Widespread Microplastic Pollution in Central Appalachian Streams: Implications for Freshwater Ecosystem Sustainability
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Environmental Variables
2.3. Sample Preparation and Digestion
2.4. Filtration and Inspection
2.5. Contamination Protocol
2.6. Data Analysis
3. Results
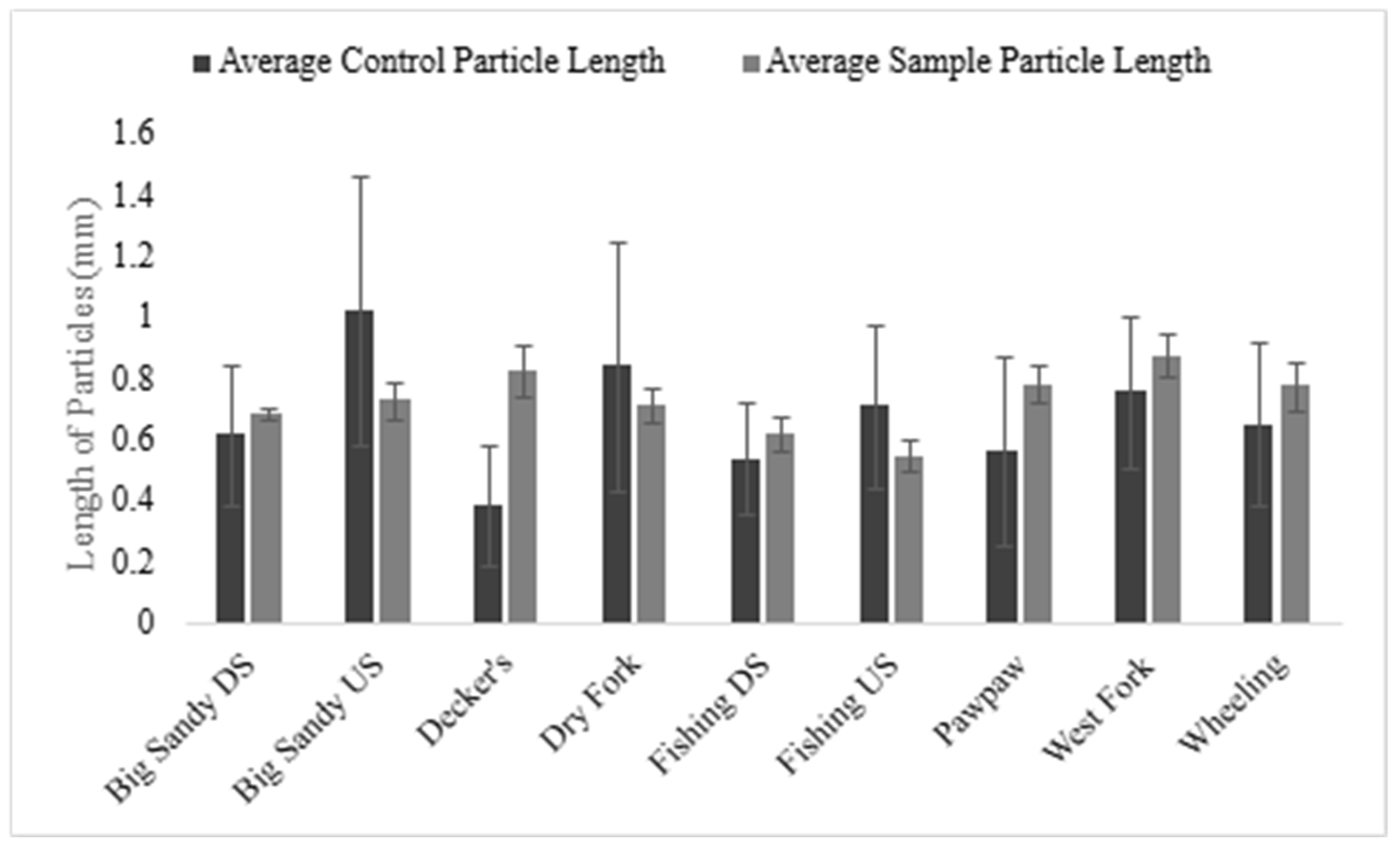
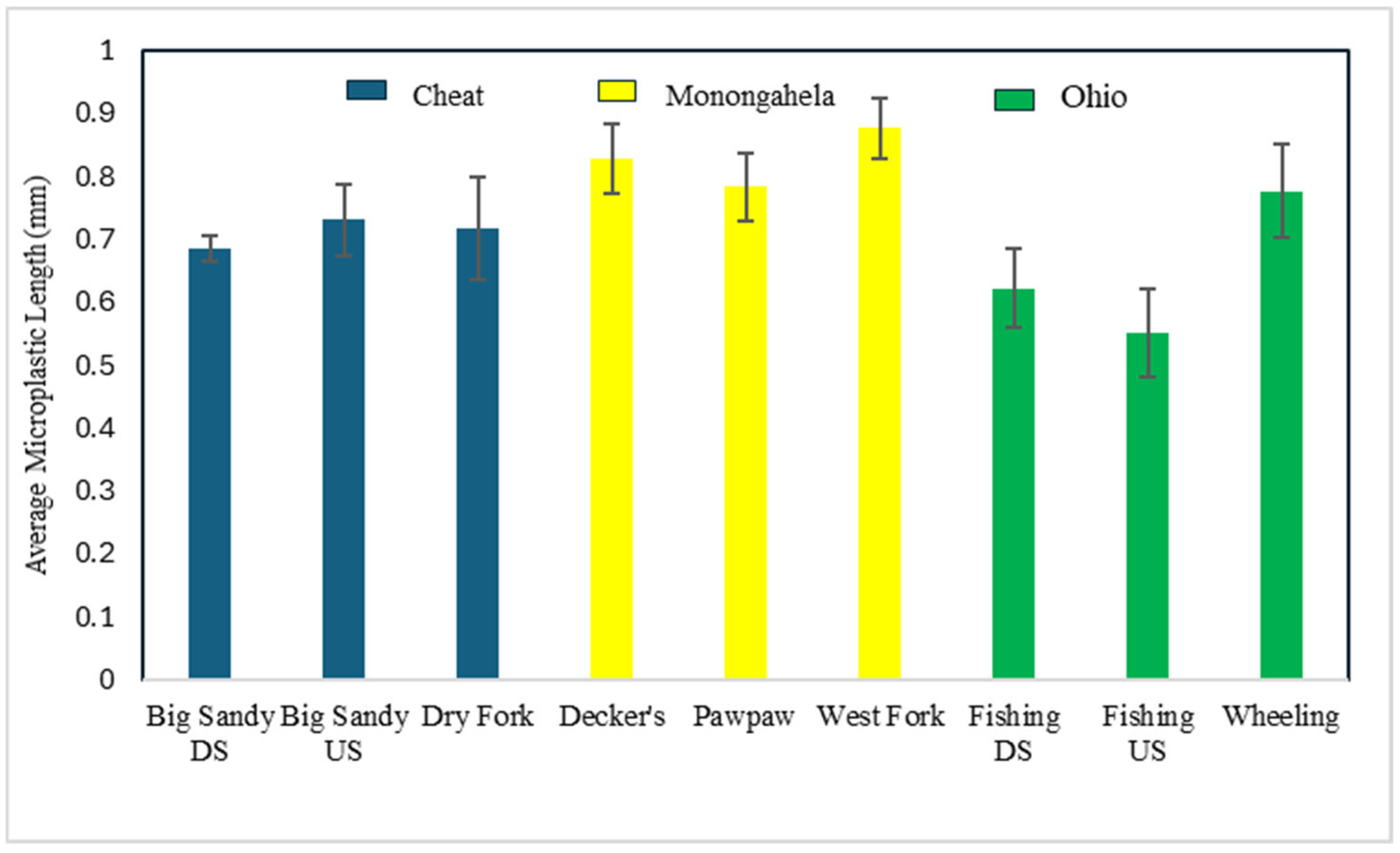
3.1. Characteristics of Microplastics in H. nigricans
3.2. Relationships Between Body Length and Microplastics in H. nigricans
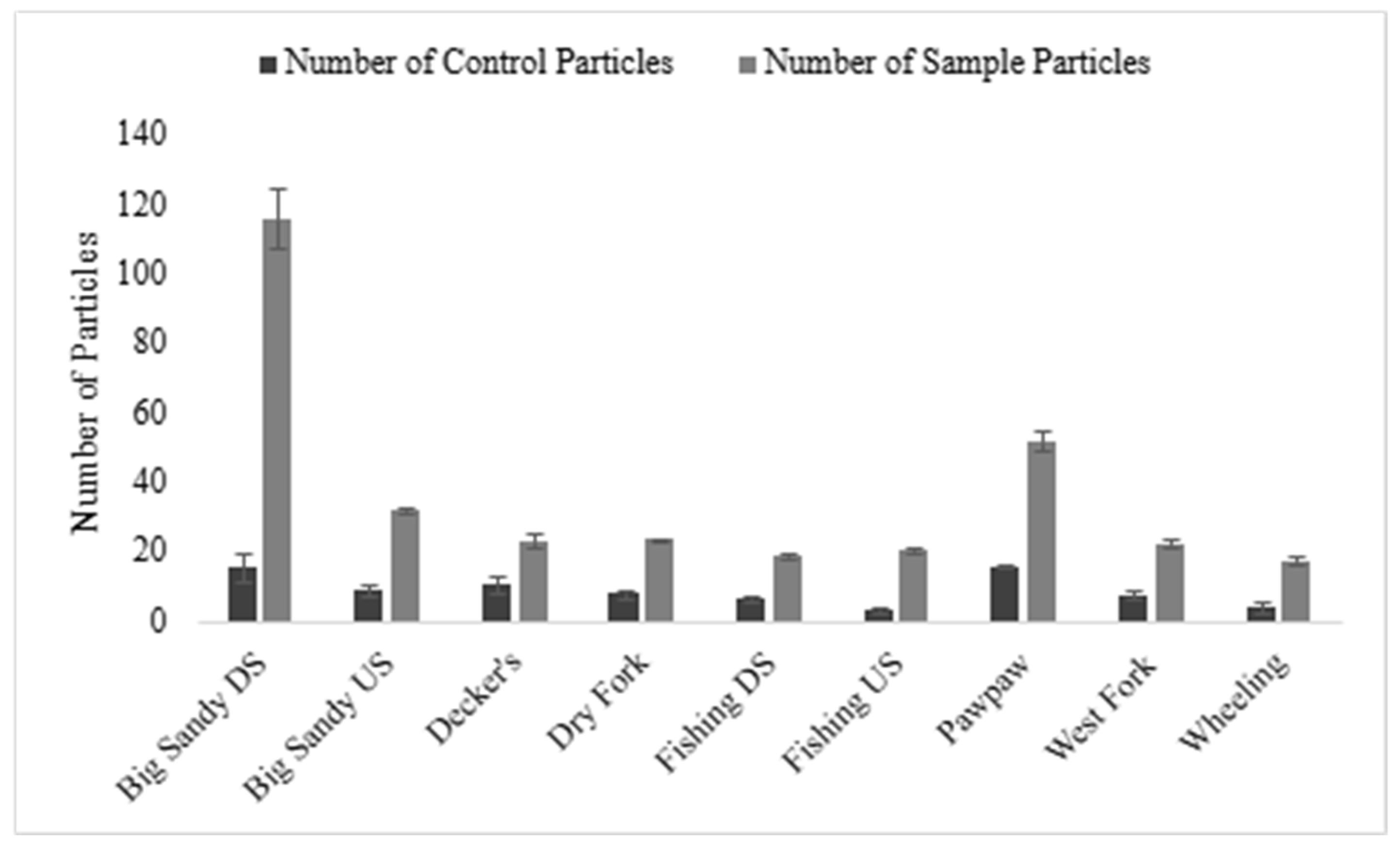
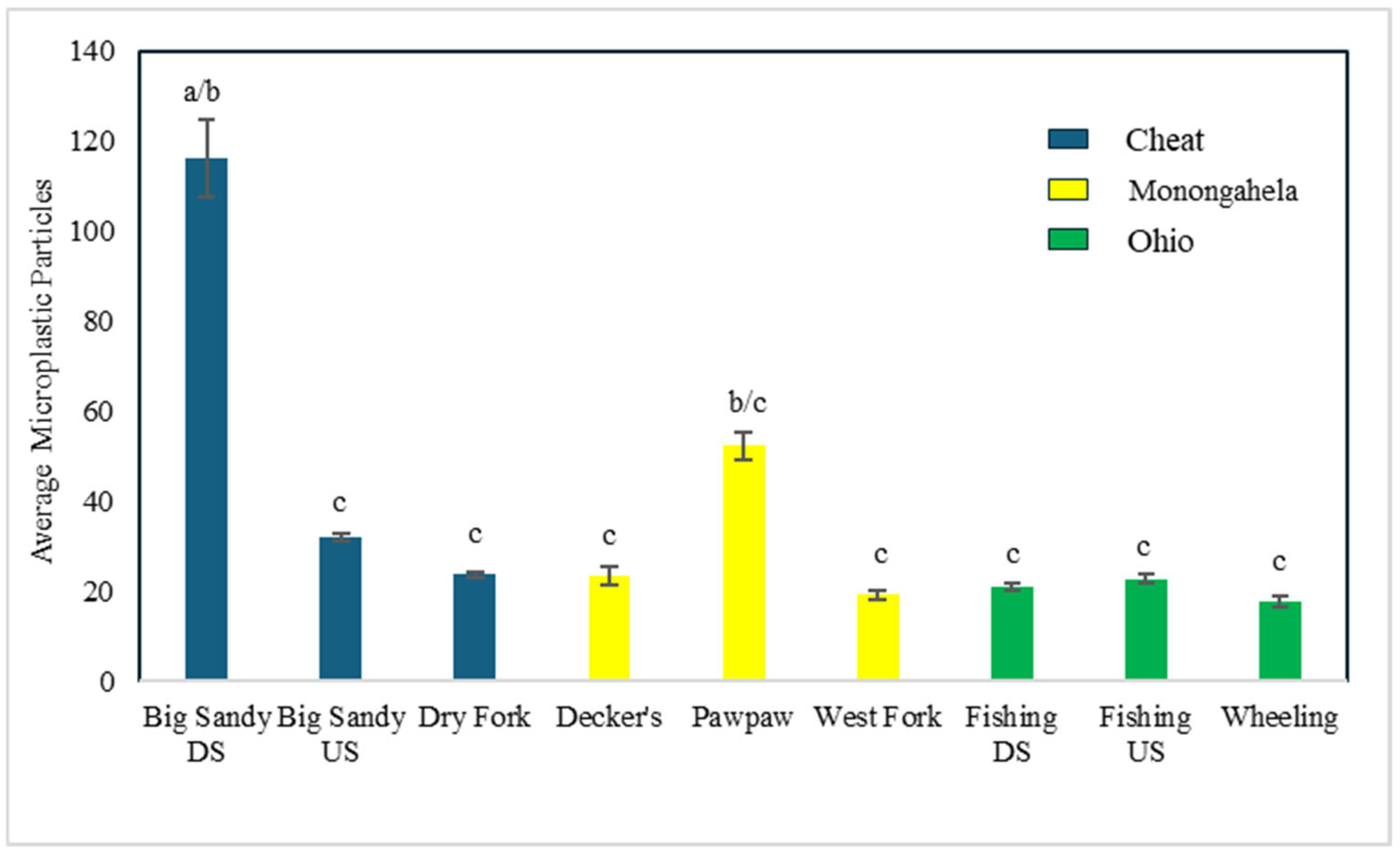
3.3. Differences in Sites, Streams, and Watersheds in Microplastic Consumption
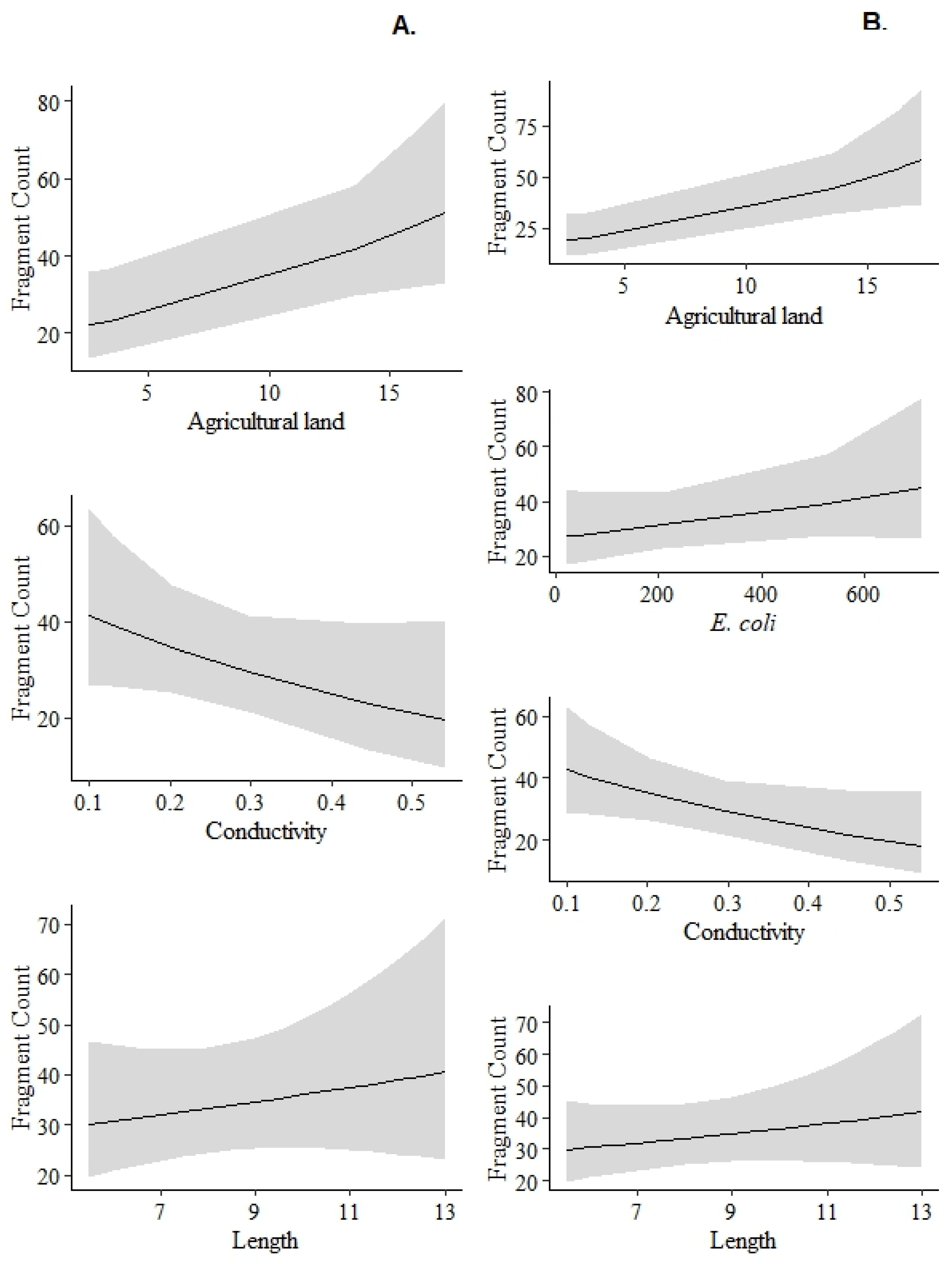
3.4. Relationship Between Microplastic Consumption and Environmental Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Model | Df | AIC | BIC | LogLi | K Deviance | Chisq | Pr(>Chisq) |
|---|---|---|---|---|---|---|---|
| MP ~ Agricultural land + Conductivity+ FishTL + (1|Site) | 6 | 414.2 | 425.55 | −201.1 | 402.2 | 2.0391 | <0.05 |
| MP ~ Agricultural land + Conductivity + E. coli+ FishTL + (1|Site) | 7 | 414.89 | 428.13 | −200.44 | 400.89 | 5.4312 | <0.05 |
| MP ~ Forest + Conductivity + (1|Site) | 5 | 415.45 | 424.91 | −202.72 | 405.45 | 0.6051 | <0.05 |
| MP ~ Area +FishTL + (1|Site) | 5 | 415.91 | 425.37 | −202.95 | 405.91 | 0.2282 | <0.05 |
| MP ~ Forest + Area + (1|Site) | 5 | 416.05 | 425.51 | −203.03 | 406.05 | 0.5367 | <0.05 |
