Utilization of Anthropogenic and Natural Waste to Produce Construction Raw Materials
Abstract
1. Introduction
- Dispersion of Si–O–Si and Al–O–Si groups in a highly concentrated alkaline solution and colloidal dispersed system formation.
- Increase in the concentration of the dispersed colloidal system.
- Compaction of the structure in the existing volume: due to autogenous shrinkage, the rings and chains of the tetrahedrons [SiO4]4− and [AlO4]5− close with the formation of three-dimensional aluminosilicate structures M·[–(Si–O)z–Al–O–]n·wH2O.
2. Materials and Methods
Materials
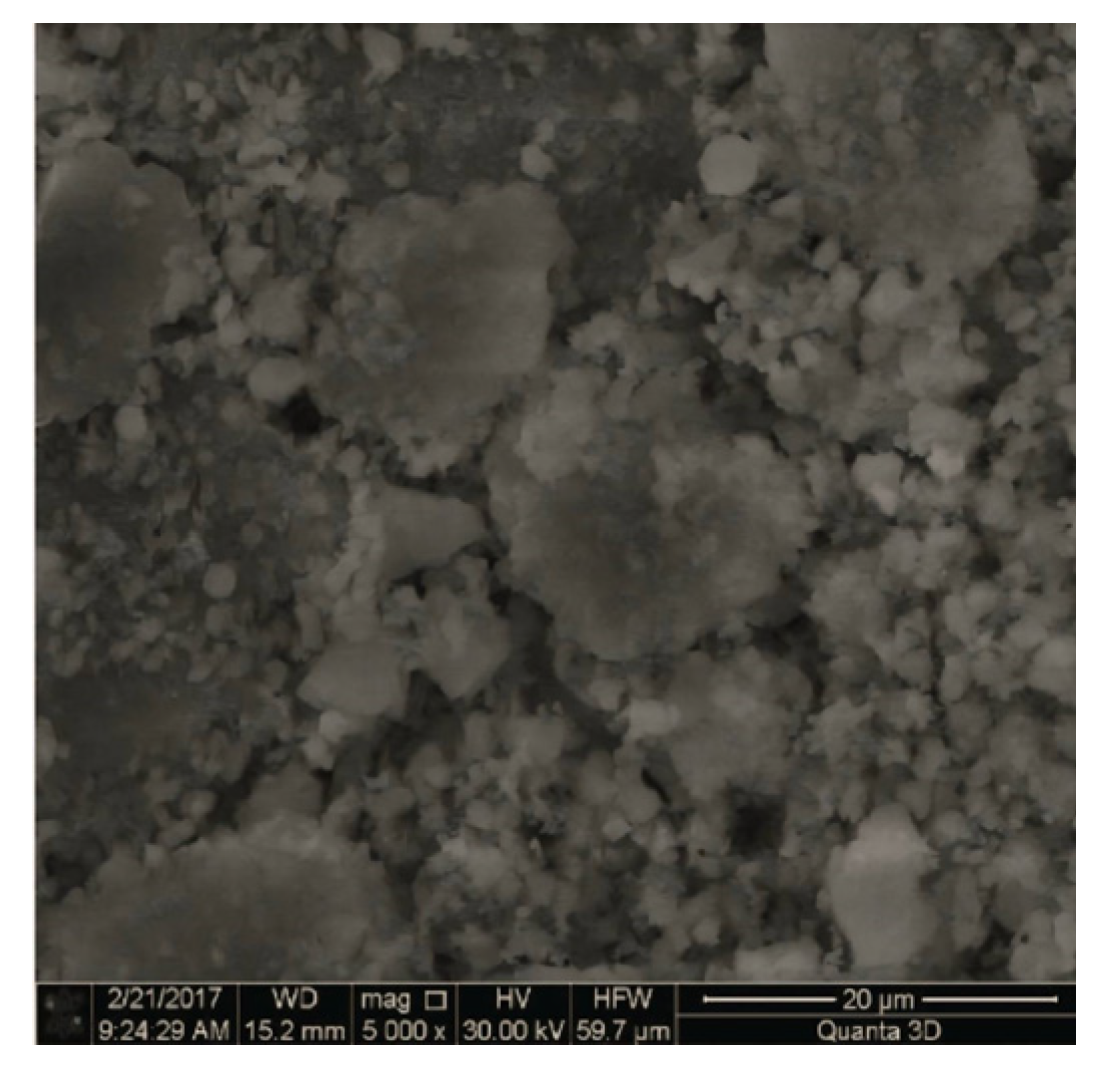
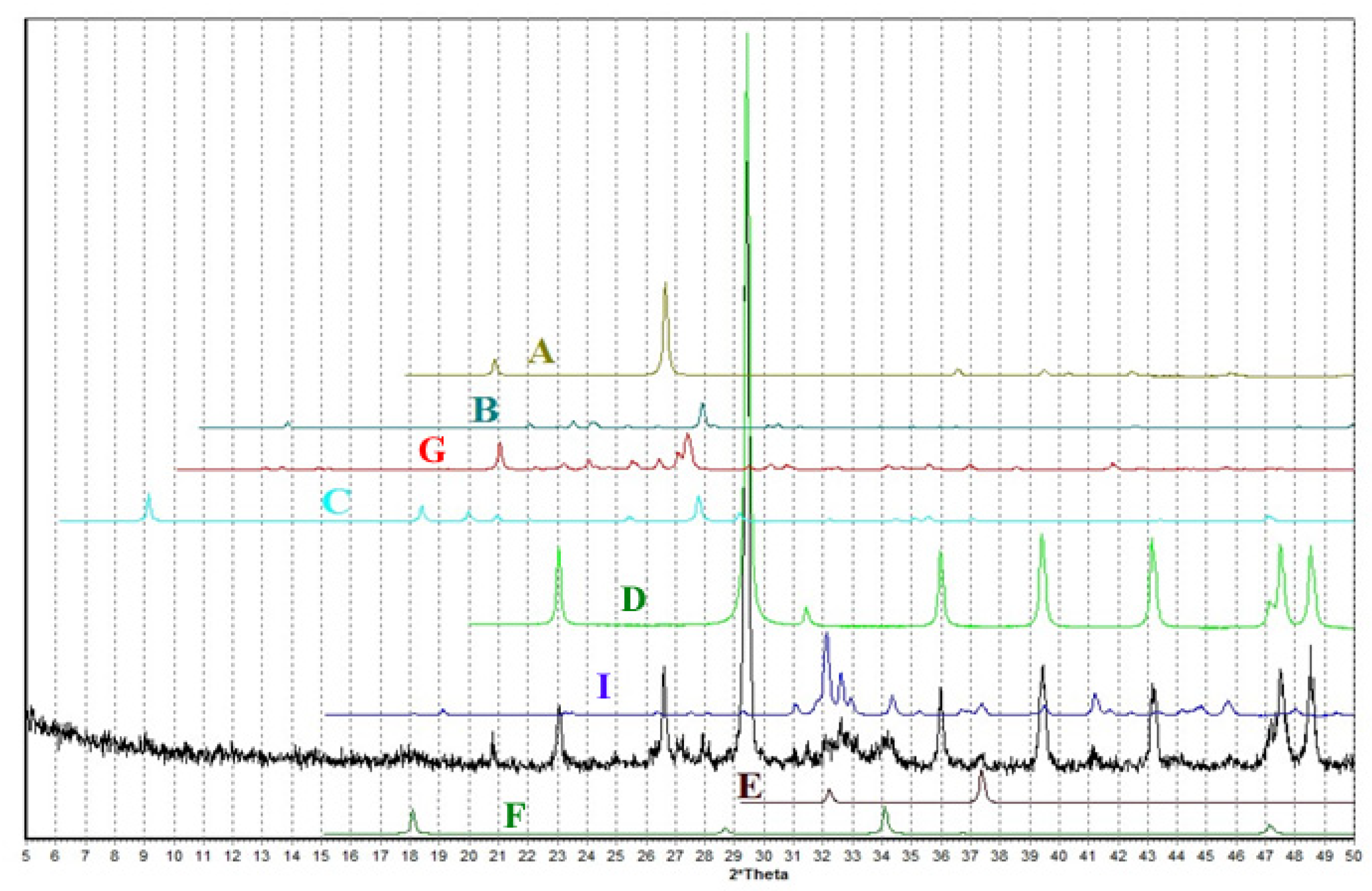
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afiq, M.; Abdullah, H.; Saifulnaz, R.; Rashid, M.; Amran, M.; Hejazii, F.; Azreen, N.; Masenwat, B.; Fediuk, R.; Voo, Y.; et al. Recent Trends in Advanced Radiation Shielding Concrete for Construction of Facilities: Materials and Properties. Polymers 2022, 14, 2830. [Google Scholar] [CrossRef] [PubMed]
- Huntingford, C.; Williamson, M.S.; Nijsse, M.M. CMIP6 climate models imply high committed warming. Clim. Change 2020, 162, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Fediuk, R.; Yushin, A. Composite binders for concrete with reduced permeability. IOP Conf. Ser. Mater. Sci. Eng. 2016, 116, 012021. [Google Scholar]
- Petrov, A.M.; Magomedov, R.M.; Savina, S.V. Ecological Safety of Construction in the Concept of Sustainable Development. Constr. Mater. Prod. 2023, 6, 5–17. [Google Scholar] [CrossRef]
- Fediuk, R.; Timokhin, R.; Mochalov, A.; Otsokov, K.; Lashina, I. Performance properties of high-density impermeable cementitious paste. J. Mater. Civ. Eng. 2019, 31, 04019013. [Google Scholar] [CrossRef]
- Rubenstein, M. Emissions from the Cement Industry. State of the Planet. 2012. Available online: https://news.climate.columbia.edu/2012/05/09/emissions-from-the-cement-industry/ (accessed on 9 May 2012).
- Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- López, F.J.; Sugita, S.; Tagaya, M.; Kobayashi, T. Metakaolin-Based Geopolymers for Targeted Adsorbents to Heavy Metal Ion Separation. J. Mater. Sci. Chem. Eng. 2014, 2, 16–27. [Google Scholar] [CrossRef]
- Semenov, P.; Uzunian, A.; Davidenko, A.; Al, E. First study of radiation hardness of lead tungstate crystals at low temperatures. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 582, 575–580. [Google Scholar] [CrossRef]
- Shang, X.; Fang, Z.; Huang, W.; Chen, Y.; Qu, N.; Zhong, R. Lightweight concrete with low-carbon artificial aggregates recycled from biomass ash and slurry waste. Constr. Build. Mater. 2024, 429, 136368. [Google Scholar] [CrossRef]
- Klyuev, A.V.; Kashapov, N.F.; Klyuev, S.V.; Lesovik, R.V.; Ageeva, M.S.; Fomina, E.V. Development of alkali-activated binders based on technogenic fibrous materials. Constr. Mater. Prod. 2023, 6, 60–73. [Google Scholar] [CrossRef]
- Salamanova, M.; Murtazaev, S.A.; Alashanov, A.; Ismailova, Z. Features of Production of Fine Concretes Based on Clinkerless Binders of Alkaline Mixing; Springer: Berlin/Heidelberg, Germany, 2019; pp. 385–388. [Google Scholar]
- Yue, Z.; Dhandapani, Y.; Adu-Amankwah, S.; Bernal, S.A. Phase evolution and performance of sodium sulfate-activated slag cement pastes. Cement 2024, 18, 100117. [Google Scholar] [CrossRef]
- You, N.; Chen, Z.; Gao, Z.; Song, X. The effect of copper slag as a precursor on the mechanical properties, shrinkage and pore structure of alkali-activated slag-copper slag mortar. J. Build. Eng. 2024, 98, 111151. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Zou, J.; Reid, A.; Wang, H. Toward an indexing approach to evaluate fly ashes for geopolymer manufacture. Cem. Concr. Res 2016, 85, 163–173. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications; Geopolymer Institute: Paris, France, 2015. [Google Scholar]
- Hardjito, D.; Wallah, S.; Sumajouw, D.; Rangan, B. On the Development of Fly Ash-Based Geopolymer Concrete. ACI Mater. J. 2004, 101, 467–472. [Google Scholar] [CrossRef]
