheat straw in anaerobic sequencing batch reactor by gradually increasing organic loading rate: Methane production and microbial community analysis. Bioresour. Technol. 2022, 354, 127232. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.J.; Qiao, W.; Xiong, L.P.; Ricci, M.; Adani, F.; Dong, R.J. Effects of organic loading rate on anaerobic digestion of chicken manure under mesophilic and thermophilic conditions. Renew. Energy 2019, 139, 242–250. [Google Scholar] [CrossRef]
- Duan, N.; Zhang, D.J.; Lin, C.; Zhang, Y.F.; Zhao, L.Y.; Liu, H.B.; Liu, Z.D. Effect of organic loading rate on anaerobic digestion of pig manure: Methane production, mass flow, reactor scale and heating scenarios. J. Environ. Manag. 2019, 231, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yang, Z.H.; Zheng, Y.; Liu, J.B.; Xiong, W.P.; Zhang, Y.R.; Lu, Y.; Xue, W.J.; Fan, C.Z. Organic loading rate and hydraulic retention time shape distinct ecological networks of anaerobic digestion related microbiome. Bioresour. Technol. 2018, 262, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Tsui, T.H.; Wu, H.; Song, B.; Liu, S.S.; Bhardwaj, A.; Wong, J.W.C. Food waste leachate treatment using an Upflow Anaerobic Sludge Bed (UASB): Effect of conductive material dosage under low and high organic loads. Bioresour. Technol. 2020, 304, 122738. [Google Scholar] [CrossRef]
- Singh, B.; Szamosi, Z.; Siménfalvi, Z. Impact of mixing intensity and duration on biogas production in an anaerobic digester: A review. Crit. Rev. Biotechnol. 2020, 40, 508–521. [Google Scholar] [CrossRef]
- Tena, M.; Perez, M.; Solera, R. Effect of hydraulic retention time on the methanogenic step of a two-stage anaerobic digestion system from sewage sludge and wine vinasse: Microbial and kinetic evaluation. Fuel 2021, 296, 120674. [Google Scholar] [CrossRef]
- Fitamo, T.; Boldrin, A.; Boe, K.; Angelidaki, I.; Scheutz, C. Co-digestion of food and garden waste with mixed sludge from wastewater treatment in continuously stirred tank reactors. Bioresour. Technol. 2016, 206, 245–254. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.P.; Delgenès, J.P.; Escudié, R. Dry anaerobic digestion of food waste and cardboard at different substrate loads, solid contents and co-digestion proportions. Bioresour. Technol. 2017, 233, 166–175. [Google Scholar] [CrossRef]
- Haider, M.R.; Zeshan; Yousaf, S.; Malik, R.N.; Visvanathan, C. Effect of mixing ratio of food waste and rice husk co-digestion and substrate to inoculum ratio on biogas production. Bioresour. Technol. 2015, 190, 451–457. [Google Scholar] [CrossRef]
- Song, Y.J.; Meng, S.Y.; Chen, G.Y.; Yan, B.B.; Zhang, Y.X.; Tao, J.Y.; Li, Y.H.; Li, J.L. Co-digestion of garden waste, food waste, and tofu residue: Effects of mixing ratio on methane production and microbial community structure. J. Environ. Chem. Eng. 2021, 9, 105901. [Google Scholar] [CrossRef]
- Li, Y.M.; Cheng, H.; Guo, G.Z.; Zhang, T.; Qin, Y.; Li, Y.Y. High solid mono-digestion and co-digestion performance of food waste and sewage sludge by a thermophilic anaerobic membrane bioreactor. Bioresour. Technol. 2020, 310, 123433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.B.; Sun, F.R.; Yu, J.D.; Cai, Y.F.; Luo, X.S.; Cui, Z.J.; Hu, Y.G.; Wang, X.F. Co-digestion of oat straw and cow manure during anaerobic digestion: Stimulative and inhibitory effects on fermentation. Bioresour. Technol. 2018, 269, 143–152. [Google Scholar] [CrossRef]
- Vats, N.; Khan, A.A.; Ahmad, K. Observation of biogas production by sugarcane bagasse and food waste in different composition combinations. Energy 2019, 185, 1100–1105. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Trably, E.; Mehariya, S.; Bernet, N.; Wong, J.W.C.; Carrere, H. Pretreatment of food waste for methane and hydrogen recovery: A review. Bioresour. Technol. 2018, 249, 1025–1039. [Google Scholar] [CrossRef]
