Biostimulants Do Not Mitigate the Effects of Pasture Dieback in the Australian Wet Subtropics
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites
2.1.1. Experiment 1
2.1.2. Experiment 2
2.1.3. Experiment 3
2.2. Assessment of Nutritional Attributes
2.3. Climatic Conditions
2.4. Statistical Analysis
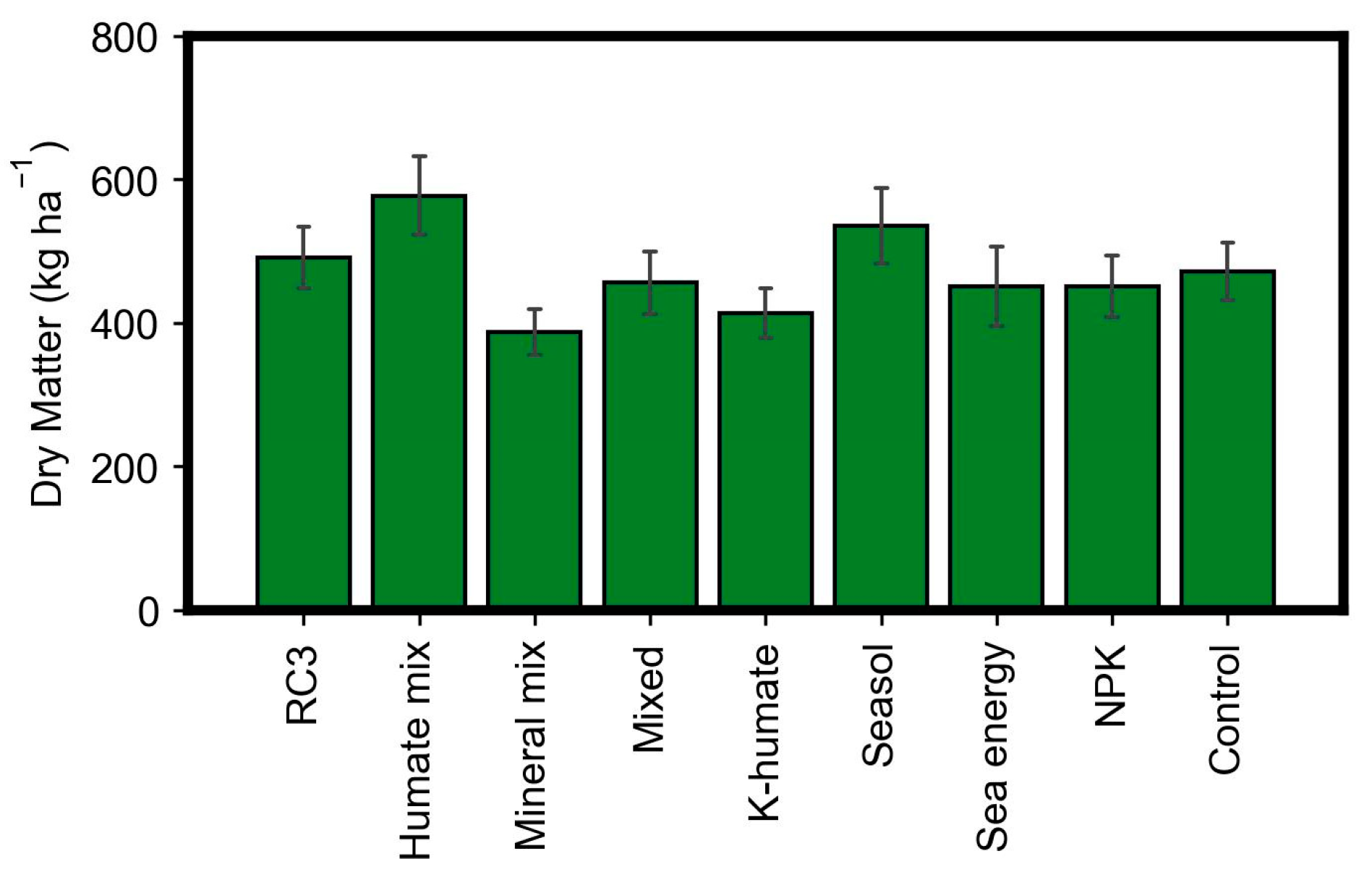
