Abstract
The transition toward sustainable conservation practices requires a scientifically ground approach to substituting traditional solvent systems with green alternatives. This study aims to facilitate the adoption of green solvents by restoration professionals by systematically evaluating their chemical compatibility and toxicological safety. By integrating Hansen solubility parameters (HSP), Relative Energy Difference (RED), and the Integrated Toxicity Index (ITI), we identified green solvents with high potential for replacing Cremonesi mixtures. The analysis revealed that ether-based solvents, such as 2,5-dimethyltetrahydrofuran and cyclopentyl methyl ether, exhibit high chemical affinity with Cremonesi mixtures, while esters and fatty acid methyl esters (FAMEs) offer a balanced combination of solubility and low toxicity. However, the study also underscores significant gaps in safety data (SDS) for many innovative solvents, highlighting the need for further toxicological evaluation before widespread implementation.
1. Introduction
1.1. Green Solvent
The cleaning of cultural heritage objects is a fundamental restoration procedure aimed at removing unwanted layers (such as aged varnishes, coatings, overpaints, or vandalism) from the surfaces of artworks. This process must be executed with great care to avoid any alteration to the original materials, including binders, pigments, and supports [1,2,3]. Historically, toxic organic solvents such as acetone and aliphatic hydrocarbons (e.g., ligroin and mineral spirits) have been extensively used due to their effectiveness. However, these solvents pose significant concerns related to their toxicity, volatility, and low biodegradability [4,5].
The increasing awareness of the detrimental effects of traditional solvents on both human health and the environment has intensified efforts to identify sustainable alternatives [6,7]. Conventional solvents contribute to the emission of volatile organic compounds (VOCs), leading to air pollution, water contamination, and health risks such as neurotoxicity and carcinogenicity [8,9].
Guided by the 12 principles of green chemistry outlined by Anastas and Warner [10], the focus has shifted toward the development of eco-friendly solvents that reduce environmental impact and enhance chemical safety [11,12,13]. According to these principles, green solvents should exhibit characteristics such as low vapor pressure, high biodegradability, and safety for both operators and the environment [12,14,15,16]. Prominent examples include fatty acid methyl esters (FAMEs) and ethyl lactate, which have emerged as promising substitutes with lower environmental footprints for various applications [17]. Melchiorre et al. highlighted the possibility of using Solketal (SOLK), γ-valerolactone (GVL), and 2-ethylhexyl pelargonate (ARGO) as solvents to replace acetone, ethanol, and isooctane for the removal of film-forming substances (Dammar, Mastic, Shellac, Paraloid® B72, and linseed oil) [18].
FAMEs, derived from renewable resources like vegetable oils, are biodegradable and produce minimal VOCs emissions, making them suitable replacements for non-polar solvents such as ligroin and white spirit [19,20,21]. Zaratti et al. demonstrated the effectiveness of FAMEs in conservation treatments, offering sustainable solutions for removing waxes and oils from artworks [22]. FAMEs have also been employed in industrial pesticide formulations as carriers and diluents, replacing hazardous volatile organic compounds and demonstrating versatility across sectors [23].
Similarly, ethyl lactate has shown considerable promise in extraction and separation processes, owing to its environmental compatibility and miscibility with water [24].
This property allows for fine-tuning the solvent properties through aqueous solutions, facilitating a range of chemical transformations, including olefin metathesis, carbonyl group reactions, and multicomponent coupling processes [25,26,27,28]. In conservation science, ethyl lactate has proven effective for cleaning varnishes and resins, providing an eco-friendly alternative to harsher solvents.
In addition to FAMEs and ethyl lactate, other green solvents like ethyl fatty acid esters (FAEEs) and 2-methyltetrahydrofuran (2-MeTHF) have gained attention for their efficiency in extracting and purifying bioactive compounds [29,30,31,32,33,34,35]. Osman et al. studied the application of a new green solvent for biodiesel purification via Solvent-Aided Crystallization (SAC) comparing the performance of a new green solvent with conventional solvents in the production of high-purity biodiesel. FAEEs have demonstrated superior extraction efficiency compared to hexane. These solvents are also used in the pharmaceutical industry, where their low toxicity and high biodegradability are critical point [36,37,38,39].
Another significant class of green solvents is deep eutectic solvents (DES), formed by combining hydrogen bond donors and acceptors such as choline chloride and urea [40,41,42]. DES are cost-effective, biodegradable, and have shown excellent performance in extracting phenols, carotenoids, and alkaloids from plant matrices [15,43,44,45,46].
Natural deep eutectic solvents (NADES), a subclass of DES composed of natural components like sugars and organic acids, have been applied in the pharmaceutical and food industries, further promoting sustainability in supply chains. These solvents have also been explored for cleaning delicate painted surfaces in artworks, showing effectiveness in removing stubborn residues without compromising original materials [47,48].
Supercritical carbon dioxide (scCO2) is a green solvent celebrated for its ability to extract thermolabile compounds without leaving harmful residues [49]. It has been used to replace solvents like hexane and methanol in industrial applications, including the extraction of pigments and resins. In pharmaceutical and cosmetic production, scCO2 has significantly reduced reliance on conventional organic solvents while ensuring high product purity [50,51,52].
Ionic liquids (ILs), characterized by low volatility and high thermal stability, have gained prominence in biomass dissolution, catalysis, and separation processes. [53]. They have been used at Tor Vergata University for the restoration of archival and library materials [54]. Several authors report that N,N′-Dialkylimidazolium-based ionic liquids are capable of completely dissolve lignocellulosic biomass and can be considered as promising green solvents for future biorefining and bio-extraction technologies [55,56,57,58,59].
Bio-based solvents such as d-limonene, p-cymene, and ethyl acetate have also demonstrated promise as replacements for petroleum-derived solvents [60]. For instance, d-limonene has been effectively used in lipid extractions and varnish removal. Many substances produced from renewable materials and the fermentation of polysaccharides are used as solvents or as precursors in catalysis due to a wide range of advantageous properties, including comparatively moderate toxicity, high polarity, and solubility in water and other solvents, such as Benzyl Alcohol [61].
Acetals are described by an R2C(OR’)2 structure that is formed by the reversible nucleophilic addition of an alcohol to a ketone or an aldehyde. They can be considered promising sustainable alternatives to many toxic organic solvents [62,63,64,65]. Macchia et al. evaluated acetals, acetal blends, and acetal–ethanol mixtures as possible alternative solvents to identify a green solvent capable of replacing more toxic organic solvents for use in the conservation of cultural heritage [66,67].
Dimethyl carbonate, categorized among the greenest solvents, holds promise as a substitute for traditional ketones, esters, and alcohols [68,69,70,71]. Dimethyl carbonate is classified in the greenest “recommended” bracket according to the solvent selection guide, and can be a potential replacement for methyl ethyl ketone, ethyl acetate, methyl isobutyl ketone, and most other ketones [72,73].
The use of green solvent represents a pivotal step toward achieving sustainable and environmentally responsible chemistry [74,75,76]. Applications in industrial chemistry have also highlighted the versatility of these solvents. For example, dimethyl carbonate has been applied as a replacement for more toxic compounds in coatings and adhesives [73]. Moreover, p-cymene has been explored for its use in olefin metathesis reactions, offering both sustainability and efficiency. NADES have demonstrated their efficacy in the extraction of bioactive compounds for pharmaceuticals and nutraceuticals, supporting their potential in a wide range of industries [40].
Despite their advantages, the adoption of green solvents in restoration treatments faces challenges such as higher initial costs, the need for technological adaptations, and limited accessibility. This is primarily due to the lack of available products from distributors specializing in the sector, resulting from insufficient demand by restorers, and the insufficient transfer of knowledge and technology from academic research to specific applications and, ultimately, to restoration practitioners [76].
1.2. Potential Use of Green Solvents for the Solubilization of Materials in Cultural Heritage
The cleaning of polychromatic artworks in cultural heritage restoration has traditionally relied on empirical practices and the expertise of restorers [77].
Solvent mixtures such as Ligroin-Acetone or Ligroin-Ethanol were commonly employed for removing varnishes and retouchings, adhering to the principle of minimal intervention. Following the principle “like-dissolves-like” rule, now more aptly described as “like seeks like”, these mixtures were chosen for their effectiveness in dissolving materials of similar polarity closed to the different mixtures. Thus, solubility tests based on the Teas chart were pivotal in this process, starting with solvents of minimal polarity and gradually increasing polarity to determine the optimal composition for removing unwanted layers [78,79].
The Teas chart integrates the three Hansen solubility parameters (dispersion forces δd, dipolar interactions δp, and hydrogen bonding δh) into a planar graph. These parameters represent fractions of the total Hildebrand solubility value (δt) and are related through a mathematical relationship (Equation (1)). By plotting the relative contributions of δd, δp, and δh as percentages, the Teas chart provides a systematic framework for assessing solubility behavior, assuming all materials share the same total Hildebrand value. This method, introduced by Nathan Stolow and refined by Robert Feller [80] enabled restorers to inductively determine the polarity of the material to be removed through empirical testing [81,82]. While effective, Feller’s method has limitations, including reliance on potentially hazardous solvents and a lack of comprehensive structure.
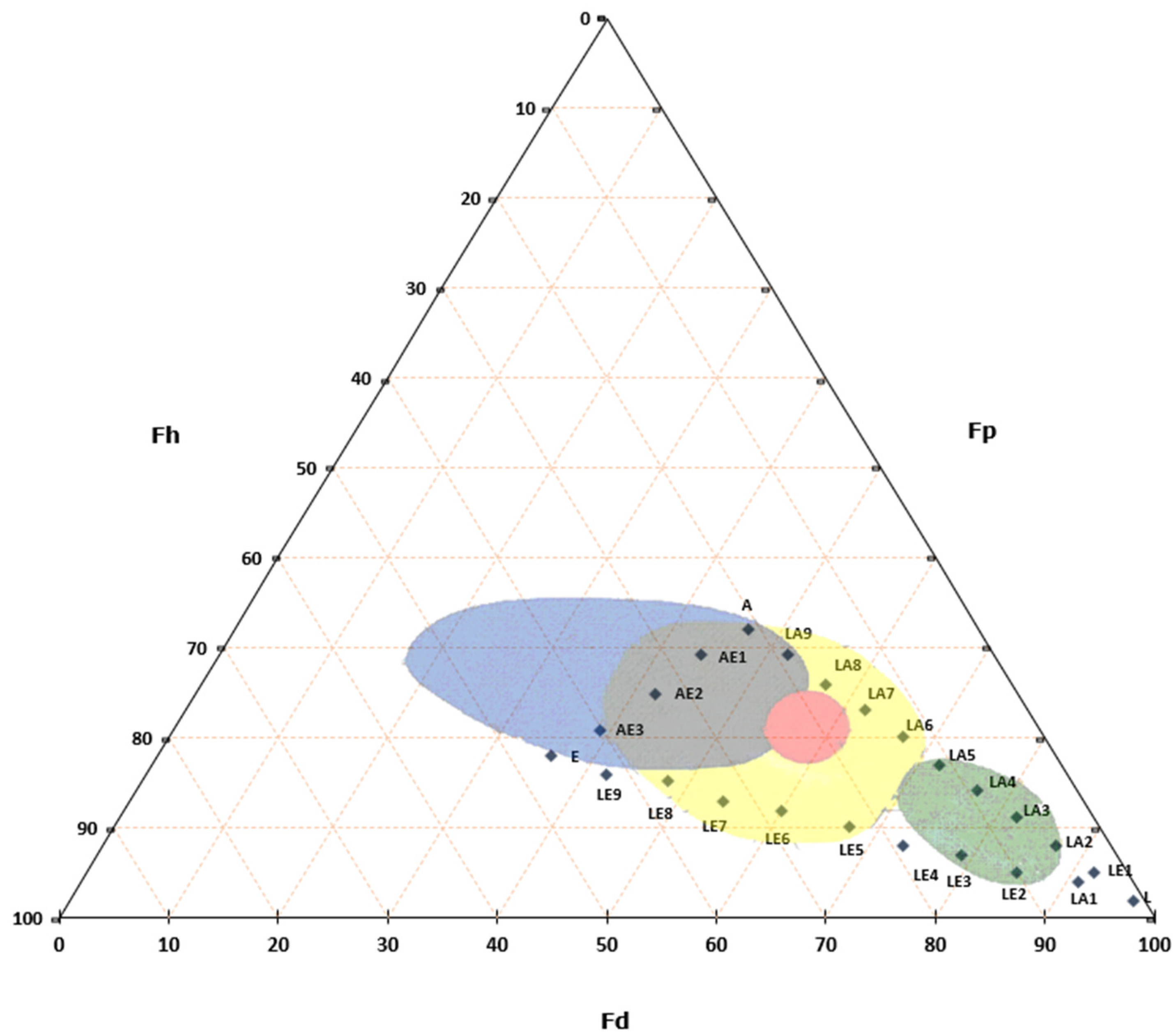
In recent years, safer and less toxic alternatives have emerged. A particularly noteworthy approach in Italy is the method developed by Paolo Cremonesi [83], which uses three primary solvents—ligroin, acetone, and ethanol (Figure 1). Cremonesi’s method not only enhances safety for operators but also reduces environmental toxicity, providing a practical framework that accommodates the wide range of solutes encountered in cultural heritage restoration [84,85,86,87,88].