- El Gnaoui, Y.; Karouach, F.; Bakraoui, M.; Barz, M.; El Bari, H. Mesophilic anaerobic digestion of food waste: Effect of thermal pretreatment on improvement of anaerobic digestion process. Energy Rep. 2020, 6, 417–422. [Google Scholar] [CrossRef]
- Tulun, S.; Bilgin, M. Enhancement of anaerobic digestion of waste activated sludge by chemical pretreatment. Fuel 2019, 254, 115671. [Google Scholar] [CrossRef]
- Paritosh, K.; Yadav, M.; Kesharwani, N.; Pareek, N.; Karthyikeyan, O.P.; Balan, V.; Vivekanand, V. Strategies to improve solid state anaerobic bioconversion of lignocellulosic biomass: An overview. Bioresour. Technol. 2021, 331, 125036. [Google Scholar] [CrossRef]
- Fdez-Güelfo, L.A.; Alvarez-Gallego, C.; Márquez, D.S.; García, L.I.R. Biological pretreatment applied to industrial organic fraction of municipal solid wastes (OFMSW): Effect on anaerobic digestion. Chem. Eng. J. 2011, 172, 321–325. [Google Scholar] [CrossRef]
- Yadav, M.; Vivekanand, V. Combined fungal and bacterial pretreatment of wheat and pearl millet straw for biogas production—A study from batch to continuous stirred tank reactors. Bioresour. Technol. 2021, 321, 124523. [Google Scholar] [CrossRef]
- Wang, J.; Ma, D.M.; Lou, Y.; Ma, J.; Xing, D.F. Optimization of biogas production from straw wastes by different pretreatments: Progress, challenges, and prospects. Sci. Total Environ. 2023, 905, 166992. [Google Scholar] [CrossRef]
- Acosta, N.; Kang, I.D.; Rabaey, K.; De Vrieze, J. Cow manure stabilizes anaerobic digestion of cocoa waste. Waste Manag. 2021, 126, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Ceotto, E.; Galletti, S. Co-digestion of pig slurry and KOH pre-treated giant reed enhances methane yield and digestate characteristics. Environ. Technol. Innov. 2023, 31, 103204. [Google Scholar] [CrossRef]
- Khanthong, K.; Kadam, R.; Kim, T.; Park, J. Synergetic effects of anaerobic co-digestion of food waste and algae on biogas production. Bioresour. Technol. 2023, 382, 129208. [Google Scholar] [CrossRef]
- Sun, J.; Kosaki, Y.; Kawamura, K.; Watanabe, N. Operational load enhancement for an anaerobic membrane bioreactor through ethanol fermentation pretreatment of food waste. Energy Convers. Manag. 2021, 249, 114840. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Zhang, Z.H.; Nghiem, L.D.; Gao, L.; Wang, Q.L. Semi-continuous anaerobic digestion of secondary sludge with free ammonia pretreatment: Focusing on volatile solids destruction, dewaterability, pathogen removal and its implications. Water Res. 2021, 202, 117481. [Google Scholar] [CrossRef]
- Vannarath, A.; Thalla, A.K. Effects of chemical pretreatments on material solubilization of L. husk: Digestion, biodegradability, and kinetic studies for biogas yield. J. Environ. Manag. 2022, 316, 115322. [Google Scholar] [CrossRef]
- Silva, A.D.E.; dos Santos, A.L.M.; Malveira, I.C.C.; Girao, B.H.A.; dos Santos, A.B. Effect of thermo-alkaline pretreatment and substrate inoculum ratio on methane production from dry and semi-dry anaerobic digestion of swine manure. Renew. Energy 2024, 231, 121015. [Google Scholar] [CrossRef]
- Hackula, A.; Shinde, R.; Hickey, D.; O’Shea, R.; Murphy, J.D.; Wall, D.M. Two-phase anaerobic digestion for enhanced valorisation of whiskey distillery by-products. Bioresour. Technol. 2023, 383, 129239. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, X.X.; Sun, H.S.; Xie, D.; Zhao, P.; Wang, Q.H.; Wu, C.F.; Gao, M. Semi-continuous mesophilic-thermophilic two-phase anaerobic co-digestion of food waste and spent mushroom substance: Methanogenic performance, microbial, and metagenomic analysis. Bioresour. Technol. 2022, 360, 127518. [Google Scholar] [CrossRef]
