Abstract
The rapid increase in electronic waste emphasizes the urgent need for low-toxicity, highly selective gold recovery methods. This study introduces a novel water-soluble organic leaching system using sodium dichloroisocyanurate (DCCNa) dissolved in water to investigate the gold leaching efficiency from the gold fingers of waste printed circuit boards (WPCBs). The pregnant leach solution (PLS) was processed using stepwise extraction. The gold oxidation states and leaching pathway were confirmed through UV–Vis, XPS, and ESI-MS analyses. Under optimal conditions (pH 2, 3-h leaching time, DCCNa concentration of 40 mmol/L, and 25 °C), the DCCNa leaching system achieved a gold leaching efficiency of 96.4%, significantly outperforming other metals and demonstrating its excellent selectivity. Stepwise extraction of the PLS using Acorga M5640 and dibutyl carbitol (DBC) resulted in a gold extraction efficiency of 95.5%. DCCNa generates the strong oxidant HClO, which reacts with HCl to produce Cl2. Both HClO and Cl2 oxidize Au(0) to Au(I) and Au(III), forming [AuCl2]− and [AuCl4]− complexes, with Cl− originating from the hydrolysis of HCl. This study presents a safe, economical, and eco-friendly approach for the efficient and selective recovery of gold from WPCB gold fingers under laboratory conditions, which achieves the sustainable utilization of precious metals.
1. Introduction
With the progress and development of science and technology, electronic equipment is rapidly updated, and the amount of e-waste generated increases year by year [1]. According to The Global E-waste Monitor 2024 by the United Nations, 62 million tons of e-waste were generated globally in 2022, growing annually by 7–10%, which is three times faster than domestic waste [2]. Printed circuit board (PCB) is an essential part of electronic devices, and waste printed circuit boards (WPCBs) serve as both waste and valuable resources. WPCBs contain heavy metals and harmful substances such as chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), and brominated flame retardants (BFRs), posing severe environmental risks if mishandled [3]. Simultaneously, they are rich in valuable metals, comprising about 30% of their total weight, making them crucial for secondary resource recovery [4]. Notably, the gold (Au) content in WPCB (~500 g/t) far exceeds that of natural ores (~3 g/t), earning them the designation of “urban mining” [5,6]. Gold, along with other metals such as Ca, Cu, Ni, Al, and Fe, is primarily concentrated in gold-plated conductive pads (gold fingers), with Au content up to 3500 g/t—more than five times the Au content in the overall WPCBs. Thus, recovering Au from the gold finger of WPCBs holds substantial economic value. Separating and processing gold fingers from other WPCB components allows for the selective recovery of Au and other recyclable metals [7].
Gold recovery from WPCBs primarily involves pyrometallurgical, hydrometallurgical, and biohydrometallurgical methods and their combinations [8]. Pyrometallurgy requires high temperatures, resulting in high energy consumption and environmental risks [9]. Bioleaching, despite being cost-effective and eco-friendly, is characterized by slow reaction rates and low efficiency, restricting its application to laboratory studies [10,11,12]. Hydrometallurgy, the most widely used method, offers simplicity, high recovery rates, and fast reactions. Leaching, a critical step in hydrometallurgy, involves using a leaching agent to dissolve metals into a pregnant leach solution (PLS). Conventional methods of leaching Au use cyanide and aqua regia. Petter et al. [13] reported a 60% recovery of Au from mobile phone WPCBs using cyanide leaching. Similarly, Sheng et al. [14] employed aqua regia to leach Au from computer chips, successfully extracting all the gold within 30 min at a temperature of 90 °C. However, it is important to emphasize that both cyanide and aqua regia present significant risks to the environment and human health. Cyanide and its decomposition products can infiltrate water sources and soil, adversely impacting soil quality and hindering plant growth. Ingestion or skin contact with cyanide can result in severe acute poisoning. The reagents in aqua regia are highly corrosive and can cause serious burns upon contact with skin, eyes, or if inhaled [15,16]. Consequently, researchers have focused on less toxic leaching agents, such as thiosulfates, thiourea, thiocyanates, and halides, but these often face challenges like high reagent consumption, complexity, and costs [17,18,19,20]. Organic leaching agents have gained attention for their low cost and high selectivity. For instance, organic aqua regia (OAR), prepared by dissolving thionyl chloride and pyridine in acetonitrile, has achieved over 95% platinum recovery from waste automotive catalysts [21,22,23]. However, the use of explosive thionyl chloride and toxic acetonitrile raises significant safety concerns, limiting its application. To address these issues, researchers have developed water-soluble organic leaching agents, using water as a solvent for safer and milder processing [24,25]. The aqueous phase, after leaching, separates easily from the organic solvent, facilitating subsequent metal separation and purification via solvent extraction. Solvent extraction is a key hydrometallurgical technology, offering simplicity, high selectivity, high recovery rates, and recyclable reagents [26]. Combining water-soluble organic leaching with solvent extraction presents an efficient, selective, and safe approach for gold recovery from WPCBs.
Currently, research on water-soluble organic leaching technologies is limited. Therefore, it is crucial to develop an effective water-soluble leaching system for gold leaching and investigate its leaching mechanism in order to recover Au from WPCBs. This approach not only conserves resources and reduces environmental pollution but also aligns with the principles of sustainable development. A water-soluble organic leaching system was developed using N-bromosuccinimide (NBS) and pyridine (Py) to leach gold ores, achieving an Au leaching efficiency of 90% [24]. However, this system showed a relatively low Au leaching efficiency (<70%) for WPCB, which is insufficient for effective gold recovery. A novel water-soluble NBS-dimethylformamide (DMF) leaching agent, WS NBS-DMF, was synthesized and achieved Au leaching efficiencies of 94.9%, 90.1%, and 96.5% from WPCBs, WPCB gold fingers, and gold ores, respectively [25]. In these two systems, NBS containing bromine was utilized [24,25]. Halogens share similar chemical properties, and chlorine-based water-soluble organic leachates offer cost advantages over bromine-containing compounds. This makes them a more economical choice for reducing reagent costs in Au leaching from WPCBs. In light of these considerations, this study proposed a novel water-soluble organic leaching agent by dissolving sodium dichloroisocyanurate (DCCNa) in water. The molecular structural formula of DCCNa is shown in Figure S1. DCCNa, a widely used disinfectant in food processing and drinking water treatment, dissolves in water at a concentration of 25 g/100 g at 25 °C, releasing Cl− and ClO− ions. This confers both coordination and oxidative properties to the leaching system, enhancing its ability to oxidize and leach Au [27]. Furthermore, since leaching agents may also extract other base metals, particularly Cu, which is abundant in WPCB, a stepwise solvent extraction method is proposed to recover Au and base metals (such as Cu) from the PLS.
This study carried out the following research: (1) investigated the effects of pH, leaching time, DCCNa concentration, temperature, stirring speed, and liquid–solid ratio on the leaching efficiency of Au and other base metals from WPCB gold fingers using DCCNa, and determine the optimal leaching conditions; (2) performed stepwise extraction of Cu and Au from the DCCNa PLS using Acorga M5640 as a copper extractant and dibutyl carbitol (DBC) as a gold extractant, evaluating the extraction efficiency and identifying optimal extraction conditions; (3) determined the oxidation state and structure of the Au leachate products through spectroscopy, energy-dispersive spectroscopy, and mass spectrometry, elucidating the leaching pathway and mechanism of Au with DCCNa. This study presents a safe, economical, and eco-friendly approach for the recovery of gold from WPCB gold fingers under laboratory conditions, which achieves sustainable utilization of precious metals.
2. Materials and Methods
2.1. Chemicals and Materials
All chemicals used in this study were of analytical grade without further purification. WPCB samples were obtained from a company in Shanghai, China. The WPCBs were rinsed with distilled water and dried in an oven at 40 °C for 24 h. The gold fingers, known for their high metal content, were separated from the other WPCB components (such as plastics and silica) using a cutter and cut into small pieces measuring 1 cm × 0.5 cm. These pieces were subsequently ground in batches for 5 min into a fine powder using a grinder (A 11 Basic Analytical Mill, IKA Guangzhou Instrument Equipment Co., Ltd., Guangzhou, China) and passed through a 100-mesh sieve to produce gold-containing material for leaching experiments. To prepare colloidal gold, 50 mg of NaBH4 was dissolved in 500 mL of 0.1 mmol/L HAuCl4 solution and stirred at 800 rpm for 24 h. The initial pH of the leachate was adjusted using 1 mol/L H2SO4 and 1 mol/L NaOH. All solutions were prepared with ultrapure water. Additional experimental details are provided in the Supporting Information (Text S1).
2.2. Determination of Au and Base Metal Content in WPCB Gold Finger Powder
Au, along with several base metals (Cu, Ca, Al, Ni, and Fe), which together account for the majority of the metallic content in the WPCB gold finger, was selected for subsequent leaching experiments. Aqua regia was employed to dissolve the gold finger powder for precise analysis. The metal content was accurately determined using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES, Optima 8000DV, PerkinElmer, Waltham, MA, USA). Leaching with aqua regia was conducted at 25 °C for 6 h, with a stirring speed of 800 rpm and a liquid-to-solid ratio of 40:1 mL/g. The PLS was vacuum-filtered, and ICP-OES quantified the concentrations of Au and base metals (Table 1), providing data for calculating leaching efficiency in subsequent experiments.

Table 1.
Content of gold and major base metals in the WPCB gold finger confirmed by aqua regia leaching.
2.3. Leaching of Au from WPCB Gold Finger
DCCNa was dissolved in ultrapure water to prepare DCCNa solutions of different concentrations. Leaching was performed by mixing different concentrations and volumes of the DCCNa solution with 1 g of WPCB gold finger powder using a temperature-controlled magnetic stirrer (HMS-1, Shanghai Huxi Co., Ltd., Shanghai, China). The leaching reaction was carried out in 100 mL stoppered glass conical flasks. After a certain time of leaching, the leach residue and the PLS were separated via vacuum filtration using a 0.45 μm hydrophilic filter membrane. The concentrations of Au and base metals in the PLS were measured using an ICP-OES. The metal leaching efficiency was calculated using Equation (1):
where Mp is the mass of metal in the sample powder (mg), determined by aqua regia leaching; CDCCNa is the concentration of metal in the PLS (mg/L); and VDCCNa is the volume of the PLS (L).
Leaching Efficiency = (CDCCNa × VDCCNa)/Mp × 100%,
The effects of initial pH, leaching time, DCCNa concentration, liquid-to-solid ratio (L/S), leaching temperature, and stirring speed on the leaching efficiency of Au and base metals were evaluated through single-factor experiments. The specific conditions for each experiment are detailed in Table 2. To ensure the reliability of the results, each experiment was performed in triplicate.

Table 2.
Experimental conditions for each influencing factor in WPCB gold finger leaching.
2.4. Recovery of Au from PLS via Solvent Extraction
Sequential extraction of copper and gold from the PLS obtained through DCCNa leaching of WPCB gold finger was performed, with copper extracted first, followed by gold extraction. For copper extraction, the aqueous phase was the PLS under optimal DCCNa leaching conditions, and the organic phase was an Acorga M5640 extractant diluted with sulfonated kerosene. Before copper extraction, the kerosene was sulfonated to remove unsaturated hydrocarbons (Text S2). The organic and aqueous phases were mixed in a water bath shaker (SHA-C, Changzhou Guohua Electric Co., Ltd., Changzhou, China) at 200 ± 2 rpm and 25 ± 0.5 °C until equilibrium was reached. The mixture was centrifuged at 2000 rpm and allowed to stand for 10 min, and the aqueous raffinate phase was analyzed using ICP-OES. The metal concentrations in the organic phase were calculated using mass balance. Single-factor experiments optimized copper extraction conditions by varying pH (2, 2.5, 3, and 3.5), extraction time (5, 15, 30, and 60 min), and organic-to-aqueous (O/A) volume ratio (1:1, 1:2, 1:5, and 1:10). Extraction efficiency was calculated using Equation (2).
For gold extraction, the aqueous phase was the raffinate after copper extraction, and the organic phase was a DBC solution. The organic and aqueous phases were mixed at a 1:1 volume ratio, with the same extraction and centrifugation procedures used for copper extraction. Single-factor experiments varied extraction time (5, 15, 30, and 60 min) and O/A ratio (1:1, 1:2, 1:5, and 1:10) to optimize conditions, evaluated using Equation (2):
where C0 is the metal concentration in the starting aqueous solution (mg/L); Ci is the metal concentration in the aqueous phase after extraction (mg/L).
