Sustainable Breakthrough in Manganese Oxide Thermochemical Energy Storage: Advancing Efficient Solar Utilization and Clean Energy Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermal Analysis
2.3. Kinetic Analysis
2.4. Material Characterization
3. Results and Discussion
3.1. Thermal Conversion Performance Evaluation of Mn3O4
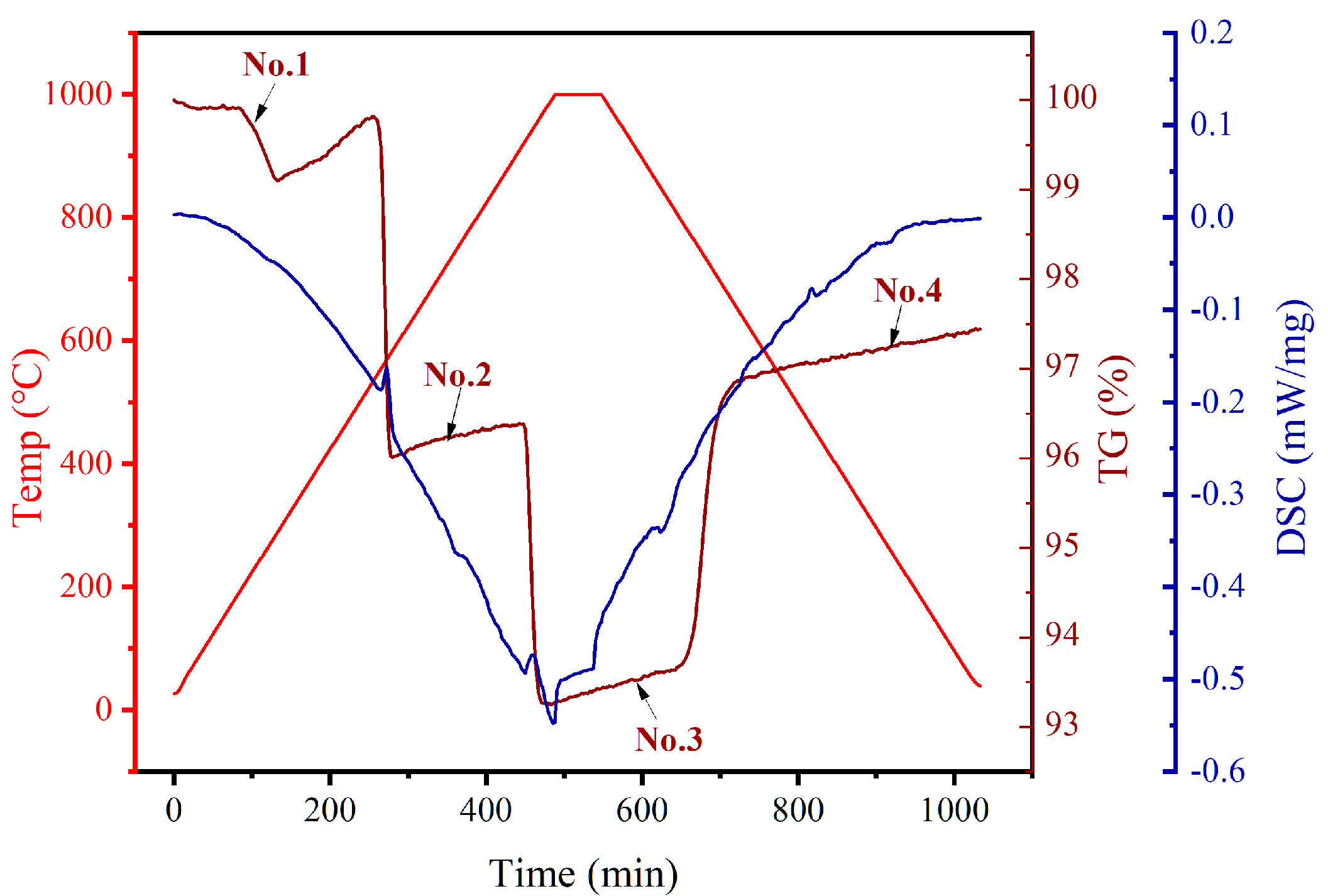
3.1.1. Thermochemical Reaction Assessment
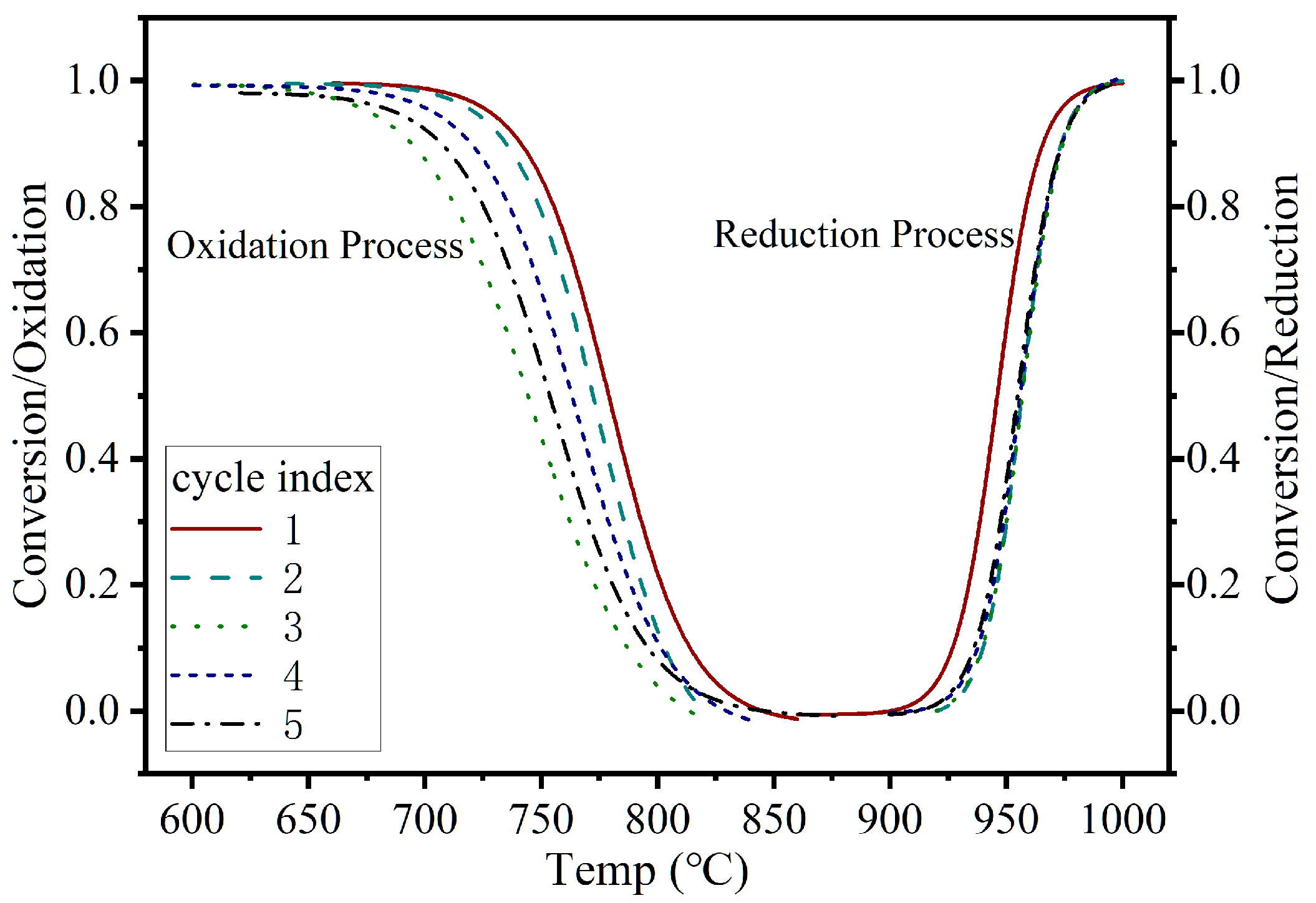
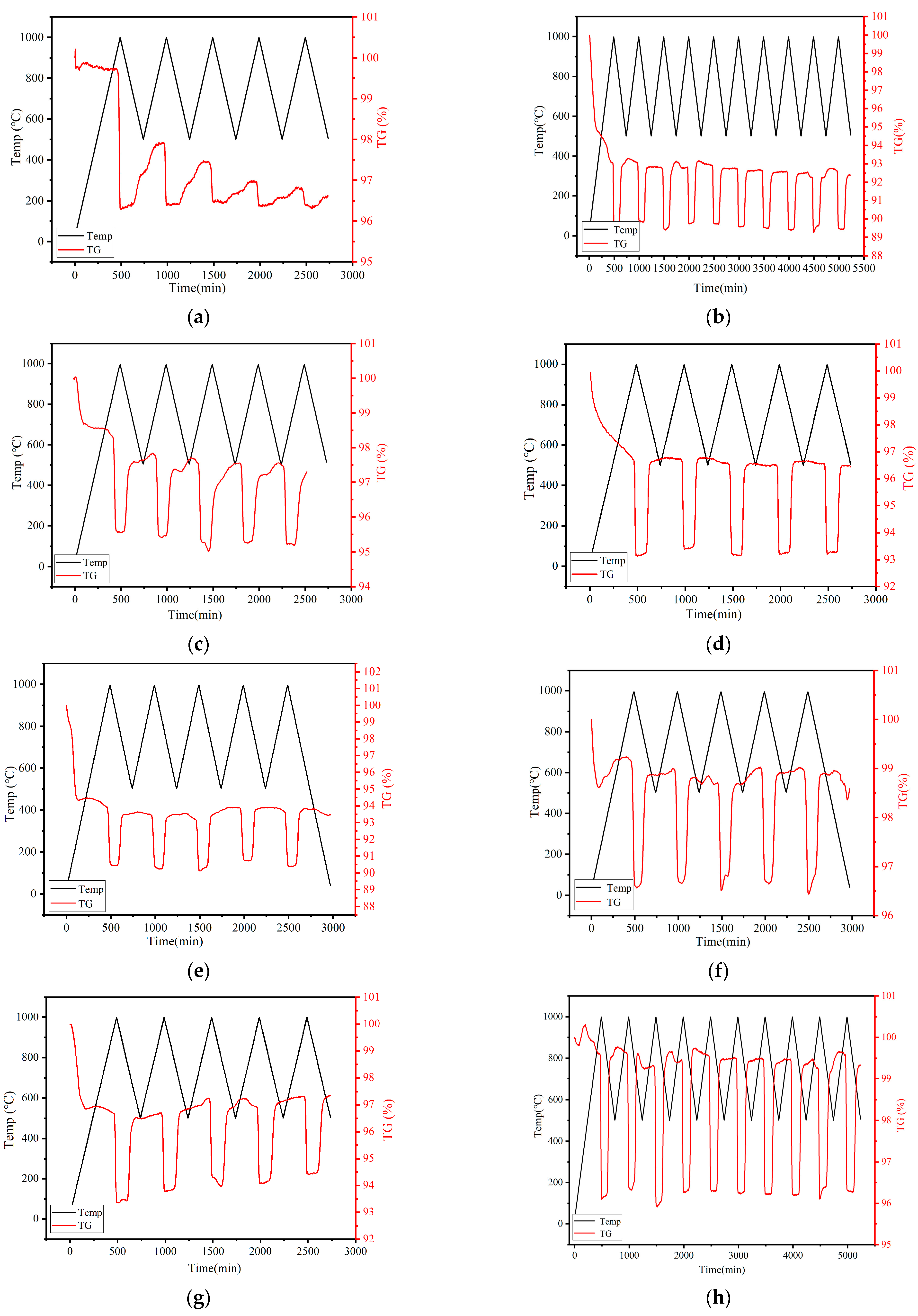
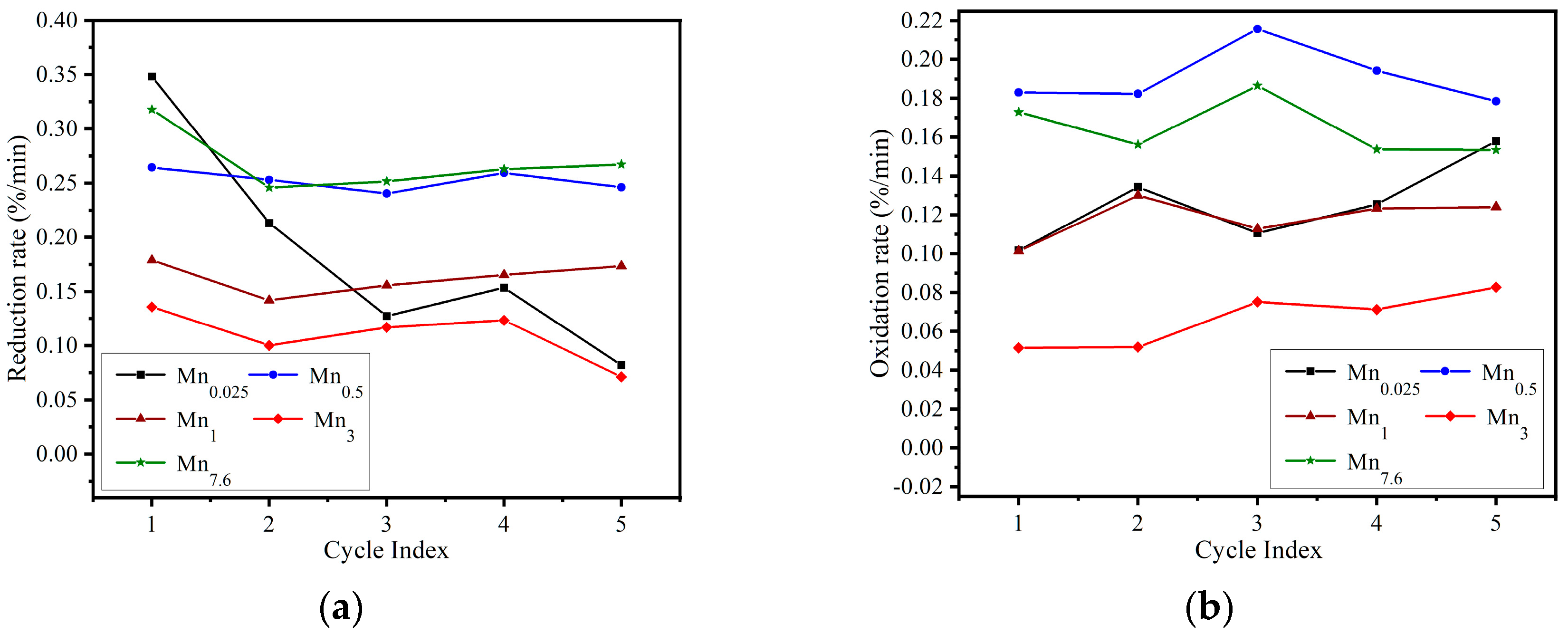
3.1.2. Cyclic Performance Assessment
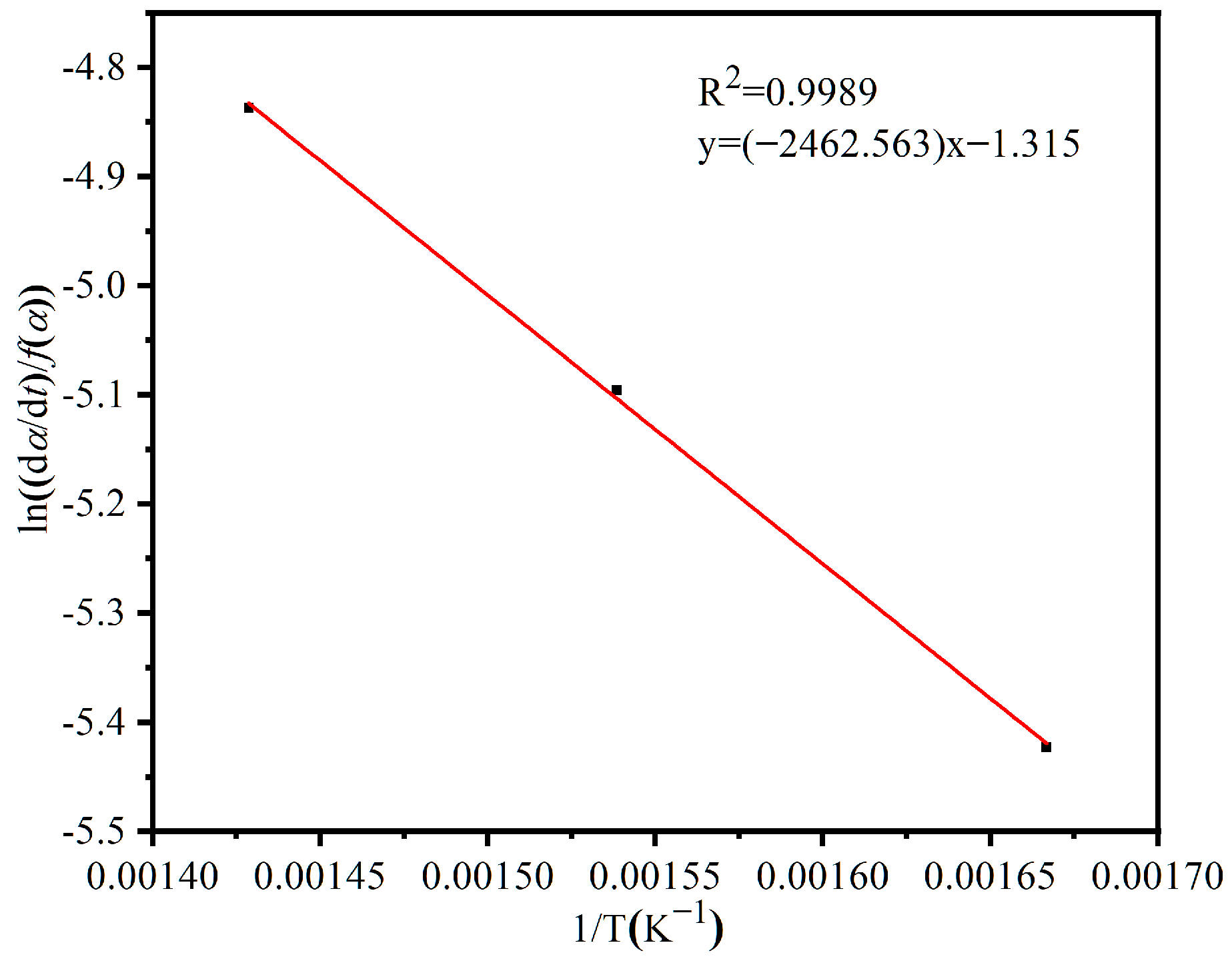
3.1.3. Reaction Kinetics Analysis
3.2. Thermal Conversion Performance Evaluation of Mn2O3
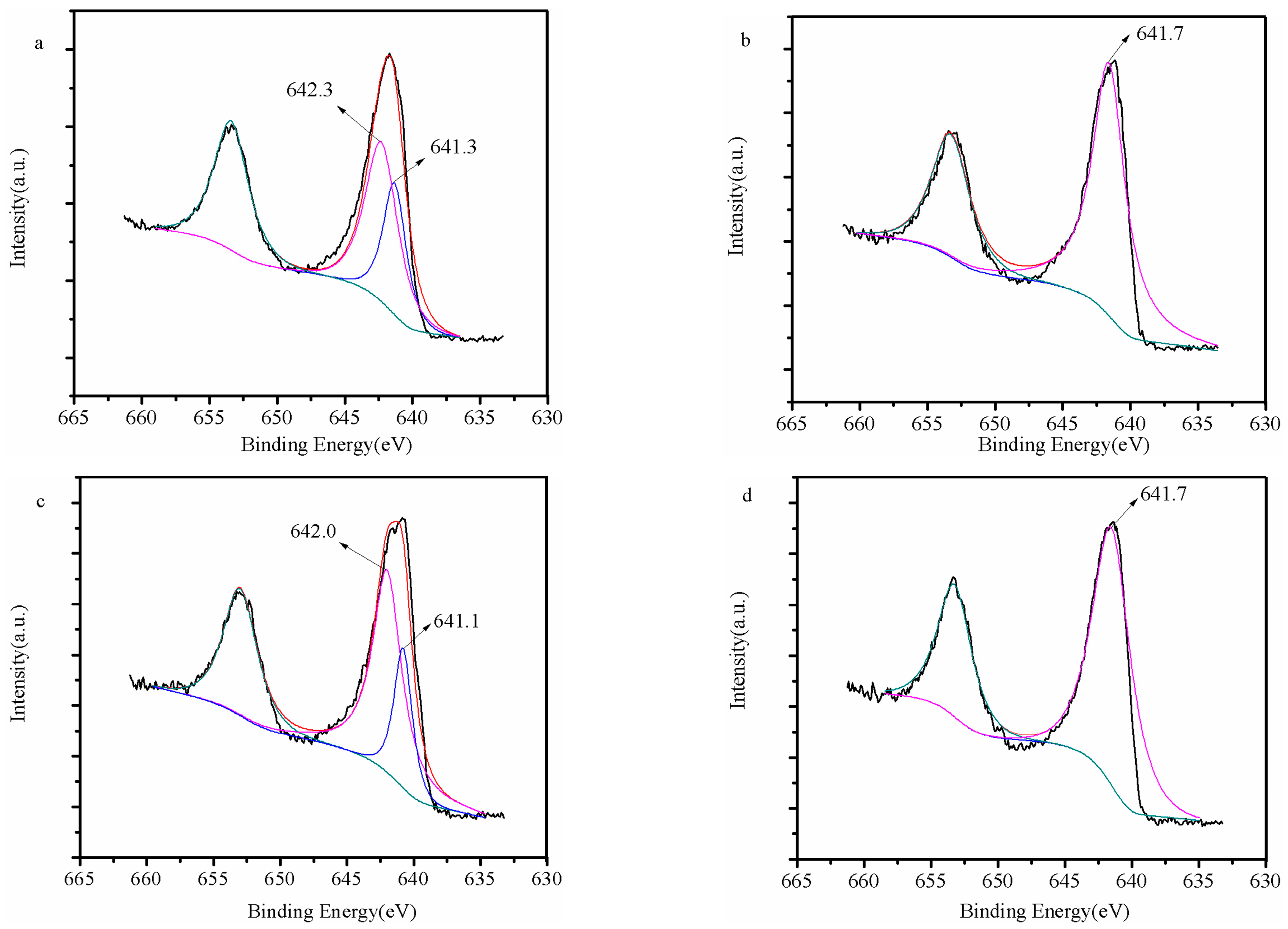
3.2.1. Thermochemical Reaction Assessment
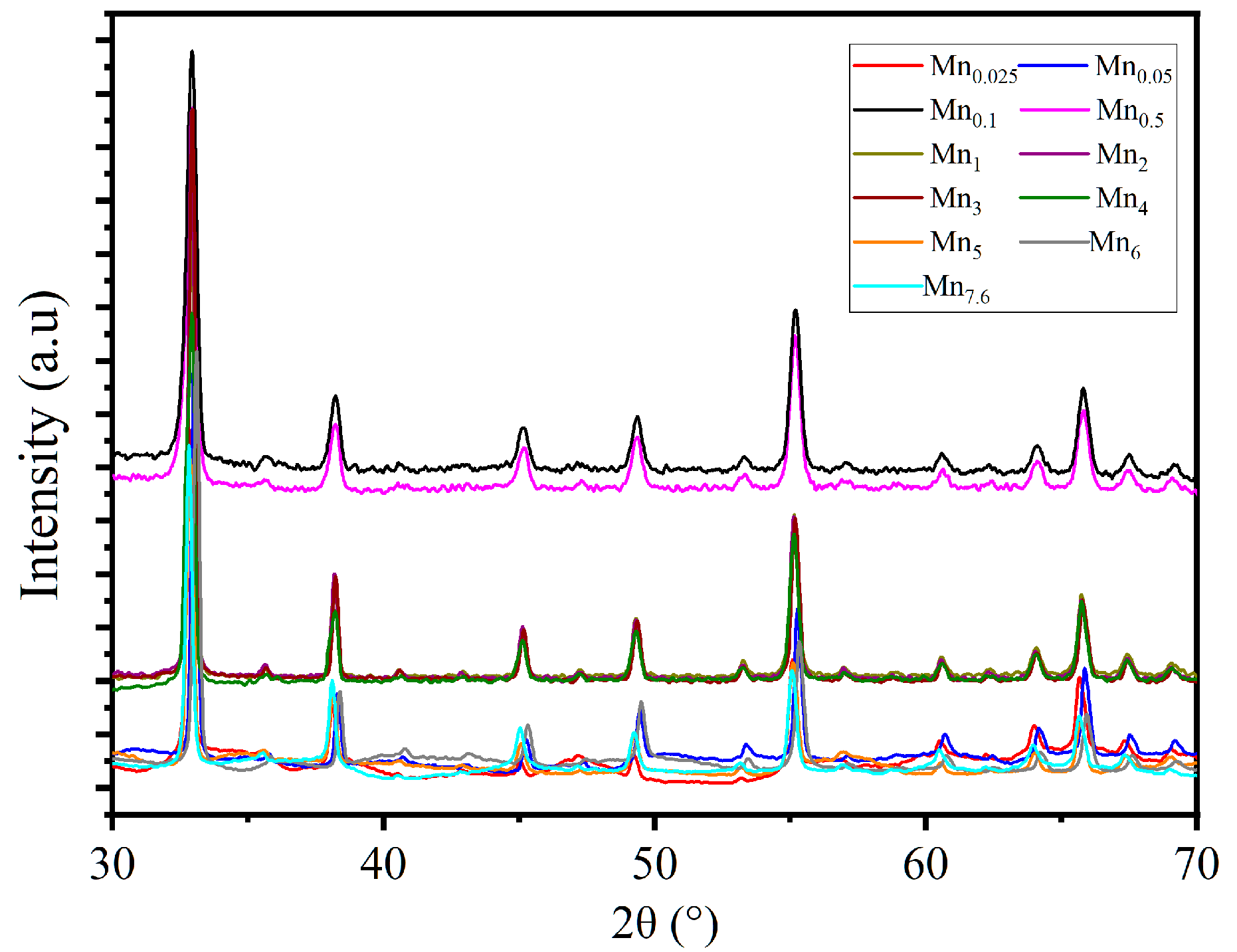
3.2.2. Synthesis Parameters Assessment on Physical Properties
3.2.3. Synthesis Parameters Assessment on Reaction Performance
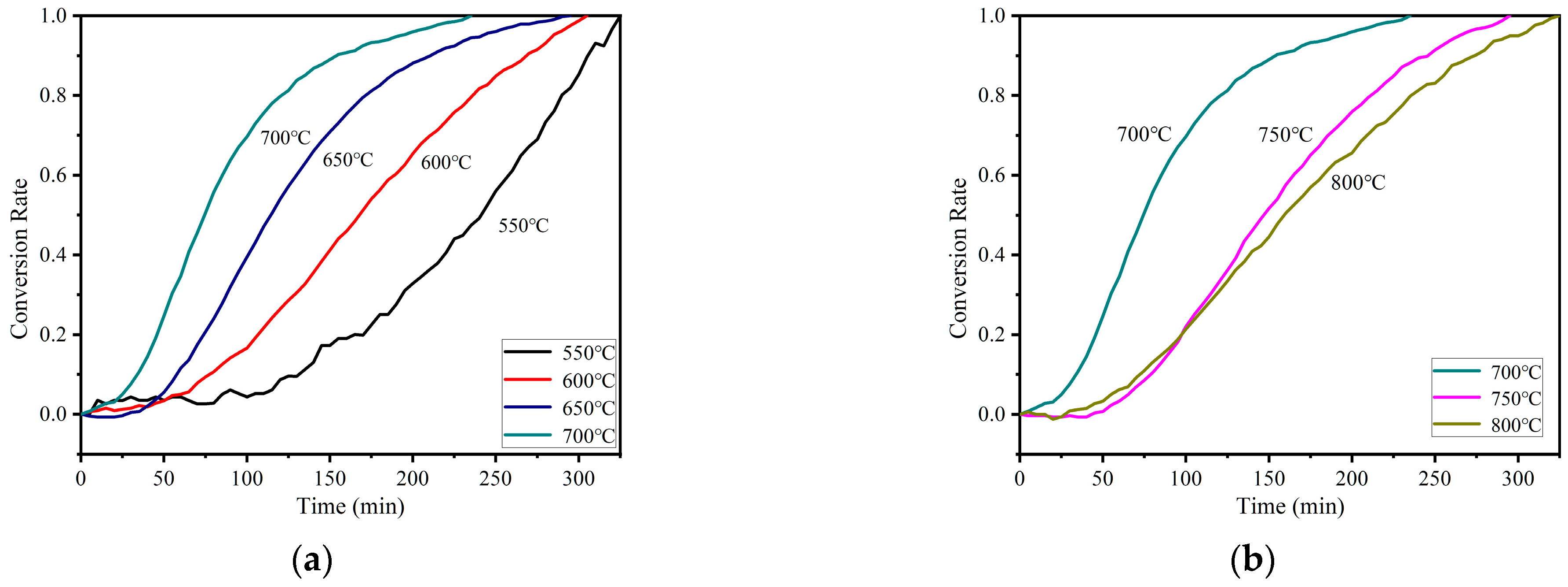
3.2.4. Reaction Kinetics Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, S.; Li, T.X.; Yan, T.; Wang, R.Z. Advanced thermochemical resorption heat transformer for high-efficiency energy storage and heat transformation. Energy 2019, 175, 1222–1233. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Andre, L.; Abanades, S. Experimental assessment of oxygen exchange capacity and thermochemical redox cycle behavior of Ba and Sr series perovskites for solar energy storage. Sol. Energy 2016, 134, 494–502. [Google Scholar] [CrossRef]
