Abstract
This paper introduces a novel method for synthesizing a cationic adhesion additive for road bitumen. The method involves condensing higher carboxylic acids and triglycerides of non-edible rapeseed oil with diethanolamine. The details of this innovative synthesis, the properties of the additive, and its action mechanism are shown in the results of this investigation. The surfactants in the synthesized products were identified by IR spectroscopy. They have significant practical implications. Different methods were used to investigate bitumen adhesion of acidic rock materials without and with additives. The results of the wetting angle study on the surface of rock materials changed from 19.81° to 80.33°, indicating a significant and remarkable improvement in its surface hydrophobicity. Moreover, the unique feature of the additive does not require dilution in organic solvents before use, which makes it highly practical for direct use.
1. Introduction
Bitumen is widely used to produce bitumen–mineral compositions, acting as a binder [1,2,3,4]. Despite the technological effectiveness of road construction materials prepared based on bitumen, it is also characterized by its disadvantages, such as sensitivity to alternating temperatures, low adhesive properties, especially acidic stone materials, and a constant tendency to change properties over time (embrittlement and aging). One of the reasons for this behavior of bitumen is that bitumen production is a year-round continuous process, and road construction and repair, especially in the northern regions, is seasonal. Under the influence of atmospheric factors, primarily due to the oxidation of bitumen components, the physical properties and chemical composition of bitumen changes. Their components change, partially passing from one type to another. Oils pass into resins, and resins into asphaltenes. As asphaltenes accumulate, the colloidal structure of bitumen changes. The association and subsequent agglomeration of asphaltene micelles leads to increased rigidity and deterioration of the performance characteristics of bitumen. The increasing brittleness of bitumen over time also makes asphalt concrete more brittle. Specifically, it loses its ability to withstand tensile stresses. Such asphalt concrete has significantly reduced fatigue resistance [5] and low temperature [6] cracking. Combined with the stress state from mechanical (dynamic) loads, aging, and increased rigidity, the continuity of the road surface structure is disrupted, leading to cracking and increased spalling of the stone material, ultimately reducing the service life of asphalt concrete [7,8]. One of the practical challenges in asphalt concrete products is the acidity of sand and gravel mixtures and the organic acids in bitumen. Traditional road construction methods introduce surface-active substances (SASs) to address this. These substances are added to bitumen to reduce surface tension, expand the plasticity range of bitumen, increase its resistance to aging, and enhance adhesion to mineral materials [1,9,10,11,12]. Various types of SASs are used to enhance the adhesion between bituminous binders and aggregates. These SASs are typically produced through the interaction of higher carboxylic acids with amine compounds [13,14,15,16]. The mechanism of action involves the migration of the adhesive molecules to the “bitumen/aggregate” interface, where the positively charged hydrophilic amine groups bind to the negatively charged surface of the aggregates. Meanwhile, the hydrophobic hydrocarbon tails interact with the bitumen, improving overall adhesion and cohesion.
These additives, including the one synthesized in this research, are introduced into road bitumen composition to enhance its properties significantly. They help expand the plasticity range of bitumen, increase its resistance to aging properties, and enhance the degree of adhesion to the surface of mineral materials. This enhancement leads to a substantial improvement in the overall quality and durability of road surfaces, providing reassurance about the effectiveness of the new adhesive additive.
Research has consistently shown that the most effective method for achieving strong bitumen adhesion to mineral materials is using adhesion additives containing cationic surfactants (SASs). These additives work by migrating to the “bitumen/stone material” interface, where their positively charged (hydrophilic) head groups firmly attach to the negatively charged areas on the stone material’s surface. At the same time, the hydrophobic hydrocarbon tail parts react with the bitumen’s constituent parts [11,12].
These adhesion additives, often called “binding activators”, essentially act as a glue between the bitumen and the surface of the stone material. They are typically cationic surfactants, such as higher alkyl diamines, acylamido amines, and alkyl imidazolines, which are obtained by the interaction of higher carboxylic acids with polyethylene polyamines [13,14,15,16]. When added to the bitumen, these binding activators enhance its adhesion to the mineral materials, thereby improving the overall quality and durability of road surfaces.
Purified vegetable oils, which contain sufficient organic acids, are primarily used in household chemicals, cosmetology, and pharmacology due to their high price. However, this study demonstrates the economic prudence of using various wastes from the chemical, forest chemical, and food industries as precursors for producing adhesive additives for road bitumen. The cost effectiveness of this approach is further highlighted using cold-pressed rapeseed oil, an inexpensive waste plant material unsuitable for food use, in the synthesis of the new adhesive additive [17,18,19,20].
In this study, we used cold-pressed rapeseed oil, which is unsuitable for food use and several times cheaper than the purified product, as a waste plant material. We combined this with diethanolamine, a member of the amino alcohol group characterized by the manifestation of both acidic and basic properties. The synthesis of this compound was of particular interest because the surface of most mineral materials used in road construction contains active centers—Lewis acids, which have vacant electron orbitals capable of accepting electron pairs (for example, SiO2 and Bronsted acids), and bases that attach a proton [21,22,23,24]. The compound we synthesized is unique because it contains cationic, anionic, and ampholytic centers, making it a “universal compound”. Its synthesis and mechanism of action have yet to be adequately reflected in the literature, but it has the potential to be widely applicable in road construction practice, instilling optimism in the potential of this new compound.
2. Materials
2.1. Bitumen
This study’s object was bitumen grade BND 70/100, produced by Asfaltobeton-1 LLP (Almaty, Kazakhstan) by direct oxidation of tar (vacuum residue). This bitumen is used for constructing and repairing roads operated in a wide temperature range, from +30 °C to −30 °C. The main standard characteristics of bitumen are penetration at 25 °C, 73 mm; softening point, 50 °C; brittleness temperature, −22 °C; and viscosity at 60 °C, 330 Pa·s.
2.2. Rock Material
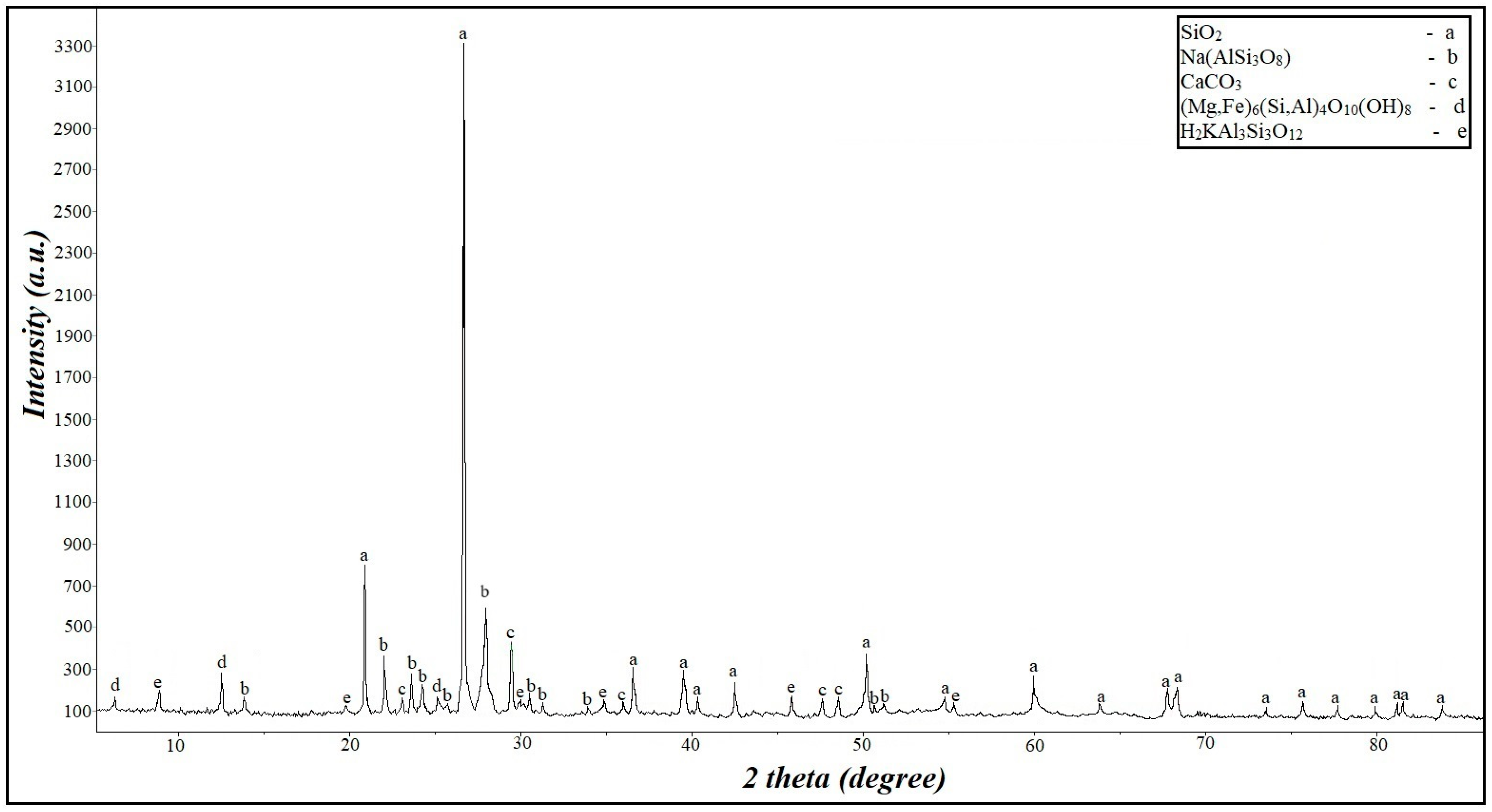
The stone material used was crushed stone from acidic rock; the phase composition of which is shown in Figure 1 and Table 1.
