Abstract
Sustainable feeding strategies incorporating alternative feed ingredients, such as insect-derived oils, play a crucial role in improving nutrient utilization in ruminants while mitigating environmental impact and methane emissions. Black soldier fly larvae oil (BSFLO) has emerged as a promising lipid source; however, its effective utilization requires protection to prevent adverse effects on rumen microbial activity. This study aimed to evaluate the effects of encapsulated BSFLO on rumen fermentation, gas production kinetics, methane estimation, and digestibility using an in vitro approach. A completely randomized design (CRD) with a 2 × 4 (+1) factorial arrangement was employed. The first factor was the type of BSFLO (intact or encapsulated), while the second factor was the product level (2%, 4%, 6%, and 8% of dry matter [DM]), with an additional negative control included. The in vitro analysis revealed that encapsulated BSFLO significantly (p < 0.05) increased gas production, with the highest value observed at the 2% level. Encapsulated BSFLO enhanced rumen digestibility, with the highest values recorded for the negative control, 2%, and 4% encapsulated BSFLO treatments. Additionally, digestibility in the abomasum was highest for the negative control and the 2% encapsulated BSFLO treatment. Encapsulated BSFLO also significantly (p < 0.05) reduced methane (CH4) production, with the lowest values observed at 2%, 4%, 6%, and 8% encapsulated BSFLO. Encapsulated BSFLO had no significant effect (p > 0.05) on total ammonia-nitrogen (NH3-N), pH, and protozoal population. Furthermore, total volatile fatty acid (VFA) values were not significantly (p > 0.05) increased by the inclusion of encapsulated BSFLO. In conclusion, encapsulated BSFLO is a promising feed additive that enhances digestibility and reduces methane emissions, contributing to sustainable animal nutrition. Its potential to lower the environmental impact of ruminant production supports efficient feeding strategies for improved livestock productivity.
1. Introduction
The growing global population, rapid urbanization, and rising incomes in developing countries are driving an increased demand for animal-based food products, placing significant pressure on livestock production systems [1,2]. By 2050, the world population is expected to reach 10 billion, necessitating a 60–70% rise in the production of milk and meat to meet food security demands [3]. However, the intensification of agricultural production is closely related to environmental challenges such as CH4 emissions, and agriculture contributes about 40% of anthropogenic methane emissions, mainly from livestock systems (32%) and rice cultivation (8%) [4]. Cattle produce about 74% of total CH4 emissions from livestock worldwide [5]. These emissions not only impact climate change but also underscore the urgent need for sustainable animal nutrition strategies that optimize feed efficiency, enhance livestock productivity, and reduce the environmental footprint of ruminant systems [6]. Implementing innovative feeding strategies, such as precision nutrition, alternative feed resources, and methane mitigation approaches, is essential to achieving a balance between productivity and environmental sustainability in livestock production [5].
The increasing demand for animal products highlights the urgent need to mitigate CH4 emissions from cattle. Reducing CH4 emissions from ruminants is essential for sustainable animal nutrition and environmental protection [7]. While several promising strategies exist, including feed additives and dietary modifications, their practical application requires careful consideration of economic, environmental, and performance impacts [8]. Continued research and development are necessary to refine these strategies and ensure their effectiveness and sustainability in diverse farming systems [9]. Several strategies have been proposed, including dietary supplementation with essential oils, tannins, saponins, and flavonoids, which possess anti-methanogenic properties and can significantly reduce CH4 emissions [10,11]. Additionally, the inclusion of oils in feed can suppress CH4 production by reducing the population of methanogenic microbes and protozoa in the rumen [12,13,14].
Black soldier fly larvae oil (BSFLO), derived from Hermetia illucens larvae, is known for its high lauric acid content [15,16] and a composition of over 60% polyunsaturated fatty acids, with linoleic acid as the primary component [17]. Prior studies, such as those by Prachumchai and Cherdthong [18], have investigated the effects of BSFLO on cattle in vitro, focusing on fermentation characteristics, degradability, and CH4 production. However, these studies did not explore rumen protection techniques, despite the potential degradation of BSFLO in the rumen, limiting its full utilization.
Feeding unprotected oil has been shown to reduce CH4 production but may also have toxic effects on rumen microbes, leading to reduced production of other fermentation products [19]. Excessive unprotected oil in the diet can impair the tolerance of rumen bacteria, disrupting the digestion process and reducing overall efficiency [20]. Unprotected oil undergoes hydrolysis in the rumen, releasing unsaturated fatty acids and glycerol, which are not fully utilized [21].
Oil supplementation challenges can be mitigated through protective treatments. Protecting oil allows it to bypass the rumen and be digested directly in the small intestine [22,23]. Various oil protection methods, such as calcium soap, have been studied. For instance, Wulandari et al. [24] successfully protected palm oil using calcium soap, and Riestanti et al. [25] applied this technique to protect corn, sunflower, palm, and sesame oils. Behan et al. [26] also demonstrated that rumen-protected fats could enhance total volatile fatty acid production, improving fermentation and animal performance.
Encapsulation is an advanced technique for oil protection that involves enclosing active agents within coatings, membranes, shells, or matrices [27,28,29]. This process forms microcapsules or nanocapsules that protect bioactive compounds, allowing them to be delivered to specific sites in the digestive system [30,31]. Encapsulation has emerged as a promising technology in animal feed nutrition, enhancing the stability and efficacy of bioactive compounds [31].
While extensive research has been conducted on oil protection for conventional lipid sources, studies on the protection of insect-derived oils, particularly BSFLO, remain limited. The potential of encapsulation as a strategy to enhance the stability and efficacy of BSFLO in ruminant nutrition has yet to be explored. Given the need for sustainable feeding strategies that optimize nutrient utilization while mitigating methane emissions and reducing environmental impact, exploring novel approaches to improve the efficiency of alternative lipid sources is crucial. The findings of this study will contribute to the development of innovative feeding strategies that support sustainable livestock production and environmental sustainability. This study aimed to evaluate the effects of encapsulated BSFLO on rumen fermentation, gas production kinetics, and digestibility using an in vitro rumen fermentation model.
2. Materials and Methods
2.1. Location, Animal Ethics, and Preparation of Black Soldier Larvae Oil
This research was conducted at Khon Kaen University (KKU), Faculty of Agriculture, Department of Animal Science, located in Khon Kaen, Thailand. The study adhered to animal ethics guidelines under record number IACUC-KKU-48/67.
BSFLO was purchased from BSFLY Company Ltd., located in Udon Thani city, Udon Thani Province, Thailand. The processing involved sanitizing and drying the larvae to 30% of their original mass weight, followed by oil extraction using a press-defatting technique with an NF-80 cold press machine. This process yielded black soldier fly larvae flour and oil, with the latter collected as the final product through separation and extraction of oil content.
2.2. Encapsulation Black Soldier Fly Larvae Oil Preparation
The encapsulation method for BSFLO was adapted from Phupaboon et al. [32] and Shetta et al. [33]. A 1% (w/v) chitosan wall-material solution was prepared by dissolving chitosan in 500 mL of 1% (v/v) acetic acid. The oil solution comprised 30% (v/v) BSFLO and 1% (v/v) Tween 80 as a surfactant in 500 mL. Encapsulation was achieved by mixing the wall and oil solutions in a 1:1 (v/v) ratio and agitating the mixture continuously at room temperature overnight. The encapsulated material was freeze-dried using a compact freeze dryer. The mixture was initially frozen at −30 °C overnight. The freeze-drying process included several steps: freezing at −60 °C, gradual warming to −60 °C for 15 min, and subsequent drying under a vacuum pressure of 0.0500 bar at −60 °C. The final drying stage was completed at 20 °C and 0.0500 bar.
