Study on the Effects of Tar Reforming and Steam Gasification of Keyaki Bark in Saitama Prefecture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Metal Contents of Waste Biomass
2.1.2. The Demineralization of Materials
2.1.3. The Addition of Catalytic Calcium Using the Impregnation Method with Ca (OH)2
2.1.4. Ultimate Analysis of the Waste Biomass
2.2. Thermogravimetric Analyses
2.3. Reactor System for Steam Gasification Experiments
2.4. Reactor System for CO2 Gasification Experiments
2.5. Reactor of Two-Stage Tar Reforming
3. Results and Discussions
3.1. Metal Analysis of Ca, Mg, K, Na Contents in Bark
3.2. The Ultimate Sample Analysis
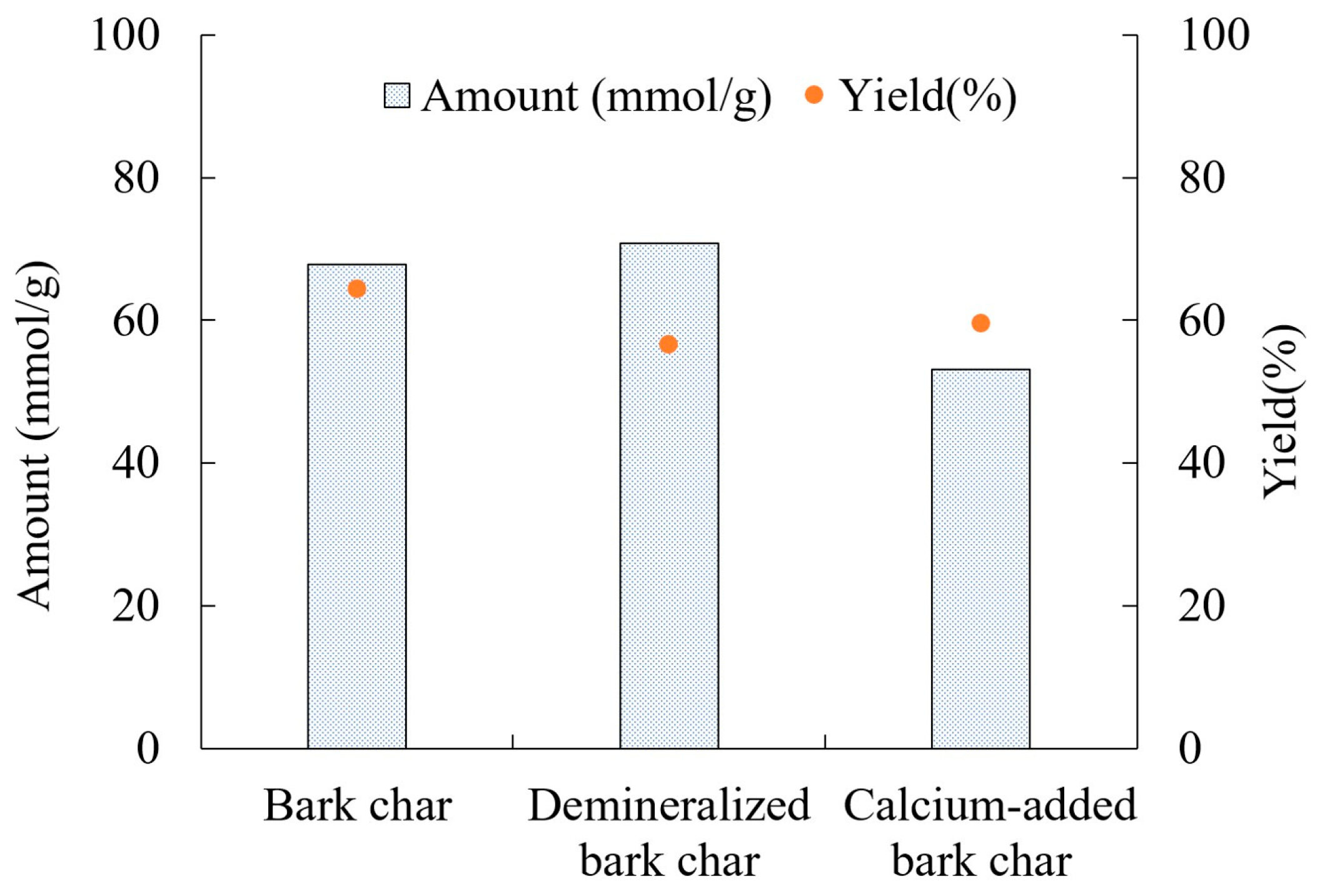
3.3. Reaction Behaviors with Different Samples at 900 °C
3.4. Gas Amount and Yields
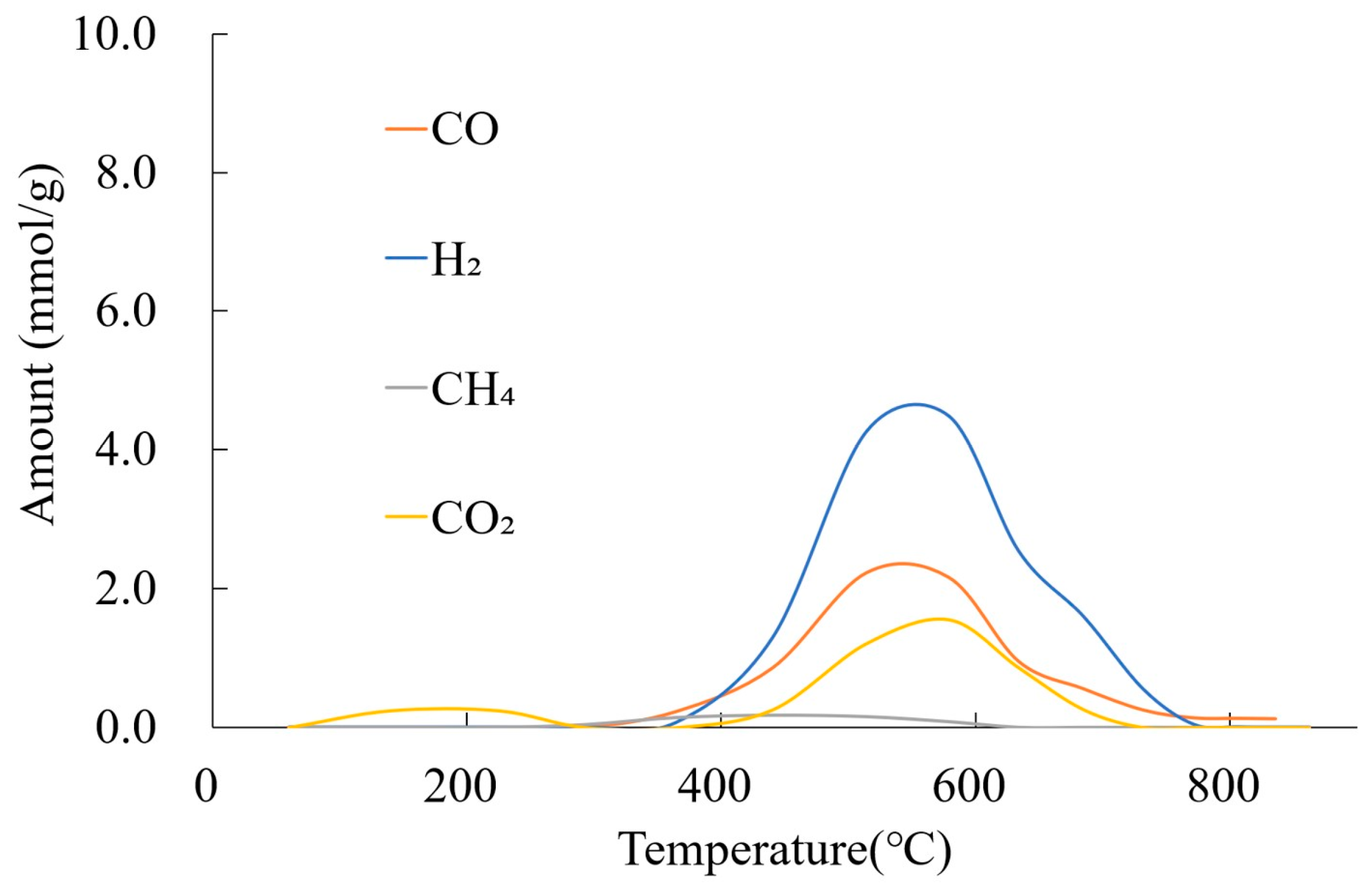
3.4.1. Gas Amount and Yields by CO2 Gasification
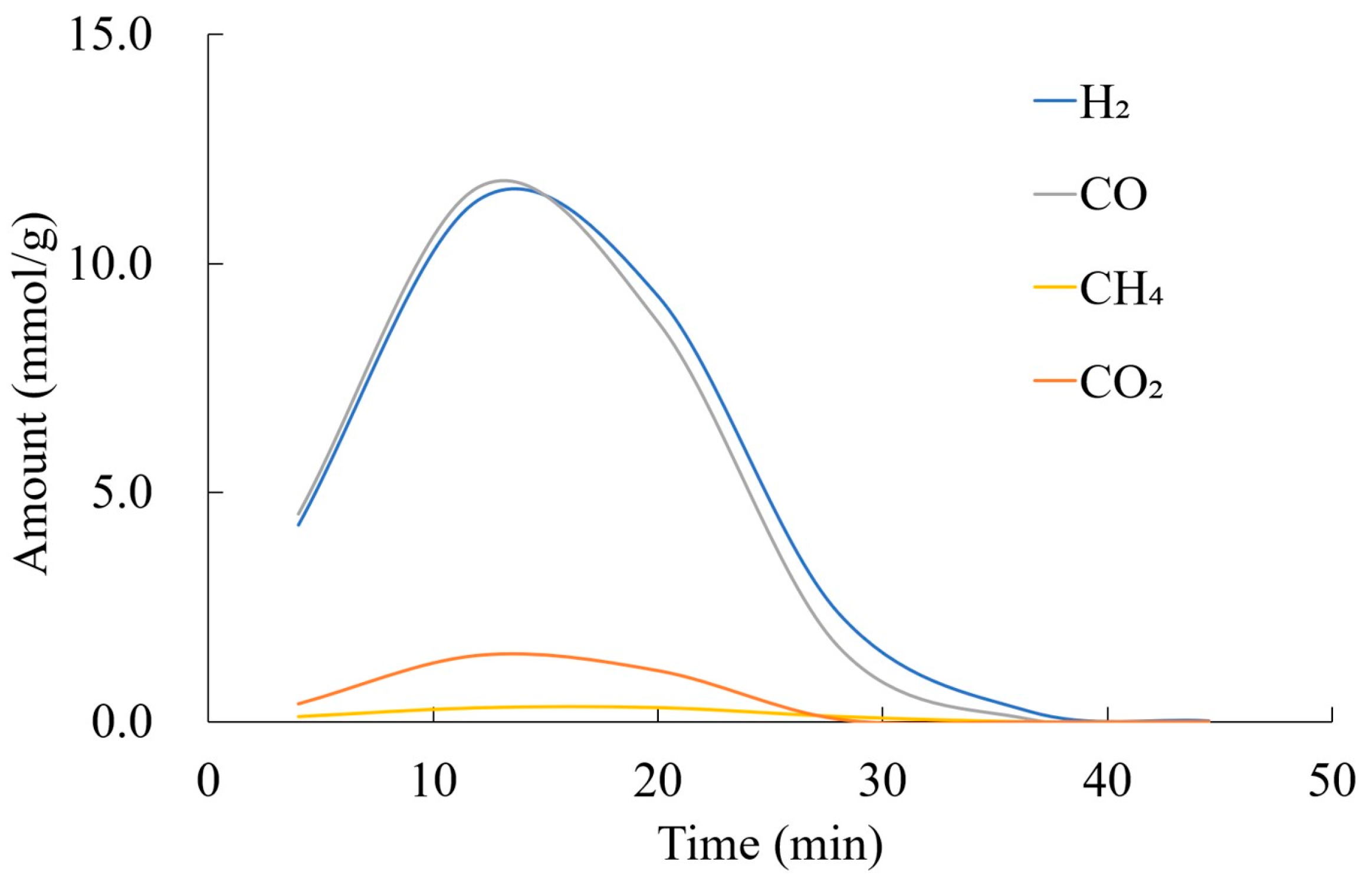
3.4.2. Gas Amount and Yields by H2O Gasification
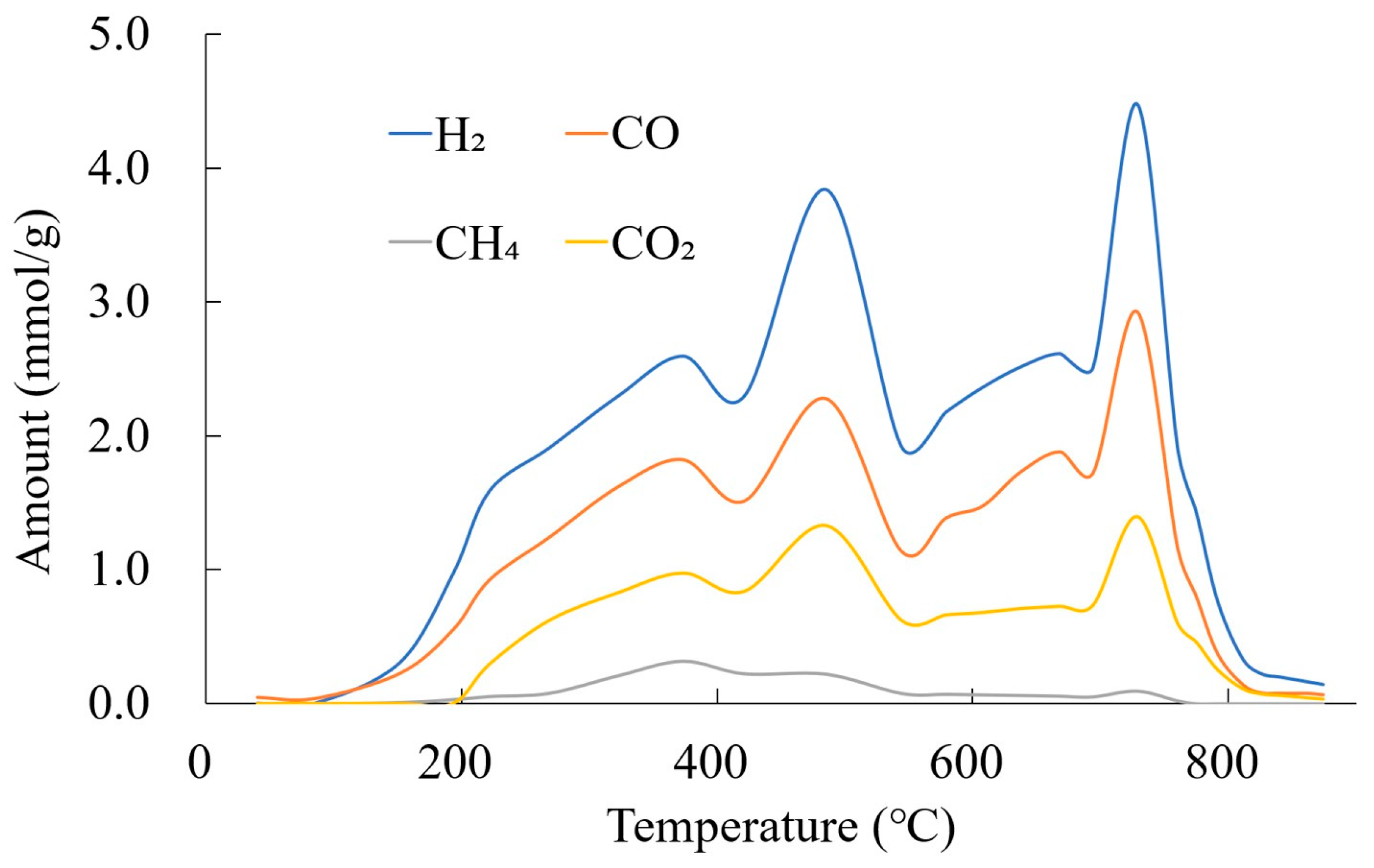
3.4.3. Gas Amount and Yields by Tar Reforming
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a Sustainable Bioeconomy: An Overview of World Biomass Production and Utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Demirbas, M.F.; Balat, M.; Balat, H. Potential Contribution of Biomass to the Sustainable Energy Development. Energy Convers. Manag. 2009, 50, 1746–1760. [Google Scholar] [CrossRef]