- Xu, J.X.; Li, T.X.; Chao, J.W.; Yan, T.S.; Wang, R.Z. High energy-density multi-form thermochemical energy storage based on multi-step sorption processes. Energy 2019, 185, 1131–1142. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Sastre, D.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Revisiting the BaO2/BaO redox cycle for solar thermochemical energy storage. Phys. Chem. Chem. Phys. 2016, 18, 8039–8048. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- Agrafiotis, C.; de Oliveira, L.; Roeb, M.; Sattler, C. A Solar Receiver-Storage Modular Cascade Based on Porous Ceramic Structures for Hybrid Sensible/Thermochemical Solar Energy Storage. In Proceedings of the 21st International Conference on Concentrating Solar Power and Chemical Energy Systems (SolarPACES), Cape Town, South Africa, 13–16 October 2015. [Google Scholar]
- Bayon, A.; Bader, R.; Jafarian, M.; Fedunik-Hofman, L.; Sun, Y.P.; Hinkley, J.; Miller, S.; Lipinski, W. Techno-economic assessment of solid-gas thermochemical energy storage systems for solar thermal power applications. Energy 2018, 149, 473–484. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Schmücker, M.; Sattler, C. Exploitation of thermochemical cycles based on solid oxide redox systems for thermochemical storage of solar heat. Part 1: Testing of cobalt oxide-based powders. Solar Energy 2014, 102, 189–211. [Google Scholar] [CrossRef]
