Assessment of Water Quality and Ecological Integrity in an Ecuadorian Andean Watershed
Abstract
:1. Introduction
2. Materials and Methods
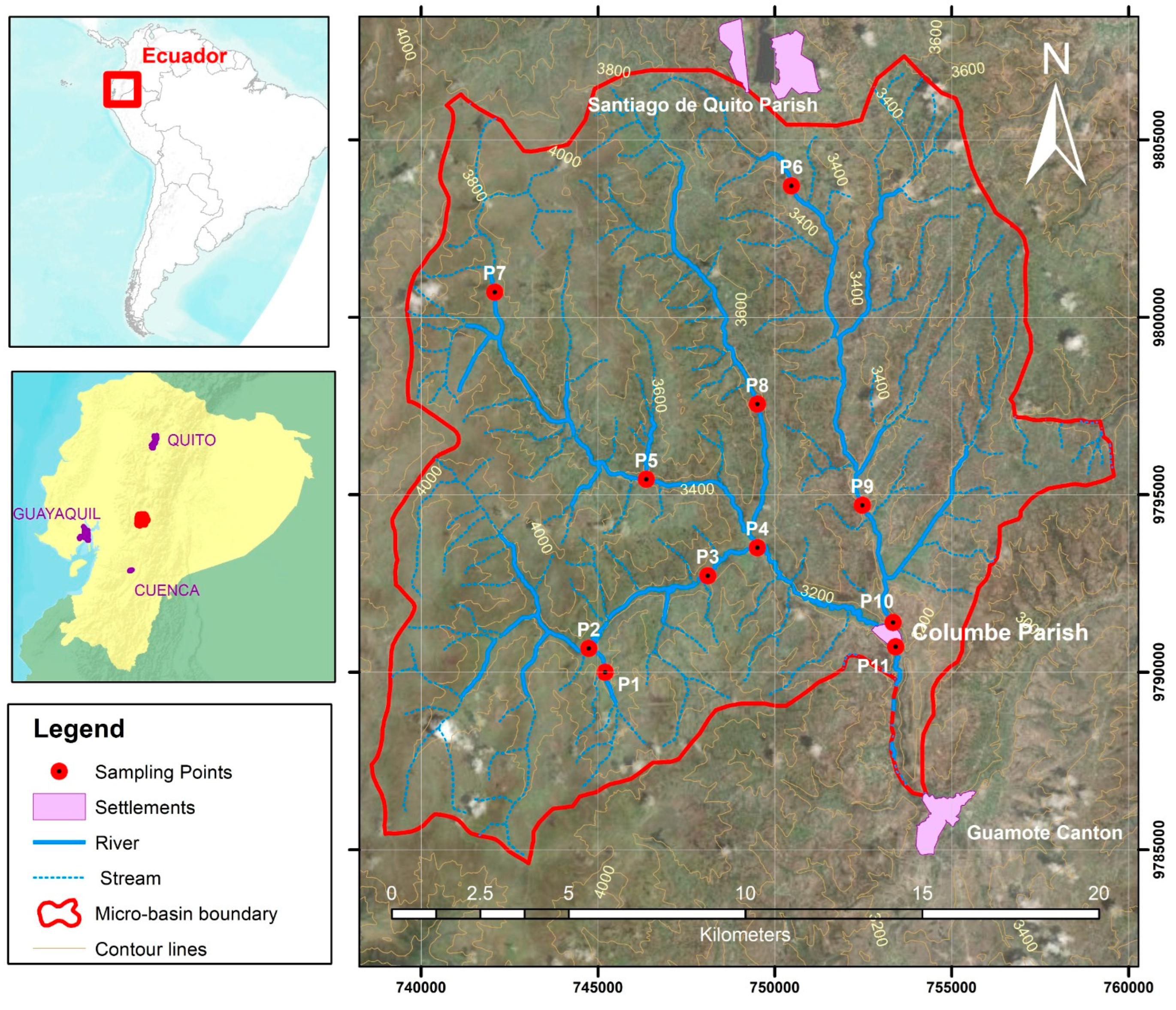
2.1. Study Area and Monitoring Samples
2.2. Water Quality Parameters
2.3. Hydrological Characterization
2.4. Sampling of Macroinvertebrates
2.5. Determination of Biotic and Abiotic Indices
2.5.1. Aquatic Biotic Biodiversity
2.5.2. Fluvial Habitat Structure
2.5.3. Physicochemical and Microbiological Water Quality
2.6. Data Processing
3. Results
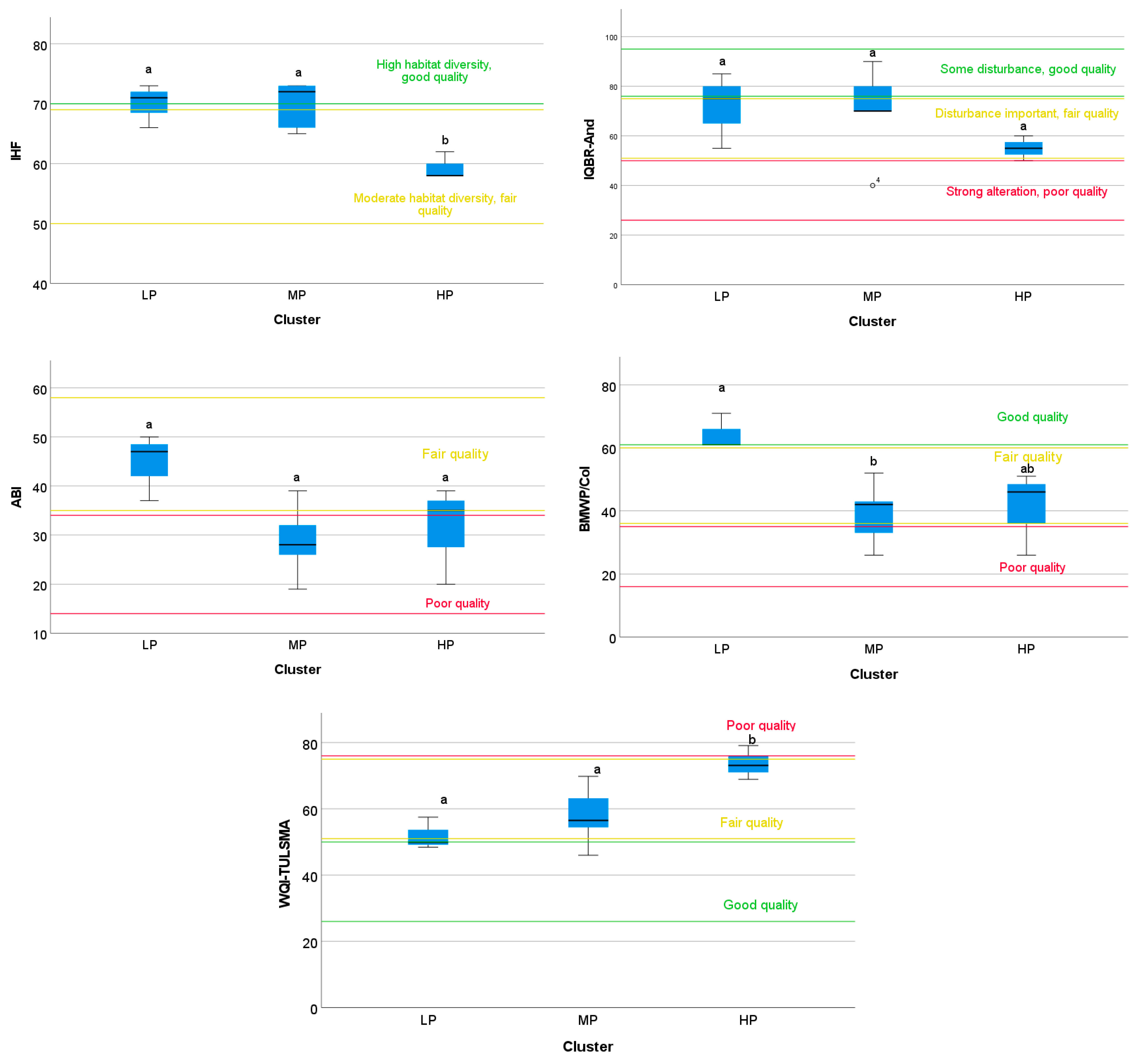
3.1. Aquatic Biotic Biodiversity
3.2. Fluvial Habitat Structure
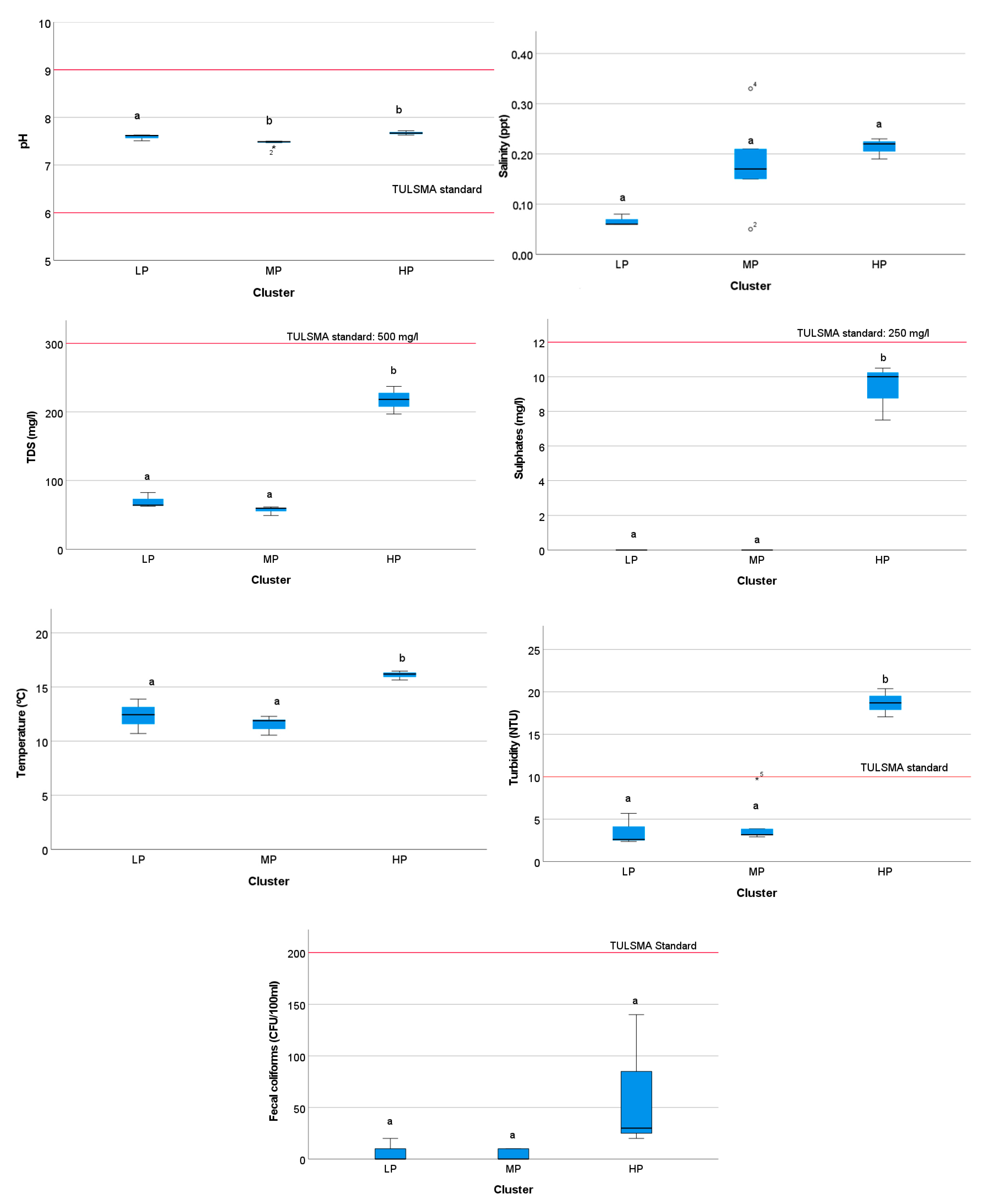
3.3. Physicochemical and Microbiological Water Quality
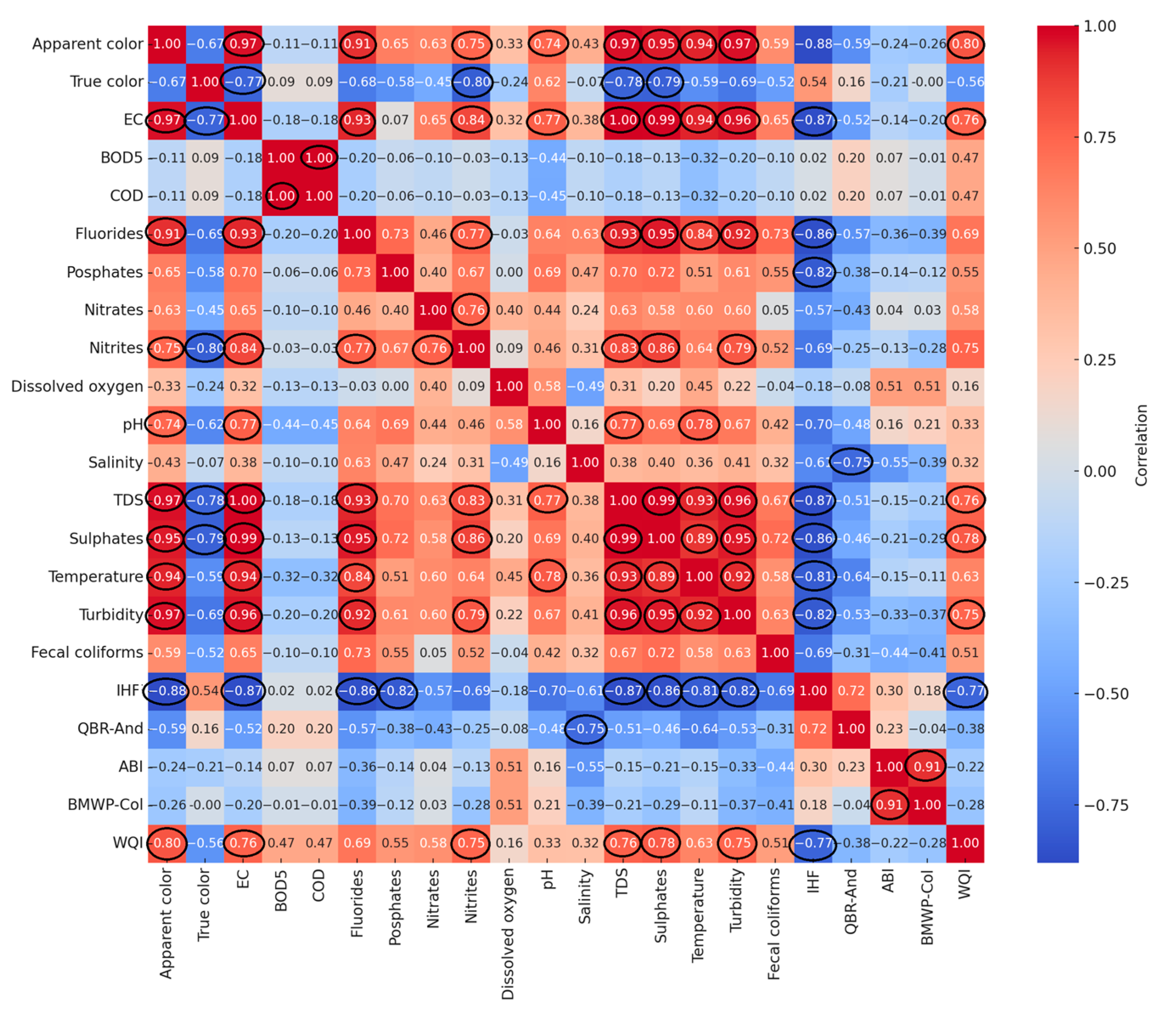
3.4. Correlation Analysis and Spatial Classification of Environmental Quality
3.4.1. Correlation Patterns Among Physicochemical and Biological Variables
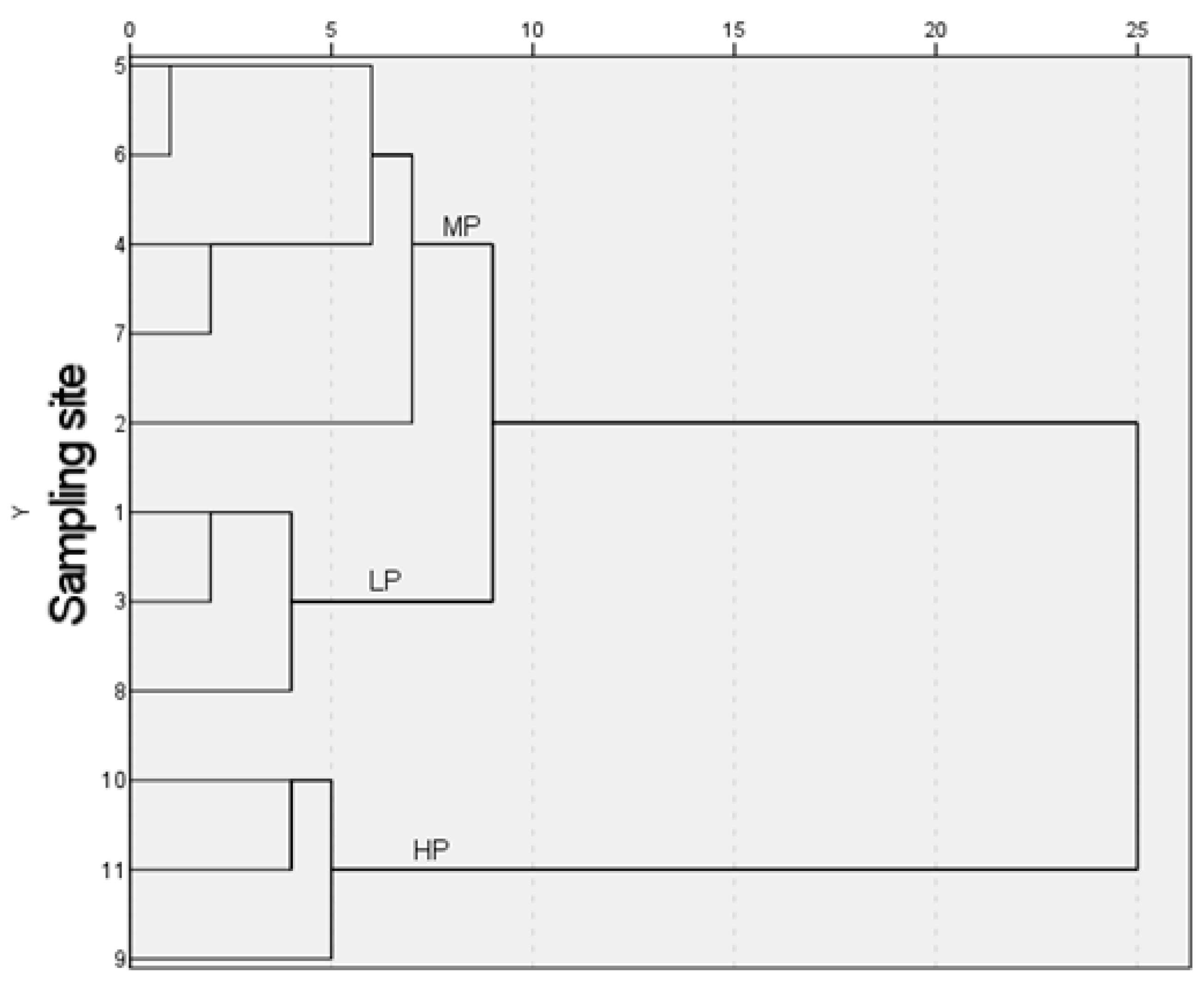
3.4.2. Cluster Analysis of Pollution Gradients
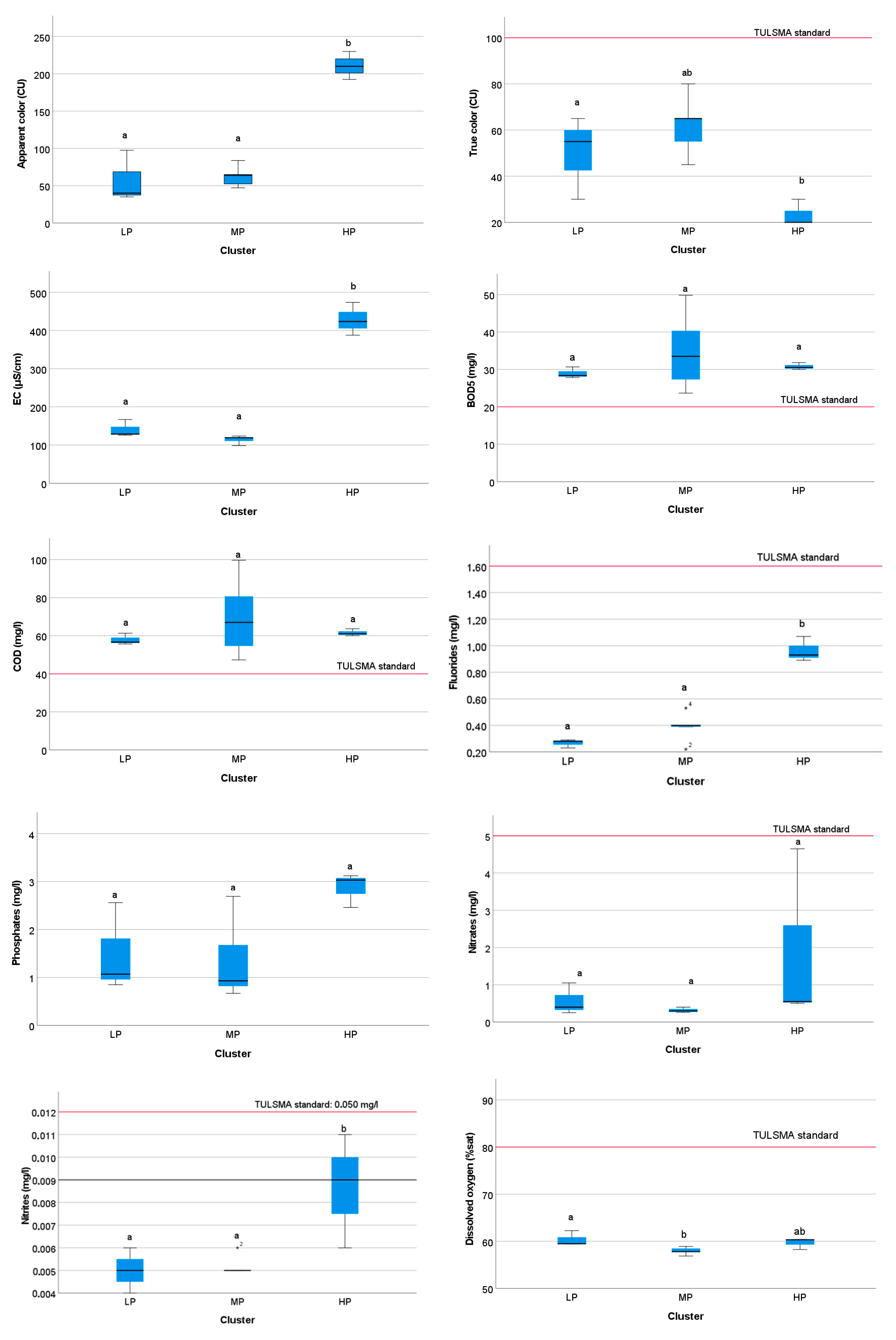
3.4.3. Spatial Variation in Environmental Quality by Cluster Group
4. Discussion
4.1. Physicochemical Parameters
4.2. Aquatic Diversity (ABI and BMWP-Col Indices)
4.3. Fluvial Habitat Structure (IHF and QBR Indices)
4.4. Correlation Patterns Between Environmental and Biological Metrics
4.5. Spatial Distribution of Physicochemical and Biological Indices
4.6. Implications for Sustainability and Integrated Watershed Management
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Villamarín, C.; Prat, N.; Rieradevall, M. Physical, chemical and hydromorphological characterization of Ecuador and Peru tropical highland Andean rivers. Lat. Am. J. Aquat. Res. 2014, 42, 1072–1086. [Google Scholar] [CrossRef]
