Anaerobic Bioremediation of Acid Mine Drainage Using Sulphate-Reducing Bacteria: Current Status, Challenges, and Future Directions
Abstract
:1. Introduction
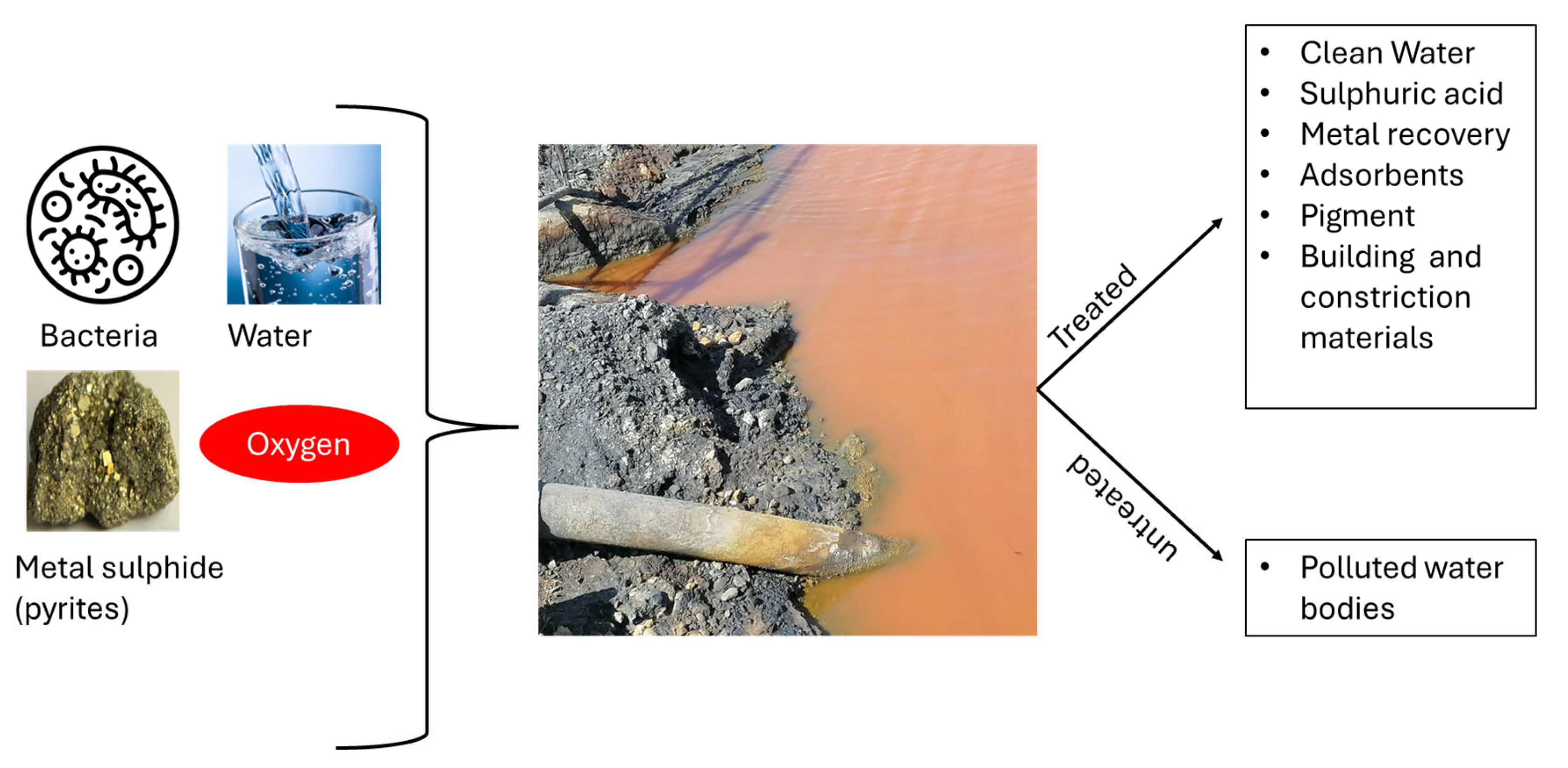
2. Overview of Acid Mine Drainage
Environmental Problem Caused by AMD
3. Current Status
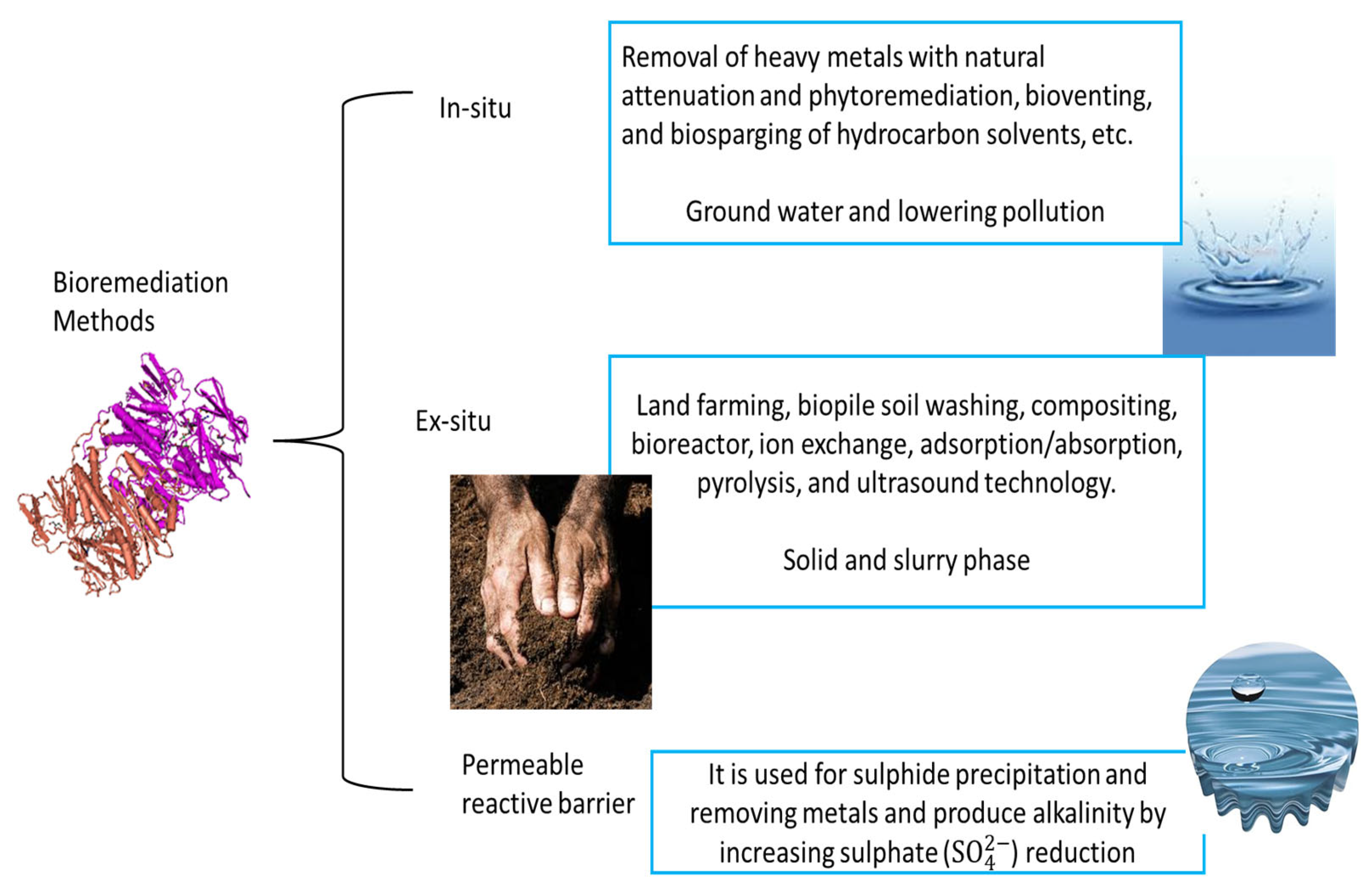
3.1. AMD Bioremediation Options
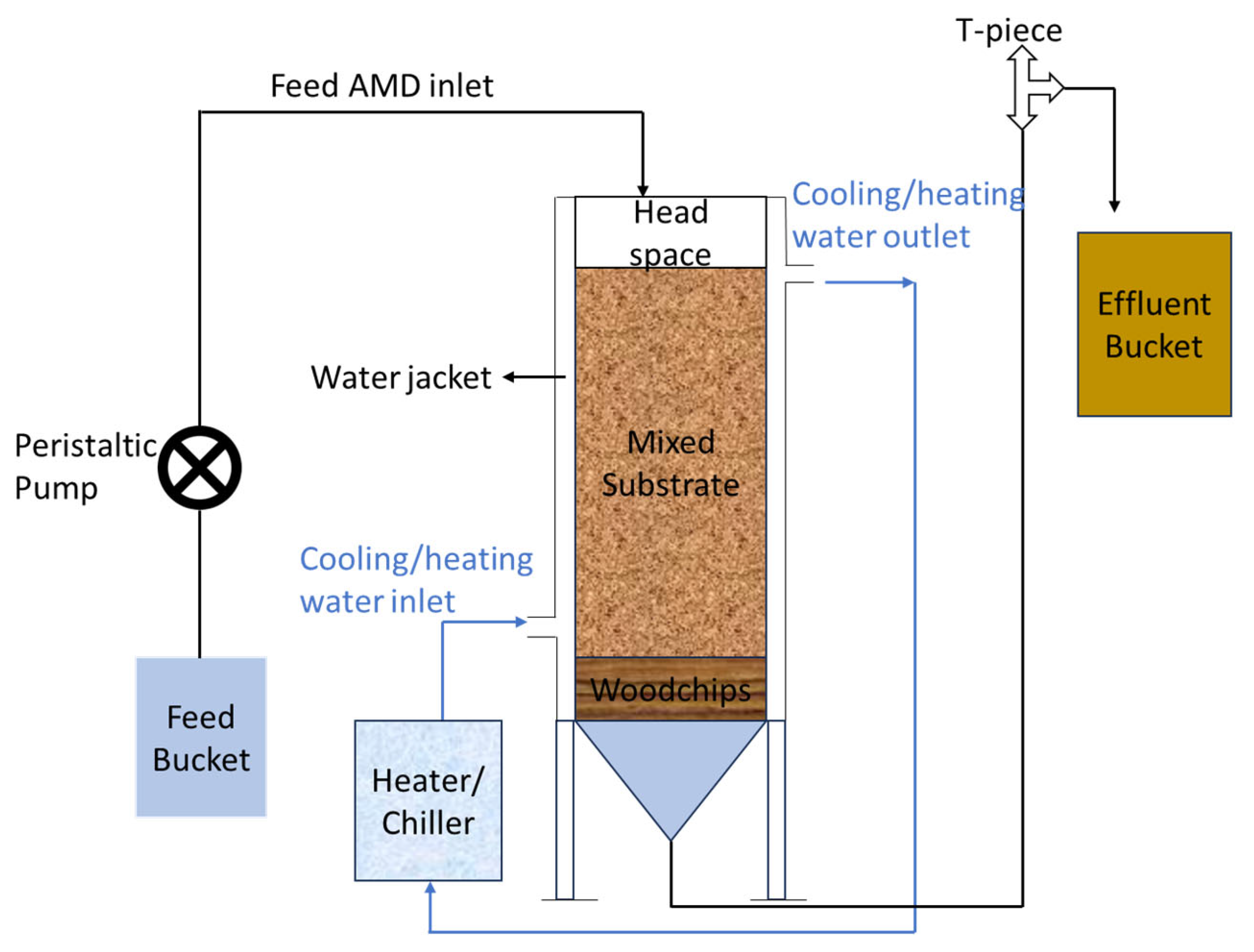
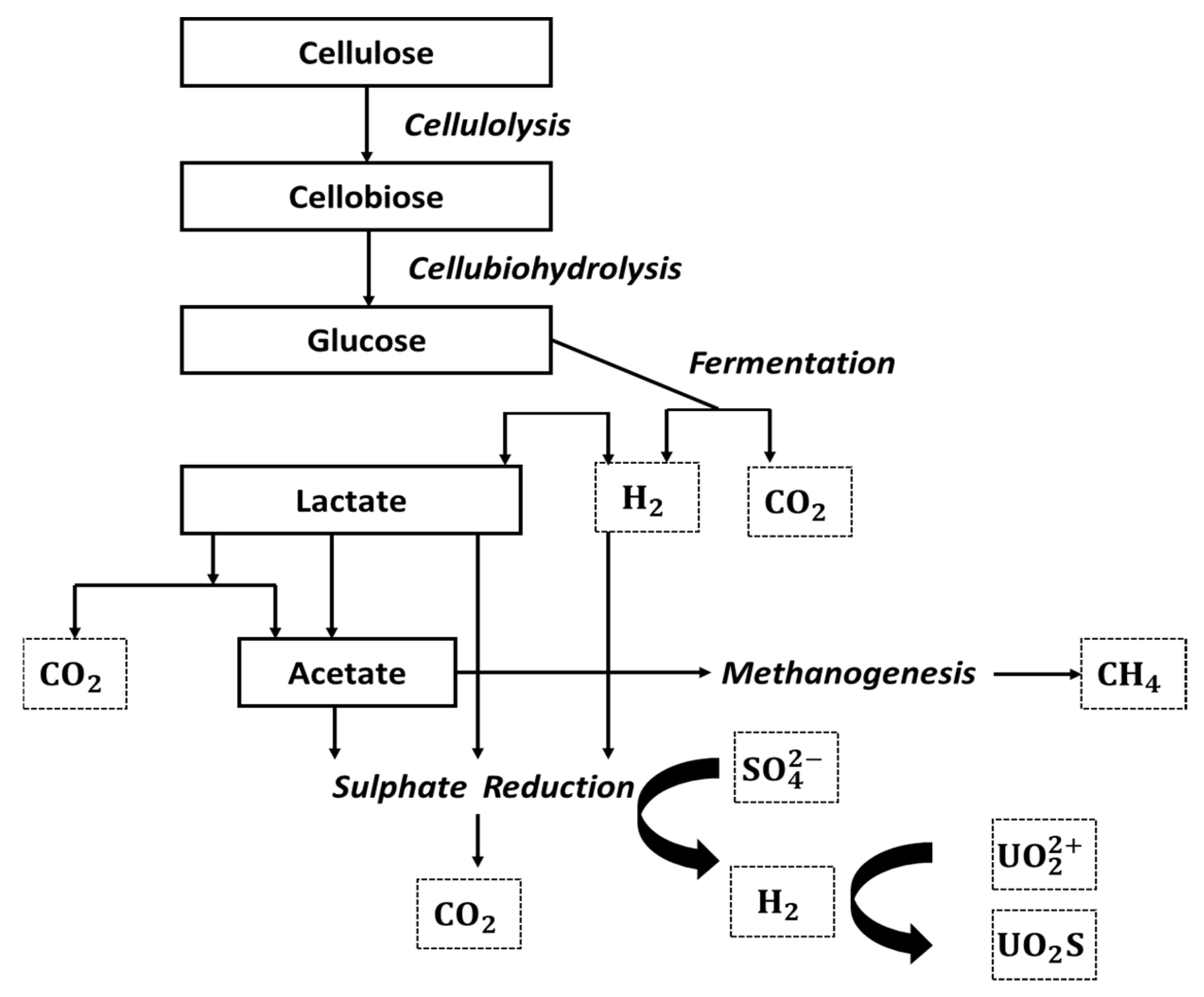
3.2. Bioremediation Process Using Sulphate-Reducing Bioreactors
3.3. Effectiveness of Current Bioremediation Methods Using Sulphate-Reducing Bioreactors
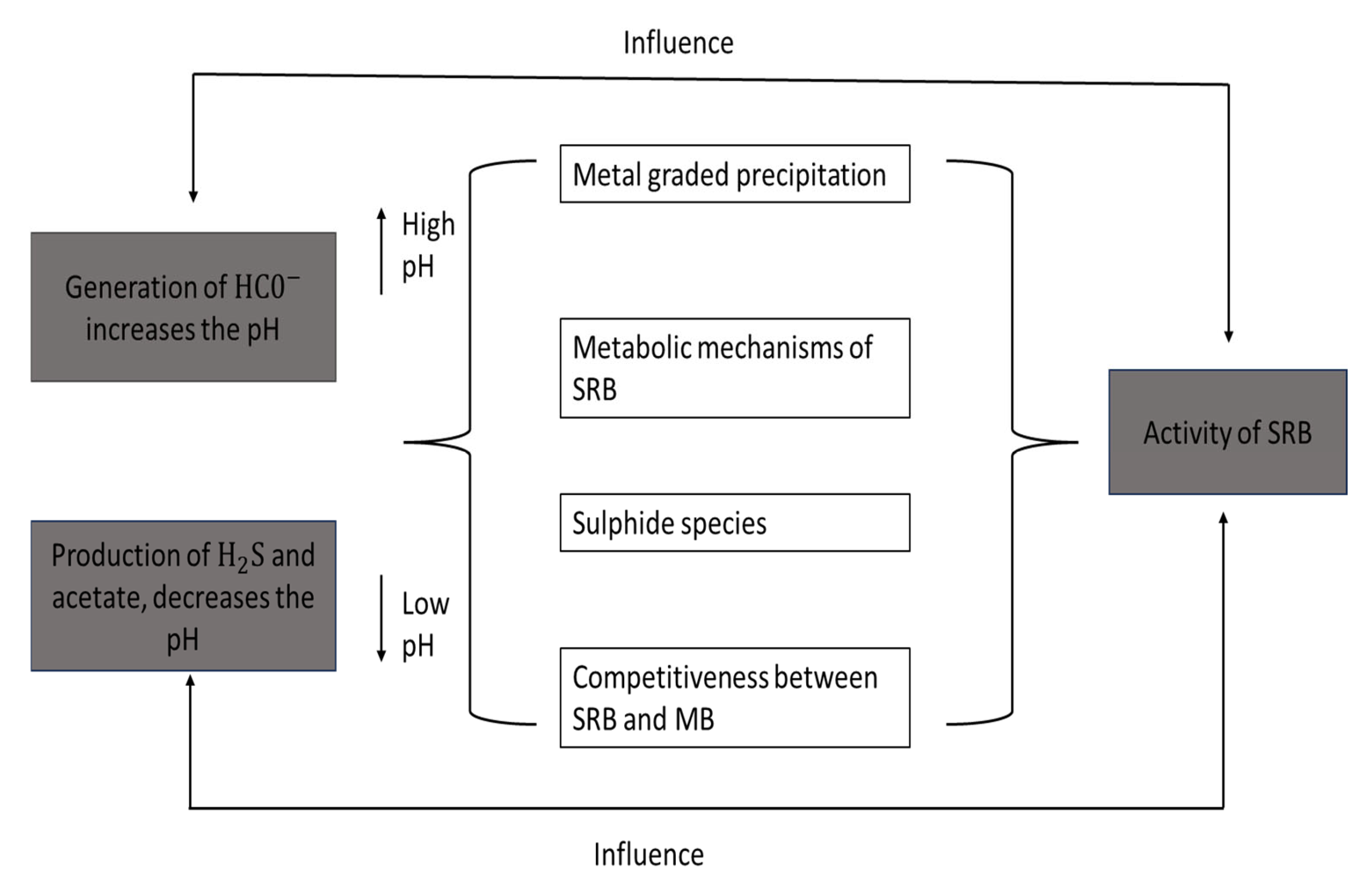
3.4. Factors That Affect the Capacity of the Bioreactor That Reduces Sulphate
3.4.1. pH
3.4.2. Substrate
3.4.3. Sulphide Concentration and the Ratio of COD to Sulphate
3.4.4. Metal Concentrations
3.4.5. Hydraulic Retention Time
3.4.6. Temperature
4. Challenges, Future Perspective, and Research Potential
4.1. Challenges Faced in the Bioremediation of Acid Mine Wastewater
4.2. Potential and Technologically Innovative Solutions to Consider
4.2.1. Capital Investments
4.2.2. Operational and Maintenance Costs
4.2.3. Treatment Efficiency and Scalability
4.3. Future Perspectives and Research Potential
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anekwe, I.M.S.; Yusuf, M.I. Bioremediation of acid mine drainage–Review. Alex. Eng. J. 2023, 65, 1047–1075. [Google Scholar] [CrossRef]
- Masindi, V. Recovery of drinking water and valuable minerals from acid mine drainage using an integration of magnesite, lime, soda ash, CO2 and reverse osmosis treatment processes. J. Environ. Chem. Eng. 2017, 5, 3136–3142. [Google Scholar] [CrossRef]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.H.; Ren, N.Q.; Show, P.L. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef] [PubMed]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Almeida, Â.; Cotas, J.; Pereira, L.; Carvalho, P. Potential Role of Spirogyra sp. and Chlorella sp. in bioremediation of mine drainage: A review. Phycology 2023, 3, 186–201. [Google Scholar] [CrossRef]
- Anawar, H.M. Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction, and climate knowledge. J. Environ. Manag. 2015, 158, 111–121. [Google Scholar] [CrossRef]
- Gupta, A.; Sar, P. Treatment options for acid mine drainage: Remedial achievements through microbial-mediated processes. In Combined Application of Physico-Chemical & Microbiological Processes for Industrial Effluent Treatment Plant; Springer Nature: Singapore, 2020; pp. 145–185. [Google Scholar]
