The Variations in Soil Microbial Communities and Their Mechanisms Along an Elevation Gradient in the Qilian Mountains, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Study Site Establishment and Sample Collection and Treatment
2.3. Sample Determination
2.3.1. Determination of Soil Physiochemical Properties
2.3.2. Determination of Soil Microorganisms
2.4. Statistical Analyses
3. Results
3.1. Soil Properties Along an Elevation Gradient
3.2. Plant Diversity Along an Elevation Gradient
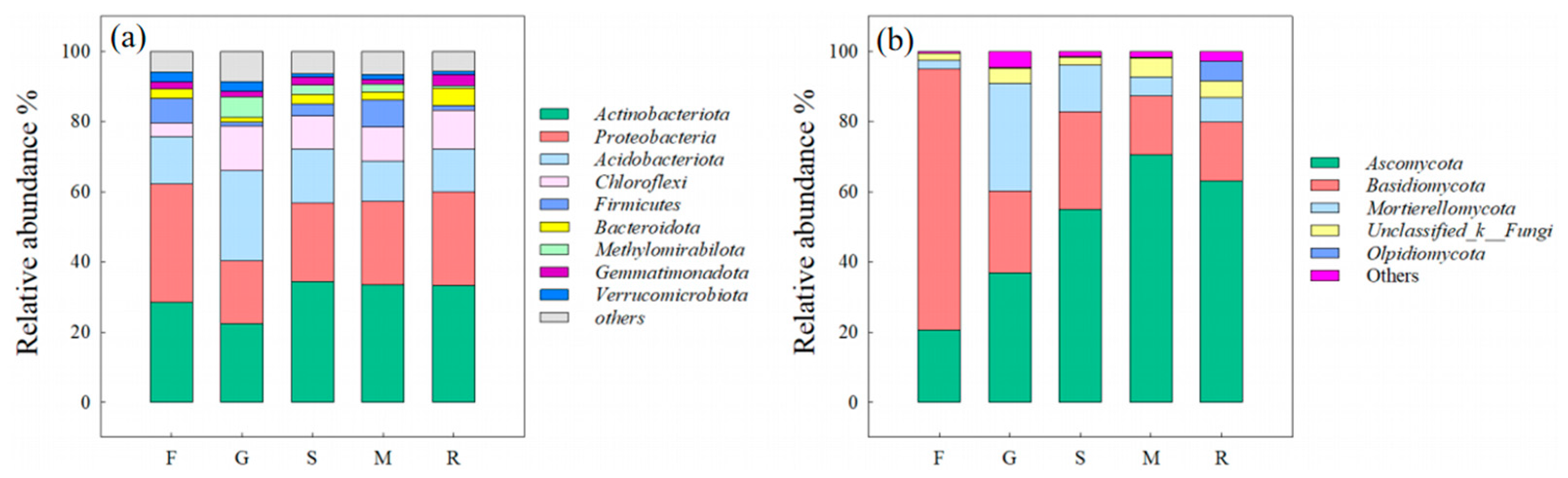
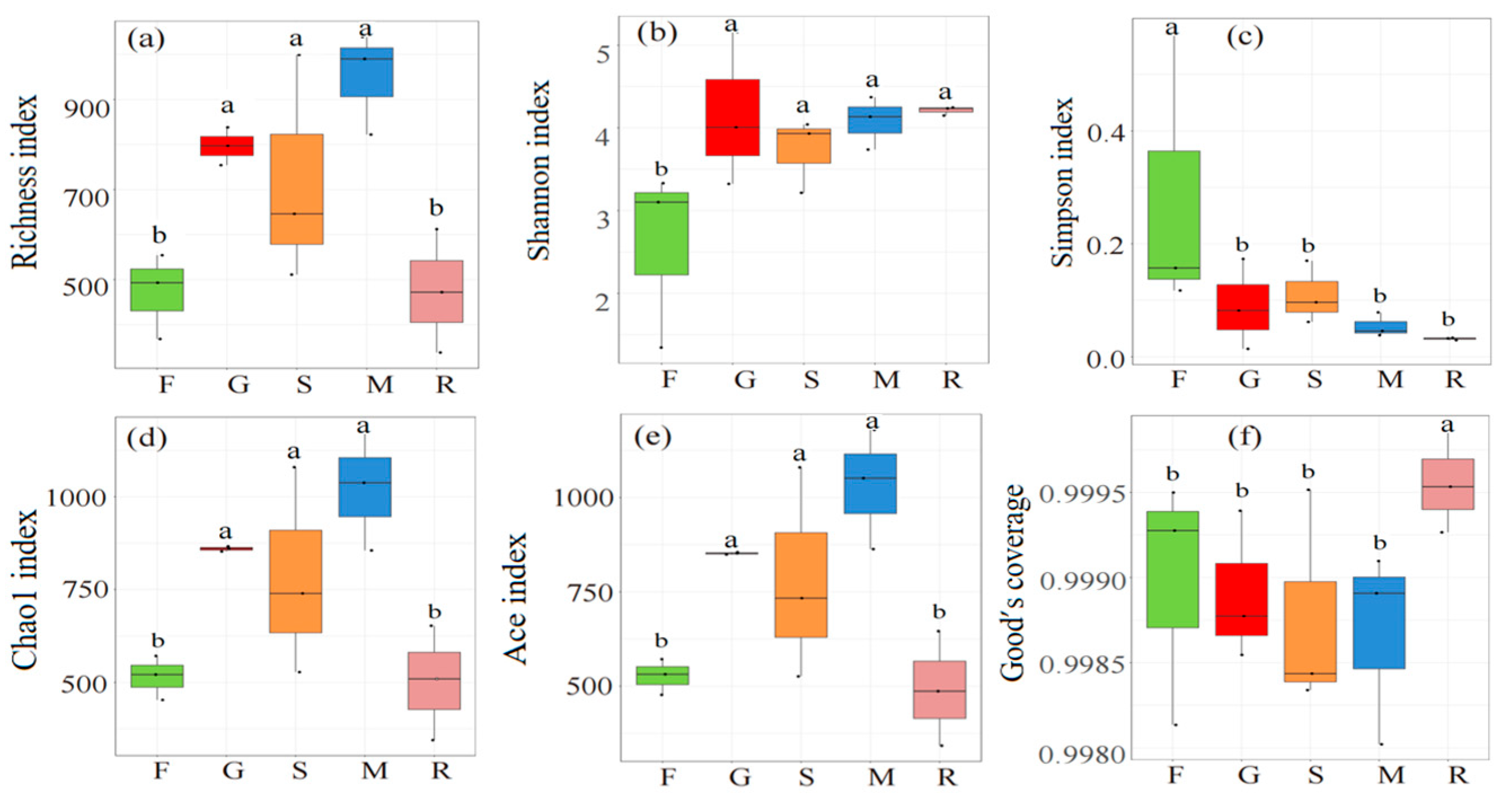
3.3. Microbial Diversity and Composition Along an Elevation Gradient
3.3.1. Composition of Bacterial and Fungal Communities
3.3.2. Diversity of Bacterial and Fungal Communities
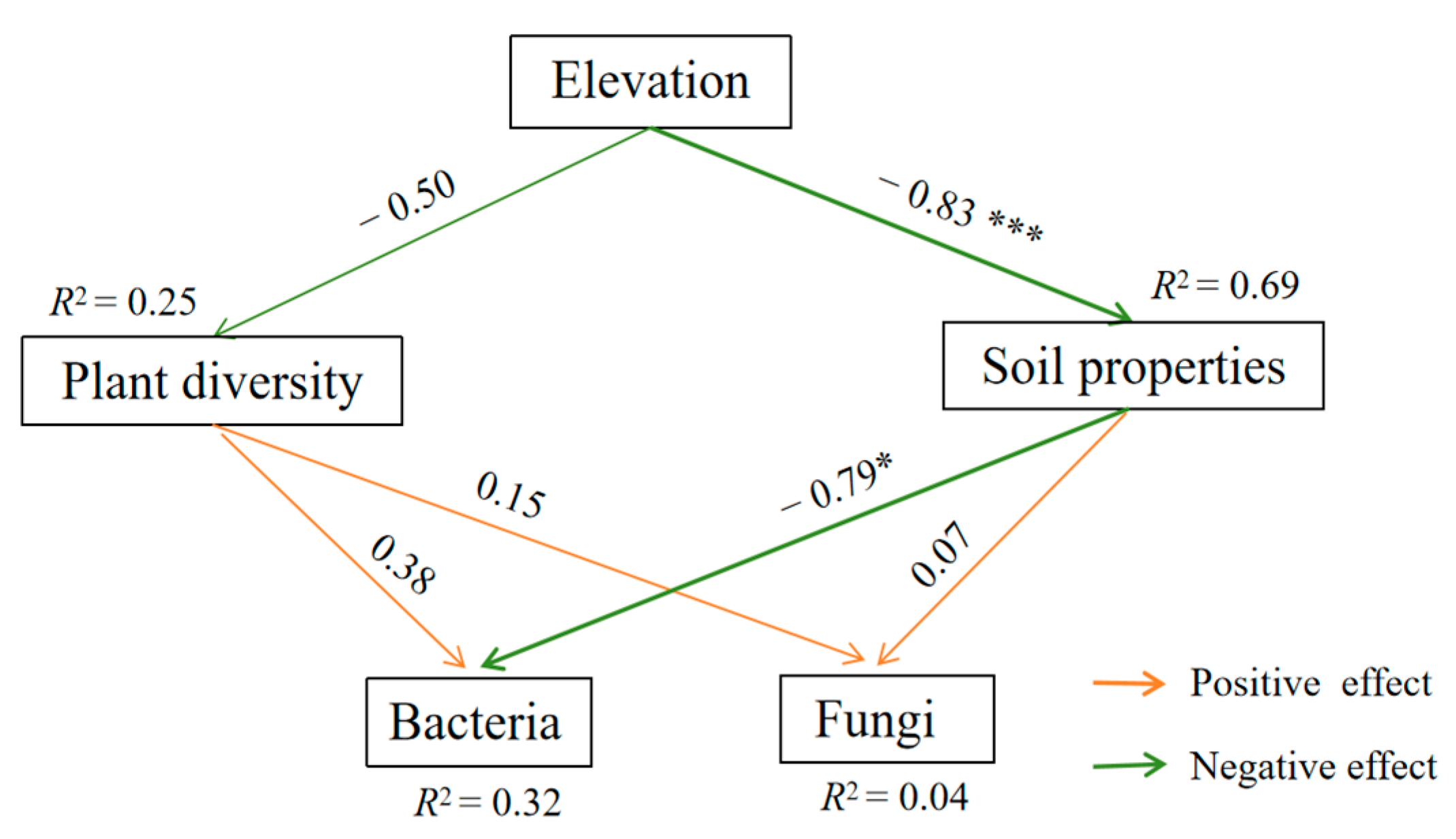
3.3.3. Relationships Between Soil Properties, Plant Diversity, and Soil Microbial Communities
4. Discussion
4.1. Elevational Soil Microbial Composition and Diversity
4.2. Relationships Between Soil Microorganisms and Environmental Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falkowski, P.G.; Frenchel, T.; Delong, E.F. The microbial engines that drive Earth’s biogeochemical cycles. Science 2008, 320, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; van Bodegom, P.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Eldridge, D.J.; Liu, Y.R.; Sokoya, B.; Wang, J.T.; Hu, H.W.; He, J.Z.; Bastida, F.; Moreno, J.L.; Bamigboye, A.R.; et al. Global homogenization of the structure and function in the soil microbiome of urban greenspaces. Sci. Adv. 2021, 7, eabg5809. [Google Scholar] [CrossRef]
- Mukhtar, H.; Wunderlich, R.F.; Muzaffar, A.; Ansari, A.; Shipin, O.V.; Cao, T.N.; Lin, Y.P. Soil microbiome feedback to climate change and options for mitigation. Sci. Total Environ. 2023, 882, 163412. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Zou, Z.; Yuan, K.; Ming, L.; Li, Z.; Yang, Y.; Yang, R.; Chen, B. Changes in alpine soil bacterial communities with altitude and slopes at mount Shergyla, Tibetan Plateau: Diversity, structure, and influencing factors. Front. Microbiol. 2022, 13, 839499. [Google Scholar] [CrossRef]
