Dissolved Oxygen Changes in Wastewater During Sulfamethoxazole Degradation by Photo-Fenton Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results
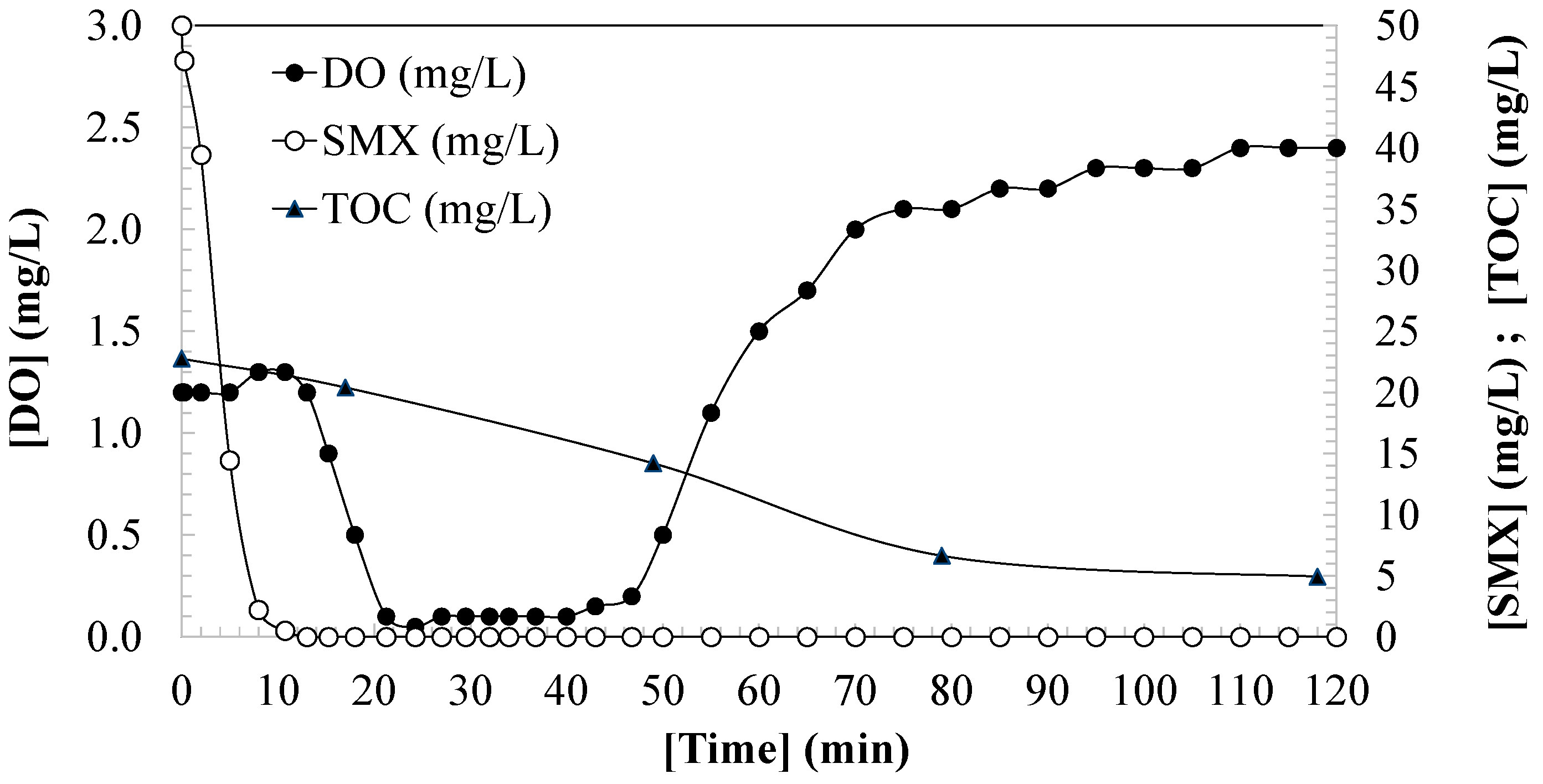
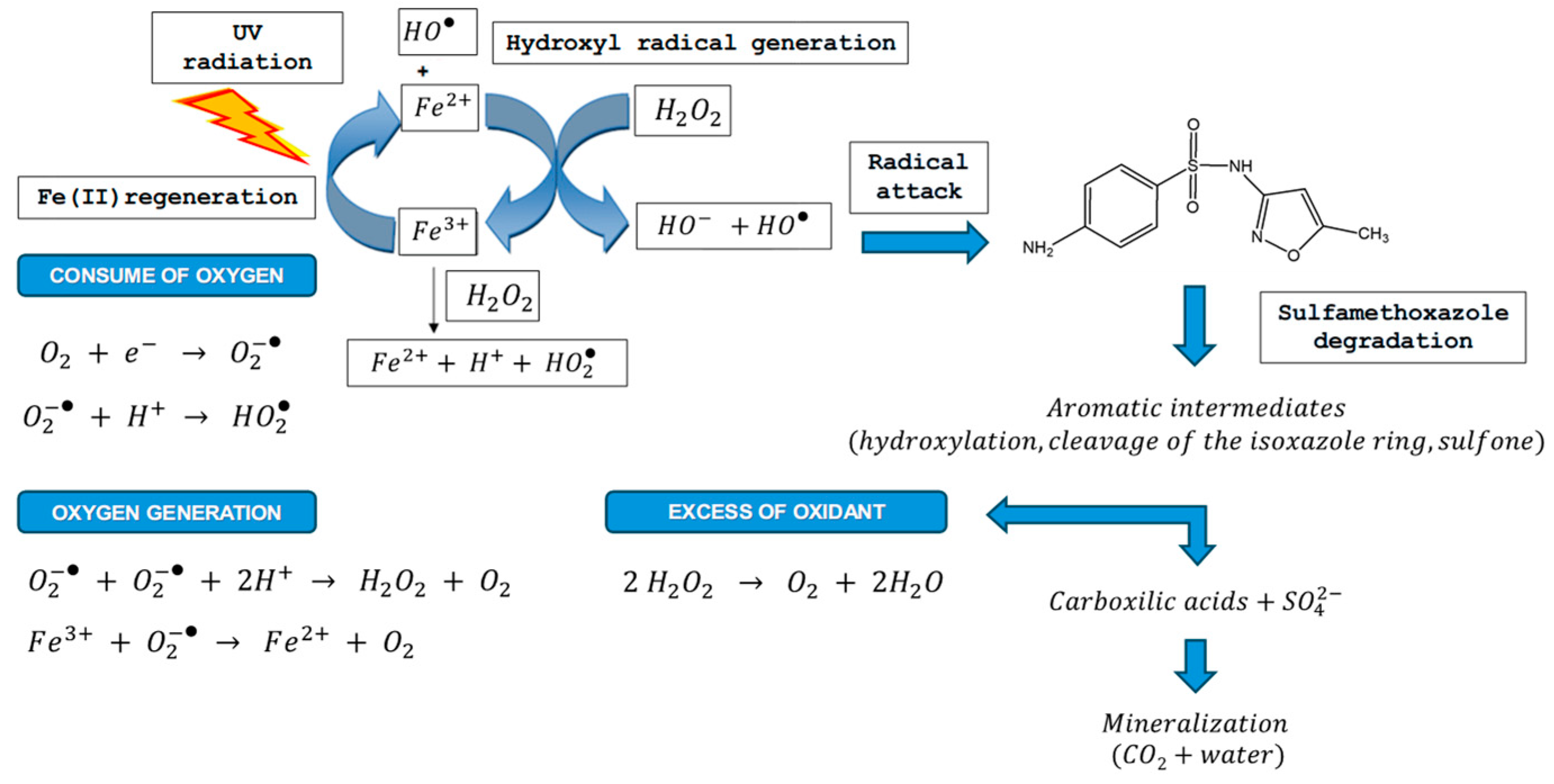
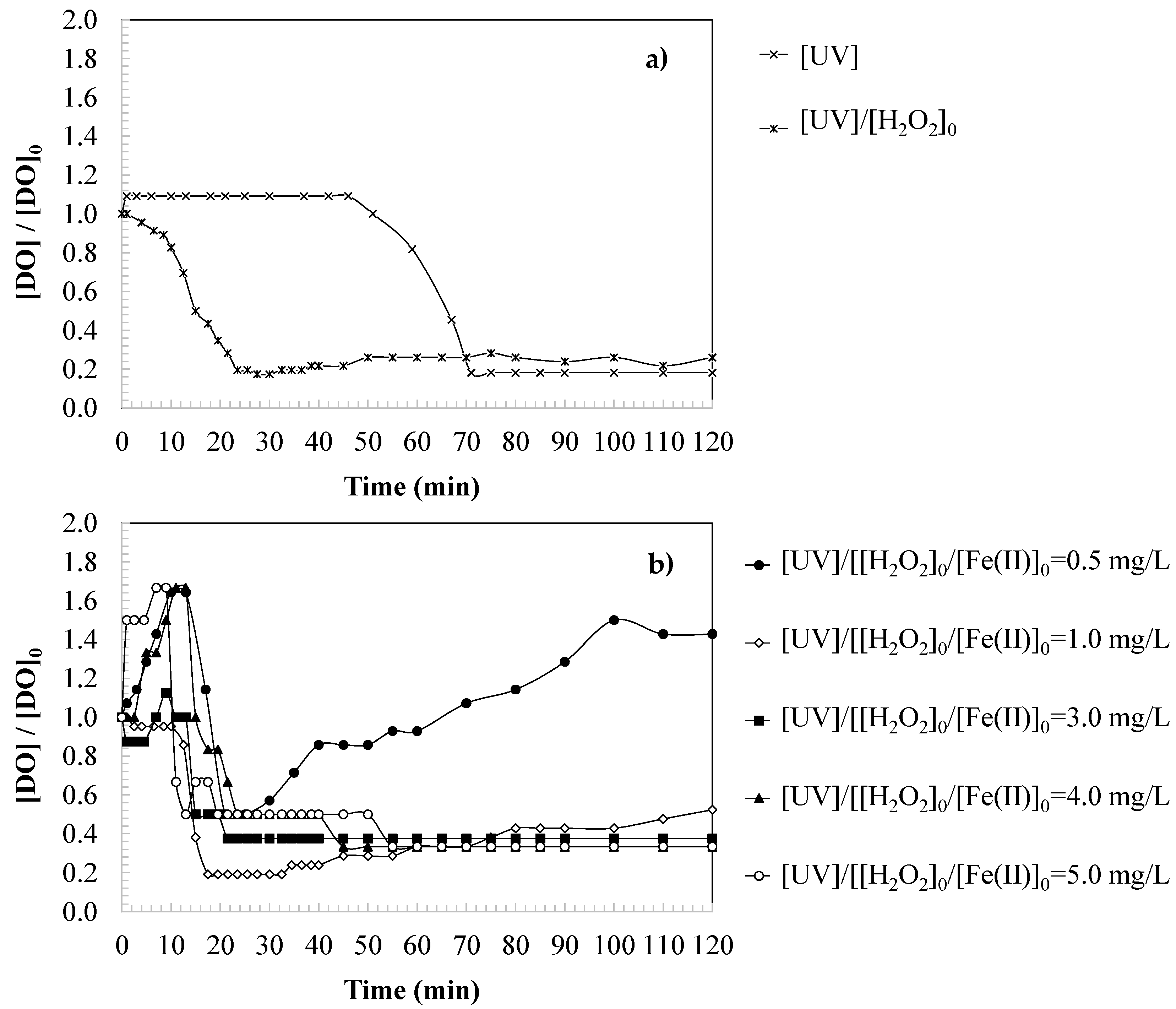
3.1. Changes in Dissolved Oxygen During SMX Oxidation
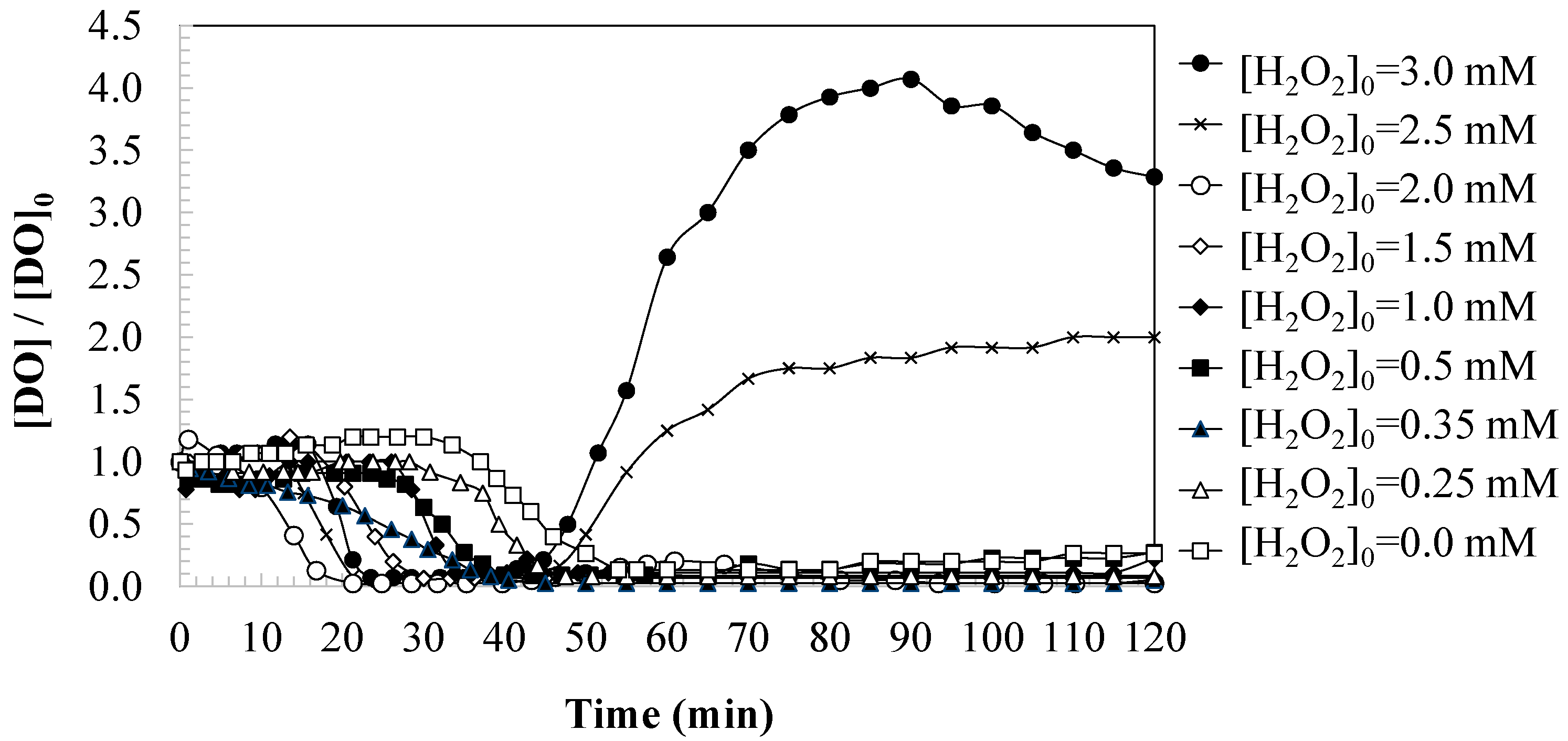
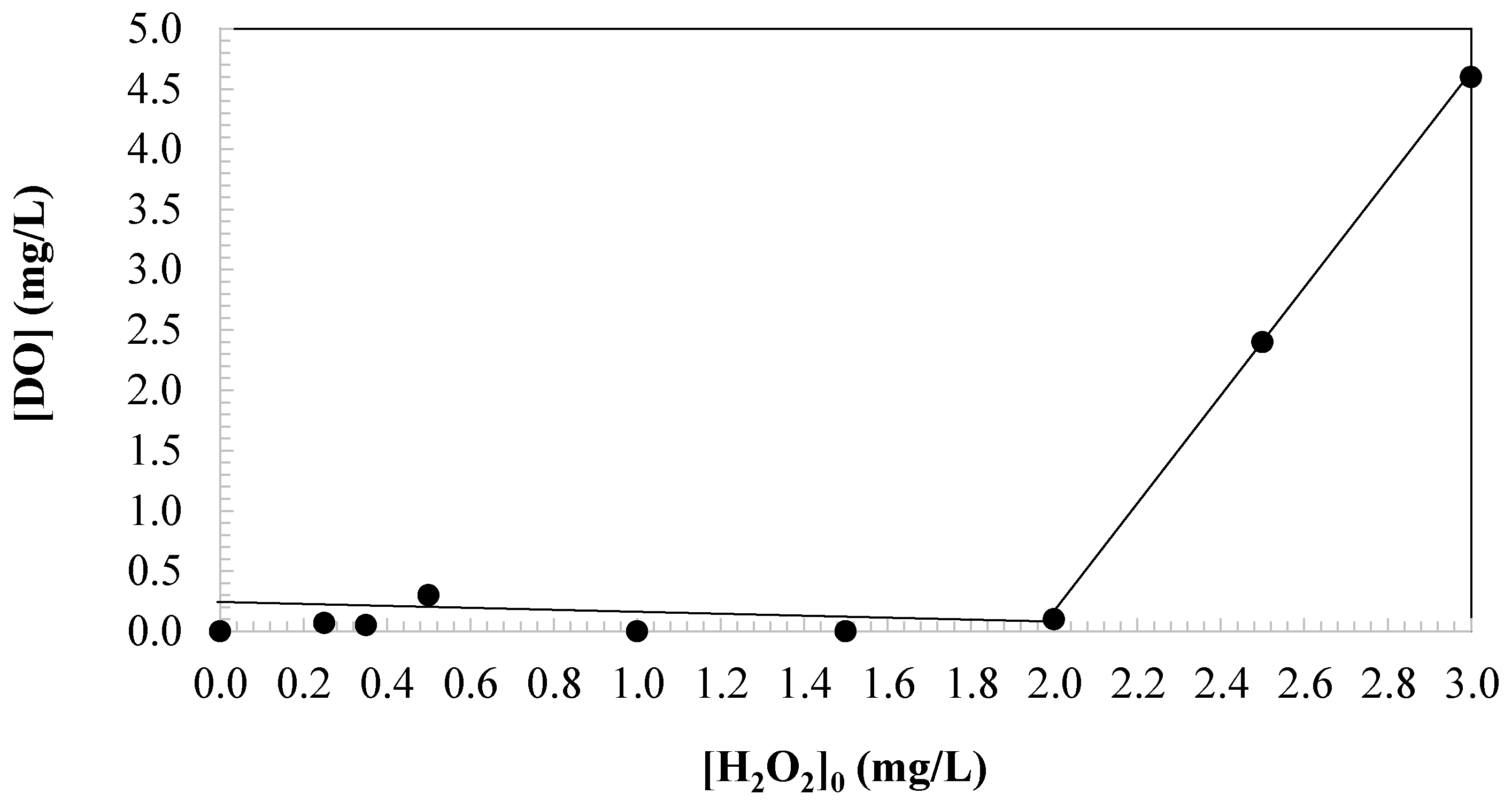
3.2. Effect of Oxidant Dosage on Changes in Dissolved Oxygen
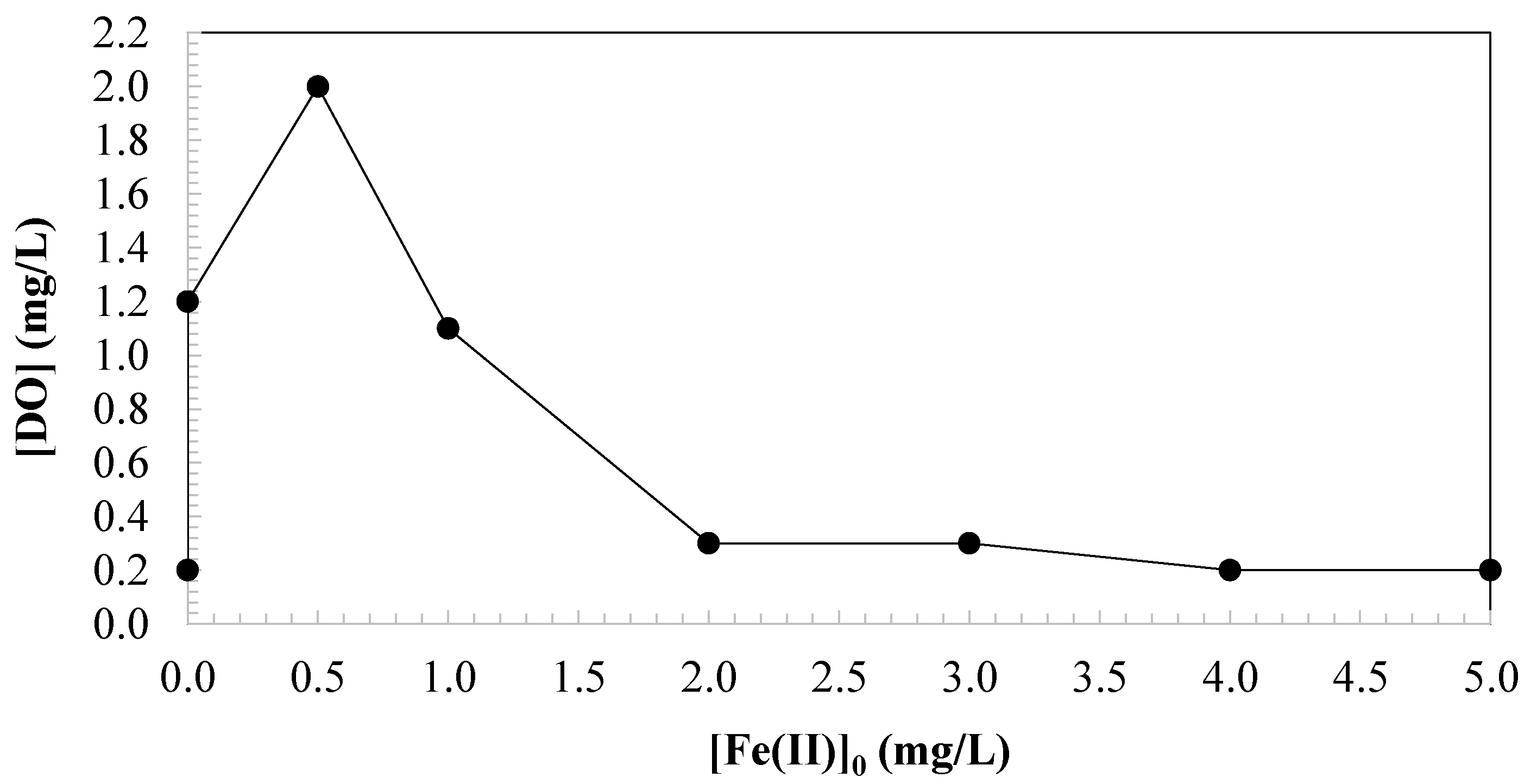
3.3. Effect of Iron Catalyst Dosage on Changes in Dissolved Oxygen
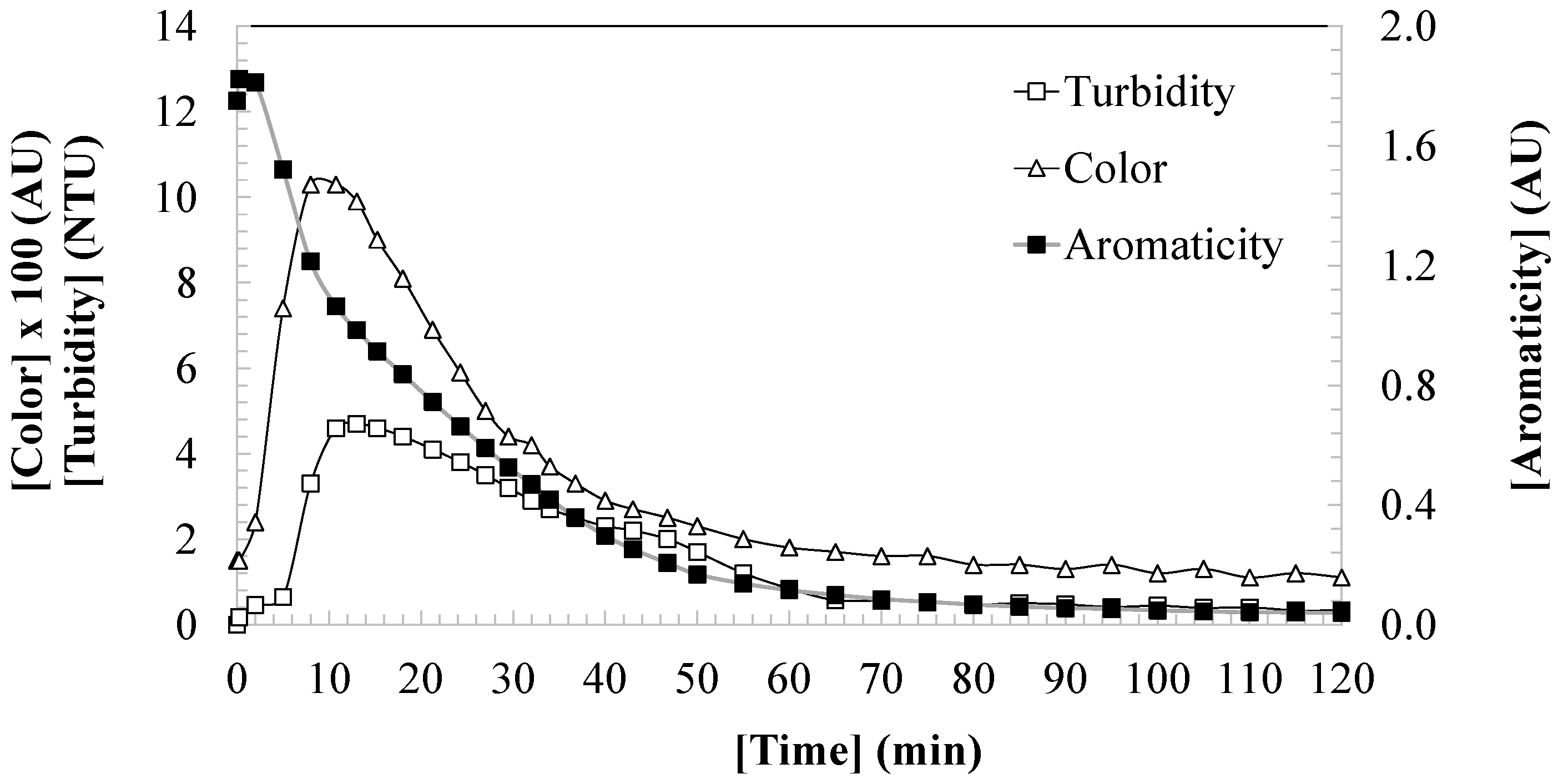
3.4. Analysis of the Quality of Treated Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, W.; Chen, H.; Liu, X.; Zhang, L.; Ding, H.; Deng, C. Enhanced sulfamethoxazole degradation in aqueous media using a synergistic system with dielectric barrier discharge plasma, biochar-enriched iron concentrate, and persulfate. J. Water Process Eng. 2025, 69, 106579. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Liang, L.; Duan, X.; Li, R.; Lu, X.; Yan, B.; Li, N.; Wang, S. Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. J. Hazard. Mater. 2021, 408, 124461. [Google Scholar] [CrossRef]
- Cai, D.; Ding, J.; Li, F.; Zhuang, G.; Li, M.; Guo, L. Sulfonamide disinfection byproducts exhibited severe toxicity to human commensal bacteria. Water Res. 2024, 256, 121551. [Google Scholar] [CrossRef]
- Szymańska, U.; Wiergowski, M.; Sołtyszewski, I.; Kuzemko, J.; Wiergowska, G.; Woźniak, M.K. Presence of antibiotics in the aquatic environment in Europe and their analytical monitoring: Recent trends and perspectives. Microchem. J. 2019, 147, 729–740. [Google Scholar] [CrossRef]