| MP ~ Forest +FishTL + (1|Site) | 5 | 416.14 | 425.6 | −203.07 | 406.14 | 0.4234 | <0.05 |
| MP ~ Agricultural land + Area+ Conductivity + E. coli+ FishTL + (1|Site) | 8 | 416.81 | 431.94 | −200.4 | 400.81 | 5.3509 | <0.05 |
References
- Shahul Hamid, F.; Bhatti, M.S.; Anuar, N.; Anuar, N.; Mohan, P.; Periathamby, A. Worldwide distribution and abundance of microplastic: How dire is the situation? Waste Manag. Res. J. A Sustain. Circ. Econ. 2018, 36, 873–897. [Google Scholar] [CrossRef]
- Bajt, O. From plastics to microplastics and organisms. FEBS Open Bio 2021, 11, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wu, J.; Wang, H.; Bao, Y.; Xing, H.; Ye, W.; Li, X.; Huang, M. Interaction of microplastics with perfluoroalkyl and polyfluoroalkyl substances in water: A review of the fate, mechanisms and toxicity. Sci. Total Environ. 2024, 948, 175000. [Google Scholar] [CrossRef] [PubMed]
- Salawu, O.A.; Olivares, C.I.; Adeleye, A.S. Adsorption of PFAS onto secondary microplastics: A mechanistic study. J. Hazard. Mater. 2024, 470, 134185. [Google Scholar] [CrossRef]
- An, L.; Liu, Q.; Deng, Y.; Wu, W.; Gao, Y.; Ling, W. Sources of Microplastic in the Environment. In Microplastics in Terrestrial Environments: Emerging Contaminants and Major Challenges; He, D., Luo, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 143–159. [Google Scholar] [CrossRef]