- Ma, X.X.; Yu, M.; Yang, M.; Zhang, S.; Gao, M.; Wu, C.F.; Wang, Q.H. Effect of liquid digestate recirculation on the ethanol-type two-phase semicontinuous anaerobic digestion system of food waste. Bioresour. Technol. 2020, 313, 123534. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, H.P.; Hua, D.L.; Zhao, B.F.; Mu, H.; Jin, F.Q.; Meng, G.F.; Fang, X. Two-phase anaerobic digestion of lignocellulosic hydrolysate: Focusing on the acidification with different inoculum to substrate ratios and inoculum sources. Sci. Total Environ. 2020, 699, 134226. [Google Scholar] [CrossRef] [PubMed]
- Detman, A.; Bucha, M.; Treu, L.; Chojnacka, A.; Plesniak, L.; Salamon, A.; Lupikasza, E.; Gromadka, R.; Gawor, J.; Gromadka, A.; et al. Evaluation of acidogenesis products’ effect on biogas production performed with metagenomics and isotopic approaches. Biotechnol. Biofuels 2021, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.X.; Yu, M.; Song, N.; Xu, B.H.; Gao, M.; Wu, C.F.; Wang, Q.H. Effect of ethanol pre-fermentation on organic load rate and stability of semi-continuous anaerobic digestion of food waste. Bioresour. Technol. 2020, 299, 122587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Hu, Q.; Qu, Y.Y.; Dai, Y.J.; He, Y.L.; Wang, C.H.; Tong, Y.W. Integrating food waste sorting system with anaerobic digestion and gasification for hydrogen and methane co-production. Appl. Energy 2020, 257, 113988. [Google Scholar] [CrossRef]
- Silva, F.M.S.; Mahler, C.F.; Oliveira, L.B.; Bassin, J.P. Hydrogen and methane production in a two-stage anaerobic digestion system by co-digestion of food waste, sewage sludge and glycerol. Waste Manag. 2018, 76, 339–349. [Google Scholar] [CrossRef]
- Yan, B.H.; Selvam, A.; Wong, J.W.C. Bio-hydrogen and methane production from two-phase anaerobic digestion of food waste under the scheme of acidogenic off-gas reuse. Bioresour. Technol. 2020, 297, 122400. [Google Scholar] [CrossRef]
- Demichelis, F.; Pleissner, D.; Fiore, S.; Mariano, S.; Gutiérrez, I.M.N.; Schneider, R.; Venus, J. Investigation of food waste valorization through sequential lactic acid fermentative production and anaerobic digestion of fermentation residues. Bioresour. Technol. 2017, 241, 508–516. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Ye, X.M.; Chen, K.Q.; Xiao, Q.B.; Zhang, Z.Y.; Cao, C.H.; Han, T.; Kong, X.P.; Xi, Y.L. The new strategy of using humic acid loaded biochar to enhance the anaerobic digestion of cow manure for methane production. J. Clean. Prod. 2023, 428, 139353. [Google Scholar] [CrossRef]
- Qi, Q.X.; Sun, C.; Zhang, J.X.; He, Y.L.; Tong, Y.W. Internal enhancement mechanism of biochar with graphene structure in anaerobic digestion: The bioavailability of trace elements and potential direct interspecies electron transfer. Chem. Eng. J. 2021, 406, 126833. [Google Scholar] [CrossRef]
- Tian, W.J.; Li, J.H.; Zhu, L.R.; Li, W.; He, L.Y.; Gu, L.; Deng, R.; Shi, D.Z.; Chai, H.X.; Gao, M. Insights of enhancing methane production under high-solid anaerobic digestion of wheat straw by calcium peroxide pretreatment and zero valent iron addition. Renew. Energy 2021, 177, 1321–1332. [Google Scholar] [CrossRef]
- Cerrillo, M.; Burgos, L.; Ruiz, B.; Barrena, R.; Moral-Vico, J.; Font, X.; Sánchez, A.; Bonmatí, A. In-situ methane enrichment in continuous anaerobic digestion of pig slurry by zero-valent iron nanoparticles addition under mesophilic and thermophilic conditions. Renew. Energy 2021, 180, 372–382. [Google Scholar] [CrossRef]
- Charalambous, P.; Vyrides, I. In situ biogas upgrading and enhancement of anaerobic digestion of cheese whey by addition of scrap or powder zero-valent iron (ZVI). J. Environ. Manag. 2021, 280, 111651. [Google Scholar] [CrossRef]