- Sun, F.; Jiao, Y.; Liang, S.; Zhuang, L.; Zhang, J. The effect of sulfamethoxazole on the growth of microalgae biofilms and the internal transportation and transformation of nutrients in the biofilm. Environ. Res. 2025, 273, 121232. [Google Scholar] [CrossRef]
- Caruncho-Pérez, S.; Bernárdez, N.; Pazos, M.; Sanromán, M.Á.; González-Romero, E. Voltammetric methodology for the quality control and monitoring of sulfamethoxazole removal from water. Talanta 2025, 284, 127255. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ali, A.; Su, J.; Liu, S.; Yan, H.; Xu, L. Removal of sulfamethoxazole from water by biosurfactant-modified sludge biochar: Properties and mechanism. J. Environ. Chem. Eng. 2024, 12, 114200. [Google Scholar] [CrossRef]
- Yang, G.; Zhen, Z.; Wu, W.; Yang, C.; Li, Q.; Li, X.; Yin, J.; Zhong, X.; Lin, Z.; Zhang, D. Mechanisms of Sulfamethoxazole biodegradation in mangrove rhizosphere by metagenomic and metabolic pathways. Environ. Technol. Innov. 2025, 37, 103970. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Jin, Q.; Zhu, F. Effects of the aquatic pollutant sulfamethoxazole on the innate immunity and antioxidant capacity of the mud crab Scylla paramamosain. Chemosphere 2024, 349, 140775. [Google Scholar] [CrossRef]
- Tian, S.; You, L.; Huang, X.; Liu, C.; Su, J. Efficient sulfamethoxazole biotransformation and detoxification by newly isolated strain Hydrogenophaga sp. SNF1 via a ring ortho-hydroxylation pathway. J. Hazard. Mater. 2024, 480, 136113. [Google Scholar] [CrossRef] [PubMed]