- Hampel, H.; Vázquez, R.F.; González, H.; Acosta, R. Evaluating the ecological status of fluvial networks of tropical Andean catchments of Ecuador. Water 2023, 15, 1742. [Google Scholar] [CrossRef]
- Fierro, P.; Bertrán, C.; Tapia, J.; Hauenstein, E.; Peña-Cortés, F.; Vergara, C.; Cerna, C.; Vargas-Chacoff, L. Effects of local land-use on riparian vegetation, water quality, and the functional organization of macroinvertebrate assemblages. Sci. Total Environ. 2017, 609, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morales, M.; Acevedo-Novoa, D.; Machado, D.; Ablan, M.; Dugarte, W.; Dávila, F. Ecohydrology of the Venezuelan páramo: Water balance of a high Andean watershed. Plant Ecol. Divers. 2019, 12, 573–591. [Google Scholar] [CrossRef]
- Espinosa, J.; Rivera, D. Variations in water resources availability at the Ecuadorian páramo due to land-use changes. Environ. Earth Sci. 2016, 75, 1173. [Google Scholar] [CrossRef]
- Patiño, S.; Hernández, Y.; Plata, C.; Domínguez, I.; Daza, M.; Oviedo-Ocaña, R.; Ochoa-Tocachi, B.F. Influence of land use on hydro-physical soil properties of Andean páramos and its effect on streamflow buffering. Catena 2021, 202, 105227. [Google Scholar] [CrossRef]
- Villa-Achupallas, M.; Rosado, D.; Aguilar, S.; Galindo-Riaño, M.D. Water quality in the tropical Andes hotspot: The Yacuambi river (southeastern Ecuador). Sci. Total Environ. 2018, 633, 50–58. [Google Scholar] [CrossRef]
- Salazar Flores, C.; Zambrano, C.; Parreno, C. Microbiological assessment of the quality of water supplied to the Latacunga water treatment plant (Republic of Ecuador). Int. Res. J. 2023, 2, 128. [Google Scholar] [CrossRef]
