The Significance of Herbicide–Humin Interactions in Sustainable Agroecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Humin Extraction
2.2. Sample Preparation
- Solvent Evaporation: The evaporation of hexane and ethyl acetate under a fume hood may not be fully efficient. If not completely evaporated, residual solvents could interfere with the analysis or impact the interaction between the pesticides and humin. The differing evaporation times for hexane (24 h) and ethyl acetate (96 h) might suggest that the solvents have very different evaporation rates. This discrepancy could affect the timing and effectiveness of solvent removal and introduce inconsistency in pesticide concentrations in the final samples.
- Koc Coefficients: The experiment’s reliance on the Koc partition coefficients is a good starting point for determining pesticide absorption, but Koc values are general indicators that may not perfectly reflect the actual behavior of the pesticides in humin samples under the specific experimental conditions. Factors such as humin composition, moisture content, and temperature might affect sorption, leading to deviations from predicted values based on Koc alone.
- Humin Sample Heterogeneity: Humin samples can be quite heterogeneous in terms of their chemical and physical properties; such variability might affect the reproducibility of the results. Careful sampling homogenization before saturation helps reduce the likelihood of this potential issue.
- Effect of Hexane and Ethyl Acetate on Active Substances: Hexane and ethyl acetate may not be inert solvents for all the active substances and their commercial formulations. Some chemicals might degrade, react, or change their behavior in these solvents. The effect of these solvents on the pesticides should be further validated.
- Measurement Errors/Bias: Systematic errors and random errors were eliminated by optimization of the saturation process as determined by a sorption experiment [27] and quantitative evaluation of the residual test substance in solution after saturation therefore the unpredictable variations in measurement that can increase variability in the data were eradicated.
- Instrument limitations: The tools used for measurement may have inherent limitations; therefore, the appropriate quality control standards are regularly applied and equipment used for testing is constantly maintained and supervised. The Sheward cards are maintained.
2.3. Analytical Methods
2.3.1. Elemental Analysis
2.3.2. CP MAS 13C NMR
2.3.3. FTIR
2.3.4. EPR
2.3.5. UV-Vis
3. Results and Discussion
3.1. Elemental Analyses and Atomic Ratios of Investigated Samples
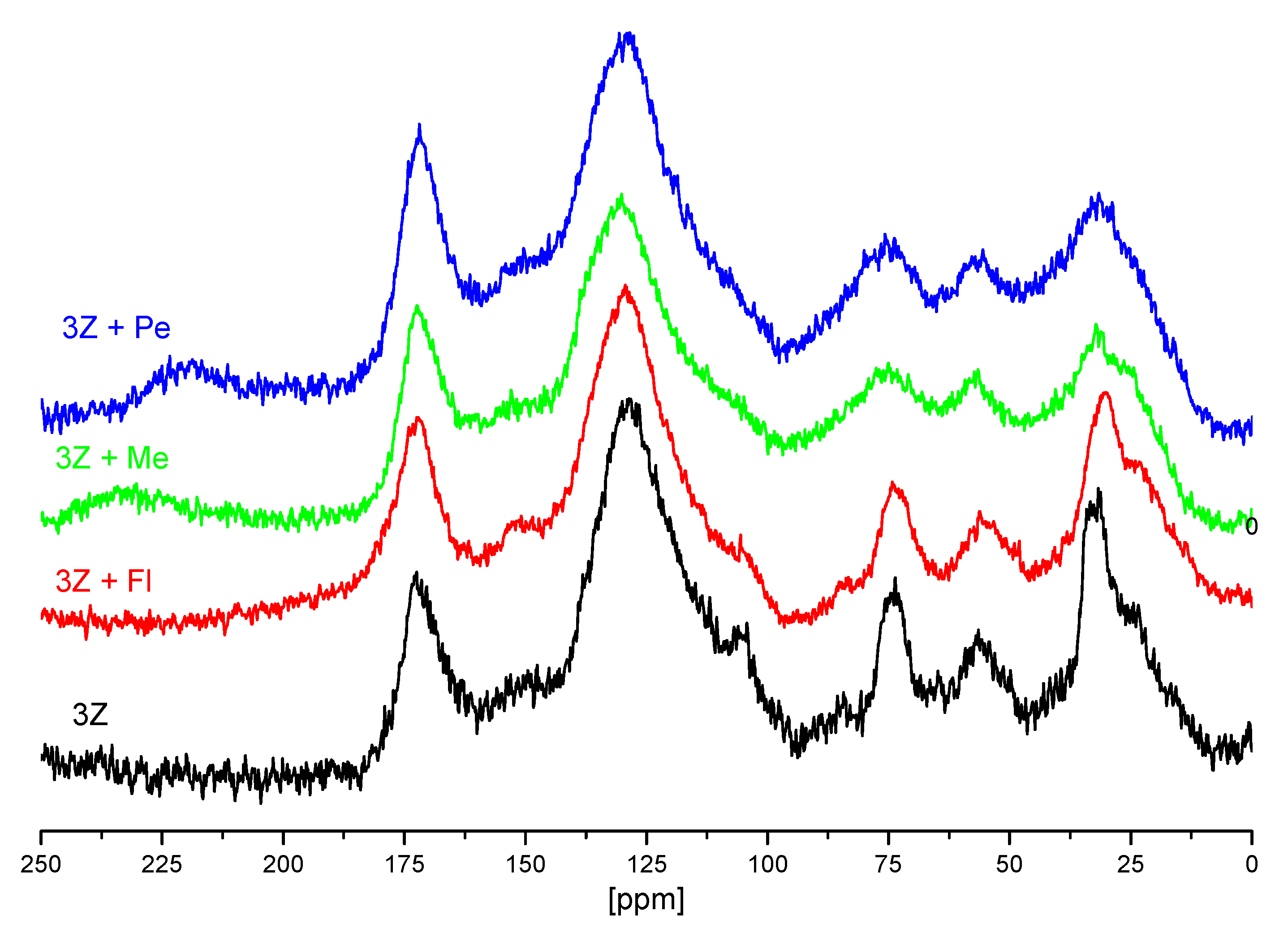
3.2. NMR Measurements of Unsaturated and Saturated Humin with Pesticides
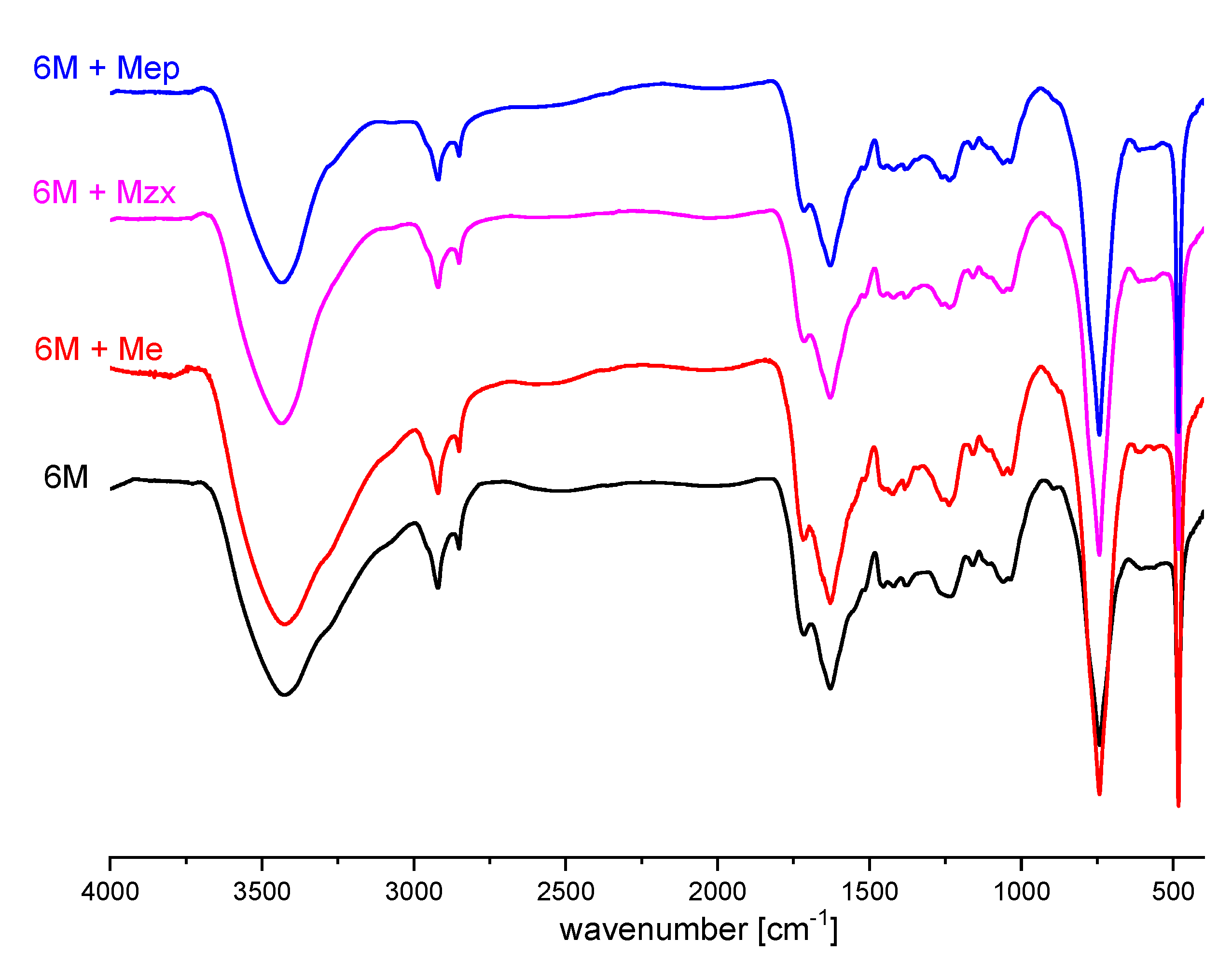
3.3. Functional Groups Analysis of Investigated Humin
3.4. UV-Vis Studies
3.5. Radical Structures in Humin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample No | GPS Coordinates | WRB Soil Group | Cultivated Plant |
|---|---|---|---|
| 1Ps | N 51°11′27.79″; E 17°02′08.24″ | Gleyic/Stagnic Phaeozems | triticale |
| 3Z | N 50°34′30.50″; E 17°55′59.81″ | Rendzic Phaeozems | maize |
| 6M | N 50°59′00.04″; E 16°56′52.48″ | Gleyic/Stagnic Phaeozems | maize |
| 7T | N 50°49′11.87″; E 16°52′39.38″ | Calcic/Haplic Chernozems | sugar beets |
| 8C | N 50°40′53.98″; E 16°55′47.78″ | Gleyic/Stagnic Phaeozems | maize |
| 9H | N 50°43′32.91″; E 23°50′05.94″ | Calcic/Haplic Chernozems | wheat |
| 10Py | N 53°09′57.87″; E 14°55′15.19″ | Gleyic/Stagnic Phaeozems | sugar beets |
| 11K | N 54°03′53.67″; E 21°21′09.66″ | Gleyic/Stagnic Phaeozems | triticale |
| Sample No | pH (KCl) | TOC g kg−1 | N | C/N | CaCO3 g kg−1 | CEC cmol kg−1 | % Particles >0.002 mm | USDA Textural Class |
|---|---|---|---|---|---|---|---|---|
| 1Ps | 7.71 | 13.3 | 1.06 | 12.5 | 1.46 | 28.3 | 16 | sandy loam |
| 3Z | 7.45 | 24.4 | 2.14 | 11.4 | 3.43 | 50.0 | 41 | clay |
| 6M | 7.52 | 21.2 | 1.60 | 13.2 | 1.53 | 33.4 | 22 | loam |
| 7T | 5.64 | 41.7 | 3.39 | 12.3 | 0.51 | 53.2 | 24 | silt loam |
| 8C | 7.39 | 26.1 | 2.03 | 12.8 | 1.03 | 21.6 | 19 | silt loam |
| 9H | 7.52 | 39.9 | 2.90 | 13.7 | 3.26 | 52.5 | 21 | silt loam |
| 10Py | 7.48 | 24.6 | 2.12 | 11.6 | 1.54 | 34.4 | 24 | loam |
| 11K | 6.66 | 37.7 | 2.80 | 13.4 | 0.61 | 25.8 | 47 | clay |