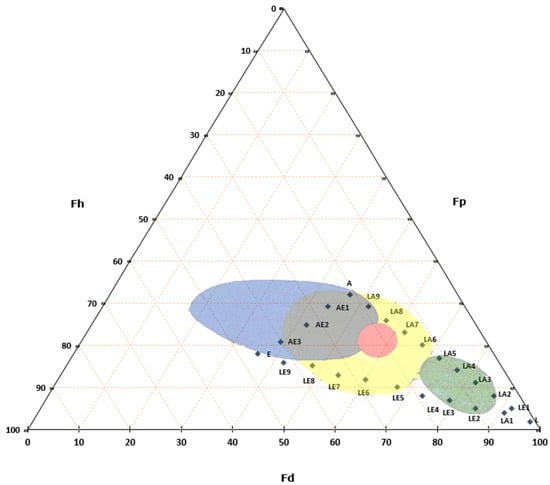
Figure 1.
Teas Triangle with Cremonesi mixtures (L, LA1….AE3) in solubility areas of common substances used in cultural heritage. Yellow represents resins, green represent waxes, red represent dry oils and blue represent proteins and polysaccharides.
This study aims to collect and systematize the solubility, toxicity, and application parameters of green organic solvents available in literature, with the goal of transferring this knowledge to the field of cultural heritage conservation. By analysing the solubility parameters of these solvents, the research seeks to provide practical guidance to restorers on which traditional solvent mixtures can be replaced with green alternatives and which solutes can be dissolved using these new formulations. To facilitate the application of green solvents in the sector, the chemical affinity of these solvents with Cremonesi mixtures was calculated using Hansen solubility parameters (HSP) and the Relative Energy Difference (RED) value. This approach enabled the identification of green solvents that can effectively substitute the traditional mixtures used [89,90]. The assessment of solvent toxicity is crucial for ensuring both environmental and human safety. Traditionally, toxicity evaluations rely on LD50 (lethal dose 50), GHS (Globally Harmonized System) classifications, and precautionary statements (P-statements), as commonly reported in Safety Data Sheets (SDS). To address this issue, several methodologies have been developed to refine chemical classification and risk assessment [91,92].
In this study, an integrated approaches like the Integrated Toxicity Index (ITI), was used to combine multiple data sources to enhance risk assessment. To ensure the reliability and consistency of the data collected from literature and technical data sheets, an artificial intelligence-based approach was employed. AI was used exclusively as a support system to validate the consistency and accuracy of the collected data, leveraging advanced validation frameworks that ensure dependability and reduce human error [93,94,95,96,97].
2. Materials and Methods
2.1. Green Solvents Dataset
The dataset for this study was compiled from a combination of scientific literature, technical data sheets (SDS), and online resources. It includes key parameters of green solvents such as CAS Number, Chemical Formula, Family, Potentially Replaced Solvent, Common Solute or Application. The assignment of each solvent to a chemical family (e.g., Alcohols, Acetals, Ionic Liquids) was based on its chemical structure and functional groups. For example, alcohol solvents were categorized by the presence of a hydroxyl (-OH) group, acetals by two -OR groups attached to a single carbon, and ionic liquids by the typical presence of an organic cation and an inorganic/organic anion. The selection of families was also guided by classifications found in the scientific literature. Additionally, solutes dissolved by these solvents and their potential applications were identified based on literature sources. The physicochemical properties of the solvents were collected from SDS available on product websites such as Merck and Sigma Aldrich (uploaded until November 2024). Only data consistently reported across multiple SDS were included, and these values were further validated using the ECHA (European Chemicals Agency) database using AI. This approach was chosen to align with real-world practices of conservators, who typically rely on SDS provided by suppliers when purchasing solvents and are less likely to independently verify data through the ECHA website [98,99,100]. The Hansen parameters were obtained and calculated using the HSPiP software (HSPiP, version 6.1.02) developed by Professor Steven Abbott. The Teas parameters were obtained through the following equations:
- Fractional parameters developed by Teas in 1968 [101]:
- fd = δd/(δd + δp + δh);
- fp = δp/(δd + δp + δh);
- fh = δh/(δd + δp + δh).
- Final relationship between fractional parameters:fd + fp + fh = 1
2.2. Toxicity Parameters
The Integrated Toxicity Index (ITI) was developed as a quantitative and comparative measure to assess the toxicity of green solvents by integrating multiple toxicological parameters into a standardized metric. The primary objective of the ITI is to provide a consistent and reproducible ranking of solvent hazards, facilitating the selection of safer alternatives for both environmental and occupational health applications. The ITI was based on a weighted scoring system that incorporates acute and chronic toxicological data, regulatory classifications, and safety information. The core toxicological parameters integrated into the ITI framework include:
- GHS Hazard Statements (H-statements): Indicators of both acute and chronic toxicity risks, weighted according to severity.
- Precautionary Statements (P-statements): Recommendations for handling, exposure control, and risk mitigation.
- LD50 Data (if available): The lethal dose required to kill 50% of a test population, used as a standard measure of acute toxicity.
- Derived No-Effect Levels (DNELs) and Occupational Exposure Limits (OELs): Regulatory benchmarks for safe exposure limits, used to validate and adjust hazard severity scores when available.
To ensure a balanced and objective classification of solvent toxicity, weighting factors have been standardized based on their relative impact on human health and environmental safety. The revised weighting structure was developed in alignment with methodologies established in the studies of Pilon et al. and Prat et al. [102,103].
2.2.1. Hazard Statement Weights (H-Values)
H-statements provide critical information regarding the toxicological risks of a solvent based on the following criteria:
- Quantitative Toxicity Measures. LD50 values serve as reference points for adjusting acute toxicity weights, ensuring consistency with traditional toxicity thresholds.
- GHS Classification and Toxicity Thresholds. Higher weights are assigned to substances with severe acute toxicity (Category 1: H300, H310, H330) and those causing significant long-term health effects (e.g., organ-specific damage H370, H372). Lower weights are applied to substances with moderate or localized toxicity, such as mild skin irritants (H315).
- Toxicological Benchmarks and Health Risks. Weights for systemic toxicity (STOT), reproductive toxicity, and carcinogenicity were determined by the literature. Carcinogens classified as Category 1A or 1B (H350, H340) are assigned the highest weight (10), reflecting their severe long-term health impacts.
The standardized weighting system for H-statements is presented in the following Table 1:

Table 1.
Weight used in the article to define Hazard Statement Weights (H-values).
2.2.2. Precautionary Statement Weights (p-Values)
Precautionary statements (P-statements) weight in the ITI was based on:
- Regulatory Guidelines and Hazard Control Standards.
- Criticality of Exposure Prevention. Higher weights are assigned to P-statements that require immediate medical attention or the use of personal protective equipment (PPE).
- Impact on Safety and Exposure Control. P-statements associated with life-threatening exposure risks receive higher weights, whereas handling and disposal precautions receive lower weights.
The assigned weights for P-statements are as follows (Table 2):

Table 2.
Weight used in the article to define precautionary Statement Weights (p-values).
2.2.3. Uncertainty Adjustment
To prevent underestimation of toxicity due to missing data, an uncertainty factor (Uf) is applied. This adjustment accounts for incomplete toxicological information and ensures a conservative toxicity estimation.
The following uncertainty factors are applied:
- Uf = 1.0 → When full toxicological data is available.
- Uf = 1.5 → When LD50 values are missing, requiring estimation from structurally similar substances.
- Uf = 2.0 → When only partial H-statement or P-statement data is available, necessitating conservative extrapolation.
2.2.4. Final ITI Calculation
The ITI score is calculated using the following formula:
where:
ITI = (Σ (Hi × WHi) + Σ (Pj × WPj)) × Uf
- Hi = Individual hazard statement
- Pj = Precautionary statement
- WHi, WPj = Adjusted weights based on DNELs/OELs
- Uf = Uncertainty factor
Due to the limited availability of DNELs and OELs for many green solvents, weight factors (WHi, WPj) were set to 1 in the current application of the ITI. The index was applied to a selection of well-characterized solvents, including water, acetone, ethanol, ligroin, toluene, xylene, and methanol. Additionally, the index was compared with the toxicity assessment system proposed by Ito and Yamamoto [91] to predict acute oral toxicity using HSP parameters. In this study, evaporation rate was not considered in the toxicity assessment, as many of these green solvents have to be use, in conservation treatments, in gel formulations, where volatility is significantly reduced to limit the penetration of the solvent inside the artwork’s support [104,105,106].
2.3. Cremonesi Re-Style with Hansen Style
The Cremonesi mixtures (Table S1), widely used in the conservation of cultural heritage, were define on a geometric approach utilizing the Teas diagram. While this method has demonstrated practical value, the growing need for a deeper understanding of chemical interactions has led to the integration in Hansen space. The analysis began with the characterization of the three primary solvents in the Cremonesi method through their Hansen parameters by HSPiP software: ligroin (δD = 16.0, δP = 0.0, δH = 0.0), acetone (δD = 15.5, δP = 10.4, δH = 7.0), and ethanol (δD = 15.8, δP = 8.8, δH = 19.4) with a standard deviation < 2.5%. To analyse the distribution and effectiveness of the existing Cremonesi mixtures and identified areas for potential improvement, a global solubility sphere in Hansen space was created, encompassing the complete range of solubility parameters accessible through combinations of the three primary solvents. The center of the Hansen Solubility Sphere was calculated as the arithmetic mean of the Hansen coordinates of the three solvents, while the radius of the sphere was determined as half the maximum distance between the three solvents in Hansen space. This approach ensures that the sphere encompasses all three Hansen points, representing the minimum compatibility region containing them. The optimization of the Cremonesi method was based on calculating Relative Energy Difference (RED). Each mixture was treated as an independent solvent entity, with its area of influence defined by its Hansen parameters. For each mixture (e.g., LA1, LE3, AE2, etc.), a “local sphere” was generated to describe its expected interactions. The radius of each sphere was calculated considering the intrinsic variability of the Hansen parameters of the solvents that compose the mixture. This radius represents the mixture’s capacity to dissolve solutes within a neighbouring area of Hansen space.
For instance, the radius for the mixture LE3 (70% ligroin, 30% ethanol) was determined by incorporating the influence of hydrogen bonding (δH) and dispersion forces (δD) specific to ligroin and ethanol. This “local sphere” approach allows for the mapping of the solubility regions of each mixture, verifying their ability to represent the system of interest. Recent studies in solvent selection methodology have demonstrated that a RED value greater than 0.9 between adjacent mixtures provides optimal coverage while maintaining minimal necessary overlap [107,108]. Based on this criterion, a set of 13 mixtures was developed. The compositions were selected to maintain RED values < 0.9 between adjacent mixtures while ensuring continuous coverage of the solubility space. The Hansen parameters (δD, δP, δH) were calculated for each mixture using weighted averages of the pure solvent parameters [109].
Finally, a critical aspect emerged from the analysis concerning the evaporation dynamics of the mixtures. The three solvents exhibit significantly different relative evaporation rates (RER): acetone (RER = 560), ligroin (RER ≈ 390), and ethanol (RER = 150). This disparity causes substantial composition changes during application. For example, in an equal-proportion mixture (33% each), the rapid evaporation of acetone leads to a dramatic shift in composition over time.
To address this issue, new mixtures were proposed where acetone was replaced with methyl ethyl ketone (MEK). MEK has an evaporation rate closer to that of ligroin, ensuring greater stability in the mixture composition during application.
2.4. Replacing Traditional Solvents in Restoration Applications
To evaluate the potential substitution of Cremonesi mixtures by green solvents, we adopted an approach based on chemical compatibility and toxicological safety. The selection of replacement solvents was carried out by calculating the RED value, which measures the energy distance between a solvent and a Cremonesi mixture, and the Integrated Toxicity Index (ITI), which provides an indication of the solvent’s toxicity.
To visualize the results HSPiP software was used and RED matrix was created, using a customized colour scale. The RED value was represented with a colour gradient ranging from dark green (high compatibility, low RED) to red and purple (low compatibility, high RED).
Subsequently, solvents with RED < 0.5 were correlated with their Integrated Toxicity Index (ITI) to identify the most suitable substitutes for Cremonesi mixtures. This approach allowed for the selection of alternatives that not only exhibit optimal chemical compatibility, as defined by Hansen solubility parameters, but also ensure lower toxicity, making them safer for both operators and the environment.
2.5. AI Validation Section
To ensure the reliability and consistency of the data collected from literature and technical data sheets, this study employed artificial intelligence (AI) frameworks for comprehensive data validation. The AI methodologies used included anomaly detection, logical consistency checks, and imputation of missing values.