- Jamil, I.N.; Clarke, W.P. Bioremediation for acid mine drainage: Organic solid waste as carbon sources for sulfate-reducing bacteria: A review. J. Mech. Eng. Sci. 2013, 5, 569–581. [Google Scholar] [CrossRef]
- Mosai, A.K.; Ndlovu, G.; Tutu, H. Improving acid mine drainage treatment by combining treatment technologies: A review. Sci. Total Environ. 2024, 919, 170806. [Google Scholar] [CrossRef]
- Bwapwa, J.K.; Jaiyeola, A.T.; Chetty, R. Bioremediation of acid mine drainage using algae strains: A review. S. Afr. J. Chem. Eng. 2017, 24, 62–70. [Google Scholar] [CrossRef]
- Wang, X.; Yang, M.; Chen, H.; Cai, Z.; Fu, W.; Zhang, X.; Li, Y. Monitoring and Prevention Strategies for Iron and Aluminum Pollutants in Acid Mine Drainage (AMD): Evidence from Xiaomixi Stream in Qinling Mountains. Minerals 2025, 15, 59. [Google Scholar] [CrossRef]
- Mafane, D.; Ngulube, T.; Mphahlele-Makgwane, M. Recovery of Al (iii) and Fe (iii) from Acid Mine Drainage using CaO and their Subsequent Use in Fluoride Removal from Water. Chem. Biol. Environ. Eng. 2023, 39, 183–188. [Google Scholar] [CrossRef]
- Brar, K.K.; Etteieb, S.; Magdouli, S.; Calugaru, L.; Brar, S.K. Novel approach for the management of acid mine drainage (AMD) for the recovery of heavy metals along with lipid production by Chlorella vulgaris. J. Environ. Manag. 2022, 308, 114507. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, T.; Uçar, D. Utilization of excess microorganisms as carbon and electron sources in the sulphate reduction process. J. Chem. Technol. Biotechnol. 2024, 99, 601–608. [Google Scholar] [CrossRef]
- Wibowo, Y.G.; Taher, T.; Khairurrijal, K.; Ramadan, B.S.; Safitri, H.; Sudibyo, S.; Yuliansyah, A.T.; Petrus, H.T.B.M. Recent advances in the adsorptive removal of heavy metals from acid mine drainage by conventional and novel materials: A review. Bioresour. Technol. Rep. 2024, 25, 101797. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Manyatshe, A.; Waanders, F. Mobility potential of metals in acid mine drainage occurring in the Highveld area of Mpumalanga Province in South Africa: Implication of sediments and efflorescent crusts. Int. Biodeterior. Biodegrad. 2017, 119, 661–670. [Google Scholar] [CrossRef]