Extraction efficiency = (C0 − Ci)/C0 × 100%,
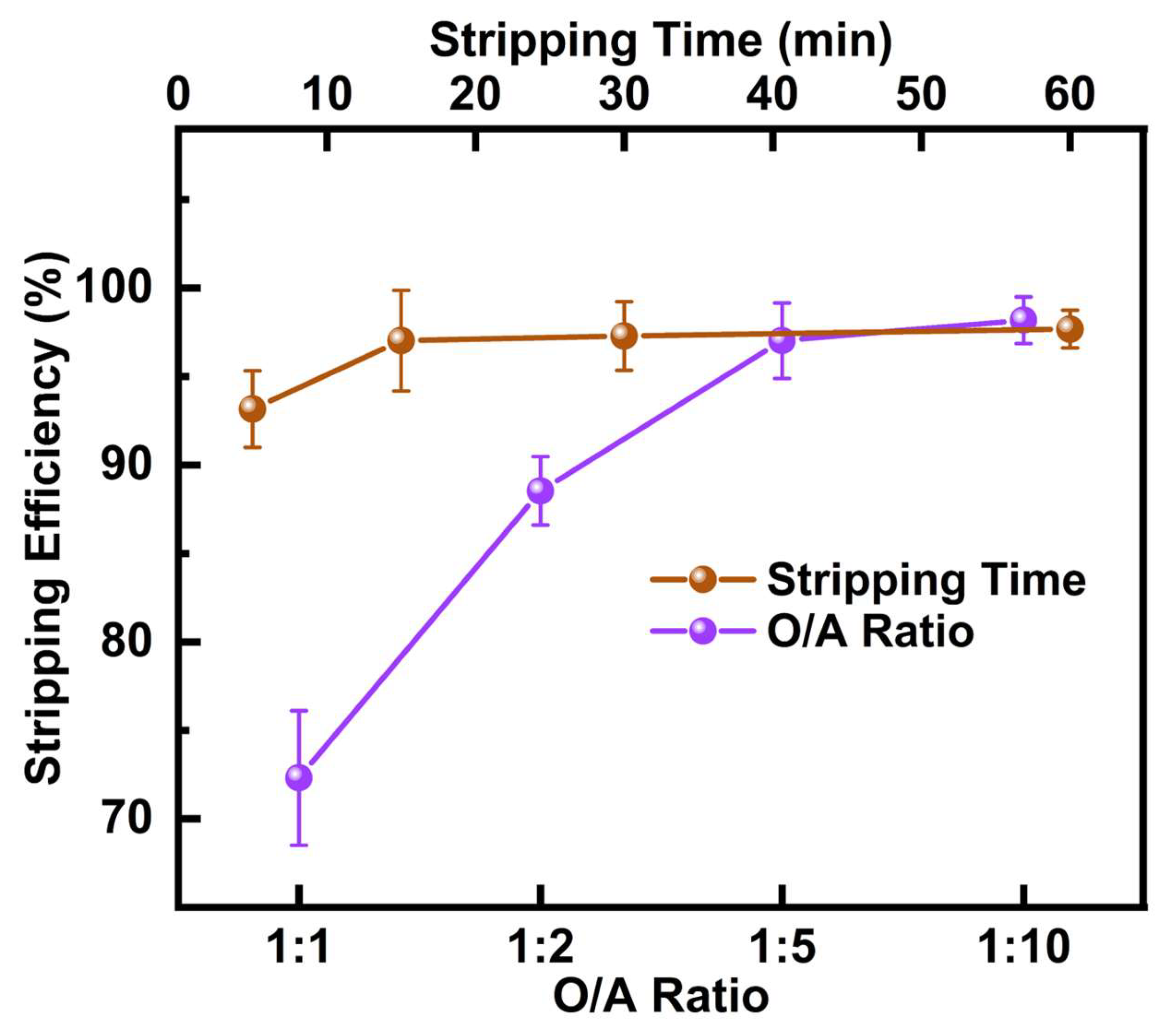
Stripping experiments used 2mol/L H2SO4 as the aqueous phase and the extracted organic phase from the optimal copper extraction as the organic phase. The variables included stripping time (5, 15, 30, and 60 min) and organic-to-aqueous phase volume ratios (O/A) (1:1, 1:2, 1:5, and 1:10). Stripping efficiency was calculated using Equation (3). Each experiment was performed in triplicate for reliability.
where M0 is the mass of metal ions in the starting leach solution (g); M1 is the mass of metal ions in the aqueous phase after extraction (g); and M2 is the mass of metal ions in the aqueous phase after stripping (g).
Stripping efficiency = M2/(M0 − M1) × 100%,
2.5. Experimental Au Leaching Pathway and Leaching Mechanism
The absorption band changes during the leaching of colloidal gold (zero-valent gold) with DCCNa solution were analyzed using ultraviolet–visible spectrophotometry (UV–Vis, UV-2401PC, Shimadzu, Kyoto, Japan). The chemical states of elements in the evaporated products of the gold powder PLS were examined using X-ray photoelectron spectroscopy (XPS, ESCALAB Xi+, Thermo Fisher Scientific, Waltham, MA, USA). The product structures in the gold powder PLS were characterized via electrospray ionization mass spectrometry (ESI-MS, Q Exactive Mass Spectrometer, Thermo Fisher Scientific). Detailed analytical methods are provided in Text S3.
3. Results and Discussion
3.1. Gold Leaching from WPCB Gold Finger Using DCCNa
To investigate the optimal leaching conditions for Au from WPCB gold fingers using DCCNa, various experiments were conducted on factors affecting leaching, including initial pH, leaching time, DCCNa concentration, leaching temperature, liquid-to-solid ratio, and stirring speed.
3.1.1. Effect of Initial pH and Leaching Time
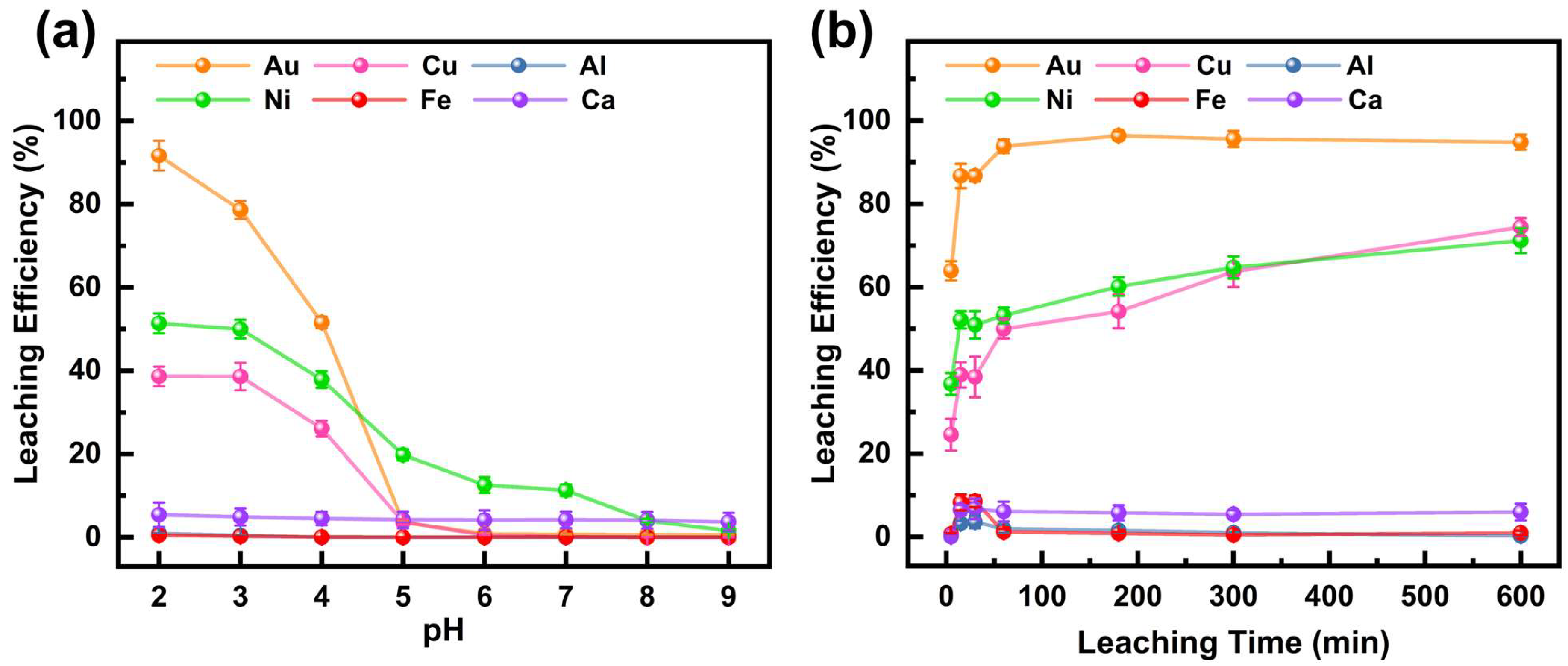
The effect of the initial DCCNa solution pH on the leaching efficiency of six metals from the WPCB gold finger is shown in Figure 1a (experimental conditions are shown in Table 2). The Au leaching efficiency peaked at 92.2% at pH 2 but sharply declined to 51.3% at pH 4 and declined to 3.6% at pH 5. When the initial pH exceeded 5, the leaching efficiency of Au was below 1%. DCCNa releases reactive chlorine species (Cl2 and Cl−) in solution, which can react with Au to form Au-chlorine complexes, such as [AuCl4]−. As reported by previous researchers, the speciation of Au complexes in solution is highly pH-dependent [28,29]. [AuCl4]− predominantly exists under acidic conditions, while at higher pH, Au tends to precipitate as hydroxide (Au(OH)3), leading to a decline in leaching efficiency. Therefore, the leaching efficiency of Au is significantly higher under low-pH conditions. The leaching efficiency of Ni was 51.4% at pH 2, decreasing to 1.5% at pH 9. The Cu leaching efficiency remained similar at pH 2 and 3 (38.7% and 38.6%, respectively) but dropped sharply to 3.6% at pH 5. Under the studied conditions, Al, Fe, and Ca leaching efficiencies were all below 6%, with Ca reaching a maximum of 5.4% at pH 2. Au showed significantly higher leaching efficiency under low-pH conditions, achieving 92.2% at pH 2, while other metals remained relatively low, indicating the high selectivity of DCCNa for Au. Therefore, pH 2 was selected for subsequent leaching experiments.
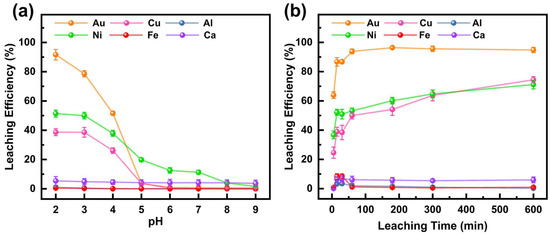
Figure 1.
Effect of (a) initial pH of the DCCNa solution and (b) leaching time on leaching efficiencies of metals in WPCB gold finger.
The effect of leaching time on metal leaching efficiencies is shown in Figure 1b (experimental conditions shown in Table 2). The Au leaching efficiency rose rapidly to 63.9% within 5 min, exceeding 93% at 60 min and reaching equilibrium at 96.4% after 3 h. This indicates the fast leaching of the DCCNa solution due to the rapid release of active chlorine species [30]. Ni and Cu showed similar trends, with a rapid initial increase followed by slower growth. After 5 min, Cu and Ni leaching efficiencies were 24.6% and 36.7%, respectively, reaching 74.4% and 71.2% after 10 h. Al, Fe, and Ca leaching efficiencies remained low, peaking at 3.7%, 8.6%, and 6.7% at 30 min. In summary, the Au leaching efficiency was significantly higher than that of other metals, peaking at 96.4% within 3 h. As time increased, the leaching efficiencies of Au did not increase much, but those of Ni and Cu increased obviously, which complicated subsequent metal separation. Therefore, 3 h was selected as the optimal leaching time for further experiments.
3.1.2. Effect of DCCNa Concentration
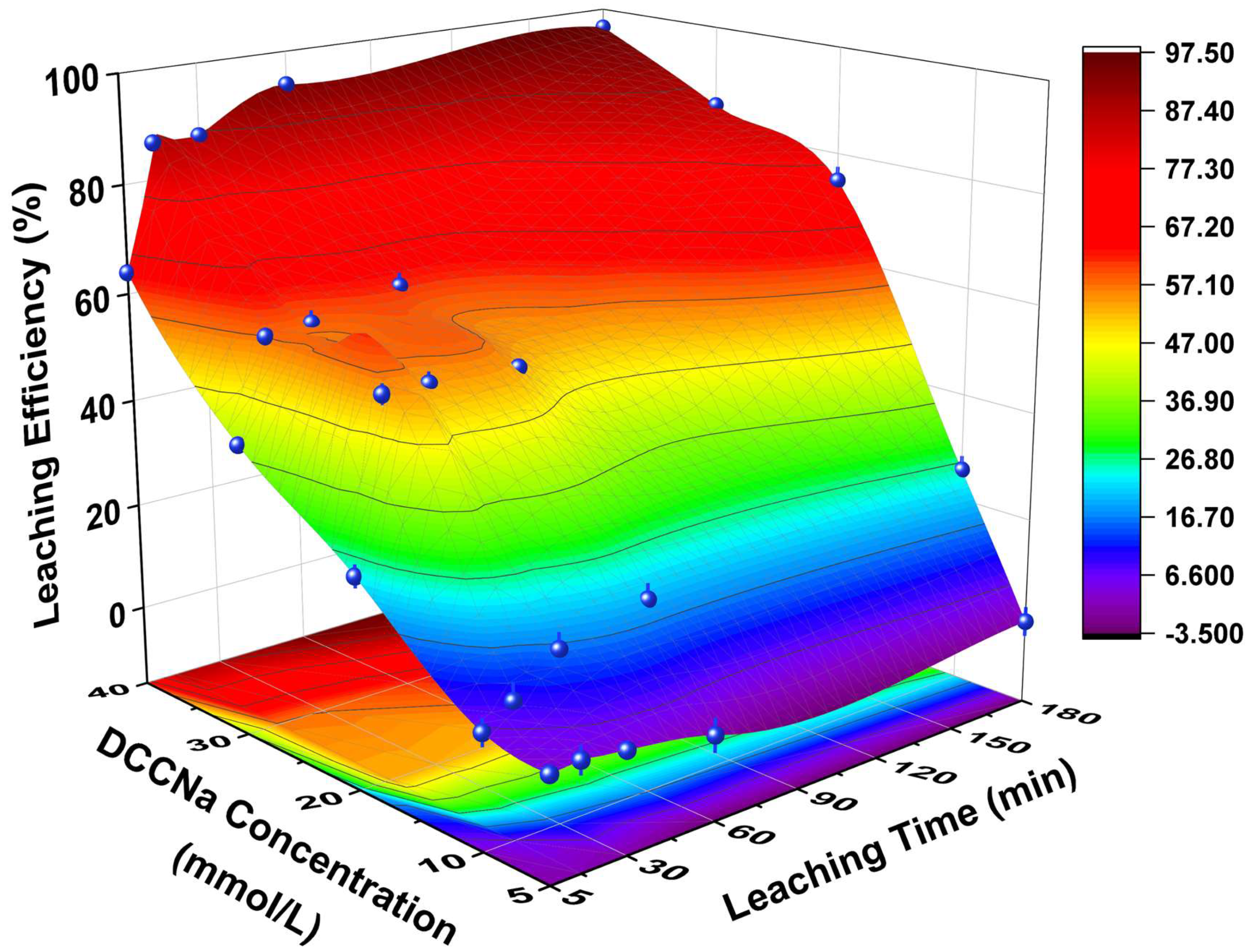
The effect of DCCNa concentration on Au leaching efficiency from WPCB gold finger powder is shown in Figure 2 (experimental conditions are shown in Table 2). At fixed DCCNa concentrations, Au leaching efficiency increased over time, with higher concentrations significantly enhancing efficiency. At 5 mmol/L, efficiency remained low throughout the experiment. Increasing the concentration to 10 mmol/L resulted in an efficiency of 5.9% at 5 min, rising to 25.7% at 180 min. At 40 mmol/L, efficiency reached 63.9% within 5 min and 93.8% at 60 min. After 180 min, Au leaching efficiency remained stable at 96.4%. At a fixed leaching time, efficiency also increased with DCCNa concentration. For example, at 60 min, efficiency rose from 0.4% at 5 mmol/L to 53.1% at 20 mmol/L and 93.8% at 40 mmol/L. Low efficiency at lower concentrations is attributed to limited active chlorine species. Higher concentrations enhanced the release of active chlorine and coordinating Cl− ions, improving Au leaching efficiency [31].