- Liu, D.; Wu, X.; Shi, S.; Liu, H.; Liu, G. A hollow bacterial diversity pattern with elevation in Wolong Nature Reserve, Western Sichuan Plateau. J. Soils Sediments 2016, 16, 2365–2374. [Google Scholar] [CrossRef]
- Fierer, N.; McCain, C.M.; Meir, P.; Zimmermann, M.; Rapp, J.M.; Silman, M.R.; Knight, R. Microbes do not follow the elevational diversity patterns of plants and animals. Ecology 2011, 92, 797–804. [Google Scholar] [CrossRef]
- Bryant, J.A.; Lamanna, C.; Morlon, H.; Kerkhoff, A.J.; Enquist, B.J.; Green, J.L. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 11505–11511. [Google Scholar] [CrossRef]
- Fu, F.; Li, J.; Li, S.; Chen, W.; Ding, H.; Xiao, S.; Li, Y. Elevational distribution patterns and drivers of soil microbial diversity in the Sygera Mountains, southeastern Tibet, China. Catena 2023, 221, 106738. [Google Scholar] [CrossRef]
- Shen, C.; Gunina, A.; Luo, Y.; Wang, J.; He, J.Z.; Kuzyakov, Y.; Hemp, A.; Classen, A.T.; Ge, Y. Contrasting patterns and drivers of soil bacterial and fungal diversity across a mountain gradient. Environ. Microbiol. 2020, 22, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, F.; Gavazov, K.; Alexander, J.M. Above- and belowground linkages shape responses of mountain vegetation to climate change. Science 2019, 365, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Hu, Y.; Chen, B.; Zhang, Y.; Thiele, J.; Shi, R.; Liu, M.; Bu, R. Soil pH and plant diversity shape soil bacterial community structure in the active layer across the latitudinal gradients in continuous permafrost region of Northeastern China. Sci. Rep. 2018, 8, 5619. [Google Scholar] [CrossRef] [PubMed]
- Picazo, F.; Vilmi, A.; Aalto, J.; Soininen, J.; Casamayor, E.O.; Liu, Y.; Wang, J. Climate mediates continental scale patterns of stream microbial functional diversity. Microbiome 2020, 8, 92. [Google Scholar] [CrossRef]
