Ecological Assessment of Polluted Soils: Linking Ecological Risks, Soil Quality, and Biota Diversity in Contaminated Soils
Abstract
:1. Introduction
2. Materials and Methods
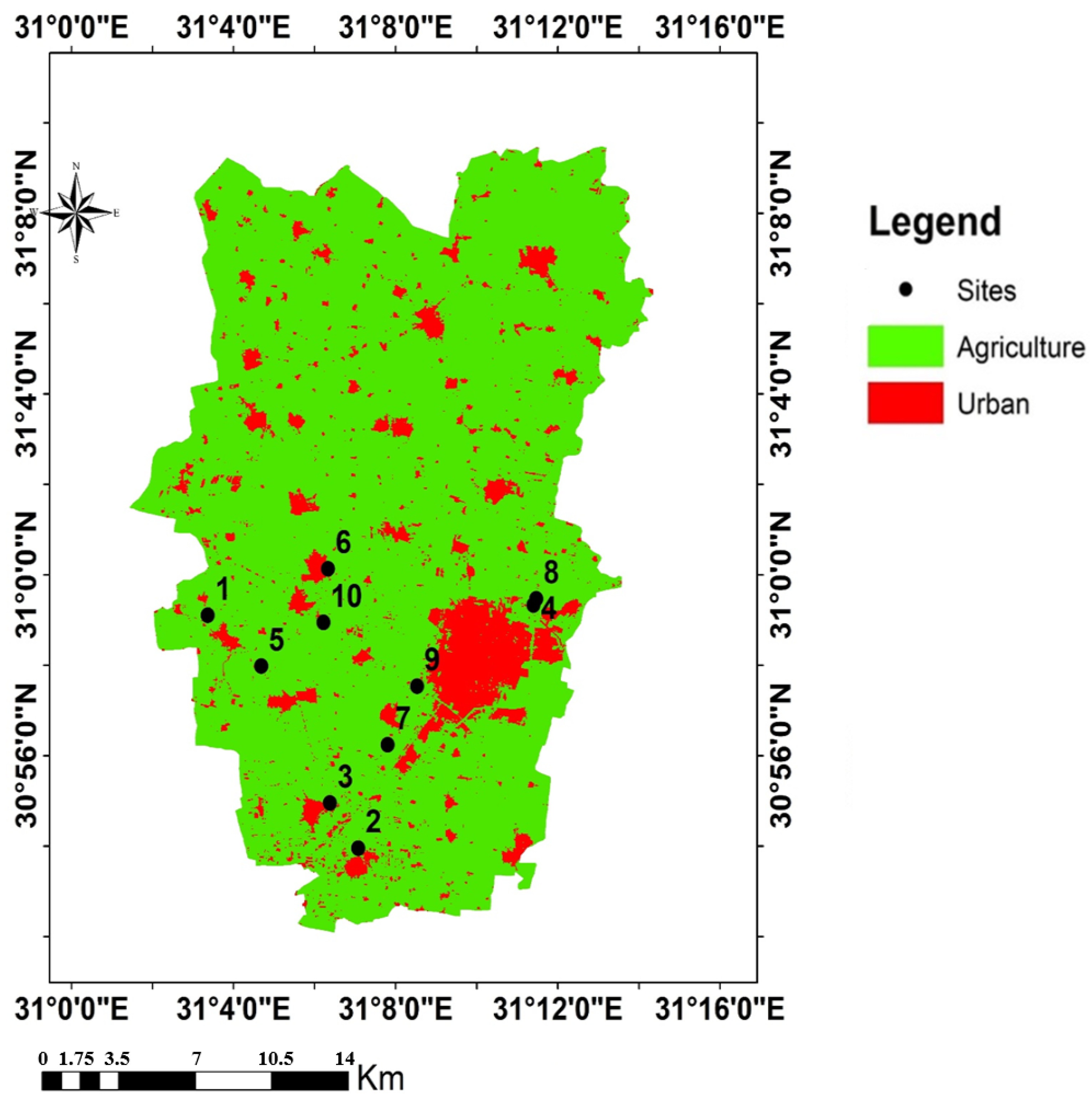
2.1. Study Area
2.2. Soil Analysis
2.3. Soil Contamination Indices
2.4. Soil Diversity Assessment Indices
2.5. Statistical and Data Analysis
2.5.1. Soil Chemical and Biological Data
2.5.2. Diversity Analysis
2.5.3. Species Similarity
2.5.4. Dominance
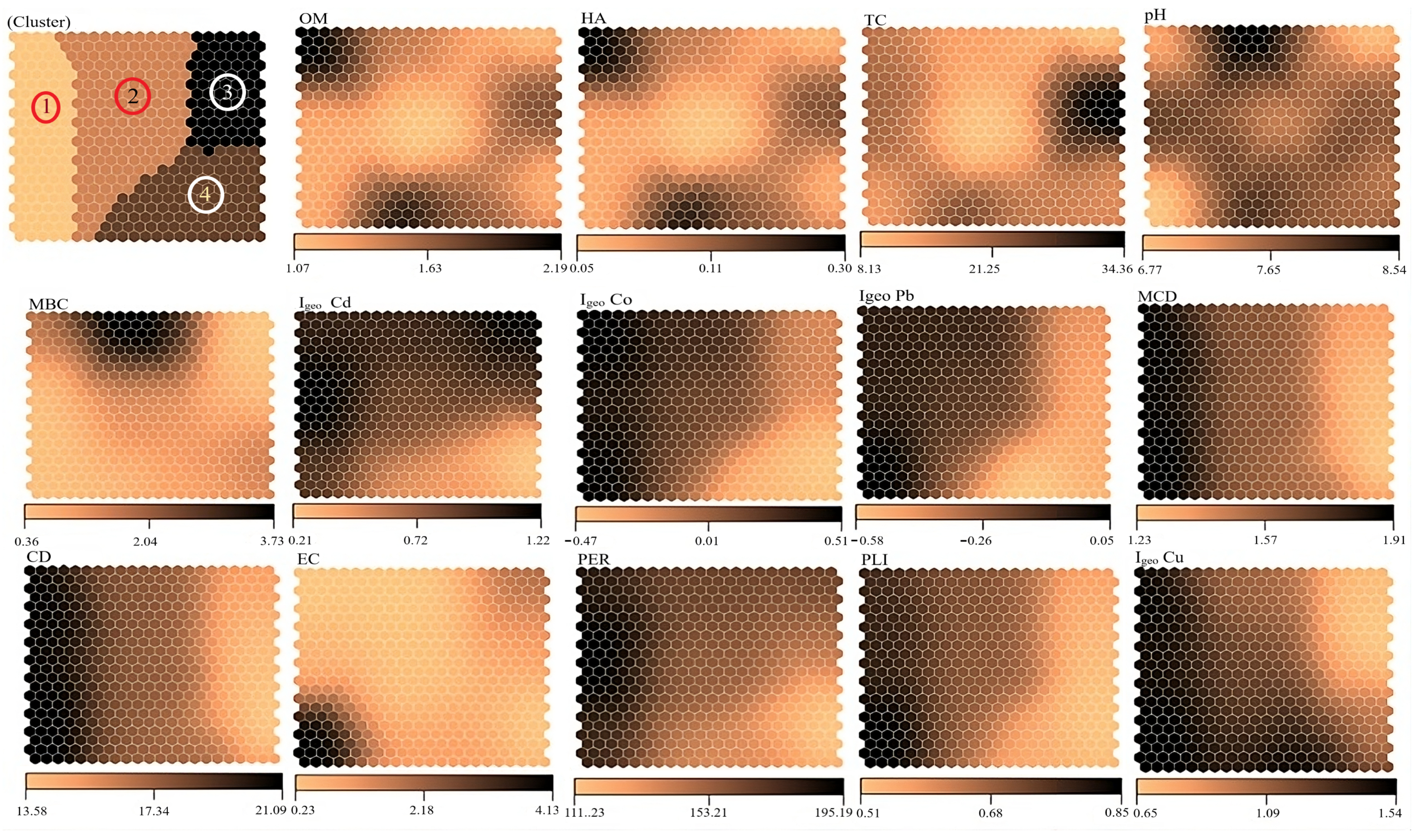
3. Results
3.1. Soil Properties
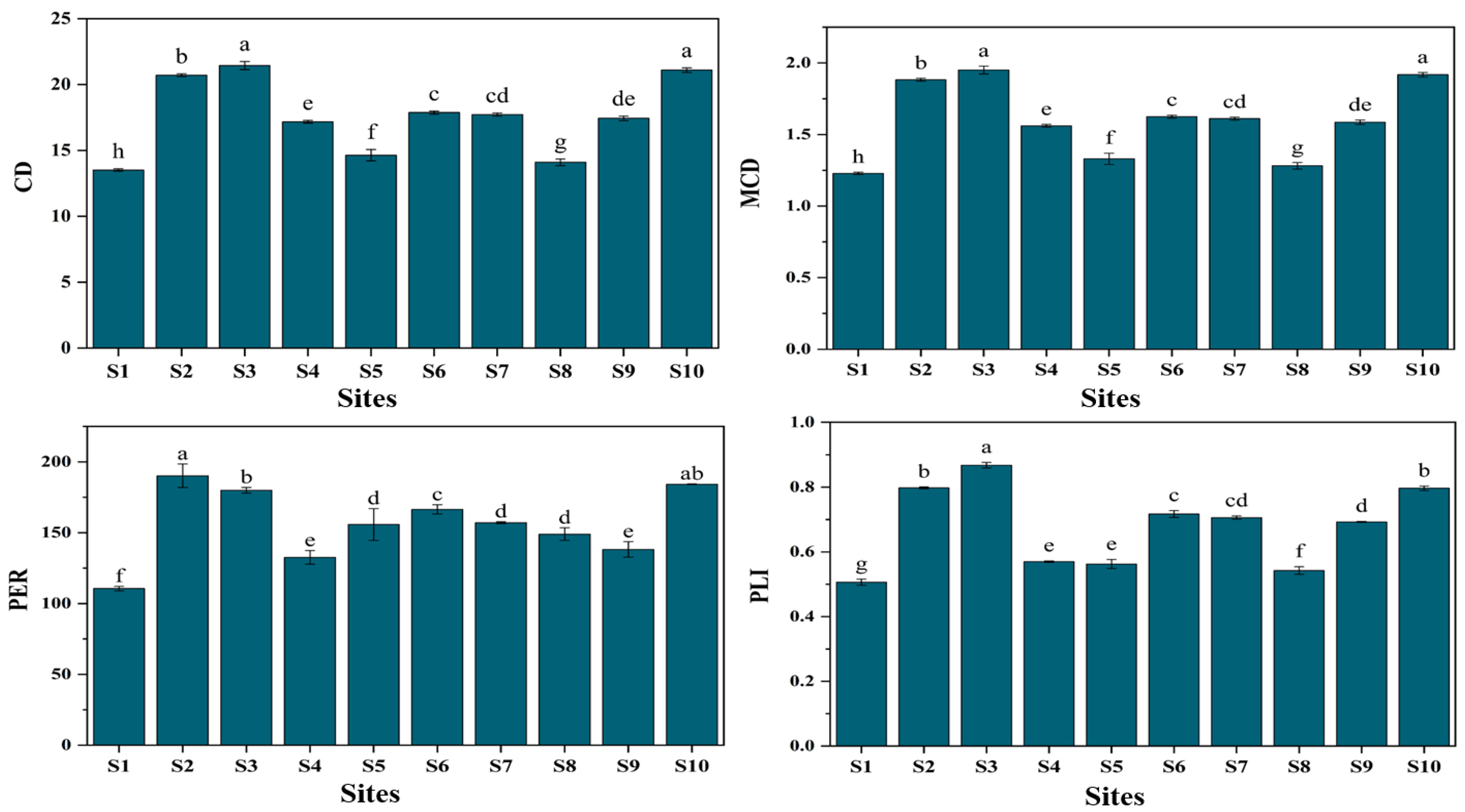
3.2. Pollution Indices
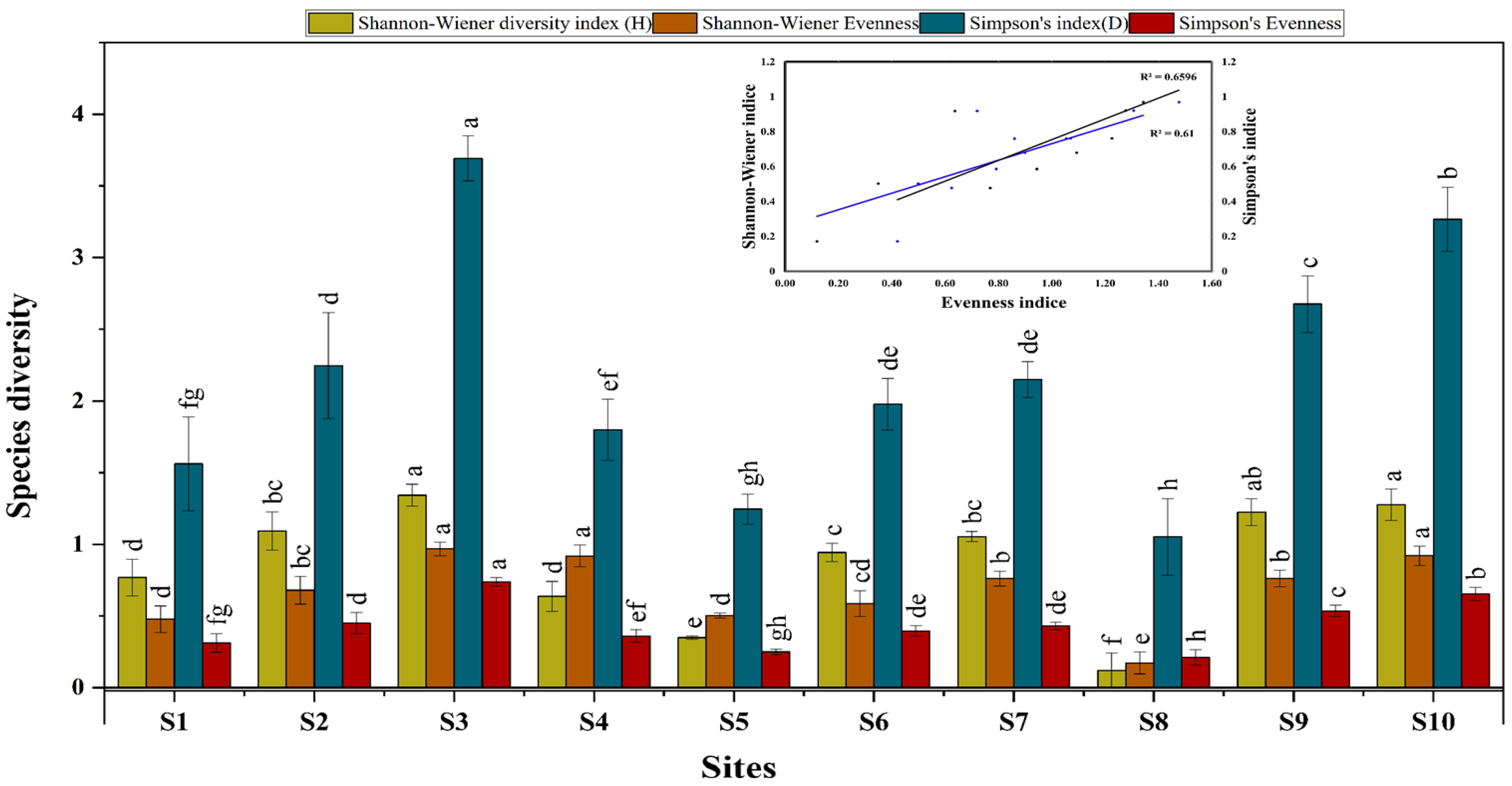
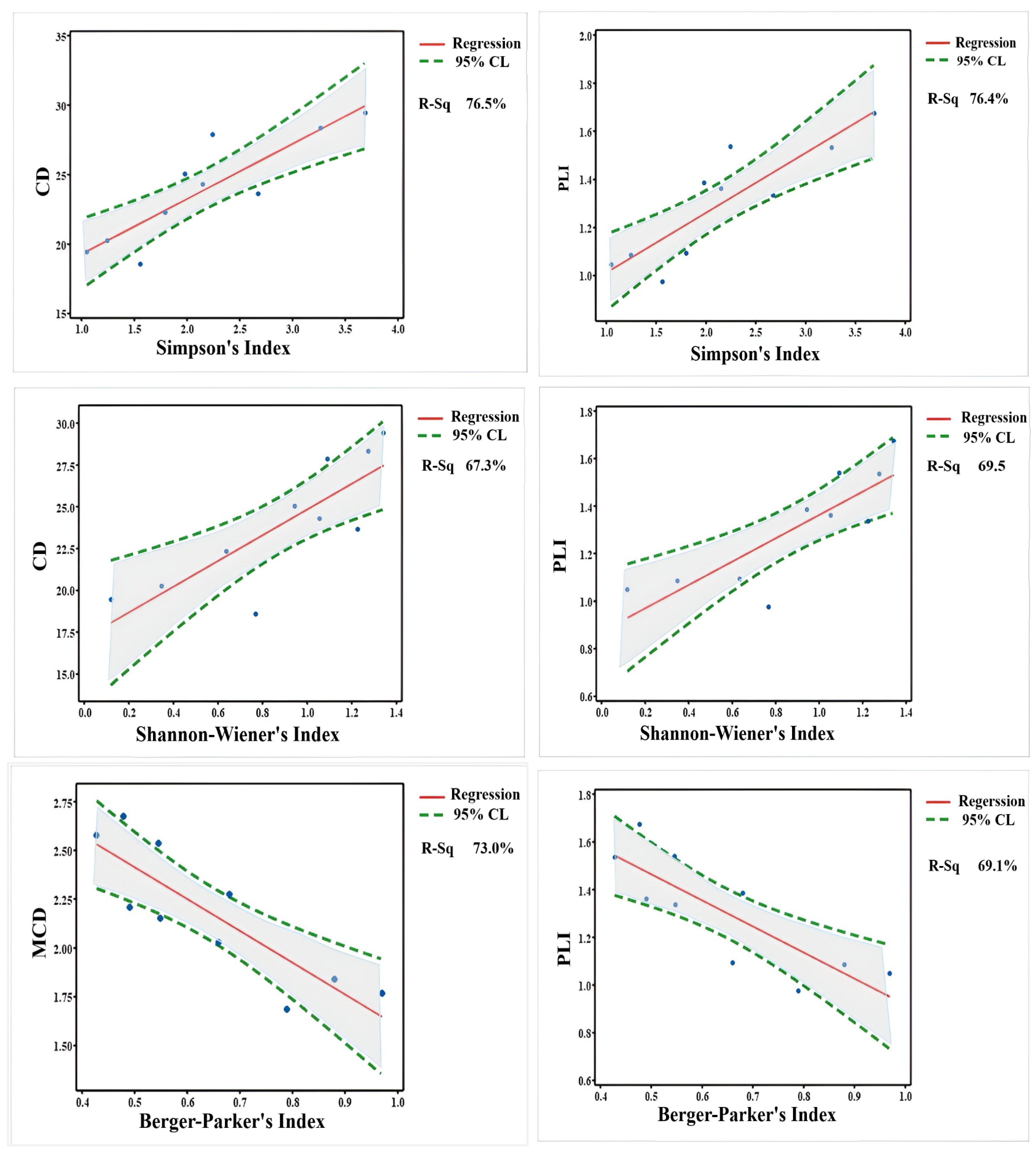
3.3. Species Diversity
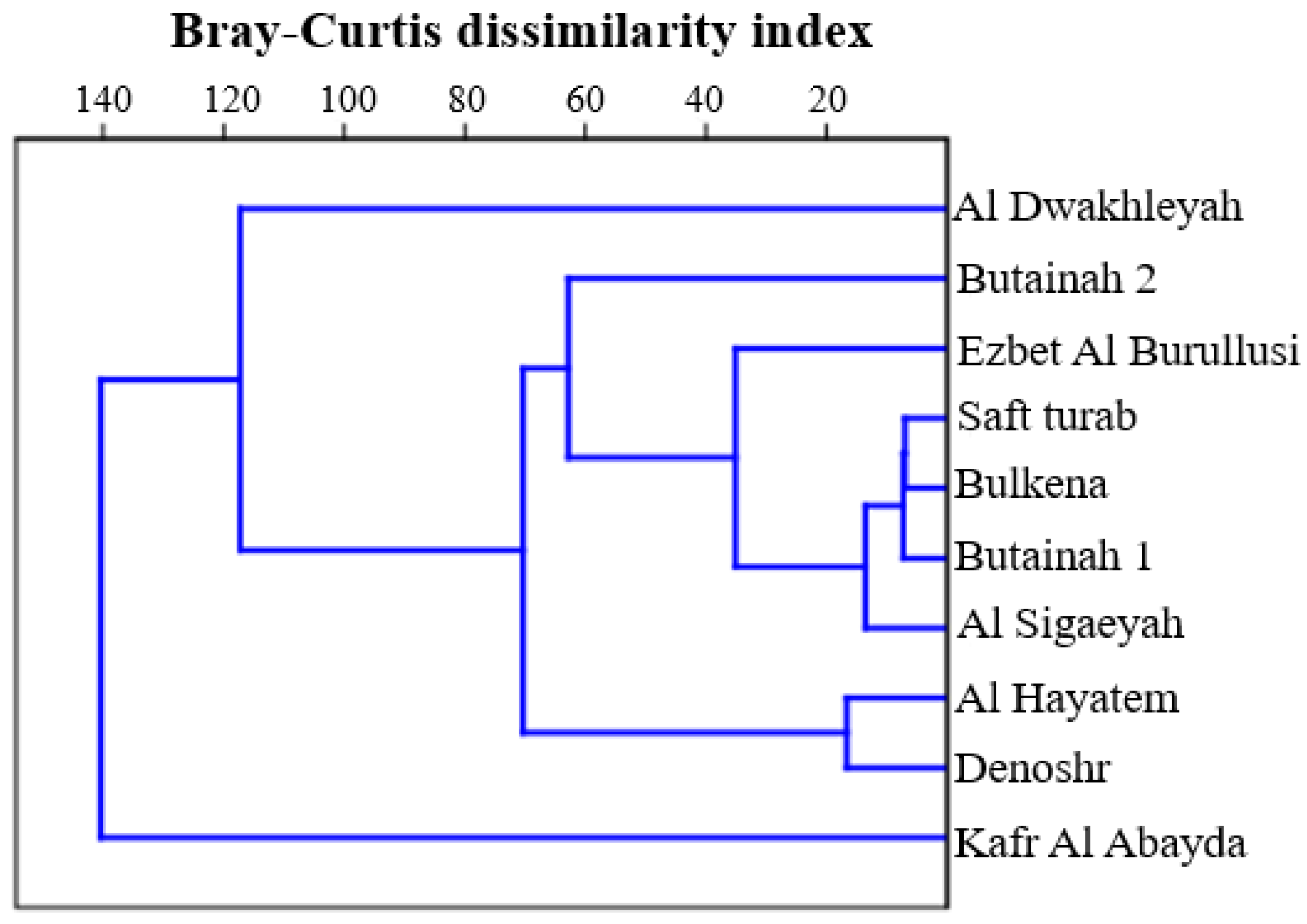
3.4. Species Similarity
3.5. Dominance and Abundance
4. Discussion
4.1. Soil Properties and Pollution Indices
4.2. Soil Fauna and Biota Diversity
4.3. Correlations Between Pollution Indices and Biota Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teschke, R. Aluminum, Arsenic, Beryllium, Cadmium, Chromium, Cobalt, Copper, Iron, Lead, Mercury, Molybdenum, Nickel, Platinum, Thallium, Titanium, Vanadium, and Zinc: Molecular Aspects in Experimental Liver Injury. Int. J. Mol. Sci. 2022, 23, 12213. [Google Scholar] [CrossRef] [PubMed]
- Fred-Ahmadu, O.H.; Ayejuyo, O.O.; Tenebe, I.T.; Benson, N.U. Occurrence and Distribution of Micro(Meso)Plastic-Sorbed Heavy Metals and Metalloids in Sediments, Gulf of Guinea Coast (SE Atlantic). Sci. Total Environ. 2022, 813, 152650. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zheng, H.; Liu, F.; Ding, Z.; Wang, Z.; Peng, Y.; Zhang, D.; Zhang, Y.; Zheng, Y.; Ding, A. Heavy Metal Contamination Assessment and Source Attribution in the Vicinity of an Iron Slag Pile in Hechi, China: Integrating Multi-Medium Analysis. Environ. Res. 2024, 263, 120206. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.H.A.; Ali, M.; Abbas, M.H.H.; Bassouny, M.A. Effects of Industrialization Processes in Giza Factories (Egypt) on Soil and Water Quality in Adjacent Territories. Egypt. J. Soil Sci. 2022, 62, 253–266. [Google Scholar] [CrossRef]
- Goher, M.E.; Mangood, A.H.; Mousa, I.E.; Salem, S.G.; Hussein, M.M. Ecological Risk Assessment of Heavy Metal Pollution in Sediments of Nile River, Egypt. Environ. Monit. Assess. 2021, 193, 703. [Google Scholar] [CrossRef]
- EL-Sharkawy, M.; Sleem, M.; Du, D.; El Baroudy, A.; Li, J.; Mahmoud, E.; Ali, N. Nano-water Treatment Residuals: Enhancing Phosphorus Kinetics and Optimization in Saline Soils. Land Degrad. Dev. 2024, 35, 3314–3329. [Google Scholar] [CrossRef]
- El-Sharkawy, M.; Li, J.; Kamal, N.; Mahmoud, E.; Omara, A.E.-D.; Du, D. Assessing and Predicting Soil Quality in Heavy Metal-Contaminated Soils: Statistical and ANN-Based Techniques. J. Soil Sci. Plant Nutr. 2023, 23, 6510–6526. [Google Scholar] [CrossRef]
- Mohammed, A.M.F.; Saleh, I.A.; Zahran, H.R.; Abdel-Latif, N.M. Ecological and Risk Assessment of Heavy Metals in a Diverse Industrial Area of Al-Akrasha, Egypt. Atmosphere 2023, 14, 1745. [Google Scholar] [CrossRef]
- El-Sharkawy, M.; Alotaibi, M.O.; Li, J.; Du, D.; Mahmoud, E. Heavy Metal Pollution in Coastal Environments: Ecological Implications and Management Strategies: A Review. Sustainability 2025, 17, 701. [Google Scholar] [CrossRef]