- Sher, F.; Hameed, S.; Smječanin Omerbegović, N.; Chupin, A.; Ul Hai, I.; Wang, B.; Heng Teoh, Y.; Joka Yildiz, M. Cutting-Edge Biomass Gasification Technologies for Renewable Energy Generation and Achieving Net Zero Emissions. Energy Convers. Manag. 2025, 323, 119213. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Al-Musawi, T.J.; Al-Jiboory, A.K.; Salman, H.M.; Ali, B.M.; Jaszczur, M. A Comprehensive Review of International Renewable Energy Growth. Energy Built Environ. 2024, in press. [Google Scholar] [CrossRef]
- Yu, H.; Wen, B.; Zahidi, I.; Chow, M.F.; Liang, D.; Madsen, D.Ø. The Critical Role of Energy Transition in Addressing Climate Change at COP28. Results Eng. 2024, 22, 102324. [Google Scholar] [CrossRef]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic Biomass as Sustainable Feedstock and Materials for Power Generation and Energy Storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Çolpan, C.Ö.; Ayol, A. Biomass Gasification for Sustainable Energy Production: A Review. Int. J. Hydrog. Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Joshua Abioye, K.; Rajamanickam, R.; Ogunjinmi, T.; Paul, S.; Selvasembian, R.; Ighalo, J.O. Advancements in Biomass Waste Conversion to Sustainable Biofuels via Gasification. Chem. Eng. J. 2025, 505, 159151. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Z.; Das, S.; Kawi, S. Reforming of Tar from Biomass Gasification in a Hybrid Catalysis-Plasma System: A Review. Appl. Catal. B 2019, 250, 250–272. [Google Scholar] [CrossRef]
- Faizan, M.; Song, H. Critical Review on Catalytic Biomass Gasification: State-of-Art Progress, Technical Challenges, and Perspectives in Future Development. J. Clean. Prod. 2023, 408, 137224. [Google Scholar] [CrossRef]
- Zeng, X.; Shen, M.; Wang, F.; Ma, X.; Hu, D.; Wang, T.; Cui, Y. Generation Features and Kinetics of Gas Products in Bio-Tar Catalytic Cracking by Char for Biomass Gasification. Int. J. Hydrog. Energy 2023, 48, 31905–31919. [Google Scholar] [CrossRef]
- Guan, G.; Kaewpanha, M.; Hao, X.; Abudula, A. Catalytic Steam Reforming of Biomass Tar: Prospects and Challenges. Renew. Sustain. Energy Rev. 2016, 58, 450–461. [Google Scholar] [CrossRef]
- Li, J.; Chang, G.; Song, K.; Hao, B.; Wang, C.; Zhang, J.; Yue, G.; Hu, S. Influence of Coal Bottom Ash Additives on Catalytic Reforming of Biomass Pyrolysis Gaseous Tar and Biochar/Steam Gasification Reactivity. Renew. Energy 2023, 203, 434–444. [Google Scholar] [CrossRef]