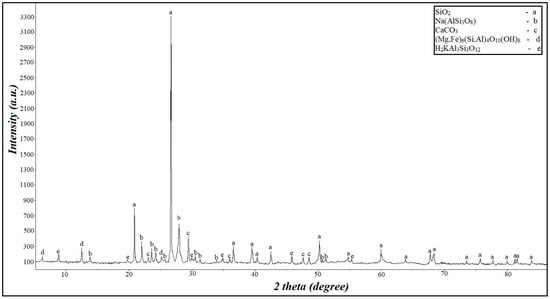
Figure 1.
XRD pattern of crushed stone.

Table 1.
Phase composition of crushed stone.
2.3. Rapeseed Oil
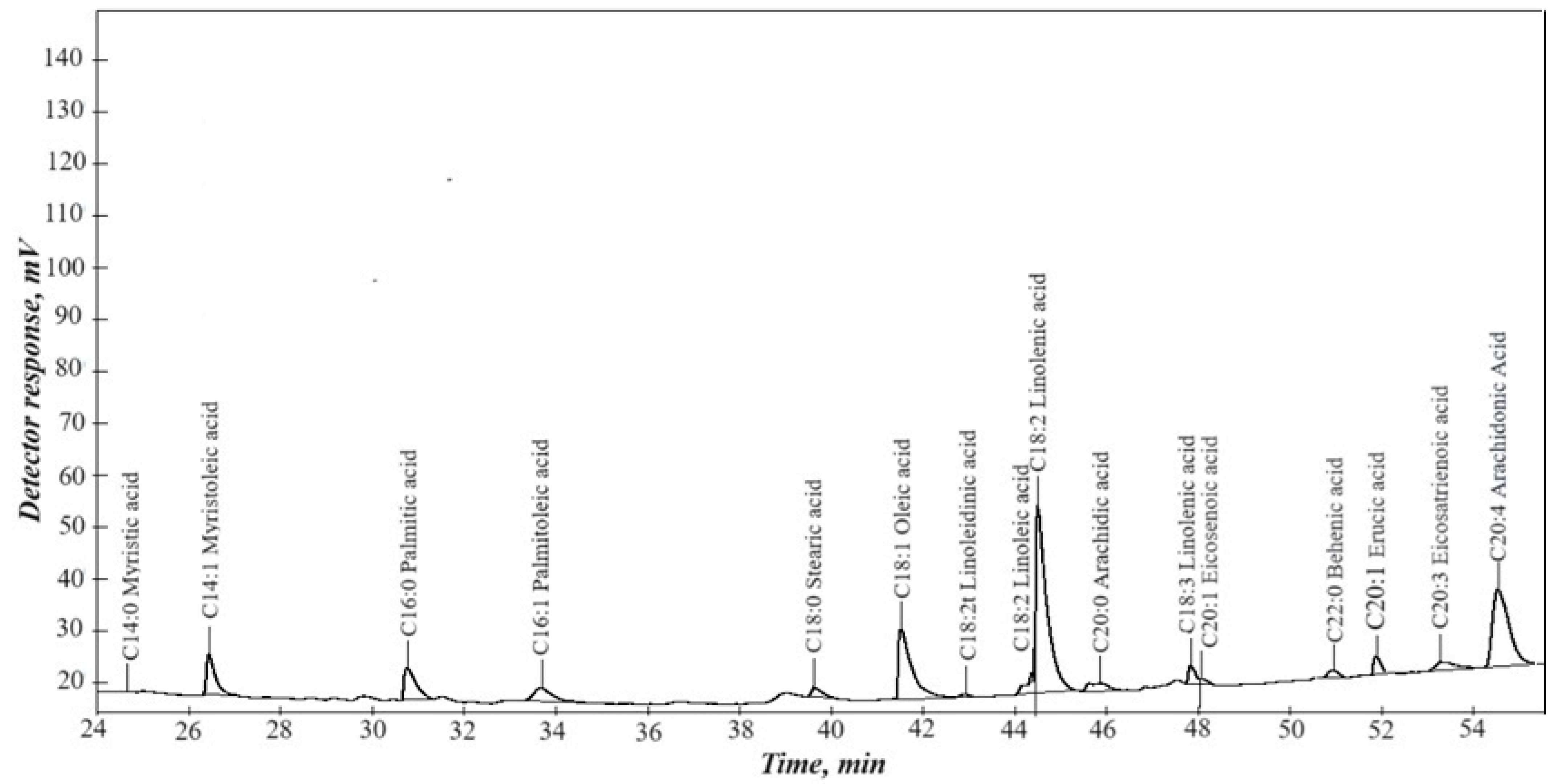
According to the chromatographic analysis (Figure 2), the waste from processing rapeseed oil was a mixture of higher carboxylic acids and their glycerides.
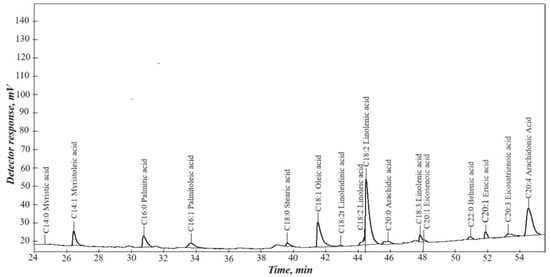
Figure 2.
Chromatographic analysis of rapeseed oil.
Plant-derived oils and fats are triglycerides of higher carboxylic acids. The fatty acid composition was determined using gas chromatography. Before analysis, the triglycerides were converted into their methyl esters. The resulting methyl esters of fatty acids were analyzed using a “Chromos GH-1000” gas chromatograph (Chromos, Russia) equipped with a flame ionization detector and a CP-Sil 88 capillary column (100 m × 0.25 mm × 0.20 μm; Agilent Technologies, Santa Clara, CA, USA). Helium served as the carrier gas, and the injection volume was 0.5 μL. The detection of signals corresponding to methyl esters of higher carboxylic acids indicates the presence of triglycerides in these acids.
The main acid components are myristic, myristoleic, palmitic, palmitoleic, stearic, oleic, linoleic, linolenic, eicosenoic, behenic, erucic, eicosatrienoic, and arachidonic acids. The presence of erucic acid makes this waste unsuitable for food purposes.
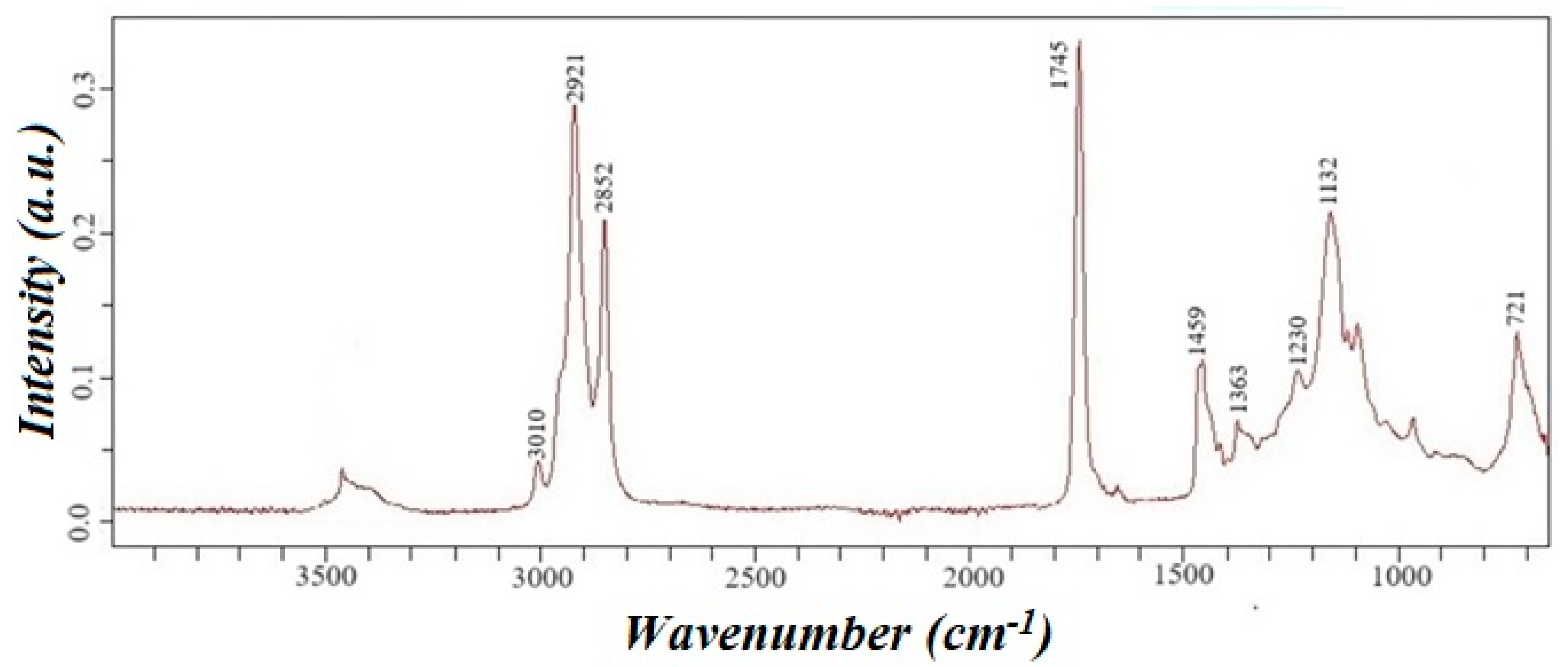
The waste’s acid number is determined under Euro-Asian council standardization 5985-59 [25], equal to 20 mg KOH/g. IR spectroscopy confirmed the presence of triglycerides in the oil, and the resulting IR spectrum of rapeseed oil is presented in Figure 3.
