Abstract
Retention forestry is the dominant practice in Northern Europe, with large-scale clear-cuts following natural disturbances becoming more frequent as the climate changes. Despite its widespread use, clear-cutting is criticized for its potential adverse effects on species diversity and ecosystem recovery, particularly in understory vegetation. This study examines early vegetation changes after large-scale clear-cutting in Latvia’s hemiboreal forests. The sampling was conducted in 2017 and 2020, three and six years post-harvest, using 210 systematically placed plots (1 × 1 m) to assess species abundance and vegetation cover across moss/lichen, herbaceous, and shrub/tree layers. The findings indicate that species diversity was initially higher following clear-cutting but declined after six years, with the herbaceous layer most affected. While clear-cutting temporarily increases species diversity, negative effects become evident over time. Recovery is prolonged, with succession progressing faster in wet areas. To fully understand the long-term impacts of clear-cutting, continued monitoring is necessary.
1. Introduction
Forests cover over half of Latvia’s territory (~53%), making them a significant part of the country’s landscape. Latvia is situated within the hemiboreal vegetation zone, which is characterized by a transitional forest composition comprising both pure and mixed stands of deciduous and coniferous tree species [1]. The predominant tree species include Scots pine (Pinus sylvestris L.), birch (Betula spp.), and Norway spruce (Picea abies L.) [2]. Among the harvesting methods, regeneration cutting—including clear-cutting—is the most prevalent, accounting for approximately 40% of all cuttings [2]. Clear-cutting, in particular, is one of the most widely applied forestry practices in boreal forests worldwide [3]. However, it has been criticized for its direct effect on vegetation species diversity at the ecosystem level [4] as it involves the complete removal of overstory trees and the partial removal of understory vegetation [5].
Large clear-cut effects are sometimes compared to those of windstorms, as following significant wind disturbances clear-cutting is often carried out as a sanitary measure [6]. Tree removal significantly influences the diversity and composition of understory vegetation, including the shrub, herb, and moss layers, which are vital components of forest ecosystems regarding biodiversity and nutrient cycling [7]. Changes resulting from natural disturbances, such as windstorms [8], bear similarities to those caused by clear-cutting. Windstorms also provide opportunities for shade-intolerant and early-successional species to thrive in the canopy openings created by fallen trees [9]. However, clear-cuts and windstorms differ significantly in several ecological aspects [10], such as the extent of ecosystem removal and the more uniform nature of this process in clear-cutting [11]. Windstorms contribute to the diversification of forest stands by creating variations in age, height, and structure [12], which is the opposite of clear-cuts.
Each silvicultural treatment acts as a sudden disturbance to the natural successional dynamics of forest vegetation, resulting in changes to the canopy closure and affecting the environmental conditions for understory plant communities [13]. These alterations lead to several consequences, such as accelerated litter decomposition, increased nutrient availability, and higher soil moisture content [14]. Following clear-cutting, the soil is typically subjected to mechanical preparation to support either natural regeneration or artificial regeneration through seeding or planting [13]. The increased light availability, nutrient levels from logging residues, and changes in soil conditions, such as surface drying and a rise in the groundwater table [15,16], create favorable conditions for plant colonization through germination from the soil seed bank, seed dispersal, or vegetative reproduction [17,18]. However, such significant changes in habitat conditions often create unfavorable environments for shade-tolerant forest bryophytes while simultaneously promoting the growth of early-successional species [14]. This shift favors colonizing species that take advantage of the open undergrowth for establishment [19]. Intense soil disturbance can be damaging to sensitive species of the forest [20], and the recovery [21] of late-successional species is primarily facilitated through local survival [22].
As a result of clear-cutting, vegetation biodiversity often increases in the first years after clear-cutting, with species abundance peaking at the beginning of succession and declining in later stages [21,23]. Previous studies have reported greater species richness in clear-cut areas compared to mature forests, with species numbers potentially doubling within 1–2 years following clear-cutting [24]. Although successional patterns are well studied in temperate forests, limited research exists on this process in hemiboreal forests, particularly following extensive clear-cutting. Gaining insight into vegetation dynamics after clear-cutting is essential for sustainable forest management and predicting ecosystem recovery trajectories. Forest ground cover vegetation is regarded as one of the most reliable and biodiversity-rich indicators of specific stages in successional forest development [21]. Therefore, this study aims to examine changes in vegetation composition and structure at early succession following large-scale clear-cutting in Latvia’s hemiboreal region.