For instance, an Isolation Forest model was applied to detect anomalies in numerical parameters such as TWA (mg/m3) and toxicity metrics (LD50 Oral, LD50 Dermal). This process identified solvents with unexpected deviations, flagging them for further review. Logical consistency checks were conducted to validate the Hansen solubility parameters (δD, δP, δH, and δT). The computed total solubility parameter (δT) was compared against the provided values, and any inconsistencies were corrected by replacing erroneous values with recalculated ones.
Additionally, toxicity data were scrutinized for logical relationships. For example, cases where LD50 Dermal values were unexpectedly lower than LD50 Oral—a deviation from typical toxicity profiles—were identified, and the data was rectified to ensure accuracy.
3. Results
3.1. Green Solvents Dataset
The collection of green solvents from the literature enabled the identification of a wide range of compounds, categorized into chemical families. Each solvent was characterized by its ability to replace traditional solvents and its specific applications for solutes. Table 3 reports the main properties and applications of green solvents, offering an overview of their solvent power. Acetals, such as 1,3-dioxolane and WAX RESCUE (commercial product by Lab4green s.r.l, Rome, Italy), stand out for their ability to replace acetone and white spirits. Based on literature, these solvents demonstrate high efficiency in removing acrylic resins, crystalline waxes, and low molecular weight synthetic resin, as Regarlez varnish, showcasing their versatility and compatibility with the principles of sustainable chemistry. Alcohols, represented by ethanol, isopropanol, and benzyl alcohol, are used for dissolving aged varnishes, alcohol-soluble compounds, and lipophilic materials. Esters, such as butyl and ethyl lactate, emerge as viable alternatives to petroleum-based solvents, showing high efficacy in dissolving non-polar compounds, including oil-based coatings. For instance, 2-ethylhexyl pelargonate proves particularly effective for applications involving oil-based coatings, contributing to the replacement of hydrocarbon-derived solvents. Ionic liquids, including 1-Butyl-3-Methylimidazolium Chloride and 1-Ethyl-3-methylimidazolium acetate, provide sustainable solutions for essential oil extraction and methanol-ethanol mixtures. These solvents are distinguished by their high chemical stability and polarity, making them ideal substitutes for traditional polar-protic and aprotic solvents. Methyl fatty acid esters (FAME), such as Methyl Cocoate and Methyl Laurate, are applied in the removal of microcrystalline waxes and non-polar varnishes, demonstrating excellent compatibility with lipophilic materials.

Table 3.
Green solvents from the literature.
These compounds offer an effective and biodegradable alternative to petroleum-derived solvents like toluene and white spirits. Lastly, deep eutectic solvents (DES), such as Choline Chloride and its combinations with lactic acid or urea, exhibit remarkable versatility in the removal of sulfides, biomolecules, and varnishes. The CAS numbers and chemical formulas for each solvent were verified against authoritative chemical databases, such as PubChem and ChemSpider, by AI. For example, the CAS number 646-06-0 was confirmed as 1,3-dioxolane. Similarly, the chemical formula C7H8O was validated for Benzyl Alcohol, aligning it with its classification as an alcohol. Furthermore, the replaced solvents were evaluated for their plausibility based on the green solvent’s polarity, hydrogen bonding capacity, and miscibility with target solutes. For example, Acetone, a commonly used polar aprotic solvent, was frequently listed as a replaced solvent for green alternatives such as Ethanol and Ethyl Lactate.
The Table S2 (available in Supplementary Materials) reported the physical-chemical properties of green solvents highlights significant gaps in the available data in SDS, particularly regarding parameters such as surface tension, vapor pressure, boiling point, and melting point. This issue is especially evident for innovative solvents. A notable example is 1,3-dioxolane, solvent already widely used in industrial applications. Several SDS provide detailed information for this solvent, such as molecular weight (74.08 g/mol), melting point (−95 °C), boiling point (75–76 °C), density (1.06 g/cm3), vapor pressure (93 hPa at 20 °C), and surface tension (71.7 mN/m at 20 °C). For other solvents, such as fatty acid methyl esters (FAME) and those derived from bio-based materials, key parameters are often missing or only partially reported. Incomplete or inconsistent reporting of key physical-chemical parameters, such as vapor pressure, surface tension, and solubility, prevents a thorough verification of the solvent properties.
The Hansen parameters analysis (Table 4) revealed distinct patterns in solute compatibility across different green solvent families, correlating with variations in dispersion forces (δD), polarity (δP), and hydrogen bonding capacity (δH):

Table 4.
Hansen parameters of green solvents. SD max < 2.5%.
- Ionic Liquids demonstrated superior compatibility with polar mixtures, particularly water-ethanol systems and essential oils. This can be attributed to their relatively high δP and δH values, which enhance their ability to solubilize highly polar and hydrogen-bonding solutes.
- FAME-family solvents exhibited remarkable versatility in handling hydrophobic compounds, such as fatty acids and plant oils. This behavior is consistent with their high δD and low δP/δH values, indicating a dominance of dispersion interactions and limited polar or hydrogen bonding capabilities.
- Deep Eutectic Solvent (DES) systems exhibit a strong solvation power, which is linked to moderate δD, high δP, and exceptionally high δH values, which enable them to engage in extensive hydrogen bonding.
3.2. Toxicity Parameters of Green Solvents
The toxicological evaluation of green solvents (Table S3, available in the Supplementary Materials) highlights key differences in their safety profiles, which are crucial for determining their suitability in various applications.
For instance, solvents such as methanol and methyl isocyanate exhibit high acute toxicity, with oral LD50 values of 100.1 mg/kg and 100 mg/kg, respectively, indicating significant health risks if not handled properly. While methanol has a relatively high LD50, it is known to cause neurotoxicity and blindness even at lower doses, making it hazardous despite its moderate acute toxicity. In contrast, substances like dimethyl sulfoxide and methyl soyate report much lower toxicity concerns, with oral LD50 values of 28,300 mg/kg and >2000 mg/kg, respectively. However, toxicity assessments must also consider long-term effects, regulatory classifications, and bioaccumulation risks, which are not fully captured by LD50 values alone.
Regarding dermal toxicity, many solvents have LD50 values above 2000 mg/kg, suggesting moderate acute dermal safety. However, compounds like methyl isocyanate, with a dermal LD50 of approximately 300 mg/kg, require enhanced precautions due to their high absorption potential and systemic toxicity. Additionally, dermal exposure risks depend not only on LD50 values but also on skin penetration rates and irritation potential, which should be further investigated for an accurate safety assessment.
The classification under the Globally Harmonized System GHS further reveals widespread risks with several solvents labelled as flammable and irritants These hazards are symbolized by GHS02 for flammability GHS07 for skin and eye irritation and GHS08 for systemic toxicity. Notably solvent such as ethyl lactate and 2-methyloxolane are both flammable and irritating while others like methyl decanoate also bear environmental hazard labels under GHS09 indicating potential ecological impacts. Precautionary statements such as P210 emphasizing the importance of avoiding heat sources and P280 recommending the use of protective gear are commonly associated with these solvents which underscores the necessity of proper handling protocols. Additionally, some solvents like glycerol anhydrous and glycerol carbonate present relatively safe profiles with high LD50 values and no significant hazard symbols making them promising candidates for safer and more sustainable applications. However critical attention must be given to highly toxic and hazardous substances such as methyl isocyanate and methanol which demand stringent compliance with safety measures to mitigate risks and prevent adverse outcomes. In this analysis of green solvents, some Safety Data Sheets (SDS) do not provide complete information regarding critical toxicological parameters such as oral and dermal LD50. This lack of data is evident for solvents such as butyl lactate, glycerol formal, glycerol triethyl ether, isosorbide dimethyl ether, levoglucosenone, methyl ricinoleate, and tetrahydropyran just to say a few.
The absence of such information in SDS may result from several factors. In some cases, toxicological data might not be available due to the recent introduction of the solvent into the market or a lack of extensive studies. In other cases, it could involve proprietary mixtures or products with complex compositions, making it challenging to determine specific toxicological parameters. It is important to note that, according to Regulation (EC) No. 1907/2006 (REACH), suppliers are required to provide updated and complete SDS to ensure the safe use of chemical substances. However, the availability and completeness of information may vary depending on the solvent and the level of existing research.
The final ITI score for each solvent is reported in Table 5. As expected, water does not exhibit toxic effects and is classified as non-toxic under the ITI framework. Acetone and Ethanol demonstrate low acute toxicity, primarily due to their irritation effects rather than systemic toxicity. Their LD50 values exceed 5000 mg/kg, further confirming their low health risk. Ligroin and Xylene are classified as toxic (highly toxic and extremely toxic respectively), given their organ-specific toxicity (STOT), potential aspiration hazards, and systemic effects upon prolonged exposure. Toluene is associated with chronic exposure risks, including central nervous system (CNS) depression and developmental toxicity, resulting in a high ITI score. Methanol exhibits high systemic toxicity, affecting the nervous system, optic nerve, and metabolic pathways, leading to its classification as toxic.

Table 5.
ITI Scores for Reference Solvents.
The ITI scores obtained for reference solvents closely align with established toxicological classifications, reinforcing the reliability of the ITI methodology in distinguishing between low-risk and high-risk solvents. To validate the ITI approach, we compared the ITI and HSP methods across reference solvents. Table 6 reports the toxicity classifications predicted by both methodologies. Both ITI and HSP correctly classify water as non-toxic, and ligroin, toluene, and xylene as toxic solvents. However, notable discrepancies arise in the classification of methanol, which the ITI correctly identifies as extremely toxic, while the HSP model erroneously labels it as non-toxic, likely due to its reliance on solubility interactions rather than systemic toxicological effects. Additionally, the ITI classifies acetone and ethanol as harmful solvents, acknowledging their potential for irritation and long-term exposure risks, whereas the HSP model falsely categorizes them as low-toxicity. This discrepancy highlights a fundamental limitation of the HSP methodology, which does not account for biological interactions, metabolic effects, or long-term toxicity risks, leading to potential false negatives in hazard assessments.

Table 6.
Comparison of ITI and HSP Toxicity Predictions for Reference Solvents.
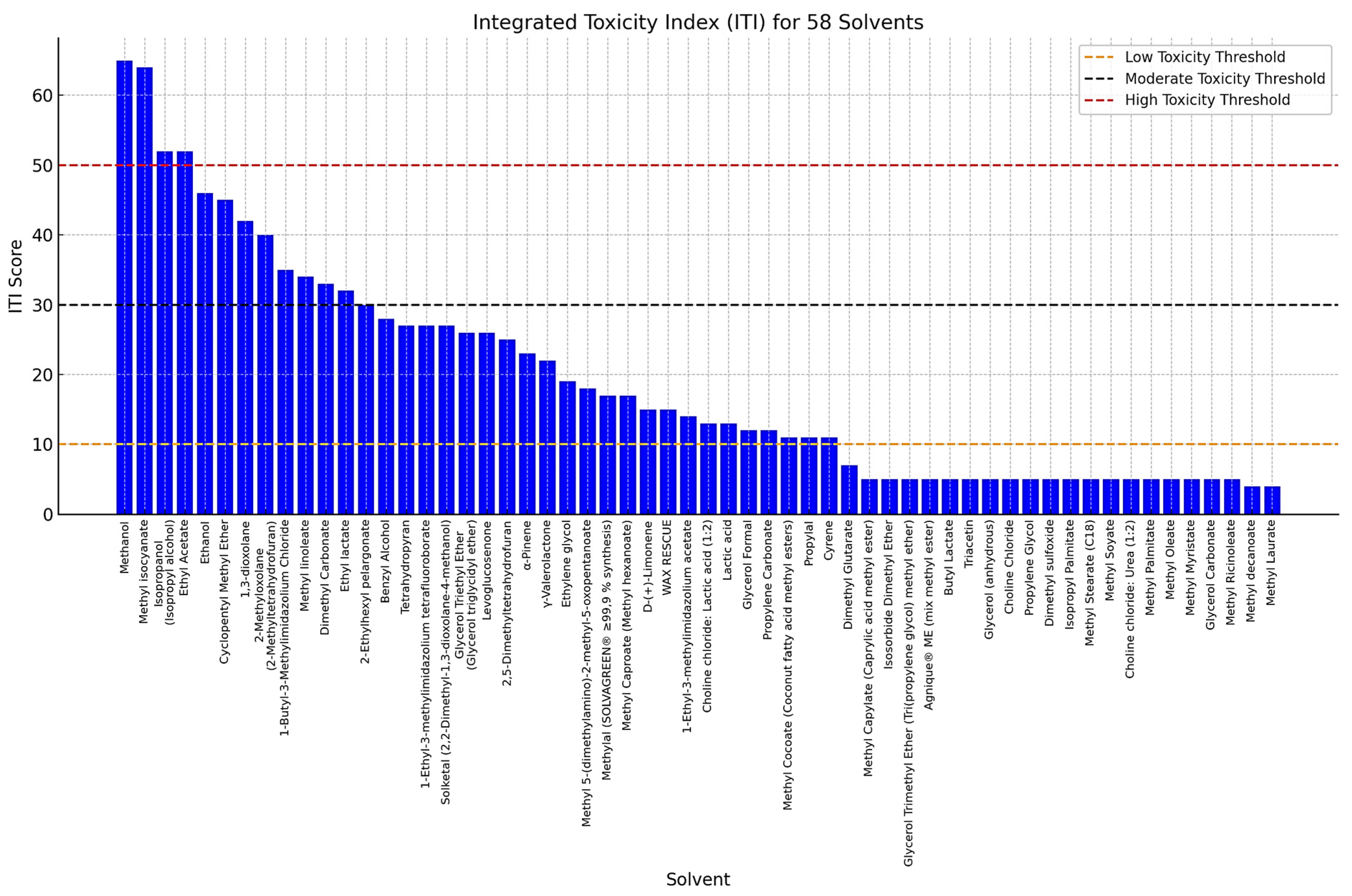
Among the green solvents, several exhibited particularly low ITI scores (Figure 2), suggesting a lower toxicological profile and a safer alternative for common applications. One of the most notable examples is dimethyl carbonate, which demonstrated a low ITI score attributed to fewer H and P statements and medium LD50 values, indicating minimal acute toxicity. Similarly, methyl soyate, a solvent derived from renewable plant-based sources, is widely recognized for its low toxicity and biodegradability, making it a promising replacement for more hazardous petrochemical-based solvents in industrial applications. Another key candidate is glycerol carbonate that maintaining a low systemic toxicity profile. Propylene carbonate follows a similar trend, exhibiting low toxicity. Several solvents in the dataset recorded an ITI score near to zero, primarily due to missing or incomplete toxicological and regulatory information. Additionally, many of these solvents were missing critical toxicological parameters, such as LD50 values for oral or dermal exposure, further limiting the ability to assess their risk using the ITI framework. This highlights the importance of comprehensive toxicological data to ensure a reliable evaluation of solvent safety and sustainability, particularly when considering these solvents as candidates for green chemistry applications comprehensive toxicological data to ensure a reliable evaluation of solvent safety and sustainability, particularly when considering these solvents as candidates for green chemistry applications.