- Khajeh, A.; Masoumi, S.; Wang, L.; Shahbazi, A. Effects of Various Carbon-Supported Iron Catalysts on Tar Removal Efficiency and Syngas Yield during Catalytic Biomass Gasification. J. Environ. Chem. Eng. 2023, 11, 110884. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Xu, D.; Zhang, H. Catalytic Activity Evaluation and Deactivation Progress of Red Mud/Carbonaceous Catalyst for Efficient Biomass Gasification Tar Cracking. Fuel 2022, 323, 124278. [Google Scholar] [CrossRef]
- Kong, G.; Liu, Q.; Ji, G.; Jia, H.; Cao, T.; Zhang, X.; Han, L. Improving Hydrogen-Rich Gas Production from Biomass Catalytic Steam Gasification over Metal-Doping Porous Biochar. Bioresour. Technol. 2023, 387, 129662. [Google Scholar] [CrossRef]
- Quan, C.; Feng, S.; Gao, N. Production of Hydrogen-Rich Syngas from Catalytic Reforming of Biomass Gasification Tar Model Compounds Coupled with in-Situ CO2 Capture. Biomass Bioenergy 2025, 193, 107569. [Google Scholar] [CrossRef]
- Hu, D.; Zeng, X.; Wang, F.; Cui, Y.; Zhou, Q.; Xu, G. Assessment of the Inherent CaO in Char on Tar Catalytic Conversion by a Micro Fluidized Bed Reaction Analyzer for Biomass Gasification. Fuel 2023, 344, 127866. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Gong, Y.; Ding, L.; Wang, J.; Yu, G. A Review of the Effects of Alkali and Alkaline Earth Metal Species on Biomass Gasification. Fuel Process. Technol. 2021, 214, 106723. [Google Scholar] [CrossRef]