2. Materials and Methods
2.1. Study Area
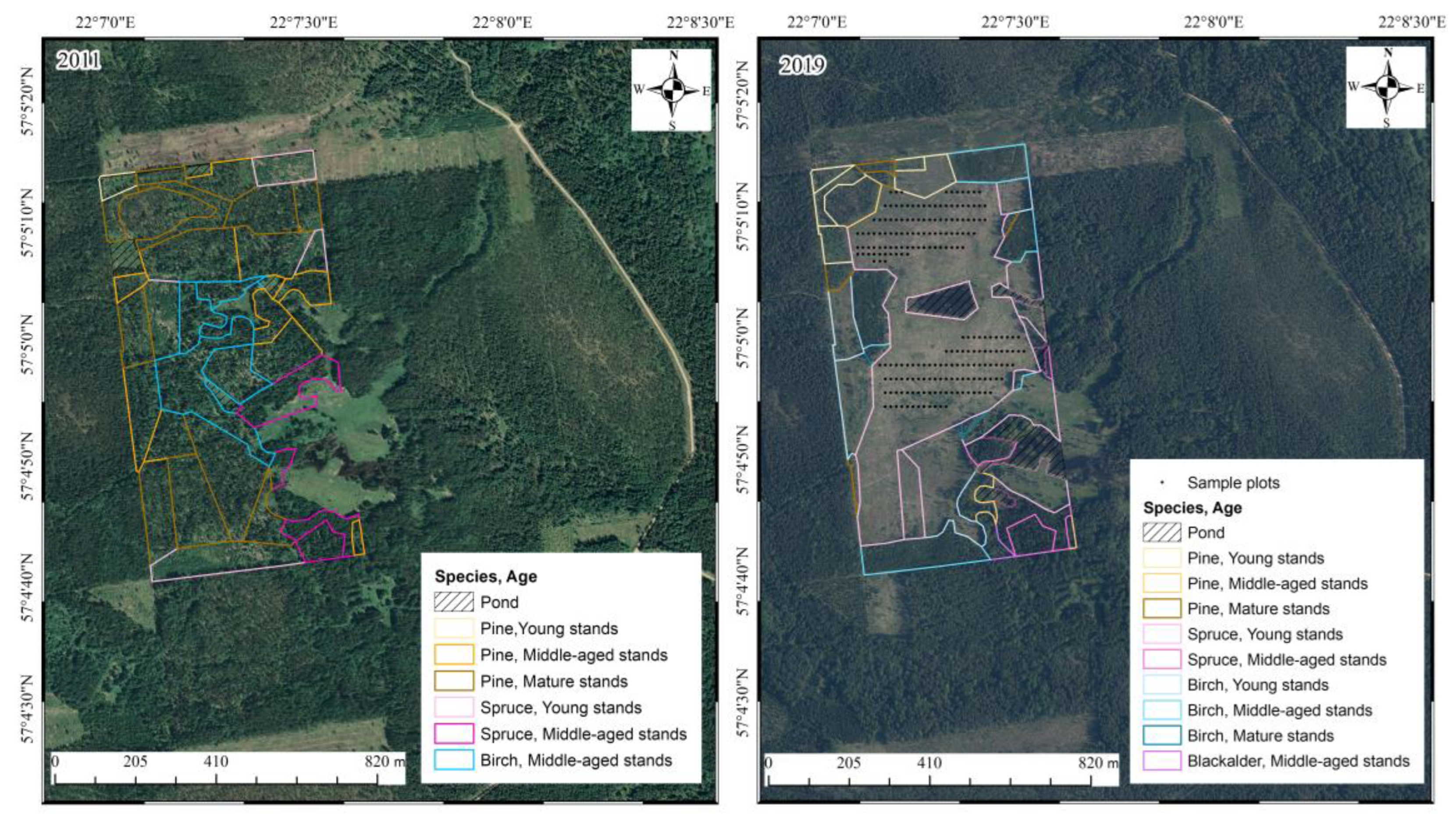
The research site was in the Kuldīga district of Latvia (57°08′ N, 22°12′ E). Before the clear-cut, the area was predominantly comprised of middle-aged birch and pine forest stands (Figure 1, first panel) (forest taxation data), which corresponded to Myrtillosa and Hylocomiosa forest types [25]. Most of the previous forest area belonged to the Hylocomiosa forest type, which supports approximately 120 ground cover vegetation species. Common herbaceous and shrub species include Oxalis acetosella L., Fragaria vesca L., Convallaria majalis L., and Anemone spp., along with species also found in Myrtillosa forests, such as Vaccinium myrtillus L., Orthilia secunda L., Pteridium aquilinum L., Maianthemum bifolium L., and Luzula pilosa L. The moss and lichen layer consists of Rhytidiadelphus squarrosus Hedw., Juniperus spp., and Plagiomnium spp., and shared species like Hylocomium splendens Hedw. and Pleurozium schreberi Brid. A distinct spring aspect can also be observed in this forest type. The understory in Hylocomiosa forests is denser than in Myrtillosa stands, commonly including Corylus avellana L., Sorbus aucuparia L., and Juniperus communis L. In the Myrtillosa forest type, approximately 100 ground cover vegetation species can be found. Unique to this forest type are Vaccinium vitis-idaea L. and Solidago virgaurea L., while it also shares several species with Hylocomiosa, including Vaccinium myrtillus, Maianthemum bifolium, Luzula pilosa, Pteridium aquilinum, and Orthilia secunda. Among mosses, Myrtillosa forests contain species such as Dicranum scoparium Hedw., Dicranum polysetum Sw., and Ptilium crista-castrensis Hedw., alongside those also present in Hylocomiosa forests. The understory is similar to Hylocomiosa but generally less dense, with Sorbus aucuparia L. and Corylus avellana L. being the most common species [25].
Figure 1.
Orthophoto imagery from 2011 (before the clear-cutting) and 2019 (post-clear-cutting), with sample plot locations marked by dots. The districts are color-coded to indicate dominant species and age classes. Note: The years of the orthophotos (2011, 2019) do not align with the study years (2014, 2017, 2020) because orthophotos are not captured annually.
In 2014, the area underwent clear-cutting over 31 hectares, an unusually large scale for Latvia. The region experiences a temperate, moist continental climate [26]. Between 1992 and 2022, the mean annual temperature was +7.7 ± 0.7 °C. February was the coldest month, with an average temperature of −1.3 ± 2.9 °C, while July was the warmest, averaging +17.8 ± 1.6 °C. The site received a mean annual precipitation of 700.5 ± 86.0 mm with an average of 13.7 ± 1.2 precipitation days per month, including 12.2 ± 2.0 days during summer [27].