- Zheng, J.; Weng, C.; Tian, C.; Zhang, W.; Qin, J.; Li, X.; Liu, W.; Zhang, J.; Lin, Z. Ingenious Approach for Retrieving Valuable Metals from Gypsum via Dehydration–Rehydration Two-Step Phase Transition. Chem. Eng. J. 2024, 491, 152122. [Google Scholar] [CrossRef]
- Behan-Pelletier, V.; Lindo, Z. Oribatid Mites: Biodiversity, Taxonomy and Ecology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2023; ISBN 1003214649. [Google Scholar]
- Neemisha. Role of Soil Organisms in Maintaining Soil Health, Ecosystem Functioning, and Sustaining Agricultural Production. In Soil Health; Giri, B., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-44364-1. [Google Scholar]
- Brown, E.D.; Williams, B.K. Ecological Integrity Assessment as a Metric of Biodiversity: Are We Measuring What We Say We Are? Biodivers. Conserv. 2016, 25, 1011–1035. [Google Scholar] [CrossRef]
- Pennekamp, F.; Pontarp, M.; Tabi, A.; Altermatt, F.; Alther, R.; Choffat, Y.; Fronhofer, E.A.; Ganesanandamoorthy, P.; Garnier, A.; Griffiths, J.I.; et al. Biodiversity Increases and Decreases Ecosystem Stability. Nature 2018, 563, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Manu, M.; Honciuc, V.; Neagoe, A.; Băncilă, R.I.; Iordache, V.; Onete, M. Soil Mite Communities (Acari: Mesostigmata, Oribatida) as Bioindicators for Environmental Conditions from Polluted Soils. Sci. Rep. 2019, 9, 20250. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.E.; Proctor, H.C. Mites in Soil and Litter Systems. In Mites: Ecology, Evolution & Behaviour: Life at a Microscale; Walter, D.E., Proctor, H.C., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 161–228. ISBN 978-94-007-7164-2. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Cottenie, A.; Verloo, M.; Kiekens, L. Chemical Analysis of Plants and Soils; RUG Laboratory of Analytical and Agrochemistry: Gent, Belgium, 1982; Volume 42. [Google Scholar]
- Ladd, J.N.; Amato, M. Relationship between Microbial Biomass Carbon in Soils and Absorbance (260 Nm) of Extracts of Fumigated Soils. Soil Biol. Biochem. 1989, 21, 457–459. [Google Scholar] [CrossRef]
- Schnitzer, M. Chapter 1 Humic Substances: Chemistry and Reactions. In Soil Organic Matter; Schnitzer, M., Khan, S.U., Eds.; Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 1978; Volume 8, pp. 1–64. ISBN 0166-2481. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; Wiley: New York, NY, USA, 1994; ISBN 9780471594741. [Google Scholar]
- Allen, O.N. Experiments in Soil Bacteriology. Soil Sci. 1958, 85, 172. [Google Scholar] [CrossRef]
- Siddique, M.A.B.; Alam, M.K.; Islam, S.; Diganta, M.T.M.; Akbor, M.A.; Bithi, U.H.; Chowdhury, A.I.; Ullah, A.K.M.A. Apportionment of Some Chemical Elements in Soils around the Coal Mining Area in Northern Bangladesh and Associated Health Risk Assessment. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100366. [Google Scholar] [CrossRef]
- Weissmannová, H.D.; Pavlovský, J. Indices of Soil Contamination by Heavy Metals–Methodology of Calculation for Pollution Assessment (Minireview). Environ. Monit. Assess. 2017, 189, 616. [Google Scholar] [CrossRef]
- IAEA AQCS Catalogue for Reference Materials and Intercomparison Exercises 1998/1999; International Atomic Energy Agency: Vienna, Austria, 1998.