- Salamanova, M.S. Production of alkali binder from silica additives by simplified technology. Bull. Mosc. State Univ. Constr. 2022, 17, 341–352. [Google Scholar] [CrossRef]
- Nuruddin, M.F.; Demie, S.; Shafiq, N. Effect of mix composition on workability and compressive strength of self-compacting geopolymer concrete. Can. J. Civ. Eng. 2011, 38, 1196–1203. [Google Scholar] [CrossRef]
- Villa, C.; Pecina, E.T.; Torres, R.; Gómez, L. Geopolymer synthesis using alkaline activation of natural zeolite. Constr. Build. Mater. 2010, 24, 2084–2090. [Google Scholar] [CrossRef]
- Alex, T.C.; Kalinkin, A.M.; Nath, S.K.; Gurevich, B.I.; Kalinkina, E.V.; Tyukavkina, V.V.; Kumar, S. Utilization of zinc slag through geopolymerization: Influence of milling atmosphere. Int. J. Miner. Process. 2013, 123, 102–107. [Google Scholar] [CrossRef]
- Bataev, D.; Salamanova, M.S.; Murtazaev, S.-A.; Viskhanov, S.S.; Murtazaev, S.-A.Y. Utilization of Cement Kiln Dust in Production of Alkali-Activated Clinker-Free Binders. In International Symposium “Engineering and Earth Sciences: Applied and Fundamental Research” Dedicated to the 85th Anniversary of HI Ibragimov (ISEES 2019); Atlantis Press: Paris, France, 2019. [Google Scholar]
- Salamanova, M.S.; Mintsaev, M.S.; Murtazaev, S.-A.Y.; Bisultanov, R.G.; Salamanova, M.S. Fine-Grained Concretes with Clinker-Free Binders on an Alkali Gauging. In International Symposium “Engineering and Earth Sciences: Applied and Fundamental Research" Dedicated to the 85th Anniversary of HI Ibragimov (ISEES 2019); Atlantis Press: Paris, France, 2019. [Google Scholar]
- Murtazaev, S.-A.Y.; Salamanova, M.S.; Saidumov, M.S. Development of geopolymer binders. Constr. Mater. Prod. 2024, 7, 4. [Google Scholar] [CrossRef]
- Khan, M.Z.N.; Shaikh, F.U.A.; Hao, Y.; Hao, H. Effects of curing conditions and sand-to-binder ratios on compressive strength development of fly ash geopolymer. J. Mater. Civ. Eng. 2018, 30, 04017267. [Google Scholar] [CrossRef]
- GOST 30744-2001; Cements. Methods of Testing with Using Polyfraction Standard Sand. Standartinform Publich: Moscow, Russia, 2001; Volume 20. (In Russian)