2.2. Sampling and Measurements
The study area was divided into two distinct sections (Figure 1). The northern part of the clear-cut featured a mix of mineral and peat soils, referred to as the “wet type”, while the southern section consisted of mineral soil with generally drier conditions, referred to as the “dry type”. Despite these differences, the same set of sampling plots was evaluated in both assessments. As a result, sampling plots from both clear-cut sections were analyzed together. Initially, a grid was applied to the clear-cut area (excluding sections at the central location where a pond had formed) to cover most of the site. However, during the first assessment, it became evident that there were no significant differences between the plots with the initially selected distance. As a result, to balance the time required for assessment with the information gained, it was decided to choose every second plot from the original grid. In 2017, six transects were established within each section of the clear-cut. Sampling plots measuring 1 × 1 m were systematically placed along each transect with 12 m spacing between plots, and their exact coordinates were recorded. In total, 105 sampling points were selected per section, resulting in 210 sampling plots overall. In each sampling plot, a plastic tube with a metal armature in the middle was installed at the plot center. This setup allowed for easy relocation during subsequent evaluations, either visually or using a metal detector. Vegetation cover and species abundance within each 1 × 1 m plot were assessed across three layers: mosses and lichens, herbaceous plants, and shrubs and trees. The initial vegetation survey was conducted in 2017, three years post-clear-cut, with a follow-up survey performed in 2020. The sampling was conducted by two individuals.
2.3. Data Analysis
Mean frequency and percentage cover were calculated for the vegetation data. The proportional distribution of species across vegetation layers and between the two observation years was also determined. The Shannon–Wiener index was calculated to describe both species richness and evenness [28]. Additionally, Pielou’s evenness index was used to measure the equitability of species abundance within the community [29]. It ranges from 0 to 1, where a value of 1 indicates perfect evenness, meaning all species have equal abundance, and a value closer to 0 suggests greater dominance by a few species, indicating low evenness. The Shannon–Wiener and Pielou’s evenness indices were compared between the observation years using a dependent samples t-test, while all other comparisons were performed using the dependent samples Wilcoxon test. The detrended correspondence analysis (DCA), implemented in R package vegan [30] was used to evaluate the primary ecological gradients and vegetation communities of ground cover in the studied clear-cut. The analysis was based on the percentage cover of species in the plots [31]. All statistical tests were performed with a significance level of p < 0.05, and all analyses were performed using R version 4.4.2 [32].
3. Results and Discussion
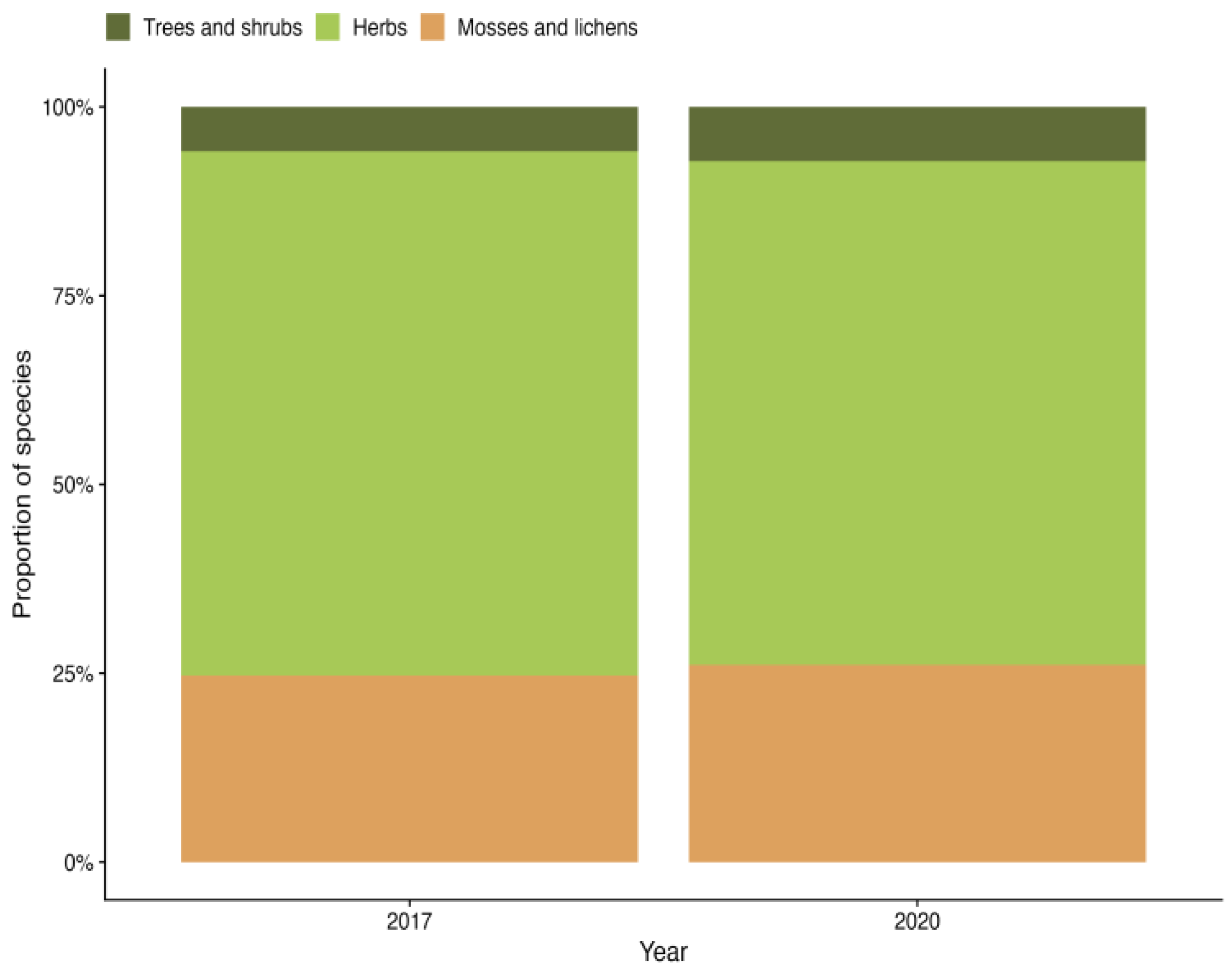
Vegetation succession starts with the removal of overstory vegetation, which triggers the establishment of stress-tolerant plant species. As stand development progresses, the forest ecosystem species composition gradually becomes more characteristic [24]. In the first evaluation (2017), 170 plant species were identified, whereas the second evaluation (2020) identified 111 species. This indicates a potential declining trend in species diversity. As the clear-cut was conducted in 2014, it is possible that the peak in species richness occurred during the three years between the clear-cut and the first evaluation, resulting in its omission. Although the proportion of species between the layers did not change considerably between the observation years (Figure 2), a slight increase was observed in the tree and shrub and moss and lichen layers in 2020, while the herb layer showed a decrease. These changes in species proportions are likely linked to the overall decline in species richness, which alters the proportion.