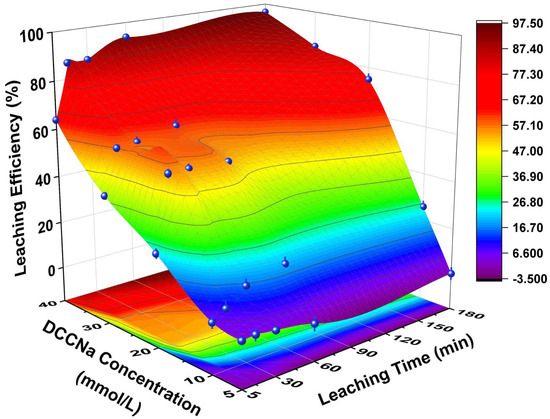
Figure 2.
Leaching efficiencies of Au in WPCB gold finger using DCCNa under different DCCNa concentrations and time.
The leaching efficiency of base metals with increasing DCCNa concentration is shown in Figure S2 (experimental conditions are shown in Table 2). Cu leaching efficiency rose from 14.2% to 50.0% at 60 min and from 16.0% to 54.2% at 180 min as the concentration increased from 5 mmol/L to 40 mmol/L. Similarly, Ni efficiency increased from 28.9% to 53.2% at 60 min and from 38.8% to 66.7% at 180 min. The leaching efficiencies of Ca, Al, and Fe remained consistently low under all conditions.
In summary, increasing DCCNa concentration can significantly enhance Au leaching efficiency, achieving near-complete recovery at 40 mmol/L. Subsequent experiments were conducted at a fixed DCCNa concentration of 40 mmol/L.
3.1.3. Effect of Leaching Temperature, Liquid-to-Solid Ratio, and Stirring Speed
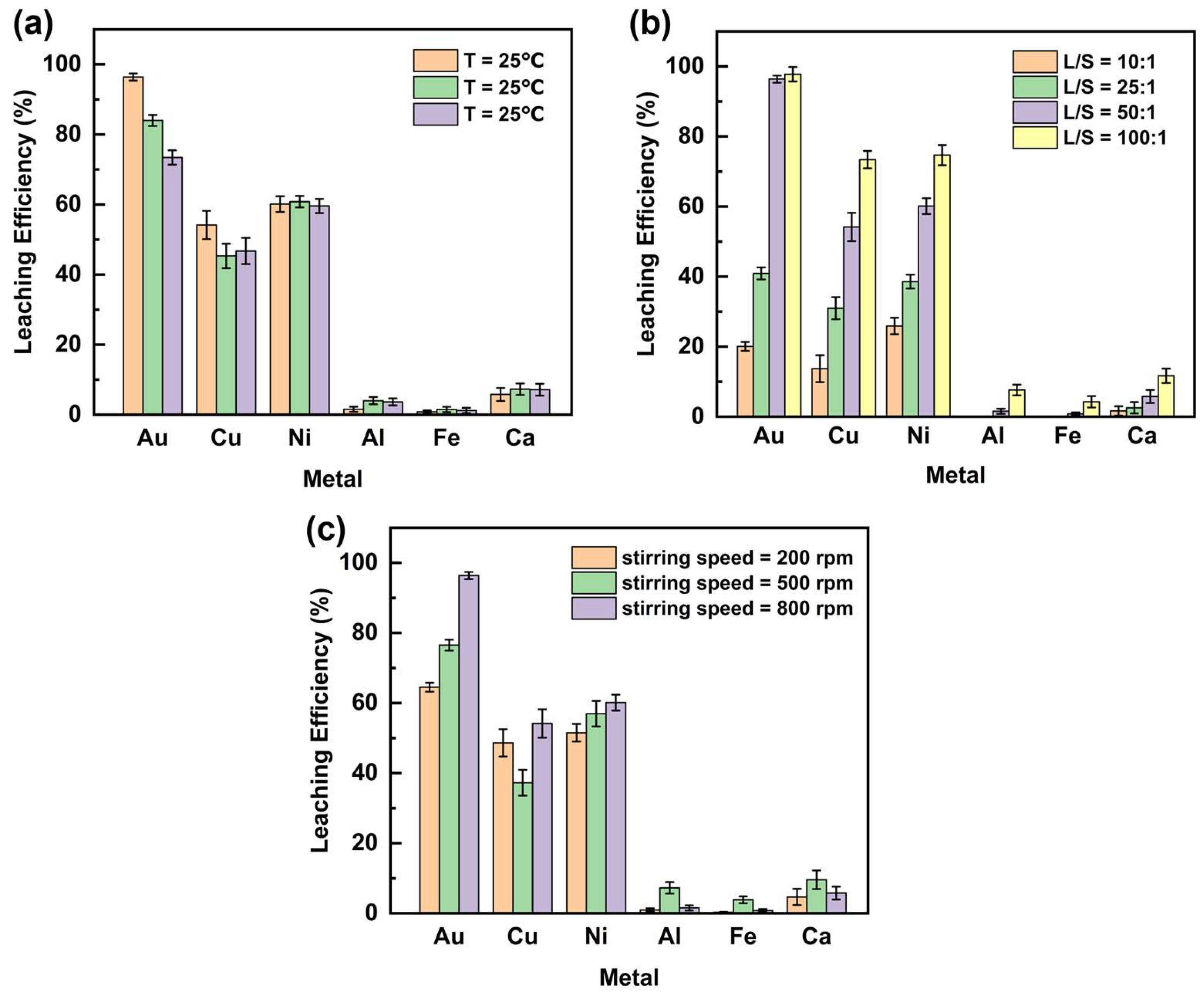
The effect of leaching temperature on the extraction efficiency of Au and base metals from WPCB gold finger using DCCNa is shown in Figure 3a (experimental conditions are provided in Table 2). Au leaching efficiency decreased with rising temperature, peaking at 96.4% at 25 °C, then dropping to 84.0% at 40 °C and 73.4% at 50 °C. This decline may be attributed to (1) increased volatilization of active chlorine species [29]; (2) thermal decomposition of intermediate trichloroisocyanuric acid (TCCA), generating nitrogen trichloride (NCl3) and lowering active chlorine content; and (3) thermal instability of Au complexes, causing decomposition. For Cu, leaching efficiency decreased from 54.2% at 25 °C to 45.3% at 40 °C but slightly rose to 46.7% at 50 °C. The efficiencies of Ni, Al, Fe, and Ca were minimally affected by temperature. Ni fluctuated around 60.0%, while Al, Fe, and Ca remained consistently low at approximately 2.0%, 1.1%, and 5.9%, respectively. In summary, Au leaching efficiency was highest at 25 °C and decreased at higher temperatures, making elevated temperatures detrimental to Au leaching. Room-temperature leaching (25 °C) is advantageous due to lower equipment requirements and operational simplicity, supporting its suitability for industrial applications. Consequently, 25 °C was selected for subsequent experiments.
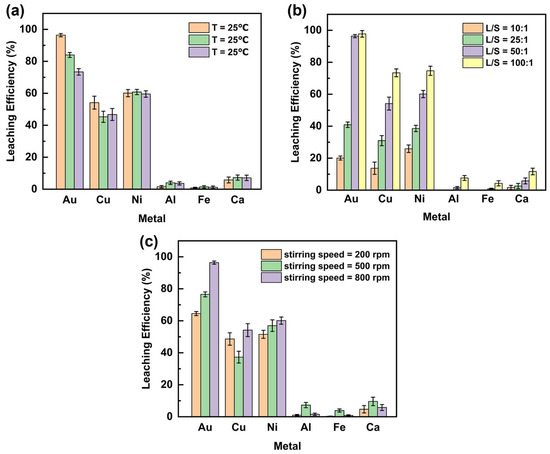
Figure 3.
Effect of (a) leaching temperatures (°C), (b) leaching liquid-to-solid ratios (mL/g), and (c) stirring speed (rpm) on the leaching efficiency of metals in WPCB gold finger.
The effect of the liquid-to-solid ratio (L/S) on the leaching efficiency of Au and base metals from WPCB gold powder using DCCNa is shown in Figure 3b (experimental conditions are provided in Table 2). Increasing the L/S ratio significantly enhanced leaching efficiency, primarily due to reduced slurry viscosity [32], which lowered mass transfer resistance and improved ion diffusion and interaction between the leaching agent and metal particles [33]. Among the metals, Au exhibited the most significant improvement in leaching efficiency. At an L/S ratio of 10:1, the leaching efficiency of Au was 20.3%, increasing rapidly to 96.4% at 50:1. A further increase to 100:1 resulted in only a slight improvement to 97.8%. Cu followed a similar trend, with efficiencies of 13.7%, 54.2%, and 73.4% at L/S ratios of 10:1, 50:1, and 100:1, respectively. Ni showed comparable behavior, with efficiencies rising from 25.9% to 74.7% over the same range. In contrast, Al, Fe, and Ca exhibited minor efficiency increases with higher L/S ratios, reaching 7.6%, 4.3%, and 11.7%, respectively, at 100:1. Notably, at an L/S ratio of 50:1, Au leaching efficiency plateaued, with further increases providing negligible benefits for Au but significant improvements for the other metals. In addition, higher L/S ratios increase leaching agent consumption and operational costs. Thus, an L/S ratio of 50:1 was selected for subsequent experiments as the optimal balance between efficiency and practicality.
The effect of stirring speed on the leaching efficiency of Au and base metals from WPCB gold finger using DCCNa is shown in Figure 3c (experimental conditions are provided in Table 2). Increasing stirring speed improved the leaching efficiencies of Au and Ni, with Au showing the most significant increase from 64.5% at 200 rpm to 96.4% at 800 rpm. Ni’s leaching efficiency rose more modestly, from 51.5% to 60.1%. This improvement is attributed to enhanced interaction and collision frequency between the gold finger powder and leaching solution at higher stirring speeds [34]. For Cu, the leaching efficiency exhibited a non-linear trend, decreasing from 48.6% at 200 rpm to 37.3% at 500 rpm before rising to 54.2% at 800 rpm. In contrast, the leaching efficiencies of Al, Fe, and Ca remained consistently low (<10%) across all stirring speeds. Overall, stirring speed significantly influenced Au leaching, with 800 rpm identified as the optimal speed for subsequent experiments.
3.1.4. Reagent Cost and Reagent Toxicity of DCCNa Leaching and Other Leaching Methods
The leaching experiments demonstrated that DCCNa exhibits excellent efficiency (96.4%) for leaching Au from the WPCB gold finger. A cost comparison between DCCNa and conventional leaching methods, including thiosulfate, thiourea, iodide, cyanide, and aqua regia leaching, is provided in the Supporting Information (Text S4, Table S1). As shown in Table S1, the reagent cost of DCCNa for leaching 1 g of Au is 10.9 USD, significantly lower than those of other methods (Thiosulfate: 23.6 USD, Thiourea: 50.2 USD, I2-KI: 52.7 USD, Cyanide: 16.2 USD, and Aqua regia: 16.1 USD). While cyanide and aqua regia leaching are relatively inexpensive, KCN is highly toxic, posing severe risks to human health and the environment. Similarly, using aqua regia leaching requires corrosion-resistant equipment and requires handling strongly acidic and corrosive waste, necessitating strict treatment to prevent environmental harm. Thus, the DCCNa leaching offers a more economically viable and environmentally friendly solution for Au leaching under laboratory conditions.
A comparison of the acute toxicity of reagents used in the aforementioned leaching methods is provided in Table S2. DCCNa, a common disinfectant widely used in agriculture, the food industry, and drinking water treatment, has an LD50 of 1420 mg/kgbw, classifying it as slightly toxic under the ecotoxicity categories defined by the US Environmental Protection Agency (EPA). In contrast, cyanide and I2 are classified as highly toxic, with LD50 values of 7.49 mg/kgbw and 14 mg/kgbw, respectively. Additionally, thiourea, CuSO4, and NH3·H2O are categorized as moderately toxic chemicals. Other toxicological data indicate that HNO3 and HCl may cause muscle contraction, spasms, and enzyme inhibition. Based on these findings, DCCNa emerges as a safer option for gold leaching.
In summary, under the optimized leaching conditions (initial pH 2, DCCNa concentration 40 mmol/L, temperature 25 °C, liquid-to-solid ratio 50:1, stirring speed 800 rpm, and leaching time 3 h), the leaching efficiency of Au reached 96.4%, while those of Cu, Ni, Al, Fe, and Ca were 54.2%, 60.1%, 1.5%, 0.8%, and 5.8%, respectively. The significantly higher efficiency for Au highlights the selectivity of DCCNa in gold leaching. And compared to other leaching agents, DCCNa leaching is both more economical and safer.
3.2. Stepwise Extraction of Metals from DCCNa PLS
Previous studies have demonstrated efficient Au leaching from WPCB gold fingers using DCCNa. However, Au remains in the PLS, requiring further separation and purification. As DCCNa is a water-soluble leaching agent, it offers the advantage of enabling the natural separation of the PLS from the organic phase extractant used in solvent extraction. Considering the inhibitory effect of high Cu concentrations on Au extraction, a sequential extraction approach (first extracting Cu, followed by Au) is recommended. Acorga M5640 is highly effective for Cu recovery from leachate [35,36], while dibutyl carbitol (DBC) exhibits excellent selectivity for Au extraction [37,38]. Thus, a two-step process is proposed: M5640 is used to extract Cu from the DCCNa leachate, followed by DBC for selective Au extraction, ensuring effective Cu and Au separation.