- Alonso, E.; Pérez-Rábago, C.; Licurgo, J.; Fuentealba, E.; Estrada, C.A. First experimental studies of solar redox reactions of copper oxides for thermochemical energy storage. Sol. Energy 2015, 115, 297–305. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Moya, J.; Bayón, A.; Jana, P.; O’Shea, V.A.D.; Romero, M.; Gonzalez-Aguilar, J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical energy storage at high temperature via redox cycles of Mn and Co oxides: Pure oxides versus mixed ones. Sol. Energy Mater. Sol. Cells 2014, 123, 47–57. [Google Scholar] [CrossRef]
- Dizaji, H.B.; Hosseini, H. A review of material screening in pure and mixed-metal oxide thermochemical energy storage (TCES) systems for concentrated solar power (CSP) applications. Renew. Sustain. Energy Rev. 2018, 98, 9–26. [Google Scholar] [CrossRef]
- Prieto, C.; Cooper, P.; Fernández, A.I.; Cabeza, L.F. Review of technology: Thermochemical energy storage for concentrated solar power plants. Renew. Sustain. Energy Rev. 2016, 60, 909–929. [Google Scholar] [CrossRef]
- Singh, A.; Tescari, S.; Lantin, G.; Agrafiotis, C.; Roeb, M.; Sattler, C. Solar thermochemical heat storage via the Co3O4/CoO looping cycle: Storage reactor modelling and experimental validation. Sol. Energy 2017, 144, 453–465. [Google Scholar] [CrossRef]
- Tamayo, A.; Rodríguez, M.A.; Arroyo, C.; Beltran-Heredia, J.; Rubio, F. Dependence of the synthetic strategy on the thermochemical energy storage capability of CuxCo3-xO4 spinels. J. Eur. Ceram. Soc. 2018, 38, 1583–1591. [Google Scholar] [CrossRef]