| Ash | C (SD) | N (SD) | H (SD) | O (SD) | S (SD) | H/C | O/C | O/H | C/N | ω | E4/E6 (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1Ps | 39.45 | 41.26 (0.45) | 1.87 (0.02) | 38.32 (0.72) | 18.17 (0.98) | 0.38 (0.04) | 0.93 | 0.44 | 0.47 | 22.04 | 0.09 | 1.016 (0.005) |
| +FL | 33.72 (0.65) | 1.66 (0.06) | 36.57 (0.31) | 32.15 (0.88) | 0.79 (0.47) | 1.26 | 1.11 | 0.88 | 19.36 | 1.11 | 1.038 (0.003) | |
| +Flt | 38.14 (2.76) | 1.84 (0.16) | 32.13 (2.16) | 25.65 (1.54) | 2.24 (2.23) | 0.84 | 0.67 | 0.8 | 20.68 | 0.65 | 1.064 (0.0002) | |
| +Cen | 32.55 (0.84) | 1.47 (0.09) | 38.13 (2.18) | 27.71 (1.67) | 0.13 (0) | 1.17 | 0.85 | 0.73 | 22.08 | 0.67 | 1.053 (0.002) | |
| 3Z | 22.89 | 39.61 (1.15) | 2.12 (0.06) | 40.10 (1.89) | 17.93 (0.87) | 0.23 (0.03) | 1.01 | 0.45 | 0.45 | 18.66 | 0.05 | 1.019 (0.003) |
| +FL | 38.84(1.63) | 2.21 (0.09) | 38.69 (1.37) | 20.18(0.65) | 0.12 (0.0) | 1 | 0.52 | 0.52 | 17.54 | 0.21 | 1.017 (0.003) | |
| +Flt | 37.78 (0.26) | 2.13 (0.13) | 40.82 (0.32) | 19.16 (0.21) | 0.11 (0) | 1.08 | 0.51 | 0.47 | 17.72 | 0.1 | 1.040 (0.001) | |
| +Cen | 40.82 (1.17) | 2.27 (0.07) | 36.75 (1.97) | 19.22 (0.88) | 0.94 (0.13) | 0.9 | 0.47 | 0.52 | 17.95 | 0.21 | 1.036 (0.0006) | |
| 6M | 38.57 | 38.98 (0.27) | 1.99 (0.05) | 38.00 (1.4) | 20.36 (1.58) | 0.67 (0.11) | 0.97 | 0.52 | 0.54 | 19.62 | 0.22 | 1.0634 (0.016) |
| +FL | 34.11(0.35) | 1.75 (0.09) | 38.46 (1.45) | 25.28(1.29) | 0.41(0.19) | 1.13 | 0.74 | 0.66 | 19.51 | 0.51 | 1.017 (0.008) | |
| +Flt | 36.09 (0.39) | 1.9 (0.01) | 34.17 (0.79) | 25.84 (0.72) | 2.01 (0.2) | 0.95 | 0.72 | 0.76 | 19.01 | 0.64 | 1.057 (0.0008) | |
| +Cen | 35.42 (1.07) | 1.77 (0.04) | 36.79 (0.46) | 24.06 (0.78) | 1.97 (0.2) | 1.04 | 0.68 | 0.65 | 20.00 | 0.47 | 1.066(0.0007) | |
| 7T | 41.67 | 30.6 (2.61) | 1.79 (0.08) | 38.48 (1.35) | 28.96 (3.03) | 0.16 (0.01) | 1.26 | 0.95 | 0.75 | 17.12 | 0.81 | 1.069 (0.004) |
| +FL | 29.84(0.66) | 1.78 (0.02) | 36.5 (0.82) | 31.74 (0.38) | 0.14(0.01) | 1.22 | 1.06 | 0.87 | 16.8 | 1.08 | 1.021 (0.006) | |
| +Flt | 30.6 (0.91) | 1.79 (0.08) | 38.48 (0.56) | 28.96 (0.71) | 0.16 (0.03) | 1.26 | 0.95 | 0.75 | 17.12 | 0.81 | 1.070 (0.001) | |
| +Cen | 29.87 (1.05) | 1.66 (0.08) | 36.4 (2.08) | 30.89 (2.66) | 1.17 (0.35) | 1.22 | 1.03 | 0.85 | 18.00 | 1.02 | 1.057 (0.0009) | |
| 8C | 28.01 | 42.84 (0.11) | 2.3 (0.01) | 37.86 (1.1) | 15.92 (0.83) | 1.08 (0.21) | 0.88 | 0.37 | 0.42 | 18.65 | 0.02 | 1.059 (0.022) |
| +FL | 38.9 (1.21) | 1.77 (0.06) | 34.2 (1.07) | 22.69 (1.65) | 2.44 (0.43) | 0.88 | 0.58 | 0.66 | 21.92 | 0.42 | 1.018 (0.006) | |
| +Flt | 38.9 (0.23) | 1.83 (0.06) | 34.43 (0.46) | 22.01 (0.25) | 2.83 (0.36) | 0.89 | 0.57 | 0.64 | 21.28 | 0.39 | 1.063 (0.0004) | |
| +Cen | 38.83 (2.10) | 1.65(0.010) | 36.15 (1.21) | 22.47(1.34) | 0.89 (0.02) | 0.93 | 0.58 | 0.62 | 23.5 | 0.35 | 1.067 (0.0002) | |
| 9 H | 43.31 | 40.14 (3.79) | 1.73 (0.13) | 35.65 (2.07) | 21.43 (1.97) | 1.06 (0.12) | 0.89 | 0.53 | 0.6 | 23.24 | 0.31 | 1.098 (0.005) |
| +FL | 37.99 (1.83) | 1.9 (0.06) | 36.26 (1.19) | 22.8 (2.73) | 1.05 (0.2) | 0.95 | 0.6 | 0.63 | 20.00 | 0.4 | 1.136 (0.002) | |
| +Flt | 36.27 (2.58) | 1.79 (0.13) | 43.21 (2.66) | 18.58 (0.2) | 1.02 (0.02) | 1.19 | 0.51 | 0.43 | 20.30 | −0.02 | 1.096 (0.0006) | |
| +Cen | 38.81 (2.06) | 1.87 (0.04) | 39.65 (0.96) | 19.46 (1.01) | 0.21 (0.2) | 1.02 | 0.5 | 0.49 | 20.75 | 0.13 | 1.126 (0.0008) | |
| 10Py | 54.5 | 38.59 (1.38) | 1.92 (0.12) | 37.44 (2.15) | 20.81 (2.96) | 1.24 (0.21) | 0.97 | 0.54 | 0.56 | 20.08 | 0.26 | 1.022 (0.0026) |
| +FL | 36.08 (0.42) | 1.87 (0.01) | 35.00 (1.51) | 24.23 (2.25) | 2.82 (0.79) | 0.97 | 0.67 | 0.69 | 19.33 | 0.53 | 1.044 (0.006) | |
| +Flt | 36.9 (0.05) | 1.96 (0.06) | 33.87 (1.19) | 24.92 (1.25) | 2.35 (0.12) | 0.92 | 0.68 | 0.74 | 18.83 | 0.59 | 1.089 (0.0009) | |
| +Cen | 35.23 (0.78) | 1.82 (0.09) | 36.55 (1.05) | 23.89 (1.79) | 2.5 (0.14) | 1.04 | 0.68 | 0.65 | 19.31 | 0.47 | 1.047 (0.0007) | |
| 11K | 48.87 | 34.89 (0.42) | 1.97 (0.05) | 39.29 (1.36) | 23.22 (1.01) | 0.63 (0.17) | 1.13 | 0.67 | 0.59 | 17.68 | 0.37 | 1.061 (0.009) |
| +FL | 32.88 (1.16) | 1.92 (0.07) | 41.35 (0.28) | 23.67 (1.13) | 2.72 (0.44) | 1.26 | 0.72 | 0.57 | 17.12 | 0.36 | 1.047 (0.007) | |
| +Flt | 31.91 (0.77) | 1.92 (0.09) | 40.26 (3.63) | 25.04 (3.56) | 0.87 (0.23) | 1.26 | 0.78 | 0.62 | 16.63 | 0.49 | 1.077 (0.0008) | |
| +Cen | 33.7 (0.83) | 1.86 (0.02) | 36.88 (0.77) | 23.28 (1.51) | 4.28 (0.69) | 1.09 | 0.69 | 0.63 | 18.16 | 0.45 | 1.039 (0.001) |