- De Falco, F.; Gullo, M.P.; Gentile, G.; Di Pace, E.; Cocca, M.; Gelabert, L.; Brouta-Agnésa, M.; Rovira, A.; Escudero, R.; Villalba, R.; et al. Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ. Pollut. 2018, 236, 916–925. [Google Scholar] [CrossRef]
- Golwala, H.; Zhang, X.; Iskander, S.M.; Smith, A.L. Solid waste: An overlooked source of microplastics to the environment. Sci. Total Environ. 2021, 769, 144581. [Google Scholar] [CrossRef]
- Collard, F.; Gasperi, J.; Gabrielsen, G.W.; Tassin, B. Plastic Particle Ingestion by Wild Freshwater Fish: A Critical Review. Environ. Sci. Technol. 2019, 53, 12974–12988. [Google Scholar] [CrossRef]
- Blettler, M.C.M.; Abrial, E.; Khan, F.R.; Sivri, N.; Espinola, L.A. Freshwater plastic pollution: Recognizing research biases and identifying knowledge gaps. Water Res. 2018, 143, 416–424. [Google Scholar] [CrossRef]
- Zandaryaa, S. Freshwater Microplastic Pollution: The State of Knowledge and Research. In Plastics in the Aquatic Environment—Part I; The Handbook of Environmental Chemistry, Volume 111; Stock, F., Reifferscheid, G., Brennholt, N., Kostianaia, E., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 255–272. [Google Scholar] [CrossRef]
- Parker, B.; Andreou, D.; Green, I.D.; Britton, J.R. Microplastics in freshwater fishes: Occurrence, impacts and future perspectives. Fish Fish. 2021, 22, 467–488. [Google Scholar] [CrossRef]