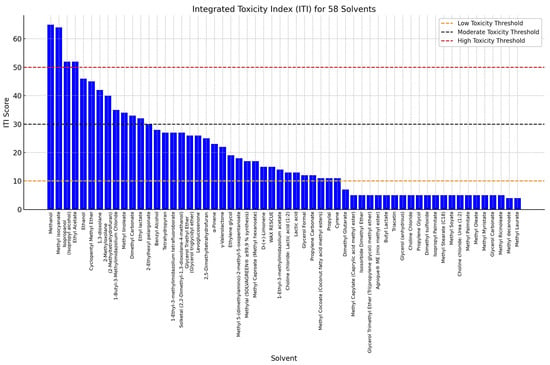
Figure 2.
Integrated toxicity index (ITI) of green solvents.
3.3. Cremonesi Restyle with Hansen Style
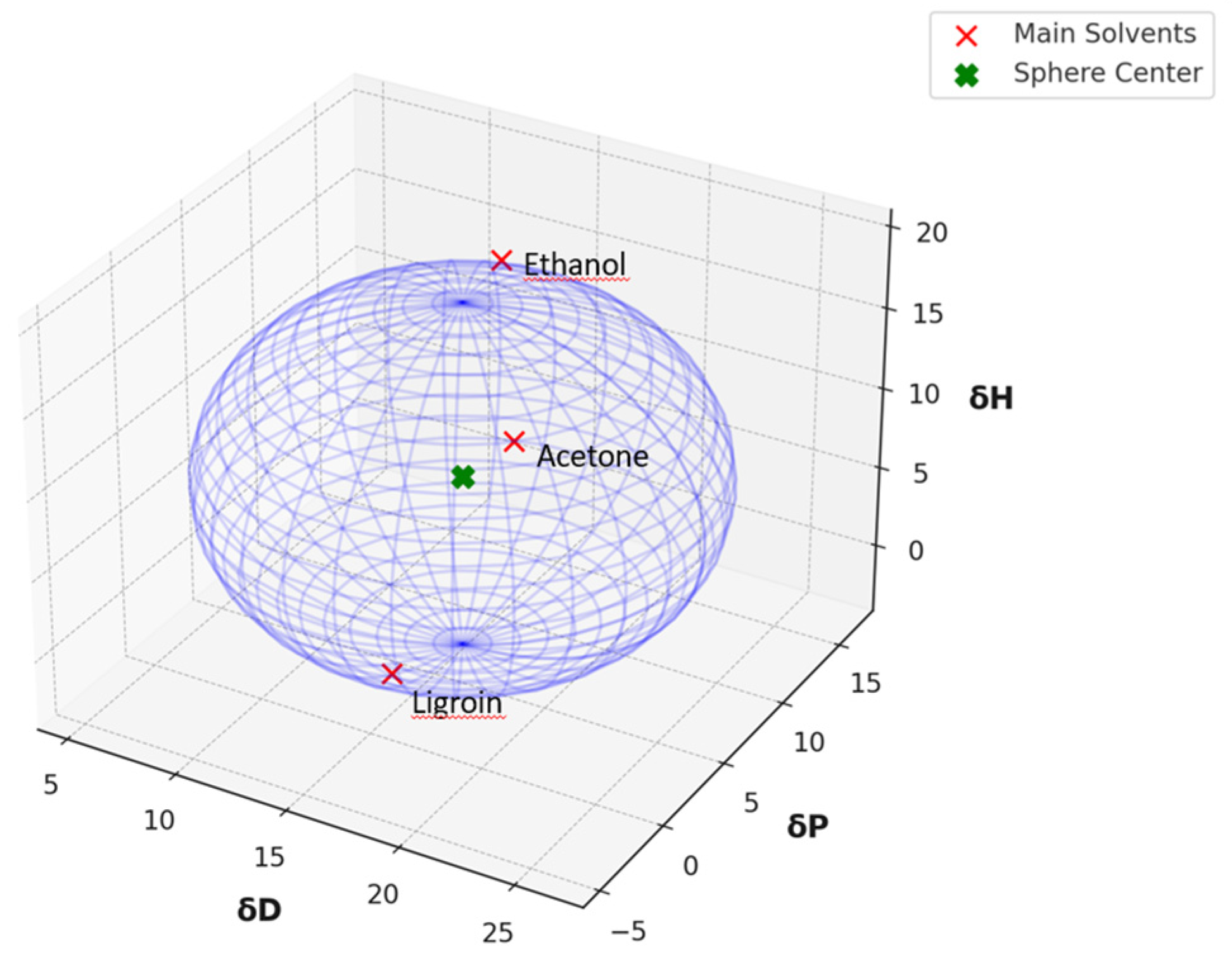
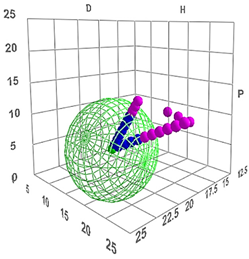
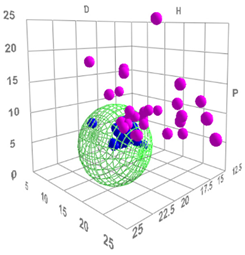
The global Hansen solubility sphere, constructed using the parameters of ligroin, acetone, and ethanol, was characterized by the center δD = 15.75 ± 0.14 MPa½, δP = 5.9 ± 1.2 MPa½, and δH = 8.0 ± 2.7 MPa½), with radius (10.65 ± 1.6 MPa½,) defining a volume that potentially overestimates the actual solubility domain achievable through real mixtures of these solvents. This geometric approach, while mathematically elegant, suggests a solubility space significantly larger than what can be practically achieved through physical mixing of the three solvents. The sphere model thus includes regions in Hansen space that, while theoretically part of the solubility domain, may not be accessible through any practical combination of these three solvents (Figure 3). This overestimation occurs because the spherical model assumes uniform solubility behaviour in all directions from the center point, whereas actual solvent-solute interactions are more complex and often constrained by the thermodynamic limitations of mixing. Therefore, while the sphere model provides a useful first approximation, it should be interpreted with the understanding that the practically achievable solubility range is more restricted than the theoretical sphere suggests.
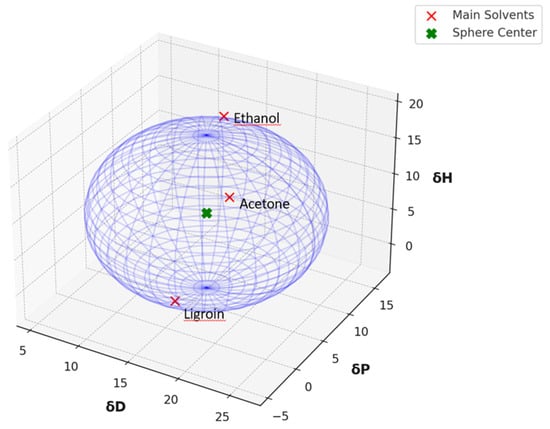
Figure 3.
Hansen solubility sphere, constructed using the parameters of ligroin, acetone, and ethanol.
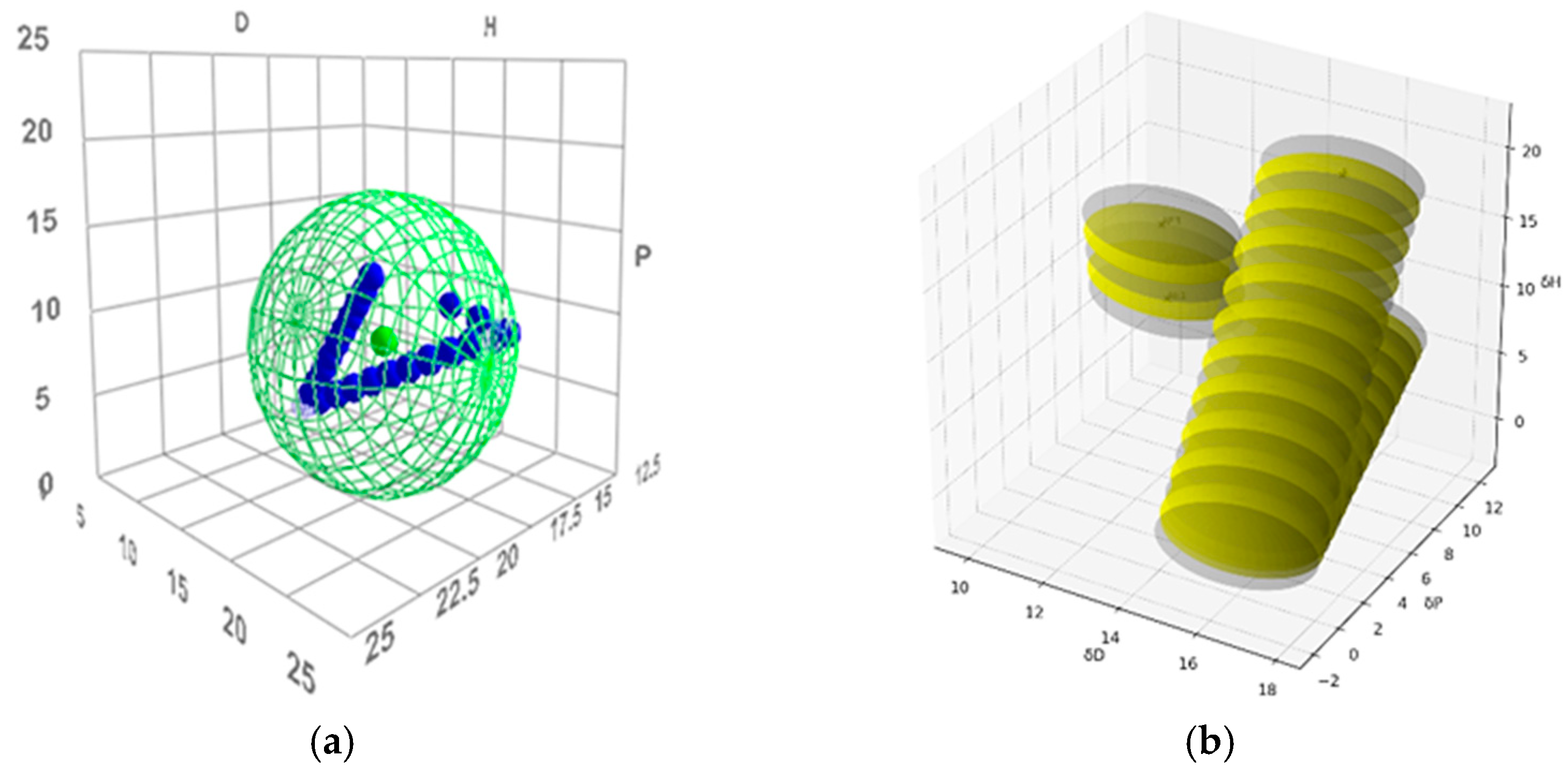
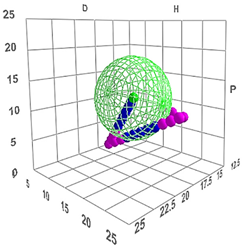
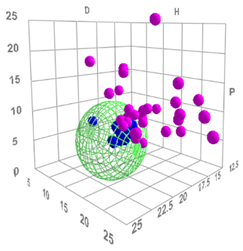
The distribution of Cremonesi mixtures in Hansen space reveals significant overlap among the local solubility spheres. The mixtures demonstrated a high degree of redundancy. This is particularly evident in the LA-series mixtures, where progressive increases in the acetone content result in closely overlapping spheres. Similarly, the LE-series mixtures, characterized by varying ethanol content relative to ligroin, exhibit overlapping solubility domains due to gradual shifts in polarity and hydrogen bonding parameters.