3.2.1. Extraction of Cu from the PLS
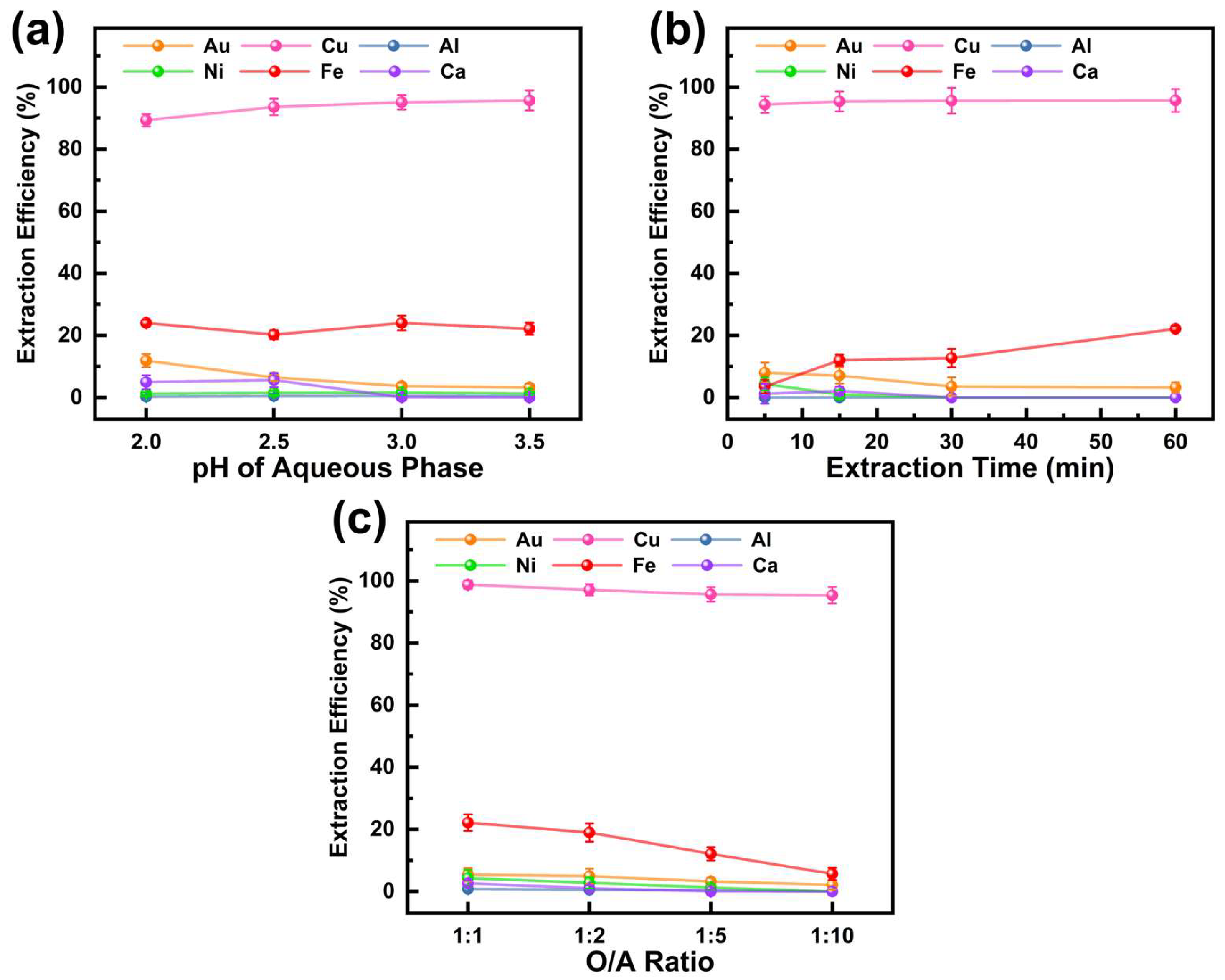
During Cu extraction, the O/A ratio was 1:5, the extraction time was 60 min, and the reaction temperature was 25 °C. The effect of aqueous phase pH on the extraction efficiency of six metals using M5640 is shown in Figure 4a. M5640 demonstrated significantly higher extraction efficiency for Cu compared to other metals, with the Cu extraction efficiency increasing as pH rose. At pH 3.5, the Cu extraction efficiency reached 95.7%, consistent with previous findings [39]. The superior Cu extraction performance of M5640 is attributed to the molecular structure of nonyl salicylaldehyde oxime. The nine-carbon R group enhances extraction efficiency, while the two hydroxyl oxygen atoms, with more negative electrostatic potential than the nitrogen atom, serve as favorable binding sites for Cu during coordination and extraction [40,41]. Within the studied pH range, M5640 exhibited an extraction efficiency of around 22.0% for Fe. At pH 2, the extraction efficiencies for Au and Ca were 11.9% and 4.9%, respectively, but both decreased to 3.2% and 0 at pH 3.5. For Al and Ni, extraction efficiency remained below 1.5%. These results indicate that higher pH favors Cu and Au separation. However, at pH 4, significant Cu2+ precipitation hindered Cu recovery from the PLS. Therefore, the optimal aqueous phase pH was set to 3.5.
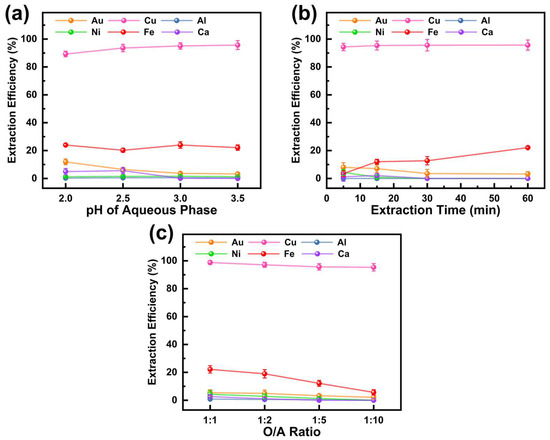
Figure 4.
Effect of (a) pH of the aqueous phase, (b) extraction time, and (c) O/A ratio on extraction efficiency of metals in PLS using M5640.
With an O/A ratio of 1:5, an aqueous phase pH of 3.5, and a reaction temperature of 25 °C, the effect of extraction time (5–60 min) on metal extraction efficiency is shown in Figure 4b. The Cu extraction efficiency reached 94.3% within 5 min and slightly increased to 95.7% at 60 min, indicating rapid equilibrium for Cu. In contrast, Fe extraction efficiency rose from 3.5% at 5 min to 22.2% at 60 min, consistent with findings from previous studies [42]. The Au extraction efficiency slightly declined over time, remaining below 8%, likely due to the instability and dissociation of the M5640–Au complex. For Al, Ni, and Ca, extraction rates remained consistently low (<4%) throughout. The increase in Cu and Fe extraction efficiency with time, coupled with the minimal Au extraction, highlights the selectivity of M5640 for Cu extraction because most of the Au remained in the raffinate. This separation is advantageous for subsequent Au recovery. Therefore, the extraction time was set at 60 min.
With an extraction time of 60 min, an initial aqueous phase pH of 3.5, and a reaction temperature of 25 °C, the effect of the O/A ratio (1:1–1:10) on metal extraction efficiency is shown in Figure 4c. The results indicate that decreasing the O/A ratio reduces extraction efficiency for all six metals, attributed to the lower availability of extractant molecules in the organic phase. For Cu, the extraction efficiency declined slightly, from 98.8% at an O/A ratio of 1:1 to 95.4% at 1:10. In contrast, the extraction efficiency of Au and Ni dropped significantly, from 5.4% and 4.3% at 1:1 to 2.1% and 0%, respectively, at 1:10. Fe exhibited the largest decrease, with its extraction efficiency falling from 32.2% at 1:1 to 12.6% at 1:10. M5640 showed negligible extraction effects for Al and Ca across the O/A ratio range. A lower O/A ratio effectively minimizes Au extraction, reduces losses, and conserves extractants. Therefore, an O/A ratio of 1:10 is considered optimal.
In summary, the optimal conditions for Cu extraction using M5640 are as follows: aqueous phase pH of 3.5, O/A ratio of 1:10, reaction temperature of 25 °C, and extraction time of 60 min. Under these conditions, the Cu extraction efficiency reached 95.4%, while Au was scarcely extracted, significantly simplifying subsequent Au recovery and extraction processes.
3.2.2. Stripping of Cu from the Organic Phase After Cu Extraction
After completing the copper extraction experiment, the organic phase contained a significant amount of copper. To further purify the product, 2 mol/L H2SO4 was used for stripping to recover Cu from the DCCNa PLS. The effect of stripping time and O/A ratio on Cu stripping efficiency was investigated at 25 °C, with the results shown in Figure 5. When the stripping time was 15 min, and the O/A ratio was 1:5, the Cu stripping efficiency reached 97.0%. At this point, the concentrations of other metals were negligible, resulting in a highly pure copper-containing solution, which is conducive to subsequent copper reduction processes. Through M5640 extraction followed by stripping with dilute H2SO4, Cu was almost entirely recovered, achieving a recovery rate of 92.5%. This efficient two-step process provides a high-purity copper product while ensuring minimal loss, supporting the sustainable recovery of valuable metals.
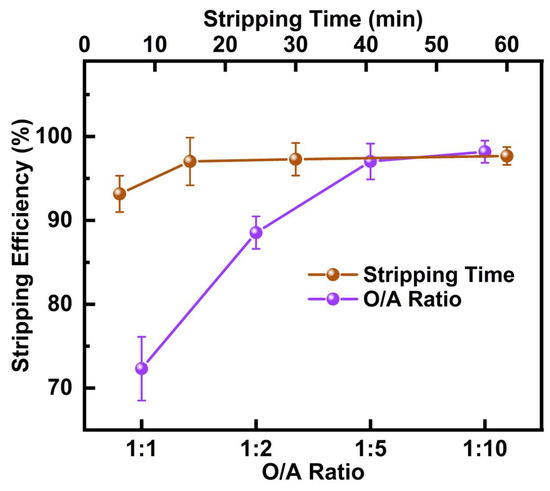
Figure 5.
Effect of stripping time and O/A ratio on Cu stripping efficiency from M5640 raffinate organic.
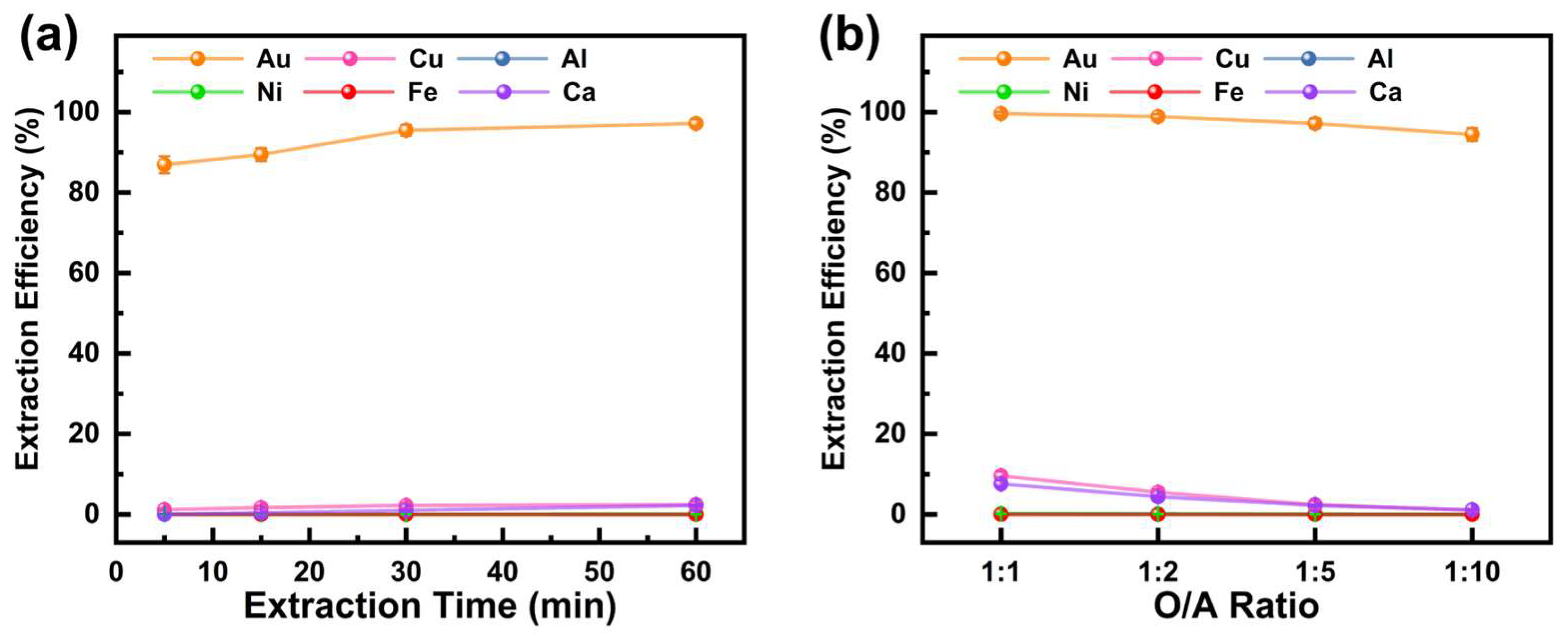
3.2.3. Extraction of Au from the Raffinate Solution After Cu Extraction
Following the extraction of copper from the PLS, DBC was used to further extract gold from the raffinate. The effect of extraction time on metal extraction efficiency was investigated under conditions of an O/A ratio of 1:5, an initial aqueous phase pH of 3.5, and a reaction temperature of 25 °C. The results, shown in Figure 6a, indicate that extraction time significantly influenced the extraction efficiency of Au but had minimal impact on other metals. The Au extraction efficiency gradually improved from 86.9% at 5 min to 97.2% at 60 min as the extraction time increased. In contrast, DBC exhibited negligible extraction effects on Cu, Al, Ca, Fe, and Ni, with extraction efficiencies for these metals consistently below 2%, aligning with findings reported by previous researchers [38]. Thus, an extraction time of 60 min is optimal for achieving the effective separation of Au from the raffinate.