- Bellasi, A.; Binda, G.; Pozzi, A.; Galafassi, S.; Volta, P.; Bettinetti, R. Microplastic Contamination in Freshwater Environments: A Review, Focusing on Interactions with Sediments and Benthic Organisms. Environments 2020, 7, 30. [Google Scholar] [CrossRef]
- Sarijan, S.; Azman, S.; Said, M.I.M.; Jamal, M.H. Microplastics in freshwater ecosystems: A recent review of occurrence, analysis, potential impacts, and research needs. Environ. Sci. Pollut. Res. 2021, 28, 1341–1356. [Google Scholar] [CrossRef]
- Talbot, R.; Chang, H. Microplastics in freshwater: A global review of factors affecting spatial and temporal variations. Environ. Pollut. 2022, 292, 118393. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Shim, W.J.; Cho, Y.; Han, G.M.; Song, Y.K.; Hong, S.H. A close relationship between microplastic contamination and coastal area use pattern. Water Res. 2020, 171, 115400. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.R.; Kim, Y.-N.; Yoon, J.-H.; Dickinson, N.; Kim, K.-H. Plastic contamination of forest, urban, and agricultural soils: A case study of Yeoju City in the Republic of Korea. J. Soils Sediments 2021, 21, 1962–1973. [Google Scholar] [CrossRef]
- Klemmensen, N.D.R.; Sobrino Blanco, M.; Vollertsen, J. Assessing microplastic contamination in Icelandic soils: Insights from remote, agricultural, and urban environments. Sci. Total Environ. 2024, 955, 177215. [Google Scholar] [CrossRef]