- Ma, L.; Liu, L.; Lu, Y.; Chen, L.; Zhang, Z.; Zhang, H.; Zhang, J. When microclimates meet soil microbes: Temperature controls soil microbial diversity along an elevational gradient in subtropical forests. Soil Biol. Biochem. 2022, 166, 108566. [Google Scholar] [CrossRef]
- De, G.B.; Van, W.H. Linking aboveground and belowground diversity. Trends Ecol. Evol. 2005, 20, 625–633. [Google Scholar]
- Tian, Q.; Jiang, Y.; Tang, Y.; Wu, Y.; Tang, Z.; Liu, F. Soil pH and organic carbon properties drive soil bacterial communities in surface and deep layers along an elevational gradient. Front. Microbiol. 2021, 12, 646124. [Google Scholar] [CrossRef]
- Praeg, N.; Seeber, J.; Leitinger, G.; Tasser, E.; Newesely, C.; Tappeiner, U. The role of land management and elevation in shaping soil microbial communities: Insights from the Central European Alps. Biochemistry 2020, 150, 107951. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, K.; Zhou, S.; Che, R.; Du, J.; Tang, L.; Pang, Z.; Wang, F.; Wang, D.; Cui, X.Y.; et al. Phosphorus mediates soil prokaryote distribution pattern along a small-scale elevation gradient in Noijin Kangsang Peak, Tibetan Plateau. FEMS Microbiol. Ecol. 2019, 95, fiz076. [Google Scholar] [CrossRef]
- Zhao, W.; Yin, Y.; Li, S.; Liu, J.; Dong, Y.; Su, S. Soil Microbial Community Varied with Vegetation Types on a Small Regional Scale of the Qilian Mountains. Sustainability 2022, 14, 7910. [Google Scholar] [CrossRef]
- Seaton, F.M.; George, P.B.; Alison, J.; Jones, D.L.; Creer, S.; Smart, S.M.; Robinson, D.A. A diversity of diversities: Do complex environmental effects underpin associations between below-and above-ground taxa? J. Ecol. 2024, 112, 1550–1564. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, H.; Qiu, L.; Cao, S.; He, Y.; Pang, G. Spatial and temporal analyses of vegetation changes at multiple time scales in the Qilian Mountains. Remote Sens. 2021, 13, 5046. [Google Scholar] [CrossRef]
- Li, T.; Kamran, M.; Chang, S.; Peng, Z.; Wang, Z.; Ran, L.; Hou, F. Climate-soil interactions improve the stability of grassland ecosystem by driving alpine plant diversity. Ecol. Indic. 2022, 141, 109002. [Google Scholar] [CrossRef]
