Research on Soil Inorganic Nitrogen Detection Technology Based on Dielectric Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
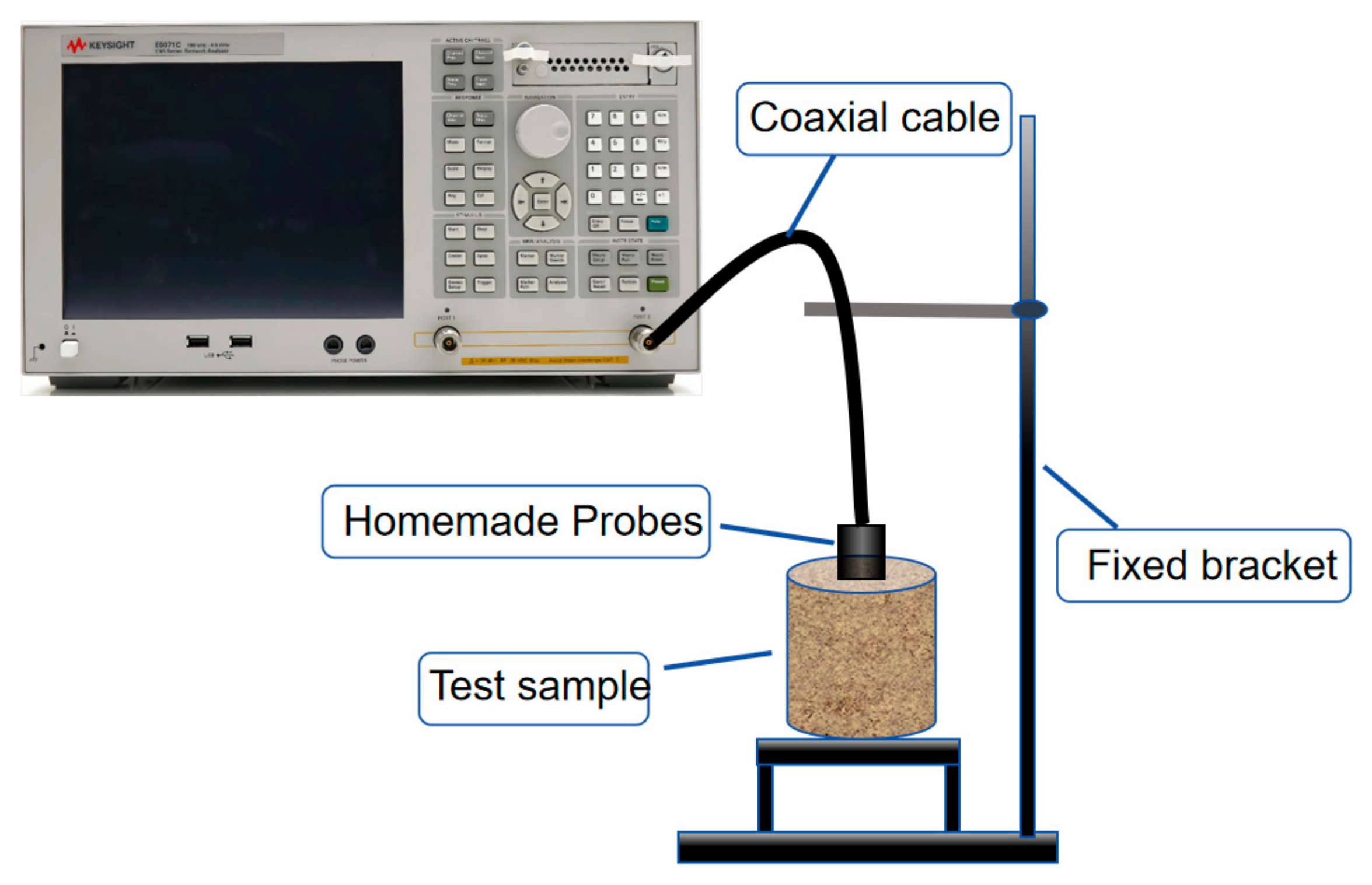
2.2. Experimental Design
2.3. Principle of Dielectric Measurement
3. Results
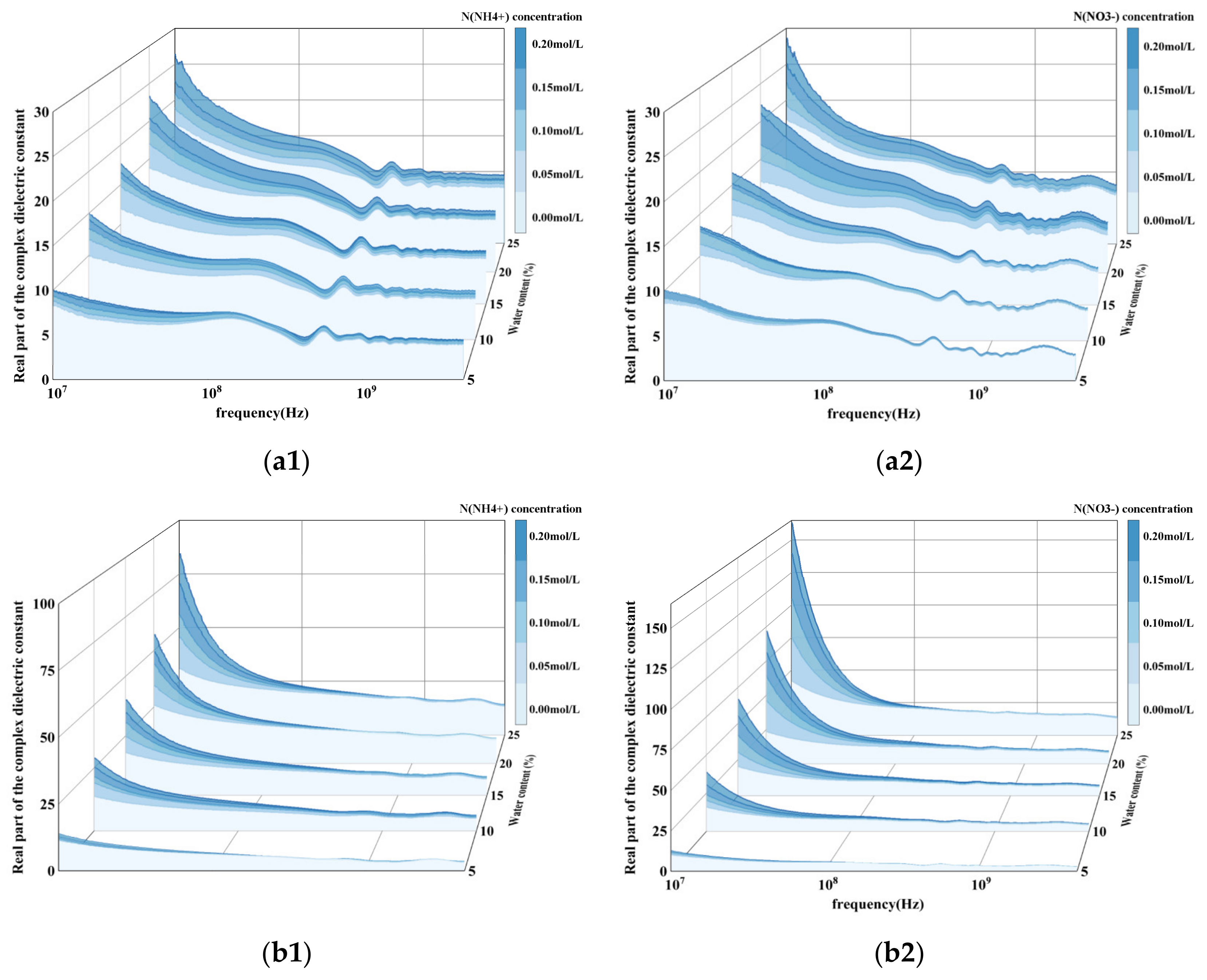
3.1. Performance of Soil Dielectric Properties Under Different Nitrogen Ion Concentrations
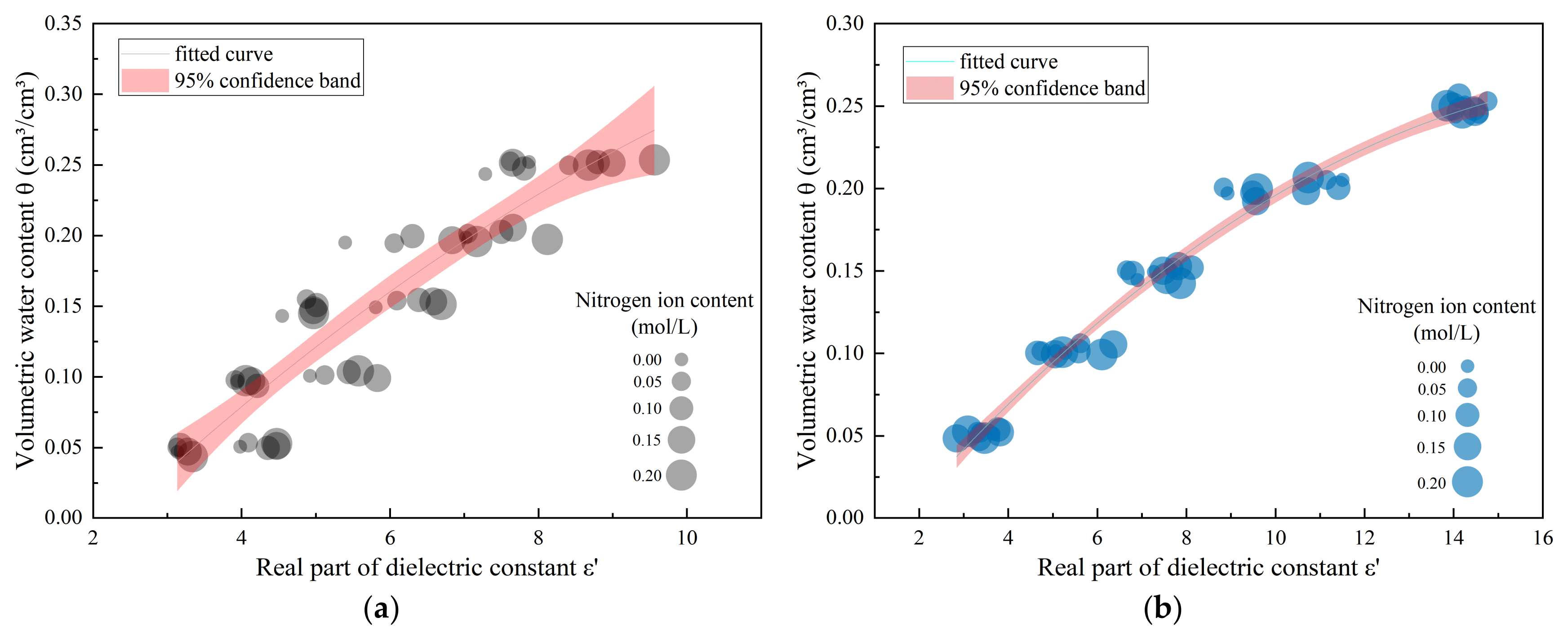
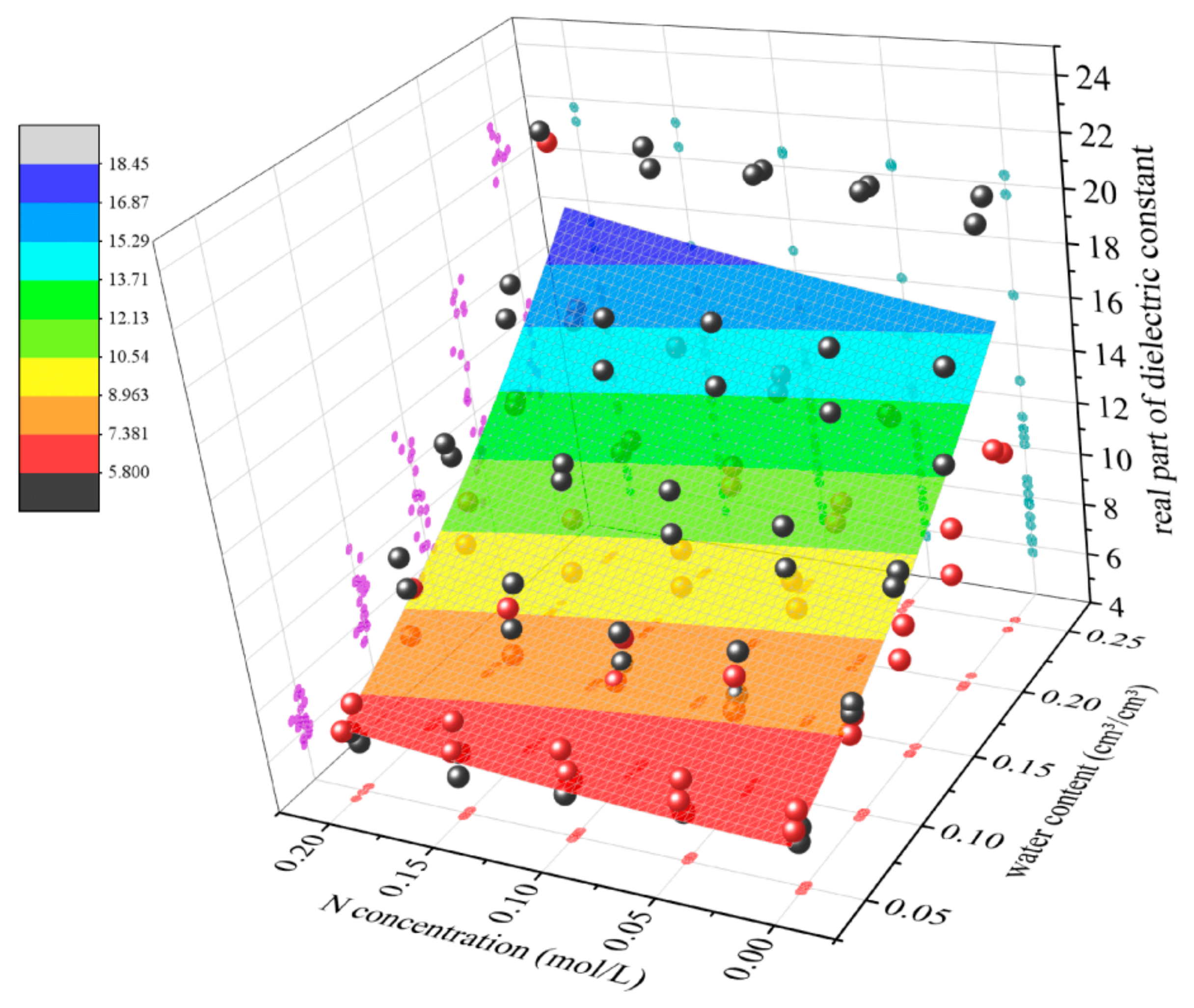
3.2. Empirical Modeling of Soil Dielectric Measurements Under Water–Nitrogen Coupling
4. Discussion
4.1. Overcoming Water Interference
4.2. Sensitivity to Nitrogen Ions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Zhao, L.; Yi, H.; Lan, S.; Chen, L.; Han, G. Nitrogen Addition Alters Plant Growth in China’s Yellow River Delta Coastal Wetland through Direct and Indirect Effects. Front. Plant Sci. 2022, 13, 1016949. [Google Scholar] [CrossRef]
- Mustafa, A.; Athar, F.; Khan, I.; Chattha, M.U.; Nawaz, M.; Shah, A.N.; Mahmood, A.; Batool, M.; Aslam, M.T.; Jaremko, M.; et al. Improving Crop Productivity and Nitrogen Use Efficiency Using Sulfur and Zinc-Coated Urea: A Review. Front. Plant Sci. 2022, 13, 942384. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Rajwar, D.; Yadav, R.K.; Chaudhari, S.K.; Sharma, D.K. Nitrogen Availability and Use Efficiency in Wheat Crop as Influenced by the Organic-Input Quality Under Major Integrated Nutrient Management Systems. Front. Plant Sci. 2021, 12, 634448. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, R.; Liu, H.; Wu, S.; Wu, H. Nitrogen Removal Responses to Biochar Addition in Intermittent-Aerated Subsurface Flow Constructed Wetland Microcosms: Enhancing Role and Mechanism. Ecol. Eng. 2019, 128, 57–65. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of Nitrogen in Soil by the Kjeldahl Method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Chang, L.; Li, H.; Liu, C.; Liang, X.; Xie, C.; Li, Z.; Li, Y.; Zhan, F.; He, Y. Effects of UV-B Radiation on the Chemical Composition of Azolla and Its Decomposition after Returning to the Field and Nitrogen Transformation in Soil. Agronomy 2023, 13, 1968. [Google Scholar] [CrossRef]
- Li, K.; Zhao, Y.; Yuan, X.; Zhao, H.; Wang, Z.; Li, S.; Malhi, S.S. Comparison of Factors Affecting Soil Nitrate Nitrogen and Ammonium Nitrogen Extraction. Commun. Soil Sci. Plant Anal. 2012, 43, 571–588. [Google Scholar] [CrossRef]
- McLeod, S. Determination of Total Soil and Plant Nitrogen Using a Micro-Distillation Unit in a Continuous Flow Analyzer. Anal. Chim. Acta 1992, 266, 113–117. [Google Scholar] [CrossRef]
- Li, L.; Ma, L.; Zhang, H.; Wu, J.; Naqvi, S.M.Z.A.; Li, Z.; Wei, W.; Awais, M.; Li, S.; Ewulo, B.S.; et al. Development of Improved Flow Analysis Prototype Method for Measuring and Understanding Agricultural Non-Point Source (NPS) Nitrogen (N) and Phosphorus (P) Pollution. Comput. Electron. Agric. 2023, 210, 107894. [Google Scholar] [CrossRef]
- Chen, H.; Feng, Q.; Jia, Z.; Song, Q. Improvement of Partial Least Squares Modelling for Determination of Soil Nitrogen by Fourier Transform Near-Infrared Spectrometry. Asian J. Chem. 2014, 26, 4839–4844. [Google Scholar] [CrossRef]