- GOST 310.4-81; Cements. Methods for Determining Bending and Compressive Strength. Standartinform Publich: Moscow, Russia, 2003; Volume 22. (In Russian)
- GOST 310.3-76; Cements. Methods for Determination of Standard Consistency, Times of Setting and Soundness. Standartinform Publich: Moscow, Russia, 2003; Volume 11. (In Russian)






| Alkaline Metal Compounds | Activity, MPa |
|---|---|
| H2O | 7.5 |
| NaOH | 80 |
| Na2CO3 | 80 |
| Na2NO2 | 55 |
| NaF | 85 |
| Na2S | 60 |
| Na2SiO3 | 130 |
| Na2O·2SiO2 | 160 |
| 4Na2O·SiO2 | 23 |
| Na2O·Al2O3·2SiO2 | 10 |
| Na2O·Al2O3·8SiO2 | 6.5 |
| Na2O·Al2O3 | 67 |
| № | Quality Indicators | Heat-Activated Porcupine Merge 700 °C | Clinker Dust | Aspiration Dust | |||
|---|---|---|---|---|---|---|---|
| Type of Grouting Fluid | |||||||
| Na2SiO3+ Na2SiF6 | H2O | Na2SiO3+ Na2SiF6 | H2O | Na2SiO3+ Na2SiF6 | H2O | ||
| 1 | Normal density of alkaline cement paste (NGCT), % | 56.5 | 40.0 | 50.0 | 30.0 | 70.0 | 42.0 |
| 2 | Setting time, start/end, hours–min. | 00–26 00–32 | 01–37 06–29 | 00–40 01–20 | 00–54 01–56 | 00–24 00–36 | 06–08 07–16 |
| 3 | Activity, 28 days, MPa | 32.1 | 9.2 | 24.0 | 6.3 | 32.6 | 5.3 |
| Mix ID | Mineral Powder | Sspecific, m2/kg | Normal Density of alkaline Cement Paste (NGCT), % | Setting Time, Hours–Minutes | Compressive Strength, MPa | |||
|---|---|---|---|---|---|---|---|---|
| 7 Days | 28 Days | 60 Days | Heat Generating Waste +27 Days | |||||
| aspiration (85%) + clinker dust (5%) + min. powder + Na2SiO3 | ||||||||
| 1 | Velvat sand | 80 | 61.2 | 00–45 01–35 | 9.8 | 8.4 | 8.9 | 1.5 |
| 2 | Velvet tuff | 76 | 62.0 | 00–32 01–21 | 2.8 | 2.6 | 7.8 | 9.3 |
| opoka 700 °C (85%) + clinker dust (5%) + min. powder (10%) + Na2SiO3 | ||||||||
| 3 | Velvat sand | 80 | 50.1 | 00–56 01–50 | 8.6 | 8.0 | 0.5 | 2.1 |
| 4 | Velvet tuff | 76 | 50.4 | 00–33 01–35 | 2.4 | 2.1 | 6.2 | 0.0 |
| Compositions | Mineral Powder | Na2B4O7·10N2O, % | C6H7NO3S, % | Destiny, kg/m3 | Water Absorption, % | Compressive Streght, MPa | ||