- Tescari, S.; Singh, A.; Agrafiotis, C.; de Oliveira, L.; Breuer, S.; Schlögl-Knothe, B.; Roeb, M.; Sattler, C. Experimental evaluation of a pilot-scale thermochemical storage system for a concentrated solar power plant. Appl. Energy 2017, 189, 66–75. [Google Scholar] [CrossRef]
- Wokon, M.; Block, T.; Nicolai, S.; Linder, M.; Schmücker, M. Thermodynamic and kinetic investigation of a technical grade manganese-iron binary oxide for thermochemical energy storage. Sol. Energy 2017, 153, 471–485. [Google Scholar] [CrossRef]
- Imponenti, L.; Albrecht, K.J.; Wands, J.W.; Sanders, M.D.; Jackson, G.S. Thermochemical energy storage in strontium-doped calcium manganites for concentrating solar power applications. Sol. Energy 2017, 151, 1–13. [Google Scholar] [CrossRef]
- Silakhori, M.; Jafarian, M.; Arjomandi, M.; Nathan, G.J. Thermogravimetric analysis of Cu, Mn, Co, and Pb oxides for thermochemical energy storage. J. Energy Storage 2019, 23, 138–147. [Google Scholar] [CrossRef]
- Block, T.; Knoblauch, N.; Schmücker, M. The cobalt-oxide/iron-oxide binary system for use as high temperature thermochemical energy storage material. Thermochim. Acta 2014, 577, 25–32. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Schmücker, M.; Sattler, C. Exploitation of thermochemical cycles based on solid oxide redox systems for thermochemical storage of solar heat. Part 2: Redox oxide-coated porous ceramic structures as integrated thermochemical reactors/heat exchangers. Sol. Energy 2015, 114, 440–458. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Solé, A.; Fontanet, X.; Barreneche, C.; Jové, A.; Gallas, M.; Prieto, C.; Fernández, A.I. Thermochemical energy storage by consecutive reactions for higher efficient concentrated solar power plants (CSP): Proof of concept. Appl. Energy 2017, 185, 836–845. [Google Scholar] [CrossRef]
- Pagkoura, C.; Karagiannakis, G.; Zygogianni, A.; Lorentzou, S.; Kostoglou, M.; Konstandopoulos, A.G.; Rattenburry, M.; Woodhead, J.W. Cobalt oxide based structured bodies as redox thermochemical heat storage medium for future CSP plants. Sol. Energy 2014, 108, 146–163. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Design of Efficient Mn-based Redox Materials for Thermochemical Heat Storage at High Temperatures. In Proceedings of the 21st International Conference on Concentrating Solar Power and Chemical Energy Systems (SolarPACES), Cape Town, South Africa, 13–16 October 2015. [Google Scholar]
- Chen, Z.P.; Jiang, Q.Q.; Tong, J.H.; Yang, M.; Jiang, Z.X.; Li, C. Influences of morphology and structure on Mn-based multi-step thermochemical H2O splitting cycle. Sol. Energy 2016, 129, 236–243. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.J.; Bian, Z.G.; Yan, X.Y.; Wang, Z.Y.; Liu, W.Q. Thermochemical energy storage performances of Ca-based natural and waste materials under high pressure during CaO/CaCO3 cycles. Energy Convers. Manag. 2019, 197, 111885. [Google Scholar] [CrossRef]