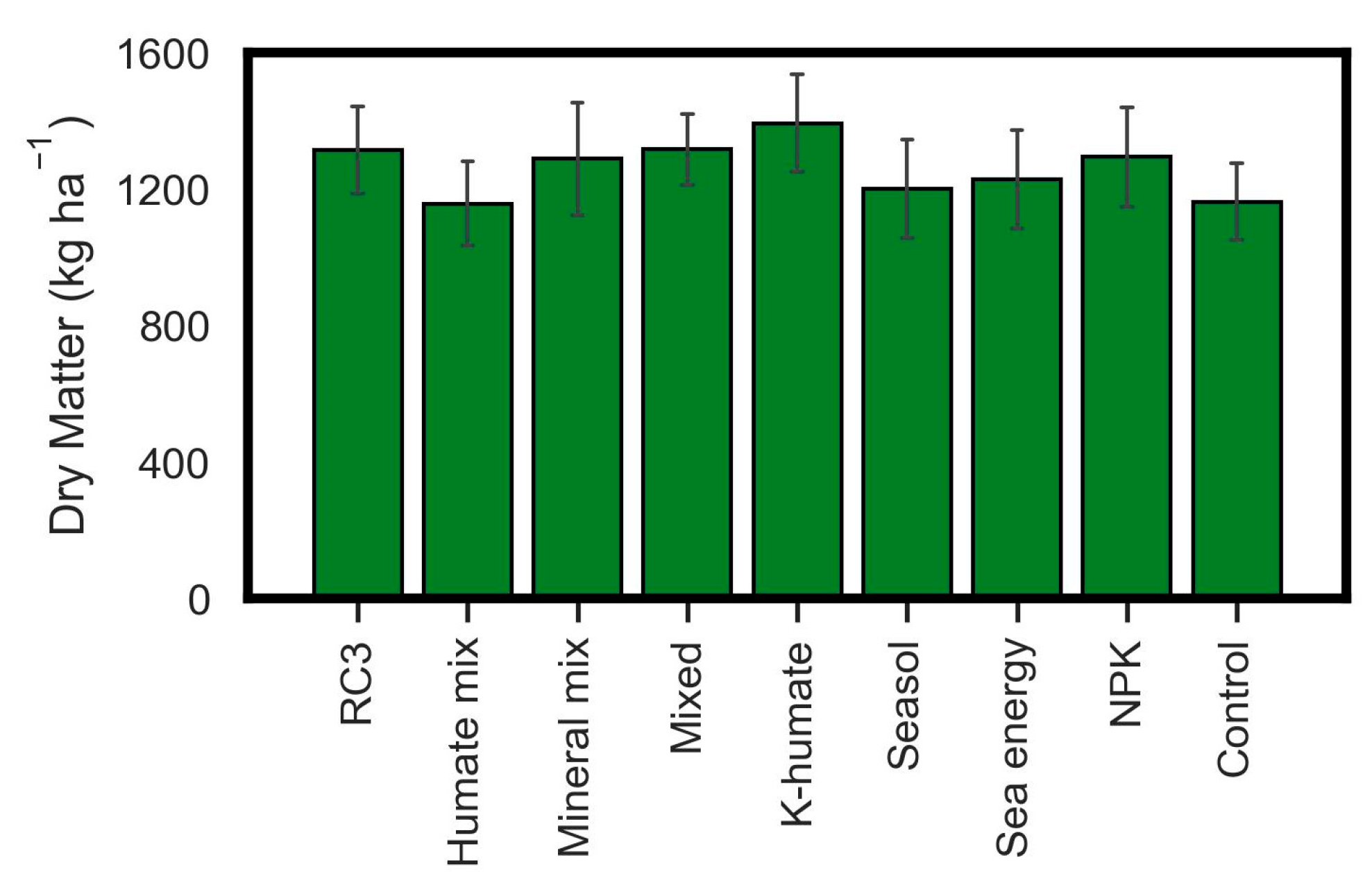
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buck, S. Pasture dieback: Past activities and current situation across Queensland. Project Report (Department of Agriculture and Fisheries: Brisbane, Qld) 2017. Available online: https://era.dpi.qld.gov.au/id/eprint/6521/ (accessed on 28 January 2025).