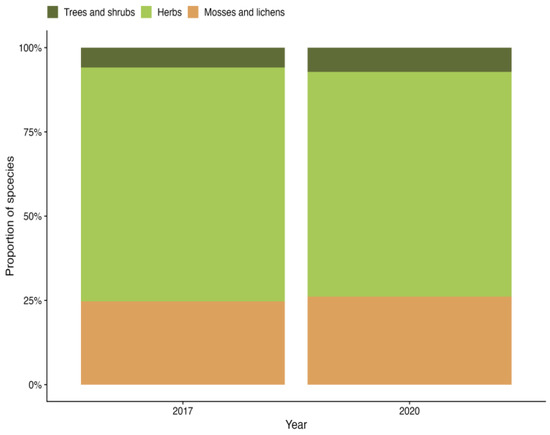
Figure 2.
Proportion of species across vegetation layers.
The overall species richness has significantly declined between the two assessment years (p < 0.05). In 2017, the average species richness was 13.5 ± 4.9 per plot, declining to 7.8 ± 2.7 in 2020. The herbaceous plant layer exhibited the highest species richness in both years, though it decreased significantly (p < 0.05) from 8.9 ± 4.2 in 2017 to 5.8 ± 2.3 in 2020. A similar significant (p < 0.05) reduction was observed in the moss and lichen layer, where species richness declined from 3.8 ± 1.8 to 1.6 ± 1.0. The most minor changes occurred in the tree and shrub layer, with the mean species number per plot decreasing from 0.7 ± 0.9 in 2017 to 0.4 ± 0.6 in 2020, with these changes being statistically non-significant (p > 0.05). The changes in the total number of species are likely linked to alterations in the environmental conditions within the clear-cut. The higher species richness observed during the first evaluation can be attributed not only to the expansion of pioneer species but also to the presence of late-successional species remaining from the previous forest stand [33].
This can be further understood by analyzing the unique species recorded exclusively during one of the observation years. In 2017, 69 unique species were recorded, but this number plummeted to just 10 in 2020. The unique species in 2017 were predominantly herbaceous plants (49 species), followed by mosses and lichens (17 species), and a small contribution from the tree and shrub layer (3 species). By 2020, the unique species comprised only five herbaceous plants, four mosses and lichens, and one species in the tree and shrub layer. The unique species recorded in 2017 were primarily associated with wetter habitats, including Carex spp., Equisetum spp., Juncus spp., and Ranunculus spp. In contrast, the 2020 evaluation featured additional Carex species, such as Carex cespitosa L., alongside species more typical of forest habitats, including Angelica sylvestris L.
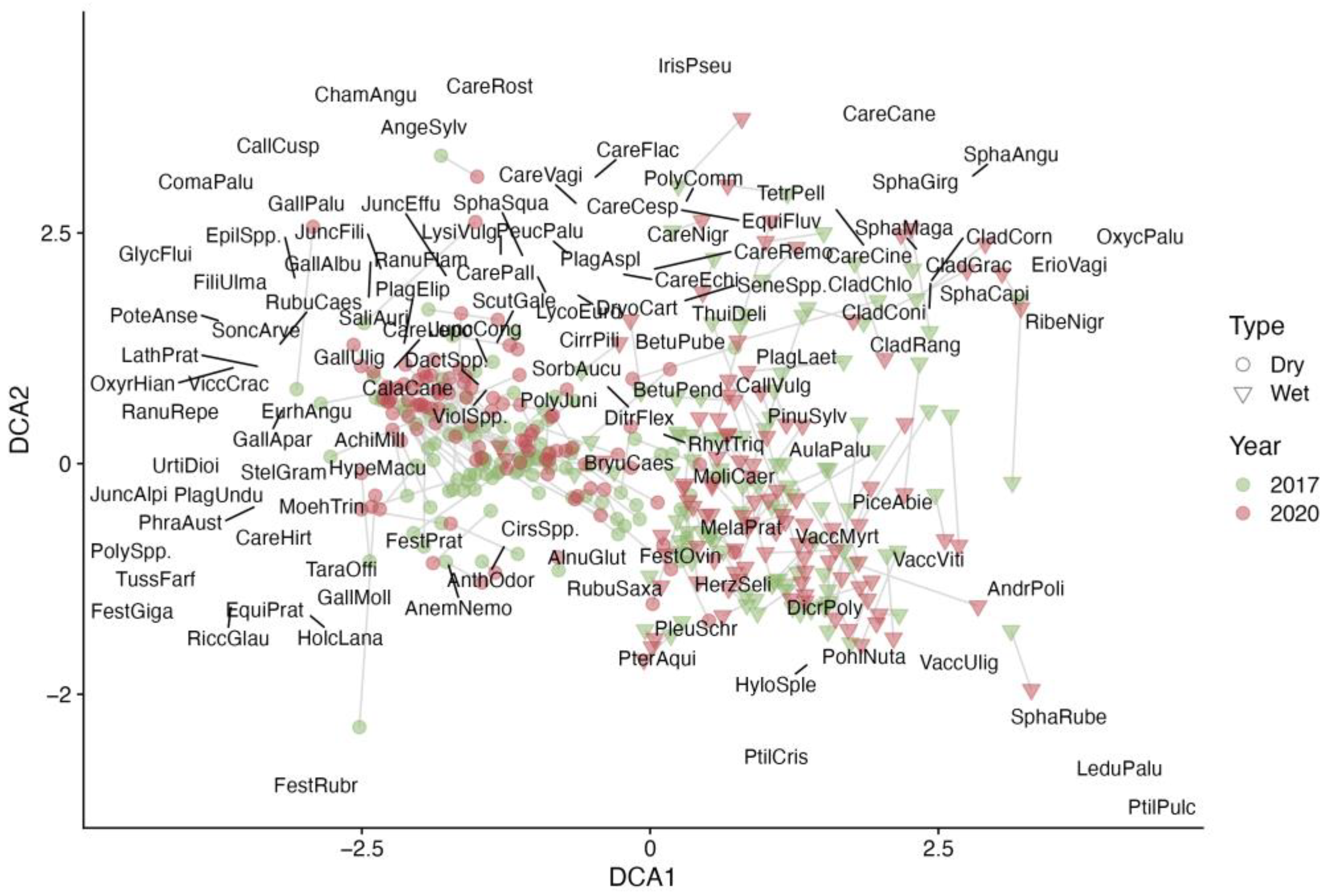
Sample plots show distinct clustering in the detrended correspondence analysis (DCA) ordination diagram (Figure 3). The “wet” sample plots on one side of the clear-cut are positioned on the right side of the ordination space, while the “dry” sample plots from the opposite side are on the left. Additionally, the sample plots on the dry side of the clear-cut form a tighter cluster, suggesting a greater uniformity in vegetation species diversity or coverage compared to the “wet” side, where the plots are more scattered. It was also evident that in 2017, the sample plots from both sides of the clear-cut were somewhat more similar to each other than in 2020. By 2020, the plots had shifted in the ordination space, with the groups becoming more distinct, possibly due to faster a succession in the wet part of the clear-cut [18].
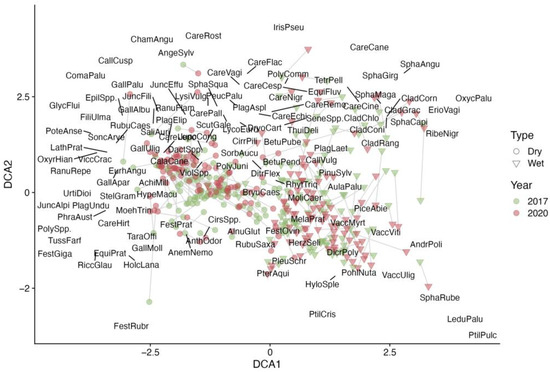
Figure 3.
The DCA ordination of ground cover vegetation species and sample plots according to their projective cover in the clear-cut in the two evaluation years. The type represents the growth conditions in a clear-cut section; refer to the methods section for a detailed explanation.