- Dirany, A.; Sirés, I.; Oturan, N.; Oturan, M.A. Electrochemical abatement of the antibiotic sulfamethoxazole from water. Chemosphere 2010, 81, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Neyrot, S.; Acha, D.; Morales-Belpaire, I. The fate of sulfamethoxazole in microcosms of the macrophyte Schoenoplectus californicus and its impact on microbial communities. Environ. Pollut. 2024, 362, 124947. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Qian, J.; Xia, J.; Li, L.; Wang, S.; Zeng, Z.; Mao, J.; Ahamad, M.; Xiao, Z.; Zhang, Q. Efficient photoelectrocatalytic degradation of pollutants over hydrophobic carbon felt loaded with Fe-doped porous carbon nitride via direct activation of molecular oxygen. Environ. Res. 2024, 249, 118497. [Google Scholar] [CrossRef]
- Saheed, I.O.; Ying, L.R.; Yaacob, S.F.F.S.; Hanafiah, M.A.K.M.; Latip, A.F.A.; Suah, F.B.M. Adsorption of sulfamethoxazole in an aqueous environment onto a novel magnetic sporopollenin–cellulose triacetate. Int. J. Biol. Macromol. 2025, 296, 139787. [Google Scholar] [CrossRef]
- Całus-Makowska, K.; Grosser, A.; Grobelak, A.; Białek, H.; Siedlecka, E. Kinetic study of the simultaneous removal of ibuprofen, carbamazepine, sulfamethoxazole, and diclofenac from water using biochar and activated carbon adsorption, and TiO2 photocatalysis. Desalination Water Treat. 2024, 320, 100817. [Google Scholar] [CrossRef]
- Bux, N.; Tumrani, S.H.; Soomro, R.A.; Ma, Q.; Zhou, J.; Wang, T. Catalytic degradation of organic pollutants in aqueous systems: A comprehensive review of peroxyacetic acid-based advanced oxidation processes. J. Environ. Manag. 2025, 373, 123989. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, Y.; Ma, C.; Sun, Y.; Ding, G. Degradation of aqueous organic pollutants by dual oxidant advanced oxidation processes: A comprehensive review. J. Environ. Chem. Eng. 2024, 12, 114174. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, A. Advanced oxidation process: A remediation technique for organic and non-biodegradable pollutant. Results Surf. Interfaces 2023, 11, 100122. [Google Scholar] [CrossRef]
- Okpara, E.C.; Wojuola, O.B.; Quadri, T.W.; Banks, C.E. An overview of advanced oxidation processes using copper-based catalytic degradation of organic pollutants in water. Appl. Mater. Today 2024, 36, 102053. [Google Scholar] [CrossRef]
- Pal, D.; Kumar, V.; Sahu, G.; Wasewar, K.L. Process intensification in wastewater treatments: Advanced oxidation processes for organic pollutants. In Contamination of Water; Ahamad, A., Siddiqui, S.I., Singh, P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 351–361. [Google Scholar]
- Li, S.; Yang, Y.; Zheng, H.; Zheng, Y.; Jing, T.; Ma, J.; Nan, J.; Leong, Y.K.; Chang, J. Advanced oxidation process based on hydroxyl and sulfate radicals to degrade refractory organic pollutants in landfill leachate. Chemosphere 2022, 297, 134214. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Shah, M.P. Advanced oxidation processes for complex wastewater treatment. In Advanced Oxidation Processes for Effluent Treatment Plants; Shah, M.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–31. [Google Scholar]
- Khan, Z.U.H.; Gul, N.S.; Sabahat, S.; Sun, J.; Tahir, K.; Shah, N.S.; Muhammad, N.; Rahim, A.; Imran, M.; Iqbal, J.; et al. Removal of organic pollutants through hydroxyl radical-based advanced oxidation processes. Ecotoxicol. Environ. Saf. 2023, 267, 115564. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Shi, J.; Nie, L.; Xue, K.; Gao, Y.; Shi, J. Photo-Fenton degradation of oxytetracycline by g-C3N4/CQDs/FeOCl with in-situ hydrogen peroxide production: Degradation pathway and toxicity analysis of intermediate products. J. Water Process Eng. 2025, 69, 106615. [Google Scholar] [CrossRef]
- Shalini; Setty, Y.P. Fenton/photo-Fenton processes for wastewater treatment and disinfection. In Novel Approaches Towards Wastewater Treatment and Resource Recovery Technologies; Mungray, A., Mungray, A., Sonawane, S., Sonawane, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 241–252. [Google Scholar]
- Peralta-Hernández, J.M.; Vijay, S.; Rodríguez-Narváez, O.; Pacheco-Álvarez, M.A. Photo and solar Fenton processes for wastewater treatment. In Electrochemical Water and Wastewater Treatment; Martínez-Huitle, C.A., Rodrigo, M.A., Scialdone, O., Eds.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 223–237. [Google Scholar]
- Çalık, Ç.; Çifçi, D.İ. Comparison of kinetics and costs of Fenton and photo-Fenton processes used for the treatment of a textile industry wastewater. J. Environ. Manag. 2022, 304, 114234. [Google Scholar] [CrossRef]
- Módenes, A.N.; Espinoza-Quiñones, F.R.; Manenti, D.R.; Borba, F.H.; Palácio, S.M.; Colombo, A. Performance evaluation of a photo-Fenton process applied to pollutant removal from textile effluents in a batch system. J. Environ. Manag. 2012, 104, 1–8. [Google Scholar] [CrossRef]
- Carbajo, J.; Silveira, J.E.; Pliego, G.; Zazo, J.A.; Casas, J.A. Increasing Photo-Fenton process efficiency: The effect of high temperatures. Sep. Purif. Technol. 2021, 271, 118876. [Google Scholar] [CrossRef]