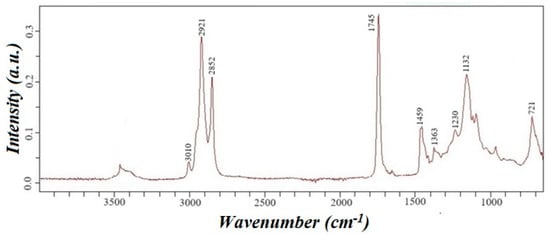
Figure 3.
IR spectrum of cold-pressed rapeseed oil waste.
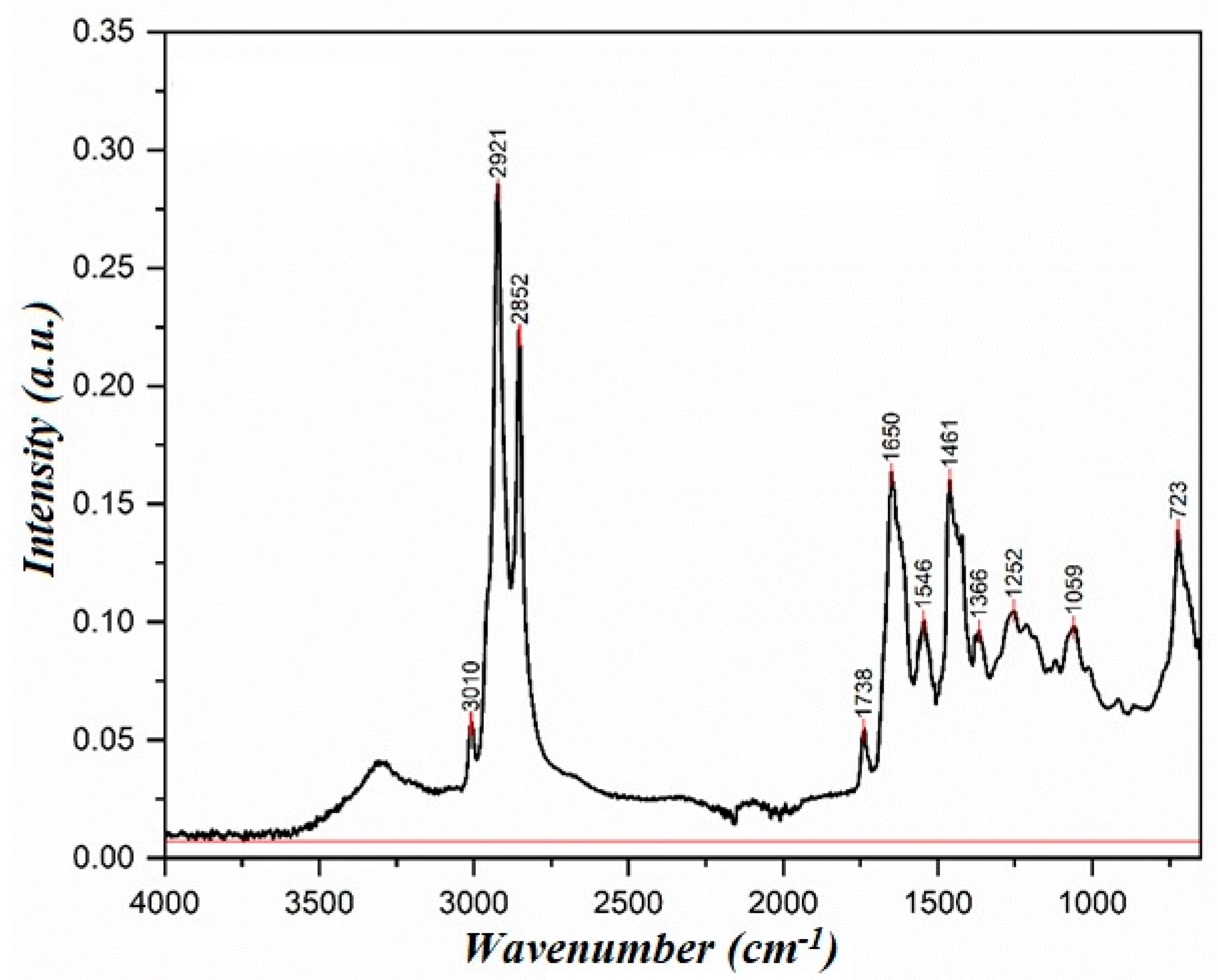
In the IR spectrum, the absorption band in the region of 3010 cm−1 corresponds to the stretching vibrations of the =CH bond, and the absorption bands in the region of 1290–1420 and 675–1005 cm−1 correspond to the in-plane and out-of-plane deformation vibrations of the =CH bond. Intense absorption bands in the region of 2840–3000 cm−1 correspond to the stretching vibrations of the C-H bond, and the absorption bands in the region of 1450–1475 cm−1 and 720–770 cm−1 correspond to the deformation of CH2—groups. The intense absorption band of 1745 cm−1 corresponds to the absorption band of the carbonyl bond of the ester group. Moreover, the absorption band in the 1050–1330 cm−1 region corresponds to the ester group’s C-O bond. Cold-pressed rapeseed oil consists of triglycerides of higher carboxylic acids with trace amounts of free higher carboxylic acids. The presence of these impurities is confirmed by a weak absorption band in the 3300–3500 cm−1 range, corresponding to the stretching vibrations of the O-H bond. Additionally, an absorption band at 1230 cm−1, attributed to the bending vibrations of the O-H bond, further supports the presence of free higher carboxylic acids.
2.4. Diethanolamine
Diethanolamine is produced in Russia and has the following physical constants: melting point, 27 °C; boiling point, 280 °C; density, 1.096 g/cm3; and refractive index, 1.4776.
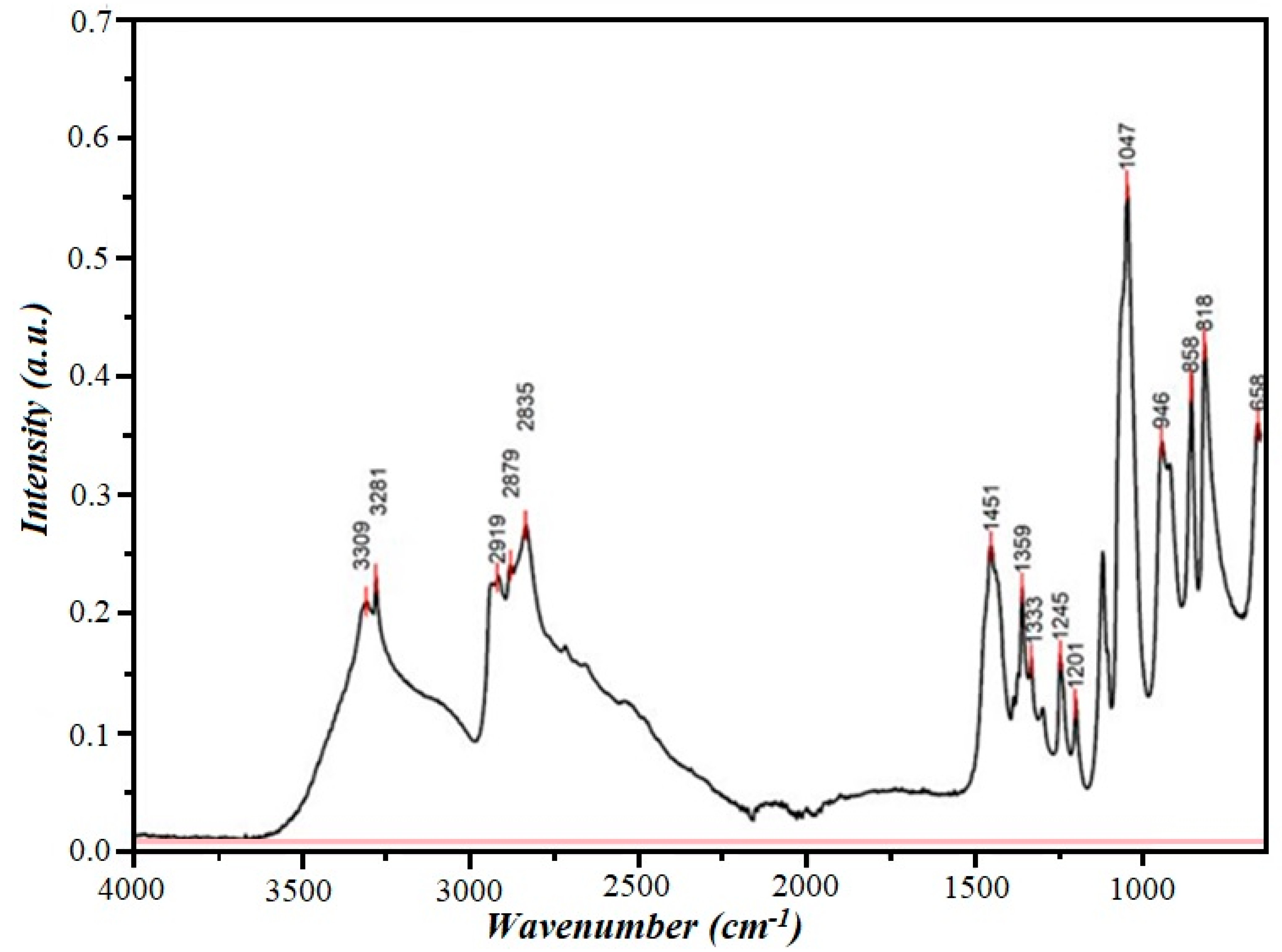
The IR spectrum of the substance is shown in Figure 4.