Early-successional species, which include colonists, stress-tolerant perennials, and pioneer species, are present in the forest only during the open-canopy phase, whereas late-successional species, classified as competitive perennials, dominate after canopy closure [34]. Previous studies have shown that many species persist and emerge during the early stages of secondary succession following stand-replacing disturbances, such as windthrow or logging [35,36]. This pattern was reflected in our findings with shade-tolerant species, such as Luzula pilosa L. and Trientalis europaea L. The first evaluation detected them in 22.9% and 48.6% of the study plots, respectively. However, by 2020 their presence had decreased to 21% and 15%, respectively (Table 1).

Table 1.
Mean percentage cover and frequency of most abundant herbaceous and moss species in clear-cut area in two separate evaluation years.
During the first observation, Deschampsia flexuosa L. exhibited the highest occurrence frequency among herbaceous plants, being present in 49.5% of the sample plots (Table 1), with an average coverage of 19.3% in the plots where it was detected. Previous studies suggest that the abundance of Deschampsia flexuosa begins to increase slightly in the second year following a clear-cut, reaching its peak after approximately five years [37] before starting to decline [38]. A great dominance was observed for grasses such as Calamagrostis canescens Weber. and Calamagrostis epigeios L. While these species were less frequent, their average coverage per plot was 26.4% and 25.1%, respectively. Notably, these species exhibited the highest frequency in the 2020 evaluation. Certain herb species, such as Epilobium angustifolium L., and grass species, like Deschampsia flexuosa, are typical pioneer species that thrive under increased light and nutrient availability [19]. As a result, these species are often promoted by clear-cutting [39]. Deschampsia flexuosa and Epilobium angustifolium seeds are wind-dispersed, facilitating their colonization of plots from surrounding areas. Furthermore, these species can reproduce vegetatively, enabling rapid coverage expansion by persisting on-site, germinating from the seed bank, or recolonizing the plots [35]. In 2017, Epilobium spp. was recorded in twenty sampling plots, but by 2020 it was present in only nine plots. This aligns with [37], which indicates that the abundance of Epilobium typically peaks 3–5 years after clear-cutting, with its percentage cover declining in sites 10 years post-clear-cut. Another species that typically peaks during the first few years after the clear-cut is Pteridium aquilinum [21]. However, in our study, neither the frequency nor the percentage cover of this species changed between the two observation years. However, our evaluation began just three years after the clear-cut. Overall, the abundance of light-demanding species remains exceptionally high in clear-cut areas during the first 10 years [21].