2.3. Design of Experiment
The experiment utilized a gas production technique across various incubation durations. The design used in the study was a 2 × 4 (+1) factorial in CRD. The experimental feed factors included (factor A) two different treatments, unprotected BSFLO and encapsulated BSFLO, together with (factor B) four distinct levels of concentration (2, 4, 6, and 8% DM). The negative control was a basal diet without the addition of BSFLO or encapsulated BSFLO. The ratio of roughage-to-concentrate (R:C) used was 60:40. Rice straw was used as a fiber source. The nine treatments were as follows: negative control or without BSFLO and encapsulation, (T1) BSFLO 2%, (T2) BSFLO 4%, (T3) BSFLO 6%, (T4) BSFLO 8%, (T5) encapsulated BSFLO 2%, (T6) encapsulated BSFLO 4%, (T7) encapsulated BSFLO 6%, and (T8) encapsulated BSFLO 8%. The formulation ratios for all treatments were provided in Table 1. The provision of BSFLO levels in this study is based on previous research by Prachumchai and Cherdthong [18], which used unprotected BSFLO at levels of 0, 2, 4, and 6%. The results showed that the administration of 6% BSFLO negatively impacted digestibility. Therefore, this study increased the levels to 8% to determine its effects at a more optimal level, especially on encapsulated BSFLO products.

Table 1.
Ingredient and chemical composition of concentrate diet (%DM).
2.4. Animals and Rumen Fluid Inoculum
Rumen fluid was collected from five Thai native bulls (4.0–4.5 years old, 450 ± 10 kg body weight). The bulls were maintained on a diet of concentrate feed (14% crude protein and 75% total digestible nutrients) for 14 days and had ad libitum access to hay. The concentrate feed consisted of cassava chips, corn meal, rice bran, soybean meal, palm kernel meal, molasses, urea, salt, minerals, and vitamins. Feeding was conducted twice daily at 07:30 and 15:30, with the concentrate provided at 1% of body weight. Clean water and mineral blocks were available ad libitum.
Ruminal fluid was collected in the morning before feeding using a suction tube inserted through the mouth into the rumen. Fluid samples from three animals were pooled to create a representative sample from the donor animals. After collection, the ruminal fluid was filtered through a cloth, transferred into an Erlenmeyer flask, and stored in a thermos at 39 °C under anaerobic conditions.
2.5. Fermentation Substrates In Vitro
The in vitro fermentation process began by mixing rumen fluid with artificial saliva at a 1:2 ratio. Rice straw and concentrate, in a 40:60 ratio, were weighed to a total of 0.5 g and placed into individual vials. Each vial was then filled with 40 mL of the rumen fluid and artificial saliva mixture. The vials were sealed with rubber stoppers and elasticated lids to maintain anaerobic condition.
The experimental setup was divided into two groups. The first group, comprising (5 bottle replications × 9 treatments) + 4 blanks, was designated for kinetic gas production analysis. The second group, consisting of (3 bottle replications × 9 treatments × 2 observation times at 24 and 48 h), was used to assess pH, VFA, NH3-N, CH4, and protozoal population.
2.6. In Vitro Digestibility
In vitro digestibility was evaluated using the DAISY II Incubator method by Tassone et al. [34]. A 0.5 g sample was placed inside an F57 nylon bag and securely sealed. Rumen fluid and artificial saliva were mixed in a 1:2 ratio, with a total volume of 2000 mL added to each DAISY II Incubator bottle. Each sample was tested in triplicate with two observation intervals: 24 h and 48 h. After incubation, the samples were removed, dried in an oven, and reweighed. Samples incubated for 48 h were further exposed to a pepsin solution in the DAISY II Incubator to determine digestibility in the abomasum.
2.7. Measurements and Chemical Analysis
The chemical composition of concentrates and roughages was analyzed using the AOAC [35] methodology. Proximate analysis included the determination of dry matter (DM), ash, ether extract (EE), and crude protein (CP). Fiber was categorized into two types, neutral-detergent fiber (NDF) and acid-detergent fiber (ADF), based on the system developed by Van Soest et al. [36], which identifies fibers as cell walls or other components insoluble in neutral bleaching chemicals.
Gas production was assessed using the first set of bottles. Measurements were taken post-incubation at regular intervals: 0, 0.5, 1, 2, 4, 6, 8, 12, 16, 24, 48, 72, and 96 h. Gas production parameters were estimated using the model proposed by Ørskov and McDonald [37].
Three additional sets of bottles were prepared for analyzing pH, VFAs, NH3-N, CH4, and protozoal populations. After incubation, a 20 mL sample from the first set was combined with 5 mL of 1 M H2SO4 and stored at −20 °C for NH3-N analysis, performed using a UV/Vis Spectrometer (PG Instruments Ltd., London, UK) following the methodology of Fawcett and Scott [38].
Ruminal fluid samples were analyzed for VFAs, including total VFA, acetic acid (A), propionic acid (P), butyric acid, and A:P ratio using a Newis GC-2030 gas chromatograph (Shimadzu Corporation, Kyoto, Japan) equipped with a DB-Wax capillary column (30 m length, 0.25 mm diameter, 0.25 µm film; Agilent, Santa Clara, CA, USA), as described by Porter and Murray [39].
For the protozoal population analysis, a 1 mL sample was diluted with 9 mL of a 10% solution. Protozoal enumeration was conducted manually using a Boeco hemocytometer (Hamburg, Germany).
CH4 gas generation was estimated based on volatile fatty acid (VFA) concentrations, following the method detailed by Moss et al. [40] and further described by Williams et al. [41]. The calculation was performed using the following formula:
CH4 (mM) = 0.50C2 − 0.25C3 + 0.50C4
In the equation, C2 preferred to acetate (mM), C3 to propionate (mM), and C4 to butyrate (mM).
Multiple samples were placed in F57 nylon bags and incubated in rumen fluid using the DAISY II Incubator for 24 and 48 h. Samples representing each experimental component were randomly selected for in vitro dry matter digestibility (IVDMD) analysis following the incubation period. The process began with the preparation of the F57 nylon bags and samples. Each nylon bag underwent a 3–5 min cleaning procedure with acetone to remove surfactants that could interfere with microbial decomposition. The bags were then either air-dried or baked at 100 °C until fully dried. The dry weight of each F57 nylon bag was recorded as (W1). A single bag was weighed and securely sealed for calibration purposes (C1). For the 24 h incubation, a 0.25 g sample was used, while a 0.5 g sample (W2) was used for the 48 h incubation. Both samples were enclosed in nylon bags and incubated. Digestibility assessment for the abomasum followed the same procedure as for the rumen, except a pepsin–HCl solution was used instead of rumen fluid. Heat was applied to secure each bag during this step.
The formula for calculating digestibility was shown below:
where W1 is the weight of bag F57, C1 is an empty bag (final dry weight in oven/weight of original empty bag), W2 is sample weight, DM is % dry matter (multiplied by the decimal equivalent), and W3 is final bag weight after in vitro measurement.