- Yin, C.K.; Shen, Y.W.; Yu, Y.M.; Yuan, H.P.; Lou, Z.Y.; Zhu, N.W. In-situ biogas upgrading by a stepwise addition of ash additives: Methanogen adaption and CO2 sequestration. Bioresour. Technol. 2019, 282, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, Q.Q.; Gong, L.L.; Liu, H.; Cui, M.H.; Zhang, J. In-situ mineral CO2 sequestration in a methane producing microbial electrolysis cell treating sludge hydrolysate. J. Hazard. Mater. 2020, 394, 122519. [Google Scholar] [CrossRef]
- Yin, C.K.; Shen, Y.W.; Dai, X.H.; Zhu, N.W.; Yuan, H.P.; Lou, Z.Y.; Yuan, R.X. Integrated anaerobic digestion and CO2 sequestration for energy recovery from waste activated sludge by calcium addition: Timing matters. Energy 2020, 199, 117421. [Google Scholar] [CrossRef]
- Liu, L.J.A.; Yun, S.N.; Ke, T.; Wang, K.J.; An, J.H.; Liu, J.Y. Dual utilization of aloe peel: Aloe peel-derived carbon quantum dots enhanced anaerobic co-digestion of aloe peel. Waste Manag. 2023, 159, 163–173. [Google Scholar] [CrossRef]
- Hua, Y.; Dai, X.H.; Chen, S.X.; Dai, L.L. High Proton Conductivity of MOF-808 Promotes Methane Production in Anaerobic Digestion. ACS Sustain. Chem. Eng. 2022, 10, 1419–1429. [Google Scholar]
- Saif, I.; Thakur, N.; Zhang, P.; Zhang, L.H.; Xing, X.H.; Yue, J.W.; Song, Z.Z.; Nan, L.; Yujun, S.; Usman, M.; et al. Biochar assisted anaerobic digestion for biomethane production: Microbial symbiosis and electron transfer. J. Environ. Chem. Eng. 2022, 10, 107960. [Google Scholar] [CrossRef]
- Sugiarto, Y.; Sunyoto, N.M.S.; Zhu, M.M.; Jones, I.; Zhang, D.K. Effect of biochar in enhancing hydrogen production by mesophilic anaerobic digestion of food wastes: The role of minerals. Int. J. Hydrogen Energy 2021, 46, 3695–3703. [Google Scholar] [CrossRef]
- Chen, J.G.; Yun, S.N.; Shi, J.; Wang, Z.Q.; Abbas, Y.; Wang, K.J.; Han, F.; Jia, B.; Xu, H.F.; Xing, T.; et al. Role of biomass-derived carbon-based composite accelerants in enhanced anaerobic digestion: Focusing on biogas yield, fertilizer utilization, and density functional theory calculations. Bioresour. Technol. 2020, 307, 123204. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Y.; Yun, S.N.; Wang, K.J.; Shah, F.A.; Xing, T.; Li, B.J. Static-magnetic-field coupled with fly-ash accelerant: A powerful strategy to significantly enhance the mesophilic anaerobic-co-digestion. Bioresour. Technol. 2021, 327, 124793. [Google Scholar] [CrossRef]
- Wang, Q.; Sha, H.; Cao, S.X.; Zhao, B.; Wang, G.; Zheng, P.F. Tourmaline enhanced methane yield via regulating microbial metabolic balance during anaerobic co-digestion of corn stover and cow manure. Bioresour. Technol. 2022, 359, 127470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Li, Y.; Quan, X.; Zhang, Y.B. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017, 115, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.G.; Bian, S.W.; Ko, J.H.; Liu, J.G.; Shi, X.Y.; Xu, Q.Y. Exploring the roles of zero-valent iron in two-stage food waste anaerobic digestion. Waste Manag. 2020, 107, 91–100. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.L.; Dong, B.; Dai, X.H. Magnetite Triggering Enhanced Direct Interspecies Electron Transfer: A Scavenger for the Blockage of Electron Transfer in Anaerobic Digestion of High-Solids Sewage Sludge. Environ. Sci. Technol. 2018, 52, 7160–7169. [Google Scholar] [CrossRef]
- Hassanein, A.; Kumar, A.N.; Lansing, S. Impact of electro-conductive nanoparticles additives on anaerobic digestion performance-A review. Bioresour. Technol. 2021, 342, 126023. [Google Scholar] [CrossRef]
- Li, Y.Q.; Ma, C.J.; Ma, J.F.; Guo, W.Y.; Liu, Y.; Jing, Z.M.; Wang, Z.X.; Feng, L.; Zhang, W.Y.; Xu, Q. Promoting potential direct interspecies electron transfer (DIET) and methanogenesis with nitrogen and zinc doped carbon quantum dots. J. Hazard. Mater. 2021, 410, 124886. [Google Scholar] [CrossRef]