- Makiela, S.; Harrower, K.M. Overview of the current status of buffel grass dieback. Australas. Plant Dis. Notes 2008, 3, 12–16. [Google Scholar] [CrossRef]
- Peck, G.; Newman, L.; Macor, J.; Buck, S.; Taylor, B. Field tolerance to pasture dieback of 26 tropical grass varieties sown into an affected paddock. In Proceedings of the 20th Australian Society of Agronomy Conference, Toowoomba Qld, Australia, 6–10 February 2022; Available online: https://www.agronomyaustraliaproceedings.org/images/sampledata/2022/Pastures/ASApeck_g_529s.pdf (accessed on 20 January 2025).
- Brookes, H.M. A new species of Heliococcus Šulc from Australia and Pakistan and a redescription of Heliococcus glacialis (Newstead) Comb.N. (Homoptera: Pseaudococcidae). Aust. J. Entomol. 1978, 17, 241–245. [Google Scholar] [CrossRef]
- Hauxwell, C. Mealybugs and pasture dieback. Institute for Future Environments, Queensland University of Technology with Meat & Livestock Australia. 2018. Available online: https://cms.qut.edu.au/__data/assets/pdf_file/0006/786066/pasture-mealybugs-technical-note.pdf (accessed on 15 January 2025).
- Agforce. Pasture Dieback Survey 2019; Agforce: Brisbane Qld, Australia, 2021. [Google Scholar]
- McKenna, P.B.; Ufer, N.; Glenn, V.; Dale, N.; Carins, T.; Nguyen, T.H.; Thomson, M.B.; Young, A.J.; Buck, S.; Jones, P.; et al. Mapping pasture dieback impact and recovery using an aerial imagery time series: A central Queensland case study. Crop Pasture Sci. 2024, 75, CP23340. [Google Scholar] [CrossRef]
- Boschma, S.P.; Daly, A.; Gillespie, P.S.; Wildman, O.; Shrivasatava, M.; Jennings, N.; Geary, J.M.; Baker, S.J. Pasture dieback on the North Coast of New South Wales. 1. Initial diagnostics to identify the causal agent. In Proceedings of the 32nd Conference of the Grassland Society of NSW Inc, virtual conference, 20–21 July 2021; Available online: https://pgnsw.com.au/documents/conference-proceedings/2021/2021%20Grassland%20Conf%20Proc%20B5%20-%20CP1%20-%20Boschma%20-%20Pasture%20dieback%20NC%20NSW%20-%20Diagnostics%20%20-%20WEB%20ver.pdf (accessed on 28 January 2025).
- Boschma, S.P.; Jennings, N.; Geary, J.M.; Baker, S.J. Pasture dieback on the North Coast of New South Wales. 2. Symptom development and current recommendations. In Proceedings of the 32nd Conference of the Grassland Society of NSW Inc., virtual conference, 20–21 July 2021; Available online: https://pgnsw.com.au/documents/conference-proceedings/2021/2021%20Grassland%20Conf%20Proc%20B5%20-%20CP2%20-%20Boschma%20-%20Pasture%20dieback%20NC%20NSW%20-%20Symptoms%20-%20WEB%20ver.pdf (accessed on 28 January 2025).
- Gibson, A.J.; McGregor, H.; Rose, T.J.; Jennings, N. The spread of mealybug-associated pasture dieback into the New South Wales wet subtropics. Aust. Geogr. 2024, 55, 527–539. [Google Scholar] [CrossRef]
- Buck, S.R.; Brazier, N.; Reid, D.J. Management solutions for pasture dieback: Outcomes of field research. In Proceedings of the 20th Agronomy Australia Conference, Toowoomba Qld, Australia, 6–10 February 2022; Available online: https://www.agronomyaustraliaproceedings.org/images/sampledata/2022/Pastures/ASAbuck_s_532s.pdf (accessed on 20 January 2025).