2.8. Statistical Analysis
The gas production estimates are derived using the Ørskov and McDonald [42] methodology. The equation was illustrated below:
where Y is gas production at time (t), a represents gas produced from the immediately soluble fraction, b represents gas produced from the insoluble fraction, c represents gas production rate constant for the insoluble fraction, and t represents the rate of non-degradation or incubation at time t, where (a + b) is the potential extent of gas production.
Y = a + b (1 − e (−ct))
Data were measured in a completely randomized design with a 2 × 4 (+1) factorial treatment by ANOVA using general linear model (GLM) procedures. The average treatment value was calculated using the least-square means (LSMEANS) function of the SAS software (version 9.00, window version 6.2.9200, Cary, NC, USA), using statistical modeling as follows:
where Yij = observation; µ = overall mean; αi = the treatment of BSFLO and encapsulated BSFLO (I, 1–2); βj = are the BSFLO levels at 2, 4, 6, and 8% of DM (j, 1–4); αβij = the interaction effects; and εij = error. When F-tests were significant, a single degree of freedom orthogonal contrast was used to determine the contrast between factors. Duncan’s new multiple-range test (DMRT) was used to determine the difference in the mean of treatments at p < 0.05.
Yij = µ + αi + βj + αβij + εij
3. Results
3.1. Nutritional Composition of Diet
Table 1 presents the ingredients and chemical composition of the nine treatments: negative control, unprotected BSFLO, and encapsulated BSFLO. The proximate analysis results indicate that the DM content ranged from 97.81% to 98.99%, while organic matter (OM) values varied between 89.70% and 92.78%. Crude protein content ranged from 15.66% to 16.96%. NDF and ADF values ranged from 52.45% to 60.79% and 52.14% to 57.54%, respectively. The EE content showed significant differences across treatments. The negative control exhibited an EE value of 3.18%. For unprotected BSFLO, the inclusion levels of 2%, 4%, 6%, and 8% corresponded to actual inclusion rates of 4.41%, 6.50%, 8.48%, and 10.24%, respectively. Encapsulated BSFLO at inclusion levels of 2%, 4%, 6%, and 8% corresponded to actual inclusion rates of 4.47%, 6.22%, 7.39%, and 9.26%, respectively.
3.2. Kinetics and Cumulative Production of Gas
Table 2 presents the effects of unprotected BSFLO and encapsulated BSFLO, combined with different utilization levels, on gas kinetics and cumulative gas production at 96 h post-incubation. For the gas production from the immediately soluble fraction (a), there was a significant cubic interaction (p < 0.05). The negative control was not significantly different from other treatments. The highest value for parameter (a) was observed with encapsulated BSFLO at 2% (−3.26 mL/0.5 g). The gas production from the insoluble fraction (b) showed a significant quadratic interaction (p < 0.05). The negative control had a significantly higher value compared to other treatments. The highest values for parameter (b) were recorded for the negative control, BSFLO at 2%, encapsulated BSFLO at 2%, and encapsulated BSFLO at 4%, with values of 78.55, 84.83, 83.79, and 79.78 mL/0.5 g, respectively.

Table 2.
Effect of unprotected black soldier fly larvae oil (BSFLO) and encapsulated BSFLO combined with the utilization level on gas kinetics and cumulative gas at 96 h after incubation.
For the gas production rate constant from the insoluble fraction (c), no significant interaction was observed (p > 0.05). The negative control was not significantly different from other treatments, and the type of product had no significant effect. However, the level of the product showed a significant linear effect, indicating that higher levels resulted in greater values for parameter (c). The highest values were observed at the 6% and 8% levels (0.06 and 0.06 mL/0.5 g, respectively). For the potential extent of gas production (∣a∣ + b), no significant interaction was observed (p > 0.05). The negative control was not significantly different from other treatments. The type of product did not significantly affect parameter (∣a∣ + b), but the level of the product showed a significant linear effect, with lower levels resulting in higher values. The highest value was recorded at the 2% level (91.52 mL/0.5 g).
For cumulative gas production, a significant cubic interaction was observed (p < 0.05). The negative control showed significantly higher values compared to other treatments. The highest cumulative gas production value was observed with encapsulated BSFLO at 2% (84.3 mL). In addition, for cumulative gas production value, an increase in the encapsulated BSFLO level will decrease the cumulative gas production value.
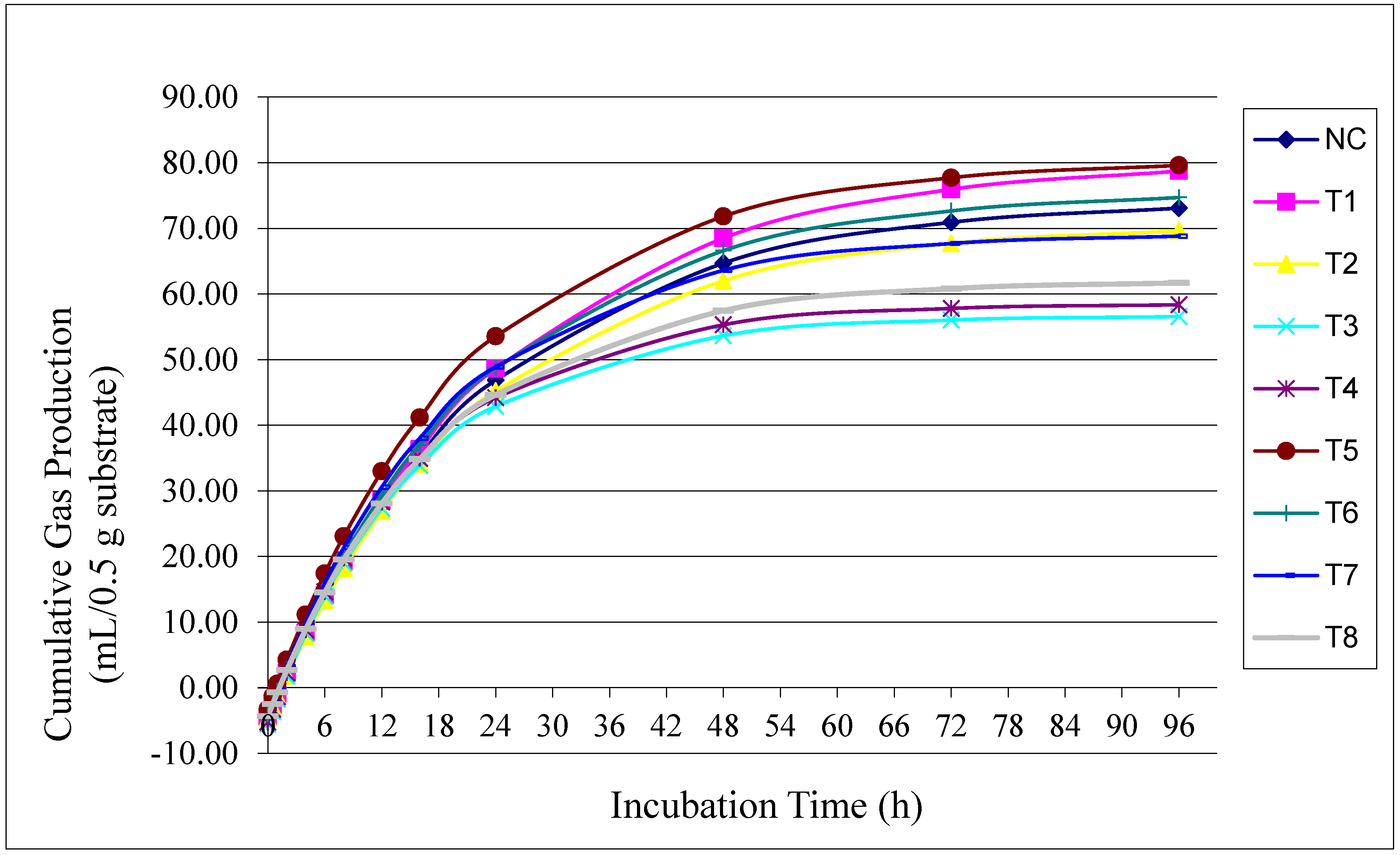
Figure 1 illustrates the effects of unprotected and encapsulated BSFLO at different utilization levels on cumulative gas production during the incubation period. From 0 to 6 h, a sharp increase in gas production was observed across all treatments. Between 72 and 96 h, gas production began to stabilize. The highest cumulative gas production at 96 h was observed with encapsulated BSFLO at 2%.