- Al-Assiuty, A.I.M.; Bayoumi, B.M.; Khalil, M.A.; Van Straalen, N.M. The Influence of Vegetational Type on Seasonal Abundance and Species Composition of Soil Fauna at Different Localities in Egypt. Pedobiologia 1993, 37, 210–222. [Google Scholar] [CrossRef]
- Silver, J.B. (Ed.) Indices of Association and Species Diversity Indices. In Mosquito Ecology: Field Sampling Methods; Springer: Dordrecht, The Netherlands, 2008; pp. 1445–1467. ISBN 978-1-4020-6666-5. [Google Scholar]
- Roberts, F.S. Measurement of Biodiversity: Richness and Evenness. In Mathematics of Planet Earth: Protecting Our Planet, Learning from the Past, Safeguarding for the Future; Kaper, H.G., Roberts, F.S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 203–224. ISBN 978-3-030-22044-0. [Google Scholar]
- Pielou, E.C. The Measurement of Diversity in Different Types of Biological Collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Sorensen, T. A Method of Establishing Groups of Equal Amplitude in Plant Sociology Based on Similarity of Species Content and Its Application to Analyses of the Vegetation on Danish Commons. Biol. Skr. 1948, 5, 1–34. [Google Scholar] [CrossRef]
- Magurran, A.E. A Variety of Diversities. In Ecological Diversity and Its Measurement; Magurran, A.E., Ed.; Springer: Dordrecht, The Netherlands, 1988; pp. 81–99. ISBN 978-94-015-7358-0. [Google Scholar]
- Engelmann, H.-D. Zur Dominanzklassifizierung von Bodenarthropoden. Pedobiologia 1978, 18, 378–380. [Google Scholar] [CrossRef]
- Berger, W.H.; Parker, F.L. Diversity of Planktonic Foraminifera in Deep-Sea Sediments. Science 1970, 168, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of Heavy Metal(Loid)s Contaminated Soils—To Mobilize or to Immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz, A.M.; Kapusta, P.; Zubek, S.; Stanek, M.; Woch, M.W. Soil Organic Matter Prevails over Heavy Metal Pollution and Vegetation as a Factor Shaping Soil Microbial Communities at Historical Zn–Pb Mining Sites. Chemosphere 2020, 240, 124922. [Google Scholar] [CrossRef]
- Gao, J.; Han, H.; Gao, C.; Wang, Y.; Dong, B.; Xu, Z. Organic Amendments for in Situ Immobilization of Heavy Metals in Soil: A Review. Chemosphere 2023, 335, 139088. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of Heavy Metals in Soils and Their Immobilization at Micro-Scale Interfaces among Diverse Soil Components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef]
- Zhuo, T.; He, L.; Chai, B.; Zhou, S.; Wan, Q.; Lei, X.; Zhou, Z.; Chen, B. Micro-Pressure Promotes Endogenous Phosphorus Release in a Deep Reservoir by Favouring Microbial Phosphate Mineralisation and Solubilisation Coupled with Sulphate Reduction. Water Res. 2023, 245, 120647. [Google Scholar] [CrossRef]
- Pan, X.-R.; Shang-Guan, P.-K.; Li, S.-H.; Zhang, C.-H.; Lou, J.-M.; Guo, L.; Liu, L.; Lu, Y. The Influence of Carbon Dioxide on Fermentation Products, Microbial Community, and Functional Gene in Food Waste Fermentation with Uncontrol PH. Environ. Res. 2025, 267, 120645. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, X.; Xiong, T.; Wang, H.; Jiang, L. Bioremediation of Co-Contaminated Soil with Heavy Metals and Pesticides: Influence Factors, Mechanisms and Evaluation Methods. Chem. Eng. J. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Khan, S.; El-Latif Hesham, A.; Qiao, M.; Rehman, S.; He, J.-Z. Effects of Cd and Pb on Soil Microbial Community Structure and Activities. Environ. Sci. Pollut. Res. 2010, 17, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Lester, L.A. Statistical Analysis: Metal Concentrations in Soil; Department of Environmental Protection and Energy, Division of Science and Research: Trenton, NJ, USA, 2020; pp. 1–40.