- Ruma, F.Y.; Munnaf, M.A.; De Neve, S.; Mouazen, A.M. Visible and near Infrared Spectroscopy for Predicting Soil Nitrogen Mineralization Rate: Effect of Incubation Period and Ancillary Soil Properties. CATENA 2024, 235, 107649. [Google Scholar] [CrossRef]
- Liu, J.; Cai, H.; Chen, S.; Pi, J.; Zhao, L. A Review on Soil Nitrogen Sensing Technologies: Challenges, Progress and Perspectives. Agriculture 2023, 13, 743. [Google Scholar] [CrossRef]
- Fan, S. Physical Properties and Basic Theory of Dielectric. IOP Conf. Ser. Earth Environ. Sci. 2021, 692, 022122. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Zheng, L.; Zhao, Y.; Pei, X. Soil Nitrogen Content Forecasting Based on Real-Time NIR Spectroscopy. Comput. Electron. Agric. 2016, 124, 29–36. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Chen, M.; Wang, M.; Zhang, M. An ISE-Based On-Site Soil Nitrate Nitrogen Detection System. Sensors 2019, 19, 4669. [Google Scholar] [CrossRef]
- Ren Haiyan, Z.M. Prediction of Soil Nitrate-Nitrogen Based on Sensor Fusion. Nongye Jixie XuebaoTransactions Chin. Soc. Agric. Mach. 2015, 46, 96–101. [Google Scholar] [CrossRef]
- Guerrero, A.; De Neve, S.; Mouazen, A.M. Current Sensor Technologies for in Situ and on-Line Measurement of Soil Nitrogen for Variable Rate Fertilization: A Review. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 168, pp. 1–38. [Google Scholar]
- Baumbauer, C.L.; Goodrich, P.J.; Payne, M.E.; Anthony, T.; Beckstoffer, C.; Toor, A.; Silver, W.; Arias, A.C. Printed Potentiometric Nitrate Sensors for Use in Soil. Sensors 2022, 22, 4095. [Google Scholar] [CrossRef]
- Palta, P.; Kumar, A. Soil Dielectric Response to Chemical Fertilizers in Northern India’s Key Agricultural Areas. Phys. Chem. Earth Parts ABC 2025, 137, 103806. [Google Scholar] [CrossRef]