Figure 1.
Effect of unprotected black soldier fly larvae oil (BSFLO) and encapsulated BSFLO combined with utilization level on cumulative gas during the incubation time.
3.3. Ruminal pH, Protozoal Population, NH3-N, and CH4
Table 3 outlines the effects of unprotected BSFLO and encapsulated BSFLO, combined with different utilization levels, on ruminal pH, protozoal population, NH3-N, and CH4 production. Ruminal pH was unaffected by unprotected or encapsulated BSFLO after 24 and 48 h of incubation. Neither product nor level factors showed any significant influence on pH. However, after 48 h, the pH value in the negative control was significantly lower compared to the other treatments. After 24 and 48 h of incubation, neither unprotected BSFLO nor encapsulated BSFLO had a significant effect on the protozoal population. However, the negative control consistently exhibited a significantly higher protozoal population compared to the treatments at both time points. At 24 h, the product factor showed no significant effect, but the level factor demonstrated a significant linear relationship (p < 0.05), with the highest protozoal populations observed at levels of 2% and 4% (0.44 and 0.47 × 105, respectively). By 48 h, neither the product nor the level factor significantly influenced the protozoal population.

Table 3.
Effect of unprotected black soldier fly larvae oil (BSFLO) and encapsulated BSFLO combined with utilization level on ruminal pH, protozoal population, ammonia-nitrogen (NH3-N), and estimation of methane (CH4).
After 24 and 48 h of incubation, NH3-N concentrations were not significantly affected by unprotected or encapsulated BSFLO. At 48 h, the negative control showed a significantly lower NH3-N value compared to the treatments. While the product factor had no effect at 24 h, the level factor displayed a significant linear relationship (p < 0.05), with the highest NH3-N concentration recorded at the 8% level (12.94 mM). By 48 h, neither the product nor the level factor significantly influenced NH3-N.
After 24 h of incubation, CH4 production exhibited a significant cubic effect (p < 0.05). The negative control was significantly different from the treatments, with the lowest CH4 values observed in unprotected BSFLO at 8% and encapsulated BSFLO at 2% and 4% (7.70, 7.46, and 7.49 mM, respectively). After 48 h, CH4 production remained significantly cubic (p < 0.05), with the negative control again differing significantly from the treatments. The lowest CH4 values were recorded for encapsulated BSFLO at 2%, 4%, 6%, and 8% (10.12, 11.02, 9.95, and 12.37 mM, respectively).
3.4. In Vitro Volatile Fatty Acid
Table 4 presents the effects of unprotected BSFLO and encapsulated BSFLO at different utilization levels on ruminal VFA concentrations. After 24 h of incubation, acetic acid levels showed a cubic pattern of significance (p < 0.05), with the highest value observed in the negative control group (60.16 mM). Propionic acid levels exhibited linear significance (p < 0.05), with the negative control also displaying the highest concentration (31.55 mM). For butyric acid, a cubic pattern of significance was identified (p < 0.05), with the highest value again in the negative control group (14.09 mM). Total VFA concentrations were significantly affected in a cubic manner (p < 0.05), with the negative control reaching the highest value (107.03 mM). However, the acetic-to-propionic acid ratio (A:P) was not significantly influenced (p > 0.05), although the negative control exhibited a higher value compared to the other treatments. Notably, the encapsulated BSFLO product showed a significant effect on the A:P ratio (1.74), while cubic effects were observed at the 6% and 8% levels, which resulted in higher ratios (1.83 and 1.79, respectively).

Table 4.
Effect of unprotected black soldier fly larvae oil (BSFLO) and encapsulated BSFLO combined with utilization level on ruminal volatile fatty acid (VFA).
After 48 h of incubation, acetic acid concentrations remained cubically significant (p < 0.05), with the highest value recorded in the negative control group (82.97 mM). Propionic acid levels followed a cubic pattern of significance (p < 0.05), with the highest concentrations observed in the negative control and unprotected BSFLO at 2% (35.77 and 34.02 mM, respectively). Butyric acid levels were not significantly affected (p > 0.05), but the negative control group still had the highest concentration compared to the other treatments. The type of product significantly influenced butyric acid levels, with unprotected BSFLO yielding the highest value (15.46 mM), while the level of product did not have a significant effect. Total VFA concentrations displayed cubic significance (p < 0.05), with the negative control and unprotected BSFLO showing the highest values (141.26 and 132.97 mM, respectively). The A:P ratio was linearly significant (p < 0.05), with the negative control exhibiting the highest ratio (2.33).
3.5. In Vitro Degradability
Table 5 presents the effects of unprotected BSFLO and encapsulated BSFLO at different utilization levels on in vitro dry matter digestibility. After 24 h of incubation, the rumen IVDMD parameter showed no significant differences among treatments (p > 0.05), but the negative control group had significantly higher values compared to the other treatments. The product factor was significant (p < 0.05), with unprotected BSFLO showing higher digestibility (37.95%) than encapsulated BSFLO. The level factor was also significant (p < 0.05), with the 2% level achieving the highest digestibility (39.60%).

Table 5.
Effect of unprotected black soldier fly larvae oil (BSFLO) and encapsulated BSFLO combined with utilization level on in vitro dry matter digestibility (IVDMD).
After 48 h of incubation, the rumen IVDMD parameters demonstrated linear significance (p < 0.05), with the highest values observed in the negative control, 2% encapsulated BSFLO, and 4% encapsulated BSFLO treatments (49.39%, 50.75%, and 48.33%, respectively). Following incubation with liquid pepsin, the IVDMD abomasum parameters also exhibited linear significance (p < 0.05), with the highest values recorded in the negative control and 2% encapsulated BSFLO treatments (60.77% and 59.24%, respectively). The results of digestibility in the rumen and abomasum found that a higher level of encapsulated BSFLO will reduce the IVDMD value in the rumen and abomasum.
4. Discussion
4.1. Kinetics and Cumulative Production of Gas
The soluble fraction values for encapsulated BSFLO at all inclusion levels were higher than those for unprotected BSFLO at corresponding levels. Protected fat reduces the hydrogenation of unsaturated fatty acids, increasing their availability for fermentation, which enhances the soluble fraction in in vitro gas kinetics in the rumen [42]. This process aligns with findings that protected fat improves the bioavailability of unsaturated fatty acids, subsequently influencing gas kinetics and soluble fractions [22]. These results are consistent with prior studies showing that supplementation with protected fats, such as corn oil, enhances in vitro gas production, indicating increased fractional fermentation of soluble components [43].