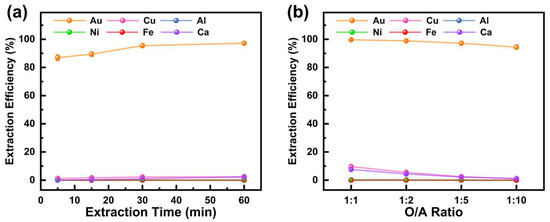
Figure 6.
Effect of (a) extraction time and (b) O/A ratio on extraction efficiency of metals from M5640 raffinate aqueous phase using DBC.
With the extraction time fixed at 60 min, the effects of the O/A ratio on metal extraction efficiency were studied at an initial aqueous phase pH of 3.5 and a reaction temperature of 25 °C. The results in Figure 6b reveal that, except for Fe, the extraction rates of all other metals decreased with a reduction in the O/A ratio. At an O/A ratio of 1:10, the Au extraction rate reached 95.5%, while the extraction efficiencies of Cu and Ca were only 1.0% and 1.2%, respectively. Within the studied O/A ratio range, DBC showed no extraction effect on Al and Ni. Therefore, a smaller O/A ratio not only minimizes the extraction of impurity metals but also conserves the DBC extractant, making an O/A ratio of 1:10 the preferred choice.
In summary, during the extraction of gold from the M5640 raffinate using DBC, the optimal conditions include an extraction time of 60 min and an O/A ratio of 1:10, under which the Au extraction efficiency reaches 95.5%. Under these conditions, DBC exhibits minimal extraction of Cu, Ca, Ni, Al, and Fe. Following the two-step extraction process, the Au purity increased to 81.7%, while the contents of Cu, Ca, Ni, Al, and Fe were reduced to 0.2%, 9.8%, 6.1%, 2.1% and 0.1%, respectively. High-purity gold can be recovered for reuse through methods such as electrodeposition, stripping, and reduction. For instance, an electrodeposition–redox replacement (EDRR) method has been utilized to recover trace amounts of gold from chloride solutions [43]. Some researchers have used water and NaOH solutions at varying concentrations for stripping and recovering gold from the organic phase [44]. Oxalic acid has been used as a reducing agent to achieve the quantitative recovery of gold from the organic phase [45].
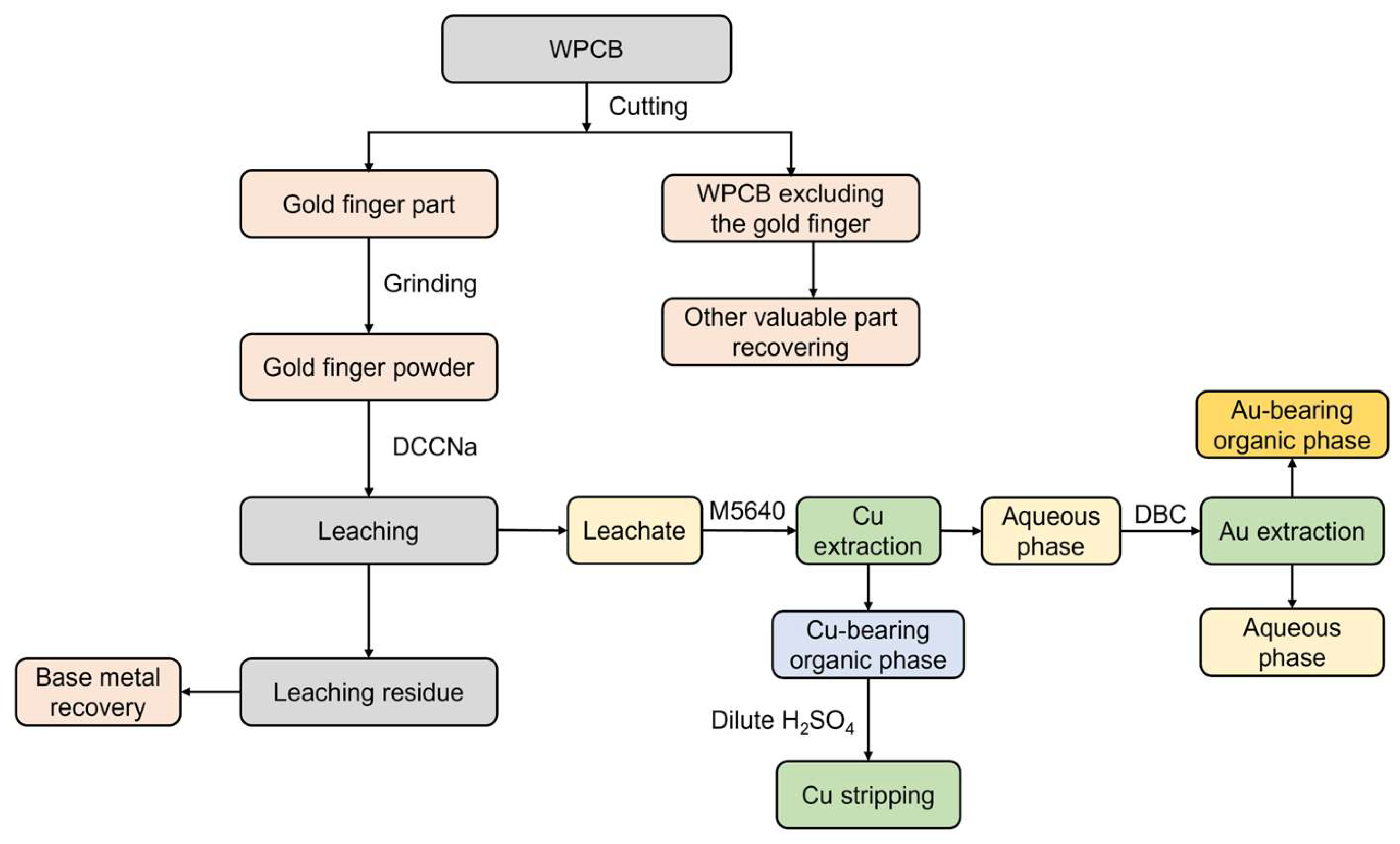
A comprehensive scheme for the recovery of Au and Cu from WPCB gold fingers, based on the findings discussed above, is proposed. This process involves aqueous organic leaching using DCCNa and stepwise extraction with M5640 + DBC, as illustrated in Figure 7. The process begins with the separation of the gold fingers from the other WPCB components through cutting. The non-gold finger parts are then processed for the recovery of base metals, as well as materials such as plastics and resins, to enhance the overall economic efficiency of the recovery process. Next, the gold fingers undergo leaching using the DCCNa leaching agent, and the resulting leaching residue is further treated for the recovery of base metals such as Cu, Ni, Fe, and Al. The leachate is then subjected to a two-stage solvent extraction process using M5640 and DBC to recover Au and Cu. Cu is extracted using M5640, followed by stripping with dilute sulfuric acid to separate Cu. The aqueous phase from the M5640 extraction is then processed with DBC to recover Au.
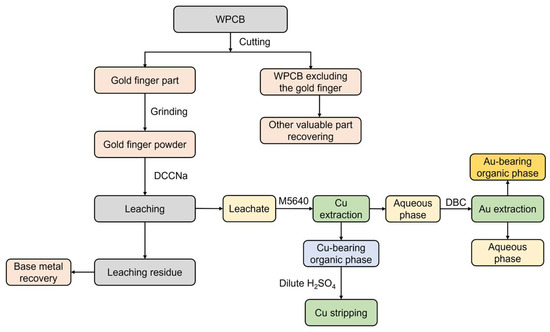
Figure 7.
Schematic diagram of Au recovery process via DCCNa leaching and two-stage extraction.
In the quantitative summary of the recovery process, the mass of the metal at each stage was calculated based on the contents of Au and the other five base metals present in the WPCB gold finger (Table 3). The total recovery rate of Au and Cu were subsequently determined. The Au content in the gold finger was 2.27 mg/g gold finger. After leaching with DCCNa, followed by Cu extraction using M5640 and Au extraction with DBC, the Au content in the Au-bearing organic phase was 2.06 mg/g gold finger, resulting in a total Au recovery of 90.7%. In contrast, the Cu content in the gold finger was 174.77 mg/g gold finger. Following the leaching process, two-step extraction, and dilute H2SO4 stripping, the Cu content in the Cu-bearing aqueous phase was 87.66 mg/g gold finger, yielding a total recovery of 50.2%.

Table 3.
Metal balance during the process of DCCNa leaching, M5640 extraction, and DBC extraction.
3.3. Au Oxidation State in Leaching Products and Leaching Pathway of DCCNa Solution
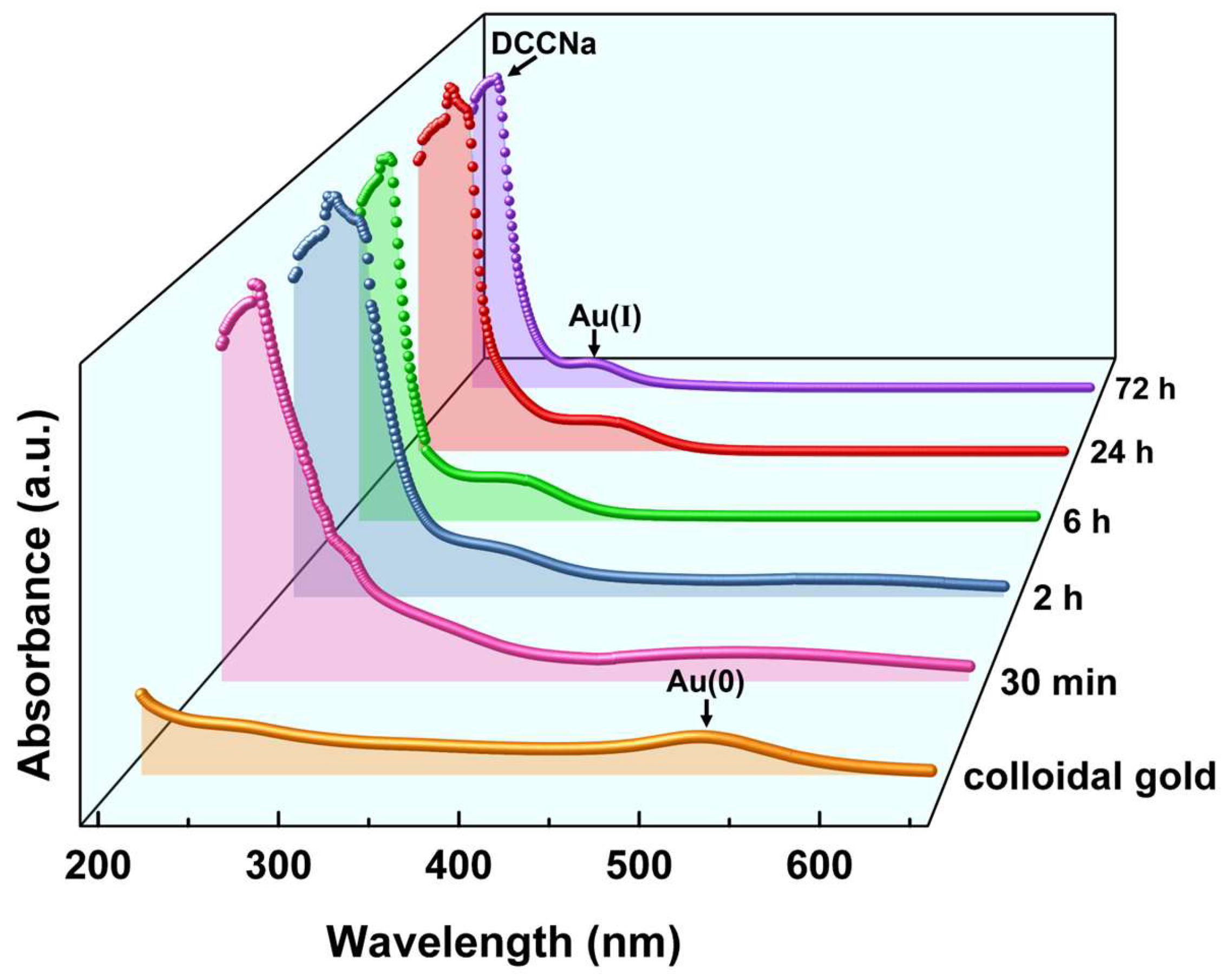
The oxidation state of Au in the leachate is critical for understanding the oxidation and coordination mechanisms, as well as the structure of Au leaching products. To eliminate interference from other metals, pure gold materials (colloidal gold and nano-gold powder) were used in the experiments to analyze the oxidation state and structural characteristics of Au leaching products. UV–Vis spectroscopy was employed to examine spectral changes in colloidal gold before and after leaching with DCCNa solution, focusing on the oxidation state transformation of Au during the leaching process. The evolution of UV–Vis absorption peaks during the leaching of colloidal gold with DCCNa solution over a period of 30 min to 72 h was investigated, and the results are shown in Figure 8.
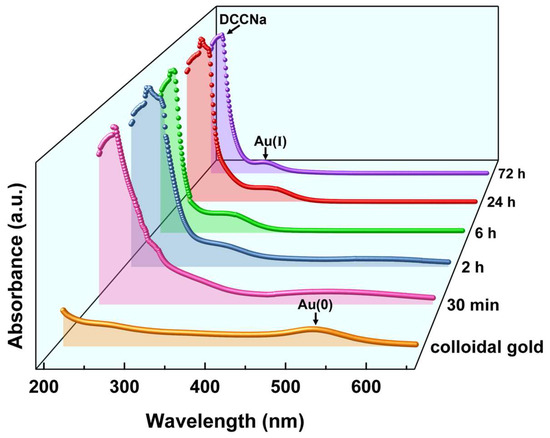
Figure 8.