- Santos-Juanes, L.; Sánchez, J.L.G.; López, J.L.C.; Oller, I.; Malato, S.; Sánchez Pérez, J.A. Dissolved oxygen concentration: A key parameter in monitoring the photo-Fenton process. Appl. Catal. B Environ. 2011, 104, 316–323. [Google Scholar] [CrossRef]
- Cabrera Reina, A.; Santos-Juanes Jordá, L.; García Sánchez, J.L.; Casas López, J.L.; Sánchez Pérez, J.A. Modelling photo-Fenton process for organic matter mineralization, hydrogen peroxide consumption and dissolved oxygen evolution. Appl. Catal. B Environ. 2012, 119–120, 132–138. [Google Scholar] [CrossRef]
- Prieto-Rodríguez, L.; Oller, I.; Zapata, A.; Agüera, A.; Malato, S. Hydrogen peroxide automatic dosing based on dissolved oxygen concentration during solar photo-Fenton. Catal. Today 2011, 161, 247–254. [Google Scholar] [CrossRef]
- Villota, N.; Duoandicoechea, U.; Lombraña, J.I.; De Luis, A.M. Kinetic modelling of aromaticity and colour changes during the degradation of sulfamethoxazole using photo-Fenton technology. Catalysts 2024, 14, 718. [Google Scholar] [CrossRef]
- Villota, N.; Jankelevitch, S.; Lomas, J.M. Kinetic modelling of colour and turbidity formation in aqueous solutions of sulphamethoxazole degraded by UV/H2O2. Environ. Technol. 2024, 45, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Kovalevskiy, N.S.; Lyulyukin, M.N.; Selishchev, D.S.; Kozlov, D.V. Analysis of air photocatalytic purification using a total hazard index: Effect of the composite TiO2/zeolite photocatalyst. J. Hazard. Mater. 2018, 358, 302–309. [Google Scholar] [CrossRef]