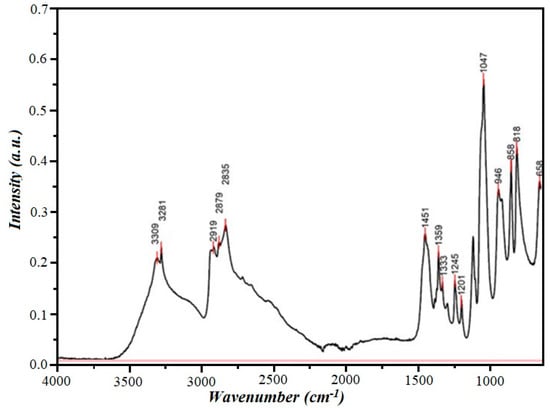
Figure 4.
IR spectrum of diethanolamine.
The IR spectrum of diethanolamine showed that the absorption band at 3309 cm−1 corresponds to the stretching vibrations of the N-H bond, and the absorption bands at 818 and 858 cm−1 correspond to the deformation vibrations of the secondary amine. The absorption band at 3281 cm−1 corresponds to the stretching vibrations of the O-H bond, and the absorption bands at 1245 cm−1 correspond to the deformation in-plane vibrations of the O-H bond. The intense absorption band at 1047 cm−1 corresponds to the stretching vibrations of the C-O bond of primary alcohols. Finally, the absorption bands (2919, 2879, 2835, 1451, and 1359 cm−1) characterize the stretching and deformation vibrations of the C-H bonds.
3. Research Methods
Boiling Method
The adhesion of the synthesized additive to the stone material was assessed using the boiling method recommended by the national standard of Kazakhstan, ST RK 1225-2013 [26], which is harmonized with the standard ASTM D 3625/D 3625M-12 [27] and ASTM D1664 [28], also known as AASHTO T 182 [29]. The essence of the method is that a sample of the filler mixture coated with bitumen is placed in a container with boiling distilled water and boiled for 10 min according to ASTM D 3625/D 3625M-12 and 30 min according to ST RK 1225-2013. After cooling the mixture, a visual inspection is carried out based on the results, and a rating is given on a five-point scale. A rating of five (5) corresponds to the complete preservation of the bitumen film on the surface of the stone material.
4. Results and Discussion
4.1. Synthesis of Adhesive Additive
The synthesis of the adhesive additive was carried out according to the following two schemes:
- The interaction of higher carboxylic acids with diethanolamine.
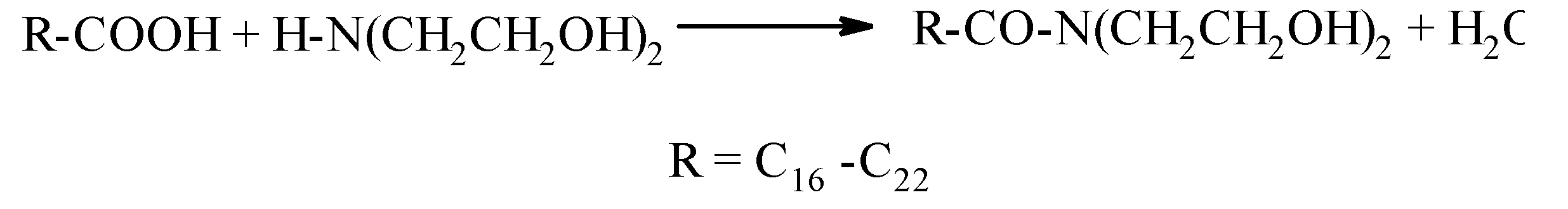
- The interaction of triglycerides of higher carboxylic acids with diethanolamine.
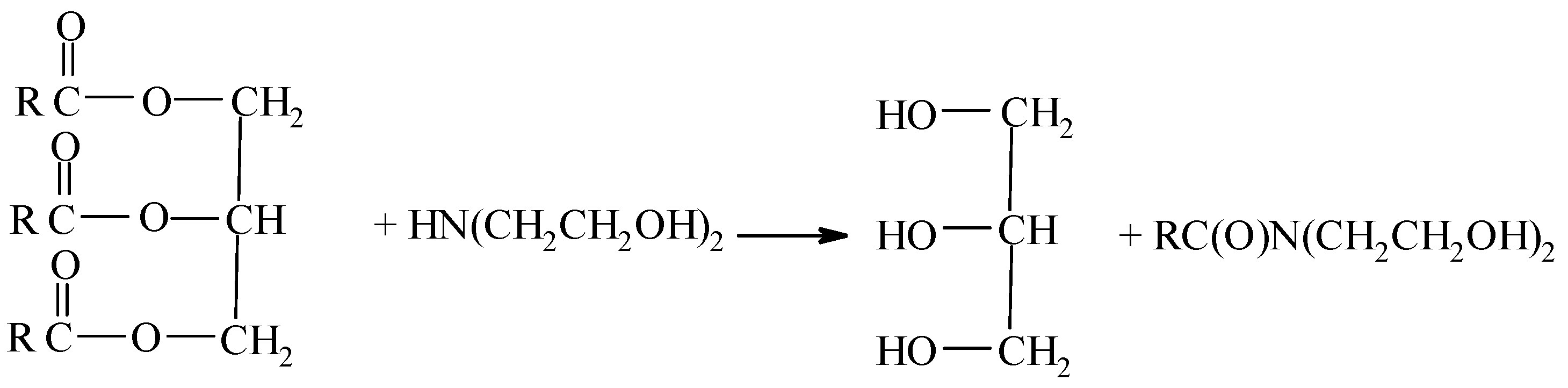
The interaction of triglycerides with diethanolamine occurs via the nucleophilic addition–elimination mechanism [30]. Due to the weak carbonyl activity of carboxylic acid triglycerides, an acid catalyst is required. Rapeseed oil contains carboxylic acids, which provide this catalyst.