- Sajjad, W.; Ilahi, N.; Kang, S.; Bahadur, A.; Banerjee, A.; Zada, S.; Ali, B.; Rafiq, M.; Zheng, G. Microbial diversity and community structure dynamics in acid mine drainage: Acidic fire with dissolved heavy metals. Sci. Total Environ. 2024, 909, 168635. [Google Scholar] [CrossRef]
- Ayora, C.; Macías, F.; Torres, E.; Lozano, A.; Carrero, S.; Nieto, J.M.; Pérez-López, R.; Fernández-Martínez, A.; Castillo-Michel, H. Recovery of rare earth elements and yttrium from passive-remediation systems of acid mine drainage. Environ. Sci. Technol. 2016, 50, 8255–8262. [Google Scholar] [CrossRef]
- Folifac, L.; Ameh, A.E.; Broadhurst, J.; Petrik, L.F.; Ojumu, T.V. Iron nanoparticles prepared from South African acid mine drainage for the treatment of methylene blue in wastewater. Environ. Sci. Pollut. Res. 2024, 31, 38310–38322. [Google Scholar] [CrossRef]
- Song, Y.; Guo, Z.; Wang, R.; Yang, L.; Cao, Y.; Wang, H. A novel approach for treating acid mine drainage by forming schwertmannite driven by a combination of bio-oxidation and electro reduction before lime neutralization. Water Res. 2022, 221, 118748. [Google Scholar] [CrossRef]
- Muliwa, A.M.; Leswifi, T.Y.; Onyango, M.S. Performance evaluation of eggshell waste material for remediation of acid mine drainage from coal dump leachate. Miner. Eng. 2018, 122, 241–250. [Google Scholar] [CrossRef]
- Brewster, E.T.; Freguia, S.; Edraki, M.; Berry, L.; Ledezma, P. Staged electrochemical treatment guided by modelling allows for targeted recovery of metals and rare earth elements from acid mine drainage. J. Environ. Manag. 2020, 275, 111266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, J.; Biswas, A.; Fang, W.; Chen, L. Acid mine wastewater treatment: A scientometrics review. J. Water Process Eng. 2024, 57, 104713. [Google Scholar] [CrossRef]
- Yuan, J.; Ding, Z.; Bi, Y.; Li, J.; Wen, S.; Bai, S. Resource utilization of acid mine drainage (AMD): A review. Water 2022, 14, 2385. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Kurniawan, S.B.; Iwuozor, K.O.; Aniagor, C.O.; Ajala, O.J.; Oba, S.N.; Iwuchukwu, F.U.; Ahmadi, S.; Igwegbe, C.A. A review of treatment technologies for the mitigation of the toxic environmental effects of acid mine drainage (AMD). Process Saf. Environ. Prot. 2022, 157, 37–58. [Google Scholar] [CrossRef]
- Masindi, V.; Chatzisymeon, E.; Kortidis, I.; Foteinis, S. Assessing the sustainability of acid mine drainage (AMD) treatment in South Africa. Sci. Total Environ. 2018, 635, 793–802. [Google Scholar] [CrossRef]