- Campo, Y.M.; Rebolledo, M.I.C.; Londoño, A.M.J. Comparison of water quality between two Andean rivers by using the BMWP/COL and ABI indices. Acta Biol. Colomb. 2019, 24, 1–9. [Google Scholar] [CrossRef]
- Cabrera-García, S.; Goethals, P.L.M.; Lock, K.; Domínguez-Granda, L.; Villacís, M.; Galárraga-Sánchez, R.; Van der Heyden, C.; Forio, M.A.E. Taxonomic and feeding trait-based analysis of macroinvertebrates in the Antisana River Basin (Ecuadorian Andean Region). Biology 2023, 12, 1386. [Google Scholar] [CrossRef]
- Vargas-Tierras, T.; Suárez-Cedillo, S.; Morales-León, V.; Vargas-Tierras, Y.; Tinoco-Jaramillo, L.; Viera-Arroyo, W.; Vásquez-Castillo, W. Ecological River Water Quality Based on Macroinvertebrates Present in the Ecuadorian Amazon. Sustainability 2023, 15, 5790. [Google Scholar] [CrossRef]
- Herrera-Martínez, J.R.; Navarro-Sining, B.A.; Torres-Cervera, K.P.; Martínez-García, N.; Royero-Ibarra, A.; Cahuana-Mojica, A. Determinación de los índices BMWP/COL, QBR, IHF e ICO en Valledupar, Colombia. Rev. Politéc. 2022, 18, 98–109. [Google Scholar] [CrossRef]
- Ríos-Touma, B.; Acosta, R.; Prat, N. The Andean Biotic Index (ABI): Revised tolerance to pollution values for macroinvertebrate families and index performance evaluation. Rev. Biol. Trop. 2014, 62, 249–273. [Google Scholar] [CrossRef]
- Roldán, G. La Bioindicación de la Calidad del Agua en Colombia: Propuesta para el uso del Método BMWP-COL; Editorial Universidad de Antioquia: Medellín, Colombia, 2003. [Google Scholar]
- Pardo, I.; Álvarez, M.; Casas, J.J.; Moreno, J.L.; Vivas, S.; Bonada, N.; Alba-Tercedo, J.; Jaimez-Cuéllar, P.; Moya, G.; Prat, N.; et al. El hábitat de los ríos mediterráneos: Diseño de un índice de diversidad de hábitat. Limnetica 2002, 21, 115–132. [Google Scholar] [CrossRef]