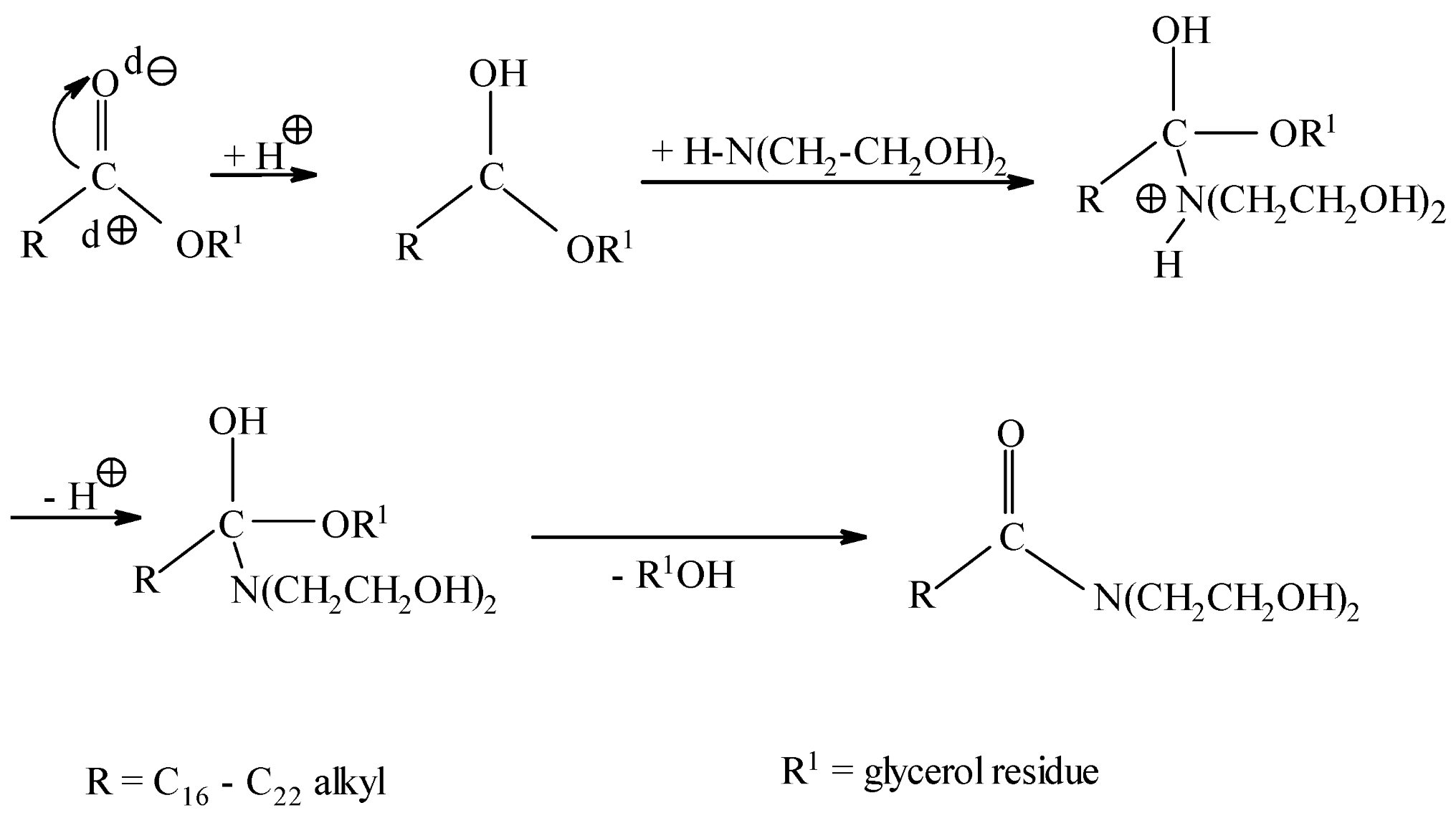
In the first stage, the hydrogen cation attacks the carbonyl group of the triglyceride of higher carboxylic acids. As a result, carbocation (1) is formed. When carbocation (1) interacts with diethanolamine, intermediate (2) is formed. After the hydrogen cation is split off, intermediate (2) is converted into intermediate (3). The latter splits off glycerol, forming diethanolamine of higher carboxylic acids.
The synthesis was carried out for 4 h with simultaneous water separation in a vacuum. The removal of water was carried out at a pressure of 200 mmHg and elevated temperature. As a result, a mixture of diethanolamine and glycerol was obtained, which was identified using IR spectroscopy. The IR spectrum of the synthesized compound is shown in Figure 5. Infrared spectra were acquired using the Agilent Cary 630 ATR-FTIR instrument (Agilent Technologies, Santa Clara, CA, USA), which enables direct sample analysis with minimal preparation. This information will be included in the revised manuscript for improved clarity.
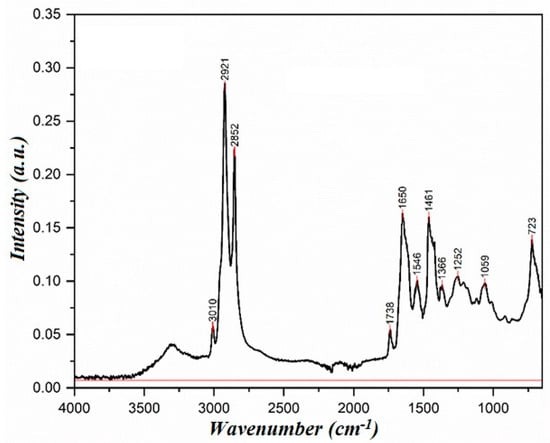
Figure 5.
IR spectrum of the synthesized compound.
In the IR spectrum of the product’s sowed, the absorption band at 3309 cm−1 corresponds to the stretching vibrations of the N-H bond, and the absorption bands in the 850-800 cm−1 region correspond to the secondary amine’s deformation vibrations.
The intense absorption band at 1650 cm−1 corresponds to the amide group. The absorption bands (2919, 2879, 2835, 1451, and 1359 cm−1) characterize the stretching and deformation vibrations of the C-H bonds. Notably, the absence of an absorption band in the 1745 cm−1 (ester group) region provides strong evidence that the reaction has been completed, offering reassurance in the synthesis process.
The comparison of the IR spectra of the synthesized compound (Figure 5) and diethanolamine (Figure 4) shows the absence of intense absorption bands in the 3309–3281 cm−1 region, indicating that the reaction has occurred. Additionally, the intensity pattern of the stretching vibration absorption bands changes, In the IR spectrum of diethanolamine, these bands are less intense, whereas in the spectrum of the synthesized compound, they are significantly more intense. The absorption bands in the 3500–3000 cm−1 region in diethanolamine indicate the presence of hydrogen bonding. In the IR spectrum of the synthesized compound, the peak in this region is less intense, suggesting a reduced degree of hydrogen bonding.
The synthesized substance, a paste-like product, is practically insoluble in water but well soluble in heated petroleum bitumen. It contains micelle-forming surfactants and is a complex mixture of amidoamines of higher fatty acids and glycerides. This substance holds great potential for enhancing the properties of asphalt concrete mixtures, offering a promising avenue for future research and application.
The positive role of glycerol in asphalt concrete mixtures was noted in the article by Saal et al. back in 1936 [31]. Modern studies [32,33,34,35,36] showed that glycerol derivatives act as SASs, reducing mixing time and increasing the homogeneity of the structure in asphalt concrete.
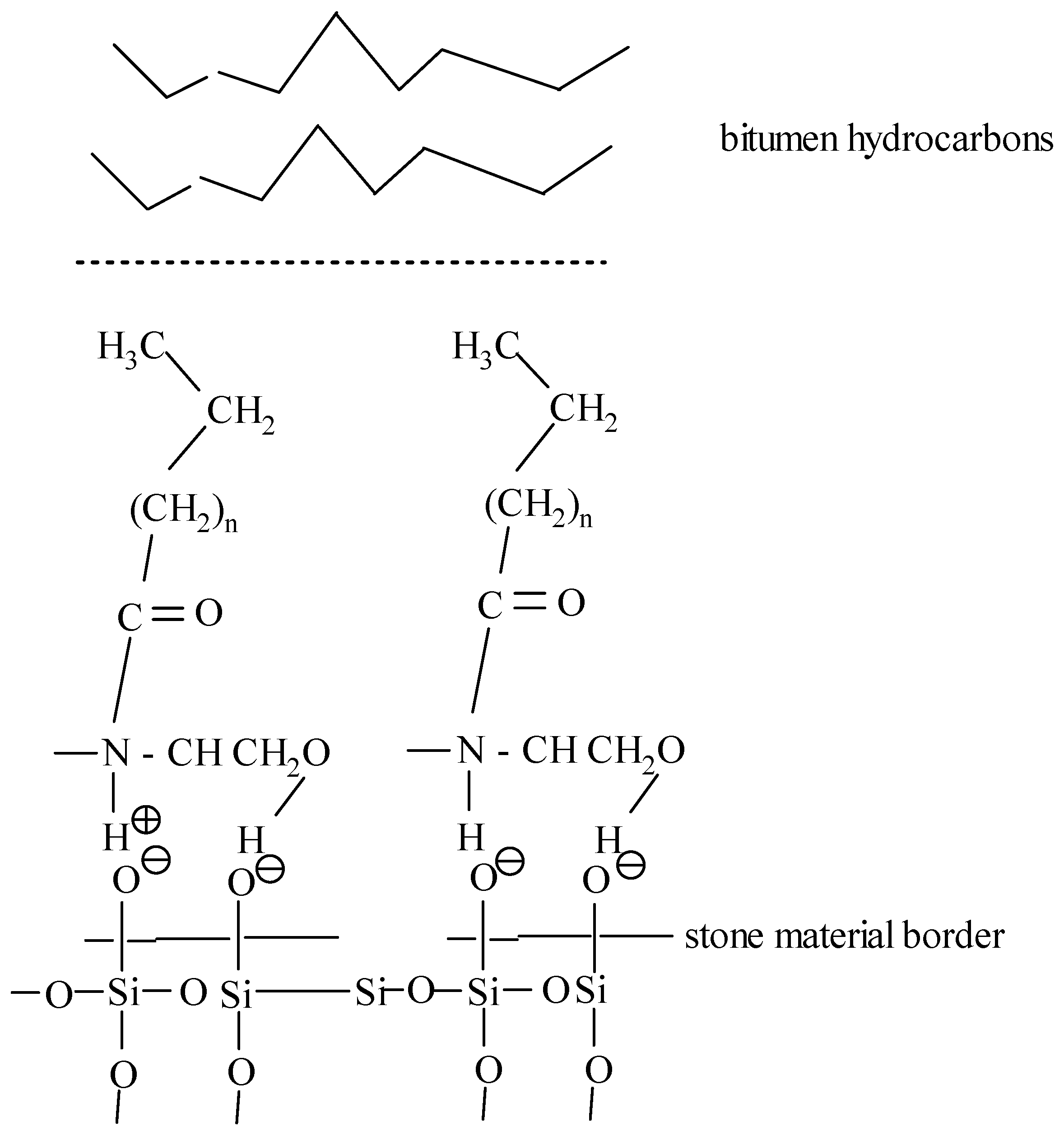
The synthesized additive is a cation-active compound containing a hydrophilic diethanolamine and a hydrophobic alkyl group. The process of action of the additive can be schematically represented as follows (Figure 6).