- Wen, J.; Qin, R.; Zhang, S.; Yang, X.; Xu, M. Effects of long-term warming on the aboveground biomass and species diversity in an alpine meadow on the Qinghai-Tibetan Plateau of China. J. Arid Land 2020, 12, 252–266. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, D.; Jiang, Z.; Sun, P.; Xiao, H.; Yuxin, W.; Chen, J. Changes in the soil microbial communities of alpine steppe at Qinghai-Tibetan Plateau under different degradation levels. Sci. Total Environ. 2019, 651, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, H.; Zhao, X.; Liu, P.; Wang, L.; Wang, W. A functional metagenomics study of soil carbon and nitrogen degradation networks and limiting factors on the Tibetan plateau. Front. Microbiol. 2023, 14, 1170806. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, E.L. Phosphorus Availability Indices. Phosphorus soluble in sodium bicarbonate. Methods of soil analysis, Part II. Chemical and microbiological properties. ASA-SSSA Agron. Ser. 1982, 9, 404–430. [Google Scholar]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic note: A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, V.E.; El Mujtar, V.A.; Falcão Salles, J.; Jia, X.; Castán, E.; Cardozo, A.G.; Tittonell, P.A. Micro-Environmental Variation in Soil Microbial Biodiversity in Forest Frontier Ecosystems—Implications for Sustainability Assessments. Sustainability 2024, 16, 1236. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, F.; Ochieng, B.; Xu, J.; Zhang, L.; Kimirei, I.A.; Wang, J. Climate and Environmental Variables Drive Stream Biofilm Bacterial and Fungal Diversity on Tropical Mountainsides. Microb. Ecol. 2024, 87, 28. [Google Scholar] [CrossRef]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Yang, D.Q. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Change 2015, 5, 424–430. [Google Scholar]
- Mayor, J.R.; Sanders, N.J.; Classen, A.T.; Bardgett, R.D.; Clement, J.C.; Fajardo, A.; Wardle, D.A. Elevation alters ecosystem properties across temperate treelines globally. Nature 2017, 542, 91–95. [Google Scholar] [CrossRef]