- Acosta, R.; Ríos, B.; Rieradevall, M.; Prat, N. Propuesta de un protocolo de evaluación de la calidad ecológica de ríos andinos (C.E.R.A.) y su aplicación en dos cuencas en Ecuador y Perú. Limnetica 2009, 28, 35–64. [Google Scholar] [CrossRef]
- Salazar Flores, C.A.; Kurbatova, A.I.; Mikhaylichenko, K.Y.; Barannikova, S.I. Comprehensive water quality assessment of surface sources in the city of Latacunga and the canton Pedro Vicente Maldonado in Ecuador. RUDN J. Ecol. Life Saf. 2023, 31, 251–264. [Google Scholar] [CrossRef]
- Custodio, M.; Chávez, E. Quality of the aquatic environment of high Andean rivers evaluated through environmental indicators: A case of the Cunas River, Peru. Ingeniare 2019, 27, 396–409. [Google Scholar] [CrossRef]
- Echeverría-Sáenz, S.; Ugalde-Salazar, R.; Guevara-Mora, M.; Quesada-Alvarado, F.; Ruepert, C. Ecological Integrity Impairment and Habitat Fragmentation for Neotropical Macroinvertebrate Communities in an Agricultural Stream. Toxics 2022, 10, 346. [Google Scholar] [CrossRef]
- Izquierdo-Tort, S.; Jayachandran, S.; Saavedra, S. Redesigning payments for ecosystem services to increase cost-effectiveness. Nat. Commun. 2024, 15, 9252. [Google Scholar] [CrossRef]
- Vasseur, L.; Andrade, A. Using the Red List of Ecosystems and the Nature-based Solutions Global Standard as an integrated process for climate change adaptation in the Andean high mountains. Philos. Trans. R. Soc. B 2024, 379, 20220326. [Google Scholar] [CrossRef]
- GAD de la Parroquia Columbe. Plan de Desarrollo y Ordenamiento Territorial de la Parroquia Columbe 2019–2023. Available online: https://es.slideshare.net/slideshow/pdotcolumbe2020pdf/252797787 (accessed on 25 January 2025).