|---|---|---|---|---|---|---|---|---|
| 7 Days | 28 Days | 60 Days | ||||||
| aspiration dust (85%) + clinker dust (5%) + min. powder 10% + Na2SiO3 + additive | ||||||||
| 1 | Volcanic tuff Sspecific = 476 m2/kg | 0.45 | - | 2162 | 4.1 | 27.3 | 38.9 | 45 |
| 2 | 0.35 | - | 2151 | 4.3 | 25.4 | 36.5 | 9.343.8 | |
| 3 | - | 0.8 | 2130 | 4.9 | 15.8 | 20.7 | 21.4 | |
| 4 | - | 1.0 | 2119 | 4.8 | 14.6 | 18.9 | 20.1 | |
| 700 °C opoka (85%) + clinker dust (5%) + min. powder 10% + Na2SiO3 + additive | ||||||||
| 5 | Volcanic tuff Sspecific = 476 m2/kg | 0.45 | - | 2135 | 4.4 | 24.9 | 36.1 | 41.1 |
| 6 | 0.35 | - | 2140 | 4.6 | 22.7 | 33.5 | 41.9 | |
| 7 | - | 0.8 | 2120 | 5.1 | 11.6 | 17.0 | 18.3 | |
| 8 | - | 1.0 | 2114 | 5.0 | 10.8 | 15.3 | 16.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarsenbayev, B.; Murtazaev, S.-A.; Salamanova, M.; Kuldeyev, E.; Saidumov, M.; Sarsenbayev, N.; Auyesbek, S.; Sauganova, G.; Abduova, A. Utilization of Anthropogenic and Natural Waste to Produce Construction Raw Materials. Sustainability 2025, 17, 2791. https://doi.org/10.3390/su17072791
Sarsenbayev B, Murtazaev S-A, Salamanova M, Kuldeyev E, Saidumov M, Sarsenbayev N, Auyesbek S, Sauganova G, Abduova A. Utilization of Anthropogenic and Natural Waste to Produce Construction Raw Materials. Sustainability. 2025; 17(7):2791. https://doi.org/10.3390/su17072791
Chicago/Turabian StyleSarsenbayev, Bakhytzhan, Said-Alvi Murtazaev, Madina Salamanova, Erzhan Kuldeyev, Magomed Saidumov, Nuraly Sarsenbayev, Sultan Auyesbek, Gaukhar Sauganova, and Aisulu Abduova. 2025. "Utilization of Anthropogenic and Natural Waste to Produce Construction Raw Materials" Sustainability 17, no. 7: 2791. https://doi.org/10.3390/su17072791
APA StyleSarsenbayev, B., Murtazaev, S.-A., Salamanova, M., Kuldeyev, E., Saidumov, M., Sarsenbayev, N., Auyesbek, S., Sauganova, G., & Abduova, A. (2025). Utilization of Anthropogenic and Natural Waste to Produce Construction Raw Materials. Sustainability, 17(7), 2791. https://doi.org/10.3390/su17072791