- Anekwe, I.M.S.; Akpasi, S.O.; Enemuo, E.M.; Ashiegbu, D.; Mustapha, S.I.; Isa, Y.M. Innovations in Catalytic Understanding: A Journey through Advanced Characterization. Mater. Today Catal. 2024, 7, 100061. [Google Scholar] [CrossRef]
- Zou, K.; Zhang, Y.; Shen, L.; Shang, H.; Chen, B.; Wen, J.; Zhao, H. Highly Efficient Resource Recovery from Polymetallic Sulfide Ores under Neutral-Alkaline Conditions: Chemical Oxidation and Coordination Leaching. Chem. Eng. J. 2025, 503, 158198. [Google Scholar] [CrossRef]
- Niu, Y.H.; Chi, Z.Y.; Li, M.; Du, J.Z.; Han, F.T. Advancements in Biomass Gasification and Catalytic Tar-Cracking Technologies. Mater. Rep. Energy 2024, 4, 100295. [Google Scholar] [CrossRef]
- Llavata, B.; García-Pérez, J.V.; Simal, S.; Cárcel, J.A. Innovative Pre-Treatments to Enhance Food Drying: A Current Review. Curr. Opin. Food Sci. 2020, 35, 20–26. [Google Scholar] [CrossRef]
- Babu, A.K.; Kumaresan, G.; Raj, V.A.A.; Velraj, R. Review of Leaf Drying: Mechanism and Influencing Parameters, Drying Methods, Nutrient Preservation, and Mathematical Models. Renew. Sustain. Energy Rev. 2018, 90, 536–556. [Google Scholar] [CrossRef]