- GAD de la Provincia de Chimborazo. Plan de Desarrollo y Ordenamiento Territorial de la Provincia de Chimborazo 2019–2023. Available online: https://chimborazo.gob.ec/principal/wp-content/uploads/2022/06/PDOT.pdf (accessed on 24 January 2025).
- Santillán, G. Macroinvertebrados Acuáticos Como Bioindicadores de Calidad de Agua en la Microcuenca Columbe, Cantón Colta. Master’s Thesis, Universidad Nacional de Chimborazo, Riobamba, Ecuador, 2024. Available online: http://dspace.unach.edu.ec/handle/51000/13571 (accessed on 25 January 2025).
- Damanik-Ambarita, M.N.; Lock, K.; Boets, P.; Everaert, G.; Nguyen, T.H.T.; Forio, M.A.E.; Musonge, P.L.S.; Suhareva, N.; Bennetsen, E.; Landuyt, D.; et al. Ecological water quality analysis of the Guayas River basin (Ecuador) based on macroinvertebrates indices. Limnologica 2016, 57, 27–59. [Google Scholar] [CrossRef]
- Paller, M.H.; Kosnicki, E.; Prusha, B.A.; Fletcher, D.E.; Sefick, S.A.; Feminella, J.W. Development of an Index of Biotic Integrity for the Sand Hills Ecoregion of the Southeastern United States. Trans. Am. Fish. Soc. 2017, 146, 112–127. [Google Scholar] [CrossRef]
- Edegbene, A.O.; Akamagwuna, F.C.; Odume, O.N.; Arimoro, F.O.; Edegbene, O.T.T.; Akumabor, E.C.; Ogidiaka, E.; Kaine, E.A.; Nwaka, K.H. A Macroinvertebrate-Based Multimetric Index for Assessing Ecological Condition of Forested Stream Sites Draining Nigerian Urbanizing Landscapes. Sustainability 2022, 14, 11289. [Google Scholar] [CrossRef]
- Raven, P.J.; Holmes, N.T.H.; Charrier, P.; Dawson, F.H.; Naura, M.; Boon, P.J. Towards a harmonized approach for hydromorphological assessment of rivers in Europe: A qualitative comparison of three survey methods. Aquat. Conserv. 2002, 12, 405–424. [Google Scholar] [CrossRef]
- Fernández, D.; Barquín, J.; Raven, P. A review of river habitat characterisation methods: Indices vs. characterisation protocols. Limnetica 2011, 30, 217–234. [Google Scholar] [CrossRef]
- Uddin, M.G.; Nash, S.; Olbert, A.I. A review of water quality index models and their use for assessing surface water quality. Ecol. Indic. 2021, 122, 107218. [Google Scholar] [CrossRef]
- Lukhabi, D.K.; Mensah, P.K.; Asare, N.K.; Pulumuka-Kamanga, T.; Ouma, K.O. Adapted Water Quality Indices: Limitations and Potential for Water Quality Monitoring in Africa. Water 2023, 15, 1736. [Google Scholar] [CrossRef]
- Hanna Instruments. HI98194 Multiparameter Waterproof Meter. Available online: https://www.hannainstruments.co.uk/multi-parameter-devices/2147-hi-98194-multiparameter-waterproof-meter (accessed on 10 January 2025).
- Valladares Pogo, V. Evaluación Ecológica del río Columbe. Bachelor’s Thesis, Escuela Superior Politécnica de Chimborazo (ESPOCH), Riobamba, Ecuador, 2024. Available online: http://dspace.espoch.edu.ec/handle/123456789/23192 (accessed on 25 January 2025).
- Instituto Privado de Investigación Sobre Cambio Climático (ICC). Manual de Medición de Caudales; Guatemala. 2017. Available online: https://icc.org.gt/wp-content/uploads/2023/03/064.pdf (accessed on 30 January 2025).
- Ministerio de Agricultura del Perú. Manual de Hidrometría; Ministerio de Agricultura del Perú: Lima, Perú, 2005. [Google Scholar]
- Pence, R.A.; Cianciolo, T.R.; Drover, D.R.; McLaughlin, D.L.; Soucek, D.J.; Timpano, A.J.; Zipper, C.E.; Schoenholtz, S.H. Comparison of benthic macroinvertebrate assessment methods along a salinity gradient in headwater streams. Environ. Monit. Assess. 2021, 193, 765. [Google Scholar] [CrossRef]
- Getachew, M.; Mulat, W.L.; Mereta, S.T.; Gebrie, G.S.; Kelly-Quinn, M. Refining benthic macroinvertebrate kick sampling protocol for wadeable rivers and streams in Ethiopia. Environ. Monit. Assess. 2022, 194, 196. [Google Scholar] [CrossRef]
- Munné, A.; Prat, N.; Solà, C.; Bonada, N.; Rieradevall, M. A simple field method for assessing the ecological quality of riparian habitat in rivers and streams: QBR index. Aquat. Conserv. 2003, 13, 147–163. [Google Scholar] [CrossRef]
- Instituto Geográfico Militar del Ecuador (IGM). Capas de Información Geográfica del IGM (Codificación UTF-8). Available online: http://www.geoportaligm.gob.ec/portal/index.php/descargas/cartografia-de-libre-acceso/registro/ (accessed on 27 January 2025).
- Gaagai, A.; Aouissi, H.A.; Bencedira, S.; Hinge, G.; Athamena, A.; Heddam, S.; Gad, M.; Elsherbiny, O.; Elsayed, S.; Eid, M.H.; et al. Application of Water Quality Indices, Machine Learning Approaches, and GIS to Identify Groundwater Quality for Irrigation Purposes: A Case Study of Sahara Aquifer, Doucen Plain, Algeria. Water 2023, 15, 289. [Google Scholar] [CrossRef]
- Yang, J.; Lv, J.; Liu, Q.; Nan, F.; Li, B.; Xie, S.; Feng, J. Seasonal and spatial patterns of eukaryotic phytoplankton communities in an urban river based on marker gene. Sci. Rep. 2021, 11, 23147. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Pacheco, B.S.; Choque-Quispe, D.; Ligarda-Samanez, C.A.; Solano-Reynoso, A.M.; Choque-Quispe, Y.; Aguirre Landa, J.P.; Agreda Cerna, H.W.; Palomino-Rincón, H.; Taipe-Pardo, F.; Zamalloa-Puma, M.M.; et al. Water Pollution Indexes Proposal for a High Andean River Using Multivariate Statistics: Case of Chumbao River, Andahuaylas, Apurímac. Water 2023, 15, 2662. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Wen, C.; Wen, G. S-type Dissolved Oxygen Distribution along Water Depth in a Canyon-shaped and Algae Blooming Water Source Reservoir: Reasons and Control. Int. J. Environ. Res. Public Health 2019, 16, 987. [Google Scholar] [CrossRef]
- Sinche, F.; Cabrera, M.; Vaca, L.; Segura, E.; Carrera, P. Determination of the Ecological Water Quality in the Orienco Stream Using Benthic Macroinvertebrates in the Northern Ecuadorian Amazon. Integr. Environ. Assess. Manag. 2022, 11, e4666. [Google Scholar] [CrossRef]
- Sánchez-Araujo, V.; Portuguez-Maurtua, M.; Palomino-Pastrana, P.; Escobar-Soldevilla, M.; Saez Huaman, W.; Chávez-Araujo, E.; Contreras López, E. Water Quality Index and Health Risks in a Peruvian High Andean River. Ecol. Eng. Environ. Technol. 2024, 25, 301–315. [Google Scholar] [CrossRef]