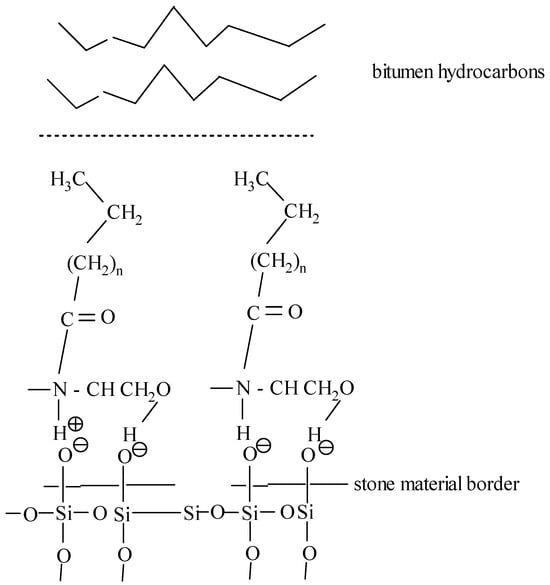
Figure 6.
The mechanism of interaction between the synthesized additive and the stone material.
Building on the findings reported in [37], its effect on bitumen is as follows. Hydrophilic amides (polyamides) have an unshared pair of nitrogen electrons, which react with acidic silica surfaces, acting as a promoter of amidoamine adhesion. In addition, the presence of oleic, linoleic, and erucic acid radicals in diethanolamine with a chain length of 18–22 carbon atoms promotes the redistribution of electron density and the formation of quaternary ammonium ions (R–NH3+), which provide electrostatic interaction with the surface of the mineral filler, in particular SiO2 [38,39]. Thus, the hydrophobic part of the additive is responsible for adhesion to bitumen hydrocarbons, and the hydrophilic part is responsible for the bond with the silicate base. The presence of acidic and basic centers, according to Brønsted and Lewis, in the diethanolamine group promotes strong adhesion of the organic part (bitumen) to the mineral part of asphalt concrete.
4.2. Adhesion Quality Assessment
Figure 7, Figure 8 and Figure 9 show samples after adhesion testing using the boiling method according to ASTM D 3625/D 3625M-12 [27].

Figure 7.
Untreated crushed stone (left) and crushed stone treated with pure bitumen after boiling (right).

Figure 8.
Untreated crushed stone (left) and crushed stone treated with bitumen with a synthesized adhesive additive after boiling (right).

Figure 9.
Untreated crushed stone (left) and crushed stone treated with bitumen with the adhesive industrial additive AlfaDob after boiling (right).
When acidic mineral material is treated with the original bitumen, i.e., without adding a modifier, the binder film remains on the crushed stone surface over an area of less than 50%, and individual droplets of bitumen are observed on the exposed surface, which corresponds to 2 points (Figure 7) [27]. When a modifier is added to the bitumen after the adhesion test, complete coverage of the mineral material surface is observed, corresponding to 5 points on a five-point scale (Figure 8).
When testing the AlfaDob industrial additive used in road construction in Kazakhstan, a similar coating of stone material with bitumen was obtained (Figure 9).
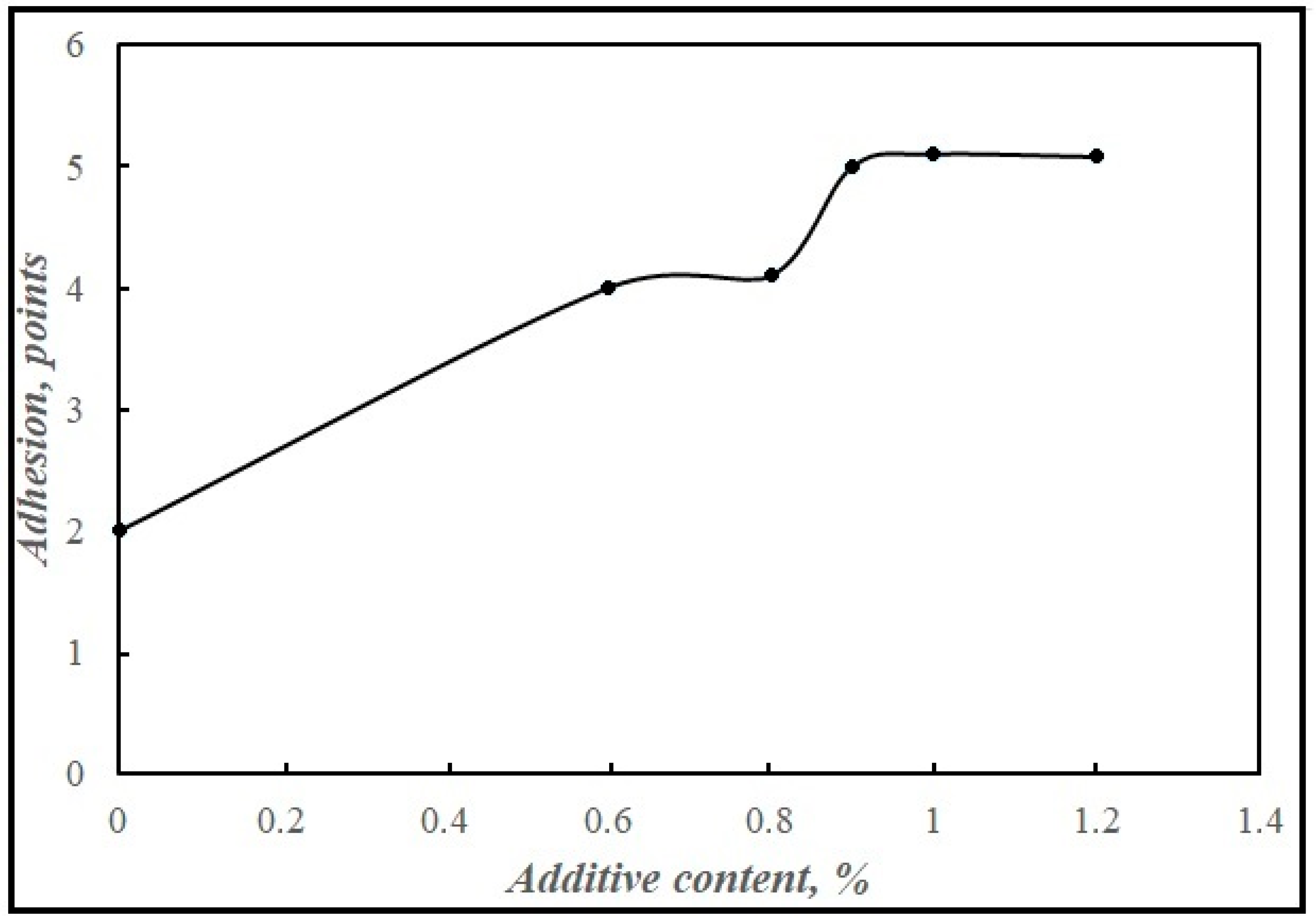
The optimal amount of the synthetic additive to bitumen was determined. Starting from 0.6 mass %, it increased the degree of coating of the stone material, reaching the maximum value at 0.9 mass % (Figure 10). A further increase in the concentration of the adhesive additive is not advisable.
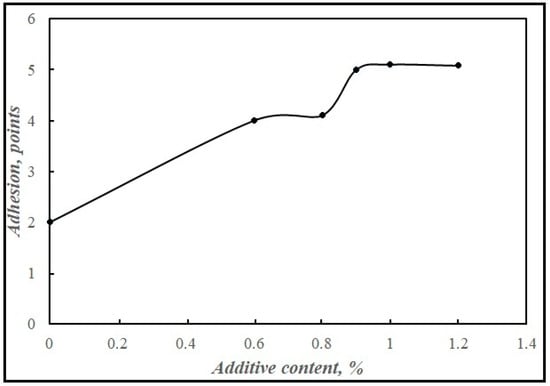
Figure 10.
Dependence of bitumen adhesion to stone material (in points) on the content of the synthesized adhesive additive in bitumen.
The fact that roads are damaged by water is widely recognized. However, the primary issue is the softening of granular materials and subgrade soils beneath the pavement, rather than the stripping of bitumen alone. Water, being a polar liquid, spreads across the surface of stone materials and can penetrate under the bitumen film, contributing to its detachment. To mitigate this, adhesive additives are needed to hydrophobize the surface of the stone materials, reducing the risk of bitumen peeling. A quantitative measure of hydrophobicity is the wetting angle.