- Greenblum, S. Microbial adaptability in changing environments. Nat. Rev. Microbiol. 2024, 22, 327. [Google Scholar] [CrossRef]
- Wang, J.T.; Cao, P.; Hu, H.W.; Li, J.; Han, L.L.; Zhang, L.M.; He, J.Z. Altitudinal distribution patterns of soil bacterial and archaeal communities along Mt. Shegyla on the Tibetan Plateau. Microb. Ecol. 2015, 69, 135–145. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, C.; Yi, J.; Doughty, R.; Zhao, F. Contrasting responses of rhizosphere bacteria, fungi and arbuscular mycorrhizal fungi along an elevational gradient in a temperate montane forest of China. Front. Microbiol. 2020, 11, 2042. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, S.; Kergunteuil, A.; Sánchez-Moreno, S.; Puissant, J.; Goodall, T.; Griffiths, R.; Rasmann, S. Relative contribution of high and low elevation soil microbes and nematodes to ecosystem functioning. Funct. Ecol. 2022, 36, 974–986. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Semenov, M.V.; Yao, F.; Ye, J.; Bu, R.; Kuzyakov, Y. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob. Change Biol. 2021, 27, 2763–2779. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Hu, L.; Wang, C.; Yang, W.; Zi, H.; Manuel, L. Herbaceous plants influence bacterial communities, while shrubs influence fungal communities in subalpine coniferous forests. For. Ecol. Manag. 2021, 500, 119656. [Google Scholar] [CrossRef]
- Nie, H.; Qin, T.; Yan, D.; Lv, X.; Wang, J.; Huang, Y.; Liu, F. How do tree species characteristics affect the bacterial community structure of subtropical natural mixed forests? Sci. Total Environ. 2021, 764, 144633. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Drivers of microbial community structure in forest soils. Appl. Microbiol. Biotechnol. 2018, 102, 4331–4338. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, X.; Bhadra, S.; Gao, Q. Distinct factors drive the assembly of quinoa-associated microbiomes along elevation. Plant Soil 2020, 448, 55–69. [Google Scholar] [CrossRef]