- Torres, M.A.; West, A.J.; Clark, K.E.; Paris, G.; Bouchez, J.; Ponton, C.; Feakins, S.J.; Galy, V.; Adkins, J.F. The acid and alkalinity budgets of weathering in the Andes–Amazon system: Insights into the erosional control of global biogeochemical cycles. Earth Planet. Sci. Lett. 2016, 450, 381–391. [Google Scholar] [CrossRef]
- Granitto, M.; Lopez, M.E.; Bursztyn Fuentes, A.L.; Maluendez Testoni, M.C.; Rodríguez, P. Relationship between riparian zones and water quality in the main watersheds of Ushuaia City, Tierra del Fuego (Argentina). Ecol. Process. 2025, 14, 18. [Google Scholar] [CrossRef]
- Vásquez-Velásquez, G. Headwaters Deforestation for Cattle Pastures in the Andes of Colombia and Its Implications for Soils Properties and Hydrological Dynamic. Open J. For. 2016, 6, 337–347. [Google Scholar] [CrossRef]
- Ye, F.; Duan, L.; Sun, Y.; Yang, F.; Liu, R.; Gao, F.; Xu, Y. Nitrogen removal in freshwater sediments of riparian zone: N-loss pathways and environmental controls. Front. Microbiol. 2023, 14, 1239055. [Google Scholar] [CrossRef]
- Weston, N.B.; Troy, C.; Kearns, P.J.; Bowen, J.L.; Porubsky, W.; Hyacinthe, C.; Meile, C.; Van Cappellen, P.; Joye, S.B. Physicochemical perturbation increases nitrous oxide production from denitrification in soils and sediments. Biogeosciences 2024, 21, 4837–4851. [Google Scholar] [CrossRef]
- Castillo, M.M.; Allan, J.D.; Brunzell, S. Nutrient Concentrations and Discharges in a Midwestern Agricultural Catchment. J. Environ. Qual. 2000, 29, 1142–1151. [Google Scholar] [CrossRef]
- Boyd, C.E. Phosphorus. In Water Quality; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Zhang, M.; Krom, M.D.; Lin, J.; Cheng, P.; Chen, N. Effects of a storm on the transformation and export of phosphorus through a subtropical river-turbid estuary continuum revealed by continuous observation. J. Geophys. Res. Biogeosci. 2022, 127, e2022JG006786. [Google Scholar] [CrossRef]
- Jin, Z.; Liao, P.; Jaisi, D.P.; Wang, D.; Wang, J.; Wang, H.; Jiang, S.; Yang, J.; Qiu, S.; Chen, J. Suspended phosphorus sustains algal blooms in a dissolved phosphorus-depleted lake. Water Res. 2023, 241, 120134. [Google Scholar] [CrossRef]
- Wilson, J.L.; Everard, M. Real-time consequences of riparian cattle trampling for mobilization of sediment, nutrients and bacteria in a British lowland river. Int. J. River Basin Manag. 2017, 16, 231–244. [Google Scholar] [CrossRef]
- Kowalkowski, T.; Pastuszak, M.; Igras, J.; Buszewski, B. Differences in emission of nitrogen and phosphorus into the Vistula and Oder basins in 1995–2008—Natural and anthropogenic causes (MONERIS model). J. Mar. Syst. 2012, 89, 48–60. [Google Scholar] [CrossRef]
- Graham, D.J.; Bierkens, M.F.P.; van Vliet, M.T.H. Impacts of droughts and heatwaves on river water quality worldwide. J. Hydrol. 2024, 629, 130590. [Google Scholar] [CrossRef]
- Pakoksung, K.; Inseeyong, N.; Chawaloesphonsiya, N.; Punyapalakul, P.; Chaiwiwatworakul, P.; Xu, M.; Chuenchum, P. Seasonal dynamics of water quality in response to land use changes in the Chi and Mun River Basins, Thailand. Sci. Rep. 2025, 15, 7101. [Google Scholar] [CrossRef]
- Jerves-Cobo, R.; Córdova-Vela, G.; Iñiguez-Vela, X.; Díaz-Granda, C.; Van Echelpoel, W.; Cisneros, F.; Nopens, I.; Goethals, P.L.M. Model-Based Analysis of the Potential of Macroinvertebrates as Indicators for Microbial Pathogens in Rivers. Water 2018, 10, 375. [Google Scholar] [CrossRef]
- Álvarez-Cabria, M.; Barquín, J.; Juanes, J.A. Spatial and seasonal variability of macroinvertebrate metrics: Do macroinvertebrate communities track river health? Ecol. Indic. 2010, 10, 2. [Google Scholar] [CrossRef]
- Heatherly, T., II; Whiles, M.R.; Royer, T.V.; David, M.B. Relationships between water quality, habitat quality, and macroinvertebrate assemblages in Illinois streams. J. Environ. Qual. 2007, 36, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, R.F.; Vimos-Lojano, D.; Hampel, H. Habitat Suitability Curves for Freshwater Macroinvertebrates of Tropical Andean Rivers. Water 2020, 12, 2703. [Google Scholar] [CrossRef]
- Palma, A.; Figueroa, R.; Ruiz, V.H. Evaluación de ribera y hábitat fluvial a través de los índices QBR e IHF. Gayana (Concepc.) 2009, 73, 57–63. [Google Scholar] [CrossRef]
- Galeano-Rendón, E.; Monsalve-Cortés, L.M.; Mancera-Rodríguez, N.J. Evaluación de la calidad ecológica de quebradas andinas en la cuenca del río Magdalena, Colombia. Rev. UDCA Act. Divulg. Cient. 2017, 20, 413–424. [Google Scholar]
- Lind, L.; Hasselquist, E.M.; Laudon, H. Towards ecologically functional riparian zones: A meta-analysis to develop guidelines for protecting ecosystem functions and biodiversity in agricultural landscapes. J. Environ. Manag. 2019, 249, 109391. [Google Scholar] [CrossRef]
- Chellaiah, D.; Kuglerová, L. Are riparian buffers surrounding forestry-impacted streams sufficient to meet key ecological objectives? A Swedish case study. For. Ecol. Manag. 2021, 499, 119591. [Google Scholar] [CrossRef]
- Kędzior, R.; Kłonowska-Olejnik, M.; Dumnicka, E.; Woś, A.; Wyrębek, M.; Książek, L.; Grela, J.; Madej, P.; Skalski, T. Macroinvertebrate habitat requirements in rivers: Overestimation of environmental flow calculations in incised rivers. Hydrol. Earth Syst. Sci. 2022, 26, 4109–4124. [Google Scholar] [CrossRef]
- Parreño, C.; Salazar Flores, C.; Slabospitskaya, A.S.; Adarchenko, I.A. Turbidity as a primary indicator of water quality from surface water sources. Int. Res. J. 2024, 3, 1–7. [Google Scholar] [CrossRef]
- Thirumalini, S.; Joseph, K. Correlation between Electrical Conductivity and Total Dissolved Solids in Natural Waters. Malays. J. Sci. 2009, 28, 55–61. [Google Scholar] [CrossRef]
- Scott, E.E.; Haggard, B.E. Natural Characteristics and Human Activity Influence Turbidity and Ion Concentrations in Streams. J. Contemp. Water Res. Educ. 2021, 172, 34–49. [Google Scholar] [CrossRef]
- Revelo-Mejía, I.A.; Gutiérrez-Idrobo, R.; López-Fernández, V.A.; López-Rosales, A.; Astaiza-Montenegro, F.C.; Garcés-Rengifo, L.; López-Ordoñez, P.A.; Hardisson, A.; Rubio, C.; Gutiérrez, Á.J.; et al. Fluoride levels in river water from the volcanic regions of Cauca (Colombia). Environ. Monit. Assess. 2022, 194, 327. [Google Scholar] [CrossRef] [PubMed]