- De Neve, S.; Hartmann, R.; Hofman, G. Temperature Effects on N Mineralization: Changes in Soil Solution Composition and Determination of Temperature Coefficients by TDR. Eur. J. SOIL Sci. 2003, 54, 49–61. [Google Scholar] [CrossRef]
- Tsegaye, T.D.; Ranatunga, T.D.; Reddy, K.C. Variation of Soil Dielectric Constant, Moisture Holding Capacity, and Nitrate-Nitrogen as Influenced by Application of Fresh and compostpPoultry Litter on a Decatur Silt Loam Soi. J. Sustain. Agric. 2003, 22, 55–73. [Google Scholar] [CrossRef]
- Payero, J.O.; Tarkalson, D.D.; Irmak, S. Use of Time Domain Reflectometry for Continuous Monitoring of Nitrate-Nitrogen in Soil and Water. Appl. Eng. Agric. 2006, 22, 689–700. [Google Scholar] [CrossRef]
- Gao, B.; Walhof, A.C.; Laird, D.A.; Toor, F.; Prineas, J.P. Analytical Evaluation of Mobile In Situ Soil Nitrate Infrared Sensor Designs for Precision Agriculture. IEEE Sens. J. 2021, 21, 20200–20209. [Google Scholar] [CrossRef]
- Maru, A.; Ahmed, O.H.; Primus, W.C.; Jeffary, A.V. Dielectric Response of Nitrogen in Soil Amended with Chicken Litter Biochar and Urea under Oryza Sativa L. Cultivation. Sci. Rep. 2021, 11, 12545. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, P.R. Variation of Dielectric Constant of Indian Soils with Their Physical Parameters at C-Band Microwave Frequency. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 15396–15400. [Google Scholar] [CrossRef]
- Bonachela, S.; Fernández, M.D.; Cabrera, F.J.; Granados, M.R. Soil Spatio-Temporal Distribution of Water, Salts and Nutrients in Greenhouse, Drip-Irrigated Tomato Crops Using Lysimetry and Dielectric Methods. Agric. Water Manag. 2018, 203, 151–161. [Google Scholar] [CrossRef]