- Masindi, V.; Foteinis, S.; Renforth, P.; Ndiritu, J.; Maree, J.P.; Tekere, M.; Chatzisymeon, E. Challenges and avenues for acid mine drainage treatment, beneficiation, and valorisation in circular economy: A review. Ecol. Eng. 2022, 183, 106740. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, C.; Su, P.; Tang, Y.; Huang, Z.; Ma, T. A review of acid mine drainage: Formation mechanism, treatment technology, typical engineering cases and resource utilization. Process Saf. Environ. Prot. 2023, 170, 1240–1260. [Google Scholar] [CrossRef]
- Waters, A.S.; Webster-Brown, J.G. Assessing aluminium toxicity in streams affected by acid mine drainage. Water Sci. Technol. 2013, 67, 1764–1772. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity, and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Ge, X.; Nie, D.; Wang, M.; Zhou, H.; Chen, M. In Vitro toxicity evaluation of heavy metals in urban air particulate matter on human lung epithelial cells. Sci. Total Environ. 2019, 678, 301–308. [Google Scholar] [CrossRef]
- Moodley, I.; Sheridan, C.M.; Kappelmeyer, U.; Akcil, A. Environmentally sustainable acid mine drainage remediation: Research developments with a focus on waste/by-products. Miner. Eng. 2018, 126, 207–220. [Google Scholar] [CrossRef]
- Cao, G.; Zhao, J.; Zhao, G.; Wan, D.; Wu, Z.; Li, R.; He, Q. Determination of the Acute and Chronic Toxicity of Sulfate from the Sulfur Autotrophic Denitrification Process to Juvenile Zebrafish (Danio rerio). ACS Omega 2022, 7, 47165–47173. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.C.; Tamburini, J.; Johns, F. Designing a mine water treatment facility to remove sulphate. In Proceedings of the 10th International Conference on Acid Rock Drainage & IMWA Annual Conference, Santiago, Chile, 21–24 April 2015; pp. 21–24. [Google Scholar]
- Bega, S. Coal Mine Acidic Water Spillage into Wilge River System Kills All Life Everything. The Mail and Guardian, 24 February 2022. Available online: https://mg.co.za/the-green-guardian/2022-02-24-coal-mine-acidic-water-spillage-into-wilge-river-system-kills-all-life-everything/ (accessed on 25 February 2024).
- Thungela Resources. Thungela Remediating Impact of Environmental Incident, Media Release. Available online: https://www.thungela.com/ (accessed on 13 April 2024).