- Simon, T.P.; Dufour, R. Development of Index of Biotic Integrity Expectations for the Ecoregions of Indiana: V. Eastern Corn Belt Plain; EPA 905-R-96-002; U.S. Environmental Protection Agency, Region V, Water Division, Watershed and Nonpoint Source Branch: Chicag, IL, USA, 1997; p. 73. [Google Scholar]
- Lau, J.K.; Lauer, T.E.; Weinman, M.L. Impacts of Channelization on Stream Habitats and Associated Fish Assemblages in East Central Indiana. Am. Midl. Nat. 2006, 156, 319–330. [Google Scholar] [CrossRef]
- Dane, H.; Şïşman, T. A morpho-histopathological study in the digestive tract of three fish species influenced with heavy metal pollution. Chemosphere 2020, 242, 125212. [Google Scholar] [CrossRef]
- Driehaus, E.R.; Landreth, J.; Adase, K.; Smith, D.; Wellman, D.; Arantes, C.C.; Murry, B.A. Length-Weight Relationships for 44 Central Appalachian Fish Species. J. Appl. Ichthyol. 2023, 2023, 5573054. [Google Scholar] [CrossRef]
- Zipp, K. Integration of Fisheries Ecology with the Assessment of Microplastic Consumption in Black Bass in the Upper Ohio River Drainage. Master’s Thesis, West Virginia University, Morgantown, WV, USA, 2022. Graduate Theses, Dissertations, and Problem Reports. Available online: https://researchrepository.wvu.edu/etd/11241 (accessed on 2 January 2025).
- Hajovsky, P.A. Microplastic Ingestion of Juvenile Fish in Corpus Christi Bay and Upper Laguna Madre, Texas. Ph.D. Thesis, Texas A&M University, Corpus Christi, TX, USA, 2019. Available online: https://hdl.handle.net/1969.6/87888 (accessed on 30 January 2025).