- Sturzenegger, M.; Nüesch, P. Efficiency analysis for a manganese-oxide-based thermochemical cycle. Energy 1999, 24, 959–970. [Google Scholar] [CrossRef]
- Gokon, N.; Ohashi, F.; Sawaguri, H.; Hayashi, K. Comparative Study of Heat-Discharging Kinetics of Fe-Substituted Mn2O3/Mn3O4 Being Subjected to Long-Term Cycling for Thermochemical Energy Storage. Energies 2023, 16, 3367. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, P.; Gu, C.; Xu, H.; Zhou, J.; Xiao, G. Investigations on substituted Si-doped Mn2O3/Mn3O4 redox pair for thermochemical energy storage. J. Energy Storage 2023, 73, 108804. [Google Scholar] [CrossRef]
- Fetisov, V.B.; Fetisov, A.V.; Korchemkina, N.V.; Ovchinnikova, L.A.; Pastukhov, E.A.; Fishman, A.Y. Kinetics of redox processes in manganese oxides. Dokl. Phys. Chem. 2002, 387, 291–293. [Google Scholar] [CrossRef]
- Müller, D.; Knoll, C.; Artner, W.; Harasek, M.; Gierl-Mayer, C.; Welch, J.M.; Werner, A.; Weinberger, P. Combining in-situ X-ray diffraction with thermogravimetry and differential scanning calorimetry—An investigation of Co3O4, MnO2 and PbO2 for thermochemical energy storage. Sol. Energy 2017, 153, 11–24. [Google Scholar] [CrossRef]
- Block, T.; Schmücker, M. Metal oxides for thermochemical energy storage: A comparison of several metal oxide systems. Sol. Energy 2016, 126, 195–207. [Google Scholar] [CrossRef]