- Mahey, S.; Kumar, R.; Sharma, M.; Kumar, V.; Bhardwaj, R. A Critical Review on Toxicity of Cobalt and Its Bioremediation Strategies. SN Appl. Sci. 2020, 2, 1279. [Google Scholar] [CrossRef]
- Barałkiewicz, D.; Siepak, J. Chromium, Nickel and Cobalt in Environmental Samples and Existing Legal Norms. Pol. J. Environ. Stud. 1999, 8, 201–208. [Google Scholar]
- Kiran; Bharti, R.; Sharma, R. Effect of Heavy Metals: An Overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar] [CrossRef]
- Khatri, N.; Tyagi, S. Influences of Natural and Anthropogenic Factors on Surface and Groundwater Quality in Rural and Urban Areas. Front. Life Sci. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Yu, K.; Chai, B.; Zhuo, T.; Tang, Q.; Gao, X.; Wang, J.; He, L.; Lei, X.; Chen, B. Hydrostatic Pressure Drives Microbe-Mediated Biodegradation of Microplastics in Surface Sediments of Deep Reservoirs: Novel Findings from Hydrostatic Pressure Simulation Experiments. Water Res. 2023, 242, 120185. [Google Scholar] [CrossRef]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of Cadmium Pollution on Food Safety and Human Health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Soleimani, H.; Mansouri, B.; Kiani, A.; Omer, A.K.; Tazik, M.; Ebrahimzadeh, G.; Sharafi, K. Ecological Risk Assessment and Heavy Metals Accumulation in Agriculture Soils Irrigated with Treated Wastewater Effluent, River Water, and Well Water Combined with Chemical Fertilizers. Heliyon 2023, 9, e14580. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 9780429191121. [Google Scholar]
- Ukhurebor, K.E.; Aigbe, U.O.; Onyancha, R.B.; Nwankwo, W.; Osibote, O.A.; Paumo, H.K.; Ama, O.M.; Adetunji, C.O.; Siloko, I.U. Effect of Hexavalent Chromium on the Environment and Removal Techniques: A Review. J. Environ. Manag. 2021, 280, 111809. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.S.; Rezania, S.; Radwan, N.; Alam, J. Chromium Contamination and Effect on Environmental Health and Its Remediation: A Sustainable Approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A Review of Soil Heavy Metal Pollution from Industrial and Agricultural Regions in China: Pollution and Risk Assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Nweke, M.O.; Ukpai, S.N. Use of Enrichment, Ecological Risk and Contamination Factors with Geoaccumulation Indexes to Evaluate Heavy Metal Contents in the Soils around Ameka Mining Area, South of Abakaliki, Nigeria. J. Geogr. Environ. Earth Sci. Int. 2016, 5, 1–13. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. Int. Sch. Res. Not. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy Metals in Agricultural Soils of the European Union with Implications for Food Safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of Heavy Metal-Contaminated Sites: Eco-Environmental Concerns, Field Studies, Sustainability Issues, and Future Prospects. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Springer International Publishing: Cham, Switzerland, 2020; Volume 249, pp. 71–131. ISBN 978-3-030-20194-4. [Google Scholar]
- Hayat, M.T.; Nauman, M.; Nazir, N.; Ali, S.; Bangash, N. Chapter 7—Environmental Hazards of Cadmium: Past, Present, and Future. In Cadmium Toxicity and Tolerance in Plants; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 163–183. ISBN 978-0-12-814864-8. [Google Scholar]
- Soni, S.; Jha, A.B.; Dubey, R.S.; Sharma, P. Mitigating Cadmium Accumulation and Toxicity in Plants: The Promising Role of Nanoparticles. Sci. Total Environ. 2024, 912, 168826. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The Relative Impact of Toxic Heavy Metals (THMs) (Arsenic (As), Cadmium (Cd), Chromium (Cr)(VI), Mercury (Hg), and Lead (Pb)) on the Total Environment: An Overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Qiao, D.; Wang, G.; Li, X.; Wang, S.; Zhao, Y. Pollution, Sources and Environmental Risk Assessment of Heavy Metals in the Surface AMD Water, Sediments and Surface Soils around Unexploited Rona Cu Deposit, Tibet, China. Chemosphere 2020, 248, 125988. [Google Scholar] [CrossRef]
- Chen, T.-B.; Zheng, Y.-M.; Lei, M.; Huang, Z.-C.; Wu, H.-T.; Chen, H.; Fan, K.-K.; Yu, K.; Wu, X.; Tian, Q.-Z. Assessment of Heavy Metal Pollution in Surface Soils of Urban Parks in Beijing, China. Chemosphere 2005, 60, 542–551. [Google Scholar] [CrossRef]
- Nawrot, N.; Wojciechowska, E.; Mohsin, M.; Kuittinen, S.; Pappinen, A.; Rezania, S. Trace Metal Contamination of Bottom Sediments: A Review of Assessment Measures and Geochemical Background Determination Methods. Minerals 2021, 11, 872. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.-K.; Noh, H.-J.; Byun, Y.J.; Chung, H.-M.; Kim, J.-I. Source Identification and Assessment of Heavy Metal Contamination in Urban Soils Based on Cluster Analysis and Multiple Pollution Indices. J. Soils Sediments 2021, 21, 1947–1961. [Google Scholar] [CrossRef]
- Proshad, R.; Abedin Asha, S.M.A.; Abedin, M.A.; Chen, G.; Li, Z.; Zhang, S.; Tan, R.; Lu, Y.; Zhang, X.; Zhao, Z. Pollution Area Identification, Receptor Model-Oriented Sources and Probabilistic Health Hazards to Prioritize Control Measures for Heavy Metal Management in Soil. J. Environ. Manag. 2024, 369, 122322. [Google Scholar] [CrossRef] [PubMed]
- Zerizghi, T.; Guo, Q.; Tian, L.; Wei, R.; Zhao, C. An Integrated Approach to Quantify Ecological and Human Health Risks of Soil Heavy Metal Contamination around Coal Mining Area. Sci. Total Environ. 2022, 814, 152653. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Rad, S.; Xu, L.; Gui, L.; Song, X.; Li, Y.; Wu, Z.; Chen, Z. Heavy Metals Distribution, Sources, and Ecological Risk Assessment in Huixian Wetland, South China. Water 2020, 12, 431. [Google Scholar] [CrossRef]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control.a Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in Soils and Groundwater: A Review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Licen, S.; Astel, A.; Tsakovski, S. Self-Organizing Map Algorithm for Assessing Spatial and Temporal Patterns of Pollutants in Environmental Compartments: A Review. Sci. Total Environ. 2023, 878, 163084. [Google Scholar] [CrossRef]