- Whitton, M.M.; Ren, X.; Yu, S.J.; Irving, A.D.; Trotter, T.; Bajagai, Y.S.; Stanley, D. Sea minerals reduce dysbiosis, improve pasture productivity and plant morphometrics in pasture dieback affected soils. Sustainability 2022, 14, 14873. [Google Scholar] [CrossRef]
- Whitton, M.M.; Ren, X.; Yu, S.J.; Trotter, T.; Stanley, D.; Bajagai, Y.S. Remediation of pasture dieback using plant growth promotant. Agronomy 2022, 12, 3153. [Google Scholar] [CrossRef]
- Whitton, M.M.; Ren, X.; Yu, S.J.; Irving, A.D.; Trotter, T.; Bajagai, Y.S.; Stanley, D. Humate application alters microbiota—mineral interactions and assists in pasture dieback recovery. Heliyon 2023, 9, e13327. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Whitton, M.M.; Yu, S.J.; Trotter, T.; Bajagai, Y.S.; Stanley, D. Application of phytogenic liquid supplementation in soil microbiome restoration in Queensland pasture dieback. Microorganisms 2023, 11, 561. [Google Scholar] [CrossRef] [PubMed]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hort. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Rayment, G.E.; Lyons, D.J. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press: Port Melbourne, Australia, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 22 January 2024).
- Coombes, N. DiGGer, a Spatial Design Program; Biometric Bulletin NSW Department of Primary Industries: Orange, NSW, Australia, 2009. [Google Scholar]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 9: One-way analysis of variance. Crit. Care. 2004, 8, 130–136. [Google Scholar] [PubMed]
- Mackiewicz-Walec, E.; Olszewska, M. Biostimulants in the Production of Forage Grasses and Turfgrasses. Agriculture 2023, 13, 1796. [Google Scholar] [CrossRef]
- Roche, D.; Rickson, J.R.; Pawlett, M. Moving towards a mechanistic understanding of biostimulant impacts on soil properties and processes: A semi-systematic review. Front. Agron. 2024, 6, 1271672. [Google Scholar] [CrossRef]
- Moody, P.W. Chemical fertility of krasnozems—A review. Aust. J. Soil Res. 1994, 32, 1015–1041. [Google Scholar] [CrossRef]
- Fulkerson, W.J.; Slack, K.; Hennessy, D.W.; Hough, G.M. Nutrients in ryegrass (Lolium spp.), white clover (Trifolium repens) and kikuyu (Pennisetum clandestinum) pastures in relation to season and stage of regrowth in a subtropical environment. Aust. J. Exp. Agric. 1998, 38, 227–240. [Google Scholar] [CrossRef]
- Anon. Management of Pasture Dieback. Department of Agriculture and Forestry, Queensland. 2024. Available online: https://futurebeef.com.au/wp-content/uploads/2024/03/2227-Pasture-dieback-A4-brochure_WEB_FINAL.pdf (accessed on 28 January 2025).