- Strauch, G.; Oyarzun, J.; Fiebig-Wittmaack, M.; González, E.; Weise, S.M. Contributions of the different water sources to the Elqui river runoff (northern Chile) evaluated by H/O isotopes. Isot. Environ. Health Stud. 2006, 42, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Cedeño, D.V.G.; Maddela, N.R. Evaluación de calidad del agua de los ríos Canuto y Carrizal en época seca, Manabí, Ecuador. Polo Conoc. 2022, 7, 537–554. [Google Scholar]
- Abdallah, K.Z.; Hammam, G. Correlation between Biochemical Oxygen Demand and Chemical Oxygen Demand for Various Wastewater Treatment Plants in Egypt to Obtain the Biodegradability Indices. Int. J. Sci. Basic Appl. Res. 2014, 13, 42–48. [Google Scholar]
- Braga, F.H.R.; Dutra, M.L.S.; Lima, N.S.; Silva, G.M.; Miranda, R.C.M.; Firmo, W.C.A.; Moura, A.R.L.; Monteiro, A.S.; Silva, L.C.N.; Silva, D.F.; et al. Study of the Influence of Physicochemical Parameters on the Water Quality Index (WQI) in the Maranhão Amazon, Brazil. Water 2022, 14, 1546. [Google Scholar] [CrossRef]
- Sotomayor, G.; Hampel, H.; Vázquez, R.; Goethals, P. Multivariate-statistics based selection of a benthic macroinvertebrate index for assessing water quality in the Paute River basin (Ecuador). Ecol. Indic. 2020, 111, 106037. [Google Scholar] [CrossRef]
- Nguyen, T.H.T.; Boets, P.; Lock, K.; Forio, M.A.E.; Van Echelpoel, W.; Van Butsel, J.; Dueñas Utreras, J.A.; Everaert, G.; Domínguez Granda, L.E.; Hoang, T.H.T.; et al. Water quality related macroinvertebrate community responses to environmental gradients in the Portoviejo River (Ecuador). Ann. Limnol. Int. J. Limnol. 2017, 53, 203–219. [Google Scholar] [CrossRef]
- Banda, K.; Ngwenya, V.; Mulema, M.; Chomba, I.; Chomba, M.; Nyambe, I. Influence of water quality on benthic macroinvertebrates in a groundwater-dependent wetland. Front. Water 2023, 5, 1177724. [Google Scholar] [CrossRef]
- Simon, A.; Rinaldi, M. Disturbance, stream incision, and channel evolution: The roles of excess transport capacity and boundary materials in controlling channel response. Geomorphology 2006, 79, 361–383. [Google Scholar] [CrossRef]
- Santikari, V.P.; Murdoch, L.C. Effects of construction-related land use change on streamflow and sediment yield. J. Environ. Manag. 2019, 252, 109605. [Google Scholar] [CrossRef]
- Cashman, M.J.; Lee, G.; Staub, L.E.; Katoski, M.P.; Maloney, K.O. Physical habitat is more than a sediment issue: A multi-dimensional habitat assessment indicates new approaches for river management. J. Environ. Manag. 2024, 371, 123139. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.M.; Malik, Z.A.; Rahman, I.U. Quantification and characterization of vegetation and functional trait diversity of the riparian zones in protected forest of Kashmir Himalaya, India. Nord. J. Bot. 2019, 37, e02438. [Google Scholar] [CrossRef]
- Mosquera, G.M.; Hofstede, R.; Bremer, L.L.; Asbjornsen, H.; Carabajo-Hidalgo, A.; Célleri, R.; Crespo, P.; Esquivel-Hernández, G.; Feyen, J.; Manosalvas, R.; et al. Frontiers in páramo water resources research: A multidisciplinary assessment. Sci. Total Environ. 2023, 892, 164373. [Google Scholar] [CrossRef]
- Cortes, V.R.M.; Hughes, S.; Rodrigues Pereira, V.; Pinto Varandas, S. Tools for bioindicator assessment in rivers: The importance of spatial scale, land use patterns and biotic integration. Ecol. Indic. 2013, 34, 460–477. [Google Scholar] [CrossRef]
- Brumberg, H.; Beirne, C.; Broadbent, E.; Almeyda Zambrano, A.M.; Almeyda Zambrano, S.L.; Quispe Gil, C.A.; Lopez Gutierrez, B.; Eplee, R.; Whitworth, A. Riparian buffer length is more influential than width on river water quality: A case study in southern Costa Rica. J. Environ. Manag. 2021, 286, 112132. [Google Scholar] [CrossRef]



| Monitoring Point | Flow (m3/s) |
|---|---|
| P1 | 0.017 |
| P2 | 0.623 |
| P3 | 0.845 |
| P4 | 0.379 |
| P5 | 0.685 |
| P6 | 0.419 |
| P7 | 0.065 |
| P8 | 0.156 |
| P9 | 0.217 |
| P10 | 0.166 |
| P11 | 0.856 |
| IHF Component | Definition | Ecological Relevance | Maximum Score |
|---|---|---|---|
| Substrate inclusion and limitation | Refers to the presence of compacted sand between coarse substrates. | Less embeddedness supports more habitat availability for macroinvertebrates. | 10 |
| Frequency of riffles | Assesses the number of riffles in the reach. | Riffles create habitat diversity and enhance oxygenation. | 10 |
| Substrate composition | Evaluates the diversity of substrate types present. | Diverse substrates support varied benthic communities. | 20 |
| Speed/depth regimes | Assesses the presence of combinations of flow velocity and depth. | A wider range of flow conditions promotes species richness. | 10 |
| Shade on the riverbed | Estimates shading from riparian vegetation. | Shading regulates temperature and supports aquatic life. | 10 |
| Riverbed heterogeneity | Considers the presence of woody debris, roots, and natural barriers. | Structural complexity improves refuge and habitat quality. | 10 |
| Aquatic vegetation cover | Quantifies aquatic vegetation types. | Vegetation supports food webs and habitats for colonization. | 30 |
| QBR-and Component | Definition | Ecological Relevance | Maximum Score |
|---|---|---|---|
| Riparian zone coverage | Evaluates vegetation cover on riverbanks. | Greater cover implies better erosion control and habitat quality. | 25 |
| Vegetation structure | Assesses vertical stratification and species diversity. | Structural complexity is associated with mature riparian systems. | 25 |
| Riparian vegetation quality | Evaluates the presence of native vs. exotic species and anthropogenic impact. | Native vegetation and minimal disturbance increase ecological value. | 25 |
| Degree of naturalness | Considers the extent of channel modification. | Natural channels offer better habitat continuity and ecological function. | 25 |
| Classification Index | Excellent | Good | Fair | Poor | Very Poor |
|---|---|---|---|---|---|
| Biotic indices | |||||
| ABI | >96 | 59–96 | 35–58 | 14–34 | <14 |