- Centeno-Bordones, G.; Jimenez, Y. Uso de Lodos Rojos como catalizador en los procesos de oxidación avanzada: Una aproximación al estado del arte. Tékhne 2018, 21, 26–40. [Google Scholar]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Bossmann, S.H.; Oliveros, E.; Göb, S.; Siegwart, S.; Dahlen, E.P.; Payawan, L., Jr.; Straub, M.; Wörner, M.; Braun, A.M. New evidence against hydroxyl radicals as reactive intermediates in the thermal and photochemically enhanced Fenton reactions. J. Phys. Chem. A 1998, 102, 5542–5550. [Google Scholar] [CrossRef]
- Goldstein, S.; Meyerstein, D.; Czapski, G. The Fenton reagents. Free Radic. Biol. Med. 1993, 15, 435–445. [Google Scholar] [CrossRef]
- Shang, K.; Feng, X.; Wang, Y. Efficient Degradation of sulfamethoxazole via reactive oxygen species produced from activated peroxymonosulfate by MgCoFe-LDO catalyst. Catalysts 2024, 14, 6606–6620. [Google Scholar] [CrossRef]
- Walling, C. Fenton’s reagent revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- De Laat, J.; Le, T.G. Kinetics and modeling of the Fe3⁺/H2O2 system in the presence of sulfate in acidic aqueous solutions. Environ. Sci. Technol. 2006, 40, 4024–4030. [Google Scholar]
- Li, Y.; He, J.; Zhang, K.; Hong, P.; Wang, C.; Kong, L.; Liu, J. Oxidative degradation of sulfamethoxazole antibiotic catalyzed by porous magnetic manganese ferrite nanoparticles: Mechanism and by-products identification. J. Mater. Sci. 2020, 55, 13767–13784. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O⁻) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Nogueira, R.F.P.; Oliveira, M.C.; Paterlini, W.C. Simple and Fast Spectrophotometric Determination of H2O2 in Photo-Fenton Reactions Using Metavanadate. Talanta 2005, 66, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Benitez, F.J.; Beltrán-Heredia, J.; Acero, J.L.; Rubio, F.J. Oxidation of Phenolic Wastes with Fenton’s Reagent. Water Res. 2001, 35, 1047–1053. [Google Scholar]