| C (SD) | N (SD) | H (SD) | O (SD) | S (SD) | H/C | O/C | O/H | C/N | ω | E4/E6 (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1Ps | 41.26 (0.45) | 1.87 (0.02) | 38.32 (0.72) | 18.17 (0.98) | 0.38 (0.04) | 0.93 | 0.44 | 0.47 | 22.04 | 0.09 | 1.016 (0.005) |
| +Me | 32.64 (0.48) | 1.72 (0.02) | 33.16 (0.65) | 31.01 (0.99) | 1.46 (0.24) | 1.02 | 0.95 | 0.94 | 18.94 | 1.04 | 1.053 (0.002) |
| +Mzx | 34.2 (0.54) | 1.78 (0.07) | 34.39 (0.75) | 28.06 (1.05) | 1.57 (0.54) | 1.01 | 0.82 | 0.82 | 19.18 | 0.79 | 1.045 (0.001) |
| +Mep | 34.19 (0.64) | 1.66 (0.03) | 33.84 (1.89) | 29.93 (2.57) | 0.37 (0.21) | 0.99 | 0.88 | 0.88 | 20.57 | 0.91 | 1.085 (0.001) |
| 3Z | 39.61 (1.15) | 2.12 (0.06) | 40.10 (1.89) | 17.93 (0.87) | 0.23 (0.03) | 1.01 | 0.45 | 0.45 | 18.66 | 0.05 | 1.019 (0.003) |
| +Me | 39.84 (1.15) | 2.34 (0.09) | 39.07 (2.03) | 17.08 (0.91) | 1.67 (0.13) | 0.98 | 0.43 | 0.44 | 17.00 | 0.05 | 1.038 (0.002) |
| +Mzx | 40.71 (1.13) | 2.29 (0.06) | 37.98 (1.39) | 17.34 (0.31) | 1.68 (0.45) | 0.93 | 0.43 | 0.46 | 17.75 | 0.09 | 1.050 (0.003) |
| +Mep | 38.35 (1.02) | 2.17 (0.06) | 40.9 (2.05) | 18.48 (1.0) | 0.1 (0) | 1.07 | 0.48 | 0.45 | 17.66 | 0.07 | 1.035 (0.000) |
| 6M | 38.98 (0.27) | 1.99 (0.05) | 38.00 (1.4) | 20.36 (1.58) | 0.67 (0.11) | 0.97 | 0.52 | 0.54 | 19.62 | 0.22 | 1.0634 (0.016) |
| +Me | 35.62 (0.12) | 1.97 (0.04) | 39.25 (0.78) | 19.73 (0.62) | 3.42 (0.2) | 1.1 | 0.55 | 0.5 | 18.04 | 0.17 | 1.088 (0.004) |
| +Mzx | 35.08 (1.12) | 1.83 (0.07) | 38.83 (0.77) | 22.37 (0.1) | 1.89 (0.34) | 1.11 | 0.64 | 0.58 | 19.15 | 0.33 | 1.080 (0.004) |
| +Mep | 37.02 (0.74) | 1.93 (0.03) | 35.38 (0.79) | 23.76 (0.67) | 1.9 (0.64) | 0.96 | 0.64 | 0.67 | 19.16 | 0.48 | 1.090 (0.001) |
| 7T | 30.6 (2.61) | 1.79 (0.08) | 38.48 (1.35) | 28.96 (3.03) | 0.16 (0.01) | 1.28 | 0.90 | 0.70 | 19.45 | 0.67 | 1.069 (0.004) |
| +Me | 30.49 (1.09) | 1.86 (0.09) | 38.95 (0.17) | 25.26 (1.33) | 3.44 (0.16) | 1.28 | 0.83 | 0.65 | 16.4 | 0.56 | 1.037 (0.007) |
| +Mzx | 29.68 (0.61) | 1.74 (0.05) | 37.49 (0.9) | 30.8 (0.67) | 0.29 (0.12) | 1.26 | 1.04 | 0.82 | 17.03 | 0.99 | 1.093 (0.005) |
| +Mep | 31.36 (0.27) | 1.78 (0.03) | 36.75 (0.64) | 28.35 (0.82) | 1.76 (0.17) | 1.17 | 0.9 | 0.77 | 17.62 | 0.81 | 1.086 (0.001) |
| 8C | 42.84 (0.11) | 2.3 (0.01) | 37.86 (1.1) | 15.92 (0.83) | 1.08 (0.21) | 0.88 | 0.37 | 0.42 | 18.65 | 0.02 | 1.059 (0.022) |
| +Me | 40.86 (4.88) | 1.54 (0.06) | 39.06 (1.23) | 17.66 (5.77) | 2.88 (0.06) | 0.96 | 0.43 | 0.45 | 26.55 | 0.02 | 1.055 (0.003) |
| +Mzx | 33.74 (1.48) | 1.66 (0.07) | 40.00 (1.18) | 24.44 (2.42) | 0.16 (0.01) | 1.19 | 0.72 | 0.61 | 20.38 | 0.41 | 1.075 (0.005) |
| +Mep | 39.45 (0.87) | 1.78 (0.03) | 36.33 (0.66) | 20.52 (0.47) | 1.91 (0.45) | 0.92 | 0.52 | 0.56 | 22.1 | 0.26 | 1.094 (0.000) |
| 9H | 40.14 (3.79) | 1.73 (0.13) | 35.65 (2.07) | 21.43 (1.97) | 1.06 (0.12) | 0.89 | 0.53 | 0.6 | 23.24 | 0.31 | 1.098 (0.005) |
| +Me | 39.33 (1.06) | 1.73 (0.03) | 37.86 (1.18) | 18.86 (0.64) | 2.22 (0.12) | 0.96 | 0.48 | 0.5 | 22.75 | 0.13 | 1.081 (0.004) |
| +Mzx | 38.93 (2.61) | 1.9 (0.11) | 39.41 (1.61) | 17.92 (1.86) | 1.84 (0.18) | 1.01 | 0.46 | 0.45 | 20.44 | 0.06 | 1.124 (0.004) |
| +Mep | 40.07 (2.23) | 2.01 (0.16) | 38.3 (3.38) | 18.34 (1.35) | 1.28 (0.31) | 0.96 | 0.46 | 0.48 | 19.89 | 0.11 | 1.120 (0.001) |
| 10Py | 38.59 (1.38) | 1.92 (0.12) | 37.44 (2.15) | 20.81 (2.96) | 1.24 (0.21) | 0.97 | 0.54 | 0.56 | 20.08 | 0.26 | 1.022 (0.0026) |
| +Me | 33.12(1.2) | 2.13 (0.1) | 43.3 (2.3) | 20.16 (1.81) | 1.29 (0.82) | 1.31 | 0.61 | 0.47 | 15.57 | 0.1 | 1.049 (0.003) |
| +Mzx | 36.22 (1.25) | 2.02 (0.06) | 37.66 (1.19) | 22.15 (2.21) | 1.95 (0.22) | 1.04 | 0.61 | 0.59 | 17.92 | 0.35 | 1.057 (0.004) |
| +Mep | 29.58 (1.48) | 1.62 (0.09) | 45.89 (3.2) | 22.81 (1.63) | 0.09 (0.01) | 1.55 | 0.77 | 0.50 | 18.22 | 0.16 | 1.093 (0.001) |
| 11K | 34.89 (0.42) | 1.97 (0.05) | 39.29 (1.36) | 23.22 (1.01) | 0.63 (0.17) | 1.13 | 0.67 | 0.59 | 17.68 | 0.37 | 1.061 (0.009) |
| +Me | 32.19 (1.09) | 2.03 (0.09) | 39.61 (2.21) | 23.82 (1.8) | 2.34 (0.77) | 1.23 | 0.74 | 0.60 | 15.85 | 0.44 | 1.048 (0.006) |
| +Mzx | 35.12 (0.55) | 2.12 (0.03) | 39.2 (2.48) | 21.7 (3.49) | 1.86 (0.65) | 1.12 | 0.62 | 0.55 | 16.58 | 0.3 | 1.075 (0.005) |
| +Mep | 33.73 (0.52) | 2.10 (0.07) | 38.10 (0.67) | 23.88 (1.22) | 2.19 (0.21) | 1.13 | 0.71 | 0.63 | 16.09 | 0.47 | 1.079 (0.000) |