The insoluble fraction values for unprotected BSFLO at 2% to 6% inclusion levels were lower compared to encapsulated BSFLO at 6% and 8%. Specifically, the use of encapsulated BSFLO at higher inclusion levels resulted in significantly reduced insoluble fraction values. The addition of protected fats reduces the rate of biohydrogenation of unsaturated fatty acids, which can influence the insoluble fraction in the rumen [44]. Microbial populations, particularly Butyrivibrio fibrisolvens, play a crucial role in lipolysis and the biohydrogenation of fatty acids. Manipulating these microbes can increase the flow of unsaturated fatty acids from the rumen, affecting the insoluble fraction [45]. These findings align with research indicating that protected fats, particularly at a 3% inclusion level, enhance gas production kinetics while modulating the insoluble fraction in rumen fermentation [46].
In this study, the cumulative gas production was higher for encapsulated BSFLO at low inclusion levels compared to unprotected BSFLO at higher levels. Rumen-protected fats can alter fermentation patterns by partially replacing carbohydrates with fats, which changes gas production dynamics [47]. The process of lipolysis may simultaneously increase total gas production by affecting ruminal microbial activity. Proper encapsulation of protected fats enhances gas production in the rumen [45], potentially increasing digestion rates without significantly altering passage rates [48].
In addition, for the cumulative gas production value, increasing the encapsulated BSFLO content decreased the cumulative gas production value. The inclusion of encapsulated BSFLO may start to have a threshold effect on rumen fermentation if exceeded. Encapsulation techniques for BSFLO could potentially have an inhibitory effect on fermentation if included at higher levels [31].
Conversely, the lowest cumulative gas production was observed in unprotected BSFLO at higher inclusion levels, particularly at 6% and 8%. At low inclusion levels, the gas production potential was higher than at moderate or high levels. This could be attributed to the resilience of rumen microbial populations to small amounts of oil [20]. High levels of unprotected fats may suppress rumen bacterial density and activity, particularly cellulolytic bacteria, due to the toxic effects of unsaturated fatty acids in the diet [21]. These findings are consistent with Prachumchai and Cherdthong [18], who reported that high levels of unprotected BSFLO can be toxic to rumen microbes. Oils can disrupt the physical characteristics of the rumen, affecting viscosity and flow rate, which in turn alters the rate and extent of ruminal digestion [18].
4.2. In Vitro Ruminal pH, Protozoal Population, NH3-N, and CH4 Production
The inclusion of unprotected BSFLO and encapsulated BSFLO at all levels had no effect on rumen pH, indicating that these fats did not disrupt the acid–base balance in the rumen [22]. Similarly, protected fat supplementation did not significantly alter rumen pH, suggesting that both protected and unprotected fats maintain stable ruminal conditions without affecting acidity [49]. The rumen pH observed in this study ranged from 6.0 to 7.0, a range that reflects stability and suggests no disruption of rumen functionality [18]. These findings are consistent with those of Bain et al. [50], Hidayah et al. [51], and Suharti et al. [52], who reported that fat protection does not influence rumen pH.
The protozoal population was also unaffected by the inclusion of encapsulated or unprotected BSFLO at all levels. While long-chain unsaturated fatty acids and medium-chain saturated fatty acids have been shown to reduce protozoal populations in cattle fed high-grain diets [53], this effect was not observed here. However, both unprotected and encapsulated BSFLO treatments resulted in slightly lower protozoal counts compared to the negative control. This reduction is likely due to the fat content in both forms of BSFLO, which can suppress rumen protozoa [22]. Unsaturated C18 fatty acids in protected fats are known to be toxic to protozoa, effectively reducing their populations [54]. These findings align with Bain et al. [50], who reported no adverse effects of protected fats on protozoal populations or rumen metabolism, as well as with Suharti et al. [52] and Behan et al. [22], who found that fat protection does not significantly alter protozoal counts.
Rumen NH3-N concentrations were not affected by the inclusion of unprotected or encapsulated BSFLO at any level. Previous studies have shown that protected fat supplementation does not significantly influence NH3-N concentrations, as it does not interfere with the core rumen fermentation process, a conclusion supported by the stability of rumen pH and protozoal populations [22]. In this study, NH3-N stability was evident, with crude protein results showing no differences despite variation in ether extract values (Table 1). This outcome is attributed to the isonitrogenous diet, which ensured comparable nitrogen intake from protein sources [55]. These findings are consistent with studies on Garut sheep, beef cattle, and dairy cows, where protected fats had no effect on NH3-N levels or nitrogen balance [22]. Similarly, in vitro studies by Bain et al. [50] and Suharti et al. [52] confirmed that NH3-N levels were unaffected by fat protection.
Encapsulated BSFLO at all levels resulted in lower CH4 production compared to unprotected BSFLO. Higher inclusion levels of protected fats led to a greater reduction in CH4 emissions. This effect is due to protected fats replacing fermentable carbohydrates in the diet, thereby reducing the substrates available for methanogenesis [50]. Additionally, protected fats limit microbial accessibility by coating feed particles, further reducing CH4 production [56]. Unsaturated fatty acids (UFAs) in the diet as well as reducing the conversion of Methanobrevibacter, the main methanogen, thereby inhibit the enzymatic activities related to methanogenesis and CH4 production [57]. However, methane reduction in encapsulated BSFLO of 8% is lower compared to 2% and 4%. This could be due to possible microbial adaptation or inhibitory effects at higher concentrations. Microbial communities can adapt to varying concentrations of substrates, which may lead to reduced efficacy in methane reduction at higher concentrations. For example, certain methanogenic species may become more resilient, diminishing the overall impact of methane inhibitors [58]. These findings align with those of Hidayah et al. [51], who demonstrated that microencapsulation of flaxseed oil reduced CH4 emissions compared to unprotected flaxseed oil.
4.3. In Vitro Volatile Fatty Acid
The total VFA concentration was higher in the negative control and unprotected BSFLO treatments compared to encapsulated BSFLO at all levels. Among the treatments, unprotected BSFLO produced higher total VFA concentrations than encapsulated BSFLO. This reduction in total VFA with encapsulated BSFLO can be attributed to the coating materials used in encapsulation, which limit the release of nutrients, thereby reducing microbial fermentation activity and subsequent VFA production in the rumen [59]. Encapsulation delays the availability of rapidly fermentable substrates, leading to a decrease in microbial fermentation and VFA production [60]. Similarly, Hidayah et al. [51] reported that unprotected flaxseed oil produced total VFA levels comparable to microencapsulated flaxseed oil, likely due to the coating process restricting substrate availability and fermentation.
In this study, acetic acid concentrations were highest in the negative control compared to all treatments. However, at lower inclusion levels, unprotected BSFLO had higher acetic acid concentrations than encapsulated BSFLO at all levels. The higher acetic acid values in the negative control may be due to the absence of high fat levels, which can interfere with rumen microbial activity. Feed without added fat supports undisturbed microbial populations, resulting in higher VFA production [61]. In contrast, protected fats reduce the fermentation of unsaturated fatty acids in the rumen, which would otherwise contribute to acetic acid production [62]. Furthermore, protected fats increase the intestinal absorption of unsaturated fatty acids, reducing the reliance on rumen microbes to convert these fats into simpler compounds such as acetic acid. Consequently, acetic acid concentrations in the rumen are reduced [62]. These findings align with those of Hidayah et al. [51], who observed higher acetic acid levels with unprotected flaxseed oil compared to microencapsulated flaxseed oil.