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of micro-plastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef]
- Estahbanati, S.; Fahrenfeld, N.L. Influence of wastewater treatment plant discharges on microplastic concentrations in surface water. Chemosphere 2016, 162, 277–284. [Google Scholar] [CrossRef]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Beckingham, B.; Apintiloaiei, A.; Moore, C.; Brandes, J. Hot or not: Systematic review and laboratory evaluation of the hot needle test for microplastic identification. Microplastics Nanoplastics 2023, 3, 8. [Google Scholar] [CrossRef]
- Lopes, C.; Raimundo, J.; Caetano, M.; Garrido, S. Microplastic ingestion and diet composition of planktivorous fish. Limnol. Oceanogr. Lett. 2020, 5, 103–112. [Google Scholar] [CrossRef]
- Gad, A.K.; Midway, S.R. Relationship of Microplastics to Body Size for Two Estuarine Fishes. Microplastics 2022, 1, 211–220. [Google Scholar] [CrossRef]
- Covernton, G.A.; Davies, H.L.; Cox, K.D.; El-Sabaawi, R.; Juanes, F.; Dudas, S.E.; Dower, J.F. A Bayesian analysis of the factors determining microplastics ingestion in fishes. J. Hazard. Mater. 2021, 413, 125405. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; Benthem KJ van Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.7. 2024. Available online: https://github.com/florianhartig/dharma (accessed on 11 March 2025).
- Suaria, G.; Musso, M.; Achtypi, A.; Bassotto, D.; Aliani, S. Textile Fibres in Mediterranean Surface Waters: Abundance and Composition. In Proceedings of the 2nd International Conference on Microplastic Pollution in the Mediterranean Sea; Cocca, M., Di Pace, E., Errico, M.E., Gentile, G., Montarsolo, A., Mossotti, R., Avella, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 62–66. [Google Scholar] [CrossRef]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic microfibres in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef]
- Sanchez-Vidal, A.; Thompson, R.C.; Canals, M.; De Haan, W.P. The imprint of microfibres in southern European deep seas. PLoS ONE 2018, 13, e0207033. [Google Scholar] [CrossRef]
- McNeish, R.E.; Kim, L.H.; Barrett, H.A.; Mason, S.A.; Kelly, J.J.; Hoellein, T.J. Microplastic in riverine fish is connected to species traits. Sci. Rep. 2018, 8, 11639. [Google Scholar] [CrossRef]
- Pazos, R.S.; Maiztegui, T.; Colautti, D.C.; Paracampo, A.H.; Gómez, N. Microplastics in gut contents of coastal freshwater fish from Río de la Plata estuary. Mar. Pollut. Bull. 2017, 122, 85–90. [Google Scholar] [CrossRef]
- Wagner, J.; Wang, Z.-M.; Ghosal, S.; Murphy, M.; Wall, S.; Cook, A.-M.; Robberson, W.; Allen, H. Nondestructive Extraction and Identification of Microplastics from Freshwater Sport Fish Stomachs. Environ. Sci. Technol. 2019, 53, 14496–14506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, Y.; Zhong, S.; Liu, J.; Qin, Y.; Gao, P. Microplastics in freshwater and wild fishes from Lijiang River in Guangxi, Southwest China. Sci. Total Environ. 2021, 755, 142428. [Google Scholar] [CrossRef] [PubMed]
- Dalu, T.; Themba, S.T.; Dondofema, F.; Wu, N.; Munyai, L.F. Assessing microplastic abundances in freshwater fishes in a subtropical African reservoir. Discov. Sustain. 2024, 5, 360. [Google Scholar] [CrossRef]
- Khan, H.S.; Hasan, J.; Manik, M.; Farukh, M.A.; Shahjahan, M. Pervasiveness of microplastics in the gastrointestinal tract of some selected fish species from Turag River alongside the capital city of Bangladesh. Emerg. Contam. 2024, 10, 100309. [Google Scholar] [CrossRef]
- Al Mudhar, M.H.I.; Sumberartha, I.W.; Hassan, Z.; Rahmansyah, M.S.; Tamalene, M.N. Examination of Microplastic Particles in Reef Fish Food in Ternate Island Waters, Indonesia. Jordan J. Biol. Sci. 2021, 14, 853–858. [Google Scholar] [CrossRef]
- Bosshart, S.; Erni-Cassola, G.; Burkhardt-Holm, P. Independence of microplastic ingestion from environmental load in the round goby (Neogobius melanostomus) from the Rhine river using high quality standards. Environ. Pollut. 2020, 267, 115664. [Google Scholar] [CrossRef]
- Corradini, F.; Casado, F.; Leiva, V.; Huerta-Lwanga, E.; Geissen, V. Microplastics occurrence and frequency in soils under different land uses on a regional scale. Sci. Total Environ. 2021, 752, 141917. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Chen, W.; Ba, Y.; Khan, K.; Chen, W.; Tu, C.; Chen, C.; Xu, L. Effects of land use and landscape on the occurrence and distribution of microplastics in soil, China. Sci. Total Environ. 2022, 847, 157598. [Google Scholar] [CrossRef]