- Pandey, G.; Weber, R.; Kumar, R. Agricultural Cyber-Physical System: In-Situ Soil Moisture and Salinity Estimation by Dielectric Mixing. IEEE Access 2018, 6, 43179–43191. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, L.; Wang, R.; Su, J.; Li, H.; Zhang, J.; Guo, H.; Chen, X. Contactless Conductivity Microfluidic Chip for Rapid Determination of Soil Nitrogen and Potassium Content. Smart Agric. 2024, 6, 18. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Hu, F.N.; Huang, X.R.; Xie, D.T.; Ni, J.P. Effects of Strong Ionic Polarization in the Soil Electric Field on Soil Particle Transport during Rainfall. Eur. J. Soil Sci. 2015, 66, 921–929. [Google Scholar] [CrossRef]
- Li, Q.; Shi, W.; Yang, Q. Polarization Induced Covalent Bonding: A New Force of Heavy Metal Adsorption on Charged Particle Surface. J. Hazard. Mater. 2021, 412, 125168. [Google Scholar] [CrossRef]
- Acar, Y.B.; Gale, R.J.; Alshawabkeh, A.N.; Marks, R.E.; Puppala, S.; Bricka, M.; Parker, R. Electrokinetic Remediation: Basics and Technology Status. J. Hazard. Mater. 1995, 40, 117–137. [Google Scholar] [CrossRef]
- Chen, Y.; Or, D. Geometrical Factors and Interfacial Processes Affecting Complex Dielectric Permittivity of Partially Saturated Porous Media. WATER Resour. Res. 2006, 42, W06423. [Google Scholar] [CrossRef]
- Datsios, Z.G.; Mikropoulos, P.N.; Karakousis, I. Laboratory Characterization and Modeling of DC Electrical Resistivity of Sandy Soil with Variable Water Resistivity and Content. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3063–3072. [Google Scholar] [CrossRef]