- Li, N.; Pan, Y.; Yan, Z.; Shi, S.; Liu, X.; Zhang, Y.; Yan, Y.; Liu, Q.; Shi, L.; Liu, Z. Cornstalk Steam Gasification for Syngas in a Two-Stage Electromagnetic Induction Reactor. Chem. Eng. J. 2024, 488, 150948. [Google Scholar] [CrossRef]
- Tanoh, T.S.; Ait Oumeziane, A.; Lemonon, J.; Escudero-Sanz, F.J.; Salvador, S. A Novel Two-Stage Gasification Strategy for Nitrogen-Free Syngas Production- Pilot-Scale Experiments. Fuel Process. Technol. 2021, 217, 106821. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Z.; Zhu, X.; Chen, X.; Luo, Z.; Xu, C.C.; Li, W. Sequential Catalysis Enables Efficient Pyrolysis of Food Waste for Syngas Production. Bioresour. Technol. 2025, 419, 132042. [Google Scholar] [CrossRef]
- Fang, J.; Xie, M.; He, X.; Zhang, J.; Hu, J.; Chen, Y.; Yang, Y.; Jin, Q. Machine Learning Accelerates the Materials Discovery. Mater. Today Commun. 2022, 33, 104900. [Google Scholar] [CrossRef]
- Al-Shetwi, A.Q.; Abidin, I.Z.; Mahafzah, K.A.; Hannan, M.A. Feasibility of Future Transition to 100% Renewable Energy: Recent Progress, Policies, Challenges, and Perspectives. J. Clean. Prod. 2024, 478, 143942. [Google Scholar] [CrossRef]
- Komatani, S.; Ohzawa, S. Development of the XGT-5000 X-Ray Analytical Microscope. 50th Anniversary Product. Japan. Available online: https://www.horiba.com/uploads/media/RE09-14-080 (accessed on 19 February 2025).