The acetate-to-propionate (A:P) ratio was higher in the negative control compared to the treatments. However, unprotected BSFLO at lower levels had a higher A:P ratio than encapsulated BSFLO at all levels. Diets supplemented with protected fats tend to increase propionate concentrations in the rumen more significantly than unprotected fats [62]. This results in a reduced A:P ratio in treatments with protected fats. The reduction in the A:P ratio is attributed to the diminished biohydrogenation of unsaturated fatty acids, allowing more fatty acids to bypass the rumen and be absorbed in the lower gut [62]. Protected fats have a more pronounced effect in lowering the A:P ratio compared to unprotected fats [48]. These findings are consistent with Hidayah et al. [51], who reported that unprotected flaxseed oil exhibited a higher A:P ratio than microencapsulated flaxseed oil. While both protected and unprotected fat sources generally decrease the A:P ratio, the extent of reduction varies depending on the type and level of fat inclusion.
4.4. In Vitro Degradability
Research according to Prachumcai et al. [63] states that the use of unprotected BSFLO in vivo on Thai native bulls has significant apparent digestibility results. The use of unprotected BSFLO on Thai native bulls is optimal at levels 1 and 2% with marked results of apparent digestibility on dry matter and organic matter which are higher, whereas if the level is increased at the level of 4%, digestibility in dry matter and organic matter drops.
The rumen IVDMD values for encapsulated BSFLO at all levels were higher than those for unprotected BSFLO, particularly at higher inclusion levels. The enhanced digestibility in the rumen can be attributed to the stable characteristics of the protected fat, which limited dissociation and altered the ruminal environment to support fermentation [64]. Unlike unprotected fats, which can be toxic to rumen microbes and reduce fiber digestibility, protected fats did not negatively affect microbial populations or fermentation parameters [22]. These findings align with Wulandari et al. [24], who reported that fat protection significantly increased feed digestibility values.
Unprotected BSFLO at higher inclusion levels had significantly lower rumen IVDMD values. This reduction in digestibility could result from physical barriers created by coating feed particles, which may have hindered microbial access. Additionally, certain toxic substances in unprotected fats could have disrupted rumen microbial populations, impairing fermentation and digestion processes [18]. These findings are consistent with Prachumchai and Cherdthong [18], who observed low digestibility values in unprotected BSFLO at high inclusion levels.
The IVDMD values in the abomasum were higher for encapsulated BSFLO at low levels compared to unprotected BSFLO, particularly at higher levels. Protected fats reduce the biohydrogenation of polyunsaturated fatty acids (PUFAs) in the rumen, resulting in higher concentrations of these fatty acids in the abomasal digesta [46]. By limiting biohydrogenation, protected fats allow a greater flow of unsaturated fatty acids to the abomasum compared to unprotected fats [48]. These results are consistent with Wulandari et al. [24], who reported higher post-ruminal IVDMD values for protected fats compared to unprotected fats. Additionally, the IVDMD values in the abomasum were higher than those in the rumen, which agrees with findings by Pramono et al. [65]. IVDMD in the rumen and abomasum decreased as the level of BSFLO encapsulation increased. This suggests that there is a threshold effect on rumen fermentation. Higher oil content, despite protection would correlate with a decrease in the altered volatile fatty acid profile, which could negatively impact overall fermentation efficiency and decrease digestibility [63].
5. Conclusions
The in vitro study demonstrated that encapsulated black soldier fly larvae oil (BSFLO) increased gas production, suggesting enhanced digestibility in both the rumen and abomasum compared to unprotected BSFLO. Additionally, compared to unprotected BSFLO, encapsulated BSFLO significantly reduced CH4 production, highlighting its potential as a sustainable methane mitigation strategy. However, it had no notable effects on NH3-N concentrations, rumen pH, or protozoal populations. In contrast, unprotected BSFLO increased total volatile fatty acids, acetic acid, and butyric acid concentrations. These findings suggest that encapsulated BSFLO can serve as a promising feed additive to improve nutrient utilization while reducing methane emissions. Reducing methane emissions may increase opportunities as a feeding strategy from a livestock nutrition perspective that can reduce environmental impacts to achieve sustainable livestock nutrition. Further in vivo research is necessary to validate its effectiveness, assess its long-term environmental benefits, side effects, economic considerations, and optimize its role in sustainable ruminant feeding strategies.
Author Contributions
Planning and design of the study, H.R.A., C.S. and A.C.; conducting and sampling, H.R.A. and A.C.; sample analysis, H.R.A. and C.S.; statistical analysis, H.R.A. and C.S.; manuscript drafting, H.R.A. and A.C.; manuscript editing and finalizing, H.R.A., C.S., S.W., C.B.I., A.J. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors express their sincere gratitude to the Fundamental Fund of Khon Kaen University, which has received funding support from the National Science, Research, and Innovation Fund (NSRF). Program on Toxic Substances, Microorganisms and Feed Additives in Livestock and Aquatic Animals for Food Safety, Khon Kaen University, is also acknowledged. Hajrian Rizqi Albarki received a grant from the ASEAN-GMS Scholarship, Khon Kaen University, Thailand.
Institutional Review Board Statement
The Institutional Animal Care and Use Committee of Khon Kaen University (KKU) confirmed all of the procedures using animals in this study’s metabolism experiments (Record No. IACUC-KKU-48/67).