The overlapping regions (Figure 4) indicate areas where multiple mixtures share similar solubility properties. These regions ensure continuity across Hansen space but suggest limited additional solubility coverage provided by certain mixtures. For example, mixtures LA6 and LA7, or LE4 and LE5, display minimal differences in their positions and solubility ranges, reinforcing their redundancy.
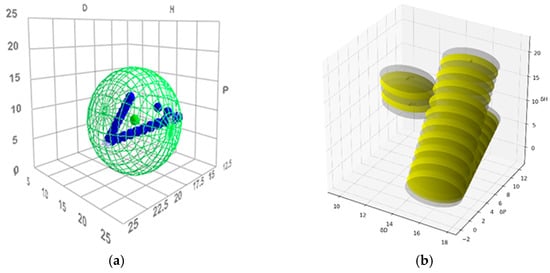
Figure 4.
(a) Cremonesi mixtures in Hansen solubility sphere, constructed using the parameters of ligroin, acetone, and ethanol; (b) The overlapping regions of Cremonesi mixtures, highlighted in yellow.
The positioning of the mixtures in Hansen space reflects their composition and the dominant contributions of the pure solvents as report in Table 7. Mixtures rich in ligroin are situated in regions characterized by high dispersion forces (δD), corresponding to ligroin’s non-polar nature and strong dispersion interactions. Conversely, mixtures with higher ethanol content are in areas with elevated hydrogen bonding values (δH), highlighting ethanol’s significant polar and hydrogen bonding characteristics.

Table 7.
Positioning of the Cremonesi’s mixtures in Hansen space. Left: ligroin; center: Ethanol; right: Acetone.
The analysis of Cremonesi mixtures examined the extent to which their local solubility spheres cover the principal solubility sphere, which represents the full solubility domain defined by the reference solvents (ligroin, acetone, and ethanol). This quantitative assessment evaluates whether the selected mixtures effectively capture the potential solubility range of the pure solvents:
- Volume of the Principal Sphere: 5062.36 units
- Total Volume of Local Spheres: 971.97 units
- Coverage Ratio: 0.192 (19.2%)
The results indicate that the Cremonesi mixtures account for only 19.2% of the total solubility domain, leaving a significant portion of Hansen space uncovered. This suggests that many potential solubility conditions, particularly those outside the local spheres, are not addressed by the existing mixtures. This finding also highlights a methodological limitation of relying exclusively on Teas solubility parameters, which provide qualitative classifications of solubility but lack the ability to quantify overlap or redundancy between mixtures and the reference solvents. In contrast, Hansen parameters offer a more precise, quantitative evaluation, revealing gaps in coverage and opportunities for refinement. To further optimize the system, a RED matrix was calculated to quantify the relative distances and overlaps among the mixtures. This analysis helps identify redundant mixtures, allowing for a more strategic selection of solvent compositions that enhance the representation of the solubility domain while maintaining efficiency.
Using RED (Relative Energy Difference) values to quantify the overlap between neighbouring mixtures, we identified significant redundancies in specific regions of the solubility space (Table S4, available in Supplementary Materials). In the LA-series (Ligroin-Acetone), the progressive increase in acetone content from LA1 to LA9 creates a series of overlapping solubility spheres with RED values < 0.5 between adjacent mixtures. This overlap is particularly pronounced between LA6-LA7 (RED = 0.3) and LA7-LA8 (RED = 0.35), indicating that these consecutive mixtures likely target nearly identical solubility ranges. The incremental 10% changes in acetone content produce smaller shifts in Hansen space than the theoretical resolution of the solubility spheres would suggest. Similarly, the LE-series (Ligroin-Ethanol) demonstrates systematic overlap patterns, but with distinct characteristics due to ethanol’s stronger hydrogen bonding capability. The RED values between consecutive LE mixtures range from 0.4 to 0.6, with the most significant redundancy observed between LE4-LE5 (RED = 0.38) and LE5-LE6 (RED = 0.42). These overlaps correspond to ethanol concentration changes from 40% to 60%, where incremental additions produce diminishing shifts in overall solubility parameters. The overlapping regions, while ensuring solubility continuity across Hansen space, suggest that the number of standard mixtures could be reduced while maintaining effective coverage of the solubility domain
Analysis of the optimized Cremonesi system using the RED > 0.9 criterion revealed significant potential for system streamlining while maintaining effective solubility coverage. The result is reported in Table 8.

Table 8.
Restyled Cremonesi mixture based on RED > 0.9. L = Ligroin, A = Acetone, E = Ethanol. δD, δP, δH = Hansen parameters, Fd, Fp, Fh = Teas parameters.
The optimization process resulted in a new practical set of 13 mixtures that effectively cover the solubility space while maintaining RED values > 0.9 between adjacent compositions. The set includes:
- Five binary mixtures of ligroin-acetone (L100A0, L75A25, L50A50, L25A75, L0A100);
- Four binary mixtures of acetone-ethanol (A75E25, A50E50, A25E75, A0E100);
- Four ternary mixtures (L50A25E25, L25A50E25, L25A25E50, L33A33E33)
This optimized selection reduces the number of standard mixtures from the original Cremonesi combinations to 13, while maintaining comprehensive coverage of the solubility space. The Hansen parameters of these mixtures show systematic variation across the solubility space: δD values range from 15.5 to 16.0 MPa½, δP from 0.0 to 10.4 MPa½, and δH from 0.0 to 19.4 MPa½. The binary mixtures provide systematic coverage along the edges of the solubility space, while the ternary mixtures ensure coverage of the internal volume. This arrangement ensures continuous solubility coverage while eliminating the redundancy observed in the original Cremonesi system.
The RED matrix (Table S5) provides a quantitative measure of the relative distances between the mixtures in the Hansen solubility parameter space. The criterion RED > 0.9 ensures that mixtures are sufficiently distinct, minimizing overlaps while maintaining practical coverage of the Hansen domain. The RED matrix for the optimized set of mixtures is reported in Supplementary Materials.
The substitution of acetone with methyl ethyl ketone (MEK) in the solvent mixtures was performed to reduce the significant compositional shifts caused by the high relative evaporation rate (RER) of acetone compared to ligroin and ethanol.
Table 9 shows the adjusted Cremonesi mixtures, where MEK replaced acetone. The ligroin-MEK binary mixtures (LM1–LM9) display a gradual decrease in Fd, accompanied by increases in Fp and Fh, reflecting the contribution of MEK’s polarity and hydrogen bonding components. Similarly, the ligroin-ethanol binary mixtures (LME1–LME9) and pure ethanol (E) maintain consistent trends in solvent parameter shifts, with Fh becoming dominant as ethanol content increases. The ternary mixtures with MEK and ethanol (ME1–ME3) demonstrate a balanced distribution of Fd, Fp, and Fh, suitable for applications requiring intermediate polarity and hydrogen bonding capabilities.

Table 9.
Adjusted Cremonesi mixtures obtained with the substitution of acetone with methyl ethyl ketone (M). L = Ligroin, E = Ethanol. Fd, Fp, Fh = Teas’ parameters.
Table 10 reports the optimized mixtures, incorporating MEK as a replacement for acetone. The ligroin-MEK-ethanol mixtures (e.g., L50M25E25, L25M50E25) and the equimolar composition (L33M33E33) exhibit stable solvent parameters, minimizing variability during evaporation. The gradual adjustments in δD, δP, and δH align with the intended solvent polarity and cohesion parameter shifts, making these mixtures more robust and reliable for conservation and restoration practice.

Table 10.
Optimized mixtures, incorporating MEK as a replacement for acetone.
3.4. Replacing Traditional Solvents in Restoration Applications
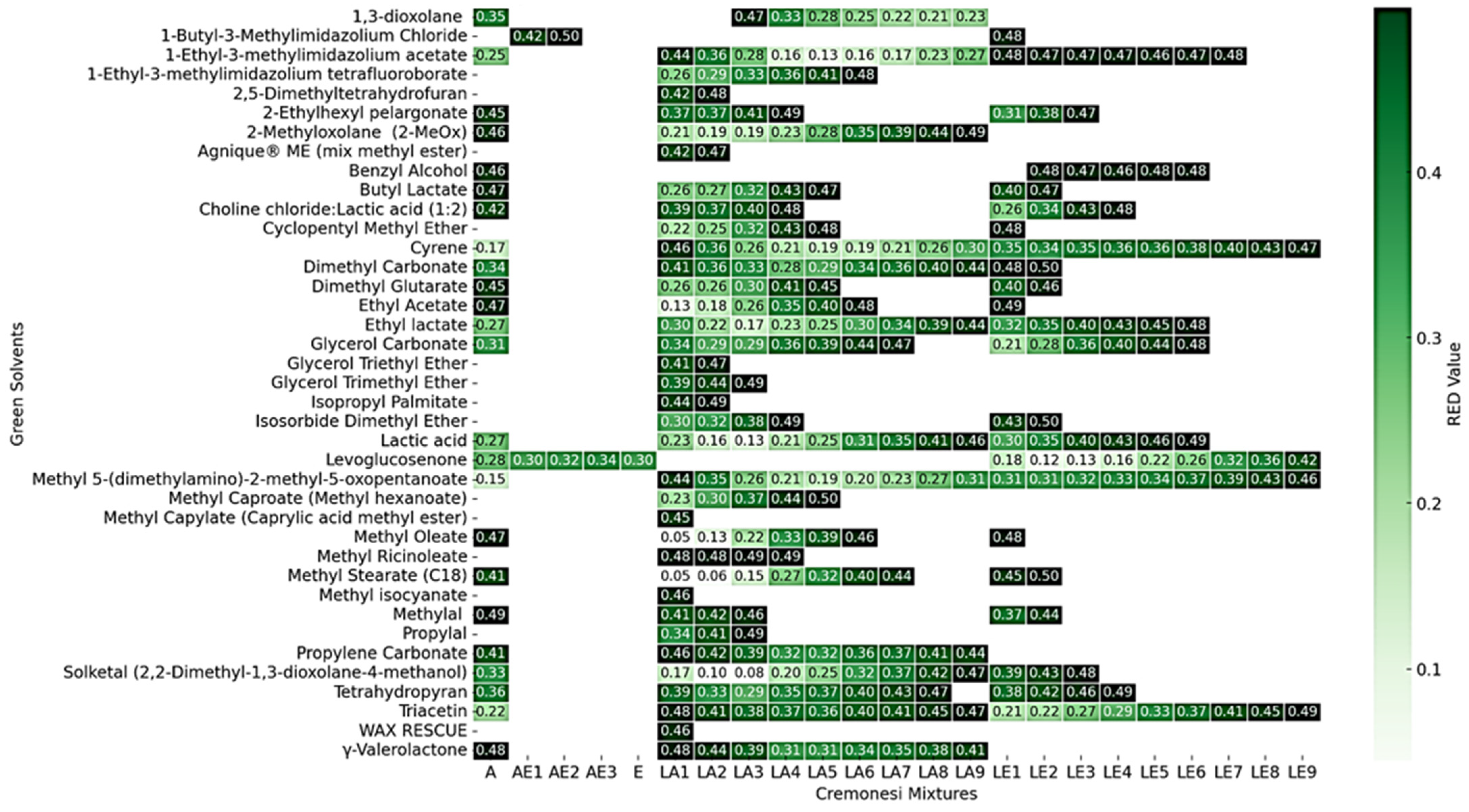
Table 11 reports an example of the compatibility between green solvents and Cremonesi mixtures, highlighting the RED values obtained from the analysis of relative energy differences. The full table (Table S6) can be found in Supplementary Materials.

Table 11.
Compatibility of green solvents with Cremonesi mixtures in Hansen space. The blue spheres indicate solvents with good compatibility, while the pink spheres represent less compatible solvents. The green sphere defines the solubility region (RED < 1), with its center marking the Cremonesi mixture for which the RED value was calculated.
The results obtained are summarized in Figure S1 (available in Supplementary Materials), which provides a comprehensive overview of the chemical compatibility between green solvents and Cremonesi’s formulations. This matrix highlights how certain solvents exhibit RED values below 1, indicating a strong affinity with the reference mixtures and, consequently, their potential as viable substitutes. Conversely, other solvents display significantly higher RED values, suggesting a substantial difference in solubility characteristics, which limits their effectiveness as replacements for traditional solvents.