- Huang, R.; Zhao, D.; Zeng, J.; Shen, F.; Cao, X.; Jiang, C.; Wu, Q.L. pH affects bacterial community composition in soils across the Huashan watershed, China. Can. J. Microbiol. 2016, 62, 726–734. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J.; Wang, J.; Li, X. Soil microbial community responds to elevation gradient in an arid montane ecosystem in Northwest China. Forests 2022, 13, 1360. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Pinto-Junior, H.V.; Villa, P.M.; Menezes, L.F.T.; Pereira, M.C.A. Effect of climate and altitude on plant community composition and richness in Brazilian inselbergs. Journal of Mountain Science 2020, 17, 1931–1941. [Google Scholar] [CrossRef]
- Lamb, E.G.; Kennedy, N.; Siciliano, S.D. Effects of plant species richness and evenness on soil microbial community diversity and function. Plant Soil 2011, 338, 483–495. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Minkina, T. Root exudates: Mechanistic insight of plant growth promoting rhizobacteria for sustainable crop production. Front. Microbiol. 2022, 13, 916488. [Google Scholar] [CrossRef] [PubMed]
- Afridi, M.S.; Kumar, A.; Javed, M.A.; Dubey, A.; de Medeiros, F.H.V.; Santoyo, G. Harnessing root exudates for plant microbiome engineering and stress resistance in plants. Microbiol. Res. 2024, 279, 127564. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Peixoto, L.; Olesen, J.E.; Elsgaard, L.; Enggrob, K.L.; Banfield, C.C.; Dippold, M.A.; Rasmussen, J. Deep-rooted perennial crops differ in capacity to stabilize C inputs in deep soil layers. Sci. Rep. 2022, 12, 5952. [Google Scholar] [CrossRef]
- Liao, W.; Tong, D.; Li, Z.; Nie, X.; Liu, Y.; Ran, F.; Liao, S. Characteristics of microbial community composition and its relationship with carbon, nitrogen and sulfur in sediments. Sci. Total Environ. 2021, 795, 148848. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, L.; Dijkstra, F.A.; Wang, X.; Delgado-Baquerizo, M.; Liu, G.; Wang, G.; Song, Z.; Gu, J.; Zhang, C. Aridity thresholds of microbiome-soil function relationship along a climatic aridity gradient in alpine ecosystem. Soil Biol. Biochem. 2024, 192, 109388. [Google Scholar] [CrossRef]