In successions following a clear-cut, the reduction in species diversity may be attributed to the elimination of certain species and the dominance of a few [40]. Although species like Deschampsia flexuosa exhibited noticeable dominance, the overall dominance of a few species within the clear-cut remained low, as reflected by the Pielou index. The index showed no significant change between the two observation years (p > 0.05), though a slight decrease in species dominance was suggested by an increase in the Pielou index from 0.76 ± 0.10 in 2017 to 0.78 ± 0.17 in 2020. When considering species richness and evenness, the Shannon–Wiener Index revealed a significant decline between the two evaluation years (p < 0.05), decreasing from 1.93 ± 0.43 in 2017 to 1.59 ± 0.48 in 2020. Other studies suggest that the Shannon–Wiener Index is typically highest in 2-year-old clear-cuts [21]. Therefore, it is possible that our evaluation missed the peak of species richness and evenness.
The first evaluation revealed that the clear-cut had a negative impact on dwarf shrubs, as most of the shrubs appeared dry and leafless, even during the vegetation season. Dwarf shrubs, adapted to shaded and consistently moist environments, struggle in clear-cut areas due to the increased sunlight, higher ground temperatures, periodic droughts, and lower air humidity [41]. The mean percentage cover of Vaccinium myrtillus increased between the observations in 2017 and 2020 (Table 1). However, its frequency declined, indicating that a portion of the population had died while the surviving individuals began regenerating or possible competition effects with graminoids [33]. The opposite was observed for Vaccinium vitis-idaea. Although its initial frequency was lower than that of Vaccinium myrtillus (2017), its frequency increased over time while the mean coverage declined slightly. The differences between the Vaccinium species may be attributed to their distinct morphological and growth characteristics. Vaccinium vitis-idaea possesses thick, leathery leaves, which provide greater resilience to high light intensity and drought conditions than the thinner leaves of Vaccinium myrtillus [42]. The response of dwarf shrubs to clear-cutting varies across studies. Some suggest a decrease in biomass [43], with biomass remaining significantly lower for the first four years after cutting [38] and only fully recovering after 6 to 80 years [44]. Conversely, other studies report an increase in the percentage cover and biomass of Vaccinium myrtillus following clear-cutting [45]. However, the tree layer exhibited lower levels of percentage cover and overall abundance compared to dwarf shrubs. The number of tree species and their percentage cover was relatively low compared to other layers. While there were no significant changes in the percentage cover of the tree layer between the years (p > 0.05), there was a notable decrease in the number of species within the tree layer (p < 0.05). In the first evaluation year 10 tree species were assessed, while this number decreased to 8 in the second evaluation. During the initial assessment, the unique species observed was Frangula alnus Mill. and Prunus padus L., whereas, in 2020 only one unique species (Ribes nigrum L.) was observed. Betula species were the most abundant tree species in both evaluations, consistently present in approximately 27% of the sampling plots during both evaluation years. This is likely attributed to their pioneer nature and the ability of their small, wind-dispersed seeds to colonize new areas rapidly [35].
As the colonization of vascular plants significantly decreased (p < 0.05), an increase in moss cover was expected [17,46]. However, the opposite trend was observed—moss cover and species diversity significantly declined (p < 0.05). This aligns with studies suggesting that moss cover is typically highest in freshly clear-cut areas and decreases gradually as the clear-cut ages [33]. The decrease could be linked to microenvironmental conditions, as precipitation primarily affects non-vascular plants [47]. Clear-cutting creates sunny conditions that accelerate soil drying, disrupting the development of non-vascular plants. In the moss and lichen layer, Pleurozium schreberi exhibited the highest frequency in 2017, recorded in 50.1% of the sampling plots, with a mean cover of 13.3%. By 2020, its occurrence had declined to 22.8%; however, it remained the species with the highest observation frequency in the moss and lichen layer. Studies have shown that both Hylocomium splendens and Pleurozium schreberi are negatively affected by clear-cutting [48] with Hylocomium splendens showing no recovery even after seven years [38]. However, our study did not reveal significant changes in this species frequency or percentage cover. A greater number of unique moss species was detected in the first evaluation year (sixteen species) compared to the second (four unique species), indicating a shift in species composition following the clear-cut. In the initial assessment, conducted three years after the clear-cut, the species composition likely reflected the remnants of the previous forest stand. The number of species decreased after the increase in sunlight, with only a few new species establishing themselves. One of these was Pohlia nutans Hedw., a species that grows on decaying wood and was identified as one of the new species following the clear-cut, consistent with observations in other studies [21].
4. Conclusions
Understanding vegetation dynamics following clear-cutting is crucial for sustainable forest management and forecasting ecosystem recovery trajectories. This study suggests that species diversity was higher in the initial years following the clear-cut but decreased as early as six years post-clear-cut. The herbaceous layer appears to be the most affected by this decline. However, assessing whether the clear-cut has significantly reduced species diversity compared to the previous forest stand is difficult, as we do not have the data from before the clear-cut. To draw more comprehensive conclusions, ongoing evaluations are necessary to assess the long-term impacts of such a large-scale clear-cut on species diversity and dominance.