Among the acetal-based solvents, 1,3-dioxolane, glycerol formal, and Solketal demonstrated moderate to high compatibility with certain Cremonesi mixtures. Solketal, in particular, emerged as a viable alternative due to its balanced solubility characteristics.
Ionic liquids, such as 1-butyl-3-methylimidazolium chloride and 1-ethyl-3-methylimidazolium acetate, displayed a different pattern of compatibility. In contrast, ether-based solvents, including 2,5-dimethyltetrahydrofuran, cyclopentyl methyl ether, and isosorbide dimethyl ether, exhibited some of the best chemical compatibility with Cremonesi mixtures. Their RED values consistently remained below 0.5, making them strong candidates for substitution. 2,5-dimethyltetrahydrofuran, in particular, stood out as an excellent alternative due to its high solubility efficiency.
The ester family, represented by solvents such as ethyl lactate, butyl lactate, and dimethyl glutarate, presented a more varied response. While some esters demonstrated RED values closer to 1, limiting their direct application as substitutes, others, such as ethyl lactate, offered a compelling balance between solubility and safety.
Fatty Acid Methyl Esters (FAMEs), including methyl oleate, methyl laurate, and methyl soyate, provided another promising avenue for solvent substitution. These bio-based solvents, derived from renewable resources, generally exhibited favorable RED values, particularly when mixed with other green solvents. Their compatibility with Cremonesi mixtures was especially notable in applications requiring non-polar solvents, positioning them as sustainable replacements for petroleum-derived solvents like ligroin.
Alcohols, such as ethanol, isopropanol, and benzyl alcohol, demonstrated a more complex relationship with Cremonesi mixtures. While ethanol is part of cremonesi mixtures, isopropanol RED values indicated lower compatibility with certain mixtures. However, when blended with other solvents, they could still contribute to effective formulations. Benzyl alcohol, on the other hand, exhibited better solubility properties and could be considered a viable alternative in some conservation scenarios.
The Deep Eutectic Solvent (DES) family, including choline chloride-based mixtures, showed less favorable RED values, indicating limited compatibility with Cremonesi formulations.
At the end, ethers and esters emerged as particularly strong candidates, with several members displaying excellent RED values. Fatty acid methyl esters also demonstrated great potential, particularly for applications requiring non-polar solvent replacements. In contrast, ionic liquids and DES solvents appeared less chemically compatible, though their advantages in terms of low volatility and environmental impact may justify their use in specific cases.
The Figure 5 reports the potential green solvents to replace the Cremonesi mixtures, based on RED values below 0.5 and the lowest ITI toxicity scores.

Figure 5.
RED Matrix (RED < 0.5) solvent green vs. Cremonesi’s mixture with ITI annotations (Green Scale).
The LA series mixtures (consisting of slight gradients of ligroin and acetone) exhibit strong compatibility with solvents such as 2,5-dimethyltetrahydrofuran and glycerol triethyl ether. Among these, glycerol triethyl ether remains a viable alternative for LA1 and LA2, with a RED value below 0.5. 2,5-dimethyltetrahydrofuran also shows good compatibility for LA1 and LA2, with RED values below 0.5, making it another promising substitute.
For mixtures with higher ethanol concentrations (LE series), glycerol triethyl ether continues to emerge as a suitable solution, particularly effective in the LE1–LE5 mixtures, with a RED below 0.5. However, further evaluation of ITI scores is needed to confirm its classification as the safest option. Mixtures containing high percentages of acetone, such as AE1, AE2, and AE3, require alternative green solvents, but anhydrous glycerol is no longer identified as a compatible substitute in the updated analysis. Additional investigation may be required to determine new low-toxicity options with optimal chemical affinity.
4. Discussion
The results of this study provide a comprehensive evaluation of the feasibility of replacing Cremonesi mixtures with green solvents, emphasizing the importance of considering not only chemical compatibility but also regulatory safety assessments. Unlike previous studies that focused primarily on solvent performance, our approach integrates Hansen Solubility Parameters (HSP), Relative Energy Difference (RED), and the Integrated Toxicity Index (ITI) to offer a multi-criteria assessment that balances solubility effectiveness with health. By combining solubility modeling (HSP, RED) with toxicity evaluations (ITI), we provide a structured method for assessing green solvents based on their ability to replace traditional mixtures used in conservation. However, our findings also highlight critical limitations in toxicity evaluation methodologies and gaps in regulatory data. While 2,5-dimethyltetrahydrofuran and glycerol triethyl ether emerged as promising substitutes due to their low RED values their ITI scores require a careful assessment in terms of risks associated with their use.
A particularly notable case is α-pinene, which was initially identified as a viable alternative based on its low RED value but was later found to be classified by ECHA as highly flammable, toxic to aquatic life, and hazardous to human health. This discrepancy highlights a fundamental limitation of data reported in Safety Data Sheets (SDS), which may not always reflect the most up-to-date regulatory classifications or consider long-term toxicological studies.
Additionally, the study indicates that many proposed “green” solvents lack sufficient toxicological evaluation. Several ethers and esters, despite exhibiting strong chemical compatibility with Cremonesi mixtures, have incomplete safety profiles. This raises concerns about their long-term viability as sustainable alternatives, as future toxicological studies may reveal risks that are currently unaccounted for.
Several solvent families demonstrated favorable properties in terms of toxicity and solubility. Fatty acid methyl esters (FAMEs), for instance, showed moderate RED values but a lower toxicity profile compared to other classes. Methyl oleate, methyl laurate, and methyl soyate, derived from renewable sources, appear to be viable options for replacing non-polar ligroin-based formulations, particularly due to their low volatility and reduced health risks.
Conversely, ionic liquids and deep eutectic solvents (DES) were found to have higher RED values, indicating substantial differences in solubility behavior. While their low volatility and environmental benefits make them attractive alternatives in some contexts, their limited compatibility with Cremonesi mixtures suggests that they may be better suited for specific conservation applications rather than broad replacement strategies. The role of alcohols in the substitution process remains particularly relevant. While ethanol is a key component of many Cremonesi mixtures, alternative alcohols such as benzyl alcohol showed better solubility properties, making them potential candidates for selected restoration treatments. However, their broader applicability remains limited due to variability in solubility depending on the formulation.
While HSP and RED values provide valuable theoretical insights into solvent compatibility, they remain predictive models that require empirical validation. Real-world cleaning scenarios involve complex interactions between solvents and multi-layered surfaces, where factors such as substrate porosity, varnish polymerization, and evaporation rates can significantly affect cleaning performance.
Thus, while this study provides a structured framework to help restorers compare solvents, the practical validation of these findings requires further empirical research. We emphasize the need for controlled experimental studies on aged coatings and historical substrates to verify the predictions made in this study and refine solvent selection guidelines for real-world conservation applications.
One of the key challenges in the transition from traditional solvents to green alternatives is their economic feasibility. While many green solvents, such as FAMEs, DES, and ionic liquids, offer clear environmental and health benefits, their production costs, availability, and market adoption remain significant barriers.
Some bio-based solvents, like methyl oleate and methyl soyate, are derived from renewable agricultural sources, making them more cost-competitive compared to synthetic alternatives. However, other green solvents, such as ionic liquids and deep eutectic solvents (DES), require complex synthesis processes, which increase production costs and limit commercial availability.
Additionally, limited large-scale production and regulatory constraints further challenge the widespread adoption of certain green solvents in conservation. The current low demand for specialized green solvents in the restoration sector means that distributors are not incentivized to supply them at competitive prices. This issue is exacerbated by insufficient knowledge transfer from academic research to restorers, which slows down implementation.
Future research should include a Life Cycle Assessment (LCA) and cost-benefit analysis to evaluate the long-term economic impact of adopting green solvents in conservation. While some bio-based solvents may become more cost-effective as production scales up, targeted incentives and policy support could help bridge the gap between research advancements and practical implementation.
Although the Integrated Toxicity Index (ITI) effectively distinguishes between solvents with varying degrees of toxicity, the relatively small differences between some values highlight the need to apply differentiated weightings for both hazards and precautionary indexes, rather than relying solely on those currently reported in the literature. The lack of toxicological data also underscores how the term “green” can sometimes be misleading, as some solvents currently considered green may later prove to have hidden risks.
Moreover, this study does not include Life Cycle Assessment (LCA), which would provide a clearer definition of what is truly “green”. LCA is an essential tool for evaluating the overall environmental impact of materials and processes used in cultural heritage conservation. Future research should focus on a more holistic sustainability assessment, incorporating not only solubility and toxicity but also economic viability, synthesis impact, and regulatory compliance.
5. Conclusions
This study was designed to facilitate the transition of green solvents into conservation practice, providing restoration professionals with a scientific framework for selecting safer and more sustainable alternatives to Cremonesi mixtures. By integrating Hansen solubility parameters (HSP), Relative Energy Difference (RED), and the Integrated Toxicity Index (ITI), the study offers a comprehensive methodology for assessing chemical compatibility and safety in the conservation field.
The results highlight that several green solvents—particularly ethers, esters, and fatty acid methyl esters (FAMEs)—demonstrate good chemical compatibility with Cremonesi mixtures while also presenting reduced toxicity risks. However, this study also underscores critical gaps in available toxicological data, as many green solvents lack comprehensive safety evaluations, particularly regarding their long-term exposure effects and biodegradability in conservation environments. The absence of standardized toxicological profiles makes it difficult to assess their full impact on health and safety, reinforcing the need for further studies and regulatory updates before widespread adoption in professional conservation settings.
Additionally, this study does not account for Life Cycle Assessment (LCA), a key factor in defining a truly green solvent, nor does it evaluate the interaction between these solvents and artwork materials. Understanding these interactions is essential to ensure that proposed green solvents do not cause unintended damage to cultural heritage objects.
Several of the green solvents reviewed in this study are still under investigation, and their practical applications in restoration require further validation. While their low volatility and tunable properties offer clear advantages, their chemical behavior in real-world conservation scenarios must be further assessed. Looking ahead, this study aims to serve as a foundation for the development of a digital tool that will allow restoration professionals to quickly and effectively evaluate the suitability of green solvents for specific applications. By combining chemical compatibility with safety considerations, such a tool could provide conservators with a practical and accessible resource, enabling a confident transition to greener practices while ensuring the protection of cultural heritage and operator health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17072944/s1, Table S1: Cremonesi mixtures with % of pure solvents and Teas solubility parameters; Table S2: Physicochemical properties most commonly reported in different SDS expressed as mean value: Surface Tension (SD) = 0.5–2 mN/m, Vapor Pressure (SD) = 5%, Boiling Point (SD) = ±1–5 °C, Melting Point (SD) = ±0.5–3 °C, Density (SD) = ±0.001–0.01 g/cm3, and Flash Point (SD) = ±1–5 °C; Table S3: Toxicity Parameters of green solvents; Table S4: Matrix of RED (Relative Energy Difference) values among all Cremonesi’s mixtures. L = Ligroin, A = Acetone, E = Ethanol; Table S5: Matrix RED of the optimized set of Cremonesi’s mixtures; Table S6: Compatibility between green solvents and Cremonesi mixtures in Hansen space. The blue spheres represent solvent with good compatibility while the pink ones represent the solvent less compatible. The green sphere represents the solubility area (RED < 1) and the center of the green sphere the Cremonesi mixture to which the RED was calculated; Figure S1: Red matrix solvent green vs. Cremonesi’s mixture.
Author Contributions
Conceptualization, A.M.; methodology, A.M.; software, A.M. and C.Z.; validation, A.M. and F.V.; formal analysis, A.M. and C.Z.; investigation, A.M.; resources, A.M.; data curation, A.M. and C.Z.; writing—original draft preparation, A.M.; writing—review and editing, A.M., C.Z., I.A.C. and F.V.; visualization, C.Z. and I.A.C.; supervision, A.M. and F.V.; project administration, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available in the article and Supplementary Materials.
Acknowledgments
The authors would like to express their most gratitude to Steven Abbot for his invaluable insight to this research.