- Dong, L.; Berg, B.; Gu, W.; Wang, Z.; Sun, T. Effects of different forms of nitrogen addition on microbial extracellular enzyme activity in temperate grassland soil. Ecol. Process. 2022, 11, 36. [Google Scholar] [CrossRef]
- Cruz-Paredes, C.; Tájmel, D.; Rousk, J. Can moisture affect temperature dependences of microbial growth and respiration? Soil Biol. Biochem. 2021, 156, 108223. [Google Scholar] [CrossRef]
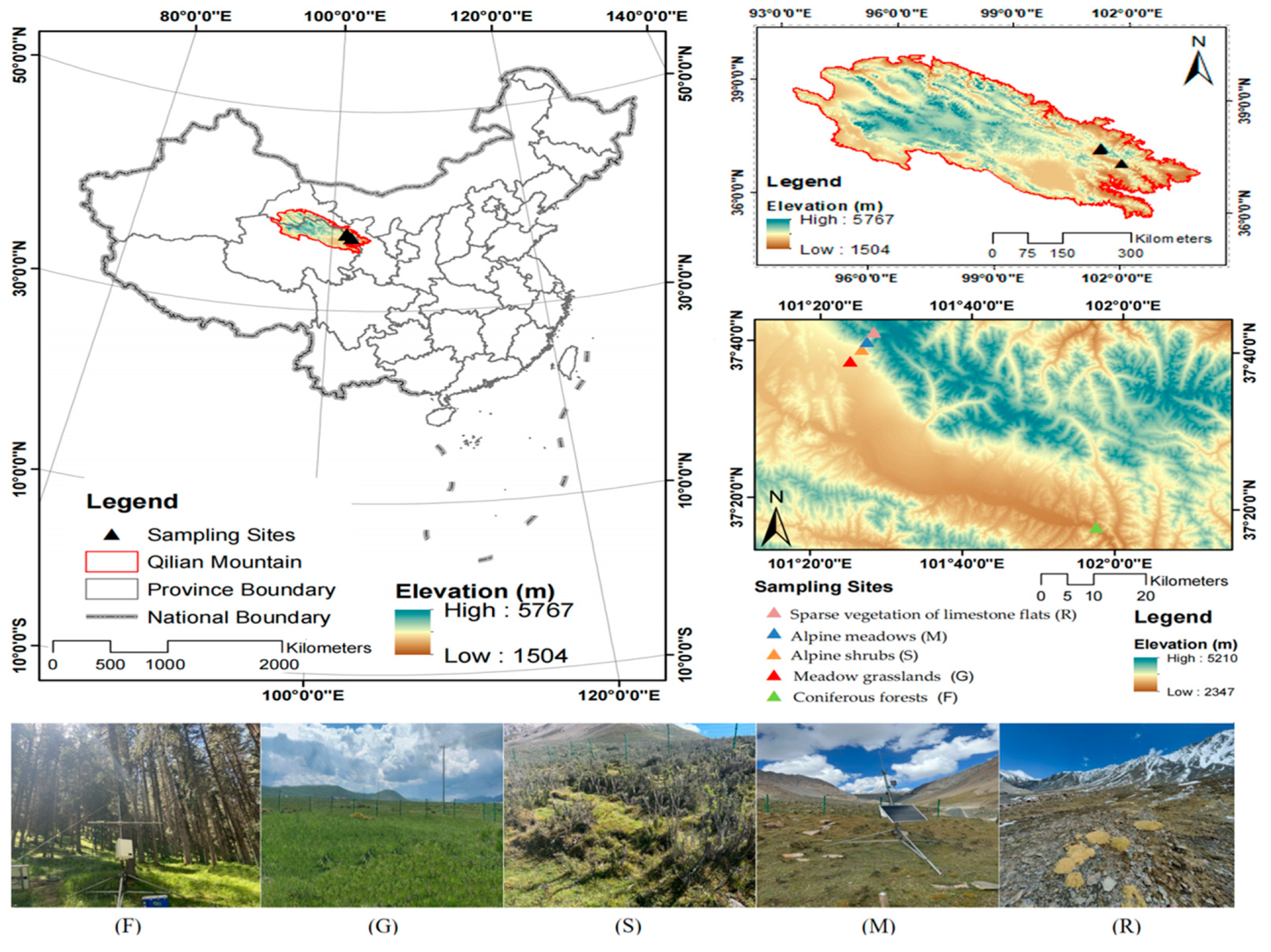




| Vegetation Types (No.) | Geographic Position | Elevation (m) | Dominant Species | Vegetation Coverage (%) | Soil Types |
|---|---|---|---|---|---|
| Coniferous forests (F) | 37°17′25″ N 101°57′29″ E | 2700 | Picea crassifolia + Poa annua L. + Anemone rivularis | 80 | Mountain gray–brown soil |
| Meadow grasslands (G) | 37°37′42″ N 101°24′11″ E | 3200 | Bistorta vivipara L. + Potentilla anserina + Kobresia pygmaea | 95 | Subalpine meadow soil |
| Alpine shrubs (S) | 37°39′17″ N 101°25′38″ E | 3500 | Potentilla fruticosa L. + Caragana jubata + Kobresia humilis | 95 | Alpine shrub–meadow soil |
| Alpine meadows (M) | 37°40′19″ N 101°26′13″ E | 3700 | Kobresia pygmaea + Poa annua L. | 70 | Alpine meadow soil |
| Sparse vegetation of limestone flats (R) | 37°41′30″ N 101°27′06″ E | 4000 | Poa bulbosa L. var. vivipara Koeler + Kobresia pygmaea + Thylacospermum caespitosum | 40 | Frigid desert soil |
| Soil Properties | Coniferous Forests (F) | Meadow Grasslands (G) | Alpine Shrubs (S) | Alpine Meadows (M) | Sparse Vegetation of Limestone Flats (R) |
|---|---|---|---|---|---|
| Soil moisture (%) | 112.39 ± 22.38 a | 64.41 ± 7.71 bc | 96.39 ± 3.32 ab | 45.09 ± 1.99 cd | 10.7 ± 5.69 d |
| Bulk density (g·cm−3) | 0.64 ± 0.04 d | 0.92 ± 0.07 bc | 0.78 ± 0.02 cd | 1.1 ± 0.08 b | 2.25 ± 0.65 a |
| pH | 7.42 ± 0.03 a | 7.35 ± 0.43 a | 7.39 ± 0.31 a | 7.32 ± 0.33 a | 7.69 ± 0.06 a |
| NH4+-N (mg·kg−1) | 16.87 ± 4.05 a | 16.41 ± 2.88 a | 14.12 ± 2.5 a | 18.13 ± 3.91 a | 14.73 ± 2.79 a |
| NO3−-N (mg·kg−1) | 14.91 ± 1.86 a | 13.95 ± 4.1 a | 9.26 ± 2.19 ab | 4.89 ± 1.42 b | 2.78 ± 0.84 b |