- Oza, J.; Rabari, V.; Yadav, V.K.; Sahoo, D.K.; Patel, A.; Trivedi, J. A Systematic Review on Microplastic Contamination in Fishes of Asia: Polymeric Risk Assessment and Future Prospectives. Environ. Toxicol. Chem. 2024, 43, 671–685. [Google Scholar] [CrossRef]
- Milne, M.H.; Helm, P.A.; Munno, K.; Bhavsar, S.P.; Rochman, C.M. Microplastics and Anthropogenic Particles in Recreationally Caught Freshwater Fish from an Urbanized Region of the North American Great Lakes. Environ. Health Perspect. 2024, 132, 077004. [Google Scholar] [CrossRef]
- Horton, A.A.; Jürgens, M.D.; Lahive, E.; Van Bodegom, P.M.; Vijver, M.G. The influence of exposure and physiology on microplastic ingestion by the freshwater fish Rutilus rutilus (roach) in the River Thames, UK. Environ. Pollut. 2018, 236, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Guevara, F.; Roy, P.D.; Kutralam-Muniasamy, D.; Shruti, V.C. Cover of microplastic data underreporting and progress toward standardization. Sci. Total Environ. 2022, 829, 154727. [Google Scholar] [CrossRef]
- Chen, H.; Jia, Q.; Zhao, X.; Li, L.; Nie, Y.; Liu, H.; Ye, J. The occurrence of microplastics in water bodies in urban agglomerations: Impacts of drainage system overflow in wet weather, catchment land-uses, and environmental management practices. Water Res. 2020, 183, 116073. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Kelkar, V.; Kumar, R.; Halden, R.U. Methods and challenges in the detection of microplastics and nanoplastics: A mini-review. Polym. Int. 2022, 71, 543–551. [Google Scholar] [CrossRef]
- Rolsky, C.; Kelkar, V.; Driver, E.; Halden, R.U. Municipal sewage sludge as a source of microplastics in the environment. Curr. Opin. Environ. Sci. Health 2020, 14, 16–22. [Google Scholar] [CrossRef]
- Klein, M.; Bechtel, B.; Brecht, T.; Fischer, E.K. Spatial distribution of atmospheric microplastics in bulk-deposition of urban and rural environments—A one-year follow-up study in northern Germany. Sci. Total Environ. 2023, 901, 165923. [Google Scholar] [CrossRef]
- Jin, S.; Dewitz, J.; Danielson, P.; Granneman, B.; Costello, C.; Smith, K.; Zhu, Z. National Land Cover Database 2019, A New Strategy for Creating Clean Leaf-On and Leaf-Off Landsat Composite Images. J. Remote Sens. 2023, 3, 0022. [Google Scholar] [CrossRef]
- Elnahas, A.; Gray, A.; Lee, J.; AlAmiri, N.; Pokhrel, N.; Allen, S.; Foroutan, H. Atmospheric Deposition of Microplastics in South Central Appalachia in the United States. ACS EST Air 2025, 2, 64–72. [Google Scholar] [CrossRef]
- Wang, F.; Lai, Z.; Peng, G.; Luo, L.; Liu, K.; Huang, X.; Xu, Y.; Shen, Q.; Li, D. Microplastic abundance and distribution in a Central Asian desert. Sci. Total Environ. 2021, 800, 149529. [Google Scholar] [CrossRef]
- Illuminati, S.; Notarstefano, V.; Tinari, C.; Fanelli, M.; Girolametti, F.; Ajdini, B.; Scarchilli, C.; Ciardini, V.; Iaccarino, A.; Giorgini, E.; et al. Microplastics in bulk atmospheric deposition along the coastal region of Victoria Land, Antarctica. Sci. Total Environ. 2024, 949, 175221. [Google Scholar] [CrossRef]
- Sun, X.; Jia, Q.; Ye, J.; Zhu, Y.; Song, Z.; Guo, Y.; Chen, H. Real-time variabilities in microplastic abundance and characteristics of urban surface runoff and sewer overflow in wet weather as impacted by land use and storm factors. Sci. Total Environ. 2023, 859, 160148. [Google Scholar] [CrossRef] [PubMed]
- Kunz, A.; Schneider, F.; Anthony, N.; Lin, H.-T. Microplastics in rivers along an urban-rural gradient in an urban agglomeration: Correlation with land use, potential sources and pathways. Environ. Pollut. 2023, 321, 121096. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L. To Build a Septic System an Analysis of EPA’s New Environmental Justice Policies for Rural Communities Lacking Adequate Wastewater Sanitation. Georget. Environ. Law Rev. 2023, 36, 131–164. [Google Scholar]
- Withers, P.J.A.; May, L.; Jarvie, H.P.; Jordan, P.; Doody, D.; Foy, R.H.; Bechmann, M.; Cooksley, S.; Dils, R.; Deal, N. Nutrient emissions to water from septic tank systems in rural catchments: Uncertainties and implications for policy. Environ. Sci. Policy 2012, 24, 71–82. [Google Scholar] [CrossRef]
- Withers, P.J.; Jordan, P.; May, L.; Jarvie, H.P.; Deal, N.E. Do septic tank systems pose a hidden threat to water quality? Front. Ecol. Environ. 2014, 12, 123–130. [Google Scholar] [CrossRef]