| C (SD) | N (SD) | H (SD) | O (SD) | S (SD) | H/C | O/C | O/H | C/N | ω | E4/E6 (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1Ps | 41.26 (0.45) | 1.87 (0.02) | 38.32 (0.72) | 18.17 (0.98) | 0.38 (0.04) | 0.93 | 0.44 | 0.47 | 22.04 | 0.09 | 1.016 (0.005) |
| +Pe | 32.57 (0.46) | 1.61 (0.05) | 31.46 (0.73) | 33.69 (1.56) | 0.67 (0.38) | 0.97 | 1.03 | 1.07 | 20.19 | 1.25 | 1.083 (0.010) |
| +Stq | 35.41 (0.44) | 1.67 (0.06) | 33.63 (1.08) | 26.93 (1.58) | 2.36 (0.14) | 0.95 | 0.76 | 0.8 | 21.24 | 0.71 | 1.087 (0.001) |
| +Pfx | 34.64 (0.19) | 1.6 (0.01) | 31.51 (1.02) | 31.78 (1.13) | 0.47 (0.11) | 0.91 | 0.92 | 1.01 | 21.69 | 1.06 | 1.056 (0.001) |
| 3Z | 39.61 (1.15) | 2.12 (0.06) | 40.10 (1.89) | 17.93 (0.87) | 0.23 (0.03) | 1.01 | 0.45 | 0.45 | 18.66 | 0.05 | 1.019 (0.003) |
| +Pe | 39.22 (1.12) | 2.23 (0.07) | 39.19 (2.17) | 19.26 (1.19) | 0.1 (0.01) | 1.00 | 0.49 | 0.49 | 17.61 | 0.15 | 1.055 (0.007) |
| +Stq | 38.17 (0.92) | 2.14 (0.05) | 41.02 (1.17) | 18.32 (0.29) | 0.35 (0.29) | 1.07 | 0.48 | 0.45 | 17.83 | 0.05 | 1.045 (0.001) |
| +Pfx | 40.79 (1.18) | 2.21 (0.11) | 36.52 (1.84) | 20.32 (0.84) | 0.15 (0.02) | 0.9 | 0.5 | 0.56 | 18.43 | 0.26 | 1.058 (0.001) |
| 6M | 38.98 (0.27) | 1.99 (0.05) | 38.00 (1.4) | 20.36 (1.58) | 0.67 (0.11) | 0.97 | 0.52 | 0.54 | 19.62 | 0.22 | 1.0634 (0.016) |
| +Pe | 36.89 (1.38) | 1.93 (0.03) | 35.43 (0.58) | 23.87 (0.59) | 1.87 (0.16) | 0.96 | 0.65 | 0.67 | 19.11 | 0.49 | 1.052 (0.018) |
| +Stq | 36.48 (0.61) | 1.77 (0.03) | 36.73 (1.55) | 22.63 (1.46) | 2.38 (0.2) | 1.01 | 0.62 | 0.62 | 20.57 | 0.38 | 1.096 (0.001) |
| +Pfx | 35.57 (0.51) | 1.73 (0.04) | 35.54 (1.01) | 27.03 (0.56) | 0.13 (0.01) | 1.00 | 0.76 | 0.76 | 20.58 | 0.67 | 1.059 (0.001) |
| 7T | 30.6 (2.61) | 1.79 (0.08) | 38.48 (1.35) | 28.96 (3.03) | 0.16 (0.01) | 1.28 | 0.9 | 0.7 | 19.45 | 0.67 | 1.069 (0.004) |
| +Pe | 30.29 (0.95) | 1.75 (0.07) | 33.54 (1.68) | 32.15 (1.69) | 2.28 (0.67) | 1.11 | 1.06 | 0.96 | 17.35 | 1.19 | 1.120 (0.033) |
| +Stq | 30.49 (0.64) | 1.63 (0.05) | 36.38 (1.08) | 30.64 (1.69) | 0.86 (0.13) | 1.19 | 1.00 | 0.84 | 18.73 | 0.98 | 1.092 (0.000) |
| +Pfx | 29.2 (0.67) | 1.58 (0.05) | 38.18 (1.33) | 30.89 (1.96) | 0.15 (0.02) | 1.31 | 1.06 | 0.81 | 18.5 | 0.97 | 1.092 (0.001) |
| 8C | 42.84 (0.11) | 2.3 (0.01) | 37.86 (1.1) | 15.92 (0.83) | 1.08 (0.21) | 0.88 | 0.37 | 0.42 | 18.65 | 0.02 | 1.059 (0.022) |
| +Pe | 37.99 (1.44) | 1.57 (0.06) | 34.27 (0.65) | 26.01 (0.91) | 0.16 (0.02) | 0.9 | 0.68 | 0.76 | 24.13 | 0.59 | 1.060 (0.009) |
| +Stq | 36.89 (0.36) | 1.65 (0.02) | 37.86 (1.02) | 22.8 (1.42) | 0.8 (0.05) | 1.03 | 0.62 | 0.6 | 22.35 | 0.34 | 1.088 (0.001) |
| +Pfx | 39.21 (2.1) | 1.64 (0.01) | 35.43 (1.21) | 23.52 (1.35) | 0.2 (0.02) | 0.9 | 0.6 | 0.66 | 23.91 | 0.42 | 1.088 (0.000) |
| 9H | 40.14 (3.79) | 1.73 (0.13) | 35.65 (2.07) | 21.43 (1.97) | 1.06 | 0.89 | 0.53 | 0.6 | 23.24 | 0.31 | 1.098 (0.005) |
| +Pe | 38.41 (2.05) | 1.82 (0.06) | 35.76 (1.1) | 22.37 (1.41) | 1.64 (0.08) | 0.93 | 0.58 | 0.63 | 21.05 | 0.38 | 1.134 (0.007) |
| +Stq | 38.53 (0.75) | 1.72 (0.03) | 39.66 (0.84) | 16.56 (1.72) | 3.53 (0.11) | 1.03 | 0.43 | 0.42 | 22.35 | −0.04 | 1.153 (0.001) |
| +Pfx | 43.48 (1.71) | 1.82 (0.16) | 34.59 (1.28) | 17.18 (1.11) | 2.93 (0.54) | 0.8 | 0.4 | 0.5 | 23.83 | 0.12 | 1.122 (0.001) |
| 10Py | 38.59 (1.38) | 1.92 (0.12) | 37.44 (2.15) | 20.81 (2.96) | 1.24 (0.21) | 0.97 | 0.54 | 0.56 | 20.08 | 0.26 | 1.022 (0.0026) |
| +Pe | 36.71 (1.48) | 1.97 (0.11) | 35.94 (3.62) | 20.8 (7.48) | 4.58 (2.43) | 0.98 | 0.57 | 0.58 | 18.62 | 0.32 | 1.075 (0.012) |
| +Stq | 34.63 (0.41) | 1.73 (0.04) | 37.67 (0.51) | 23.24 (0.08) | 2.73 (0.34) | 1.09 | 0.67 | 0.62 | 20.02 | 0.4 | 1.063 (0.001) |
| +Pfx | 36.67 (0.59) | 1.76 (0.05) | 34.44 (0.55) | 23.49 (0.15) | 3.64 (0.25) | 0.94 | 0.64 | 0.68 | 20.79 | 0.49 | 1.073 (0.001) |
| 11K | 34.89 (0.42) | 1.97 (0.05) | 39.29 (1.36) | 23.22 (1.01) | 0.63 (0.17) | 1.13 | 0.67 | 0.59 | 17.68 | 0.37 | 1.061 (0.009) |
| +Pe | 33.4 (2.15) | 1.93 (0.12) | 35.86 (2.18) | 26.85 (4.21) | 1.96 (1.44) | 1.07 | 0.8 | 0.75 | 17.29 | 0.71 | 1.069 (0.022) |
| +Stq | 29.47 (0.52) | 1.63 (0.03) | 43.04 (2.34) | 25.73 (2.0) | 0.13 (0) | 1.46 | 0.87 | 0.6 | 18.04 | 0.45 | 1.105 (0.001) |
| +Pfx | 32.33 (0.21) | 1.79 (0.03) | 34.22 (2.16) | 31.07 (2.14) | 0.59 (0.18) | 1.06 | 0.96 | 0.91 | 18.11 | 1.03 | 1.085 (0.001) |