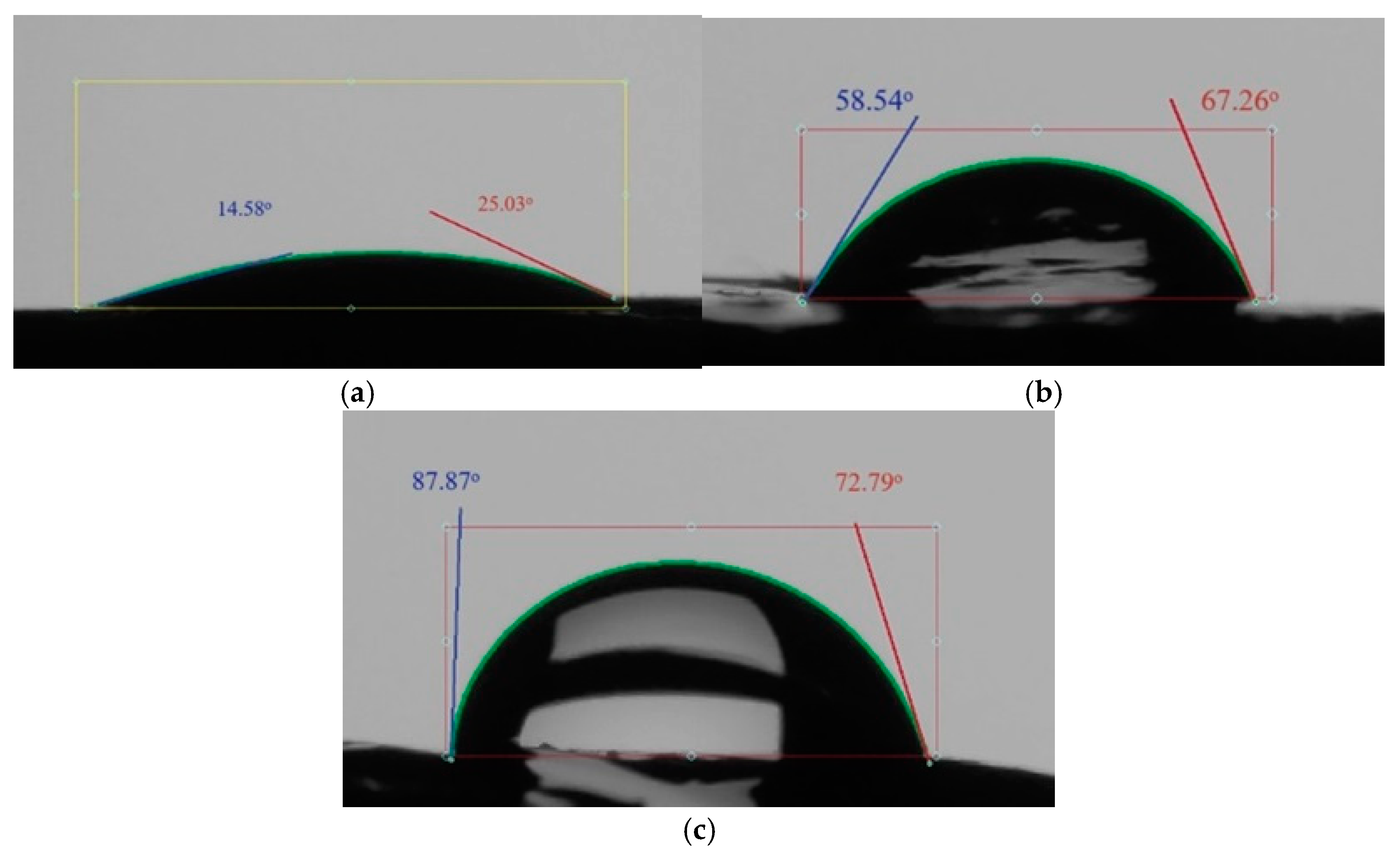
The surface tension and wettability of bitumen, both with and without the additive, were examined using the Ossila Contact Angle Goniometer (manufactured in Sheffield, UK). The results of this study, demonstrating the enhanced hydrophobic properties due to the additive, are presented in Figure 11a–c.
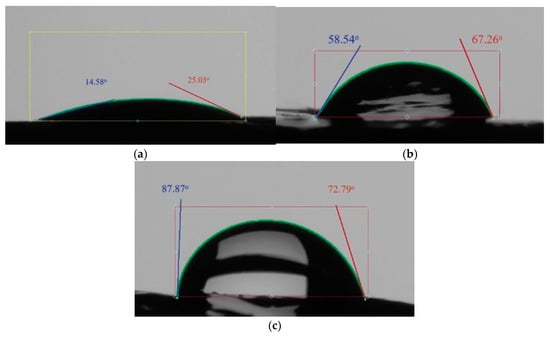
Figure 11.
Wetting angle: (a) stone material without coating; (b) stone material with bitumen; (c) stone material with an additive.
The introduction of cationic surfactants into bitumen reduced the surface tension at the “bitumen—mineral material” interphase surface, improving the wetting and coating of the mineral surface with bitumen.
The wetting angle, which measures the degree of contact between a liquid and a solid surface, of the stone material treated with bitumen was determined. The wetting angle changed from 19.81° without coating to 80.33° for the stone material coated with bitumen with an adhesive additive. Thus, the adhesive additive increases the hydrophobicity, or water-repelling properties, of bitumen and prevents its peeling off from the stone material under the influence of water.
These findings underscore the significant role of cationic surfactants and adhesive additives in enhancing the performance of road bitumen. The improved wetting and adhesion properties demonstrated in this study are a testament to the potential of these additives in the production of high-quality asphalt concrete mixtures. This potential offers a hopeful outlook for the future of road bitumen production, inspiring confidence in the industry.
4.3. Rutting Resistance Tests
Rutting resistance tests were conducted using the CRT-WTEN1 testing equipment, manufactured by Wuhan EasyTest Technology Co., Ltd. (Wuhan, China). The purpose of the test was to evaluate the rutting depth of asphalt concrete samples modified with a new adhesion additive in comparison to standard bitumen samples. The tests were performed in accordance with ST RK EN 12697-22-2012 [40], which is based on the European standard EN 12697-22 [41].
The results demonstrated that the sample modified with the new adhesion additive exhibited excellent performance, achieving a rutting depth of only 1.7 mm. In contrast, the unmodified bitumen sample showed a rutting depth of 2.77 mm. This significant reduction in rutting depth indicates a considerable improvement in the modified asphalt’s ability to resist permanent deformation under load.
These findings suggest that the newly synthesized additive not only enhances adhesion between bitumen and aggregate but also improves the overall structural stability of asphalt concrete, making it more resilient to deformation caused by heavy traffic. Such improvements are crucial for extending the service life of road surfaces and reducing maintenance costs.
5. Conclusions
The new adhesive additive for road bitumen was successfully synthesized using waste from rapeseed oil processing and diethanolamine, resulting in a complex mixture of amidoamines and glycerides. Value addition on adhesion between the bitumen and the mineral aggregate was significantly improved and was expressed by a wetting angle increase from 19.81 to 80.33°, hence increasing hydrophobicity, which keeps off water-induced bitumen stripping. From the results, it was shown that asphalt concretes modified with an additive had a higher resistance in the rutting tests, as their rut depth reached 1.7 mm in contrast to unmodified bitumen, whose rut depth reached about 2.77 mm. In this case, this additive demonstrates tremendous potential to provide enhancements in structural stability for asphalt concrete under high volumes of traffic. Additionally, the additive has practical advantages due to the fact that it is directly usable without dilution with organic solvents; hence, it is highly practical in industrial usage.