| Available P (mg·kg−1) | 8.74 ± 1.45 b | 6.69 ± 0.42 b | 8.32 ± 0.31 b | 9.18 ± 0.81 b | 13.57 ± 0.6 a |
| TC/(g·kg−1) | 96.27 ± 4.41 a | 82.58 ± 14.6 ab | 99.19 ± 5.08 a | 59.43 ± 5.85 b | 15.33 ± 2.45 c |
| TN/(g·kg−1) | 8.51 ± 0.17 a | 7.78 ± 1.21 ab | 8.9 ± 0.34 a | 6.05 ± 0.47 b | 1.63 ± 0.27 c |
| TP/(g·kg−1) | 0.55 ± 0.07 a | 0.62 ± 0.03 a | 0.62 ± 0.03 a | 0.62 ± 0.01 a | 0.55 ± 0.07 a |
| C:N | 11.3 ± 0.28 a | 10.53 ± 0.28 ab | 11.13 ± 0.16 a | 9.79 ± 0.2 b | 9.52 ± 0.69 b |
| C:P | 215.28 ± 21.43 a | 132.74 ± 20.08 b | 138.34 ± 19.96 b | 15.92 ± 3.50 b | 29.89 ± 8.46 c |
| N:P | 18.61 ± 43.79 a | 12.52 ± 1.64 ab | 12.41 ± 1.71 ab | 15.92 ± 3.50 a | 3.18 ± 0.9 b |
| Vegetation Type (No.) | S′plant | H′plant | J′plant |
|---|---|---|---|
| Coniferous forests (F) | 19.00 | 2.56 | 0.87 |
| Meadow grasslands (G) | 23.00 | 2.82 | 0.90 |
| Alpine shrubs (S) | 34.00 | 2.93 | 0.83 |
| Alpine meadows (M) | 16.00 | 2.54 | 0.92 |
| Sparse vegetation of limestone flats (R) | 18.00 | 2.22 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.; Wang, W.; Chen, Z.; Chen, X.; Xiong, Y. The Variations in Soil Microbial Communities and Their Mechanisms Along an Elevation Gradient in the Qilian Mountains, China. Sustainability 2025, 17, 1797. https://doi.org/10.3390/su17051797
Bai L, Wang W, Chen Z, Chen X, Xiong Y. The Variations in Soil Microbial Communities and Their Mechanisms Along an Elevation Gradient in the Qilian Mountains, China. Sustainability. 2025; 17(5):1797. https://doi.org/10.3390/su17051797
Chicago/Turabian StyleBai, Lili, Wenying Wang, Zhe Chen, Xiaoyue Chen, and Youcai Xiong. 2025. "The Variations in Soil Microbial Communities and Their Mechanisms Along an Elevation Gradient in the Qilian Mountains, China" Sustainability 17, no. 5: 1797. https://doi.org/10.3390/su17051797
APA StyleBai, L., Wang, W., Chen, Z., Chen, X., & Xiong, Y. (2025). The Variations in Soil Microbial Communities and Their Mechanisms Along an Elevation Gradient in the Qilian Mountains, China. Sustainability, 17(5), 1797. https://doi.org/10.3390/su17051797