References
- Carpio, M.J.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J.; Marín-Benito, J.M. Pesticide Fate in Soils Under Different Agricultural Management Practices. In Pesticides in Soils: Occurrence, Fate, Control and Remediation; Rodríguez-Cruz, M.S., Sánchez-Martín, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 251–286. [Google Scholar] [CrossRef]
- Chaplain, V.; Mamy, L.; Vieublé Gonod, L.; Mougin, C.; Benoit, P.; Barriuso, E.; Nélieu, S. Fate of Pesticides in Soils: Toward an Integrated Approach of Influential Factors. In Pesticides in the Modern World—Risks and Benefits; IntechOpen: Rijeka, Croatia, 2011; pp. 535–560. [Google Scholar] [CrossRef]
- Senesi, N. Binding mechanisms of pesticides to soil humic substances. Sci. Total Environ. 1992, 123–124, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Siek, M.; Paszko, T.; Jerzykiewicz, M.; Matysiak, J.; Wojcieszek, U. Mechanisms of Tebuconazole Adsorption in Profiles of Mineral Soils. Molecules 2021, 26, 4728. [Google Scholar] [CrossRef]
- Hsieh, T.-L.; Kao, M.-M. Adsorption of carbofuran on lateritic soils. J. Hazard. Mater. 1998, 58, 275–284. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus chemistry. In Genesis, Composition, Reactions, 2nd ed.; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Swift, R.S. Organic matter characterization (chap 35). In Methods of Soil Analysis—Part 3: Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America Book Series; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1011–1069. [Google Scholar]
- De Nobili, M.; Bravo, C.; Chen, Y. The spontaneous secondary synthesis of soil organic matter components: Acritical examination of the soil continuum model theory. Appl. Soil Ecol. 2020, 154, 103655. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Swift, R.S. Vindication of Humic Substances as a Key Component of Organic Matter in Soil and Water. Adv. Agron. 2020, 163, 1–37. [Google Scholar] [CrossRef]
- Song, G.; Simpson, A.J.; Hayes, M.H.B. Compositional changes in the humin fraction resulting from the long-term cultivation of an Irish grassland soil: Evidence from FTIR and multi-NMR spectroscopies. Sci. Total Environ. 2023, 880, 163280. [Google Scholar] [CrossRef]
- Leita, L.; De Nobili, M.; Catalano, L.; Moria, A.; Fonda, E.; Vlaic, G. Complexation of iron-cyanide by humic substances. In Understanding and Managing Organic Matter in Soils, Sediments, and Waters; Swift, R.S., Spark, K.M., Eds.; International Humic Substances Society, Hyde Park Press: Adelaide, Australia, 2001; pp. 477–482. [Google Scholar]
- Chianese, S.; Fenti, A.; Iovino, P.; Musmarra, D.; Salvestrini, S. Sorption of Organic Pollutants by Humic Acids: A Review. Molecules 2020, 25, 918. [Google Scholar] [CrossRef]
- Spark, K.M.; Swift, R.S. Effect of soil composition and dissolved organic matter on pesticide sorption. Sci. Total Environ. 2002, 298, 147–161. [Google Scholar] [CrossRef]
- Hesketh, N.; Jones, M.N.; Tipping, E. The interaction of some pesticides and herbicides with humic substances. Anal. Chim. Acta 1996, 327, 191–201. [Google Scholar] [CrossRef]
- Haberhauer, G.; Pfeiffer, L.; Gerzabek, M.H.; Kirchmann, H.; Aquino, A.J.A.; Tunega, D.; Lischka, H. Response of sorption processes of MCPA to the amount and origin of organic matter in a long-term field experiment. Eur. J. Soil Sci. 2001, 52, 279–286. [Google Scholar] [CrossRef]
- Novotny, E.H.; Turetta, A.P.D.; Resende, M.F.; Rebello, C.M. The quality of soil organic matter, accessed by 13C solid state nuclear magnetic resonance, is just as important as its content concerning pesticide sorption. Environ. Pollut. 2020, 266, 115298. [Google Scholar] [CrossRef] [PubMed]
- Alister, C.; Araya, M.; Cordova, A.; Saavedra, J.; Kogan, M. Humic Substances and their Relation to Pesticide Sorption in Eight Volcanic Soils. Planta Daninha 2020, 38, e020171636. [Google Scholar] [CrossRef]
- Helal, A.-A.; Imam, D.M.; Khalifa, S.; Aly, H. Interaction of pesticides with humic compounds and their metal complexes. Radiochemistry 2006, 48, 419–425. [Google Scholar] [CrossRef]
- Iglesias, A.; López, R.; Gondar, D.; Antelo, J.; Fiol, S.; Arce, F. Effect of pH and ionic strength on the binding of paraquat and MCPA by soil fulvic and humic acids. Chemosphere 2009, 76, 107–113. [Google Scholar] [CrossRef]