| BMWP-Col | ≥150 | 61–100 | 36–60 | 16–35 | <15 |
| Abiotic indices | |||||
| IHF | ≥90 | 71–80 | 50–70 | 31–49 | 0–30 |
| QBR-And | ≥96 | 76–95 | 51–75 | 26–50 | ≤25 |
| Physicochemical index | |||||
| WQI | ≤25 | 26–50 | 51–75 | 76–100 | >100 |
| Families | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baetidae | x | x | x | x | x | x | |||||
| Blephariceridae | |||||||||||
| Chironomidae | x | x | x | x | |||||||
| Elmidae | x | x | x | x | x | x | x | x | x | ||
| Glossiphoniidae | x | x | |||||||||
| Hyalellidae | x | x | x | x | x | x | |||||
| Hydracarina | x | ||||||||||
| Hydrobiosidae | x | x | x | x | x | ||||||
| Leptoceridae | x | x | |||||||||
| Limnephilidae | x | x | |||||||||
| Lymnaeidae | |||||||||||
| Odontoceridae | x | x | |||||||||
| Oligochaeta | x | x | x | x | x | x | x | x | x | x | |
| Perlidae | x | x | x | x | x | x | x | ||||
| Scirtidae | |||||||||||
| Simuliidae | x | x | x | x | x | x | x | x | |||
| Sphaeriidae | x | x | x | ||||||||
| Tabanidae | x | ||||||||||
| Turbellaria | x | x | x | x |
| Family | BMWP-Col Scores by Sampling Point | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | |
| Baetidae | 7 | - | 7 | 7 | - | - | 7 | 7 | - | - | 7 |
| Blephariceridae | 10 | 10 | 10 | 10 | |||||||
| Chironomidae | - | 2 | 2 | 2 | - | - | - | 2 | - | - | - |
| Elmidae | 6 | 6 | 6 | - | 6 | 6 | - | 6 | 6 | 6 | 6 |
| Glossiphoniidae | 7 | - | 7 | - | - | - | - | - | - | - | - |
| Hyalellidae | 7 | 7 | - | - | - | - | - | 7 | 7 | 7 | 7 |
| Hydrobiosidae | - | - | 9 | - | - | 9 | 9 | - | 9 | - | 9 |
| Leptoceridae | - | - | - | - | 8 | - | 8 | - | - | - | - |
| Limnephilidae | - | - | - | 7 | - | - | - | - | - | - | 7 |
| Lymnaeidae | - | - | - | 4 | 5 | - | - | 4 | 4 | 4 | 4 |
| Odontoceridae | 10 | 10 | - | - | - | - | - | - | - | - | - |
| Perlidae | 10 | 10 | 10 | 10 | - | 10 | - | 10 | - | 10 | - |
| Scirtidae | 7 | - | - | - | - | - | - | 7 | - | - | - |
| Simuliidae | - | 8 | 8 | 8 | 8 | 8 | 8 | 8 | - | 8 | - |
| Sphaeriidae | - | - | - | 4 | - | - | - | - | - | 4 | 4 |
| Tabanidae | 5 | ||||||||||
| Turbellaria | 7 | - | 7 | - | - | - | - | - | - | 7 | 7 |
| Family | ABI Scores by Sampling Point | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | |
| Baetidae | 4 | - | 4 | 4 | - | - | 4 | 4 | - | - | 4 |
| Chironomidae | - | 2 | 2 | 2 | - | - | - | 2 | - | - | - |
| Elmidae | 5 | 5 | 5 | - | 5 | 5 | - | 5 | 5 | 5 | 5 |
| Glossiphoniidae | 6 | - | 6 | - | - | - | - | - | - | - | - |
| Hyalellidae | 6 | 6 | - | - | - | - | - | 6 | 6 | 6 | 6 |
| Hydracarina | - | - | - | - | - | - | - | 4 | - | - | - |
| Hydrobiosidae | - | - | 8 | - | - | 8 | 8 | - | 8 | - | 8 |
| Leptoceridae | - | - | - | - | 8 | - | 8 | - | - | - | - |
| Limnephilidae | - | - | - | 7 | - | - | - | - | - | - | 7 |
| Odontoceridae | 10 | 10 | - | - | - | - | - | - | - | - | - |
| Oligochaeta | 1 | 1 | 1 | 1 | 1 | - | 1 | 1 | 1 | 1 | 1 |
| Perlidae | 10 | 10 | 10 | 10 | - | 10 | - | 10 | - | 10 | - |
| Simuliidae | - | 5 | 5 | 5 | 5 | 5 | 5 | 5 | - | 5 | - |
| Sphaeriidae | - | - | - | 3 | - | - | - | - | - | 3 | 3 |
| Tabanidae | - | 4 | - | - | - | - | - | - | - | - | - |
| Turbellaria | 5 | - | 5 | - | - | - | - | - | - | 5 | 5 |
| Parameter (Measure Unit) | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | TULSMA Standard |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apparent color (CU) | 40 | 52.50 | 35 | 65 | 83.75 | 47.19 | 63.98 | 97.50 | 192.50 | 230 | 210 | - |
| True color (PCU) | 30 | 45 | 55 | 55 | 65 | 65 | 80 | 65 | 20 | 20 | 30 | 100 |
| EC (uS/cm) | 128.6 | 98.4 | 125.6 | 123.3 | 119.4 | 118.9 | 110.5 | 166.7 | 423.5 | 473.8 | 387.8 | - |
| BOD5 (mg/L) | 27.8 | 49.8 | 28.3 | 33.5 | 27.3 | 23.6 | 40.3 | 30.6 | 30 | 30.5 | 31.8 | 20 |
| COD (mg/L) | 55.6 | 99.6 | 56.6 | 67 | 54.6 | 47.3 | 80.6 | 61.3 | 60 | 61 | 63.6 | 40 |
| Fluorides (mg/L) | 0.29 | 0.22 | 0.28 | 0.53 | 0.40 | 0.40 | 0.39 | 0.23 | 1.07 | 0.93 | 0.89 | 1.60 |
| Phosphates (mg/L) | 2.56 | 0.67 | 0.85 | 1.68 | 0.82 | 0.93 | 2.69 | 1.07 | 3.12 | 3.03 | 2.46 | - |
| Nitrates (mg/L) | 0.25 | 0.30 | 0.40 | 0.40 | 0.35 | 0.26 | 0.27 | 1.05 | 0.55 | 4.65 | 0.50 | 5 |
| Nitrites (mg/L) | 0.006 | 0.006 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.004 | 0.009 | 0.011 | 0.006 | 0.05 |
| Dissolved oxygen (%sat) | 59.4 | 58.9 | 59.4 | 56.8 | 57.7 | 58.4 | 57.7 | 62.2 | 58.2 | 60.3 | 60.3 | 80 |
| pH | 7.63 | 7.38 | 7.51 | 7.49 | 7.47 | 7.49 | 7.50 | 7.62 | 7.63 | 7.67 | 7.72 | 6–9 |
| Salinity (ppt) | 0.06 | 0.05 | 0.06 | 0.33 | 0.15 | 0.17 | 0.21 | 0.08 | 0.22 | 0.23 | 0.19 | - |
| TDS (mg/L) | 64.4 | 49.3 | 62.9 | 61.8 | 60 | 60 | 55.5 | 82.8 | 218.3 | 237.3 | 197 | 500 |
| Sulphates (mg/L) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10.5 | 10 | 7.5 | 250 |
| Temperature (°C) | 10.7 | 10.5 | 12.4 | 11.8 | 12.2 | 11.9 | 11.1 | 13.8 | 15.6 | 16.4 | 16.2 | - |
| Turbidity (NTU) | 2.3 | 2.9 | 2.6 | 3.8 | 9.7 | 3.1 | 3.1 | 5.6 | 18.7 | 20.3 | 17 | 10 |
| Fecal coliforms (CFU/100 mL) | 0 | 0 | 0 | 10 | 0 | 0 | 10 | 20 | 140 | 20 | 30 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armijos-Arcos, F.; Salazar, C.; Beltrán-Dávalos, A.A.; Kurbatova, A.I.; Savenkova, E.V. Assessment of Water Quality and Ecological Integrity in an Ecuadorian Andean Watershed. Sustainability 2025, 17, 3684. https://doi.org/10.3390/su17083684
Armijos-Arcos F, Salazar C, Beltrán-Dávalos AA, Kurbatova AI, Savenkova EV. Assessment of Water Quality and Ecological Integrity in an Ecuadorian Andean Watershed. Sustainability. 2025; 17(8):3684. https://doi.org/10.3390/su17083684
Chicago/Turabian StyleArmijos-Arcos, Freddy, Cristian Salazar, Andrés A. Beltrán-Dávalos, Anna I. Kurbatova, and Elena V. Savenkova. 2025. "Assessment of Water Quality and Ecological Integrity in an Ecuadorian Andean Watershed" Sustainability 17, no. 8: 3684. https://doi.org/10.3390/su17083684
APA StyleArmijos-Arcos, F., Salazar, C., Beltrán-Dávalos, A. A., Kurbatova, A. I., & Savenkova, E. V. (2025). Assessment of Water Quality and Ecological Integrity in an Ecuadorian Andean Watershed. Sustainability, 17(8), 3684. https://doi.org/10.3390/su17083684