- Villota, N.; Coralli, I.; Lomas, J.M. Changes of dissolved oxygen in aqueous solutions of caffeine oxidized by photo-Fenton reagent. Environ. Technol. 2021, 42, 609–617. [Google Scholar] [CrossRef]

| [H2O2]0 mM | [Fe(II)]0 mg/L | [DO] mg/L | [H2O2]0 mM | [Fe(II)]0 mg/L | [DO] mg/L |
|---|---|---|---|---|---|
| 0.00 | 0.50 | 0.40 | 0.00 | 0.00 | 0.20 |
| 0.25 | 0.50 | 0.10 | 2.00 | 0.00 | 1.20 |
| 0.35 | 0.50 | 0.20 | 2.00 | 0.50 | 2.00 |
| 0.50 | 0.50 | 0.30 | 2.00 | 1.00 | 1.10 |
| 1.00 | 0.50 | 0.20 | 2.00 | 2.00 | 0.30 |
| 1.50 | 0.50 | 0.10 | 2.00 | 3.00 | 0.30 |
| 2.00 | 0.50 | 0.10 | 2.00 | 4.00 | 0.20 |
| 3.00 | 0.50 | 4.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilbao-García, E.; Duoandicoechea, U.; Villota, N. Dissolved Oxygen Changes in Wastewater During Sulfamethoxazole Degradation by Photo-Fenton Treatment. Sustainability 2025, 17, 3333. https://doi.org/10.3390/su17083333
Bilbao-García E, Duoandicoechea U, Villota N. Dissolved Oxygen Changes in Wastewater During Sulfamethoxazole Degradation by Photo-Fenton Treatment. Sustainability. 2025; 17(8):3333. https://doi.org/10.3390/su17083333
Chicago/Turabian StyleBilbao-García, Elisabeth, Unai Duoandicoechea, and Natalia Villota. 2025. "Dissolved Oxygen Changes in Wastewater During Sulfamethoxazole Degradation by Photo-Fenton Treatment" Sustainability 17, no. 8: 3333. https://doi.org/10.3390/su17083333
APA StyleBilbao-García, E., Duoandicoechea, U., & Villota, N. (2025). Dissolved Oxygen Changes in Wastewater During Sulfamethoxazole Degradation by Photo-Fenton Treatment. Sustainability, 17(8), 3333. https://doi.org/10.3390/su17083333