- Senesi, N.; Loffredo, E.; D’Orazio, V.; Brunetti, G.; Miano, T.M.; La Cava, P. Adsorption of Pesticides by Humic Acids from Organic Amendments and Soils. In Humic Substances and Chemical Contaminants; ASA, CSSA, and SSSA Books: Madison, WI, USA, 2001; pp. 129–153. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Mylotte, R.; Swift, R.S. Chapter Two—Humin: Its Composition and Importance in Soil Organic Matter. Adv. Agron. 2017, 143, 47–138. [Google Scholar] [CrossRef]
- Weber, J.; Jamroz, E.; Kocowicz, A.; Debicka, M.; Bekier, J.; Ćwieląg-Piasecka, I.; Ukalska-Jaruga, A.; Mielnik, L.; Bejger, R.; Jerzykiewicz, M. Optimized isolation method of humin fraction from mineral soil material. Environ. Geochem. Health 2022, 44, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Lipczynska-Kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere 2018, 202, 420–437. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Bejger, R.; Smreczak, B.; Weber, J.; Mielnik, L.; Jerzykiewicz, M.; Ćwieląg-Piasecka, I.; Jamroz, E.; Debicka, M.; Kocowicz, A.; et al. The Interaction of Pesticides with Humin Fractions and Their Potential Impact on Non-Extractable Residue Formation. Molecules 2023, 28, 7146. [Google Scholar] [CrossRef]
- Pignatello, J. Dynamic interactions of natural organic matter and organic compounds. J. Soils Sediments 2012, 12, 1241–1256. [Google Scholar] [CrossRef]
- Test No. 106: Adsorption—Desorption Using a Batch Equilibrium Method, 2000.OECD Guidelines for the Testing of Chemicals, Section 1. OECD. Available online: https://www.oecd.org/en/publications/test-no-106-adsorption-desorption-using-a-batch-equilibrium-method_9789264069602-en.html (accessed on 19 February 2025).
- Tan, K.H. Humic Matter in Soil and the Environment: Principles and Controversies, 2nd ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2014. [Google Scholar]
- Weber, J.; Jerzykiewicz, M.; Ukalska-Jaruga, A.; Ćwieląg-Piasecka, I.; Jamroz, E.; Kocowicz, A.; Debicka, M.; Bekier, J.; Mielnik, L.; Bejger, R.; et al. Properties of humin isolated from Polish arable soils: The most recalcitrant fraction of soil organic matter that prevent soil degradation. Land Degrad. Dev. 2024, 35, 2425–2436. [Google Scholar] [CrossRef]
- Stevenson, F.J. Role and Function of Humus in Soil with Emphasis on Adsorption of Herbicides and Chelation of Micronutrients. BioScience 1972, 22, 643–650. [Google Scholar] [CrossRef]
- Crosby, D.G. Nonbiological degradation of herbicides in the soil. In Herbicides: Physiology, Biochemistry, and Ecology; Academic Press: New York, NY, USA, 1976; pp. 65–97. [Google Scholar]
- Senesi, N.; Testini, C. Spectroscopic investigation of electron donor-acceptor processes involving organic free radicals in the adsorption of substituted urea herbicides by humic acids. Pestic. Sci. 1983, 14, 79–89. [Google Scholar] [CrossRef]
- Zhang, C.; Katayama, A. Humin as an Electron Mediator for Microbial Reductive Dehalogenation. Environ. Sci. Technol. 2013, 46, 6575–6583. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Mandal, A.; Manna, S.; Singh, S.B.; Berns, A.E.; Singh, N. Effect of organic carbon chemistry on sorption of atrazine and metsulfuron-methyl as determined by 13C-NMR and IR spectroscopy. Environ. Monit. Assess. 2015, 87, 620. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Senesi, N.; Schnitzer, M. Information Provided on Humic Substances by E4/E6 Ratios. S Soil. Soil Sci. Soc. Am. J. 1977, 41, 352–358. [Google Scholar] [CrossRef]
- Jerzykiewicz, M.; Drozd, J.; Jezierski, A. Organic radicals and paramagnetic metal complexes in municipal solid waste composts. An EPR and chemical study. Chemosphere 1999, 39, 253–268. [Google Scholar] [CrossRef]
- Jezierski, A.; Czechowski, F.; Jerzykiewicz, M.; Chen, Y.; Drozd, J. Electron paramagnetic resonance (EPR) studies on stable and transient radicals in humic acids from compost, soil, peat and brown coal. Spectrochim Acta A Mol. Biomol. Spectrosc. 2000, 56, 379–385. [Google Scholar] [CrossRef]
- Gerson, F.; Huber, W. Electron Spin Resonance Spectroscopy of Organic Radicals; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2001; pp. 10–36. [Google Scholar]
- Haider, K.; Haider, M.R.; Neha, K.; Yar, M.S. Free radical scavengers: An overview on heterocyclic advances and medicinal prospects. Eur. J. Med. Chem. 2020, 204, 112607. [Google Scholar] [CrossRef]