- Jiang, L.; Hu, S.; Wang, Y.; Su, S.; Sun, L.; Xu, B.; He, L.; Xiang, J. Catalytic Effects of Inherent Alkali and Alkaline Earth Metallic Species on Steam Gasification of Biomass. Int. J. Hydrog. Energy 2015, 40, 15460–15469. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, S.; Sun, L.S.; Su, S.; Xu, K.; He, L.M.; Xiang, J. Influence of Different Demineralization Treatments on Physicochemical Structure and Thermal Degradation of Biomass. Bioresour. Technol. 2013, 146, 254–260. [Google Scholar] [CrossRef]
- Mitsuoka, K.; Hayashi, S.; Amano, H.; Kayahara, K.; Sasaoaka, E.; Uddin, M.A. Gasification of Woody Biomass Char with CO2: The Catalytic Effects of K and Ca Species on Char Gasification Reactivity. Fuel Process. Technol. 2011, 92, 26–31. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Wang, W.; Wang, Y.; Lu, D. Characterization of Waste Biomass Fuel Prepared from Coffee and Tea Production: Its Properties, Combustion, and Emissions. Sustainability 2024, 16, 7246. [Google Scholar] [CrossRef]
- Mishra, S.; Upadhyay, R.K. Review on Biomass Gasification: Gasifiers, Gasifying Mediums, and Operational Parameters. Mater. Sci. Energy Technol. 2021, 4, 329–340. [Google Scholar] [CrossRef]
- Bisen, D.; Chouhan, A.P.S.; Pant, M.; Chakma, S. Advancement of Thermochemical Conversion and the Potential of Biomasses for Production of Clean Energy: A Review. Renew. Sustain. Energy Rev. 2025, 208, 115016. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.P.; Zhao, X.Y.; Liu, Y.L. Recent Progress and Perspectives of Catalyst Design and Downstream Integration in Biomass Tar Reforming. Chem. Eng. J. 2022, 429, 132316. [Google Scholar] [CrossRef]
- Gohar, O.; Zubair Khan, M.; Bibi, I.; Bashir, N.; Tariq, U.; Bakhtiar, M.; Ramzan Abdul Karim, M.; Ali, F.; Bilal Hanif, M.; Motola, M. Nanomaterials for Advanced Energy Applications: Recent Advancements and Future Trends. Mater. Des. 2024, 241, 112930. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Wang, W.; Wang, Y. Processes of Metal Oxides Catalyst on Conversion of Spent Coffee Grounds into Rich-Synthesis Gas by Gasification. Processes 2024, 12, 2232. [Google Scholar] [CrossRef]