- Zhao, Y.; Yi, J.; Yao, R.; Li, F.; Hill, R.L.; Gerke, H.H. Dimensionality and Scales of Preferential Flow in Soils of Shale Hills Hillslope Simulated Using HYDRUS. Vadose Zone J. 2024, 23, e20367. [Google Scholar] [CrossRef]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in Soil PH and Mobility of Heavy Metals in Contaminated Soils. Eur. J. Soil Sci. 2022, 73, e13203. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, Y. A Novel Heat Pulse Method in Determining “Effective” Thermal Properties in Frozen Soil. Water Resour. Res. 2024, 60, e2024WR037537. [Google Scholar] [CrossRef]
- Ertiban, S.M. Soil Fauna as Webmasters, Engineers and Bioindicators in Ecosystems: Implications for Conservation Ecology and Sustainable Agriculture. Am. J. Life Sci. 2019, 7, 17–26. [Google Scholar] [CrossRef]
- Edwards, C.A.; Arancon, N.Q. (Eds.) Earthworms, Soil Structure, Fertility, and Productivity. In Biology and Ecology of Earthworms; Springer: New York, NY, USA, 2022; pp. 303–334. ISBN 978-0-387-74943-3. [Google Scholar]
- Zhao, L.; Zhang, F.-S.; Wang, K.; Zhu, J. Chemical Properties of Heavy Metals in Typical Hospital Waste Incinerator Ashes in China. Waste Manag. 2009, 29, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Song, B.; Dong, W.; Xin, W.; Wang, Y. A Review on the Eco-Geography of Soil Fauna in China. J. Geogr. Sci. 2010, 20, 333–346. [Google Scholar] [CrossRef]
- El-Kahawy, R.; El-Shafeiy, M.; Helal, S.; Aboul-Ela, N.; Abd El-Wahab, M. Benthic Ostracods (Crustacean) as a Nearshore Pollution Bio-Monitor: Examples from the Red Sea Coast of Egypt. Environ. Sci. Pollut. Res. 2021, 28, 31975–31993. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhu, D.; Wang, H.-T.; Lassen, S.B.; Chen, Q.-L.; Li, G.; Lv, M.; Zhu, Y.-G. Dysbiosis in the Gut Microbiota of Soil Fauna Explains the Toxicity of Tire Tread Particles. Environ. Sci. Technol. 2020, 54, 7450–7460. [Google Scholar] [CrossRef]
- Shah, K.; Dubey, R.S. Cadmium Elevates Level of Protein, Amino Acids and Alters Activity of Proteolytic Enzymes in Germinating Rice Seeds. Acta Physiol. Plant. 1998, 20, 189–196. [Google Scholar] [CrossRef]
- van Noordwijk, M.; Schoonderbeek, D.; Kooistra, M.J. Root—Soil Contact of Field-Grown Winter WheatααCommunication No. 45 of the Dutch Programme on Soil Ecology of Arable Farming Systems. In Soil Structure/Soil Biota Interrelationships; Brussaard, L., Kooistra, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 277–286. ISBN 978-0-444-81490-6. [Google Scholar]
- Roane, T.M.; Kellogg, S.T. Characterization of Bacterial Communities in Heavy Metal Contaminated Soils. Can. J. Microbiol. 1996, 42, 593–603. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement, 1st ed.; Springer: Dordrecht, The Netherlands, 1988; p. 179. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Nelson, K.; Palmer, M.A. Linking Species Diversity to the Functioning of Ecosystems: On the Importance of Environmental Context. Oikos 2000, 91, 175–183. [Google Scholar] [CrossRef]
- Cachada, A.; Rocha-Santos, T.; Duarte, A.C. Chapter 1—Soil and Pollution: An Introduction to the Main Issues. In Soil Pollution; Duarte, A.C., Cachada, A., Rocha-Santos, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–28. ISBN 978-0-12-849873-6. [Google Scholar]
- Rahman, S.H.; Khanam, D.; Adyel, T.M.; Islam, M.S.; Ahsan, M.A.; Akbor, M.A. Assessment of Heavy Metal Contamination of Agricultural Soil around Dhaka Export Processing Zone (DEPZ), Bangladesh: Implication of Seasonal Variation and Indices. Appl. Sci. 2012, 2, 584–601. [Google Scholar] [CrossRef]
- Jorfi, S.; Maleki, R.; Jaafarzadeh, N.; Ahmadi, M. Pollution Load Index for Heavy Metals in Mian-Ab Plain Soil, Khuzestan, Iran. Data Br. 2017, 15, 584–590. [Google Scholar] [CrossRef]
- Srinivasa Gowd, S.; Ramakrishna Reddy, M.; Govil, P.K. Assessment of Heavy Metal Contamination in Soils at Jajmau (Kanpur) and Unnao Industrial Areas of the Ganga Plain, Uttar Pradesh, India. J. Hazard. Mater. 2010, 174, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Shao, C.; Ju, M. Heavy Metal Contamination Assessment and Partition for Industrial and Mining Gathering Areas. Int. J. Environ. Res. Public Health 2014, 11, 7286–7303. [Google Scholar] [CrossRef] [PubMed]



| Sites | pH | EC | OM | OC | HA | FA | MBC | Total Bacterial Count × 106 |
|---|---|---|---|---|---|---|---|---|
| - | dS m−1 | % | % | % | % | % | CFU g−1 | |
| S1 | 7.40 | 0.62 | 1.33 | 0.77 | 0.15 | 0.40 | 0.55 | 15.50 |
| S2 | 7.60 | 0.27 | 2.48 | 1.44 | 0.29 | 0.74 | 1.66 | 21.50 |
| S3 | 8.03 | 4.63 | 1.22 | 0.71 | 0.14 | 0.37 | 0.44 | 21.50 |
| S4 | 8.06 | 0.21 | 1.40 | 0.81 | 0.16 | 0.42 | 3.60 | 12.50 |
| S5 | 7.96 | 0.37 | 1.09 | 0.63 | 0.13 | 0.33 | 1.35 | 8.00 |
| S6 | 7.85 | 0.79 | 2.16 | 1.25 | 0.25 | 0.65 | 0.55 | 26.00 |
| S7 | 7.70 | 1.04 | 1.71 | 0.99 | 0.20 | 0.51 | 0.99 | 18.00 |
| S8 | 7.38 | 2.16 | 1.19 | 0.69 | 0.14 | 0.36 | 0.32 | 6.00 |
| S9 | 7.69 | 1.01 | 1.81 | 1.05 | 0.21 | 0.54 | 0.48 | 39.00 |
| S10 | 8.26 | 0.21 | 1.31 | 0.76 | 0.15 | 0.39 | 1.70 | 17.00 |
| Sites | Al | Co | Cu | Fe | Mn | Ni | TI | Zn | Cd | Cr | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 136,905.60 h | 18.72 h | 76.06 f | 1398.52 f | 209.95 e | 7.11 d | 7213.81 f | 6.81 g | 0.52 e | 42.23 c | 20.27 f |
| S2 | 150,616.08 f | 35.38 b | 104.51 b | 2038.76 b | 332.52 c | 11.13 b | 13,353.51 ab | 17.77 b | 1.00 a | 29.24 f | 29.33 b |
| S3 | 209,621.93 a | 37.96 a | 110.44 a | 2259.97 a | 376.11 b | 11.36 b | 11,936.98 c | 14.98 e | 0.87 bc | 49.62 b | 31.77 a |
| S4 | 145,476.75 g | 20.89 g | 104.50 b | 1394.03 f | 171.95 f | 6.94 d | 6918.79 f | 7.25 g | 0.58 de | 83.98 a | 19.92 f |
| S5 | 129,275.75 i | 23.94 f | 61.21 g | 1532.30 e | 333.73 c | 11.25 b | 9616.85 e | 19.30 a | 0.94 ab | 6.16 h | 22.16 e |