Author Contributions
Conceptualization, D.J. and Ā.J.; methodology, D.J.; software, D.E.; validation, Ā.J.; formal analysis, D.E.; investigation, D.J. and A.A.L.; resources, Ā.J.; data curation, D.J.; writing—original draft preparation, D.J. and A.A.L.; writing—review and editing, Ā.J.; visualization, D.E.; supervision, Ā.J. and D.E.; project administration, Ā.J.; funding acquisition, Ā.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Effect of climate change on forestry and associated risks” project, Latvia’s State Forests, No 5-5.9.1_007p_101_21_78.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We thank Endijs Bāders for his contribution in creating the map.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahti, T.; Hämet-Ahti, L.; Jalas, J. Vegetation Zones and Their Sections in Northwestern Europe. Ann. Bot. Fenn. 1968, 5, 169–211. [Google Scholar]
- Ministry of Agriculture. Forest Sector in Numbers and Facts 2023; Zemkopības Ministrija: Rīga, Latvia, 2023; 54p. (In Latvian)
- Boucher, Y.; Auger, I.; Arseneault, D.; Elzein, T.; Sirois, L. Long-Term (1925–2015) Forest Structure Reorganization in an Actively Managed Temperate-Boreal Forest Region of Eastern North America. For. Ecol. Manag. 2021, 481, 118744. [Google Scholar] [CrossRef]
- Dieler, J.; Uhl, E.; Biber, P. Effect of Forest Stand Management on Species Composition, Structural Diversity, and Productivity in the Temperate Zone of Europe. For. Ecol. Manag. 2017, 391, 364–374. [Google Scholar] [CrossRef]
- Česonienė, L.; Daubaras, R.; Tamutis, V.; Kaškonienė, V.; Kaškonas, P.; Stakėnas, V.; Zych, M. Effect of Clear-Cutting on the Understory Vegetation, Soil and Diversity of Litter Beetles in Scots Pine-Dominated Forest. J. Sustain. For. 2019, 38, 791–808. [Google Scholar] [CrossRef]
- Meža likums 2000 (The Forest Law of the Republic of Latvia 2000). Available online: http://www.likumi.lv/doc.php?id=2825 (accessed on 27 February 2025). (In Latvian).
- Gilliam, F.S. The Ecological Significance of the Herbaceous Layer in Temperate Forest Ecosystems. Bioscience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Angelstam, K.P. Maintaining and Restoring Biodiversity in European Boreal Forests by Developing Natural Disturbance Regimes. J. Veg. Sci. 1998, 9, 593–602. [Google Scholar]
- Peterson, C.J.; Pickett, S.T.A. Forest Reorganization: A Case Study in an Old-Growth Forest Catastrophic Blowdown. Ecology 1995, 76, 763–774. [Google Scholar] [CrossRef]
- Esseen, A.P.; Ehnström, B.; Ericson, L.; Sjöberg, K. Boreal Forests. Ecol. Bull. 2015, 46, 16–47. [Google Scholar]
- Franklin, J.F.; Lindenmayer, D.; Macmahon, J.A.; Mckee, A.; Perry, D.A.; Waide, R.; Foster, D. Threads of Continuity: Ecosystem Disturbance, Recovery, and the Theory of Biological Legacies. Conserv. Pract. 2000, 1, 8–17. [Google Scholar] [CrossRef]
- Dobrowolska, D. Forest Regeneration in Northeastern Poland Following a Catastrophic Blowdown. Can. J. For. Res. 2015, 45, 1172–1182. [Google Scholar] [CrossRef]
- Tonteri, T.; Salemaa, M.; Rautio, P.; Hallikainen, V.; Korpela, L.; Merilä, P. Forest Management Regulates Temporal Change in the Cover of Boreal Plant Species. For. Ecol. Manag. 2016, 381, 115–124. [Google Scholar] [CrossRef]
- Hannerz, M.; Hånell, B. Effects on the flora in Norway spruce forests following clearcutting and shelterwood cutting. For. Ecol. Manag. 1997, 90, 29–49. [Google Scholar] [CrossRef]
- 15Prévost, M.; Raymond, P. Effect of Gap Size, Aspect and Slope on Available Light and Soil Temperature after Patch-Selection Cutting in Yellow Birch-Conifer Stands, Quebec, Canada. For. Ecol. Manag. 2012, 274, 210–221. [Google Scholar] [CrossRef]
- Schelker, J.; Kuglerová, L.; Eklöf, K.; Bishop, K.; Laudon, H. Hydrological Effects of Clear-Cutting in a Boreal Forest—Snowpack Dynamics, Snowmelt and Streamflow Responses. J. Hydrol. 2013, 484, 105–114. [Google Scholar] [CrossRef]
- Hannerz, M.; Hånell, B. Changes in the Vascular Plant Vegetation after Different Cutting Regimes on a Productive Peatland Site in Central Sweden. Scand. J. For. Res. 2008, 23, 37–41. [Google Scholar] [CrossRef]
- Walker, L.R.; del Moral, R. PrimarySuccession. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2011. [Google Scholar] [CrossRef]
- Bergstedt, J.; Hagner, M.; Milberg, P. Effects on Vegetation Composition of a Modified Forest Harvesting and Propagation Method Compared with Clear-Cutting, Scarification, and Planting. Appl. Veg. Sci. 2008, 11, 159–168. [Google Scholar] [CrossRef]
- Nelson, C.R.; Halpern, C.B. Short-Term Effects of Timber Harvest and Forest Edges on Ground-Layer Mosses and Liverworts. Can. J. Bot. 2005, 83, 610–620. [Google Scholar] [CrossRef]
- Gustienė, D.; Varnagirytė-Kabašinskienė, I.; Stakėnas, V. Ground Vegetation, Forest Floor and Mineral Topsoil in a Clear-Cutting and Reforested Scots Pine Stands of Different Ages: A Case Study. J. For. Res. 2022, 33, 1247–1257. [Google Scholar] [CrossRef]
- Hylander, K. No Increase in Colonization Rate of Boreal Bryophytes Near Propagule Sources. Ecology 2009, 90, 160–169. [Google Scholar] [CrossRef]
- Karazija, S. Age-Related Dynamics of Pine Forest Communities In Lithuania. Balt. For. 2002, 9, 50–62. [Google Scholar]
- Pykälä, J. Immediate Increase in Plant Species Richness after Clear-Cutting of Boreal Herb-Rich Forests. Appl. Veg. Sci. 2004, 7, 29–34. [Google Scholar] [CrossRef]
- AS Latvijas Valsts Meži. Handbook for Forest Type Identification; Latvijas Valsts meži: Rīga, Latvia, 2013; pp. 1–67. (In Latvian) [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS Monthly High-Resolution Gridded Multivariate Climate Dataset. Sci. Data 2020, 7, 1–18. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communications. Bell. Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. Ecological Diversity. Limnol. Oceanogr. 1975, 22, 165. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘Vegan’, Version 2.0-5. Community Ecol. Package 2013, 2, 1–295. [Google Scholar]
- Correa-Metrio, A.; Garcia, S.L.; Caballero, M. Detrended Correspondence Analysis: A Useful Tool to Quantify Ecological Changes from Fossil Data Sets. Bol. Soc. Geol. Mex. 2014, 66, 135–143. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 20 December 2024).