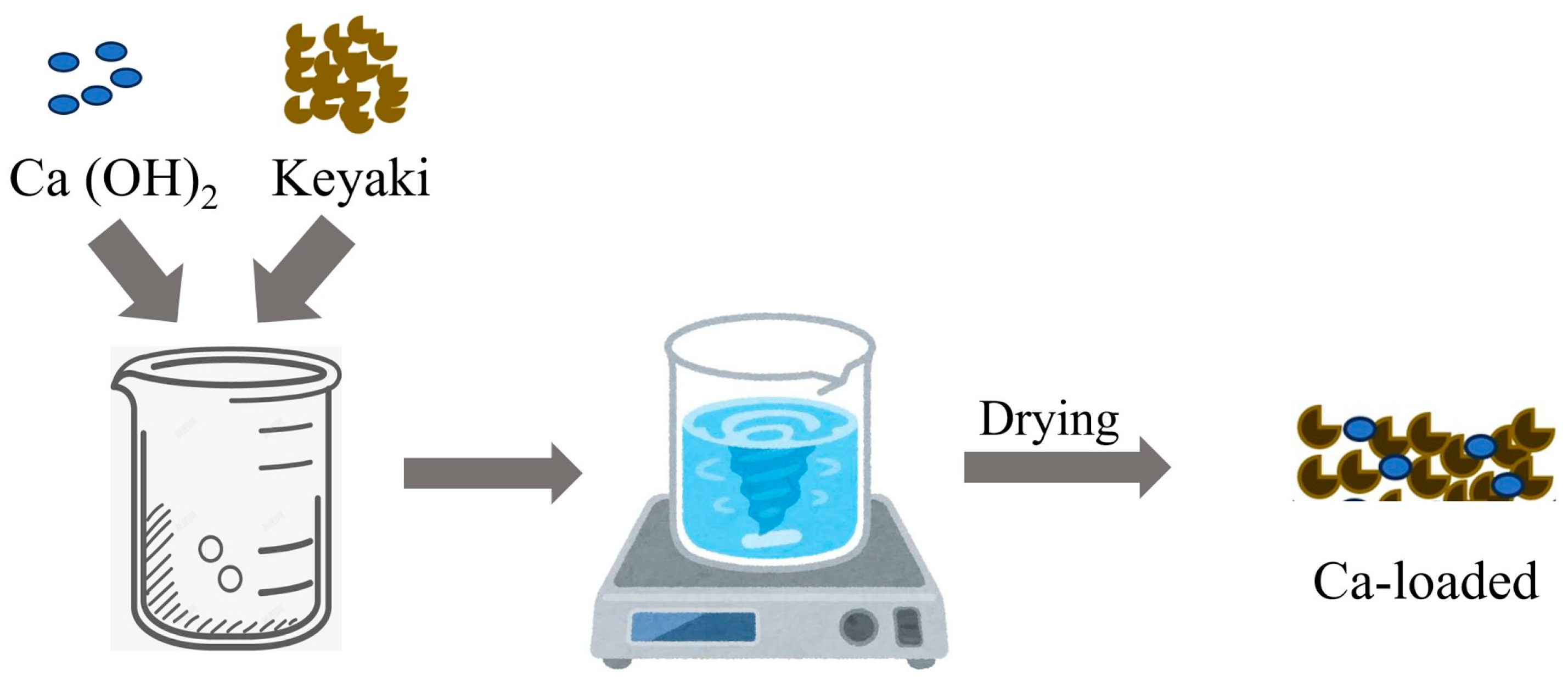







| Ca (Weight %) | Mg (Weight %) | K (Weight %) | Na (Weight %) | |
|---|---|---|---|---|
| Bark | 9.19 | 0.17 | 0.04 | 0.15 |
| Ultimate Analysis (Weight %) | |||||
|---|---|---|---|---|---|
| C | H | N | O | Ash | |
| Bark Raw | 44.50 | 5.81 | 0.25 | 38.69 | 10.76 |
| Bark Char | 60.41 | 1.22 | 0.94 | 1.76 | 35.67 |
| Demineralized Bark Char | 75.01 | 1.09 | 0.91 | 14.31 | 8.68 |
| Calcium-added Bark Char | 53.53 | 1.06 | 0.85 | 0.18 | 44.38 |
| H2 (mmol/g) | CO (mmol/g) | CH4 (mmol/g) | CO2 (mmol/g) | Syngas (mmol/g) | |
|---|---|---|---|---|---|
| Bark Raw Gasification | 14.82 | 7.61 | 0.60 | 4.63 | 22.43 |
| Bark Char Gasification | 27.60 | 26.64 | 0.85 | 3.02 | 54.24 |
| Bark Raw and Char Total | 42.42 | 34.25 | 1.44 | 7.65 | 76.67 |
| Tar Reform Gasification | 40.90 | 26.28 | 1.64 | 12.13 | 67.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Wang, Q.; Ryota, I. Study on the Effects of Tar Reforming and Steam Gasification of Keyaki Bark in Saitama Prefecture. Sustainability 2025, 17, 2215. https://doi.org/10.3390/su17052215
Wu S, Wang Q, Ryota I. Study on the Effects of Tar Reforming and Steam Gasification of Keyaki Bark in Saitama Prefecture. Sustainability. 2025; 17(5):2215. https://doi.org/10.3390/su17052215
Chicago/Turabian StyleWu, Shangrong, Qingyue Wang, and Isobe Ryota. 2025. "Study on the Effects of Tar Reforming and Steam Gasification of Keyaki Bark in Saitama Prefecture" Sustainability 17, no. 5: 2215. https://doi.org/10.3390/su17052215
APA StyleWu, S., Wang, Q., & Ryota, I. (2025). Study on the Effects of Tar Reforming and Steam Gasification of Keyaki Bark in Saitama Prefecture. Sustainability, 17(5), 2215. https://doi.org/10.3390/su17052215