| S6 | 153,285.47 d | 32.29 c | 80.31 e | 2044.30 b | 487.32 a | 12.73 a | 13,106.47 b | 16.86 bc | 0.91 b | 10.61 g | 28.56 b |
| S7 | 162,324.01 b | 29.60 d | 89.05 d | 1898.77 c | 328.13 c | 10.45 b | 10,565.50 d | 11.84 f | 0.81 c | 34.07 d | 27.22 c |
| S8 | 114,825.60 j | 23.60 f | 57.13 h | 1530.00 e | 365.35 b | 11.53 b | 9708.53 e | 15.60 de | 0.90 b | 6.16 h | 21.61 e |
| S9 | 160,880.06 c | 27.76 e | 96.40 c | 1775.64 d | 258.05 d | 8.90 c | 9504.86 e | 16.48 cd | 0.63 d | 49.86 b | 25.13 d |
| S10 | 151,972.13 e | 37.75 a | 109.14 a | 2026.64 b | 328.33 c | 11.02 b | 13,523.25 a | 15.27 e | 0.92 ab | 32.20 e | 29.38 b |
| LSD (0.05) | 1025.86 | 1.05 | 1.60 | 74.42 | 11.79 | 1.08 | 334.02 | 0.99 | 0.08 | 1.43 | 0.78 |
| Sites | Al | Co | Cu | Fe | Mn | Ni | TI | Zn | Cd | Cr | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 2.91 h | 2.103 h | 6.915 f | 0.054 f | 0.333 e | 0.730 d | 2.40 f | 0.065 g | 2.003 e | 0.704 c | 0.338 f |
| S2 | 3.20 f | 3.976 b | 9.501 b | 0.079 b | 0.527 c | 1.143 b | 4.45 ab | 0.171 b | 3.861 a | 0.487 f | 0.489 b |
| S3 | 4.46 a | 4.265 a | 10.040 a | 0.088 a | 0.596 b | 1.166 b | 3.98 c | 0.144 e | 3.351 bc | 0.827 b | 0.530 a |
| S4 | 3.10 g | 2.347 g | 9.500 b | 0.054 f | 0.273 f | 0.712 d | 2.31 f | 0.070 g | 2.223 de | 1.400 a | 0.332 f |
| S5 | 2.75 i | 2.690 f | 5.565 g | 0.060 e | 0.529 c | 1.155 b | 3.21 e | 0.186 a | 3.630 ab | 0.103 h | 0.369 e |
| S6 | 3.26 d | 3.628 c | 7.301 e | 0.080 b | 0.772 a | 1.307 a | 4.37 b | 0.162 bc | 3.501 b | 0.177 g | 0.476 b |
| S7 | 3.45 b | 3.326 d | 8.095 d | 0.074 c | 0.520 c | 1.073 b | 3.52 d | 0.114 f | 3.109 c | 0.568 d | 0.454 c |
| S8 | 2.44 j | 2.652 f | 5.194 h | 0.060 e | 0.579 b | 1.183 b | 3.24 e | 0.150 de | 3.470 b | 0.103 h | 0.360 e |
| S9 | 3.42 c | 3.119 e | 8.764 c | 0.069 d | 0.409 d | 0.913 c | 3.17 e | 0.158 cd | 2.405 d | 0.831 b | 0.419 d |
| S10 | 3.23 e | 4.242 a | 9.922 a | 0.079 b | 0.520 c | 1.132 b | 4.51 a | 0.147 e | 3.545 ab | 0.537 e | 0.490 b |
| LSD (0.05) | 0.02 | 0.12 | 0.15 | 0.002 | 0.01 | 0.11 | 0.1 | 0.009 | 0.31 | 0.02 | 0.01 |
| Sites | Al | Co | Cu | Fe | Mn | Ni | TI | Zn | Cd | Cr | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 0.10 h | −0.49 h | 1.02 f | −5.23 f | −2.22 e | −2.88 d | −0.19 e | −4.05 f | 0.21 e | −1.59 c | −0.57 f |
| S2 | 0.24 f | 0.43 b | 1.48 b | −4.69 b | −1.55 c | −2.24 b | 0.69 a | −2.66 ab | 1.16 a | −2.12 f | −0.03 b |
| S3 | 0.72 a | 0.53 a | 1.56 a | −4.54 a | −1.37 b | −2.21 b | 0.53 b | −2.91 d | 0.95 bc | −1.36 b | 0.08 a |
| S4 | 0.19 g | −0.33 g | 1.48 b | −5.23 f | −2.50 f | −2.92 d | −0.25 f | −3.96 f | 0.36 de | −0.60 a | −0.59 f |
| S5 | 0.02 i | −0.13 f | 0.71 g | −5.10 e | −1.55 c | −2.22 b | 0.22 d | −2.54 a | 1.07 ab | −4.37 h | −0.44 e |
| S6 | 0.27 d | 0.30 c | 1.10 e | −4.68 b | −1.00 a | −2.04 a | 0.67 a | −2.74 bc | 1.02 ab | −3.59 g | −0.07 b |
| S7 | 0.35 b | 0.17 d | 1.25 d | −4.79 c | −1.57 c | −2.33 b | 0.36 c | −3.25 e | 0.84 c | −1.90 d | −0.14 c |
| S8 | −0.15 j | −0.15 f | 0.61 h | −5.10 e | −1.42 b | −2.19 ab | 0.23 d | −2.85 cd | 1.00 ab | −4.37 h | −0.47 e |
| S9 | 0.34 c | 0.08 e | 1.36 c | −4.89 d | −1.92 d | −2.56 c | 0.20 d | −2.77 bcd | 0.47 d | −1.35 b | −0.26 d |
| S10 | 0.25 e | 0.52 a | 1.54 a | −4.70 b | −1.57 c | −2.25 b | 0.71 a | −2.88 cd | 1.03 ab | −1.99 e | −0.03 b |
| LSD (0.05) | 0.009 | 0.05 | 0.02 | 0.06 | 0.04 | 0.15 | 0.04 | 0.14 | 0.14 | 0.06 | 0.05 |
| Site | Orbatida | Prostigmata | Mesostigmata | Collembola | Nematode | Total No. of Sp. |
|---|---|---|---|---|---|---|
| S1 | 129 | 13 | 3 | 5 | 13 | 163 |
| S2 | 45 | 65 | 1 | 7 | 1 | 119 |
| S3 | 10 | 11 | 5 | 7 | 0 | 33 |
| S4 | 1 | 2 | 0 | 0 | 0 | 3 |
| S5 | 8 | 1 | 0 | 0 | 0 | 9 |
| S6 | 15 | 8 | 0 | 5 | 60 | 88 |
| S7 | 54 | 52 | 2 | 2 | 0 | 110 |
| S8 | 0 | 38 | 0 | 1 | 0 | 39 |
| S9 | 57 | 21 | 10 | 131 | 20 | 239 |
| S10 | 4 | 2 | 6 | 2 | 0 | 14 |
| Sites | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | Similarity Degree Keys | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 1 | >0.75 | ||||||||||
| S2 | 0.460993 | 1 | 0.5–0.75 | |||||||||
| S3 | 0.295918 | 0.381579 | 1 | 0.25–0.5 | ||||||||
| S4 | 0.036145 | 0.04918 | 0.166667 | 1 | <0.25 | |||||||
| S5 | 0.104651 | 0.140625 | 0.428571 | 0.333333 | 1 | |||||||
| S6 | 0.326693 | 0.280193 | 0.380165 | 0.065934 | 0.185567 | 1 | ||||||
| S7 | 0.520147 | 0.873362 | 0.34965 | 0.053097 | 0.151261 | 0.252525 | 1 | |||||
| S8 | 0.138614 | 0.493671 | 0.333333 | 0.024793 | 0.072581 | 0.141732 | 0.52349 | 1 | ||||
| S9 | 0.452736 | 0.418994 | 0.242647 | 0.095238 | 0.041667 | 0.293578 | 0.452722 | 0.158273 | 1 | |||
| S10 | 0.124294 | 0.135338 | 0.553191 | 0.352941 | 0.434783 | 0.156863 | 0.16129 | 0.113208 | 0.110672 | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sharkawy, G.; Alotaibi, M.O.; Zuhair, R.; Mahmoud, E.; El Baroudy, A.; Omara, A.E.-D.; El-Sharkawy, M. Ecological Assessment of Polluted Soils: Linking Ecological Risks, Soil Quality, and Biota Diversity in Contaminated Soils. Sustainability 2025, 17, 1524. https://doi.org/10.3390/su17041524
El-Sharkawy G, Alotaibi MO, Zuhair R, Mahmoud E, El Baroudy A, Omara AE-D, El-Sharkawy M. Ecological Assessment of Polluted Soils: Linking Ecological Risks, Soil Quality, and Biota Diversity in Contaminated Soils. Sustainability. 2025; 17(4):1524. https://doi.org/10.3390/su17041524
Chicago/Turabian StyleEl-Sharkawy, Ghada, Modhi O. Alotaibi, Raghda Zuhair, Esawy Mahmoud, Ahmed El Baroudy, Alaa El-Dein Omara, and Mahmoud El-Sharkawy. 2025. "Ecological Assessment of Polluted Soils: Linking Ecological Risks, Soil Quality, and Biota Diversity in Contaminated Soils" Sustainability 17, no. 4: 1524. https://doi.org/10.3390/su17041524
APA StyleEl-Sharkawy, G., Alotaibi, M. O., Zuhair, R., Mahmoud, E., El Baroudy, A., Omara, A. E.-D., & El-Sharkawy, M. (2025). Ecological Assessment of Polluted Soils: Linking Ecological Risks, Soil Quality, and Biota Diversity in Contaminated Soils. Sustainability, 17(4), 1524. https://doi.org/10.3390/su17041524