- Kinnunen, P.; Kyllönen, H.; Kaartinen, T.; Mäkinen, J.; Heikkinen, J.; Miettinen, V. Sulphate removal from mine water with chemical, biological and membrane technologies. Water Sci. Technol. 2018, 2017, 194–205. [Google Scholar] [CrossRef]
- Radelyuk, I.; Tussupova, K.; Zhapargazinova, K.; Yelubay, M.; Persson, M. Pitfalls of wastewater treatment in oil refinery enterprises in Kazakhstan—A system Approach. Sustainability 2019, 11, 1618. [Google Scholar] [CrossRef]
- Novair, S.B.; Atigh, Z.B.Q.; Lajayer, B.A.; Shu, W.; Price, G.W. The role of sulphate-reducing bacteria (SRB) in bioremediation of sulphate-rich wastewater: Focus on the source of electron donors. Process. Saf. Environ. Prot. 2024, 184, 190–207. [Google Scholar] [CrossRef]
- Li, J.; Tabassum, S. Synergism of hydrolytic acidification and sulphate reducing bacteria for acid production and desulfurization in the anaerobic baffled reactor: High sulphate sewage wastewater treatment. Chem. Eng. J. 2022, 444, 136611. [Google Scholar] [CrossRef]
- Kuppan, N.; Padman, M.; Mahadeva, M.; Srinivasan, S.; Devarajan, R. A comprehensive review of sustainable bioremediation techniques: Eco-friendly solutions for waste and pollution management. Waste Manag. Bull. 2024, 2, 154–171. [Google Scholar] [CrossRef]
- King, R.B.; Sheldon, J.K.; Long, G.M. Practical Environmental Bioremediation: The Field Guide; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Hussain, A.; Rehman, F.; Rafeeq, H.; Waqas, M.; Asghar, A.; Afsheen, N.; Rahdar, A.; Bilal, M.; Iqbal, H.M. In-Situ, Ex-Situ, and nano-remediation strategies to treat polluted soil, water and air A review. Chemosphere 2022, 289, 133252. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Palanisami, T.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Ex-situ remediation technologies for environmental pollutants: A critical perspective. Rev. Environ. Contam. Toxicol. 2016, 236, 117–192. [Google Scholar]
- Besha, A.T.; Gebreyohannes, A.Y.; Tufa, R.A.; Bekele, D.N.; Curcio, E.; Giorno, L. Removal of emerging micropollutants by activated sludge process and membrane bioreactors and the effects of micropollutants on membrane fouling: A review. J. Environ. Chem. Eng. 2017, 5, 2395–2414. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Chattopadhyay, D. Coal and Other Mining Operations: Role of Sustainability; Fossil Energy; Springer: New York, NY, USA, 2020; pp. 333–356. [Google Scholar] [CrossRef]
- Reese, J.T. Cost comparison of commercial atmospheric and pressurized fluidized-bed power plants to a conventional coal-fired power plant with flue gas desulfurization. In Proceedings of the National Conference on Health, Environmental Effects, and Control Technology of Energy Use, Washington, DC, USA, 9–11 February 1976; p. 220. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Ali, H.E.B.; Neculita, C.M.; Molson, J.W.; Maqsoud, A.; Zagury, G.J. Efficiency of batch biochemical reactors for mine drainage treatment at low temperature and high salinity. Appl. Geochem. 2019, 103, 40–49. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Albarico, F.P.J.B.; Pandey, A.; Chen, C.W.; Dong, C.D. Organic wastes bioremediation and its changing prospects. Sci. Total Environ. 2022, 824, 153889. [Google Scholar] [CrossRef]
- Frederico, T.D.; Nancucheo, I.; Santos, W.C.B.; Oliveira, R.R.M.; Buzzi, D.C.; Pires, E.S.; Bitencourt, J.A.P. Comparison of two acidophilic sulfidogenic consortia for the treatment of acidic mine water. Front. Bioeng. Biotechnol. 2022, 10, 1048412. [Google Scholar] [CrossRef]
- Gu, Q.; Cui, X.; Shang, H. Optimization of a modular continuous flow bioreactor system for acid mine drainage treatment using Plackett–Burman design. Asia-Pac. J. Chem. Eng. 2020, 15, e2469. [Google Scholar] [CrossRef]
- Bhavya, K.; Begum, S.; Gangagni Rao, A. Anaerobic Bioreactor Technology (ABT) for the Treatment of Acid Mine Drainage (AMD). In Biotechnological Innovations in the Mineral-Metal Industry; Springer International Publishing: Cham, Switzerland, 2024; pp. 161–178. [Google Scholar] [CrossRef]
- Lago, A.; Rocha, V.; Barros, O.; Silva, B.; Tavares, T. Bacterial biofilm attachment to sustainable carriers as a clean-up strategy for wastewater treatment: A review. J. Water Process Eng. 2024, 63, 105368. [Google Scholar] [CrossRef]
- Qi, Z.; Jia, T.; Cong, W.; Xi, J. Mitigation of hydrogen sulphide production in sewer systems by inhibiting sulphate-reducing bacteria: A review. Front. Environ. Sci. Eng. 2025, 19, 39. [Google Scholar] [CrossRef]
- Aoyagi, T.; Hamai, T.; Hori, T.; Sato, Y.; Kobayashi, M.; Sato, Y.; Inaba, T.; Ogata, A.; Habe, H.; Sakata, T. Hydraulic retention time and pH affect the performance and microbial communities of passive bioreactors for treatment of acid mine drainage. Amb Express 2017, 7, 142. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Hussain, I.; Rasheed, R.; Iqbal, M.; Riaz, M.; Arif, M.S. Advances in microbe-assisted reclamation of heavy metal contaminated soils over the last decade: A review. J. Environ. Manag. 2017, 198, 132–143. [Google Scholar] [CrossRef]
- Adekunle, A.A.; Adekunle, I.M.; Badejo, A.A.; Alayaki, F.M.; Olusola, A.O. Laboratory scale bioremediation of crude oil-impacted soil using animal waste compost. Teh. Glas. 2017, 11, 45–49. [Google Scholar]
- Hiibel, S.R.; Pereyra, L.P.; Breazeal, M.V.R.; Reisman, D.J.; Reardon, K.F.; Pruden, A. Effect of organic substrate on the microbial community structure in pilot-scale sulphate-reducing biochemical reactors treating mine drainage. Environ. Eng. Sci. 2011, 28, 563–572. [Google Scholar] [CrossRef]
- Skousen, J.G.; Ziemkiewicz, P.F.; McDonald, L.M. Acid mine drainage formation, control, and treatment: Approaches and strategies. Extr. Ind. Soc. 2019, 6, 241–249. [Google Scholar] [CrossRef]
- Yildiz, M.; Yilmaz, T.; Arzum, C.S.; Yurtsever, A.; Kaksonen, A.H.; Ucar, D. Sulphate reduction in acetate-and ethanol-fed bioreactors: Acidic mine drainage treatment and selective metal recovery. Miner. Eng. 2019, 133, 52–59. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Yang, Y.; Zhang, Z.; Tang, Y.; Su, P.; Lin, Z. A review of sulphate-reducing bacteria: Metabolism, influencing factors and application in wastewater treatment. J. Clean. Prod. 2022, 376, 134109. [Google Scholar] [CrossRef]
- Mukwevho, M.J.; Maharajh, D.; Chirwa, E.M.N. Evaluating the effect of pH, temperature, and hydraulic retention time on biological sulphate reduction using response surface methodology. Water 2020, 12, 2662. [Google Scholar] [CrossRef]
- Di, J.; Ma, Y.; Wang, M.; Gao, Z.; Xu, X.; Dong, Y.; Fu, S.; Li, H. Dynamic experiments of acid mine drainage with Rhodopseudomonas spheroides activated lignite immobilized sulphate-reducing bacteria particles treatment. Sci. Rep. 2022, 12, 8783. [Google Scholar] [CrossRef]
- Zhang, T.; Tu, Z.; Lu, G.; Duan, X.; Yi, X.; Guo, C.; Dang, Z. Removal of heavy metals from acid mine drainage using chicken eggshells in column mode. J. Environ. Manag. 2017, 188, 1–8. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Lavonen, L.; Kuusenaho, M.; Kolli, A.; Närhi, H.; Vestola, E.; Puhakka, J.A.; Tuovinen, O.H. Bioleaching and recovery of metals from final slag waste of the copper smelting industry. Miner. Eng. 2011, 24, 1113–1121. [Google Scholar] [CrossRef]