UV–Vis spectra of colloidal gold and PLS after DCCNa leaching over time.
In Figure 8, the orange line represents the spectrum of colloidal gold (Au(0)), with an absorption peak near 520 nm [46]. Significant spectral changes occurred within the first 30 min of leaching. The intensity of the 520 nm peak decreased markedly, the peak broadened, and two new absorption peaks emerged near 220 nm and 295 nm. The peak at 220 nm was relatively strong, while the 295 nm peak was weaker. These changes indicate rapid oxidation of colloidal gold during the initial stage of leaching, consistent with the fast reaction kinetics observed in Au leaching experiments. As leaching progressed, the 520 nm peak diminished further, while the 295 nm peak became more pronounced. The 220 nm peak showed minimal variation. After 24 h of leaching, the 520 nm peak nearly disappeared (red line), and the 295 nm peak reached maximum intensity. Extending the leaching time to 72 h produced no further spectral changes, indicating the completion of the leaching process. The 220 nm absorption peak corresponds to the DCCNa molecule, attributed to π → σ* electronic transitions of the N-Cl group, a characteristic feature of DCCNa [47]. The 295 nm peak likely represents signals from Au(I) complexes, as Au(0) typically exhibits absorption peaks above 500 nm (e.g., the 520 nm peak observed for colloidal Au in this study). During leaching, Au(0) was oxidized to higher-valence Au complexes. Au(I) complexes generally show absorption peaks in the range of 200–470 nm [48], consistent with the 295 nm peak observed. This suggests the formation of [AuCl2]− complexes through coordination between Au(I) and Cl− ions released during DCCNa leaching. Although Au(III) complexes are another common form of Au coordination compounds, their absorption peaks typically appear in the 350–600 nm range [49], which was not detected in this study. Thus, UV–Vis results indicate that Au(I) complexes were the primary leaching products formed during the reaction between DCCNa and colloidal gold.
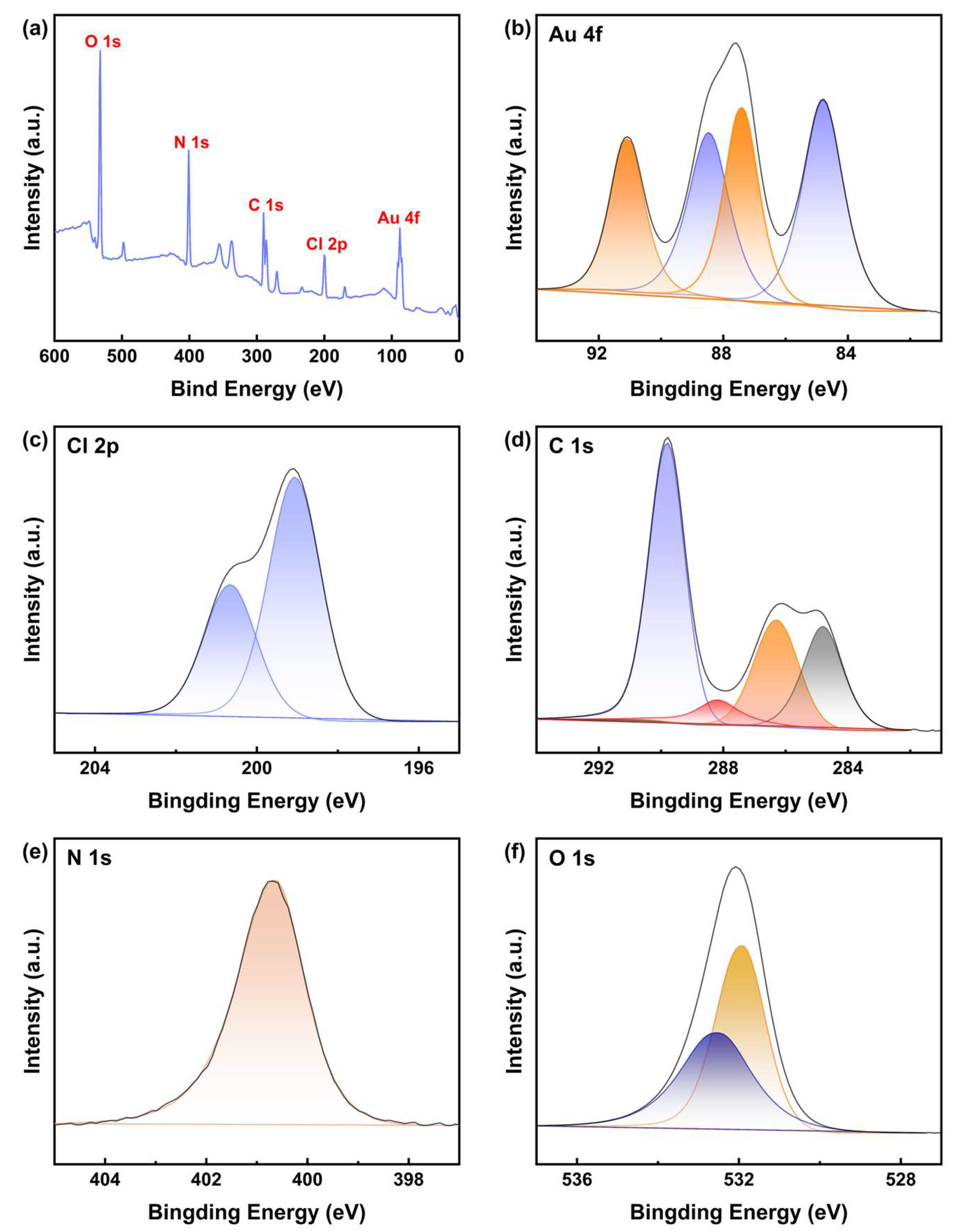
To further investigate the structural characteristics of the Au leaching products obtained with DCCNa, the powder evaporated from the PLS of gold powder was analyzed using XPS. High-resolution spectra for Au, Cl, C, N, and O were calibrated using the C 1s peak of adventitious carbon at 284.80 eV, and peak differentiation and imitation were performed. The results are shown in Figure 9.
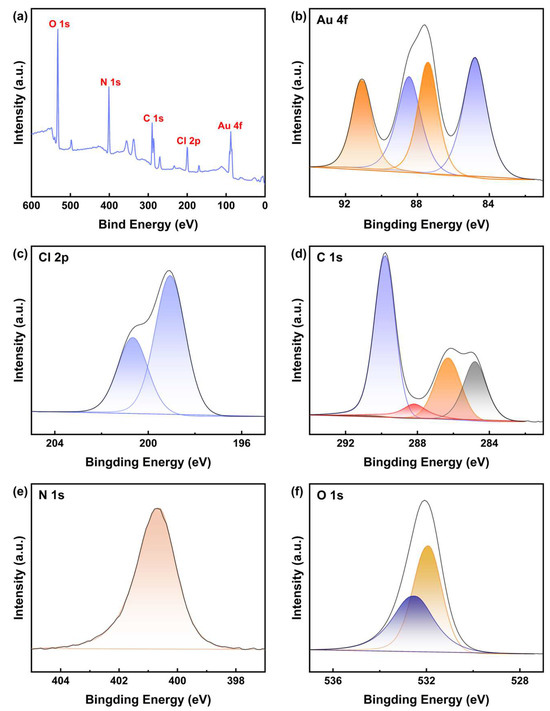
Figure 9.
(a) XPS spectrum of the evaporated substance of nano-gold powder PLS using DCCNa, and corresponding high-resolution XPS spectra for (b) Au 4f, (c) Cl 2p, (d) C 1s, (e) N 1s, and (f) O 1s orbitals.
The high-resolution spectrum of Au 4f provides compelling evidence for the oxidation states of Au (Figure 9b). The Au 4f7/2 peak at a binding energy of 84.79 eV (Δ = 3.63 eV) confirms the presence of Au(I), while the Au 4f7/2 peak at 87.40 eV (Δ = 3.63 eV) suggests the presence of Au(III) species [50,51,52]. This indicates that the leaching product contains both Au(I) and Au(III) compounds, likely in the form of [AuCl2]− and [AuCl4]− complexes. This result differs from the UV–Vis analysis, which did not show the presence of Au(III), possibly because the polar solvent (water) used in the UV–Vis experiments masked the signal of Au(III) complexes.
The high-resolution spectrum of Cl 2p is shown in Figure 9c. The doublet, arising from the spin–orbit splitting of the Cl 2p orbital, corresponds to a single chemical environment for chlorine atoms. The binding energy at 199.06 eV (Δ = 1.60 eV) is typically attributed to chloride ions (Cl−) [50,51]. This peak, located in the higher-energy region of Cl 2p, suggests that chlorine atoms are chemically bonded to more electronegative atoms, such as metals, indicating coordination between Au and Cl.
Figure 9d displays the high-resolution spectrum of C 1s. Peaks at 286.29 eV, 288.19 eV, and 289.80 eV correspond to C-N, C-Cl, and C=O bonds, respectively [50,51,53]. These functional groups likely represent the basic triazine ring structure of DCCNa after its reaction with Au, as well as functional groups associated with reaction byproducts outside the Au complexes. The N 1s spectrum (Figure 9e) exhibits a single peak at 400.63 eV, which may correspond to the C-N bond [50,51], which aligns with the molecular structure of DCCNa. Additionally, the O 1s spectrum (Figure 9f) shows peaks at 531.91 eV and 532.48 eV, representing binding energies of C=O bonds and sulfate ions (SO42−), respectively [50,51]. This suggests that amide-like compounds may be present in the product.
Following the analysis of the UV–Vis and XPS spectra, the leaching reaction products of Au were preliminarily identified as Au(I) and Au(III) compounds. To further confirm the chemical composition of the Au leachate, the PLS of gold powder was subjected to ESI-MS analysis. The results are presented in Figure 10.
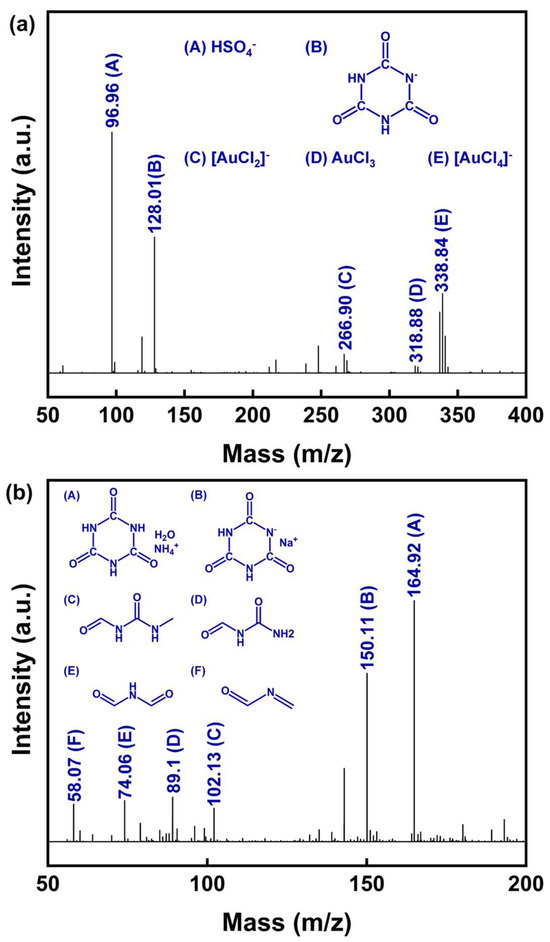
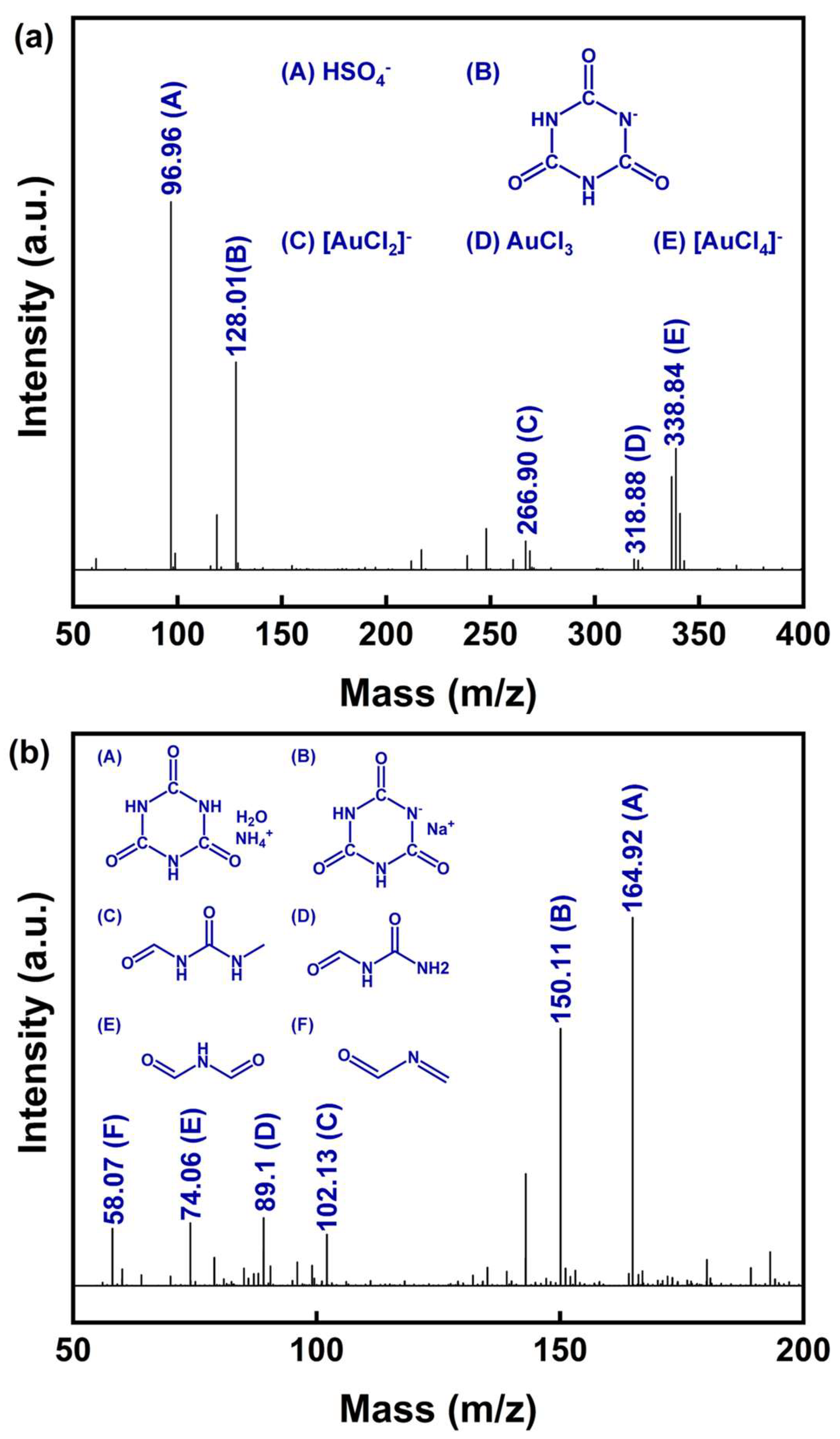
Figure 10.