- Liu, H.Y.; Xu, Y.; Geng, H.; Chen, Y.D.; Dai, X.H. Contributions of MOF-808 to methane production from anaerobic digestion of waste activated sludge. Water Res. 2022, 220, 118653. [Google Scholar] [CrossRef]
- Wang, C.C.; Ye, X.M.; Liu, Y.; Jia, Z.Y.; Cao, C.H.; Xiao, Q.B.; Du, J.; Kong, X.P.; Wu, X.Y.; Chen, Z.B.; et al. Enhanced anaerobic digestion for degradation of swine wastewater through a Fe/Ni-MOF modified microbial electrolysis cell. J. Clean. Prod. 2022, 380, 134773. [Google Scholar] [CrossRef]
- Wahid, R.; Mulat, D.G.; Gaby, J.C.; Horn, S.J. Effects of H2:CO2 ratio and H2 supply fluctuation on methane content and microbial community composition during in-situ biological biogas upgrading. Biotechnol. Biofuels 2019, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Diaz, I.; Fdz-Polanco, F.; Mutsvene, B.; Fdz-Polanco, M. Effect of operating pressure on direct biomethane production from carbon dioxide and exogenous hydrogen in the anaerobic digestion of sewage sludge. Appl. Energy 2020, 280, 115915. [Google Scholar] [CrossRef]
- Jiang, J.F.; Li, L.H.; Li, Y.; He, Y.; Wang, C.R.; Sun, Y.M. Bioaugmentation to enhance anaerobic digestion of food waste: Dosage, frequency and economic analysis. Bioresour. Technol. 2020, 307, 123256. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Achinas, S.; Zhang, Z.H.; Krooneman, J.; Euverink, G.J.W. The biomethanation of cow manure in a continuous anaerobic digester can be boosted via a bioaugmentation culture containing. Sci. Total Environ. 2020, 745, 141042. [Google Scholar] [CrossRef]
- Li, Y.; Yang, G.X.; Li, L.H.; Sun, Y.M. Bioaugmentation for overloaded anaerobic digestion recovery with acid-tolerant methanogenic enrichment. Waste Manag. 2018, 79, 744–751. [Google Scholar] [CrossRef]
- Omar, B.; El-Gammal, M.; Abou-Shanab, R.; Fotidis, I.A.; Angelidaki, I.; Zhang, Y.F. Biogas upgrading and biochemical production from gas fermentation: Impact of microbial community and gas composition. Bioresour. Technol. 2019, 286, 121413. [Google Scholar] [CrossRef]
- Cayetano, R.D.A.; Park, J.; Kim, G.B.; Jung, J.H.; Kim, S.H. Enhanced anaerobic digestion of waste-activated sludge via bioaugmentation strategy-Phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt2) analysis through hydrolytic enzymes and possible linkage to system performance. Bioresour. Technol. 2021, 332, 125014. [Google Scholar] [CrossRef]
- Yu, Z.S.; Leng, X.Y.; Zhao, S.; Ji, J.; Zhou, T.Y.; Khan, A.; Kakde, A.; Liu, P.; Li, X.K. A review on the applications of microbial electrolysis cells in anaerobic digestion. Bioresour. Technol. 2018, 255, 340–348. [Google Scholar] [CrossRef]
- Park, J.; Lee, B.; Tian, D.; Jun, H. Bioelectrochemical enhancement of methane production from highly concentrated food waste in a combined anaerobic digester and microbial electrolysis cell. Bioresour. Technol. 2018, 247, 226–233. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, W.Z.; Shi, Y.J.; Wang, B.; Huang, C.; Liu, C.S.; Wang, A.J. Microbial electrolysis enhanced bioconversion of waste sludge lysate for hydrogen production compared with anaerobic digestion. Sci. Total Environ. 2021, 767, 144344. [Google Scholar] [CrossRef]
- Dong, H.Q.; Yue, L.C.; Cheng, J.; Xia, R.X.; Zhou, J.H. Microbial electrochemical degradation of lipids for promoting methane production in anaerobic digestion. Bioresour. Technol. 2022, 345, 126467. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Yu, Q.L.; Sun, C.; Wang, Z.X.; Jin, Z.; Zhu, Y.H.; Zhao, Z.Q.; Zhang, Y.B. Additional electric field alleviates acidity suppression in anaerobic digestion of kitchen wastes via enriching electro-active methanogens in cathodic biofilms. Water Res. 2022, 212, 118118. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.; Zielinska, M.; Cydzik-Kwiatkowska, A.; Rusanowska, P.; Debowski, M. Effect of static magnetic field on microbial community during anaerobic digestion. Bioresour. Technol. 2021, 323, 124600. [Google Scholar] [CrossRef]
- Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Bakare, B.F. Effect of an Electromagnetic Field on Anaerobic Digestion: Comparing an Electromagnetic System (ES), a Microbial Electrolysis System (MEC), and a Control with No External Force. Molecules 2022, 27, 3372. [Google Scholar] [CrossRef]
- Yu, Q.L.; Mao, H.H.; Zhao, Z.Q.; Zhang, Y.B. Electro-polarization of the sludge with dynamic magnetic field enhanced the interspecies electron transfer in ZVI-added anaerobic digesters. Renew. Energy 2023, 215, 119012. [Google Scholar] [CrossRef]
- Wang, S.L.; Wang, Z.; Usman, M.; Zheng, Z.H.; Zhao, X.L.; Meng, X.Y.; Hu, K.; Shen, X.; Wang, X.F.; Cai, Y.F. Two microbial consortia obtained through purposive acclimatization as biological additives to relieve ammonia inhibition in anaerobic digestion. Water Res. 2023, 230, 119583. [Google Scholar] [CrossRef]
- Wang, N.; Xiao, M.Y.; Hu, P.P.; Shi, J.J.; Zhang, S.Y.; Shi, J.P.; Liu, L. Unveiling the bioaugmentation mechanism of Clostridium thermopalmarium HK1 enhancing methane production in thermophilic anaerobic digestion of food waste. J. Environ. Chem. Eng. 2024, 12, 112620. [Google Scholar] [CrossRef]
- Tartakovsky, B.; Lebrun, F.; Guiot, S.R.; Bock, C. A comparison of microbial and bioelectrochemical approaches for biogas upgrade through carbon dioxide conversion to methane. Sustain. Energy Technol. 2021, 45, 101158. [Google Scholar] [CrossRef]
- Ning, X.; Lin, R.C.; Mao, J.; Deng, C.; Ding, L.K.; O’Shea, R.; Wall, D.M.; Murphy, J.D. Improving the efficiency of anaerobic digestion and optimising CO2 bioconversion through the enhanced local electric field at the microbe-electrode interface. Energy Convers. Manag. 2024, 304, 118245. [Google Scholar] [CrossRef]
- Zheng, X.M.; Lin, R.J.; Xu, J.; He, Y.Y.; Zhang, X.Y.; Xie, L. Enhanced methane production by bimetallic metal-organic frameworks (MOFs) as cathode in an anaerobic digestion microbial electrolysis cell. Chem. Eng. J. 2022, 440, 135799. [Google Scholar]
- Khan, A.; Akbar, S.; Okonkwo, V.; Smith, C.; Khan, S.; Shah, A.A.; Adnan, F.; Ijaz, U.Z.; Ahmed, S.; Badshah, M. Enrichment of the hydrogenotrophic methanogens for, in-situ biogas up-gradation by recirculation of gases and supply of hydrogen in methanogenic reactor. Bioresour. Technol. 2022, 345, 126219. [Google Scholar] [CrossRef] [PubMed]
- Hafuka, A.; Fujino, S.; Kimura, K.; Oshita, K.; Konakahara, N.; Takahashi, S. In-situ biogas upgrading with H2 addition in an anaerobic membrane bioreactor (AnMBR) digesting waste activated sludge. Sci. Total Environ. 2022, 828, 154573. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.P.; Chen, L.M.; Chen, Y.C.; Cao, Q.; Liu, X.F.; Li, D. Effect of H2 addition on the microbial community structure of a mesophilic anaerobic digestion system. Energy 2020, 198, 117368. [Google Scholar] [CrossRef]
- Akimoto, S.; Tsubota, J.; Angelidaki, I.; Hidaka, T.; Fujiwara, T. Pilot-scale in-situ biomethanation of sewage sludge: Effects of gas recirculation method. Bioresour. Technol. 2024, 413, 131524. [Google Scholar] [CrossRef]
- Zhao, J.M.; Hou, T.T.; Lei, Z.F.; Shimizu, K.; Zhang, Z.Y. Effect of biogas recirculation strategy on biogas upgrading and process stability of anaerobic digestion of sewage sludge under slightly alkaline condition. Bioresour. Technol. 2020, 308, 123293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).