- Sato, Y.; Hamai, T.; Hori, T.; Habe, H.; Kobayashi, M.; Sakata, T. Year-round performance of a passive sulphate-reducing bioreactor that uses rice bran as an organic carbon source to treat acid mine drainage. Mine Water Environ. 2018, 37, 586–594. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H. Organic wastes as carbon sources to promote sulphate-reducing bacterial activity for biological remediation of acid mine drainage. Miner. Eng. 2014, 69, 81–90. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, G.; Liu, S.; Fu, Z.; Chen, J.; Ma, C. Bio removal of arsenic and antimony from wastewater by a mixed culture of sulphate-reducing bacteria using lactate and ethanol as carbon sources. Int. Biodeterior. Biodegrad. 2018, 126, 152–159. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Y.; Yang, M.; Li, W.; Deng, L. Enhanced bioremediation of heavy metal from effluent by sulphate-reducing bacteria with copper–iron bimetallic particles support. Bioresour. Technol. 2013, 136, 413–417. [Google Scholar] [CrossRef]
- Vasquez, Y.; Escobar, M.C.; Saenz, J.S.; Quiceno-Vallejo, M.F.; Neculita, C.M.; Arbeli, Z.; Roldan, F. Effect of hydraulic retention time on microbial community in biochemical passive reactors during treatment of acid mine drainage. Bioresour. Technol. 2018, 247, 624–632. [Google Scholar] [CrossRef]
- Nogueira, E.W.; de Godoi, L.A.G.; Yabuki, L.N.M.; Brucha, G.; Damianovic, M.H.R.Z. Sulphate and metal removal from acid mine drainage using sugarcane vinasse as electron donor: Performance and microbial community of the down-flow structured-bed bioreactor. Bioresour. Technol. 2021, 330, 124968. [Google Scholar] [CrossRef]
- Bertolino, S.M.; Melgaço, L.A.; Sá, R.G.; Leão, V.A. Comparing lactate and glycerol as a single-electron donor for sulphate reduction in fluidized bed reactors. Biodegradation 2014, 25, 719–733. [Google Scholar] [CrossRef]
- Chen, J.; Gan, L.; Han, Y.; Owens, G.; Chen, Z. Ferrous sulphide nanoparticles can be biosynthesized by sulphate-reducing bacteria: Synthesis, characterization, and removal of heavy metals from acid mine drainage. J. Hazard. Mater. 2024, 466, 133622. [Google Scholar] [CrossRef]
- Chai, G.; Wang, D.; Zhang, Y.; Wang, H.; Li, J.; Jing, X.; Meng, H.; Wang, Z.; Guo, Y.; Jiang, C.; et al. Effects of organic substrates on sulphate-reducing microcosms treating acid mine drainage: Performance dynamics and microbial community comparison. J. Environ. Manag. 2023, 330, 117148. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Nguyen, H.L.; Nguyen, M.H.; Nguyen, T.K.N.; Dinh, H.T. Sulfate Reduction for Bioremediation of AMD Facilitated by an Indigenous Acid-and Metal-Tolerant Sulfate-Reducer. J. Microbiol. Biotechnol. 2020, 30, 1005–1012. [Google Scholar] [CrossRef]
- Alexandrino, M.; Macías, F.; Costa, R.; Gomes, N.C.; Canário, A.V.; Costa, M.C. A bacterial consortium isolated from an Icelandic fumarole displays exceptionally high levels of sulfate reduction and metals resistance. J. Hazard. Mater. 2011, 187, 362–370. [Google Scholar] [CrossRef]
- Gu, S.; Fu, B.; Ahn, J.W. Simultaneous removal of residual sulfate and heavy metals from spent electrolyte of lead-acid battery after precipitation and carbonation. Sustainability 2020, 12, 1263. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, M.; Li, B.; Dong, Y. Mechanisms, application advances and future perspectives of microbial-induced heavy metal precipitation: A review. Int. Biodeterior. Biodegrad. 2023, 178, 105544. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, Z.; Di, J.; Wang, D.; Yang, Z.; Guo, X.; Zhu, X. Study on the effectiveness of sulphate-reducing bacteria to remove Pb (II) and Zn (II) in tailings and acid mine drainage. Front. Microbiol. 2024, 15, 1352430. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Mainardis, M.; Buttazzoni, M.; Goi, D. Up-flow anaerobic sludge blanket (UASB) technology for energy recovery: A review on state-of-the-art and recent technological advances. Bioengineering 2020, 7, 43. [Google Scholar] [CrossRef]
- Sharma, K.; Derlon, N.; Hu, S.; Yuan, Z. Modeling the pH effect on sulfidogenesis in anaerobic sewer biofilm. Water Res. 2014, 49, 175–185. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Dev, S.; Roy, S.; Bhattacharya, J. Optimization of the operation of packed bed bioreactor to improve the sulphate and metal removal from acid mine drainage. J. Environ. Manag. 2017, 200, 135–144. [Google Scholar] [CrossRef]
- Xu, Y.N.; Chen, Y. Advances in heavy metal removal by sulphate-reducing bacteria. Water Sci. Technol. 2020, 81, 1797–1827. [Google Scholar] [CrossRef]
- Yang, S.; Li, Q.; Chen, L.; Chen, Z.; Hu, B.; Wang, H.; Wang, X. Synergistic removal and reduction of U (VI) and Cr (VI) by Fe3S4 micro-crystal. Chem. Eng. J. 2020, 385, 123909. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.Q.; Sanz, J.L.; Bijmans, M.F.; Stams, A.J. Sulphate reduction at low pH to remediate acid mine drainage. J. Hazard. Mater. 2014, 269, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, H. Preparation of immobilized sulphate reducing bacteria (SRB) granules for effective bioremediation of acid mine drainage and bacterial community analysis. Miner. Eng. 2016, 92, 63–71. [Google Scholar] [CrossRef]
- Jong, T.; Parry, D.L. Microbial sulphate reduction under sequentially acidic conditions in an up flow anaerobic packed bed bioreactor. Water Res. 2006, 40, 2561–2571. [Google Scholar] [CrossRef]
- Meier, J.; Piva, A.; Fortin, D. Enrichment of sulphate-reducing bacteria and resulting mineral formation in media mimicking pore water metal ion concentrations and pH conditions of acidic pit lakes. FEMS Microbiol. Ecol. 2012, 79, 69–84. [Google Scholar] [CrossRef]
- Genty, T.; Bussière, B.; Benzaazoua, M.; Neculita, C.M.; Zagury, G.J. Iron removal in highly contaminated acid mine drainage using passive biochemical reactors. Water Sci. Technol. 2017, 76, 1833–1843. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, Q.; Chen, Y.; An, H.; Zhang, L.; Wu, Z.; Xiao, E. Nitrate reduction pathway of iron-sulphides-based MFC-CWs purifying low C/N wastewater: Competitive mechanism to inorganic and organic electrons. Chem. Eng. J. 2024, 479, 147379. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-derived materials for green electronics, biological devices, and energy applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef]
- Valdez-Nuñez, L.F.; Kappler, A.; Ayala-Muñoz, D.; Chávez, I.J.; Mansor, M. Acidophilic sulphate-reducing bacteria: Diversity, ecophysiology, and applications. Environ. Microbiol. Rep. 2024, 16, 70019. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Baloo, L.; Birniwa, A.H.; Lawal, I.M.; Aliyu, M.K.; Yaro, N.S.A.; Usman, A.K. Combined treatment of domestic and pulp and paper industry wastewater in a rice straw-embedded activated sludge bioreactor to achieve sustainable development goals. Case Stud. Chem. Environ. Eng. 2020, 6, 100261. [Google Scholar] [CrossRef]