- Richards, S.; Paterson, E.; Withers, P.J.A.; Stutter, M. Septic tank discharges as multi-pollutant hotspots in catchments. Sci. Total Environ. 2016, 542, 854–863. [Google Scholar] [CrossRef]


| Site | % Developed | % Forested | % Agricultural | Area (km2) | Mean Specific Conductance (S) | Mean E. coli Levels (CFU/100 mL) |
|---|---|---|---|---|---|---|
| Dry Fork | 4.66 | 89.52 | 3.44 | 129.06 | 0.0996 | 20.9 |
| Big Sandy | 6.3 | 75.28 | 17.26 | 538.323 | 0.1283 | 208.73 |
| Pawpaw | 7.53 | 81.01 | 13.55 | 108.484 | 0.4472 | 526.45 |
| Wheeling | 11.07 | 70.94 | 16.38 | 770.944 | 0.5406 | 43.85 |
| Deckers | 17.13 | 64.75 | 16.49 | 163.487 | 0.2969 | 114.7 |
| Fishing | 4.64 | 91.75 | 2.57 | 565.447 | 0.2026 | 711.25 |
| Location | Species | Mean Count (Items/Individual) | Range (Items/Individual) | Occurrence (%) | Separation | Identification | Source |
|---|---|---|---|---|---|---|---|
| Lake Huron, US | Salvelinus fontinalis | 0.4 | 0–2 | 37 | Pulsed Ultrasonic Extraction | VI, FTIR | Wagner et al., 2019 [38] |
| Lake Ontario, US | Oncorhynchus mykiss | 0.5 | |||||
| Lake Erie, US | Micropterus dolomieu | 0.7 | |||||
| Lijiang River, CN | Cyprinus carpio | 0.4 | 0.3–1 | 81 | 30% H2O2 | VI, μ-FTIR | Zhang et al., 2021 [39] |
| Pelteobagrus fulvidraco | 0.3 | ||||||
| Mystus macropterus | 1 | ||||||
| Pelteobagrus vachelli | 0.4 | ||||||
| Point Marion (Spring 2020), US | Micropterus dolomieu | 60 | 17–170 | 100 | 10% KOH | VI, HN | Zipp, 2022 [22] |
| Micropterus salmoides | 69 | 12–227 | |||||
| Micropterus punctulatus | 27 | 5–102 | |||||
| Morgantown (Spring 2020), US | Micropterus dolomieu | 54 | 16–110 | ||||
| Micropterus salmoides | 37 | 11–75 | |||||
| Micropterus punctulatus | 31 | 7–76 | |||||
| Opekiska (Spring 2020), US | Micropterus dolomieu | 44 | 12–101 | ||||
| Micropterus salmoides | 57 | 22–165 | |||||
| Micropterus punctulatus | 24 | 7–52 | |||||
| Point Marion (Fall 2019), US | Micropterus dolomieu | 58 | 5–281 | ||||
| Micropterus salmoides | 63 | 20–191 | |||||
| Micropterus punctulatus | 44 | 11–153 | |||||
| Nandoni Resevoir, ZA | Micropterus punctulatus | 5.6 | 0–25 | 86.6 | 55% HNO3 | VI, CM | Dalu et al., 2024 [40] |
| Oreochromis mossambicus | 12.3 | 0–39 | |||||
| Coptodon rendalli | 11.4 | 1–68 | |||||
| Micropterus salmoides | 29.3 | 11–40 | |||||
| Micropterus punctulatus | 13 | 5–23 | |||||
| Tilapia sparrmanii | 5.3 | 0–19 | |||||
| Chiloglanis paratus | 0.5 | 0–3 | |||||
| Labeo cylindricus | 25.4 | 2–47 | |||||
| Lake Ontario, CA | Brown bullhead | 93 | 0–1400 | 100 | 20% KOH, density separation, Fe(II)SO4, Fe(II)SO4 heptahydrate, 30% H2O2, density separation with CaCl2, 10% Alcojet detergent solution | VI, RS, μ-FTIR | Milne et al., 2024 [47] |
| White sucker | |||||||
| Micropterus salmoides | |||||||
| Micropterus dolomieu | |||||||
| Ambloplites rupestris | |||||||
| Esox lucius | |||||||
| Basel, DE | Negobius melanstomus | 0 | 0 | 0 | ATL Buffer | VI, FTIR | Bosshart et al., 2020 [43] |
| Thames River, UK | Rutilus rutilus | 0.6 | 0–6 | 33 | None | VI, RS | Horton et al., 2018 [48] |
| Big Sandy downstream, US | Hypentelium nigricans | 116.22 | 27–274 | 100 | 10% KOH | VI, HN | this paper |
| Big Sandy upstream, US | 32 | 25–84 | |||||
| Dry Fork, US | 23.75 | 17–31 | |||||
| Decker’s, US | 23.5 | 17–35 | |||||
| Pawpaw, US | 52.33 | 44–62 | |||||
| West Fork, US | 22.67 | 14–30 | |||||
| Fishing downstream, US | 19.13 | 13–30 | |||||
| Fishing upstream, US | 20.86 | 8–27 | |||||
| Wheeling, US | 17.75 | 13–24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuzzio, I.M.; Murry, B.A.; Arantes, C.C. Widespread Microplastic Pollution in Central Appalachian Streams: Implications for Freshwater Ecosystem Sustainability. Sustainability 2025, 17, 2926. https://doi.org/10.3390/su17072926
Tuzzio IM, Murry BA, Arantes CC. Widespread Microplastic Pollution in Central Appalachian Streams: Implications for Freshwater Ecosystem Sustainability. Sustainability. 2025; 17(7):2926. https://doi.org/10.3390/su17072926
Chicago/Turabian StyleTuzzio, Isabella M., Brent A. Murry, and Caroline C. Arantes. 2025. "Widespread Microplastic Pollution in Central Appalachian Streams: Implications for Freshwater Ecosystem Sustainability" Sustainability 17, no. 7: 2926. https://doi.org/10.3390/su17072926
APA StyleTuzzio, I. M., Murry, B. A., & Arantes, C. C. (2025). Widespread Microplastic Pollution in Central Appalachian Streams: Implications for Freshwater Ecosystem Sustainability. Sustainability, 17(7), 2926. https://doi.org/10.3390/su17072926