(a) Negative ion mode and (b) positive ion mode of ESI-MS spectra from the nano-gold powder PLS using DCCNa.
In the negative ion spectrum (Figure 10a), the peak at m/z = 96.96 (labeled A) corresponds to HSO4−, originating from the H2SO4 added during the preparation of the DCCNa leaching solution. The peak at m/z = 128.01 (labeled B) is attributed to the cyanuric acid anion, a product of the rapid hydrolysis of DCCNa in water [54]. Based on the UV–Vis and XPS analyses, the leaching products likely include Au(I) and Au(III) complexes. Combined with the findings of Gross et al. [55], the peak at m/z = 266.90 (labeled C) likely represents the [AuCl2]− complex formed by Au(I) coordinated with two Cl− ions. Similarly, the peak at m/z = 338.84 (labeled D) likely corresponds to the [AuCl4]− complex, in which Au(III) is coordinated with four Cl− ions. These results suggest that the active oxidizing chlorine species generated using DCCNa in aqueous solutions, such as Cl2 and ClO−, serve as oxidizing agents for Au, while Cl− acts as a coordinating ligand. Together, these species facilitate the oxidation of metallic Au (Au(0)) into Au(I) and Au(III) complexes.
The positive ion spectrum (Figure 10b) provides additional insights into the byproducts of DCCNa hydrolysis and oxidation. Peaks labeled A (m/z = 164.92) and B (m/z = 150.11) both correspond to derivatives of cyanuric acid, which is produced by the hydrolysis of sodium dichloroisocyanurate. Peak C (m/z = 102.94) is likely generated via the ring-opening of cyanuric acid to N-(methylcarbamoyl)formamide. Additional peaks include D (m/z = 89.11), corresponding to N-carbamoylformamide, and E (m/z = 74.06), attributed to N-formylformamide, which is formed via the deamination of N-carbamoylformamide. Lastly, the peak at F (m/z = 58.07) represents N-methyleneformamide, a product derived from further oxidation or degradation of the aforementioned intermediates.
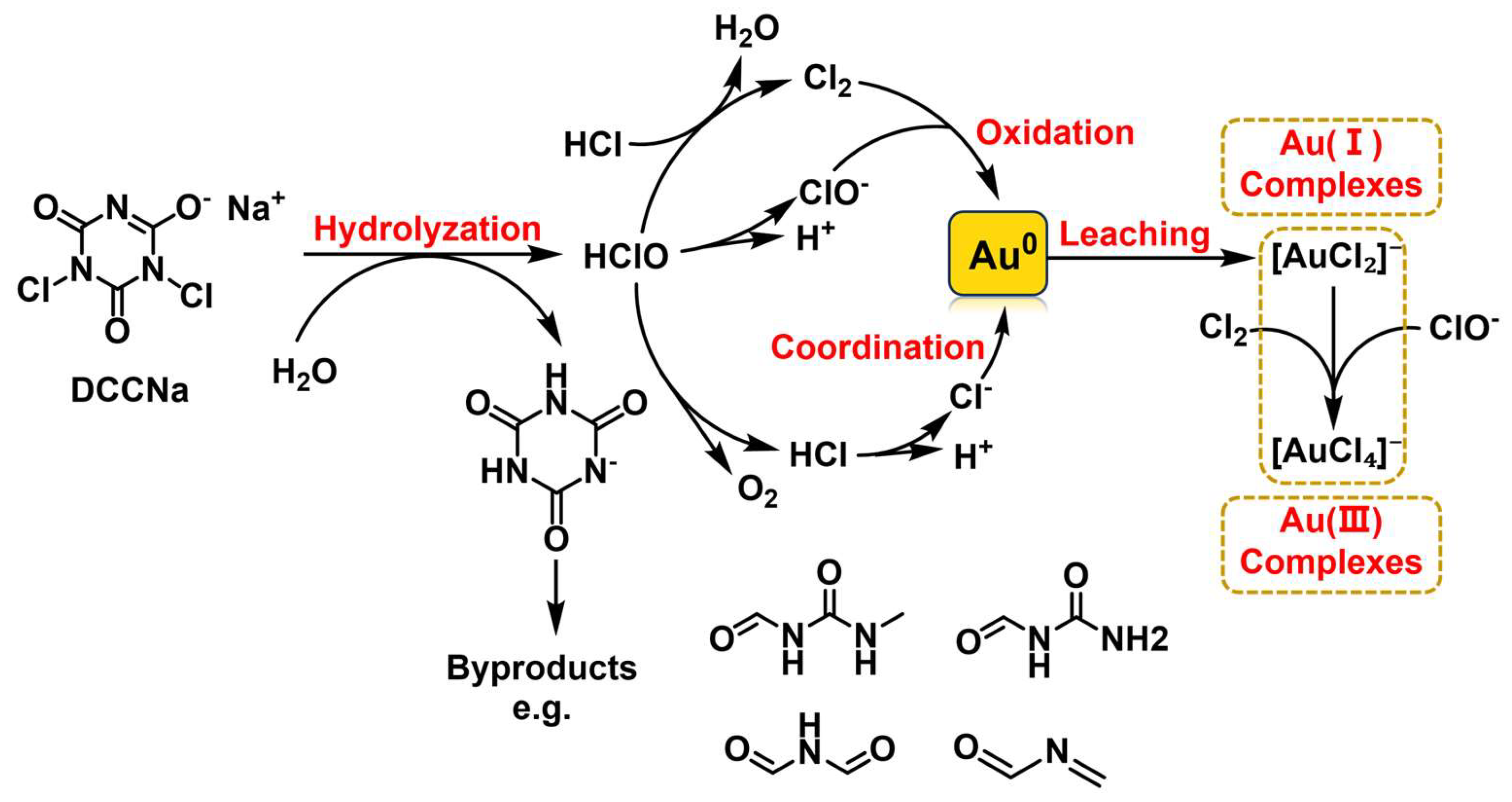
Based on the analyses of UV–Vis, XPS, and ESI-MS, the main reaction process for Au leaching using DCCNa is proposed to be represented by Equations (4)–(10). The leaching pathway and mechanism are illustrated in Figure 11. Upon dissolution in water, DCCNa generates the strong oxidant HClO, which reacts with HCl to produce Cl2. HClO and Cl2 then oxidize Au(0) to Au(I) and Au(III), forming [AuCl2]− and [AuCl4]− complexes, with Cl− derived from the hydrolysis of HCl. As the reaction progresses, DCCNa hydrolyzes into cyanuric acid, which is susceptible to oxidation by active chlorine in the solution. This oxidation induces ring-opening reactions, generating a series of organic byproducts such as N-(methylcarbamoyl)formamide, N-carbamoylformamide, N-formylformamide, and N-methyleneformamide.
DCCNa+ 3H2O ⟶ C3H2N3O3− + 2HClO + Na+
2HClO ⟶ HCl + O2
HClO + HCl ⟶ H2O + Cl2
2Au + 2HClO + 2Cl− ⟶ 2[AuCl2]− + 2OH−
2Au + Cl2 + 2Cl− ⟶ 2[AuCl2]−
2[AuCl2]− + 4HClO ⟶ 2[AuCl4]− + O2 + H2O
2[AuCl2]− + 2 Cl2 ⟶ 2[AuCl4]−
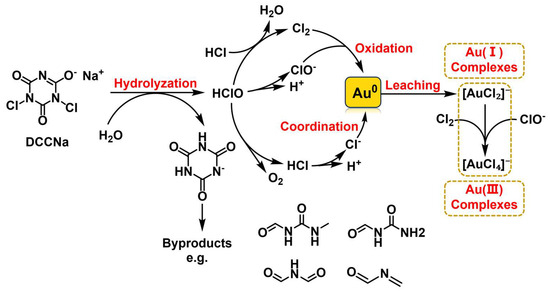
Figure 11.
Au leaching pathway and mechanism of DCCNa leaching.
While we believe that DCCNa leaching offers advantages in terms of efficiency, safety, and cost-effectiveness, the necessity for highly corrosion-resistant reactors and the incorporation of acid gas collection systems for industrial applications should be addressed. Furthermore, introducing automated control systems will help regulate reaction parameters and reduce operational complexity. However, under the current laboratory conditions, we are unable to fully assess the safety, economic viability, and environmental impact of this method due to the absence of larger-scale pilot and industrial-scale experiments. Furthermore, potential side effects associated with the leaching process have yet to be verified. Nevertheless, we believe that the DCCNa leaching agent demonstrates considerable potential for industrial application under the present conditions. To confirm its viability, further large-scale studies are required, and this is an ongoing area of research that we are actively pursuing. It is well established that in addition to the precious metal gold and primary base metals such as copper and nickel, WPCB also contains other valuable recyclable materials, including plastics and resins. These materials add further recycling value, underscoring the importance of the comprehensive recovery of all valuable resources from WPCB. The efficient extraction of these resources can be integrated with processes such as gold leaching using DCCNa, copper extraction with M5640, and gold extraction via DBC, thereby establishing a complete, closed-loop recycling system. This approach not only reduces secondary waste but also promotes sustainable development.
4. Conclusions
WPCB, as a rapidly growing source of electronic waste, contains metal components, particularly gold, at concentrations significantly higher than those in natural gold ores, offering considerable economic recovery potential. However, the widely used cyanidation method and other emerging recovery techniques (e.g., sulfate, thiourea, and halide-based methods) suffer from drawbacks such as high toxicity, large reagent consumption, and elevated process costs. This study presents a novel, efficient, low-toxicity, and environmentally friendly gold leaching method developed under laboratory conditions using a water-soluble organic leaching agent based on dissolved DCCNa. The leaching performance of DCCNa on gold from the WPCB gold finger area was systematically evaluated, along with the stepwise extraction of copper and gold from the DCCNa leachate using Acorga M5640 and DBC extractants, respectively. The oxidation states of gold during the leaching process and the gold leaching pathway were also analyzed. The main conclusions of this study are as follows:
- Optimal Leaching Conditions: The optimal conditions for the DCCNa aqueous organic leaching system were pH 2, a reaction time of 3 h, a DCCNa concentration of 40 mmol/L, a temperature of 25 °C, a liquid-to-solid ratio of 50:1, and a stirring speed of 800 rpm. Under these conditions, the gold leaching efficiency reached 96.4%, significantly higher than for other metals, demonstrating notable selectivity for gold.
- Gold Leaching Mechanism: Mechanistic studies showed that upon dissolution in water, DCCNa generates the strong oxidant HClO, which reacts with HCl to produce Cl2. HClO and Cl2 then oxidize Au(0) to Au(I) and Au(III), forming [AuCl2]− and [AuCl4]− complexes, with Cl− originating from the hydrolysis of HCl.
- Stepwise Solvent Extraction of Cu and Au: Copper and gold in the DCCNa leachate were recovered through stepwise solvent extraction. The optimal conditions for copper extraction using Acorga M5640 were pH 3.5, an extraction time of 60 min, and an O/A ratio of 1:10. For gold extraction using DBC, the optimal conditions were an extraction time of 60 min and an O/A ratio of 1:10. The stepwise extraction process achieved copper and gold extraction rates of 95.4% and 95.5%, respectively.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/su17062415/s1, Text S1: Chemical and materials; Text S2: Extraction experimental methods; Text S3: Analytical methods; Text S4: Reagent costs of the DCCNa and other leaching methods; Table S1: Reagent cost estimation of DCCNa and other leaching methods; Table S2: Acute toxicity information for reagents used in DCCNa and other leaching methods; Figure S1: Molecular formula of sodium dichloroisocyanurate; Figure S2: Effect of DCCNa concentrations on the leaching efficiencies of (a) Cu (b) Ni (c) Al (d) Fe, and (e) Ca from WPCB gold finger using DCCNa with time.
Author Contributions
Conceptualization, Y.H.; methodology, G.Z., Z.X., and Y.L.; software, R.Z.; formal analysis, G.Z., Z.X., F.G., and Y.L.; data curation, F.G.; resources, Y.H.; writing—original draft preparation, G.Z.; writing—review and editing, Y.H.; visualization, G.Z. and J.T.; supervision, Y.H.; project administration, Y.H.; funding acquisition, Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 21106019, and the Jiangsu Provincial Key Research and Development Program, grant number BE2019108.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article.