- Wu, J.; Lu, J.; Chen, T.; He, Z.; Su, Y.; Jin, X.; Yao, X. In Situ biotreatment of acidic mine drainage using straw as the sole substrate. Environ. Earth Sci. 2010, 60, 421–429. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Han, X. Preparation of metal-resistant immobilised sulphate-reducing bacteria beads for acid mine drainage treatment. Chemosphere 2016, 154, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Habe, H.; Sato, Y.; Aoyagi, T.; Inaba, T.; Hori, T.; Hamai, T.; Sato, N. Design, application, and microbiome of sulphate-reducing bioreactors for treatment of mining-influenced water. Appl. Microbiol. Biotechnol. 2020, 104, 6893–6903. [Google Scholar] [CrossRef] [PubMed]
- Oztemur, G.; Basaran, S.T.; Tayran, Z.; Sahinkaya, E. Fluidized bed membrane bioreactor achieves high sulphate reduction and filtration performances at moderate temperatures. Chemosphere 2020, 252, 126587. [Google Scholar] [CrossRef]
- Sheng, Y.; Cao, H.; Li, Y.; Zhang, Y. Effects of sulphide on sulphate reducing bacteria in response to Cu (II), Hg (II) and Cr (VI) toxicity. Chin. Sci. Bull. 2011, 56, 862–868. [Google Scholar] [CrossRef]
- Lefticariu, L.; Walters, E.R.; Pugh, C.W.; Bender, K.S. Sulfate reducing bioreactor dependence on organic substrates for remediation of coal-generated acid mine drainage: Field experiments. Appl. Geochem. 2015, 63, 70–82. [Google Scholar] [CrossRef]
- Lu, X.; Zhen, G.; Ni, J.; Hojo, T.; Kubota, K.; Li, Y.Y. Effect of influent COD/SO42− ratios on the biodegradation behaviours of starch wastewater in an up flow anaerobic sludge blanket (UASB) reactor. Bioresour. Technol. 2016, 214, 175–183. [Google Scholar] [CrossRef]
- Qian, Z.; Tianwei, H.; Mackey, H.R.; van Loosdrecht, M.C.; Guanghao, C. Recent advances in dissimilatory sulfate reduction: From metabolic study to application. Water Res. 2019, 150, 162–181. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Khan, M.A.; Xu, H.; Wang, F.; Xia, M. In-depth study of heavy metal removal by an etidronic acid-functionalized layered double hydroxide. ACS Appl. Mater. Interfaces 2022, 14, 7450–7463. [Google Scholar] [CrossRef]
- Zampieri, B.D.B.; Nogueira, E.W.; de Oliveira, A.J.F.C.; Sánchez-Andrea, I.; Brucha, G. Effects of metals on activity and community of sulfate-reducing bacterial enrichments and the discovery of a new heavy metal-resistant SRB from Santos Port sediment (São Paulo, Brazil). Environ. Sci. Pollut. Res. 2022, 29, 922–935. [Google Scholar] [CrossRef]
- Vieira, B.F.; Couto, P.T.; Sancinetti, G.P.; Klein, B.; van Zyl, D.; Rodriguez, R.P. The effect of acidic pH and presence of metals as parameters in establishing a sulfidogenic process in anaerobic reactor. J. Environ. Sci. Health Part A 2016, 51, 793–797. [Google Scholar] [CrossRef]
- Cunha, M.P.; Ferraz, R.M.; Sancinetti, G.P.; Rodriguez, R.P. Long-term performance of a UASB reactor treating acid mine drainage: Effects of sulphate loading rate, hydraulic retention time, and COD/SO42− ratio. Biodegradation 2019, 30, 47–58. [Google Scholar] [CrossRef]
- Chang, I.S.; Shin, P.K.; Kim, B.H. Biological treatment of acid mine drainage under sulphate-reducing conditions with solid waste materials as substrate. Water Res. 2000, 34, 1269–1277. [Google Scholar] [CrossRef]
- Barbera, E.; Sforza, E.; Grandi, A.; Bertucco, A. Uncoupling solid and hydraulic retention time in photobioreactors for microalgae mass production: A model-based analysis. Chem. Eng. Sci. 2020, 218, 115578. [Google Scholar] [CrossRef]
- Yao, Y.; Shi, K.; Li, Y.; Wang, J.; Cheng, D.; Jiang, Q.; Gao, Y.; Qiao, Y.; Zhu, N.; Xue, J. Mechanism of sulphate reduction hampered in anaerobic biosystem under the progressive decrease of chemical oxygen demand to sulphate ratios: Long-term performance and key microbial community dynamics. J. Water Process Eng. 2024, 65, 105782. [Google Scholar] [CrossRef]
- Akinpelu, E.A.; Fosso-Kankeu, E.; Waanders, F.; Angadam, J.O.; Ntwampe, S.K. Diversity and performance of sulphate-reducing bacteria in acid mine drainage remediation systems. In Frontiers in Water-Energy Nexus: Nature-Based Solutions, Advanced Technologies, and Best Practices for Environmental Sustainability, Proceedings of the 2nd Water Energy NEXUS Conference, Salerno, Italy, 14–17 November 2018; Springer: Cham, Switzerland, 2020; pp. 121–123. [Google Scholar]
- Willis, G.; Nancucheo, I.; Hedrich, S.; Giaveno, A.; Donati, E.; Johnson, D.B. Enrichment and isolation of acid-tolerant sulphate-reducing microorganisms in the anoxic, acidic hot spring sediments from Copahue volcano, Argentina. FEMS Microbiol. Ecol. 2019, 95, fiz175. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Sulaymon, A.H.; Khaliefa, Q.M. A review of permeable reactive barrier as passive sustainable technology for groundwater remediation. Int. J. Environ. Sci. Technol. 2018, 15, 1123–1138. [Google Scholar] [CrossRef]
- Sarkar, A.; Bhattacharjee, S. Biofilm-mediated bioremediation of xenobiotics and heavy metals: A comprehensive review of microbial ecology, molecular mechanisms, and emerging biotechnological applications. 3 Biotech 2025, 15, 1–30. [Google Scholar] [CrossRef]
- Elazzazy, A.M.; Baeshen, M.N.; Alasmi, K.M.; Alqurashi, S.I.; Desouky, S.E.; Khattab, S.M. Where Biology Meets Engineering: Scaling up microbial nutraceuticals to bridge nutrition, therapeutics, and global impact. Microorganisms 2025, 13, 566. [Google Scholar] [CrossRef]
- Panda, S.; Mishra, S.; Akcil, A. Bioremediation of acidic mine effluents and the role of sulfidogenic biosystems: A mini-review. Euro-Mediterr. J. Environ. Integr. 2016, 1, 8. [Google Scholar] [CrossRef]
- Yadav, M.; Gupta, R.; Sharma, R.K. Green and sustainable pathways for wastewater purification. In Advances in Water Purification Techniques; Elsevier: Amsterdam, The Netherlands, 2019; pp. 355–383. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Qiu, Y.; Sun, J.; Jiang, F. Realising a high-rate sulfidogenic reactor driven by sulphur-reducing bacteria with organic substrate dosage minimisation and cost-effectiveness maximisation. Chemosphere 2019, 236, 124381. [Google Scholar] [CrossRef]
- Hessler, T.; Harrison, S.T.; Banfield, J.F.; Huddy, R.J. Harnessing Fermentation May Enhance the Performance of Biological Sulphate-Reducing Bioreactors. Environ. Sci. Technol. 2024, 58, 2830–2846. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Olanrewaju, O.S.; Babalola, O.O. Sulphate-reducing bacteria as an effective tool for sustainable acid mine bioremediation. Front. Microbiol. 2018, 9, 1986. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, Z.; Di, J.; Wang, D.; Yang, Z.; Wang, Y.; Xie, Z. Study on the effectiveness of sulphate-reducing bacteria to remove heavy metals (Fe, Mn, Cu, Cr) in acid mine drainage. Sustainability 2023, 15, 5486. [Google Scholar] [CrossRef]


| pH | Sulphate (mg/L) | Iron (mg/L) | Zinc (mg/L) | Manganese (mg/L) | Aluminium (mg/L) | Copper (mg/L) | Reference |
|---|---|---|---|---|---|---|---|
| 3.22 | 29.9 | 4.66 | 0.401 | - | 0.28 | - | [11] |
| 2.32 | 471.75 | 169.22 | - | 0.016 | 21.04 | - | [12] |
| 2.9 | 3500 | 750 | - | 100 | 50 | 10 | [9] |
| 2.29 | 4520 | 788 | 0.25 | 19.4 | 310 | 3.42 | [13] |
| 2 | 5800 | 70 | 320 | 5.5 | 210 | - | [14] |
| 2.53 | 5880 | 2143 | 8.71 | 43.2 | 3735 | 17.4 | [15] |