| Soil Type | Clay Grains (%) | Powder Grains (%) | Sand Grains (%) | Ammonium Nitrogen (mg/kg) | Nitrate Nitrogen (mg/kg) | Soil PH |
|---|---|---|---|---|---|---|
| laterite | 69 | 16 | 15 | 1.288 | 0.391 | 7.2 |
| loess | 9 | 54 | 36 | 0.487 | 0.096 | 8.2 |
| Soil Type | Fitting the Relational Equation | R2 | RMSE |
|---|---|---|---|
| laterite | 0.8535 | 0.0280 | |
| loess | 0.9804 | 0.0101 |
| Frequency (MHz) | Fitting Formula | R2 |
|---|---|---|
| 76 | 0.70112 | |
| 97 | 0.71483 | |
| 108 | 0.71883 | |
| 125 | 0.71991 | |
| 127 | 0.72055 | |
| 129 | 0.72014 | |
| 136 | 0.71927 | |
| 152 | 0.71587 | |
| 201 | 0.69582 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Z.; Han, X.; Hu, R.; Yu, J.; Yan, X.; Xu, J. Research on Soil Inorganic Nitrogen Detection Technology Based on Dielectric Response. Sustainability 2025, 17, 2491. https://doi.org/10.3390/su17062491
Jia Z, Han X, Hu R, Yu J, Yan X, Xu J. Research on Soil Inorganic Nitrogen Detection Technology Based on Dielectric Response. Sustainability. 2025; 17(6):2491. https://doi.org/10.3390/su17062491
Chicago/Turabian StyleJia, Zhenyu, Xuan Han, Ri Hu, Jiangyang Yu, Xiaoqing Yan, and Jinghui Xu. 2025. "Research on Soil Inorganic Nitrogen Detection Technology Based on Dielectric Response" Sustainability 17, no. 6: 2491. https://doi.org/10.3390/su17062491
APA StyleJia, Z., Han, X., Hu, R., Yu, J., Yan, X., & Xu, J. (2025). Research on Soil Inorganic Nitrogen Detection Technology Based on Dielectric Response. Sustainability, 17(6), 2491. https://doi.org/10.3390/su17062491