| Site 1 | Site 2 | Site 3 | |
|---|---|---|---|
| Location | 28°52′02″ S, 153°26′10″ E | 28°52′01″ S, 153°26′14″ E | 28°58′31″ S, 153°04′12″ E |
| Topsoil texture | Clay–loam | Clay–loam | Clay–loam |
| pH (water) | 5.7 | 5.4 | 6.2 |
| Total carbon (%) | 6.3 | 5.5 | 6.4 |
| Total nitrogen (%) | 0.56 | 0.48 | 0.48 |
| Phosphorus (mg kg−1) (Bray 1) | 16 | 7 | 21 |
| Sulfur (mg kg−1) | 20 | 32 | 9 |
| Exchangeable cations (cmol+ kg−1) | |||
| Potassium | 0.27 | 0.17 | 1.10 |
| Calcium | 3.5 | 1.8 | 16.0 |
| Magnesium | 2.3 | 1.5 | 8.1 |
| Sodium | 0.18 | 0.12 | 0.32 |
| Aluminum | 0.10 | 0.35 | 0.01 |
| Product Details | RC3 | Humate Mix | Sea Mineral Mix | Seasol Commercial | Sea Energy | K Humate | RC3 + Humate Mix + Sea Mineral Mix (Mixed) | Express® NPK |
|---|---|---|---|---|---|---|---|---|
| Active ingredients | Trimercapto-S-triazine and humate | Humate | Marine minerals | Seaweed extract | Marine minerals | Humate | Trimercapto-S-triazine, humate, marine minerals | Macro- and micro-nutrients |
| Application rate (mL plot−1) | 3 | 45 | 10 | 15 | 15 | 30 | 3, 45 & 10 | 22.5 |
| Nutrients applied (g ha−1) | ||||||||
| Nitrogen | 2.2 | 41 | 0.01 | 9.2 | 8.4 | 52 | 43 | 975 |
| Phosphorus | 18 | 85 | 0.2 | 31 | 9.5 | 314 | 103 | 2250 |
| Potassium | 2.0 | 41 | 48 | 74 | 9.4 | 1108 | 92 | 2250 |
| Calcium | 0.1 | 2.7 | 0.2 | 0.7 | 44 | 8.5 | 2.9 | 105 |
| Magnesium | * | 1.4 | 523 | 2.1 | 568 | 17 | 525 | 7.5 |
| Sulfur | 5.8 | 4.8 | 76 | 3.2 | 27 | 9.3 | 87 | 0 |
| Copper | * | 0.002 | 0.0003 | 0.0004 | 0.02 | 0.005 | * | 5.4 |
| Iron | 0.008 | 0.1 | 0.01 | 0.3 | 0.03 | 6.5 | 0.1 | 7.5 |
| Manganese | 0.0001 | 0.03 | 0.0004 | 0.006 | 0.0009 | 3.1 | 0.03 | 7.9 |
| Zinc | 0.0005 | 0.008 | 0.001 | 0.003 | 0.01 | 2.5 | 0.01 | 7.6 |
| Boron | 0.0006 | 0.02 | 1.8 | 0.02 | 1.1 | 1.2 | 1.8 | 1.9 |
| Experiment 1 | Experiment 2 | Experiment 3 | |
|---|---|---|---|
| Duration | 1 November 2023–7 December 2023 | 8 March 2024–11 April 2024 | 26 April 2024–29 May 2024 |
| Total Rainfall (mm) | 149 | 223 | 106 |
| LTA Rain (mm) | 97 | 182 | 71 |
| Average Minimum Temperature (°C) | 16 | 17 | 13 |
| LTA Minimum Temperature (°C) | 18 | 17 | 14 |
| Average Maximum Temperature (°C) | 28 | 27 | 23 |
| LTA Maximum Temperature (°C) | 30 | 28 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mark, E.N.; Gibson, A.J.; Boschma, S.P.; Rose, T.J. Biostimulants Do Not Mitigate the Effects of Pasture Dieback in the Australian Wet Subtropics. Sustainability 2025, 17, 3013. https://doi.org/10.3390/su17073013
Mark EN, Gibson AJ, Boschma SP, Rose TJ. Biostimulants Do Not Mitigate the Effects of Pasture Dieback in the Australian Wet Subtropics. Sustainability. 2025; 17(7):3013. https://doi.org/10.3390/su17073013
Chicago/Turabian StyleMark, Eric N., Abraham J. Gibson, Suzanne P. Boschma, and Terry J. Rose. 2025. "Biostimulants Do Not Mitigate the Effects of Pasture Dieback in the Australian Wet Subtropics" Sustainability 17, no. 7: 3013. https://doi.org/10.3390/su17073013
APA StyleMark, E. N., Gibson, A. J., Boschma, S. P., & Rose, T. J. (2025). Biostimulants Do Not Mitigate the Effects of Pasture Dieback in the Australian Wet Subtropics. Sustainability, 17(7), 3013. https://doi.org/10.3390/su17073013