- Zhao, Q.; Shih, W.Y.; Shih, W.H. Effects of Processing Parameters on the Morphology of Precipitated Manganese Oxide Powders. Ind. Eng. Chem. Res. 2009, 48, 1490–1494. [Google Scholar] [CrossRef]
- Liu, L.; Xu, W.; Liu, H. Redox and melting characteristics of Mn-based ores for high-temperature thermochemical energy storage. Front. Energy Res. 2024, 12, 1361460. [Google Scholar] [CrossRef]




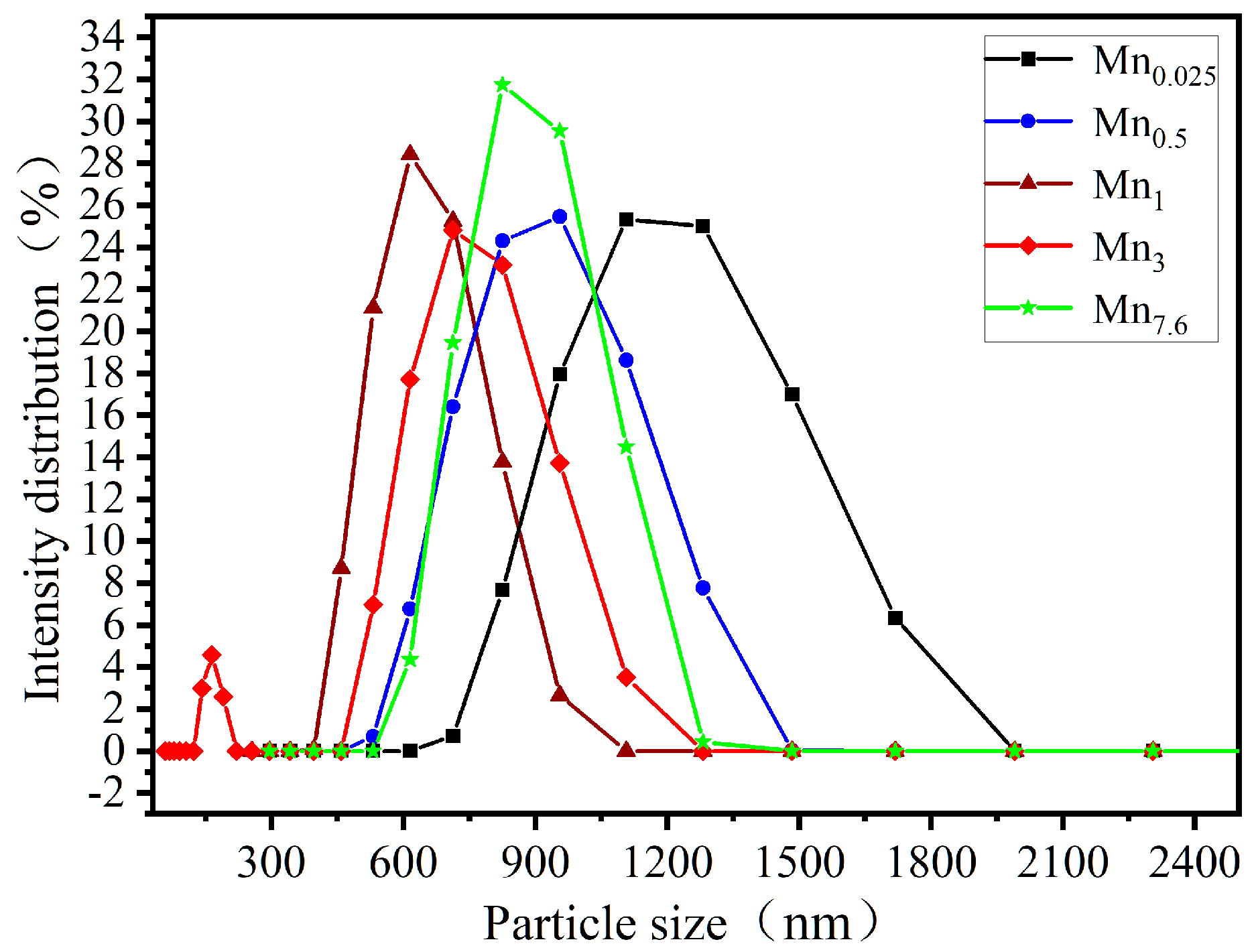
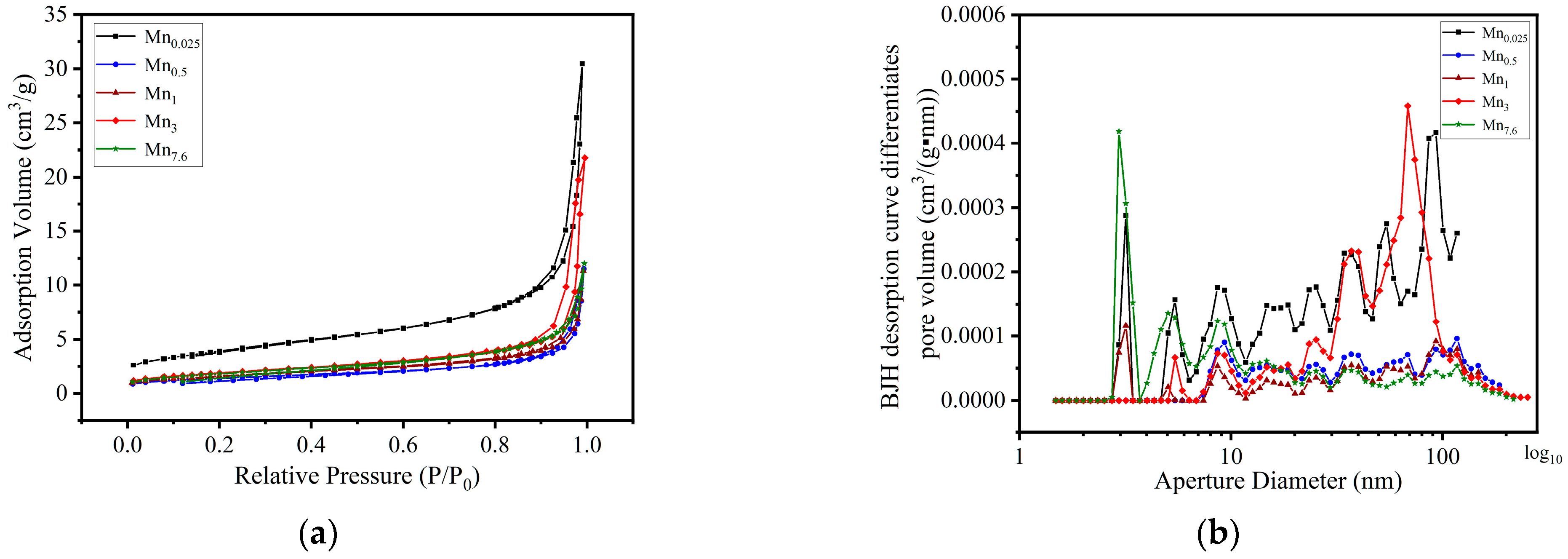
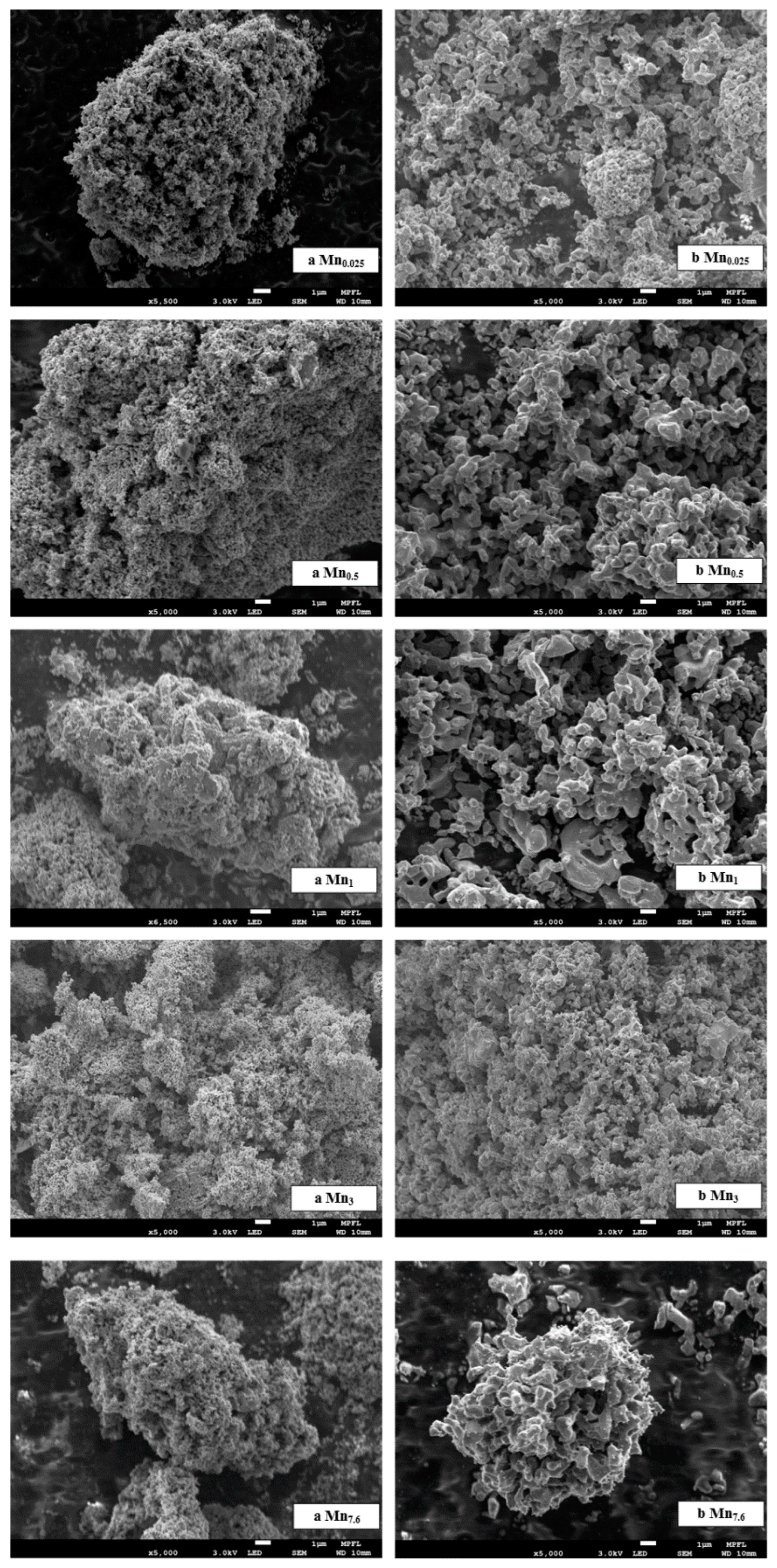

| Sample Name | [Mn(NO3)2]/M | Crystal Phase | d/nm | d50 */nm | SBET/(m2/g) | Pore Volume/(cm3/g) | Average Hole Diameter/nm |
|---|---|---|---|---|---|---|---|
| Mn0.025 | 0.025 | Mn2O3 | 40.357 | 1099 | 14.063 | 0.047 | 17.580 |
| Mn0.5 | 0.5 | Mn2O3 | 26.964 | 833 | 4.952 | 0.018 | 16.227 |
| Mn1 | 1 | Mn2O3 | 42.513 | 591 | 5.724 | 0.018 | 14.755 |
| Mn3 | 3 | Mn2O3 | 46.255 | 675 | 6.724 | 0.032 | 21.640 |
| Mn7.6 | 7.6 | Mn2O3 | 36.268 | 807 | 6.574 | 0.018 | 8.573 |
| α | Heating Rate (°C/min) | ||
|---|---|---|---|
| 2 | 5 | 10 | |
| T/K | |||
| 0.1 | 954.80 | 957.72 | 960.72 |
| 0.2 | 959.80 | 962.00 | 964.57 |
| 0.3 | 962.12 | 965.14 | 967.69 |
| 0.4 | 965.03 | 967.64 | 970.42 |
| 0.5 | 966.66 | 970.19 | 973.06 |
| 0.6 | 969.41 | 972.83 | 975.98 |
| 0.7 | 970.19 | 975.82 | 979.05 |
| 0.8 | 976.93 | 979.16 | 982.63 |
| 0.9 | 981.80 | 984.17 | 987.82 |
| α | Ozawa | KAS | ||
|---|---|---|---|---|
| Activation Energy E/(kJ/mol) | ||||
| 0.1 | 236.93 | 247.19 | 237.55 | 247.22 |