Conflicts of Interest
The authors Andrea Macchia and Camilla Zaratti are employed by Lab4green s.r.l. The remaining authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SDS | Safety Data Sheets |
| RED | Relative Energy Difference |
| ITI | Integrated Toxicity Index |
| HSP | Hansen Solubility Parameters |
| FAMEs | Fatty Acids methyl esters |
| ILs | Ionic liquids |
| NADES | Natural deep eutectic solvents |
| FAEEs | Fatty acid ethyl esters |
| SAC | Solvent-aided crystallization |
References
- Ormsby, B.; Keefe, M.; Phenix, A.; von Aderkas, E.; Learner, T.; Tucker, C.; Kozak, C. Mineral Spirits-Based Microemulsions: A Novel Cleaning System for Painted Surfaces. J. Am. Inst. Conserv. 2016, 55, 12–31. [Google Scholar] [CrossRef]
- Casini, A.; Chelazzi, D.; Baglioni, P. Advanced methodologies for the cleaning of works of art. Sci. China Technol. Sci. 2023, 66, 2162–2182. [Google Scholar] [CrossRef]
- Romero-Noguera, J.; Pérez-Villares, N.; Bolívar-Galiano, F.; Bailón-Moreno, R. Response Surface Model Applied to Fine Arts: The Case of the Restoration of Paintings. In Response Surface Methodology—Research Advances and Applications; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Filly, A.; Fabiano-Tixier, A.S.; Fernandez, X.; Chemat, F. Alternative solvents for extraction of food aromas. Experimental and COSMO-RS study. LWT-Food Sci. Technol. 2015, 61, 33–40. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Parikh, S.; Shah, M.; Dharaskar, S. A holistic review on application of green solvents and replacement study for conventional solvents. Biomass Convers. Biorefinery 2022, 12, 1985–1999. [Google Scholar] [CrossRef]
- Nanda, B.; Sailaja, M.; Mohapatra, P.; Pradhan, R.; Nanda, B.B. Green solvents: A suitable alternative for sustainable chemistry. Mater. Today Proc. 2021, 47, 1234–1240. [Google Scholar] [CrossRef]
- Scott, R.S.; Frame, S.R.; Ross, P.E.; Loveless, S.E.; Kennedy, G.L. Inhalation Toxicity of 1,3-Propanediol in the Rat. Inhal. Toxicol. 2005, 17, 487–493. [Google Scholar] [CrossRef]
- Mangotra, A.; Singh, S.K. Volatile organic compounds: A threat to the environment and health hazards to living organisms—A review. J. Biotechnol. 2024, 382, 51–69. [Google Scholar] [CrossRef]
- Warner, J.C.; Cannon, A.S.; Dye, K.M. Green chemistry. Environ. Impact Assess. Rev. 2004, 24, 775–799. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Monteagudo-Arrebola, M.J.; Dobado, J.A.; Isac-García, J. Green and Bio-Based Solvents. Top. Curr. Chem. 2018, 376, 18. [Google Scholar] [CrossRef]
- Mallakpour, S.; Rafiee, Z. Green Solvents Fundamental and Industrial Applications. In Green Solvents I; Springer: Dordrecht, The Netherlands, 2012; pp. 1–66. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, N.; Singh, A.P.; Bhardwaj, P.; Sachan, K.; Singh, S. Mixed Solvency Concept to Replace Harmful Organic Solvent: Recent Trends and Future Challenges in Formulation Development. Comb. Chem. High Throughput Screen. 2025, 28, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Pistikopoulos, E.; Stefanis, S. Optimal solvent design for environmental impact minimization. Comput. Chem. Eng. 1998, 22, 717–733. [Google Scholar] [CrossRef]
- Lakshmipraba, J.; Prabhu, R.N. Deep Eutectic Solvents, Bio-Based Solvents, and Surfactant for Green Sample Pretreatment and Determination. In Green Chemical Analysis and Sample Preparations; Springer International Publishing: Cham, Switzerland, 2022; pp. 353–378. [Google Scholar] [CrossRef]
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Yu, D.; Luo, H.; Li, C. Green Solvents for Lipid Extraction from Microalgae to Produce Biodiesel. Front. Chem. 2022, 10, 884274. [Google Scholar] [CrossRef]
- Melchiorre, C.; Melchiorre, M.; Marra, M.; Rizzo, E.; Fatigati, G.; Rossi, P.; Cerruti, P.; Improta, I.; Amoresano, A.; Marino, G.; et al. Green solvents and restoration: Application of biomass-derived solvents in cleaning procedures. J. Cult. Herit. 2023, 62, 3–12. [Google Scholar] [CrossRef]
- Márquez-Román, V.A.; Mena-Cervantes, V.Y.; Hernández-Altamirano, R.; Pineda-Flores, G.; González-Espinosa, M.A. Fatty Acid Methyl Esters from Jatropha Curcas L. Seeds as a Green Solvent for Remediation of Soil Contaminated with Anthracene. In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions; Springer: Cham, Switzerland, 2024; pp. 205–208. [Google Scholar] [CrossRef]
- Salehpour, S.; Dubé, M.A. Biodiesel: A green polymerization solvent. Green Chem. 2008, 10, 321–326. [Google Scholar] [CrossRef][Green Version]
- Osman, W.N.A.W.; Badrol, N.A.I.; Samsuri, S. Biodiesel Purification by Solvent-Aided Crystallization Using 2-Methyltetrahydrofuran. Molecules 2023, 28, 1512. [Google Scholar] [CrossRef]
- Zaratti, C.; Marinelli, L.; Colasanti, I.A.; Barbaccia, F.I.; Aureli, H.; Prestileo, F.; de Caro, T.; La Russa, M.F.; Macchia, A. Evaluation of Fatty Acid Methyl Esters (FAME) as a Green Alternative to Common Solvents in Conservation Treatments. Appl. Sci. 2024, 14, 1970. [Google Scholar] [CrossRef]
- Purkait, A.; Hazra, D.K. Biodiesel as a carrier for pesticide formulations: A green chemistry approach. Int. J. Pest Manag. 2020, 66, 341–350. [Google Scholar] [CrossRef]
- Planer, S.; Jana, A.; Grela, K. Ethyl Lactate: A Green Solvent for Olefin Metathesis. ChemSusChem 2019, 12, 4655–4661. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Nikles, S.M.; Piao, M.; Lane, A.M.; Nikles, D.E. Ethyl lactate: A green solvent for magnetic tape coating. Green Chem. 2001, 3, 109–113. [Google Scholar] [CrossRef]
- Dolzhenko, A.V. Ethyl lactate and its aqueous solutions as sustainable media for organic synthesis. Sustain. Chem. Pharm. 2020, 18, 100322. [Google Scholar] [CrossRef]
- Abdullah, M.A.; Hussein, H.A.; Alshajrawi, O.M.S. Ethyl lactate as a green solvent in the pharmaceutical industry. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 185–194. [Google Scholar] [CrossRef]
- Hu, J.; Du, Z.; Tang, Z.; Min, E. Study on the Solvent Power of a New Green Solvent: Biodiesel. Ind. Eng. Chem. Res. 2004, 43, 7928–7931. [Google Scholar] [CrossRef]
- Mehdi, H.; Fábos, V.; Tuba, R.; Bodor, A.; Mika, L.T.; Horváth, I.T. Integration of Homogeneous and Heterogeneous Catalytic Processes for a Multi-step Conversion of Biomass: From Sucrose to Levulinic Acid, γ-Valerolactone, 1,4-Pentanediol, 2-Methyl-tetrahydrofuran, and Alkanes. Top. Catal. 2008, 48, 49–54. [Google Scholar] [CrossRef]
- Veith, C.; Diot-Néant, F.; Miller, S.A.; Allais, F. Synthesis and polymerization of bio-based acrylates: A review. Polym. Chem. 2020, 11, 7452–7470. [Google Scholar] [CrossRef]
- Smoleń, M.; Kędziorek, M.; Grela, K. 2-Methyltetrahydrofuran: Sustainable solvent for ruthenium-catalyzed olefin metathesis. Catal. Commun. 2014, 44, 80–84. [Google Scholar] [CrossRef]
- Slater, C.S.; Savelski, M.J.; Hitchcock, D.; Cavanagh, E.J. Environmental analysis of the life cycle emissions of 2-methyl tetrahydrofuran solvent manufactured from renewable resources. J. Environ. Sci. Health Part A 2016, 51, 487–494. [Google Scholar] [CrossRef]
- Pace, V.; Hoyos, P.; Castoldi, L.; de María, P.D.; Alcántara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef]
- Rapinel, V.; Claux, O.; Abert-Vian, M.; McAlinden, C.; Bartier, M.; Patouillard, N.; Jacques, L.; Chemat, F. 2-Methyloxolane (2-MeOx) as Sustainable Lipophilic Solvent to Substitute Hexane for Green Extraction of Natural Products. Properties, Applications, and Perspectives. Molecules 2020, 25, 3417. [Google Scholar] [CrossRef]
- Zelner, I.; Matlow, J.N.; Natekar, A.; Koren, G. Synthesis of fatty acid ethyl esters in mammalian tissues after ethanol exposure: A systematic review of the literature. Drug Metab. Rev. 2013, 45, 277–299. [Google Scholar] [CrossRef]
- Winterton, N. The green solvent: A critical perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef]
- Bhatia, S.C. Biofuels: A review. In Advanced Renewable Energy Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 403–425. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.G.C.; Farias, F.O.; Gaioto, R.C.; Kaspchak, E.; da Costa, M.C.; Igarashi-Mafra, L.; Mafra, M.R. Thermophysical characterization of deep eutectic solvents composed by D-sorbitol, xylitol or D(+)xylose as hydrogen bond donors. J. Mol. Liq. 2022, 354, 118801. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Rodríguez-Ramos, R.; Herrera-Herrera, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Deep eutectic solvents. The new generation of green solvents in analytical chemistry. TrAC Trends Anal. Chem. 2021, 134, 116108. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Afshari, R.; Ramón, D.J.; Varma, R.S. Deep eutectic solvents: Cutting-edge applications in cross-coupling reactions. Green Chem. 2020, 22, 3668–3692. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef]
- Ristivojević, P.; Ristivojević, M.K.; Stanković, D.; Cvijetić, I. Advances in Extracting Bioactive Compounds from Food and Agricultural Waste and By-Products Using Natural Deep Eutectic Solvents: A Circular Economy Perspective. Molecules 2024, 29, 4717. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Pei, Z.; Fang, Z.; Yang, S.; Li, H. Bio-based deep eutectic solvent of enhanced lignin solubility for wheat straw fractionation and full-component utilization. Ind. Crops Prod. 2025, 223, 120054. [Google Scholar] [CrossRef]
- Breil, C.; Meullemiestre, A.; Vian, M.; Chemat, F. Bio-Based Solvents for Green Extraction of Lipids from Oleaginous Yeast Biomass for Sustainable Aviation Biofuel. Molecules 2016, 21, 196. [Google Scholar] [CrossRef]
- Jin, S.; Byrne, F.; McElroy, C.R.; Sherwood, J.; Clark, J.H.; Hunt, A.J. Challenges in the development of bio-based solvents: A case study on methyl(2,2-dimethyl-1,3-dioxolan-4-yl)methyl carbonate as an alternative aprotic solvent. Faraday Discuss. 2017, 202, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.S.; Römpp, H.; Schmidt, P.C. Pharmaceutical applications of supercritical carbon dioxide. Pharmazie 2001, 56, 907–926. [Google Scholar]
- Soren, S.; Sahoo, T.; Panda, J.; Senapati, D.K.; Sahu, J.R.; Rath, C.K.; Sahu, R. Carbon Dioxide-Based Green Solvents; Springer: Cham, Switzerland, 2022; pp. 323–333. [Google Scholar]
- Wu, T.; Han, B. Supercritical Carbon Dioxide (CO2) as Green Solvent. In Green Chemistry and Chemical Engineering; Springer: New York, NY, USA, 2019; pp. 173–197. [Google Scholar] [CrossRef]
- Besharati, Z.; Hashemi, S.H. Prediction of CO2 solubility in aqueous and organic solvent systems through machine learning techniques. Model. Earth Syst. Environ. 2024, 11, 4. [Google Scholar] [CrossRef]
- Patil, C.M.; Borse, A.U.; Meshram, J.S. Ionic liquid: Green solvent for the synthesis of cellulose/guar gum/PVA biocomposite. Green Mater. 2018, 6, 23–29. [Google Scholar] [CrossRef]
- Ceres, G.; Conte, V.; Mirruzzo, V.; Kolar, J.; Strlič, M. Imidazolium-Based Ionic Liquids for the Efficient Treatment of Iron Gall Inked Papers. ChemSusChem 2008, 1, 921–926. [Google Scholar] [CrossRef]
- Triolo, A.; Celso, F.L.; Perez, J.; Russina, O. Solubility and solvation features of native cyclodextrins in 1-ethyl-3-methylimidazolium acetate. Carbohydr. Polym. 2022, 291, 119622. [Google Scholar] [CrossRef]
- Lopes, A.M.d.C.; João, K.G.; Morais, A.R.C.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquids as a tool for lignocellulosic biomass fractionation. Sustain. Chem. Process. 2013, 1, 3. [Google Scholar] [CrossRef]