- Xu, J.; Dai, Y.; Shi, Y.; Zhao, S.; Tiana, H.; Zhu, K.; Jia, H. Mechanism of Cr(VI) reduction by humin: Role of environmentally persistent free radicals and reactive oxygen species. Sci. Total Environ. 2020, 725, 138413. [Google Scholar] [CrossRef]
- Senesi, N.; Testini, C.; Miano, T.M. Interaction mechanisms between humic acids of different origin and nature and electron donor herbicides: A comparative IR and ESR study. Org Geochem. 1987, 11, 25–30. [Google Scholar] [CrossRef]
- Ćwieląg-Piasecka, I.; Witwicki, M.; Jerzykiewicz, M.; Jezierska, J. Can Carbamates Undergo Radical Oxidation in the Soil Environment? A Case Study on Carbaryl and Carbofuran. Environ Sci Technol. 2017, 51, 14124–14134. [Google Scholar] [CrossRef] [PubMed]
- Bollag, J.-M.; Loll, M.J. Incorporation of xenobiotics into soil humus. Experientia 1983, 39, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.E.; Chesters, G.; Harris, R.F. Atrazine Hydrolysis in Soil. Soil Sci. Soc. Am. J. 1967, 31, 61–66. [Google Scholar] [CrossRef]
- Pham, D.M.; Kasai, T.; Yamaura, M.; Katayama, A. Humin: No longer inactive natural organic matter. Chemosphere 2021, 269, 128697. [Google Scholar] [CrossRef] [PubMed]

| Active Herbicide | Formula | Commercial Product |
|---|---|---|
| Flufenacet (Fl) CAS 142459-58-3 |  | Fluent—500SC (Flt) Cevino–500SC (Cen) |
| IUPAC: N-(4-fluorophenyl)-N-propan-2-yl-2-[[5-(trifluoromethyl)-1,3,4-thiadiazol-2yl]oxy]acetamide | ||
| Metazachlor (Me) CAS 67129-08-2 |  | Metazanex–500SC (Mzx) Metozop–500SC (Mep) |
| IUPAC: 2-chloro-N-(2,6-dimethylphenyl)-N-[(1H-pyrazol-1-yl)methyl]acetamide | ||
| Pendimethalin (Pe) CAS 40487-42-1 |  | StompAqua–455SC (Stq) Penfox–330EC (Pfx) |
| IUPAC: 3,4-Dimethyl-2,6-dinitro-N-(pentan-3-yl)aniline | ||
| Sample | 1Ps | 3Z | 6M | 7T | 8C | 9H | 10Py | 11K |
|---|---|---|---|---|---|---|---|---|
| Untreated | 0.44 | 0.45 | 0.52 | 0.90 | 0.37 | 0.53 | 0.54 | 0.67 |
| +Me | 0.95 | 0.43 | 0.55 | 0.83 | 0.43 | 0.48 | 0.61 | 0.74 |
| +Mzx | 0.82 | 0.42 | 0.64 | 1.04 | 0.72 | 0.46 | 0.61 | 0.62 |
| +Mep | 0.88 | 0.48 | 0.64 | 0.90 | 0.52 | 0.46 | 0.77 | 0.71 |
| +Fl | 1.11 | 0.52 | 0.74 | 1.06 | 0.58 | 0.60 | 0.67 | 0.72 |
| +Flt | 0.67 | 0.51 | 0.72 | 0.95 | 0.57 | 0.51 | 0.67 | 0.78 |
| +Cen | 0.85 | 0.47 | 0.68 | 1.03 | 0.58 | 0.50 | 0.68 | 0.69 |
| +Pe | 1.03 | 0.49 | 0.65 | 1.06 | 0.68 | 0.58 | 0.57 | 0.80 |
| +Stq | 0.76 | 0.48 | 0.62 | 1.00 | 0.62 | 0.43 | 0.67 | 0.87 |
| +Ptx | 0.92 | 0.50 | 0.76 | 1.06 | 0.60 | 0.39 | 0.64 | 0.96 |
| Range of Integrated Area [ppm] | |||||||
|---|---|---|---|---|---|---|---|
| Sample | -COOH | C(Ar)-O/N | C(Aromatic) | O-C-O | CH-OH | -OCH3 | -CH3 |
| 167–188 | 149–167 | 116–151 | 102–116 | 74–91 | 57–73 | 18–57 | |
| 1Ps | 7.7 | 8.5 | 36.1 | 7.6 | 11.2 | 8.8 | 17.3 |
| 1Ps + Fl | 8.7 | 8.3 | 32.0 | 5.4 | 9.0 | 7.5 | 22.1 |
| 1Ps + Me | 10.0 | 8.7 | 30.9 | 5.4 | 8.8 | 7.3 | 20.4 |
| 1Ps + Pe | 10.1 | 9.9 | 33.1 | 5.4 | 7.2 | 6.4 | 23.2 |
| 3Z | 9.9 | 6.5 | 38.3 | 5.9 | 8.5 | 7.7 | 21.8 |
| 3Z + Fl | 12.2 | 8.7 | 32.7 | 3.8 | 7.7 | 6.6 | 18.7 |
| 3Z + Me | 10.1 | 8.1 | 31.3 | 6.4 | 9.3 | 8.4 | 19.6 |
| 3Z + Pe | 11.7 | 8.6 | 29.8 | 5.5 | 8.6 | 7.5 | 18.6 |
| 6M | 10.6 | 9.2 | 32.5 | 4.8 | 11.4 | 8.2 | 19.8 |
| 6M + Fl | 9.8 | 7.7 | 23.6 | 4.2 | 7.3 | 6.5 | 17.8 |
| 6M + Me | 7.8 | 7.9 | 31.0 | 7.8 | 11.7 | 9.3 | 16.8 |
| 6M + Pe | 10.6 | 9.5 | 31.0 | 4.5 | 9.8 | 7.7 | 19.4 |
| 7T | 9.7 | 8.5 | 32.4 | 6.2 | 15.6 | 9.8 | 19.1 |
| 7T + Fl | 8.9 | 7.4 | 28.0 | 7.0 | 13.1 | 9.2 | 19.9 |
| 7T + Me | 13.9 | 7.3 | 29.6 | 2.8 | 10.2 | 7.9 | 21.1 |
| 7T + Pe | 9.5 | 8.3 | 27.7 | 6.7 | 12.7 | 9.2 | 17.3 |
| 8C | 12.8 | 9.6 | 41.4 | 3.5 | 9.2 | 7.2 | 17.8 |
| 8C + Fl | 7.8 | 8.4 | 32.2 | 6.6 | 10.1 | 8.2 | 16.5 |
| 8C + Me | 9.4 | 9.8 | 32.5 | 5.3 | 9.7 | 7.5 | 16.4 |
| 8C + Pe | 7.3 | 9.1 | 35.3 | 5.8 | 9.5 | 7.9 | 17.1 |
| 9H | 13.3 | 9.5 | 52.4 | 2.6 | 6.8 | 5.0 | 16.1 |
| 9H + Fl | 11.0 | 8.2 | 39.2 | 5.3 | 7.5 | 6.2 | 15.4 |
| 9H + Me | 21.6 | 5.9 | 35.2 | 1.7 | 5.9 | 2.5 | 26.0 |
| 9H + Pe | 13.0 | 10.9 | 35.5 | 0.5 | 5.2 | 4.3 | 18.2 |
| 10Py | 6.6 | 7.7 | 34.9 | 8.4 | 14.2 | 9.6 | 15.6 |
| 10Py + Fl | 9.1 | 8.7 | 31.5 | 5.9 | 11.1 | 8.1 | 17.4 |
| 10Py + Me | 10.3 | 10.0 | 30.6 | 7.3 | 11.3 | 7.7 | 13.1 |
| 10Py + Pe | 10.5 | 9.2 | 29.1 | 5.7 | 10.7 | 8.0 | 18.5 |
| 11K | 11.1 | 8.7 | 29.3 | 5.6 | 15.1 | 9.8 | 19.9 |
| 11K + Fl | 9.5 | 8.4 | 28.9 | 6.4 | 13.2 | 9.0 | 16.9 |
| 11K + Me | 11.9 | 9.2 | 28.6 | 5.0 | 11.9 | 8.2 | 16.2 |
| 11K + Pe | 16.6 | 9.4 | 29.1 | 0.2 | 10.8 | 6.5 | 21.3 |