Informed Consent Statement
This study did not involve human subjects; therefore, informed consent was not required.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to express our sincere thanks to the Tropical Feed Resources Research and Development Center (TROFREC), Department of Animal Science, Faculty of Agriculture, Khon Kaen University (KKU), for the use of the research facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Steinfeld, H. The livestock revolution—A global veterinary mission. Vet. Parasitol. 2004, 125, 19–41. [Google Scholar] [PubMed]
- Herrero, M.; Thornton, P.K. Livestock and global change: Emerging issues for sustainable food systems. Proc. Natl. Acad. Sci. USA 2013, 110, 20878–20881. [Google Scholar] [PubMed]
- Burrow, H.M. Overcoming major environmental and production challenges in cattle owned by smallholder farmers in the tropics. Caraka Tani J. Sustain. Agric. 2022, 37, 161. [Google Scholar]
- FAO. World Food and Agriculture—Statistical Yearbook 2021; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Guo, H.; Su, Z.; Yang, X.; Xu, S.; Pan, H. Greenhouse gas emissions from beef cattle breeding based on the ecological cycle model. Int. J. Environ. Res. Public Health 2022, 19, 9481. [Google Scholar] [CrossRef]
- Kozicka, K.; Žukovskis, J.; Wójcik-Gront, E. Explaining global trends in cattle population changes between 1961 and 2020 directly affecting methane emissions. Sustainability 2023, 15, 10533. [Google Scholar] [CrossRef]
- Hristov, A.N.; Melgar, A.; Wasson, D.; Arndt, C. Symposium review: Effective nutritional strategies to mitigate enteric methane in dairy cattle. J. Dairy Sci. 2022, 105, 8543–8557. [Google Scholar] [CrossRef]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animals 2010, 4, 351–365. [Google Scholar] [CrossRef]
- Patra, A.K. Enteric methane mitigation technologies for ruminant livestock: A synthesis of current research and future directions. Environ. Monit. Assess. 2012, 184, 1929–1952. [Google Scholar] [CrossRef]
- Canul-Solis, J.; Campos-Navarrete, M.; Piñeiro-Vázquez, A.; Casanova-Lugo, F.; Barros-Rodríguez, M.; Chay-Canul, A.; Cárdenas-Medina, J.; Castillo-Sánchez, L. Mitigation of rumen methane emissions with foliage and pods of tropical trees. Animals 2020, 10, 843. [Google Scholar] [CrossRef]
- Bačėninaitė, D.; Džermeikaitė, K.; Antanaitis, R. Global warming and dairy cattle: How to control and reduce methane emission. Animals 2022, 12, 2687. [Google Scholar] [CrossRef]
- Smith, S.B.; Gill, C.A.; Lunt, D.K.; Brooks, M.A. Regulation of fat and fatty acid composition in beef cattle. Asian-Australas. J. Anim. Sci. 2009, 22, 1225–1233. [Google Scholar] [CrossRef]
- Mapato, C.; Wanapat, M.; Cherdthong, A. Effects of urea treatment of straw and dietary level of vegetable oil on lactating dairy cows. Trop. Anim. Health Prod. 2010, 42, 1635–1642. [Google Scholar]
- Patra, A.K.; Yu, Z. Effects of essential oils on methane production and fermentation by, and abundance and diversity of, rumen microbial populations. Appl. Environ. Microbiol. 2012, 78, 4271–4280. [Google Scholar] [PubMed]
- Li, S.; Ji, H.; Zhang, B.; Tian, J.; Zhou, J.; Yu, H. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2016, 465, 43–52. [Google Scholar] [CrossRef]
- Van Heugten, E.; Martinez, G.; McComb, A.; Koutsos, L. Improvements in performance of nursery pigs provided with supplemental oil derived from black soldier fly (Hermetia illucens) larvae. Animals 2022, 12, 3251. [Google Scholar] [CrossRef]
- Phongpradist, R.; Semmarath, W.; Kiattisin, K.; Jiaranaikulwanitch, J.; Chaiyana, W.; Chaichit, S.; Phimolsiripol, Y.; Dejkriengkraikul, P.; Ampasavate, C. The in vitro effects of black soldier fly larvae (Hermetia illucens) oil as a high-functional active ingredient for inhibiting hyaluronidase, anti-oxidation benefits, whitening, and UVB protection. Front. Pharmacol. 2023, 14, 1243961. [Google Scholar] [CrossRef]
- Prachumchai, R.; Cherdthong, A. Black soldier fly larva oil in diets with roughage to concentrate ratios on fermentation characteristics, degradability, and methane generation. Animals 2023, 13, 2416. [Google Scholar] [CrossRef]
- Abubakr, A.; Alimon, A.R.; Yaakub, H.; Abdullah, N.; Ivan, M. Effect of feeding palm oil by-products based diets on total bacteria, cellulolytic bacteria and methanogenic archaea in the rumen of goats. PLoS ONE 2014, 9, e95713. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Alimon, A.R.; Yaakub, H.; Samsudin, A.A.; Candyrine, S.C.L.; Wan Mohamed, W.N.; Md Noh, A.; Fuat, M.A.; Mookiah, S. Effects of vegetable oil supplementation on rumen fermentation and microbial population in ruminant: A review. Trop. Anim. Health Prod. 2021, 53, 422. [Google Scholar] [CrossRef]
- Jenkins, T.C. Lipid metabolism in the rumen. J. Dairy Sci. 1993, 76, 3851–3863. [Google Scholar]
- Behan, A.A.; Loh, T.C.; Fakurazi, S.; Kaka, A.; Samsudin, A.A.; Kaka, U. Effects of supplementation of rumen protected fats on rumen ecology and digestibility of nutrients in sheep. Animals 2019, 9, 400. [Google Scholar] [CrossRef]
- Albarki, H.R.; Kusuma, R.I.; Daulai, M.S.; Suntara, C.; Iwai, C.B.; Jayanegara, A.; Cherdthong, A. Effects of rumen-protected fat on rumen fermentation products, meat characteristics, cattle performance, and milk quality: A meta-analysis. Anim. Feed Sci. Technol. 2024, 318, 116137. [Google Scholar] [CrossRef]
- Wulandari, W.; Widyobroto, B.P.; Noviandi, C.T.; Agus, A. In vitro digestibility and ruminal fermentation profile of pangola grass (Digitaria decumbens) supplemented with crude palm oil protected by sodium hydroxide. Livest. Res. Rural Dev. 2020, 32, 1–7. [Google Scholar]
- Riestanti, L.U.; Despal; Oktavianti, B.P.; Toharmat, T.; Retnani, Y. Effects of ca-soap protected vegetables oil in dairy ration on rumen fermentability and in vitro digestibility. IOP Conf. Ser. Earth Environ. Sci. 2023, 1168, 012023. [Google Scholar] [CrossRef]
- Behan, A.A.; Chwen, L.T.; Kaka, U.; Muhammad, A.I.; Samsudin, A.A. Effect of rumen-protected fat on in vitro rumen fermentation and apparent biohydrogenation of fatty acids. J. Indones. Trop. Anim. Agric. 2024, 49, 252–263. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulation: An overview on concepts, methods, properties and applications in foods. Food Front. 2021, 2, 426–442. [Google Scholar]
- Akonjuen, B.M.; Aryee, A.N.A. Novel extraction and encapsulation strategies for food bioactive lipids to improve stability and control delivery. Food Chem. Adv. 2023, 2, 100278. [Google Scholar]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of active ingredients in polysaccharide–protein complex coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef]
- Muslykhah, U.; Phupaboon, S.; Suriyapha, C.; Matra, M.; Wanapat, M. Encapsulation of protein-based bioactive from black soldier fly for ruminant feeding. J. Agric. Food Res. 2024, 18, 101325. [Google Scholar] [CrossRef]
- Phupaboon, S.; Matra, M.; Prommachart, R.; Totakul, P.; Supapong, C.; Wanapat, M. Extraction, characterization, and chitosan microencapsulation of bioactive compounds from Cannabis sativa L., Cannabis indica L., and Mitragyna speiosa K. Antioxidants 2022, 11, 2103. [Google Scholar] [CrossRef]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [PubMed]