| 0.2 | 295.82 | 309.16 | 295.79 | 309.20 |
| 0.3 | 256.40 | 267.70 | 256.12 | 267.75 |
| 0.4 | 265.38 | 277.13 | 264.81 | 277.18 |
| 0.5 | 225.36 | 235.05 | 224.90 | 235.10 |
| 0.6 | 220.71 | 230.16 | 220.23 | 230.13 |
| 0.7 | 162.12 | 168.54 | 161.29 | 168.61 |
| 0.8 | 251.15 | 262.16 | 250.81 | 262.20 |
| 0.9 | 240.88 | 251.34 | 240.48 | 251.40 |
| Ave. | 239.42 | 249.82 | 239.11 | 249.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhao, M.; Wang, D. Sustainable Breakthrough in Manganese Oxide Thermochemical Energy Storage: Advancing Efficient Solar Utilization and Clean Energy Development. Sustainability 2025, 17, 3752. https://doi.org/10.3390/su17083752
Wang Z, Zhao M, Wang D. Sustainable Breakthrough in Manganese Oxide Thermochemical Energy Storage: Advancing Efficient Solar Utilization and Clean Energy Development. Sustainability. 2025; 17(8):3752. https://doi.org/10.3390/su17083752
Chicago/Turabian StyleWang, Zhizhen, Mengjiao Zhao, and Denghui Wang. 2025. "Sustainable Breakthrough in Manganese Oxide Thermochemical Energy Storage: Advancing Efficient Solar Utilization and Clean Energy Development" Sustainability 17, no. 8: 3752. https://doi.org/10.3390/su17083752
APA StyleWang, Z., Zhao, M., & Wang, D. (2025). Sustainable Breakthrough in Manganese Oxide Thermochemical Energy Storage: Advancing Efficient Solar Utilization and Clean Energy Development. Sustainability, 17(8), 3752. https://doi.org/10.3390/su17083752