- New, E.K.; Tnah, S.K.; Voon, K.S.; Yong, K.J.; Procentese, A.; Shak, K.P.Y.; Subramonian, W.; Cheng, C.K.; Wu, T.Y. The application of green solvent in a biorefinery using lignocellulosic biomass as a feedstock. J. Environ. Manag. 2022, 307, 114385. [Google Scholar] [CrossRef]
- Belesov, A.V.; Mazur, D.M.; Faleva, A.V.; Varsegov, I.S.; Pikovskoi, I.I.; Ulyanovskii, N.V.; Kosyakov, D.S. 1-Butyl-3-methylimidazolium-Based Ionic Liquid in Biomass Fractionation—Green Solvent or Active Reagent Toward Lignin Compounds? Int. J. Mol. Sci. 2024, 25, 12623. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Ionic Liquids as Green Solvents: Progress and Prospects. In Green Solvents II; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Wickramasinghe, R. Recent progress on sustainable membrane manufacturing with green solvents and biopolymers. Curr. Opin. Chem. Eng. 2025, 47, 101092. [Google Scholar] [CrossRef]
- Corcoran, G.; Ray, S. Benzyl Alcohol. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 429–432. [Google Scholar] [CrossRef]
- Moity, L.; Benazzouz, A.; Molinier, V.; Nardello-Rataj, V.; Elmkaddem, M.K.; de Caro, P.; Thiébaud-Roux, S.; Gerbaud, V.; Marion, P.; Aubry, J.-M. Glycerol acetals and ketals as bio-based solvents: Positioning in Hansen and COSMO-RS spaces, volatility and stability towards hydrolysis and autoxidation. Green Chem. 2015, 17, 1779–1792. [Google Scholar] [CrossRef]
- Yu, L.; Lin, C.; Liao, C.; Zeng, X.; Chen, X.; Zhu, Z.; Huang, Y.; Li, Y.; Chen, L. Green chemistry: Efficient acetalization of aldehydes with alcohols using the acid red 52 photocatalyst. Environ. Chem. Lett. 2020, 18, 1353–1359. [Google Scholar] [CrossRef]
- Kob, N.E. Chapter 17—Clean Solvents. In Dibasic Ester: A Low Risk, Green Organic Solvent Alternative; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2002; Volume 819, pp. 238–253. [Google Scholar] [CrossRef]
- Nikitas, N.F.; Triandafillidi, I.; Kokotos, C.G. Photo-organocatalytic synthesis of acetals from aldehydes. Green Chem. 2019, 21, 669–674. [Google Scholar] [CrossRef]
- Macchia, A.; Zaratti, C.; Biribicchi, C.; Colasanti, I.A.; Barbaccia, F.I.; Favero, G. Evaluation of Green Solvents’ Applicability for Chromatic Reintegration of Polychrome Artworks. Heritage 2023, 6, 3353–3364. [Google Scholar] [CrossRef]
- Macchia, A.; Rivaroli, L.; Gianfreda, B. The GREEN RESCUE: A ‘green’ experimentation to clean old varnishes on oil paintings. Nat. Prod. Res. 2019, 35, 2335–2345. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; McElroy, C.R.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Granato, A.V.; Santos, A.G.; dos Santos, E.N. p-Cymene as Solvent for Olefin Metathesis: Matching Efficiency and Sustainability. ChemSusChem 2017, 10, 1832–1837. [Google Scholar] [CrossRef]
- Virot, M.; Tomao, V.; Ginies, C.; Chemat, F. Total Lipid Extraction of Food Using d-Limonene as an Alternative to n-Hexane. Chromatographia 2008, 68, 311–313. [Google Scholar] [CrossRef]
- Abdo, D.M.; Mangialardi, T.; Medici, F.; Piga, L. D-Limonene as a Promising Green Solvent for the Detachment of End-of-Life Photovoltaic Solar Panels under Sonication. Processes 2023, 11, 1848. [Google Scholar] [CrossRef]
- Pyo, S.-H.; Park, J.H.; Chang, T.-S.; Hatti-Kaul, R. Dimethyl carbonate as a green chemical. Curr. Opin. Green Sustain. Chem. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Rivetti, F. The role of dimethylcarbonate in the replacement of hazardous chemicals. C. R. Acad. Sci.-Ser. IIC-Chem. 2000, 3, 497–503. [Google Scholar] [CrossRef]
- Tanzi, C.D.; Vian, M.A.; Ginies, C.; Elmaataoui, M.; Chemat, F. Terpenes as Green Solvents for Extraction of Oil from Microalgae. Molecules 2012, 17, 8196–8205. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Hall, C.G.J.; Thorp, L.R.; Sneddon, H.F. Replacement of Less-Preferred Dipolar Aprotic and Ethereal Solvents in Synthetic Organic Chemistry with More Sustainable Alternatives. Chem. Rev. 2022, 122, 6749–6794. [Google Scholar] [CrossRef]
- Qiao, W.; Zhang, R.; Wen, Y.; Wang, X.; Wang, Z.; Tang, G.; Liu, M.; Kang, H.; Said, Z.; Hwang, J.-Y.; et al. Green solvents in battery recycling: Status and challenges. J. Mater. Chem. A 2024, 12, 11235–11265. [Google Scholar] [CrossRef]
- Signorini, E. Surface Cleaning of Paintings and Polychrome Objects in Italy: The Last 15 Years. 2013. Available online: https://api.semanticscholar.org/CorpusID:145025739 (accessed on 12 December 2024).
- Phenix, A.; Wolbers, R.C.; Townsend, J.; Zumbühl, S.; Bartoletti, A.; Lee, J.; Ormsby, B. Removal of varnish: Organic solvents as cleaning agents. In Conservation of Easel Paintings; Taylor & Francis Ltd.; Routledge: London, UK, 2020; pp. 549–573. [Google Scholar] [CrossRef]
- Baij, L.; Hermans, J.; Ormsby, B.; Noble, P.; Iedema, P.; Keune, K. A review of solvent action on oil paint. Heritage Sci. 2020, 8, 43. [Google Scholar] [CrossRef]
- Feller, R.L. On Picture Varnishes and Their Solvents; Feller, R.L., Stolow, N., Jones, E.H., Eds.; National Gallery of Art: Washington, DC, USA, 1985. [Google Scholar]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Cleaning of Wall Paintings and Stones. In Nanotechnologies in the Conservation of Cultural Heritage; Springer: Dordrecht, The Netherlands, 2015; pp. 61–82. [Google Scholar] [CrossRef]
- Renda, V.; Saladino, M.L.; Caramanna, S.; Chirco, G.; Castello, A.F.; Conti, C.; Marco, A.; SALI, D.; Caponetti, E. Investigation on a low environmental impact solvent mixture applied to a wooden painted slab. Int. J. Conserv. Sci. 2016, 7, 789. [Google Scholar]
- L_Uso_dei_Solventi_Organici_Nella_Pulitura di Opera Policrome; Paolo Cremonesi, il Prato Casa Editrice: Padova, Italy, January 2004.
- Balliana, E.; Ricci, G.; Pesce, C.; Zendri, E. International Journal of Conservation Science Assessing the Value of Green Conservation for Cultural Heritage: Positive and Critical Aspects of Already Available Methodologies. Available online: www.ijcs.uaic.ro (accessed on 17 November 2024).
- Müller, L.; Henniges, U.; Schultz, J.; Wöllner, A.; Zumbühl, S.; Brückle, I. Pressure-sensitive Tape Removal in Paper Conservation: A Review. J. Pap. Conserv. 2022, 22, 59–75. [Google Scholar] [CrossRef]
- Sawicki, M.; Rouse, E.; Bianco, S.L.; Kautto, S. An Investigation of the Feasibility of the Use of Gels and Emulsions in Cleaning of Gilded Wooden Surfaces. Part B: Cleaning of Soiled Oil-Gilding. In Heritage Wood; Springer: Cham, Switzerland, 2019; pp. 37–64. [Google Scholar] [CrossRef]
- Michalski, S. A Physical Model of the Cleaning of Oil Paint. Stud. Conserv. 1990, 35, 85–92. [Google Scholar] [CrossRef]
- Hook, J. The Use of Immiscible Solvent Combinations for the Cleaning of Paintings. J. Am. Inst. Conserv. 1988, 27, 100–104. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.d.P.; Bueno, M.; Parada-Alfonso, F.; Cifuentes, A.; Ibáñez, E. Hansen solubility parameters for selection of green extraction solvents. TrAC Trends Anal. Chem. 2019, 118, 227–237. [Google Scholar] [CrossRef]
- Fardi, T.; Stefanis, E.; Panayiotou, C.; Abbott, S.; van Loon, S. Artwork conservation materials and Hansen solubility parameters: A novel methodology towards critical solvent selection. J. Cult. Heritage 2014, 15, 583–594. [Google Scholar] [CrossRef]
- Ito, L.; Yamamoto, H. Prediction of acute oral toxicity using the Hansen solubility parameter. Toxicol. In Vitro 2022, 83, 105408. [Google Scholar] [CrossRef] [PubMed]
- Hamm, J.; Allen, D.; Ceger, P.; Flint, T.; Lowit, A.; O’Dell, L.; Tao, J.; Kleinstreuer, N. Performance of the GHS Mixtures Equation for Predicting Acute Oral Toxicity. Regul. Toxicol. Pharmacol. 2021, 125, 105007. [Google Scholar] [CrossRef]
- Myllyaho, L.; Raatikainen, M.; Männistö, T.; Mikkonen, T.; Nurminen, J.K. Systematic literature review of validation methods for AI systems. J. Syst. Softw. 2021, 181, 111050. [Google Scholar] [CrossRef]
- Kenig, N.; Echeverria, J.M.; Vives, A.M. Artificial Intelligence in Surgery: A Systematic Review of Use and Validation. J. Clin. Med. 2024, 13, 7108. [Google Scholar] [CrossRef]
- Ivcevic, Z.; Grandinetti, M. Artificial intelligence as a tool for creativity. J. Creat. 2024, 34, 100079. [Google Scholar] [CrossRef]
- Gao, C.A.; Howard, F.M.; Markov, N.S.; Dyer, E.C.; Ramesh, S.; Luo, Y.; Pearson, A.T. Comparing scientific abstracts generated by ChatGPT to real abstracts with detectors and blinded human reviewers. NPJ Digit. Med. 2023, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Breck, E.; Cai, S.; Nielsen, E.; Salib, M.; Sculley, D. The ML Test Score: A Rubric for ML Production Readiness and Technical Debt Reduction. Available online: https://pair-code.github.io/facets/ (accessed on 27 December 2024).
- Kato, Y.; Osawa, T.; Yoshihara, M.; Fujii, H.; Tsutsumi, S.; Yamamoto, H. Evaluation of the Antifoaming Effect Using Hansen Solubility Parameters. ACS Omega 2020, 5, 5684–5690. [Google Scholar] [CrossRef]
- Molina, J.; Laroche, A.; Richard, J.-V.; Schuller, A.-S.; Rolando, C. Neural Networks Are Promising Tools for the Prediction of the Viscosity of Unsaturated Polyester Resins. Front. Chem. 2019, 7, 375. [Google Scholar] [CrossRef]
- De La Peña-Gil, A.; Toro-Vazquez, J.F.; Rogers, M.A. Simplifying Hansen Solubility Parameters for Complex Edible Fats and Oils. Food Biophys. 2016, 11, 283–291. [Google Scholar] [CrossRef]
- Teas, J.P. Graphic analysis of resin solubilities. J. Paint. Technol. 1968, 40, 19–25. [Google Scholar]
- Pilon, L.; Day, D.; Maslen, H.; Stevens, O.P.J.; Carslaw, N.; Shaw, D.R.; Sneddon, H.F. Development of a solvent sustainability guide for the paints and coatings industry. Green Chem. 2024, 26, 9697–9711. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 Selection Guide of Classical- and Less Classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Macchia, A.; Zaratti, C.; Ciogli, D.; Rivici, G.; Pilati, S.; Sbiri, N.; de Caro, T.; Navarra, M.A. Developing Innovative Apolar Gels Based on Cellulose Derivatives for Cleaning Metal Artworks. Gels 2024, 10, 747. [Google Scholar] [CrossRef]
- Chelazzi, D.; Fratini, E.; Giorgi, R.; Mastrangelo, R.; Rossi, M.; Baglioni, P. Gels for the Cleaning of Works of Art; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018; Volume 1296, Chapter 15; pp. 291–314. [Google Scholar] [CrossRef]
- Giraud, T.; Gomez, A.; Lemoine, S.; Pelé-Meziani, C.; Raimon, A.; Guilminot, E. Use of gels for the cleaning of archaeological metals. Case study of silver-plated copper alloy coins. J. Cult. Herit. 2021, 52, 73–83. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Alsarra, I.A.; Alshehri, S. Solubility, Hansen Solubility Parameters and Thermodynamic Behavior of Emtricitabine in Various (Polyethylene Glycol-400 + Water) Mixtures: Computational Modeling and Thermodynamics. Molecules 2020, 25, 1559. [Google Scholar] [CrossRef] [PubMed]
- Novo, L.P.; Curvelo, A.A.S. Hansen Solubility Parameters: A Tool for Solvent Selection for Organosolv Delignification. Ind. Eng. Chem. Res. 2019, 58, 14520–14527. [Google Scholar] [CrossRef]
- Hughes, J.M.; Aherne, D.; Coleman, J.N. Generalizing solubility parameter theory to apply to one- and two-dimensional solutes and to incorporate dipolar interactions. J. Appl. Polym. Sci. 2013, 127, 4483–4491. [Google Scholar] [CrossRef]
- Durand, M.; Molinier, V.; Kunz, W.; Aubry, J. Classification of Organic Solvents Revisited by Using the COSMO-RS Approach. Chemistry 2011, 17, 5155–5164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).