- Tassone, S.; Fortina, R.; Peiretti, P.G. In vitro techniques using the DaisyII incubator for the assessment of digestibility: A review. Animals 2020, 10, 775. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar]
- Fawcett, J.K.; Scott, J.E. A rapid and precise method for the determination of urea. J. Clin. Pathol. 1960, 13, 156–159. [Google Scholar]
- Porter, M.G.; Murray, R.S. The volatility of components of grass silage on oven drying and the inter-relationship between dry-matter content estimated by different analytical methods. Grass Forage Sci. 2001, 56, 405–411. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar]
- Williams, S.R.O.; Hannah, M.C.; Jacobs, J.L.; Wales, W.J.; Moate, P.J. Volatile fatty acids in ruminal fluid can be used to predict methane yield of dairy cows. Animals 2019, 9, 1006. [Google Scholar] [CrossRef]
- Tiven, N.C.; Hartati, L.; Simanjorang, T.M. Liquid smoke as fat protector and its effect on rumen fermentation characteristics and microbial activity. Trop. Anim. Sci. J. 2021, 44, 152–159. [Google Scholar]
- Kim, T.-B.; Lee, J.-S.; Cho, S.-Y.; Lee, H.-G. In vitro and in vivo studies of rumen-protected microencapsulated supplement comprising linseed oil, vitamin E, rosemary extract, and hydrogenated palm oil on rumen fermentation, physiological profile, milk yield, and milk composition in dairy cows. Animals 2020, 10, 1631. [Google Scholar] [CrossRef] [PubMed]
- Beam, T.M.; Jenkins, T.C.; Moate, P.J.; Kohn, R.A.; Palmquist, D.L. Effects of amount and source of fat on the rates of lipolysis and biohydrogenation of fatty acids in ruminal contents. J. Dairy Sci. 2000, 83, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Clement Hawke, J. The in vitro lipolysis and biohydrogenation of monogalactosyldiglyceride by whole rumen contents and its fractions. J. Sci. Food Agric. 1979, 30, 603–612. [Google Scholar]
- Álvarez-Torres, J.N.; Ramírez-Bribiesca, J.E.; Bautista-Martínez, Y.; Crosby-Galván, M.M.; Granados-Rivera, L.D.; Ramírez-Mella, M.; Ruiz-González, A. Stability and effects of protected palmitic acid on in vitro rumen degradability and fermentation in lactating goats. Fermentation 2024, 10, 110. [Google Scholar] [CrossRef]
- Machmuller, A.; Ossowski, D.A.; Wanner, M.; Kreuzer, M. Potential of various fatty feeds to reduce methane release from rumen fermentation in vitro. Anim. Feed Sci. Technol. 1998, 71, 117–130. [Google Scholar]
- Bettero, V.P.; Valle, T.A.D.; Barletta, R.V.; de Araújo, C.E.; de Jesus, E.F.; de Almeida, G.F.; Takiya, C.S.; Zanferari, F.; de Paiva, P.G.; Júnior, J.E.; et al. Use of protected fat sources to reduce fatty acid biohydrogenation and improve abomasal flow in dry dairy cows. Anim. Feed Sci. Technol. 2017, 224, 30–38. [Google Scholar] [CrossRef]
- Simionatto, M.; Maeda, E.M.; Da Silveira, M.F.; Macedo, V.D.P.; De Paula, F.L.M.; Hill, J.A.G. Effect of adding different levels of palm oil-protected fat in the diet of lambs concerning rumen parameters. Anim. Feed Sci. Technol. 2024, 310, 115929. [Google Scholar] [CrossRef]
- Bain, A.; Wiryawan, K.G.; Astuti, D.; Suharti, S.; Arman, C.; Nasiu, F. Characteristics of in vitro fermentation and nutrient digestibility of ration supplemented with different level of soybean oil calcium soap. IOP Conf. Ser. Earth Environ. Sci. 2020, 465, 012020. [Google Scholar] [CrossRef]
- Hidayah, N.; Suharti, S.; Wiryawan, K.G. In-vitro ruminal ecosystem in buffaloes on concentrates and fat supplementation. Media Peternak. 2014, 37, 129–135. [Google Scholar]
- Suharti, S.; Nasution, A.R.; Wiryawan, K.G. In vitro rumen fermentation characteristics and fatty acid profiles added with calcium soap of canola/flaxseed oil. Media Peternak. 2017, 40, 171–177. [Google Scholar]
- Hristov, A.N.; Ivan, M.; McAllister, T.A. In vitro effects of individual fatty acids on protozoal numbers and on fermentation products in ruminal fluid from cattle fed a high-concentrate, barley-based diet. J. Anim. Sci. 2004, 82, 2693–2704. [Google Scholar] [CrossRef]
- Hegarty, R.S. Reducing rumen methane emissions through elimination of rumen protozoa. Aust. J. Agric. Res. 1999, 50, 1321. [Google Scholar] [CrossRef]
- Monteyne, A.J.; Dunlop, M.V.; Machin, D.J.; Coelho, M.O.C.; Pavis, G.F.; Porter, C.; Murton, A.J.; Abdelrahman, D.R.; Dirks, M.L.; Stephens, F.B.; et al. A mycoprotein-based high-protein vegan diet supports equivalent daily myofibrillar protein synthesis rates compared with an isonitrogenous omnivorous diet in older adults: A randomized controlled trial. Br. J. Nutr. 2021, 126, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Machmüller, A.; Ossowski, D.A.; Kreuzer, M. Comparative evaluation of the effects of coconut oil, oilseeds and crystalline fat on methane release, digestion and energy balance in lambs. Anim. Feed Sci. Technol. 2000, 85, 41–60. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, Y.; Liu, S.; Xie, T.; Wang, Q.; Wang, Z.; Li, S.; Wang, W. Rumen metagenome reveals the mechanism of mitigation methane emissions by unsaturated fatty acid while maintaining the performance of dairy cows. Anim. Nutr. 2024, 18, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Habtewold, J.; Gordon, R.; Sokolov, V.; VanderZaag, A.; Wagner-Riddle, C.; Dunfield, K. Reduction in methane emissions from acidified dairy slurry is related to inhibition of Methanosarcina species. Front. Microbiol. 2018, 9, 2806. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Zhang, M.; Yang, H.; Zhao, F.; Jiang, N.; Zhang, A. In situ and in vitro evaluation of the bioavailability of rumen-protected methionine with coating prototypes. J. Mech. Behav. Biomed. Mater. 2022, 133, 105355. [Google Scholar] [CrossRef]
- Gawad, R.; Fellner, V. Evaluation of glycerol encapsulated with alginate and alginate-chitosan polymers in gut environment and its resistance to rumen microbial degradation. Asian-Australas. J. Anim. Sci. 2019, 32, 72–81. [Google Scholar] [CrossRef]
- Amanullah, S.M.; Lee, S.-S.; Paradhipta, D.H.V.; Joo, Y.-H.; Kim, D.-H.; Seong, P.-N.; Jeong, S.-M.; Kim, S.-C. Impact of oil sources on in vitro fermentation, microbes, greenhouse gas, and fatty acid profile in the rumen. Fermentation 2022, 8, 242. [Google Scholar] [CrossRef]
- Mattos, W.; Palmquist, D.L. Increased polyunsaturated fatty acid yields in milk of cows fed protected fat. J. Dairy Sci. 1974, 57, 1050–1054. [Google Scholar] [CrossRef]
- Prachumchai, R.; Suntara, C.; Kanakai, N.; Cherdthong, A. Inclusion of black soldier fly larval oil in ruminant diets influences feed consumption, nutritional digestibility, ruminal characteristics, and methane estimation in Thai-indigenous steers. J. Anim. Physiol. Anim. Nutr. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Sukhija, P.S.; Palmquist, D.L. Dissociation of calcium soaps of long-chain fatty acids in rumen fluid. J. Dairy Sci. 1990, 73, 1784–1787. [Google Scholar] [CrossRef] [PubMed]
- Pramono, A.; Hadi, R.F.; Sutrisno, J.; Cahyadi, M. The effect of protected soybean oil and soybean groats base on in vitro dry matter digestibility, in vitro organic matter digestibility in the rumen and post rumen. IOP Conf. Ser. Earth Environ. Sci. 2019, 347, 012016. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).