| Sample | Range of Integrated Areas [cm−1] | ||||
|---|---|---|---|---|---|
| -C-O; -OH (COOH) | (Ar)C=C | -C=O (COOH) | sCH2 | asCH2 | |
| 1190–1300 | 1570–1677 | 1677–1800 | 2796–2850 | 2877–2985 | |
| 1Ps | 5.22 | 8.60 | 5.58 | 1.07 | 4.03 |
| 1Ps + Fl | 3.95 | 6.62 | 3.61 | 0.54 | 2.59 |
| 1Ps + Me | 3.51 | 6.40 | 4.32 | 1.02 | 3.40 |
| 1Ps + Pe | 3.74 | 7.32 | 4.18 | 0.37 | 1.79 |
| 3Z | 6.72 | 9.17 | 6.64 | 1.16 | 3.87 |
| 3Z + Fl | 5.21 | 8.05 | 6.14 | 1.31 | 4.31 |
| 3Z + Me | 5.97 | 8.51 | 5.96 | 1.24 | 3.96 |
| 3Z + Pe | 5.43 | 7.89 | 6.21 | 1.30 | 4.08 |
| 6M | 4.80 | 8.47 | 5.08 | 0.54 | 3.10 |
| 6M + Fl | 4.52 | 7.81 | 4.83 | 1.03 | 3.71 |
| 6M + Me | 4.38 | 7.45 | 4.40 | 0.69 | 3.06 |
| 6M + Pe | 2.21 | 6.89 | 3.91 | 0.92 | 3.35 |
| 7T | 3.64 | 6.86 | 3.82 | 0.76 | 3.12 |
| 7T + Fl | 4.02 | 7.81 | 4.26 | 0.47 | 2.11 |
| 7T + Me | 3.44 | 6.61 | 3.96 | 0.89 | 3.36 |
| 7T + Pe | 4.07 | 7.46 | 4.59 | 0.94 | 3.64 |
| 8C | 6.32 | 9.04 | 6.11 | 1.03 | 3.67 |
| 8C + Fl | 4.74 | 7.91 | 4.62 | 0.78 | 2.79 |
| 8C + Me | 4.62 | 7.63 | 4.49 | 1.07 | 3.85 |
| 8C + Pe | 5.07 | 8.92 | 5.42 | 0.66 | 2.56 |
| 9H | 4.98 | 8.68 | 5.75 | 0.72 | 2.73 |
| 9H + Fl | 4.69 | 8.20 | 5.63 | 0.79 | 2.90 |
| 9H + Me | 4.61 | 7.82 | 5.15 | 1.21 | 3.63 |
| 9H + Pe | 3.36 | 8.57 | 5.91 | 0.39 | 2.49 |
| 10Py | 3.12 | 6.23 | 3.20 | 0.95 | 3.02 |
| 10Py + Fl | 3.53 | 6.79 | 3.45 | 0.26 | 1.99 |
| 10Py + Me | 2.99 | 6.09 | 3.63 | 0.79 | 2.89 |
| 10Py + Pe | 3.64 | 7.05 | 4.32 | 0.44 | 2.61 |
| 11K | 4.77 | 8.20 | 4.92 | 0.96 | 3.79 |
| 11K + Fl | 4.38 | 7.48 | 4.52 | 0.55 | 2.87 |
| 11K + Me | 3.34 | 6.44 | 3.52 | 0.89 | 3.31 |
| 11K + Pe | 4.32 | 7.58 | 4.48 | 0.56 | 3.02 |
| Humin | Radical Concentration (SD) [×1016spin/g] | |||||||
|---|---|---|---|---|---|---|---|---|
| 1Ps | 3Z | 6M | 7T | 8C | 9H | 10Py | 11K | |
| Untreated | 8.32 (0.42) | 9.14 (0.50) | 12.45 (0.2) | 12.17 (0.3) | 11.67 (0.3) | 21.3 (0.5) | 7.59 (0.3) | 16.18 (0.5) |
| +Me | 4.54 (0.11) | 4.16 (0.2) | 3.59 (0.3) | 3.96 (0.2) | 5.48 (0.6) | 13.07 (0.2) | 2.19 (0.7) | 3.27 (0.2) |
| +Mzx | 2.46 (0.11) | 5.25 (0.13) | 4.44 (0.2) | 4.36 (0.1) | 4.24 (0.33) | 18.7 (0.1) | 1.43 (0.5) | 4.05 (0.11) |
| +Mep | 3.72 (0.05) | 3.53 (0.12) | 5.34 (0.11) | 3.09 (0.15) | 4.96 (0.4) | 10.57 (0.7) | 2.17 (0.2) | 2.84 (0.22) |
| +Fl | 3.82 (0.2) | 5.99 (0.22) | 3.49 (0.12) | 3.24 (0.1) | 3.69 (0.2) | 17.28 (0.2) | 3.74 (0.2) | 3.43 (0.3) |
| +Flt | 5.17 (0.09) | 5.38 (0.2) | 4.71 (0.3) | 3.74 (0.4) | 7.7 (0.1) | 15.44 (0.1) | 3.07 (0.1) | 3.14 (0.4) |
| +Cen | 4.88 (0.11) | 3.71 (0.14) | 4.59 (0.4) | 4.38 (0.2) | 5.29 (0.2) | 12.64 (0.2) | 3.00 (0.4) | 3.39 (0.4) |
| +Pe | 4.77 (0.03) | 4.77 (0.15) | 4.64 (0.2) | 6.61 (0.2) | 6.99 (0.22) | 15.29 (0.4) | 3.01 (0.3) | 3.56 (0.10) |
| +Stq | 5.36 (0.04) | 5.96 (0.22) | 3.71 (0.2) | 3.68 (0.5) | 5.13 (0.14) | 14.77 (0.6) | 2.69 (0.10 | 3.26 (0.2) |
| +Ptx | 5.80 (0.1) | 5.86 (0.2) | 4.99 (0.2) | 6.00 (0.4) | 6.62 (0.13) | 17.25 (0.1) | 4.16 (0.02) | 3.60 (0.08) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerzykiewicz, M.; Ćwieląg-Piasecka, I.; Weber, J.; Ukalska-Jaruga, A.; Jamroz, E.; Kocowicz, A.; Debicka, M.; Bekier, J.; Mielnik, L.; Bejger, R.; et al. The Significance of Herbicide–Humin Interactions in Sustainable Agroecosystems. Sustainability 2025, 17, 3449. https://doi.org/10.3390/su17083449
Jerzykiewicz M, Ćwieląg-Piasecka I, Weber J, Ukalska-Jaruga A, Jamroz E, Kocowicz A, Debicka M, Bekier J, Mielnik L, Bejger R, et al. The Significance of Herbicide–Humin Interactions in Sustainable Agroecosystems. Sustainability. 2025; 17(8):3449. https://doi.org/10.3390/su17083449
Chicago/Turabian StyleJerzykiewicz, Maria, Irmina Ćwieląg-Piasecka, Jerzy Weber, Aleksandra Ukalska-Jaruga, Elżbieta Jamroz, Andrzej Kocowicz, Magdalena Debicka, Jakub Bekier, Lilla Mielnik, Romualda Bejger, and et al. 2025. "The Significance of Herbicide–Humin Interactions in Sustainable Agroecosystems" Sustainability 17, no. 8: 3449. https://doi.org/10.3390/su17083449
APA StyleJerzykiewicz, M., Ćwieląg-Piasecka, I., Weber, J., Ukalska-Jaruga, A., Jamroz, E., Kocowicz, A., Debicka, M., Bekier, J., Mielnik, L., Bejger, R., Banach-Szott, M., & Grabusiewicz, A. (2025). The Significance of Herbicide–Humin Interactions in Sustainable Agroecosystems. Sustainability, 17(8), 3449. https://doi.org/10.3390/su17083449