Author Contributions
Conceptualization, C.O.R.; Methodology, A.B. and T.O.; Software, T.O.; Validation, K.K.; Formal analysis, S.K. and Z.A.; Investigation, R.N., T.K., A.B., S.K., B.T. and P.C.; Data curation, P.C.; Writing—original draft, K.K.; Writing—review & editing, Z.A.; Supervision, R.N., T.K., B.T. and C.O.R.; Project administration, R.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (grant No. BR18574084).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Porto, M.; Caputo, P.; Loise, V.; Eskandarsefat, S.; Teltayev, B.; Oliviero Rossi, C. Bitumen and Bitumen Modification: A Review on Latest Advances. Appl. Sci. 2019, 9, 742. [Google Scholar] [CrossRef]
- Peden, R.A. Sustainability of bitumen use in highways. Proceedings of the Institution of Civil Engineers. Eng. Sustain. 2003, 156, 95–99. [Google Scholar] [CrossRef]
- Ingrassia, L.P.; Lu, X.; Ferrotti, G.; Canestrari, F. Chemical, morphological and rheological characterization of bitumen partially replaced with wood bio-oil: Towards more sustainable materials in road pavements. J. Traffic Transp. Eng. (Engl. Ed.) 2019, 7, 192–204. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Shukla, A.; Gangopadhyay, S.; Sharma, S. Recent trends of the emission characteristics from the road construction industry. Environ. Sci. Pollut. Res. 2011, 19, 301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teltayev, B. A new failure criterion for asphalt mixtures under fatigue loading. Int. J. Pavement Res. Technol. 2015, 8, 276–282. [Google Scholar] [CrossRef]
- Teltayev, B.; Radovskiy, B. Low temperature cracking problem for asphalt pavements in Kazakhstan. RILEM Bookseries 2016, 13, 139–144. [Google Scholar] [CrossRef]
- Petersen, J.C. Chapter 14 Chemical Composition of Asphalt as Related to Asphalt Durability. Asph. Asph. 2000, 2, 363–399. [Google Scholar] [CrossRef]
- Branthaver, J.F.; Petersen, J.C.; Robertson, R.E.; Duvall, J.J.; Kim, S.S.; Harnsberger, P.M. Aging Studies of Asphalt. In Binder Characterization and Evaluation; Vol. 2: Chemistry; Strategic Highway Research Program Report SHRP-A-368; National Research Council: Washington, DC, USA, 1993; pp. 189–285. [Google Scholar]
- Remišová, E.; Holý, M. Changes of Properties of Bitumen Binders by Additives Application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 245, 032003. [Google Scholar] [CrossRef]
- Gawel, I.; Czechowski, F.; Kosno, J. An environmental friendly anti-ageing additive to bitumen. Constr. Build. Mater. 2016, 110, 42–47. [Google Scholar] [CrossRef]
- Shatnawi, S.R.; Van Kirk, J. Premature Asphalt Concrete Pavement Distress Caused by Moisture Induced Damage. In Transportation Research Record 1417; TRB, National Research Council: Washington, DC, USA, 1993; pp. 168–177. [Google Scholar]
- Ramaswamy, S. The effects of Amino antistrip additives on stripping of bituminous mixes. Highw. Transp. 1990, 37, 9–13. [Google Scholar]
- Lesueur, D. The colloidal structure of bitumen: Consequences on the rheology and on the mechanisms of bitumen modification. Adv. Colloid Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef]
- Liang, M.; Guo, M.; Tan, Y.; He, S.; Du, X. Evaluation of anti-ageing performance of bitumen based on rheological and chemical characterization. Int. J. Pavement Eng. 2023, 2213, 385. [Google Scholar] [CrossRef]
- Hung, A.M.; Mousavi, M.; Pahlavan, F.; Fini, E.H. Intermolecular Interactions of Isolated Bio-Oil Compounds and Their Effect on Bitumen Interfaces. ACS Sustain. Chem. Eng. 2017, 5, 7920–7931. [Google Scholar] [CrossRef]
- Werkovits, S.; Bacher, M.; Mirwald, L.; Theiner, L.; Rosenau, T.; Hofko, B.; Grothe, H. The impact of field ageing on molecular structure and chemistry of bitumen. Fuel 2023, 343, 127904. [Google Scholar] [CrossRef]
- Köfteci, S.; Ahmedzade, P.; Kultayev, B. Performance evaluation of bitumen modified by various types of waste plastics. Constr. Build. Mater. 2014, 73, 592–602. [Google Scholar] [CrossRef]
- Turobov, M.A.; Danilo, V.E.; Ayzenshtadt, A.M. Use of wood processing industry waste for bitumen modification. IOP Conf. Ser. Mater. Sci. Eng. 2020, 945, 012061. [Google Scholar] [CrossRef]
- Riccardi, C.; Losa, M. Recent advances and perspectives in circular bio-binder extender to substitute part of the fossil based binder in asphalt mixture. Constr. Build. Mater. 2024, 410, 134222. [Google Scholar] [CrossRef]
- Wong, T.L.X.; Hasan, M.R.M.; Peng, L.C. Recent development, utilization, treatment, and performance of solid wastes additives in asphaltic concrete worldwide: A review. J. Traffic Transp. Eng. (Engl. Ed.) 2022, 9, 693–724. [Google Scholar] [CrossRef]
- Wu, L.M.; Zhou, C.H.; Keeling, J.; Tong, D.S.; Yu, W.H. Towards an understanding of the role of clay minerals in crude oil formation, migration, and accumulation. Earth-Sci. Rev. 2012, 115, 373–386. [Google Scholar] [CrossRef]
- Billingham, J.; Breen, C.; Yarwood, J. In situ determination of Brønsted/Lewis acidity on cation-exchanged clay mineral surfaces by ATR-IR. Clay Miner. 1996, 31, 513–522. [Google Scholar] [CrossRef]
- Komadel, P.; Madejová, J. Chapter 7.1 Acid Activation of Clay Minerals. Handb. Clay Sci. 2006, 1, 263–287. [Google Scholar] [CrossRef]
- Djomgoue, P.; Njopwouo, D. FT-IR Spectroscopy Applied for Surface Clays Characterization. J. Surf. Eng. Mater. Adv. Technol. 2013, 3, 275–282. [Google Scholar] [CrossRef]
- GOST 5985-59; Petroleum Products. Method for Determining Acidity and Acid Number (Nefteprodukty. Metod opredeleniya kislotnosti i kislotnogo chisla). Available online: https://www.russiangost.com/p-251162-gost-5985-59.aspx (accessed on 11 December 2024). (In Russian).
- National Standard of Republic Kazakhstan 1225-2013; Asphalt Concrete Road, Airport and Asphalt Concrete Mixtures (Smesi asfal’tobetonnye dorozhnye, aerodromnye i asfal’tobeton). Committee for Technical Regulation and Metrology: Astana, Kazakhstan, 2013. (In Russian)
- ASTM D3625/D3625M-12; Standard Practice for Effect of Water on Bituminous-Coated Aggregate Using Boiling Water. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM D1664-80; Standard Test Method for Coating and Stripping of Bitumen-Aggregate Mixtures. ASTM International: West Conshohocken, PA, USA, 1980.
- AASHTO T 182-84; Standard Method of Test for Coating and Stripping of Bitumen-Aggregate Mixtures. American Association of State Highway and Transportation Officials: Washington, DC, USA, 1984.
- Sykes, P. A Guidebook to Mechanism in Organic Chemistry, 6th ed.; Crist’s College: Cambridge, UK, 1991; 417p. [Google Scholar]
- Saal, R.N.J.; Labout, J.W.A. The Relation Between Absolute Viscosity and Penetration of Asphaltic Bitumens. Physics 1936, 7, 408–412. [Google Scholar] [CrossRef]
- Sihombing, A.V.R.; Utami, R.; Somantri, A.K.; Febriansya, A.; Sihombing, R.P.; Mulyadi, A.M. Stone Matrix Asphalt Performance with Glycerin Pitch as Asphalt Binder Extender. Period. Polytech. Civ. Eng. 2023, 67, 582–595. [Google Scholar] [CrossRef]
- Ahmed, R.B.; Hossain, K.; Hajj, R.M. Chemical, Morphological, and Fundamental Properties of Rejuvenated Asphalt Binders. J. Mater. Civ. Eng. 2020, 33, 04020461. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Dong, Y.; Chen, J. Preparation and Road Performance of Solvent-Based Cold Patch Asphalt Mixture. Int. J. Pavement Res. Technol. 2022, 15, 1155–1165. [Google Scholar] [CrossRef]
- Haghshenas, H.F.; Rea, R.; Gerald Reinke, G.; Yousefi, A.; Haghshenas, D.F.; Pooyan Ayar, P. Effect of Recycling Agents on the Resistance of Asphalt Binders to Cracking and Moisture Damage. J. Mater. Civ. Eng. Arch. 2021, 33, 04021292. [Google Scholar] [CrossRef]
- Osman, H.; Hasan, M.R.M.; Mukhtar, N.; Ghazali, M.F.H.M.; Raman, N.A.A. Effects of Alkylamines-Based and Polyalkylene Glycol-Based Bonding Enhancers on the Performance of Asphalt Binders. IOP Conf. Ser. Earth Environ. Sci. 2021, 920, 012021. [Google Scholar] [CrossRef]
- Levin, J.O.; Andersson, K.; Hallgren, C. Exposure to low molecular polyamines during road paving. Ann. Occup. Hyg. 1994, 38, 257–264. [Google Scholar] [CrossRef]
- Syroezhko, A.M.; Baranov, M.A.; Ivanov, S.N.; Maidanova, N.V. Influence of natural additives and those synthesized by the Fischer-Tropsch method on the properties of petroleum bitumen and quality of floated asphalt. Coke Chem. 2011, 54, 26–31. [Google Scholar] [CrossRef]
- Oliviero Rossi, C.; Teltayev, B.; Angelico, R. Adhesion Promoters in Bituminous Road Materials: A Review. Appl. Sci. 2017, 7, 524. [Google Scholar] [CrossRef]
- ST RK EN 12697-22-2012; Standard Test Methods for Asphalt Concrete Road, Airport, and Asphalt Mixtures (Metody ispytanii na asfaltobetonnykh smesey dorozhnykh, aerodromnykh i asfal’tobeton). Committee for Technical Regulation and Metrology: Astana, Kazakhstan, 2012. (In Russian)
- EN 12697-22; Standard Test Method for Wheel Tracking Test of Bituminous Mixtures. European Committee for Standardization (CEN): Brussels, Belgium, 2003.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).