- Marozas, V. Early Succession of Ground Vegetation After Clear-Cuttings in Spruce Forests in a Boreonemoral Zone, Lithuania. Acta Biol. Univ. Daugavp. 2005, 5, 127–136. [Google Scholar]
- Tefańska-Krzaczek, E.S.; Staniaszek-Kik, M. Positive Aspects of Clear-Cut Logging? Ground Bryophyte Diversity along the Age Gradient of Managed Pinus Sylvestris Stands. Cryptogam. Bryol. 2016, 37, 181–197. [Google Scholar] [CrossRef]
- Heinrichs, S.; Schmidt, W. Short-Term Effects of Selection and Clear Cutting on the Shrub and Herb Layer Vegetation during the Conversion of Even-Aged Norway Spruce Stands into Mixed Stands. For. Ecol. Manag. 2009, 258, 667–678. [Google Scholar] [CrossRef]
- Zobel, M. Secondary Forest Succession in Jarvselja, Southern Estonia: Changes in Field Layer Vegetation. Ann. Bot. Fenn. 1989, 26, 171–182. [Google Scholar]
- Genikova, N.; Mamontov, V. Decade-Long Dynamics of the Ground Vegetation in an Ecotone between Coniferous Forest and Clear-Cut Site. Environ. Sci. Proc. 2022, 22, 15. [Google Scholar] [CrossRef]
- Palviainen, M.; Finér, L.; Mannerkoski, H.; Piirainen, S.; Starr, M. Responses of Ground Vegetation Species to Clear-Cutting in a Boreal Forest: Aboveground Biomass and Nutrient Contents during the First 7 Years. Ecol. Res. 2005, 20, 652–660. [Google Scholar] [CrossRef]
- Nykvist, N. Changes in species occurrence and phytomass after clearfelling, prescribed burning and slash removal in two Swedish spruce forests. Stud. For. Suec. 1997, 201, 1–33. [Google Scholar]
- Jalonen, J.; Vanha-Majamaa, I. Immediate Effects of Four Different Felling Methods on Mature Boreal Spruce Forest Understorey Vegetation in Southern Finland. For. Ecol. Manag. 2001, 146, 25–34. [Google Scholar] [CrossRef]
- Kellomäki, S.; Hari, P.; Väisänen, E. Annual Production of Some Forest Mosses as a Function of Light Available for Photosynthesis. Silva Fenn. 1977, 11, 81–86. [Google Scholar] [CrossRef]
- Janke, A. Transpiration resistance in Vaccinium Myrtillus. Amer. J. Bot. 1970, 57, 1051–1054. [Google Scholar]
- Miina, J.; Hotanen, J.; Salo, K. Modelling the Abundance and Temporal Variation in the Production of Bilberry (Vaccinium myrtillus L.) in Finnish Mineral Soil Forests. Silva Fenn. 2009, 43, 577–593. [Google Scholar] [CrossRef]
- Lõhmus, A.; Remm, L. Disentangling the Effects of Seminatural Forestry on an Ecosystem Good: Bilberry (Vaccinium myrtillus) in Estonia. For. Ecol. Manag. 2017, 404, 75–83. [Google Scholar] [CrossRef]
- Nielsen, A.; Totland, Ø.; Ohlson, M. The Effect of Forest Management Operations on Population Performance of Vaccinium myrtillus on a Landscape-Scale. Basic Appl. Ecol. 2007, 8, 231–241. [Google Scholar] [CrossRef]
- Saetre, P. Spatial Patterns of Ground Vegetation, Soil Microbial Biomass and Activity in a Mixed Spruce-Birch Stand. Ecography 1999, 22, 183–192. [Google Scholar] [CrossRef]
- Strengbom, J.; Walheim, M.; Näsholm, T.; Ericson, L. Regional Differences in the Occurrence of Understorey Species Reflect Nitrogen Deposition in Swedish Forests. Ambio 2003, 32, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Mäkipää, R.; Heikkinen, J. Large-Scale Changes in Abundance of Terricolous Bryophytes and Macrolichens in Finland. J. Veg. Sci. 2003, 14, 497–508. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).