| 3 | 7550 | 2516.7 | - | 104.9 | 257 | - | [16] |
| 2.2 | 10,845 | 3867 | 410 | 120 | 216 | 515 | [17] |
| 2.3 | 11,700 | 744 | 976 | 467 | 251 | 165 | [18] |
| 2.6 | 13,200 | 4420 | 13.1 | 126 | 460 | 0.11 | [19] |
| 2.5 | 24,530 | 2490 | 500 | 6590 | - | 2670 | [20] |
| 2.1 | 28,980 | 6120 | - | 155 | 506 | - | [21] |
| 2.7 | 29,530 | 66 | 55 | 245 | 2317 | 65 | [22] |
| 2 | 30,000 | 8000 | - | 75 | 300 | - | [2] |
| 2.2 | 42,862 | 3867 | 410 | 120 | 216 | 515 | [23] |
| Metals | WHO and EPA Limits (mg/L) | SANS 241 (2015) Limits (mg/L) |
|---|---|---|
| Fe | 0.8 | 0.4 |
| Cu | 1.3–2 | 2 |
| Mn | 0.4 | 0.1 |
| Al | 0.1–0.2 | 0.3 |
| Zn | 0.3 | 5 |
| 250 | 250 | |
| Ca | 30 | 30 |
| Mg | 10 | 0.05 |
| As | 0.01 | 0.01 |
| Cr | 0.05–0.1 | 0.05 |
| Ni | 0.02 | 0.07 |
| Hg | 0.006 | 0.006 |
| Parameter | SRB-Based Bioremediation | Conventional Treatment |
|---|---|---|
| Capital costs | Moderate to High | Low to Moderate |
| Operational costs | Lower (with passive systems) | Higher (requires continuous chemical dosing) |
| Metal Recovery Potential | High (metal sulphide) | Low |
| Energy requirement | Low (with passive bioreactors and bio-electrochemical systems) | Moderate to High |
| Long-term sustainability | High (self-sustaining systems) | Low (continuous cost of reagents) |
| Environmental Impact | Low substrates (biodegradable substrates) | High (generates sludge and secondary waste) |
| Bioreactor Type | Environmental Conditions | Advantages | Limitations | Reference |
|---|---|---|---|---|
| Continuous flow bioreactor (Bio I and II) | Operated at 30 °C, pH 2.5, stirred at 40 rpm, nitrogen sparging, uses acidophilic SRB consortium | Higher efficiency (reduced sulphate to 4.7–19 nM from the initial 30 nM) under acidic conditions and stable operations over 302 days of operations. | Requires strict controlled conditions, and there is a moderate energy input for stirring and sparging. | [51] |
| Modular continuous flow bioreactor | Plackett-Burman bioreactor design, tested for both low and high concentrations of metals and sulphate | Enhanced tolerance for heavy metals (with efficiency of up to 99%), the modular is designed for optimization. | Limited sulphate removal of up to 58.89% compared to metal removal, the modular design is complex. | [52] |
| Passive-field bioreactor | Observed over 6 months with varying flow rates (6–130 L/h) | Effective for arsenic removal varied between 3–97%, stable biofilm formation, and suitable for sludge management. | Efficiency depends on flow rates and physicochemical parameters, and the treatment process is slow. | [3] |
| Passive bioreactors | Focusses on anaerobic SRB and reactive mixture compositions | Low operational costs, stable sludge formation, and minimal energy consumption. | Large land area required, slower reaction rates, and less effective in high-flow or acidic environment. Highly dependent on the composition of the organic carbon source. | [3] |
| Active bioreactors | Uses renewable material for greener mitigation solutions | Environmentally sustainable approach, not fully quantified but promising for advancements in the efficiency of sulphate and heavy metal removal. | Requires further research to optimise the use of renewable materials in bioreactors. | [3,53] |
| Fluidized-bed bioreactor | The product uses carrier materials for the formation of biofilms and recycles the effluent for fluidization | Improved biomass retention, suitable for both mesophilic and thermophilic conditions. | Requires strict control of fluoridation and biofilm formation, potential clogging issues. | [54] |
| Up-flow anaerobic sludge blanket bioreactor | Requires a granular sludge bed, upward flow of wastewater through the sludge blanket, and | Maintain stable pH for the effluent, can remove nearly 100% Fe, Zn, Co, and Cu, and can be used simultaneously with other treatment technologies. | Requires careful management of granular sludge, potential for washout if overloaded. | [55] |
| Treatment Technology | Constant Variable | Electron Donor | Effectiveness of Technology | Reference |
|---|---|---|---|---|
| Biological sulphate-reducing column | Temperature = 30 °C, HRT = 14 days and pH = 5.5 | Sodium lactate | Sulphate reduction of 79.04%; 64.78%; and 50.27% using chicken dairy manure and sawdust as organic substrates, respectively. 5% of sulphide precipitation and over 95% of heavy metals. | [69] |
| Sulfidogenic fixed-bed column bioreactor | Temperature = 30 °C, HRT = 7 days and pH = 4.5 | Ethanol | Recovery of Sb at 97.8%; As 98.2%; and Fe(ii) was recovered at 85%. | [70] |
| Sulphate-Reducing Bacteria Cu/Fe Reactor | Temperature = 37–45 °C; pH = 1, HRT = 48 h COD rate of 27.4 mg COD/(Lh) and nitrate rate of 17.4 mg N/(Lh) | Sodium lactate | 99.67; and Cu at 7.5–10% pH increase rate = 5–9. | [71] |
| Biochemical Passive Bioreactor | Temperature = 35 °C, pH = 4, and HRT = 2 days | Mushroom compost, limestone, and cow manure | of around 95%. | [72] |
| Sulphate-Reducing Wetland Bioreactors | pH = 6.5 HRT of 6 days Lignocellulosic waste as a substrate | Lactate | Sulphate reduction of 60.7%, with COD removal of 70.6% and recovery of valuable metals such as Fe 99.6%; Zn 99.4%; Mn 9.3%; Cd 99.9% and Cu 94.5%. pH increase rate = 6.5–7.7. | [21] |
| Downflow Structure Bed Bioreactor | The temperature was kept at 30 °C, pH = 4, and HRT = 19 h | Sugar (sugarcane) | Sulphate removal rate of 55–91%, 80% removal for Co, Ni and Zn; Cu 73%, Fe 70%, and Mn at 60%. pH increase rate = 6.7–7.5. | [73] |
| Fluidized bed bioreactor | Temperature = 20 °C, pH = 7–8, HRT = 10 h, and COD/sulphate rate = 2.5 − 1.7 | Glycerol | Sulphate removal rate of 80–92%, COD removal rate of 58%, and recovery of metals such as Ni, Mn, and Cu at a rate of 90%. | [74] |
| Sulphate Reducing Bioreactor | Temperature = 30 °C, pH = 5, and HRT = 6 h | Iron sulphate | Metal iron recovery such as Zn(ii) 88.5%; Pb(ii) 92.6%; Cu(ii) 76.0%; Mn(ii) 62.2%; Fe(iii) 56.9; Cd (ii) 78.7% and Ni(ii) 62.5. pH increase rate = 6.5. | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafane, D.; Ngulube, T.; Mphahlele-Makgwane, M.M. Anaerobic Bioremediation of Acid Mine Drainage Using Sulphate-Reducing Bacteria: Current Status, Challenges, and Future Directions. Sustainability 2025, 17, 3567. https://doi.org/10.3390/su17083567
Mafane D, Ngulube T, Mphahlele-Makgwane MM. Anaerobic Bioremediation of Acid Mine Drainage Using Sulphate-Reducing Bacteria: Current Status, Challenges, and Future Directions. Sustainability. 2025; 17(8):3567. https://doi.org/10.3390/su17083567
Chicago/Turabian StyleMafane, Ditiro, Tholiso Ngulube, and Mamasegare Mabel Mphahlele-Makgwane. 2025. "Anaerobic Bioremediation of Acid Mine Drainage Using Sulphate-Reducing Bacteria: Current Status, Challenges, and Future Directions" Sustainability 17, no. 8: 3567. https://doi.org/10.3390/su17083567
APA StyleMafane, D., Ngulube, T., & Mphahlele-Makgwane, M. M. (2025). Anaerobic Bioremediation of Acid Mine Drainage Using Sulphate-Reducing Bacteria: Current Status, Challenges, and Future Directions. Sustainability, 17(8), 3567. https://doi.org/10.3390/su17083567