Acknowledgments
We appreciate Chengxiu He and Shijun Chou from Shiyanjia Lab (www.shiyanjia.com, (accessed on 6 June 2023)) for the XPS and ESI-MS analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BFRs | brominated flame retardants |
| CFCs | chlorofluorocarbons |
| DBC | dibutyl carbitol |
| DCCNa | sodium dichloroisocyanurate |
| DMF | dimethylformamide |
| ESI-MS | electrospray ionization mass spectrometry |
| HCFCs | hydrochlorofluorocarbons |
| L/S | liquid-to-solid ratio |
| NBS | N-bromosuccinimide |
| O/A | organic-to-aqueous ratio |
| OAR | organic aqua regia |
| PCB | printed circuit board |
| PLS | pregnant leach solution |
| Py | pyridine |
| TCCA | trichloroisocyanuric acid |
| USD | United States Dollar |
| UV-Vis | ultraviolet–visible spectrophotometry |
| WPCB | waste printed circuit boards |
| XPS | X-ray photoelectron spectroscopy |
References
- Rezaee, M.; Abdollahi, H.; Saneie, R.; Mohammadzadeh, A.; Rezaei, A.; Darvanjooghi, M.H.K.; Brar, S.K.; Magdouli, S. A cleaner approach for high-efficiency regeneration of base and precious metals from waste printed circuit boards through stepwise oxido-acidic and thiocyanate leaching. Chemosphere 2022, 298, 134283. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Monteiro, J.; Futuro, A.; Regufe, M.J.; Soeiro, J.; Sousa, R. Recycling PCBs for nanoparticles production with potential applications in cosmetics, cement manufacturing, and CO2 capture. Waste Manag. 2025, 191, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Bao, P.; Liu, A.; Wang, M.; Shen, L.; Yu, R.; Liu, Y.; Chen, M.; Li, J.; Wu, X.; et al. Bioleaching of low-grade waste printed circuit boards by mixed fungal culture and its community structure analysis. Resour. Conserv. Recycl. 2018, 136, 267–275. [Google Scholar] [CrossRef]
- Batnasan, A.; Haga, K.; Shibayama, A. Recovery of Precious and Base Metals from Waste Printed Circuit Boards Using a Sequential Leaching Procedure. J. Miner. Met. Mater. Soc. 2018, 70, 124–128. [Google Scholar] [CrossRef]
- Ongondo, F.; Williams, I.; Whitlock, G. Distinct Urban Mines: Exploiting secondary resources in unique anthropogenic spaces. Waste Manag. 2015, 45, 4–9. [Google Scholar] [CrossRef]
- Trivedi, A.; Vishwakarma, A.; Saawarn, B.; Mahanty, B.; Hait, S. Fungal biotechnology for urban mining of metals from waste printed circuit boards: A review. J. Environ. Manag. 2022, 323, 116133. [Google Scholar] [CrossRef]
- Lekka, M.; Masavetas, I.; Benedetti, A.; Moutsatsou, A.; Fedrizzi, L. Gold recovery from waste electrical and electronic equipment by electrodeposition: A feasibility study. Hydrometallurgy 2015, 157, 97–106. [Google Scholar] [CrossRef]
- Arab, B.; Hassanpour, F.; Arshadi, M.; Yaghmaei, S.; Hamedi, J. Optimized bioleaching of copper by indigenous cyanogenic bacteria isolated from the landfill of e-waste. J. Environ. Manag. 2020, 261, 110124. [Google Scholar] [CrossRef]
- Becci, A.; Amato, A.; Fonti, V.; Karaj, D.; Beolchini, F. An innovative biotechnology for metal recovery from printed circuit boards. Resour. Conserv. Recycl. 2020, 153, 104549. [Google Scholar] [CrossRef]
- Chu, H.; Qian, C.; Tian, B.; Qi, S.; Wang, J.; Xin, B. Pyrometallurgy coupling bioleaching for recycling of waste printed circuit boards. Resour. Conserv. Recycl. 2022, 178, 106018. [Google Scholar] [CrossRef]
- Thacker, S.C.; Nayak, N.S.; Tipre, D.R.; Dave, S.R. Multi-Metal Mining from Waste Cell Phone Printed Circuit Boards using Lixiviant Produced by a Consortium of Acidophilic Iron Oxidizers. Environ. Eng. Sci. 2022, 39, 287–295. [Google Scholar] [CrossRef]
- Ji, X.; Yang, M.; Wan, A.; Yu, S.; Yao, Z. Bioleaching of Typical Electronic Waste—Printed Circuit Boards (WPCBs): A Short Review. Int. J. Environ. Res. Public Health 2022, 19, 7508. [Google Scholar] [CrossRef] [PubMed]
- Petter, P.; Veit, H.; Bernardes, A. Evaluation of gold and silver leaching from printed circuit board of cellphones. Waste Manag. 2013, 34, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Sheng, P.P.; Etsell, T.H. Recovery of gold from computer circuit board scrap using aqua regia. Waste Manag. Res. 2007, 25, 380–383. [Google Scholar] [CrossRef]
- Hao, J.; Wang, X.; Wang, Y.; Guo, F.; Wu, Y. Study of gold leaching from pre-treated waste printed circuit boards by thiosulfate-cobalt-glycine system and separation by solvent extraction. Hydrometallurgy 2023, 221, 106141. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Challenges and opportunities in the recovery of gold from electronic waste. RSC Adv. 2020, 10, 4300–4309. [Google Scholar] [CrossRef]
- Merli, G.; Becci, A.; Amato, A.; Beolchini, F. Non-toxic, high selectivity process for the extraction of precious metals from waste printed circuit boards. Front. Environ. Sci. Eng. 2023, 17, 123. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, S.; Liu, J.; Wu, Y.; Yu, J. Research progress of hydrometallurgy technology for leaching precious metals in waste printed circuit board. Environ. Chem. 2021, 40, 886–895. [Google Scholar] [CrossRef]
- Ning, C.; Lin, C.S.K.; Hui, D.C.W.; McKay, G. Waste Printed Circuit Board (PCB) Recycling Techniques. Top. Curr. Chem. 2017, 375, 43. [Google Scholar] [CrossRef]
- Anwer, S.; Panghal, A.; Majid, I.; Mallick, S. Urban mining: Recovery of metals from printed circuit boards. Int. J. Environ. Sci. Technol. 2022, 19, 9731–9740. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, R.; Jang, S.; Wong, C.; Hong, J. “Organic Aqua Regia”—Powerful Liquids for Dissolving Noble Metals. Angew. Chem. Int. Ed. 2010, 49, 7929–7932. [Google Scholar] [CrossRef] [PubMed]
- Serpe, A.; Marchiò, L.; Artizzu, F.; Mercuri, M.L.; Deplano, P. Effective One-Step Gold Dissolution Using Environmentally Friendly Low-Cost Reagents. Chem. Eur. J. 2013, 19, 10111–10114. [Google Scholar] [CrossRef] [PubMed]
- Lin, W. Recovery of high-purity Pt from Pt-Au bimetallic nanoparticles using organic aqua regia. Rare Met. 2012, 31, 92–95. [Google Scholar] [CrossRef]
- Yue, C.; Sun, H.; Liu, W.; Guan, B.; Deng, X.; Zhang, X.; Yang, P. Environmentally Benign, Rapid, and Selective Extraction of Gold from Ores and Waste Electronic Materials. Angew. Chem. Int. Ed. 2017, 56, 9331–9335. [Google Scholar] [CrossRef]
- Xiong, Z.; Huang, Y.; Li, Y.; Sheng, C.; Sun, L.; Ma, J.; Chi, B.; Tan, J.; Tang, X.; Zha, R.; et al. Selective recovery of Au from waste printed circuit board by water-soluble organic leaching: The key role of in situ bromine mediation and identificationof pathway. J. Hazard. Mater. 2024, 480, 136255. [Google Scholar] [CrossRef]
- Kim, J.; Kim, R.; Han, K.N. Advances in Hydrometallurgical Gold Recovery through Cementation, Adsorption, Ion Exchange and Solvent Extraction. Minerals 2024, 14, 607. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, J.; Guo, R.; Liu, B.; Qu, R.; Huo, Z.; Zhu, F. The transformation and interaction of diallyl phthalate (DAP) in the three kinds of plastic under ultraviolet/sodium dichloroisocyanurate (UV/DCCNa) disinfection process. Chem. Eng. J. 2023, 467, 143401. [Google Scholar] [CrossRef]
- He, Y.; Xu, Z. Recycling gold and copper from waste printed circuit boards using chlorination process. RSC Adv. 2015, 5, 8957–8964. [Google Scholar] [CrossRef]
- Pak, K.-S.; Zhang, T.-A.; Kim, C.-S.; Kim, G.-H. Research on chlorination leaching of pressure-oxidized refractory gold concentrate. Hydrometallurgy 2020, 194, 105325. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, L.; Wang, S.; Cui, W.; Peng, J. Synergistic extraction of gold from the refractory gold ore via ultrasound and chlorination-oxidation. Ultrason. Sonochem. 2017, 37, 471–477. [Google Scholar] [CrossRef]
- Niu, H.; Yang, H.; Tong, L. Research on gold leaching of carbonaceous pressure-oxidized gold ore via a highly effective, green and low toxic agent trichloroisocyanuric acid. J. Clean. Prod. 2023, 419, 138062. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Cheng, G.; Xue, X.; Yang, H. Kinetics and mechanism of hydrochloric acid leaching of rare earths from Bayan Obo slag and recovery of rare earth oxalate and high purity oxides. Hydrometallurgy 2022, 208, 105782. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, C.; Liu, R.; Xie, Z.; Liu, Z.; Cen, S.; Tao, C.; Guo, S. Leaching kinetics of manganese from pyrolusite using pyrite as a reductant under microwave heating. Sep. Purif. Technol. 2021, 277, 119472. [Google Scholar] [CrossRef]
- Meng, Q.; Yan, X.; Li, G. Eco-friendly and reagent recyclable gold extraction by iodination leaching-electrodeposition recovery. J. Clean. Prod. 2021, 323, 129115. [Google Scholar] [CrossRef]
- Agarwal, S.; Reis, M.T.A.; Ismael, M.R.C.; Carvalho, J.M.R. Extraction of Cu(II) with Acorga M5640 using hollow fibre liquid membrane. Chem. Pap. 2015, 69, 679–689. [Google Scholar] [CrossRef]
- Nozari, I.; Azizi, A. An Investigation into the Extraction Behavior of Copper from Sulfate Leach Liquor Using Acorga M5640 Extractant: Mechanism, Equilibrium, and Thermodynamics. Min. Metall. Explor. 2020, 37, 1673–1680. [Google Scholar] [CrossRef]
- Oshima, T.; Koyama, T.; Otsuki, A.N. A Comparative Study on the Extraction of Au(III) Using Cyclopentyl Methyl Ether, Dibutyl Carbitol, and Methyl Isobutyl Ketone in Acidic Chloride Media. Solvent Extr. Ion Exch. 2021, 39, 477–490. [Google Scholar] [CrossRef]
- Oshima, T.; Matsuzaki, K.; Inada, A.; Ohe, K. Extraction of Au(III) using aromatic ethers via ion solvation from acidic chloride media: Structural factors that influence extraction. Sep. Purif. Technol. 2021, 258, 118008. [Google Scholar] [CrossRef]
- Alguacil, F.; Lopez, F.; Garcia-Diaz, I. Copper removal from acidic wastewaters using 2-hydroxy-5-nonylbenzaldehyde oxime as ionophore in pseudo-emulsion membrane with strip dispersion (PEMSD) technology. J. Ind. Eng. Chem. 2012, 18, 255–259. [Google Scholar] [CrossRef]
- Deep, A.; Kumar, P.; Carvalho, J.M. Recovery of copper from zinc leaching liquor using ACORGA M5640. Sep. Purif. Technol. 2010, 76, 21–25. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; An, W.; Bao, S. Effect of the Structure of Alkyl Salicylaldoxime on Extraction of Copper(II). Minerals 2017, 7, 61. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Sun, X.; Wang, L. Separation and recovery of copper from waste printed circuit boards leach solution using solvent extraction with Acorga M5640 as extractant. Sep. Sci. Technol. 2019, 54, 1302–1311. [Google Scholar] [CrossRef]
- Korolev, I.; Altınkaya, P.; Halli, P.; Hannula, P.-M.; Yliniemi, K.; Lundström, M. Electrochemical recovery of minor concentrations of gold from cyanide-free cupric chloride leaching solutions. J. Clean. Prod. 2018, 186, 840–850. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Recycling copper and gold from e-waste by a two-stage leaching and solvent extraction process. Sep. Purif. Technol. 2021, 263, 118400. [Google Scholar] [CrossRef]
- Oshima, T.; Miyake, K. Au(III) extraction using ketone compounds with physical properties superior to current commercial extractants. AIChE J. 2021, 67, e17214. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Chen, D. Study of Gold Leaching from PCB by Thiocyanate Process. Precious Metals 2008, 29, 11–14. [Google Scholar] [CrossRef]
- Ding, X.; Tang, H. Determination of Trichloroisocyanuric Acid by HPLC. Pest. Sci. Admin. 2003, 24, 7–9. [Google Scholar] [CrossRef]
- Rawashdeh-Omary, M.A.; Omary, M.A.; Patterson, H.H. Oligomerization of Au(CN)2− and Ag(CN)2− Ions in Solution via Ground-State Aurophilic and Argentophilic Bonding. J. Am. Chem. Soc. 2000, 122, 10371–10380. [Google Scholar] [CrossRef]
- White-Morris, R.L.; Stender, M.; Tinti, D.S.; Balch, A.L.; Rios, D.; Attar, S. New Structural Motifs in the Aggregation of Neutral Gold(I) Complexes: Structures and Luminescence from (Alkyl isocyanide)AuCN. Inorg. Chem. 2003, 42, 3237–3244. [Google Scholar] [CrossRef]
- Naumkin, A.V. NIST Standard Reference Database 20; National Institute of Standards and Technology, U.S. Department of Commerce: Gaithersburg, MD, USA, 2012; Version 4.1.
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of Xray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Vernon, C.; Fawell, P.; Klauber, C. XPS investigation of the states of adsorption of aurocyanide onto crosslinked polydiallylamine and commercial anionexchange resins. React. Polym. 1992, 18, 35–45. [Google Scholar] [CrossRef]
- Fan, R.; Xie, F.; Guan, X.; Zhang, Q.; Luo, Z. Selective adsorption and recovery of Au(III) from three kinds of acidic systems by persimmon residual based bio-sorbent: A method for gold recycling from e-wastes. Bioresour. Technol. 2014, 163, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Luo, H.; Wu, H.; Zhu, Z.; Huang, F.; Lin, X. Determination of Aquaculture Disinfectants Residues in Aquatic Products by Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry. J. Instrum. Anal. 2012, 31, 639–643. [Google Scholar]
- Gross, J.; Todd, P. Mass Spectrometry: A Textbook. Phys. Today 2005, 58, 59–60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).